Abstract
The sensitivity of Vibrio anguillarum to the bactericidal effect of rainbow trout serum was investigated with different strains of serogroups O1 and O2a, which are the most frequently found serogroups in clinical outbreaks of vibriosis. All of the V. anguillarum strains were able to activate complement in rainbow trout serum, but smooth strains of V. anguillarum serogroup O1 were resistant to complement-mediated killing in the absence of specific antibodies. In the case of V. anguillarum serogroup O2a strains, 80% of the analyzed strains were resistant to rainbow trout serum even when specific antibodies were present. Analysis of the lipopolysaccharide structures of the tested V. anguillarum strains showed a positive correlation between the O-antigen size of the lipopolysaccharide and resistance to serum killing. The classical complement pathway was responsible for the antibody-dependent serum killing of susceptible V. anguillarum strains. When serum-resistant V. anguillarum serogroup O2a strains were grown in glucose-enriched Lennox L broth, they produced lipopolysaccharide molecules with fewer high-molecular-weight O-antigen units than did strains grown in broth without the addition of glucose. Strains grown in glucose-enriched medium became sensitive to rainbow trout serum killing, indicating that the high-molecular-weight O-antigen side chains prevented the activated complement from damaging the bacterium.
Complement activity in fish is known to play an important role in the defense against bacterial pathogens (33). Rainbow trout use two complement activation pathways, the classical and the alternative, comparable to those of mammals (33). The classical or the alternative pathway of the complement system kills susceptible gram-negative bacteria. The classical pathway requires antibodies (Ab) to recognize bacterial surface antigens before activation is initiated, whereas the alternative pathway can be initiated and amplified in the absence of antigen-Ab interactions. The complement system can kill the target cell directly or opsonize the bacterium and thereby facilitate phagocytosis. However, some gram-negative bacteria resist the bactericidal effect of serum and frequently cause bacteremia (23).
Bacterial resistance to complement-mediated killing by either of the two pathways may occur because the bacterium avoids initiating complement activation or because activated complement fails to damage the bacterium. Smooth strains of gram-negative bacteria carry long polysaccharide side chains (the O antigen) in their lipopolysaccharide (LPS) structures. They are more resistant to serum complement-mediated killing than rough strains, which lack the O-antigen side chains (18). The LPS structure of gram-negative bacteria which functions as a molecular and physical barrier for the cell may thus influence the bactericidal effect of the complement system and cause resistance to serum killing (serum resistance) (17, 18, 26). In an immune animal, Ab may bind to surface components of the bacteria and, in this way, may overcome serum resistance.
Most studies on the effect of the LPS structure on serum resistance have been carried out with bacterial pathogens and human serum as the source of complement (4, 14, 15, 20, 26), and knowledge of how the LPS structure of gram-negative bacterial fish pathogens affects sensitivity to fish serum is very limited. Vibrio anguillarum is an important marine fish pathogen and has been shown to exist in several serogroups, of which serogroups O1, O2, and O3 seem to be the most pathogenic (1). With a panel of V. anguillarum serogroup O1 and O2a strains with different LPS profiles, the aim of the present work was to investigate the effect of O-antigen size on complement activation and susceptibility to complement-mediated killing in rainbow trout serum in the presence or absence of V. anguillarum-specific Ab.
MATERIALS AND METHODS
Bacteria and culture media.
The strains used in this study are listed in Table 1. A total of 42 V. anguillarum strains were studied, with 17 belonging to serogroup O1 and 25 belonging to serogroup O2a. Further details about the strains are given by Austin et al. (1). Stock cultures were maintained at −80°C in 15% (vol/vol) glycerol–Lennox L broth base (LB; Gibco BRL, Paisley, Scotland) supplemented with 0.5% NaCl. Bacteria were grown with agitation for 17 h at 20°C in LB with 0.5% NaCl in the presence or absence of 2% glucose.
TABLE 1.
Sensitivity of V. anguillarum to rainbow trout serum in the presence and absence of specific Ab
| V. anguillarum serogroup | Strain | Sensitivity
|
No. of sensitive strains/total no. of strains tested (%) | LPS profile | |
|---|---|---|---|---|---|
| NS | Ab-NS | ||||
| O1 | NCMB 1873, RVAU 850610-1/6a, 840606-2/5 | + | + | 3/17 (18) | A |
| ATCC 43305, AVL 90-9-22, HWU 44, HWU 48, HWU 50, AVL 27.2, AVL 28.2, UB 01/91, UB 261/91, UB 601/91, LMG 10939, RVAU 830422-1/1, RVAU 775, NCMB 1875 | − | + | 14/17 (82) | B | |
| O2a | ATCC 14181, RVAU 910614-1/1 | + | + | 2/25 (8) | C and D |
| UB A078, RVAU 91-7-175, RVAU V2 1/2 | − | + | 3/25 (12) | D and Fa | |
| ATCC 43306, AVL 89-2-54, NCMB 828, UB 417/90, UB 498/90, UB 258/91, UB 578/90, NCMB 6, LMG 11684, LMG 12098, LMG 12099, LMG 12102, RVAU 2228/80, RVAU 2887, RVAU 850617-1/1, RVAU 860908-3, RVAU 89-2-62, RVAU 88-6-73/2, RVAU 92-8-161, RVAU 92-8-163 | − | − | 20/25 (80) | E and Fa | |
Profile D: RVAU 910614-1/1, RVAU 91-7-175, and RVAU V2 1/2; profile E: LMG 12099.
Serum. (i) Rainbow trout NS.
Blood was collected by caudal venipuncture from rainbow trout with an average body weight of 3 kg, and normal serum (NS) was obtained by allowing the blood to clot for 1.5 h at 5°C, followed by centrifugation. Serum samples were pooled and stored at −80°C in aliquots of 1 ml. Although the fish were raised and maintained in freshwater and presumably had never been exposed to V. anguillarum, serum was absorbed before use to remove potential natural Ab directed against V. anguillarum. Each aliquot of serum was incubated at 0°C with 109 live cells of V. anguillarum of either serogroup O2a (NCMB 6) or serogroup O1 (ATCC 43305) previously washed in phosphate-buffered saline (PBS). After 1.5 h of absorption, serum was centrifuged (13,800 × g), and the supernatant was filtered through a 0.22-μm-pore-size membrane filter (MILLEX-GP; Millipore, Bedford, Mass.). Absorbed serum had the same complement activity as unabsorbed serum when tested in a hemolytic assay with rabbit erythrocytes (RaRBC) (see below).
(ii) Heat-inactivated serum.
Serum was heated to 44°C for 20 min to inactivate complement activity (24).
Rainbow trout Ab to V. anguillarum.
Overnight cultures of the reference strains V. anguillarum serogroup O1 ATCC 43305 and serogroup O2a ATCC 43306 were inactivated with 0.9% formaldehyde for 2 h at room temperature, washed with PBS, adjusted to an optical density corresponding to approximately 1010 cells/ml, and emulsified with an equal volume of Freund’s incomplete adjuvant (Sigma, St. Louis, Mo.). Fish were immunized by intraperitoneal injections with 0.1 ml of formalin-killed bacterial suspension. Six weeks after injection of the antigen, the animals were bled, and a pool of antiserum was obtained. Antiserum was always heat inactivated before use.
Ab titers.
The levels of specific Ab in trout serum were determined by agglutination tests performed with 96-well microtiter plates. Serum (50 μl) was serially diluted in PBS, and 50 μl of V. anguillarum suspension (109 bacteria per ml) was added to each well. After incubation for 1 h at 35°C and overnight at 5°C, titers were read as the highest serum dilutions giving positive agglutination.
Plate plaque assay for classical complement-mediated killing.
The serum bactericidal assay was adopted from that of Holmgren et al. (7). Logarithmic-phase bacterial cultures were washed twice and suspended in sterile PBS to an optical density corresponding to about 4 × 105 or 1 × 105 cells per ml. Samples were spread on petri plates containing Trypticase soy agar (TSA; Difco Laboratories, Detroit, Mich.) with 0.5% NaCl and DEAE-dextran (1 mg/ml) (Pharmacia, Uppsala, Sweden). The bacterial cultures were allowed to dry on the plates for 1 h at 10°C. Aliquots of 3 μl of NS, heat-inactivated NS (as a control for the role of complement), or antiserum were placed in drops on the surface of the agar and allowed to bind to the bacteria at 10°C. After 1 h, 3 μl of NS (as a source of complement) was added to the previously applied drops of antiserum. The plates were then incubated for 48 h at 20°C and examined for the presence of clear, bacterium-free plaques. Plaques were recorded as positive if there was complete inhibition of bacterial growth or if only a few discrete colonies were observed.
Bacterial survival in NS after incubation with Ab.
Logarithmic-phase cultures of V. anguillarum were suspended in PBS and adjusted to an optical density corresponding to approximately 5 × 108 cells/ml. The suspensions were incubated with Ab (0.05%) for 1 h at 20°C, and NS (or PBS as a control) was added to the suspensions (bacterial suspension/NS ratio, 1:4). After 1.5 h, counts of viable bacteria were determined at 20°C after serial dilutions in PBS and plating on TSA with 0.5% NaCl. Results are expressed as the percentage bacteria surviving in Ab-NS compared to Ab-PBS. Classical complement activity in NS was selectively inhibited by chelation of Ca2+ with 10 mM (final concentration) EGTA plus 10 mM MgCl2. Both the alternative and the classical complement activities were inactivated by chelating Ca2+ and Mg2+ from NS with 10 mM (final concentration) EDTA or by heating NS at 44°C for 20 min. NS was diluted with PBS as a positive control.
LPS profiling.
LPS was extracted by a proteinase K method modified from that of Hitchcock and Brown (6). Overnight bacterial cultures were harvested with 1 ml of PBS from petri plates containing TSA with 0.5% NaCl, incubated for 20 min at 60°C, and centrifuged at 13,800 × g for 10 min. An aliquot of 50 μl of supernatant was mixed with 50 μl of sample buffer (4% sodium dodecyl sulfate, 1% dithiothreitol, 20% glycerol, 0.1 M Tris [pH 6.8], bromophenol blue) and heated to 100°C for 10 min. Ten microliters of proteinase K solution (2.5 mg/ml; Sigma) per 50 μl of sample solution was added, and samples were incubated at 60°C for 1 h and then subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gels (12% [wt/vol]) at 125 V for 1.5 h as described by Laemmli (12). LPS was silver stained by the method of Tsai and Frasch (30) with a silver stain kit (Bio-Rad Laboratories, Richmond, Calif.).
Measurement of complement consumption by V. anguillarum.
Complement consumption was measured by mixing equal volumes of NS and V. anguillarum (optical density corresponding to approximately 1010 bacteria/ml) suspended in PBS or NS and PBS alone as a positive control; the suspension was incubated for 1 h at 20°C with agitation. After centrifugation (1,400 × g), residual complement activity in the NS supernatant of both samples and positive control was measured as described by Yano (32) with slight modifications. RaRBC (26 μl of 1.5 × 108 cells per ml of EGTA-Mg-gelatin-Veronal buffer [EGTA-Mg-GVB; 0.1% gelatin, 0.14 M NaCl, 1.2 mM sodium barbiturate, 3.5 mM HCl, 10 mM EGTA, 10 mM MgCl2 · 6H2O, 18 mM NaOH; pH 7.5]) were added to a serial twofold dilution of the NS supernatant (26 μl in EGTA-Mg-GVB) in a microtiter plate and incubated at 20°C. After 1 h, 150 μl of ice-cold saline was added, cells were pelleted by centrifugation, and the absorbance of the supernatant was measured at 405 nm. One hundred percent hemolysis was produced by mixing 26 μl of RaRBC with 176 μl of distilled water, and spontaneous lysis was produced by mixing 26 μl of RaRBC with 26 μl of EGTA-Mg-GVB and after 1 h adding 150 μl of saline. Complement-induced hemolysis of RaRBC by the test sera was defined by the following calculation: percent hemolysis = {[A405(sample) − A405(spontaneous lysis)]/[A405(100% hemolysis) − A405(spontaneous lysis)]} × 100%.
RESULTS
Ab recognition of strains of V. anguillarum serogroups O1 and O2a.
The agglutination titers of the rainbow trout antisera were determined with a microtiter agglutination assay to confirm that all tested strains of V. anguillarum were recognized by Ab. To test for the serum sensitivity of different homologous serogroups of V. anguillarum, one antiserum was used for each serogroup, as the agglutination titers of the antisera to all homologous V. anguillarum strains were never lower than 26 (results not shown).
Sensitivity of V. anguillarum to rainbow trout NS in the presence or absence of Ab.
Seventeen strains of V. anguillarum serogroup O1 and 25 strains of V. anguillarum serogroup O2a were tested for their ability to resist the bactericidal activity of rainbow trout NS in the presence or absence of Ab in a plate plaque assay. Three of 17 V. anguillarum serogroup O1 strains (NCMB 1873, RVAU 850610-1/6a, and 840606-2/5) were sensitive to NS alone, whereas all serogroup O1 strains were sensitive to Ab-NS (Table 1). In the case of V. anguillarum serogroup O2a, 80% of the strains were resistant to NS, even in the presence of Ab. Three strains (UB A078, RVAU 91-7-175, and RVAU V2 1/2) were sensitive to NS when Ab were present, and only two strains (ATCC 14181 and RVAU 910614-1/1) were sensitive to NS alone (Table 1). Thus, more strains of V. anguillarum serogroup O2a than of V. anguillarum serogroup O1 were resistant to serum killing, even in the presence of Ab. None of the bacteria were sensitive to Ab–heat-inactivated NS.
Correlation of LPS profiles of the V. anguillarum strains with serum sensitivity.
To test if there was any association between the LPS structures of V. anguillarum and the serum sensitivity of the bacteria, the LPS profiles of the V. anguillarum strains were analyzed. Figure 1 shows a panel of LPS profiles representing the different types. On the basis of these profiles (with some minor differences in banding patterns), the selected strains were classified into LPS types (Fig. 1 and Table 1). On the basis of previous work (2), it was assumed that bands with different electrophoretic mobilities represented molecular species with different numbers of repeating O-antigen units. The results of the LPS profiling showed that, except for ATCC 14181, all strains of V. anguillarum serogroup O2a had an LPS profile with a ladder of both high-molecular-weight (HMW) and low-molecular-weight (LMW) O-antigen bands (profiles D, E, and F; Fig. 1 and Table 1). In contrast, most strains of V. anguillarum serogroup O1 had only a few HMW O-antigen bands (profile B; Fig. 1 and Table 1); moreover, the three NS-sensitive strains of V. anguillarum serogroup O1 (Table 1) were rough strains with only the LPS core present (profile A; Fig. 1). Thus, the lack of HMW O-antigen bands of V. anguillarum serogroup O1 strains (rough type) coincided with sensitivity to NS, whereas NS-resistant V. anguillarum serogroup O1 or O2a strains had some or many, respectively, HMW O-antigen bands. Only one strain of V. anguillarum serogroup O2a (ATCC 14181) was found to have a rough (or semirough) phenotype with a few O-antigen bands in the LPS profile (profile C; Fig. 1); this strain was sensitive to killing by rainbow trout NS (Table 1). However, the other serogroup O2a strain which was sensitive to NS (RVAU 910614-1/1) had an LPS profile with many HMW bands (profile D; Fig. 1), like two other strains (Table 1) which were resistant to NS. All Ab-NS-resistant V. anguillarum strains of serogroup O2a had LPS profiles with many bands of both HMW and LMW O antigens (profile F, 19 strains, and profile E, 1 strain; Fig. 1 and Table 1). On the other hand, three V. anguillarum strains of serogroup O2a were sensitive to Ab-NS despite LPS profiles consisting of bands of both HMW and LMW O antigens (profiles D and F; Fig. 1 and Table 1). No V. anguillarum strains of serogroup O1 were resistant to Ab-NS, and strains sensitive to Ab-NS had LPS profiles with only a few HMW bands and mostly LMW bands (profile B; Fig. 1 and Table 1). Thus, for most strains, there was a positive correlation between O-antigen size and serum resistance.
FIG. 1.
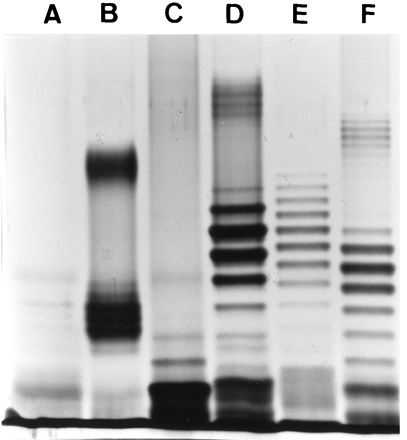
Silver-stained LPS profiles of V. anguillarum serogroup O1 and O2a strains. Lanes: A, NCMB 1873 (O1, rough strain); B, ATCC 43305 (O1, smooth strain); C, ATCC 14181 (O2a); D, RVAU 910614-1/1 (O2a); E, LMG 12099 (O2a); F, UB 258/91 (O2a).
Correlation of changes in LPS profiles under different culture conditions with serum sensitivity.
When strains of V. anguillarum serogroup O2a were grown in medium enriched with 2% glucose prior to being tested in the bactericidal plate plaque assay, 13 of 20 strains previously shown to be Ab-NS-resistant became sensitive to Ab-NS, whereas 7 strains remained resistant to serum killing in the presence of Ab (Table 2). In addition, the V. anguillarum serogroup O2a strain RVAU V2 1/2, previously found to be NS resistant but sensitive to Ab-NS, became NS sensitive when grown in glucose-enriched medium. When NS-resistant V. anguillarum serogroup O1 strains were grown in glucose-enriched medium, they remained resistant to NS alone and sensitive to Ab-NS (results not shown).
TABLE 2.
Sensitivity to rainbow trout serum of V. anguillarum serogroup O2a grown in glucose-enriched medium
| V. anguillarum strain(s) | Sensitivity in medium
|
|||
|---|---|---|---|---|
| Without extra glucose
|
With extra glucose
|
|||
| NS | Ab-NS | NS | Ab-NS | |
| RVAU V2 1/2 | − | + | + | + |
| ATCC 43306, AVL 89-2-54, UB 498/90, UB 258/91, UB 578/90, NCMB 6, LMG 12099, LMG 12102, RVAU 2887, RVAU 850617-1/1, RVAU 88-6-73/2, RVAU 92-8-161, RVAU 92-8-163 | − | − | − | + |
| NCMB 828, UB 417/90, LMG 11684, LMG 12098, RVAU 2228/80, RVAU 860908-3, RVAU 89-2-62 | − | − | − | − |
A correlation between serum sensitivity and O-antigen LPS banding patterns appeared to exist when the LPS profiles of V. anguillarum serogroup O2a strains which became sensitive to Ab-NS killing when grown under glucose-enriched conditions were examined. In all cases, when these strains were grown under glucose-enriched conditions, a reduction in the number of HMW O-antigen bands was observed compared with the pattern obtained for bacteria grown under normal culture conditions. Representative patterns are shown in Fig. 2. In contrast, two V. anguillarum serogroup O2a strains (UB 417/90 and NCMB 828) which remained serum resistant under glucose-enriched conditions did not change their LPS profiles (Fig. 2). The addition of glucose to the growth medium had no effect on the LPS profiles of the serogroup O1 strains (results not shown).
FIG. 2.
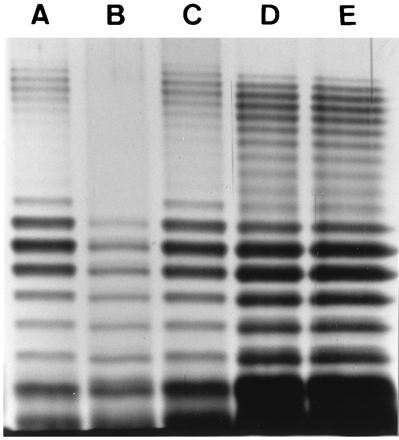
Silver-stained LPS profiles of a V. anguillarum serogroup O2a strains grown in medium with or without extra glucose. Lanes: A, UB 258/91 (serum resistant); B, UB 258/91 grown in glucose-enriched medium (serum sensitive); C, UB 258/91 grown in glucose-enriched medium and thereafter transferred to medium without extra glucose (serum resistant); D, NCMB 828 (serum resistant); E, NCMB 828 grown in glucose-enriched medium (serum resistant).
The reduced production of HMW O antigens and the shift in the serum sensitivity of V. anguillarum serogroup O2a strains grown in glucose-enriched medium were reversible. Figure 2, lane A, shows the LPS profile of an Ab-NS-resistant V. anguillarum serogroup O2a strain which lost the HMW bands when the bacterium was grown in glucose-enriched medium (Fig. 2, lane B) and became Ab-NS sensitive. Following transfer back to conventional medium without extra glucose, the strain again became resistant to Ab-NS (results not shown), and the LPS profile reverted to that seen with conventional medium (Fig. 2, lane C).
Analysis of the pathway of complement-mediated killing of V. anguillarum strains.
The classical pathway requires Ca2+ and is inhibited by EGTA, which chelates Ca2+. Both the classical and the alternative pathways require Mg2+ and are inhibited by the Ca2+- and Mg2+-chelating agent EDTA. Treatment of rainbow trout NS with EGTA-Mg2+ inhibited NS killing of NS-sensitive O2a strains and Ab-NS killing of O2a strains grown under glucose supplementation (Fig. 3 and Table 3). These results indicated that serum killing of sensitive V. anguillarum serogroup O2a strains in the presence or absence of Ab was mediated by the classical complement pathway. In contrast, killing of NS-sensitive serogroup O1 strains by NS was not inhibited by EGTA-Mg2+, while treatment of NS with EDTA or heat did inhibit NS killing, indicating that killing was mediated by the alternative pathway. On the other hand, killing of Ab-NS-sensitive serogroup O1 strains was inhibited by EGTA-Mg2+, indicating that killing was mediated by the classical pathway (Table 3).
FIG. 3.
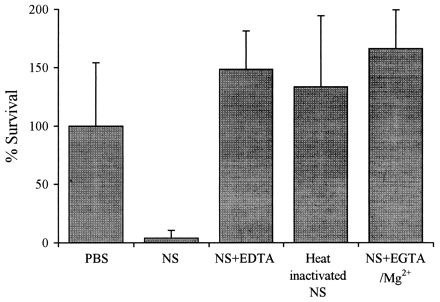
Pathway for complement-mediated killing of Ab-coated V. anguillarum serogroup O2a UB 258/91 grown in glucose-enriched medium. The bacteria were incubated with PBS, NS with or without the chelating agents EDTA and EGTA-Mg2+, or heated serum. Values shown are means ± standard deviations (n = 4).
TABLE 3.
Pathway of complement-mediated killing of V. anguillarum
| V. anguillarum strains | Presence of Ab | Sensitivity
|
Complement pathway responsible for killing | |||
|---|---|---|---|---|---|---|
| NS | NS-EDTA | Heat-inactivated NS | NS-EGTA-Mg2+ | |||
| NS-sensitive O1 | − | + | − | − | + | Alternative |
| Ab-NS-sensitive O1 | + | + | − | − | − | Classical |
| NS-sensitive O2a | − | + | − | − | − | Classical |
| Ab-NS-sensitive O2a grown in glucose-enriched medium | + | + | − | − | − | Classical |
Complement consumption by V. anguillarum.
The consumption of serum complement by V. anguillarum was measured to determine whether sensitivity to serum was due to an ability to avoid the activation of complement or to avoid the killing effect of complement despite its being activated. Consumption of complement was monitored by measuring the hemolysis of RaRBC by NS after the serum had been preincubated with strains of V. anguillarum. All the strains tested consumed complement (Fig. 4a), and V. anguillarum serogroup O2a grown in glucose-enriched medium had a higher complement-consuming activity than V. anguillarum grown in conventional medium (Fig. 4a). Rough strains of V. anguillarum serogroup O1 (sensitive to NS) consumed more complement than smooth strains (Fig. 4b). Treatment of NS with Ab-coated smooth V. anguillarum serogroup O1 strains resulted in a dramatic increase in complement consumption, compared to when no Ab were present (Fig. 4b). Coating of serogroup O2a strains grown in the presence or absence of glucose supplementation with Ab had little effect on NS complement consumption (Fig. 4c).
FIG. 4.
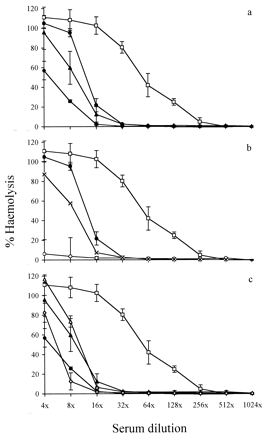
Complement consumption by V. anguillarum evaluated by a reduction in complement-mediated hemolysis of RaRBC. RaRBC were incubated in rainbow trout NS as a control (□) or with NS pretreated with smooth V. anguillarum serogroup O1 ATCC 43305 with (○) or without (•) Ab coating, rough V. anguillarum serogroup O1 840606-2/5 without Ab coating (×), V. anguillarum serogroup O2a UB 258/91 with (▵) or without (▴) Ab coating, and V. anguillarum serogroup O2a UB 258/91 grown in glucose-enriched medium and with (◊) or without (⧫) Ab coating. Values shown are means ± standard deviations (n = 4).
Antigenic structures important for Ab-dependent serum killing of V. anguillarum serogroup O1 strains.
The specificity of Ab important for serum killing of V. anguillarum serogroup O1 was tested in a bactericidal assay with antiserum raised against a rough strain, antiserum raised against a smooth strain, and anti-smooth strain antiserum absorbed with the rough strain. Antiserum raised against the rough strain of V. anguillarum (expected to react only with the LPS core, as O antigen is absent) had a high agglutination titer against the rough strain but a low titer against the smooth strain. Furthermore, it had no bactericidal effect on the smooth strain in the presence of NS (Table 4). As stated earlier, the anti-smooth strain antiserum agglutinated both groups of strains and killed the smooth strain in the presence of NS. When the anti-smooth strain antiserum was absorbed with the rough strain, it was assumed that antiserum to the core of LPS and other membrane components shared by the two strains were removed, leaving only Ab with specificity for components not shared by the strains, namely, O antigens of LPS. Western blotting verified that Ab to O antigens remained in the anti-smooth strain antiserum absorbed with the rough strain (results not shown). This absorbed antiserum did not agglutinate the rough strain but agglutinated the smooth strain well and retained its bactericidal activity (Table 4). NS complement consumption by the smooth strain was evaluated after coating of the strain with the different types of antiserum. Only strains coated with Ab with specificity for O antigens showed increased complement consumption compared with consumption by the smooth strain without Ab coating (Fig. 5).
TABLE 4.
Antigen structures important for Ab-specific serum killing of V. anguillarum serogroup O1
| Anti-V. anguillarum serogroup O1 Ab | Ab specificity for LPS |
V. anguillarum serogroup O1
|
||
|---|---|---|---|---|
| Smooth
|
Rougha agglutination titer | |||
| Agglutination titer | Serum sensitivityb | |||
| Roughc | LPS core | 22 | − | 29 |
| Smoothd | LPS core and O antigen | 28 | + | 26 |
| Smooth absorbed with rough | O antigen | 27 | + | − |
Rough strains were sensitive to NS alone.
Serum sensitivity to NS in the presence of Ab.
840606-2/5.
ATCC 43305.
FIG. 5.
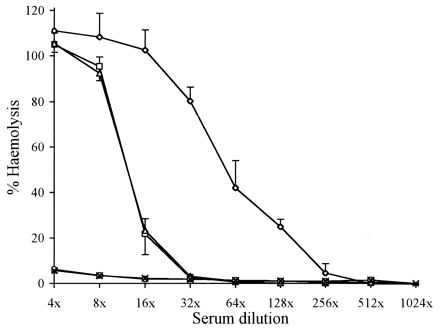
Complement consumption by V. anguillarum serogroup O1 evaluated by a reduction in complement-mediated hemolysis of RaRBC. RaRBC cells were incubated in rainbow trout NS as a control (◊) or with NS pretreated with a smooth strain (ATCC 43305) (□) or with a smooth strain coated with Ab raised against a rough strain (840606-2/5) (▵), a smooth strain (○), or a smooth strain for which the serum was preabsorbed with a rough strain (×). Values shown are means ± standard deviations (n = 4).
DISCUSSION
This study examined the importance of LPS composition and structure for the resistance of the fish pathogen V. anguillarum to direct complement-mediated killing by rainbow trout serum. V. anguillarum strains of serogroups O1 and O2a and with different LPS structures, as demonstrated by their LPS profiles in SDS-PAGE, were selected for analysis. This study demonstrated that most strains of both serogroup O1 and serogroup O2a were resistant to rainbow trout NS. The same serum-resistant strains were, in a recent study, found to be pathogenic for Atlantic salmon, whereas all of the serum-sensitive strains, except for RVAU 910614-1/1, were found to be weakly pathogenic or nonpathogenic (1). These results indicated that the serum resistance of V. anguillarum contributes to its ability to survive and induce disease in infected fish. Similarly, previous studies concluded that the resistance of V. anguillarum to the bactericidal action of rainbow trout NS (29) and serum from striped bass (28) may be an important virulence factor. In this study, a few of the V. anguillarum serogroup O1 strains were killed by rainbow trout NS, and these strains were, when analyzed by SDS-PAGE, all found to have rough LPS forms, i.e., lacking in O-antigen side chains. In agreement with these findings, previous serum sensitivity analysis of gram-negative bacteria defined rough variants to be generally more susceptible to the bactericidal action of NS than smooth forms (18). We also demonstrated that the resistance to NS killing of all of the V. anguillarum serogroup O1 strains and a few of the serogroup O2a strains was overcome when V. anguillarum-specific Ab were present, whereas the majority of V. anguillarum serogroup O2a strains were resistant to rainbow trout serum even in the presence of specific Ab.
In agreement with a previous SDS-PAGE analysis of V. anguillarum LPS (3), we found that V. anguillarum serogroup O1 had few HMW O-antigen bands, whereas the majority of strains of serogroup O2a (resistant to the bactericidal effect of rainbow trout NS in presence of Ab) had many HMW O-antigen bands. The results suggested that there may be a connection between the length of the O antigens of V. anguillarum and the serum sensitivity of these bacteria, providing evidence in support of the hypothesis that resistance to the bactericidal activity of complement is mediated by LPS, especially by the O-antigen polysaccharide chains (20, 26, 27). The O-antigen structures may protect the bacteria by sterically hindering complement from gaining access to and damaging the cytoplasmic membrane (4, 9–11).
During the present study, it was found that when extra glucose was added to the medium, 13 of 20 of the previously Ab-NS-resistant V. anguillarum serogroup O2a strains showed a marked decrease in the amount of HMW O antigens, and this change correlated with the bacteria becoming Ab-NS sensitive. This finding allowed a far more precise evaluation of the role of LPS in resistance to killing by rainbow trout serum. The advantage of culturing one strain which demonstrates different LPS profiles when grown under different culture conditions is that the different forms are isogenic. Reeves (22) considered that bacteria may lose their O antigens when grown in the laboratory due to mutations that affect either the synthesis of the O antigens themselves or the synthesis of the LPS core and proposed that the propensity to become rough during cultivation in vitro is a result of the outer core and O antigens being needed only in natural environments. The change in the O-antigen profile observed in the present work did not seem to be the result of a mutation, as it was reversible, and it is tempting to speculate that if the synthesis of HMW O antigens is energetically costly and if they are necessary only in natural environments, their synthesis may be down-regulated in artificial environments. In contrast to this down-regulation of HMW O antigens, a previous study demonstrated that a strain of V. anguillarum serogroup O2a grown on agar in the presence of fresh rainbow trout blood expressed LPS with HMW O antigens and an extracellular capsular layer and that these changes correlated with increased resistance to normal fish serum (19). An increase in the amount of HMW O antigens in the presence of rainbow trout blood might be a result of growth in an environment which mimics that in vivo.
The mechanism of killing of Ab-NS-sensitive V. anguillarum strains of serogroups O1 and O2a grown in glucose-enriched medium appeared to be the classical complement pathway, as it required V. anguillarum-specific Ab and was abolished when EGTA was present. In agreement with these findings, Ourth and Bachinski (21) demonstrated that for catfish serum, Ab-initiated classical complement activation was most important for killing of an unspecified V. anguillarum strain. On testing the pathway of complement-mediated killing of V. anguillarum serogroup O1 rough strains by NS, the alternative complement pathway was found to be involved, whereas the killing of NS-sensitive V. anguillarum serogroup O2a strains (ATCC 14181 and RVAU V2 1/2 grown in glucose-enriched medium) involved an Ab-independent classical complement pathway. Studies by others have shown that the classical pathway may be activated by the lipid A region of LPS, involving binding of the classical complement component C1 directly, without the participation of Ab (16). This observation may explain how the classical pathway can be responsible for NS killing of NS-sensitive O2a strains. Morrison and Kline (16) also postulated that the presence of polysaccharide may prevent lipid A from activating complement, perhaps explaining why V. anguillarum strains grown under different glucose conditions and producing different lengths of O-antigen polysaccharides differ significantly in their capacity to activate complement.
We have clearly demonstrated that all V. anguillarum strains were able to activate complement in NS, as evidenced by the consumption of complement. However, the activation of complement in NS usually failed to kill the bacteria. Recent studies have shown that some bacterial species with smooth LPS exposed on the cell surface are able to bind the C3b complement component but that formation of the membrane attack complex seems to occur in a way which does not cause lysis (13, 14). It therefore seems reasonable to hypothesize that complement binds more closely to the cytoplasmic membrane of sensitive V. anguillarum because its LPS lacks HMW O antigens. In agreement with a previous study on Serratia marcescens (8), we found that NS-sensitive V. anguillarum strains consumed more complement than NS-resistant strains. When specific Ab were present, V. anguillarum serogroup O1 strains sensitive to NS in the presence of Ab consumed even more complement, presumably because the classical pathway was activated by the Ab. In contrast, the presence of specific Ab did not enhance complement activation by serogroup O2a strains grown in either conventional or glucose-enriched medium, although in the latter case the presence of Ab resulted in bacterial killing.
The present findings provide further evidence that Ab to polysaccharide O antigens are important for the effect of rainbow trout complement on serogroup O1 strains of V. anguillarum. Absorption of an anti-smooth V. anguillarum serogroup O1 serum with a rough strain of V. anguillarum serogroup O1 created an antiserum with specificity for the O antigens of V. anguillarum serogroup O1. This antiserum was active against smooth strains of V. anguillarum serogroup O1, and this activity was associated with enhanced complement consumption. In contrast, an antiserum to a rough strain of V. anguillarum serogroup O1 without specificity for O antigens was unable to kill smooth strains of V. anguillarum serogroup O1 or to enhance complement consumption. This anti-rough strain serum had low agglutination activity for smooth strains, presumably because the O antigens sterically hindered the binding of Ab to the core LPS. This observation is in agreement with the findings of others which showed that Ab with specificity for the core polysaccharide of LPS have only weak bactericidal and opsonic activities for smooth strains (34).
Ab have been demonstrated to contribute to protection against V. anguillarum in rainbow trout passively immunized with V. anguillarum antiserum (5, 31). However, the present results, showing that V. anguillarum serogroup O2a strains are resistant to serum killing even in the presence of Ab, suggest that Ab are unlikely to provide the fish with protective immunity against virulent strains through activation of the complement system per se. However, the protective effect of Ab against O2a strains may be associated with an opsonizing effect of Ab and complement (25), thus facilitating the elimination of the bacteria by macrophages or neutrophils.
ACKNOWLEDGMENTS
The technical assistance of Bente Østergård and Vivi Andersen is gratefully appreciated. We thank colleagues for donations of bacteria.
Part of this work was supported by a grant from the Danish Agricultural and Veterinary Research Council (grant 9503658).
REFERENCES
- 1.Austin B, Alsina M, Austin D A, Blanch A R, Grimont F, Grimont P A D, Jofre J, Koblavi S, Larsen J L, Pedersen K, Tiainen T, Verdonck L, Swings J. Identification and typing of Vibrio anguillarum: a comparison of different methods. Syst Appl Microbiol. 1995;18:285–302. [Google Scholar]
- 2.Goldman R C, Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli O111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107:145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- 3.Grisez L, Ollevier F. Comparative serology of the marine fish pathogen Vibrio anguillarum. Appl Environ Microbiol. 1995;61:4367–4373. doi: 10.1128/aem.61.12.4367-4373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman N, Schmetz M A, Foulds J, Klima E N, Jeminez V, Leive L L, Joiner K A. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987;169:856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrell L W, Etlinger H M, Hodgins H O. Humoral factors important in resistance of salmonid fish to bacterial disease. I. Serum antibody protection of rainbow trout (Salmo gairdneri) against vibriosis. Aquaculture. 1975;6:211–219. [Google Scholar]
- 6.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren J, Svennerholm A-M, Ouchterlony Ö. Quantitation of vibriocidal antibodies using agar plaque techniques. Acta Pathol Microbiol Scand Sect B. 1971;79:708–714. [PubMed] [Google Scholar]
- 8.Jessop H L, Lambert P A. The role of surface polysaccharide in determining the resistance of Serratia marcescens to serum killing. J Gen Microbiol. 1986;132:2505–2514. doi: 10.1099/00221287-132-9-2505. [DOI] [PubMed] [Google Scholar]
- 9.Joiner K A, Grossman N, Schmetz M, Leive L. C3 binds preferentially to long-chain lipopolysaccharide during alternative pathway activation by Salmonella montevideo. J Immunol. 1986;136:710–715. [PubMed] [Google Scholar]
- 10.Joiner K A, Hammer C H, Brown E J, Cole R J, Frank M M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982;155:797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joiner K A, Hammer C H, Brown E J, Frank M M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982;155:809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Merino S, Albertí S, Tomás J M. Aeromonas salmonicida resistance to complement-mediated killing. Infect Immun. 1994;62:5483–5490. doi: 10.1128/iai.62.12.5483-5490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merino S, Camprubí S, Albertí S, Benedí V-J, Tomás J M. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect Immun. 1992;60:2529–2535. doi: 10.1128/iai.60.6.2529-2535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merino S, Camprubí S, Tomás J M. The role of lipopolysaccharide in complement-killing of Aeromonas hydrophila strains of serotype O:34. J Gen Microbiol. 1991;137:1583–1590. doi: 10.1099/00221287-137-7-1583. [DOI] [PubMed] [Google Scholar]
- 16.Morrison D C, Kline L F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS) J Immunol. 1977;118:362–368. [PubMed] [Google Scholar]
- 17.Munn C B, Ishiguro E E, Kay W W, Trust T J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982;36:1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muschel L H, Larsen L J. The sensitivity of smooth and rough Gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970;133:345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- 19.Mutharia L M, Amor P A. Monoclonal antibodies against Vibrio anguillarum O2 and Vibrio ordalii identify antigenic differences in lipopolysaccharide O-antigens. FEMS Microbiol Lett. 1994;123:289–298. doi: 10.1111/j.1574-6968.1994.tb07238.x. [DOI] [PubMed] [Google Scholar]
- 20.Odumeru J A, Wiseman G M, Ronald A R. Role of lipopolysaccharide and complement in susceptibility of Haemophilus ducreyi to human serum. Infect Immun. 1985;50:495–499. doi: 10.1128/iai.50.2.495-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ourth D D, Bachinski L M. Bactericidal response of channel catfish (Ictalurus punctatus) by the classical and alternative complement pathways against bacterial pathogens. J Appl Ichthyol. 1987;3:42–45. [Google Scholar]
- 22.Reeves P. Role of O-antigen variation in the immune response. Trends Microbiol. 1995;3:381–386. doi: 10.1016/s0966-842x(00)88983-0. [DOI] [PubMed] [Google Scholar]
- 23.Roantree R J, Rantz L A. A study of the relationship of the normal bactericidal activity of human serum to bacterial infection. J Clin Investig. 1960;39:72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai D K. Heat inactivation of complement and immune hemolysis reactions in rainbow trout, masu salmon, coho salmon, goldfish and tilapia. Bull Jpn Soc Sci Fish. 1981;47:565–571. [Google Scholar]
- 25.Secombes C J, Fletcher T C. The role of phagocytes in the protective mechanisms of fish. Annu Rev Fish Dis. 1992;2:53–71. [Google Scholar]
- 26.Tomás J M, Benedí V J, Ciurana B, Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986;54:85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomás J M, Ciurana B, Benedí V J, Juarez A. Role of lipopolysaccharide and complement in susceptibility of Escherichia coli and Salmonella typhimurium to non-immune serum. J Gen Microbiol. 1988;134:1009–1016. doi: 10.1099/00221287-134-4-1009. [DOI] [PubMed] [Google Scholar]
- 28.Toranzo A E, Barja J L, Potter S A, Colwell R R, Hetrick F M, Crosa J H. Molecular factors associated with virulence of marine vibrios isolated from striped bass in Chesapeake Bay. Infect Immun. 1983;39:1220–1227. doi: 10.1128/iai.39.3.1220-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trust T J, Courtice I D, Khouri A G, Crosa J H, Schiewe M H. Serum resistance and hemagglutination ability of marine vibrios pathogenic for fish. Infect Immun. 1981;34:702–707. doi: 10.1128/iai.34.3.702-707.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 31.Viele D, Kerstetter T H, Sullivan J. Adoptive transfer of immunity against Vibrio anguillarum in rainbow trout, Salmo gairdneri Richardson, vaccinated by the immersion method. J Fish Biol. 1980;17:379–386. [Google Scholar]
- 32.Yano T. Assays of hemolytic complement activity. In: Stolen J S, Fletcher T C, Anderson D P, Kaattari S L, Rowley A F, editors. Techniques in fish immunology. Fair Haven, N.J: SOS Publications; 1992. pp. 131–141. [Google Scholar]
- 33.Yano T. The nonspecific immune system: humoral defense. In: Iwama G, Nakanishi T, editors. The fish immune system: organism, pathogen, and environment. San Diego, Calif: Academic Press, Inc.; 1996. pp. 106–157. [Google Scholar]
- 34.Young L S, Stevens P, Ingram J. Functional role of antibody against “core” glycolipid of Enterobacteriaceae. J Clin Investig. 1975;56:850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]


