Abstract
The types and proportions of soluble sugar and organic acid in fruit significantly affect flavor quality. However, there are few reports on the crosstalk regulation between metabolism of organic acid and sugar in fruit. Here, we found that the overexpression of cytoplasmic malate dehydrogenase genes (MdcyMDHs) not only increased the malate content but also increased the sucrose concentration in transgenic apple calli and mature fruit. Enzyme activity assays indicated that the overexpression of MdcyMDH1 and MdcyMDH5 enhanced sucrose phosphate synthase (SPS) activity in transgenic materials. RNA-seq and expression analysis showed that the expression levels of SPS genes were up-regulated in MdcyMDH1-overexpressed apple fruit and MdcyMDH5-overexpressed apple calli. Further study showed that the inhibition of MdSPSB2 or MdSPSC2 expression in MdcyMDH1 transgenic fruit could reduce or eliminate, respectively, the positive effect of MdcyMDH1 on sucrose accumulation. Moreover, some starch cleavage-related genes (MdBAM6.1/6.2, MdBMY8.1/8.2, MdISA1) and the key gluconeogenesis-related phosphoenolpyruvate carboxykinase MdPEPCK1 gene were significantly up-regulated in the transcriptome differentially expressed genes of mature fruit overexpressing MdcyMDH1. These results indicate that alteration of malate metabolism mediated by MdcyMDH might regulate the expression of MdSPSs and SPS activity via affecting starch metabolism or gluconeogenesis, and thus accelerate sucrose synthesis and accumulation in fruit.
Introduction
The composition and ratio of soluble sugars and organic acids are essential factors determining the flavor and organoleptic quality of fruit, which is widely believed to be a crucial driver to boost consumption [1]. Physiological studies have revealed that during the ripening of fleshy fruit, the organic acid content decreases and the accumulation of soluble sugar increases [2–5], suggesting an interaction between sugar and organic acid metabolism in fruits. Although considerable progress has been made to understand the regulatory mechanism of sugar and malic acid metabolism and transport, our knowledge about the potential crosstalk between organic acid and sugar metabolism in fruit remains rather limited. Comprehending the regulation pathway between soluble sugar and organic acid metabolism is thus of vital significance for improving fruit quality and enhancing the production of high-quality fruit.
Organic acid levels are involved in maintaining pH and contribute to fruit sensorial quality [6]. Malate is a primary organic acid accumulated in the vacuole to form the acidity of the fruit cell [7]. Whether malate can massively accumulate in the fruit cell relies highly upon the activity of enzymes related to malic acid synthesis and transportation [6]. Cytoplasmic malate dehydrogenase (cyMDH) primarily catalyzes the conversion from oxaloacetic acid (OAA) to malic acid in pineapple (Ananas comosus) [8], alfalfa (Medicago sativa) [9], wheat (Triticum aestivum) [10], cordifolia (Aptenia cordifolia) [11] and apple (Malus domestica) [12]. Malate, as an indispensable precursor of many metabolic pathways in fruit cells, can be used as a respiratory substrate in the tricarboxylic acid (TCA) cycle in mitochondria [3], or be imported into the gluconeogenesis pathway and be converted into soluble sugars [13].
The metabolism and transportation of organic acids and soluble sugars are mostly independent from each other. However, some studies have shown an intersection between sugar and acid metabolism and regulation. Echeverria pointed out that changes in organic acid content affect sucrose synthesis and catabolism in fruit [14]. In apple, when more photoassimilates were converted to malate, the overall distribution of assimilates to soluble sugar and starch would be changed at late fruit ripening [4]. When the mitochondrial malate dehydrogenase gene mMDH was targeted by RNA interference in tomato fruit, malate content was increased and soluble sugar content was decreased through a change in the redox state of cells [15]. In strawberry, overexpression of FaMYB44.2 decreased sucrose accumulation by inhibiting the expression of the sucrose phosphate synthase (SPS) gene FaSPS and decreased the malate content in fruit [16]. These studies above indicate that organic acids and sugars in fruit are not metabolically isolated from each other. This is helpful to improve the fruit quality and elucidate the potential mechanisms of the crosstalk between carbohydrates and organic acids in fruit.
Apple is one of the world’s best-selling and most popular fleshy fruits. Unlike citrus, tomato and some other fruits, approximately 85% of organic acids in apple fruits are malic acid, which is mainly present as malate, while the soluble sugars are mainly fructose, sucrose, and glucose [7]. In the fruit of 155 apple accessions, the malic acid content showed a negative correlation with glucose content, whereas glucose content negatively correlated with sucrose content [5]. Interestingly, overexpression of a cytoplasmic malate dehydrogenase MdcyMDH1 in apple calli or tomato fruit increased not only malic acid content but also sucrose content [12]. A recent study showed that the transcription factor MdbHLH3 directly activated the transcriptional expression of MdcyMDH1 to coordinate carbohydrate distribution and malate accumulation in apple [17]. These results suggest that cytoplasmic malate dehydrogenase might be a node connecting sugar and acid in apple, while knowledge is scarce about how malate contributes to sugar metabolism, accumulation, and related processes.
In present study, MdcyMDH1 overexpressed transgenic ‘Gala’ apples were used to explore the mechanism of malate affecting fruit soluble sugar. It was found that MdcyMDH1 accelerated sucrose anabolism in the cytoplasm by affecting both the expression of the apple sucrose phosphate synthase gene MdSPSB2/C2 and the SPS activity, and thus increased the sucrose content in fruit. Our findings improve our insight into the crosstalk between malate and sucrose in apple and provide potential pathways for optimizing the sugar and acid ratio inside fruit, which could improve fruit flavor quality.
Results
Overexpression of MdcyMDH1 altered sugar content of apple fruit
Two stable transgenic lines overexpressing MdcyMDH1 driven by the CaMV35S promoter in the ‘Gala’ apple (MDH1–2, MDH1–3) were generated on the basis of previous experiments [12] (Fig. 1a), and no significant changes of per fruit weight between WT and two MdcyMDH1-overexpressed transgenic apples were observed (Fig. S1, see online supplementary material). To further explore how MdcyMDH1 affects malate and sugar content in apple fruit, wild-type (WT) ‘Gala’ and transgenic apple fruits were harvested at different stages of fruit development (30, 60, and 90 days after bloom) for analysis of the MdcyMDH1 expression level, MDH activity, and malate content.
Figure 1.
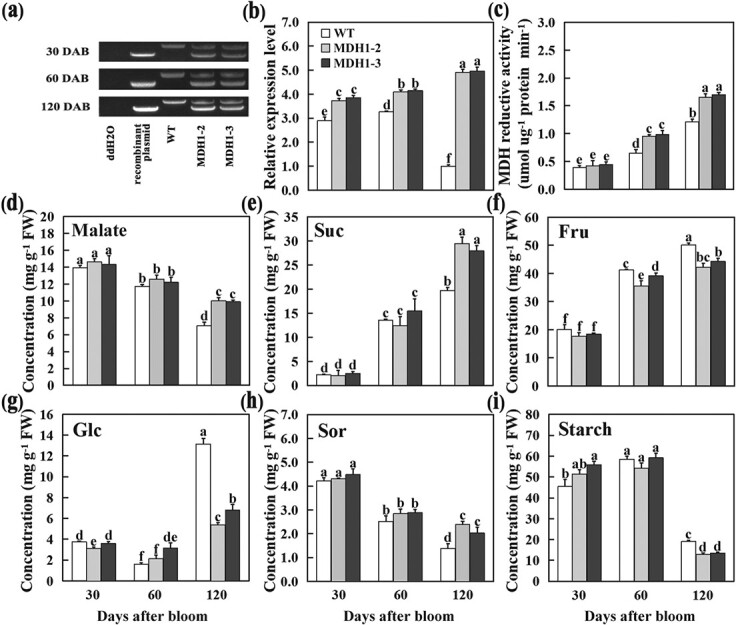
Functional analysis of MdcyMDH1. a Identification of MdcyMDH1 overexpression in apple (MDH1–2 and MDH1–3) by PCR amplification. Cross-intron primer pairs were crafted to amplify coding sequences and genome sequences using WT and transgenic fruit DNA as templates. ddH2O and recombinant plasmid were used as a negative and positive control, respectively. b The mRNA relative expression levels of MdcyMDH1 in wild-type (WT) and MdcyMDH1-overexpressed apple fruits at different stages of fruit development (30, 60, and 90 days after bloom). c–i MDH reductive activity, malate and carbohydrate levels in WT and MdcyMDH1-overexpressed apple fruits at different stages of fruit development. c MDH reductive activity. d Malate content. e Sucrose (Suc) content. f Fructose (Fru) content. g Glucose (Glc) content. h Sorbitol (Sor) content. i Starch content. The error bars show the standard deviation (SD) of data from three independent replicates. Different letters denote a significant difference (P < 0.05).
In WT apple fruit, the expression of MdcyMDH1 was high at the early stage of fruit development, and sharply decreased by 120 days after bloom (DAB), while in the fruits of the MdcyMDH1-overexpressed lines, the expression of MdcyMDH1 remained high throughout fruit development (Fig. 1b). MDH reductive activity was significantly increased in the fruit overexpressing MdcyMDH1 at 60 and 120 DAB compared with WT (Fig. 1c). In WT fruit, malate content continued to decrease with fruit development, while the overexpression of MdcyMDH1 significantly increased the malate content only in mature fruit at 120 DAB, but had little effect on the malate content during early fruit development (Fig. 1d). In MdcyMDH1-overexpressed fruit, as malate content began to increase compared to WT, sucrose and sorbitol content were also increased significantly, while glucose and starch content were decreased at 120 DAB (Fig. 1e–i). These results suggest that MdcyMDH1 functions to influence malate content and alter sugar content in apple fruit.
MdcyMDH1 overexpression changes activity of enzymes involved in sugar metabolism
To explore the reasons for the altered sugar accumulation in MdcyMDH1-overexpressed apple fruit, the activities of enzymes involved in sugar metabolism were evaluated at various stages of fruit development. Sorbitol dehydrogenase (SDH) activity dropped continuously during fruit development and was significantly higher in MdcyMDH1-overexpressed fruit than in control (Fig. 2g). The activities of vacuolar acid invertase (AINV) and neutral invertase (NINV), both involved in sucrose breakdown, declined in MdcyMDH1 transgenic fruit compared with control at 30 and 60 DAB, while the activity differences were not apparent between control and transgenic fruits at 120 DAB (Fig. 2c and d). Cell wall invertase (CWINV) activity decreased sharply from 30 to 60 DAB and then maintained relative stability to maturity in WT fruit, while decreasing slightly in transgenic fruit compared with the control only at 60 DAB (Fig. 2h). Sucrose synthase (SUSY), another crucial enzyme for sucrose decomposition in cell, showed a relatively stable activity in MdcyMDH1-overexpressed fruit but a sharp decrease in WT fruit over the fruit development period (Fig. 2b). The activity of SPS, associated with sucrose synthesis, continued to be elevated throughout fruit development and was significantly higher in MdcyMDH1-overexpressed fruit at 120 DAB than in control (Fig. 2a). The activity of hexokinase (HK) was significantly higher in MdcyMDH1-overexpressed fruit than in WT fruit at 60 and 120 DAB (Fig. 2f). Fructokinase (FRK) activity continued to decrease and was significantly higher in transgenic fruit than in control at 30 DAB (Fig. 2e).
Figure 2.

Measurement of enzyme activities related to sugar metabolism during fruit development in WT and MdcyMDH1-overexpressed apple fruit. a SPS, sucrose phosphate synthase; b SUSY, sucrose synthase; c AINV, vacuolar acid invertase; d NINV, neutral invertase; e FRK, fructokinase; f HK, hexokinase; g SDH, sorbitol dehydrogenase; h CWINV, cell wall invertase. Error bars show the standard deviation (SD) on the basis of three independent replicates. Different letters denote a significant difference (P < 0.05).
MdSPSs were up-regulated in MdcyMDH1-overexpressed apple fruit
To determine key genes during the process that up-regulated malate affected sugar content in MdcyMDH1-overexpressed apple, RNA-seq was conducted to profile gene expression in the WT and two MdcyMDH1 transgenic apple fruits at 120 DAB. A total of 3815 differentially expressed genes (DEGs) (P-value <0.05 and |log2 (fold change)| ≥1) including 2508 up-regulated genes and 1307 down-regulated genes were found between control and two MdcyMDH1 transgenic apple fruits (Fig. S2a, see online supplementary material). Among these DEGs, 24 genes were involved in major carbohydrate metabolism, and 19 genes were relevant to the tricarboxylic acid cycle (Fig. S2b, see online supplementary material).
To verify the credibility of the RNA-seq expression profiling data, some important DEGs related to cytoplasmic malate metabolism were picked for expression analysis by quantitative real-time polymerase chain reaction (qRT-PCR). The expression levels of MdcyMDH1, MdMDH2, MdPK1/2, and MdPEPCK1 were much higher in the two MdcyMDH1 transgenic apple fruit (MDH1–2 and MDH1–3) compared to their levels in WT, while the expression levels of MdPEPC and MdPEPCK2 in transgenic fruit were lower (Fig. S3, see online supplementary material; Fig. 1b). This qRT-PCR data was in agreement with the RNA-seq data (SI Appendix, Fig. S3, see online supplementary material). In addition, the overexpression of MdcyMDH1 in the cytoplasm increased the expression of MdNAD-ME in mitochondria, leading to the up-regulation of MdCS gene expression in the tricarboxylic acid cycle pathway (Fig. 3). Taken together, these results indicate that the overexpression of MdcyMDH1 accelerates malate metabolism in both cytoplasm and mitochondria.
Figure 3.

Effect of overexpression of MdcyMDH1 on the expression of genes related to major sugar and acid metabolism in transgenic apple fruit. In each heatmap, the left-most box represents the expression level in WT ‘Gala’ fruits, set to 1. α-OGDH, α-oxoglutarate dehydrogenase; AINV, acid invertase; cMDH, cytoplasmic malate dehydrogenase; CS, citrate synthase; FBPase, fructose-1, 6-bisphosphatase; FRK, fructokinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HK, hexokinase; ICDH, isocitrate dehydrogenase; NAD-ME, NAD-malate enzyme; NADP-ME, NADP-malate enzyme; NINV, neutral invertase; PEPC, phosphoenolpyruvate carboxylase; PEPCK, phosphoenolpyruvate carboxykinase; PFK, phosphofructokinase; PGI, phosphoglucoisomerase; PGK, phosphoglycerate kinase; PGM, phosphoglucose mutase; PK, pyruvate kinase; PPDK, pyruvate phosphate dikinase; SCS, succinyl-coa synthase; SDH, sorbitol dehydrogenase; SPP, sucrose-phosphate phosphatase; SPS, sucrose phosphate synthase; SuDH, succinate dehydrogenase; SUSY, sucrose synthase; UGP, uridine diphosphate glucose pyrophosphorylase.
For the DEGs involved in major carbohydrate metabolism, the expression of two sucrose synthase genes (MdSUSYs) increased, while two neutral invertases (MdNINVs) decreased. The expression of two of the three fructokinases (MdFRKs) were decreased (Fig. 3). It is worth noting that three key genes in sucrose synthesis, the MdSPSs, were significantly up-regulated in the transgenic lines (Fig. 3), which was consistent with the increased SPS enzyme activity in the MdcyMDH1-overexpressed apple fruit (Fig. 2a). This result may be an important reason for the increase of sucrose levels in mature MdcyMDH1-overexpressed apple fruit.
Functional verification of MdSPSs in affecting sucrose content
The apple MdSPS family genes were obtained from the Apple Genome Database (https://www.rosaceae.org), and phylogenetic analysis was carried out with the four SPS proteins in Arabidopsis thaliana [18]. The MdSPS proteins were named based on their similarity to Arabidopsis SPS protein (Fig. S4, see online supplementary material). Subsequently, qRT-PCR was conducted to verify the expression levels of these three MdSPS genes in MdcyMDH1 transgenic ‘Gala’ fruit. The results show that the expression levels of MdSPSB2 and MdSPSC2 in transgenic fruit increase by about four times as their levels in WT (Fig. S5, see online supplementary material).
To confirm the function of MdSPS in altering sugar accumulation, the MdSPSB2 and MdSPSC2 overexpression vectors pCAMBIA2300-MdSPSB2 and pCAMBIA2300-MdSPSC2 were transformed into apple calli, obtaining MdSPSB2- and MdSPSC2-overexpressed calli lines. qRT-PCR analysis showed markedly elevated transcription levels of MdSPSB2 and MdSPSC2 in transgenic calli compared with the control (Fig. 4a). Overexpression of MdSPSB2/MdSPSC2 significantly enhanced sucrose levels in transgenic calli, while a drastic decrease in glucose content was also observed in MdSPSC2-overexpressed calli (Fig. 4a).
Figure 4.
Carbohydrate levels in MdSPSB2 and MdSPSC2 transgenic lines. a The mRNA relative expression level of MdSPSB2/C2 and concentrations of sucrose, fructose, and glucose in control (P2300c) and MdSPSB2- and MdSPSC2-overexpressed apple calli (SPSB2c-1,2,3 and SPSC2c-1,2,3). b The mRNA relative expression level of MdSPSB2/C2 and concentrations of sucrose, fructose, glucose, and sorbitol in control (P2300) and MdSPSB2- and MdSPSC2-overexpressed apple flesh (SPSB2–1,2,3 and SPSC2–1,2,3). The error bars show the standard deviation (SD) of three independent replicates. Different letters denote a significant difference (P < 0.05).
To further characterize the effect of MdSPSB2 and MdSPSC2 on sugar levels in apple fruit, the overexpression vectors pCAMBiA2300-MdSPSB2/MdSPSC2 were transiently transformed into ‘Gala’ apple fruit via an Agrobacterium-mediated method. The overexpression of MdSPSB2 or MdSPSC2 elevated the accumulation of sucrose while the overexpression of MdSPSC2 decreased the glucose content in ‘Gala’ apple flesh (Fig. 4b), which was similar with the trend of altered sugar content in apple calli (Fig. 4a). Additionally, increased sorbitol content was observed in MdSPSB2- and MdSPSC2-overexpressed apple flesh (Fig. 4b). These results indicate that MdSPS genes affect the changes of soluble sugars seen in apple calli and fruit.
Sucrose concentration in the sps silencing of MdcyMDH1-transgenic apple
To genetically clarify if the effect of MdcyMDH1 overexpression on the sucrose level is dependent on the high expression of MdSPSB2 and MdSPSC2, Agrobacterium strain GV3101 carrying recombinant virus plasmids pTRV2-MdSPSB2 or pTRV2-MdSPSC2 were infiltrated individually into WT and MdcyMDH1 transgenic mature ‘Gala’ fruits, with the empty vector pTRV2 as control, obtaining MdSPSB2 and MdSPSC2 silenced lines in the background of WT and MdcyMDH1-overexpressed fruits (WT-spsB2/C2 and MDH1-spsB2/C2). In this study, silencing of MdSPSB2/MdSPSC2 in both WT and MdcyMDH1-overexpressed fruits decreased the sucrose concentration (Fig. 5d).
Figure 5.

Effects of VIGS-mediated silencing of MdSPSB2 and MdSPSC2 on the soluble sugar content in WT and MdcyMDH1-overexpressed apple fruits. a The mRNA relative expression levels of MdcyMDH1, MdSPSB2, and MdSPSC2 in WT and transgenic apple flesh (WT-spsB2, WT-spsC2, MDH1, MDH1-spsB2, MDH1-spsC2). b–f Malate and carbohydrate levels in WT and transgenic apple flesh. b Malate content. c Fructose content. d Sucrose content. e Glucose content. f Sorbitol content. The error bars show the standard deviation (SD) of three independent replicates. Different letters denote the significant difference (P < 0.05).
Notably, the 50% ~ 60% reduction of the MdSPSB2/MdSPSC2 transcripts in the WT fruits resulted in about a 20% reduction in sucrose content compared with that in the WT apple (Fig. 5a and d). Sucrose content in MDH1-spsB2/C2 apples decreased by 30% ~ 35% compared with MdcyMDH1-overexpressed fruit, while the difference in sucrose content between WT-spsB2/C2 and MDH1-spsB2/C2 transgenic fruit was not significant (Fig. 5d). Furthermore, the silencing of MdSPSB2/MdSPSC2 did not cause significant changes in the malate contents in either the WT or MdcyMDH1-overexpressed fruits (Fig. 5b). These results indicate that upregulated MdSPSB2/MdSPSC2 expression is the primary contributor altering the sucrose content in the mature apple fruit overexpressing MdcyMDH1.
Accumulation of malate in cytoplasm increased the sucrose concentrations in apple fruit
Previous studies have shown that MdWRKY126 enhances malate accumulation in apple calli and fruits by directly activating the expression of the cytoplasmic malate dehydrogenase gene MdcyMDH5 [19]. In this study, the overexpression of MdcyMDH5 resulted in the increased contents of sucrose, glucose, and fructose in transgenic apple calli and fruit (Fig. 6). These malate and sucrose content trends in MdcyMDH5 transgenic lines were consistent with that in MdcyMDH1-overexpressed fruit. These results suggest that an increase of malate content in the cytoplasm can promote the accumulation of sucrose.
Figure 6.

Malate and soluble sugar levels in MdcyMDH5 transgenic lines. a The concentrations of malate, sucrose, fructose, and glucose in control (P2300c) and MdcyMDH5-overexpressed apple calli (MDH5c-1,2,3). b The concentrations of malate, sucrose, fructose, and glucose in control (P2300a) and MdcyMDH5-overexpressed apple fruit (MDH5a-1,2,3). The error bars show the standard deviation (SD) of three independent replicates. Asterisks indicate significant difference at P < 0.05.
The enzyme activities and expression levels of eight genes in the SPS family were determined in MdcyMDH5 transgenic calli. The SPS and SUSY activities in MdcyMDH5 transgenic calli were significantly increased compared with the control, while the activities of CWINV and FRK decreased (Fig. S6, see online supplementary material). Meanwhile, the overexpression of MdcyMDH5 significantly increased the transcript levels of MdSPSA2.3 and MdSPSB1 in transgenic calli (Fig. S7, see online supplementary material). These results further prove that the accumulation of malate in the cytoplasm increases sucrose content by up-regulating the expression of SPS genes.
Discussion
The contents of soluble sugars and organic acids and their ratio largely affect the taste, quality, and commercial value of fresh apple [1]. Therefore, it is of great importance to explore the mutual regulatory mechanism of sugar and acid metabolism in apple fruit. Soluble sugars and organic acids in apple are not isolated from each other [4, 17]. Yao’s study showed that overexpression of cytoplasmic malate dehydrogenase MdcyMDH1 in apple calli improved malate accumulation and also caused a change of sugar content [12], but the specific mechanism underlying this phenomenon was not pursued. To explore how acid metabolism affects sugar content, here, apple fruit stably overexpressing MdcyMDH1 were used as material to investigate the effect of cytoplasmic malate dehydrogenase gene on sugar content.
MDH plays a vital role in seed germination [20], cell growth [21], embryo development [22], plant development and maturation [23], and stress resistance [24, 25]. Studies have showed that the MDH genes encoded a family of oxidoreductases localized to different organelles (cytoplasm, chloroplast, mitochondria, peroxisome) and use NAD+ or NADP+ as a coenzyme [9]. Cytoplasmic MDH (cyMDH) has been demonstrated to mainly catalyze the conversion from OAA to malate in most plants [8–10]. Overexpression of MdcyMDH1 or MdcyMDH5 increased malate accumulation in apple calli [12, 19]. Omics analysis indicated that mitochondrial MDH (mMDH) mainly catalyzed malate degradation during fruit ripening [3]. However, some studies have shown that mMDH could also catalyze the synthesis of malate in the presence of TCA reversible reaction conditions [26]. Overexpression of MdmMDH12 in apple calli accelerated malate synthesis [27].
Most studies on the functions of acid metabolism genes have relied on transient transformation of fruit or stable expression of apple calli or a model plant due to the difficulty of stable transformation in apple and the 4–6 year juvenile phase for fruit set [17, 27]. In this study, the malate content in fruits grown on stable MdcyMDH1-overexpressed apple trees was determined. During fruit development, malate accumulated during the early stage and decreased by the late stage. This decrease of malate content may be caused by the increase of basal metabolism or the formation of sugars or secondary compounds during fruit ripening. The content of malate in mature MdcyMDH1-overexpressed fruit was significantly higher than that in WT, indicating the role of MdcyMDH1 in malate accumulation of fruit. However, there was no significant difference in malate content at other periods, which may be because the expression level of MdcyMDH1 in both transgenic and WT fruit was maintained at a high state before fruit ripening and MdcyMDH1 expression level sharply decreased only in WT mature fruit. It is noteworthy that the overexpression of MdcyMDH5 and MdcyMDH1 all increased the accumulation of malate and sucrose in apple fruit, so we believed that the accumulation of malate might cause the change of sucrose content.
The content of soluble sugars in sink organs may be related to the accumulation of photosynthetic assimilates, but it was detected that the overexpression of MdcyMDH1 did not significantly increase leaf photosynthesis [24], and the transgenic trees do not show difference in growth and development compared with wild type, which excluded the possibility to some extent that the overexpression of MdcyMDH1 accelerated the accumulation of carbohydrates in source tissues. On the other hand, change of soluble sugar content in fruit vacuoles is highly regulated by sugar metabolism through various sugar synthesis and degradation genes [28, 29]. Transcriptomic differential expression analysis of MdcyMDH1-overexpressed apple revealed that the expression levels of MdSPSB2 and MdSPSC2, which are homologous to the sucrose phosphate synthase genes AtSPS3F and AtSPS4F in A. thaliana [18], were significantly up-regulated (Fig. 3, Fig. S5, see online supplementary material). SPS is a key rate-limiting enzyme in the cytoplasmic sucrose synthesis pathway, catalyzing the synthesis of sucrose-6-phosphate from UDPG and F6P, followed by dephosphorylation to sucrose in the presence of sucrose-phosphate phosphatase (SPP) [30]. Studies showed that SPS gene expression was significantly up-regulated when sucrose content increased greatly during fruit development of bananas [31], melon [32], citrus [33], and sugarcane [34]. Similarly, in our study, SPS activity increased with fruit ripening. In mature apple fruit, the overexpression of MdSPSB2 and MdSPSC2 significantly accelerated sucrose accumulation (Fig. 4), which indicated that MdSPSB2 and MdSPSC2 were involved in the synthesis and accumulation of sucrose in apple. In addition, the silencing of MdSPSB2 and MdSPSC2 in the MdcyMDH1-overexpressed background of transgenic apple fruit significantly reduced sucrose level (Fig. 5). These results show that the overexpression of MdcyMDH1 affects both the expression of MdSPSB2 and MdSPSC2 genes and the SPS activity, accelerating the accumulation of sucrose content in apple fruit.
The homeostasis of sugars and organic acids in the cytoplasm are regulated by multiple metabolism and transcription [6, 35]. Therefore, it is not unexpected that a large number of sugar- and acid-associated DEGs are present in the transcriptome of MdcyMDH1-overexpressed apple fruit (Fig. 3). In addition to MdSPSB2 and MdSPSC2, several genes related to starch degradation (BAM6.1/6.2, BMY8.1/8.2, ISA1) were also up-regulated (Fig. 3), which might be another reason for the increased sucrose content in MdcyMDH1 transgenic fruit. Studies have shown that SPS played an important role in carbon source allocation [36]. Higher SPS activity was associated with lower starch accumulation and higher sucrose content [37, 38]. Interestingly, the expression of the cytoplasmic localized phosphoenolpyruvate carboxykinase MdPEPCK was up-regulated (Fig. 3). Radiolabelling studies have clearly indicated that a portion of malate in ripe grape skins was converted to sugar via the gluconeogenic pathway, and PEPCK was considered the key enzyme converting malate to sugar [39]. In general, organic acid stored in ripe fruit provides only a small part of the substrate for fruit metabolism, while most of the carbon was sourced from sugar [40]. However, studies on peach and grape have shown that enhanced malate efflux from the vacuole induced gluconeogenesis, increasing the conversion from malic acid to sugar under certain circumstances [41, 42], which supported our result that the overexpression of MdcyMDH1 greatly accelerated the accumulation of malate in the cytoplasm and induced the up-regulated expression of MdPEPCK, promoting the accumulation of sucrose.
Biochemical studies have shown that sugar homeostasis in cytoplasm is essential for basic cellular function, so the levels of various sugar components in the cytoplasm need to be strictly controlled within a certain range [35]. The synthesis of sucrose in pulp cells of MdcyMDH1-overexpressed apple consumed a large amount of glucose and fructose, but the reduction of fructose content in the fruit was slight. Unique to Rosaceae fruit, about 80% of fructose in apple fruit is produced by sorbitol degradation under the catalysis of SDH [43]. Among the DEGs in the MdcyMDH1-overexpressed fruit, we found three predicted sorbitol dehydrogenase genes that were significantly up-regulated (Fig. 3). These may accelerate sorbitol cleavage to balance fructose content in the cytoplasm in MdcyMDH1 transgenic apple fruit. Because glucose, a direct carbon source, enters the glycolysis pathway to provide energy and serves as an intermediate product for cell metabolism, the glucose content in mature MdcyMDH1-overexpressed fruit was significantly reduced.
In summary, our study suggests that the cytoplasmic malate dehydrogenase gene MdcyMDH1 acidifies apple fruit by accelerating the conversion of OAA to malate in cytoplasm. Meanwhile, MdcyMDH1 overexpression upregulated the expression of the sucrose phosphosynthase genes MdSPSB2 and MdSPSC2, leading to significantly enhanced accumulation of sucrose in apple fruit. This phenomenon was also observed in transgenic apple calli overexpressing the cytoplasmic MdcyMDH5 gene, suggesting that the effect of cytoplasmic malate dehydrogenase on sucrose accumulation might be widespread. These findings are an initial exploration of the mechanism through which organic acid metabolism affects sugar content in apple fruit.
Materials and methods
Plant materials and growth conditions
‘Orin’ apple calli (Malus × domestica) were subcultured every 20 days on Murashige and Skoog (MS) solid medium supplemented with 1.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) and 1.0 mg/L 6-benzyla-minopurine (6-BA) at 23°C in dark conditions.
WT and MdcyMDH1-overexpressed ‘Gala’ apple trees [MDH1–2 (4 trees), MDH1–3 (3 trees)] were planted in 2013 in a garden belonging to Shandong Agricultural University, Tai’an (36°16’N, 117°6’E), Shandong, China at a spacing of 2.5 m × 1 m. The WT and transgenic trees are growing in a net room covered with 10-μm translucent nylon fabric to avoid genetic contamination for surrounding trees by pollen spread. The trees were trained as a spindle system. The crop-load was adjusted by hand-thinning to one fruit per 15 cm in crown to achieve a 10-mm king fruit size. During the growing season, fungicides and pesticides were sprayed at regular intervals throughout the growing season. The apple fruits were sampled in 2018. WT and MdcyMDH1 transgenic ‘Gala’ apple fruit were collected at 30, 60, and 120 DAB for qRT-PCR, RNA-Seq and virus-induced vector injection assay. Each sample was sampled from six fruits with similar growth state on three trees and frozen immediately in liquid nitrogen and then stored at −80°C.
Determination of organic acid, sugar, and starch content
Organic acid and sugar content were evaluated using GC–MS (gas chromatography–mass spectrometry) following the procedures as described by Li et al. [29]. Soluble sugars and organic acids were extracted with 1.4 mL of 75% (V/V) methanol for 0.1 g of sample, with 400 ppm ribitol was used as internal standard, by shaking at 900 rpm at 70°C for 30 min. The supernatants were separated and transferred into a mixture of 750 μL chloroform (CHCl3) and 1.4 mL ddH2O. After mixing and centrifugation, samples of 2 μL and 50 μL of the supernatant were dried and then derivatized with 40 μL methoxyamine hydrochloride and 60 μL N-methyl-N-trimethylsilyl-trifluoroace-tamide (MSTFA). Analysis of soluble sugar and organic acid contents was conducted on a GCMS-2010SE instrument (Shimadzu Corporation, Kyoto, Japan).
The determination of starch content was performed as described by Li et al. [28]. The remaining precipitate after the extraction with 75% methanol for the determination of sugar and acid was repeatedly cleaned with 80% (v/v) ethanol three times, mixed with 0.1 M KOH and boiled for 30 min to gelatinize the starch. α-amylase at pH 4.5 was added and incubated at 55°C for 1 h. Reducing sugar content was determined by 3, 5-dinitrosalicylic acid (DNS), and then converted to starch content.
RNA-Seq and data assays
Total RNAs were extracted from WT and MdcyMDH1-overexpressed apple fruits at 120 DAB using RNAprep plant kit (Tiangen, Beijing, China). The RNA-Seq analysis was according to the methods as described by Zhu et al. [44].
Phylogenetic analysis of the MdSPS family
MdSPS gene sequences were identified from Malus × domestica genome, GDDH13 v1.1 (https://www.rosaceae.org) by a protein–protein BLAST (BLASTp) analysis with the four AtSPS gene sequences reported in A. thaliana as templates. Phylogenetic analyses were performed with MEGA6.0 software (http://www.megasoftware.net/) via maximum likelihood method based on 1000 bootstrap replicates.
Vector constructs and apple calli transformation
To generate the MdSPSB2 and MdSPSC2 transgenic apple calli, the complete CDS of MdSPSB2 and MdSPSC2 were cloned into the pCAMBIA2300 expression vector activated by CaMV35S promoter, forming the resulting vectors pCAMBIA2300-MdSPSB2 and pCAMBIA2300-MdSPSC2, which were then transformed into ‘Orin’ apple calli via an Agrobacterium-mediated method [45].
RNA extraction and qRT-PCR reactions
Total RNA was extracted from apple calli and fruit flesh using RNAprep Plant Kit (Tiangen, Beijing, China) following the manufacturer’s instructions. Transcriptional expression of essential genes associated with sugar and acid metabolism was detected by quantitative real-time polymerase chain reaction (qRT-PCR) according to a previous report [46]. Each reaction was repeated three times to minimize error, and the transcript level was calculated via 2-△△Ct method with MdActin (MDP0000752428) as internal reference. Primer sequences were designed through web software (http://biotools.nubic.northwestern.edu/OligoCalc.html), and their sequences are listed in Table S1, see online supplementary material.
Determination of sugar and acid metabolism enzyme activities
MDH and sugar-related enzymes were extracted following the method of Wang et al. [24] and Li et al. [29], respectively. The whole process of enzyme extraction was performed at 4°C. Apple flesh (2 g) was extracted with 5 mL enzyme extraction buffer. The supernatant was treated by Sephadex G25 PD-10 desalination column (GE Healthcare, Buckinghamshire, UK) and collected for use.
For MDH reducing activity, the reaction system consisted of 50 mM Tris–HCl (pH 7.8), 2 mM MgCl2, 0.5 mM EDTA, 0.2 mM NADH, and 50 μL desalted extract. The reaction at 30°C was initiated after adding 2 mM oxaloacetate (OAA). The absorbance value at 340 nm was measured at 30 s intervals for 5 min by spectrophotometer.
For SDH activity, the reaction system consisted of 100 mM Tris–HCl (pH 9.6), 300 mM sorbitol, 1 mM NAD+ and 0.2 mL desalted extract. The absorbance value at 340 nm was measured by spectrophotometer after 3 min. The SDH activity was calculated according to the produced NADH amount.
For SPS activity, the reaction system consisted of 50 mM Hepes-KOH (pH 7.4), 1 mM EDTA, 4 mM MgCl2, 20 mM glucose 6-phsophate (G6P), 4 mM fructose 6-phosphate (F6P), 3 mM UDP-glucose (UDPG) and 250 μL desalted extract and was held at 27°C in a waterbath for 30 min, boiled for 3 min, then centrifuged. Supernatant (75 μL) was placed into a 1 mL mixture containing 50 mM Hepes-KOH (pH 7.0), 5 mM MgCl2, 0.3 mM NADH, 0.8 mM PEP, 14 U LDH, and 4 U PK. The absorbance value at 340 nm was measured by spectrophotometer.
For CWINV and AINV activity, the reaction system, consisting of 100 mM phosphate–citrate buffer (pH 4.8), 0.1 M sucrose and 125 μL desalted extract, was placed in a 37°C waterbath for 1 h, then 0.5 mL DNS was added and boiled for 5 min. The absorbance value at 540 nm was determined by spectrophotometer. The NINV activity was determined in a similar way to that of CWINV, except that the phosphate–citrate buffer was replaced by 100 mM Hepes-KOH (pH 7.2). Enzyme activity was calculated according to the produced glucose amount.
For SUSY activity, the reaction system consisted of 80 mM MES (pH 5.5), 5 mM UDP, 100 mM sucrose and 100 μL desalted extract was incubated at 27°C in a waterbath for 30 min. Then, the mixture was boiled for 5 min with 0.5 mL DNS. The absorbance value at 340 nm was measured by spectrophotometer.
For HK activity, the reaction system consisted of 50 mM Tris–HCl (pH 8.0), 2.5 mM ATP, 0.33 mM NAD+, 4 mM MgCl2, 1 U of G6PDH, 1 mM glucose, and 25 μL desalted extract was incubated at 30°C in a waterbath for 5 min. For FRK activity, the reaction system consisted of 50 mM Tris–HCl (pH 8.0), 2.5 mM ATP, 0.33 mM NAD+, 4 mM MgCl2, 1 U of G6PDH, 0.4 mM fructose, 1 U PGI and 60 μL desalted extract was incubated at 30°C in a waterbath for 5 min. The absorbance value at 340 nm was measured by spectrophotometer.
Virus-induced silencing of genes in apple fruit
Specific antisense sequences of MdSPSB2 (256 bp) and MdSPSC2 (229 bp) were cloned and introduced into the tobacco rattle virus vector pTRV2, forming resulting vectors pTRV2-MdSPSB2 and pTRV2-MdSPSC2. These recombinant vectors were transformed into Agrobacterium strain GV3101 and individually injected into WT and MdcyMDH1-overexpressed apple fruit according to previous description [19]. The treated apple fruits were initially kept out of light for 24 hours to allow Agrobacterium penetration and then transferred to natural light for three days at room temperature. The area surrounding the fruit injection site was then sampled to analyse gene expression and the contents of malate and soluble sugar.
Statistical analysis
Data analysis was carried out via one-way ANOVA or independent t-tests with significance level accepted at P < 0.05 using IBM SPSS Statistics 21. The values were presented as the mean ± standard deviation (SD) of biological triplicates.
Acknowledgements
This work was supported by the Program for the Shaanxi science and technology innovation team project (2022TD-12), the National Natural Science Foundation of China (Grant Numbers 32072527) and the National Natural Science Foundation of Shaanxi Province (No. 2020JC-21). We thank the Horticulture Science Research Center at the College of Horticulture, NWAFU for their technical support in this work.
Author contributions
M.L., F.M., Y.Y., and B.M. designed and supervised this research; L. Zha. and C.W. performed this research; L. Zhu, R.J., N.Y., and L.J. analysed data; L. Zha wrote the manuscript; M.L., F.M., Y.Y., B.M., L. Zha, and C.W. discussed this study and revised the manuscript.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Conflict of interests
The authors declare no competing interests.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Lihua Zhang, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China; The Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (Northeast Region), Ministry of Agriculture and Rural Affairs, College of Horticulture & Landscape Architecture, Northeast Agricultural University, Harbin 150030, China.
Changzhi Wang, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China.
Runpu Jia, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an, 271018, China.
Nanxiang Yang, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China.
Ling Jin, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China.
Lingcheng Zhu, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China.
Baiquan Ma, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China.
Yu-xin Yao, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an, 271018, China.
Fengwang Ma, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China.
Mingjun Li, State Key Laboratory of Crop Stress Biology for Arid Areas/Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling 712100, Shaanxi, China.
References
- 1. Jaeger SR, Andani Z, Wakeling INet al. Consumer preferences for fresh and aged apples: a cross-cultural comparison. Food Qual Prefer. 1998;9:355–66. [Google Scholar]
- 2. Ruffner HP, Hawker JS. Control of glycolysis in ripening berries of Vitis vinifera. Phytochemistry. 1977;16:1171–5. [Google Scholar]
- 3. Sweetman C, Deluc LG, Cramer GRet al. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry. 2009;70:1329–44. [DOI] [PubMed] [Google Scholar]
- 4. Berüter J. Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J Plant Physiol. 2004;161:1011–29. [DOI] [PubMed] [Google Scholar]
- 5. Mignard P, Beguería S, Giménez Ret al. Effect of genetics and climate on apple sugars and organic acids profiles. Agronomy. 2022;12:827. [Google Scholar]
- 6. Etienne A, Génard M, Lobit Pet al. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J Exp Bot. 2013;64:1451–69. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Li PCheng L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010;123:1013–8. [Google Scholar]
- 8. Cuevas IC, Podestá FE. Purification and physical and kinetic characterization of an NAD + −dependent malate dehydrogenase from leaves of pineapple (Ananas comosus). Physiol Plant. 2000;108:240–8. [Google Scholar]
- 9. Miller SS, Driscoll BT, Gregerson RGet al. Alfalfa malate dehydrogenase (MDH): molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J. 1998;15:173–84. [DOI] [PubMed] [Google Scholar]
- 10. Ding Y, Ma QH. Characterization of a cytosolic malate dehydrogenase cDNA which encodes an isozyme toward oxaloacetate reduction in wheat. Biochimie. 2004;86:509–18. [DOI] [PubMed] [Google Scholar]
- 11. KEJ T, Podestá FE. Purification and characterization of an NAD-dependent malate dehydrogenase from leaves of the crassulacean acid metabolism plant Aptenia cordifolia. Plant Physiol Biochem. 2003;41:97–105. [Google Scholar]
- 12. Yao YX, Li M, Zhai Het al. Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis. J Plant Physiol. 2011;168:474–80. [DOI] [PubMed] [Google Scholar]
- 13. Gibson N, McAlister-Henn L. Physical and genetic interactions of cytosolic malate dehydrogenase with other gluconeogenic enzymes. J Biol Chem. 2003;278:25628–36. [DOI] [PubMed] [Google Scholar]
- 14. Echeverria E, Burns JK. Vacuolar acid hydrolysis as a physiological mechanism for sucrose breakdown. Plant Physiol. 1989;90:530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centeno DC, Osorio S, Nunes-Nesi Aet al. Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell. 2011;23:162–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei L, Mao W, Jia Met al. FaMYB44.2, a transcriptional repressor, negatively regulates sucrose accumulation in strawberry receptacles through interplay with FaMYB10. J Exp Bot. 2018;69:4805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu J, Gu KD, Sun CHet al. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate. Plant Biotechnol J. 2021;19:285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun J, Zhang J, Larue CTet al. Decrease in leaf sucrose synthesis leads to increased leaf starch turnover and decreased RuBP regeneration-limited photosynthesis but not Rubisco-limited photosynthesis in Arabidopsis null mutants of SPSA1: Roles of SPS isoforms in photosynthesis. Plant Cell Environ. 2011;34:592–604. [DOI] [PubMed] [Google Scholar]
- 19. Zhang L, Ma B, Wang Cet al. MdWRKY126 modulates malate accumulation in apple fruit by regulating cytosolic malate dehydrogenase (MdMDH5). Plant Physiol. 2022;188:2059–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selinski J, König N, Wellmeyer Bet al. The plastid-localized NAD-dependent malate dehydrogenase is crucial for energy homeostasis in developing Arabidopsis thaliana Seeds. Mol Plant. 2014;7:170–86. [DOI] [PubMed] [Google Scholar]
- 21. Schreier TB, Cléry A, Schläfli Met al. Plastidial NAD-dependent malate dehydrogenase: a moonlighting protein involved in early chloroplast development through its interaction with an FtsH12-FtsHi protease complex. Plant Cell. 2018;30:1745–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beeler S, Liu HC, Stadler Met al. Plastidial NAD-dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis. Plant Physiol. 2014;164:1175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teng X, Zhong M, Zhu Xet al. FLOURY ENDOSPERM16 encoding a NAD-dependent cytosolic malate dehydrogenase plays an important role in starch synthesis and seed development in rice. Plant Biotechnol J. 2019;17:1914–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Q, Sun H, Dong QLet al. The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotechnol J. 2016;14:1986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nan N, Wang J, Shi Yet al. Rice plastidial NAD -dependent malate dehydrogenase 1 negatively regulates salt stress response by reducing the vitamin B6 content. Plant Biotechnol J. 2020;18:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ollat N, Gaudillère J. Carbon balance in developing grapevine berries. Acta Hortic. 2000;526:345–50. [Google Scholar]
- 27. Gao M, Zhao H, Zheng Let al. Overexpression of apple Ma12, a mitochondrial pyrophosphatase pump gene, leads to malic acid accumulation and the upregulation of malate dehydrogenase in tomato and apple calli. Hortic Res. 2022;9:uhab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li M, Feng FCheng L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS One. 2012;7:e33055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li M, Li P, Ma Fet al. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 2018;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stitt M. Limitation of photosynthesis by carbon metabolism: I. evidence for excess electron transport capacity in leaves carrying out photosynthesis in saturating light and CO2. Plant Physiol. 1986;81:1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nascimento JRO, Cordenunsi BR, Lajolo FMet al. Banana sucrose-phosphate synthase gene expression during fruit ripening. Planta. 1997;203:283–8. [DOI] [PubMed] [Google Scholar]
- 32. Yu X, Wang X, Fan Jet al. Cloning and characterization of a sucrose phosphate synthase-encoding gene from muskmelon. J Am Soc Hortic Sci. 2007;132:557–62. [Google Scholar]
- 33. Komatsu A, Moriguchi T, Koyama Ket al. Analysis of sucrose synthase genes in citrus suggests different roles and phylogenetic relationships. J Exp Bot. 2002;53:61–71. [PubMed] [Google Scholar]
- 34. Verma AK, Upadhyay SK, Verma PCet al. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars: Sugarcane functional sucrose enzyme analysis. Plant Biol. 2011;13:325–32. [DOI] [PubMed] [Google Scholar]
- 35. Ruan YL. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol. 2014;65:33–67. [DOI] [PubMed] [Google Scholar]
- 36. Baxter CJ, Foyer CH, Rolfe SAet al. A comparison of the carbohydrate composition and kinetic properties of sucrose phosphate synthase (SPS) in transgenic tobacco (Nicotiana tabacum) leaves expressing maize SPS protein with untransformed controls. Ann Appl Biol. 2001;138:47–55. [Google Scholar]
- 37. Zhu YJ, Komor EMoore PH. Sucrose accumulation in the sugarcane stem is regulated by the difference between the activities of soluble acid invertase and sucrose phosphate synthase. Plant Physiol. 1997;115:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Worrell AC, Bruneau J-M, Summerfelt Ket al. Expression of a maize sucrose phosphate synthase in tomato alters leaf carbohydrate partitioning. Plant Cell. 1991;3:1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruffner HP, Kliewer WM. Phosphoenolpyruvate carboxykinase activity in grape berries. Plant Physiol. 1975;56:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Famiani F, Farinelli D, Palliotti Aet al. Is stored malate the quantitatively most important substrate utilised by respiration and ethanolic fermentation in grape berry pericarp during ripening? Plant Physiol Biochem. 2014;76:52–7. [DOI] [PubMed] [Google Scholar]
- 41. Famiani F, Farinelli D, Moscatello Set al. The contribution of stored malate and citrate to the substrate requirements of metabolism of ripening peach (Prunus persica L. Batsch) flesh is negligible. Implications for the occurrence of phosphoenolpyruvate carboxykinase and gluconeogenesis. Plant Physiol Biochem. 2016;101:33–42. [DOI] [PubMed] [Google Scholar]
- 42. Famiani F, Farinelli D, Frioni Tet al. Malate as substrate for catabolism and gluconeogenesis during ripening in the pericarp of different grape cultivars. Biol Plant. 2016;60:155–62. [Google Scholar]
- 43. Chen Q, Qi W, Reiter RJet al. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J Plant Physiol. 2009;166:324–8. [DOI] [PubMed] [Google Scholar]
- 44. Zhu L, Li B, Wu Let al. MdERDL6-mediated glucose efflux to the cytosol promotes sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato. Proc Natl Acad Sci. 2021;118:e2022788118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun HJ, Uchii S, Watanabe Set al. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006;47:426–31. [DOI] [PubMed] [Google Scholar]
- 46. Zhang L, Sun S, Liang Yet al. Nitrogen levels regulate sugar metabolism and transport in the shoot tips of crabapple plants. Front Plant Sci. 2021;12:626149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.



