Abstract
Purpose: Heparin-based regimens are recommended for anticoagulation in hospitalized patients with COVID-19 though a study reported similar mortality with apixaban in critically ill hospitalized COVID-19 patients. Our pilot study sought to determine the differences in all-cause mortality, venous thromboembolism (VTE), and bleeding events between apixaban and therapeutic heparin-based regimens in hospitalized non-critically ill COVID-19 patients. Methods: We conducted a retrospective analysis of non-critically ill COVID-19 patients aged ≥ 18 years admitted to 3 campuses of Montefiore Medical Center during the first (March 2020 to May 2020) and second (January 2021 to February 2021) COVID-19 surges, who received within 48 hours of admission and continued for ≥72 hours a therapeutic dose of low-molecular-weight heparin (LMWH), unfractionated heparin (UFH), or any apixaban dose for VTE prophylaxis. Outcomes data analyzed included mortality, suspected or imaging-confirmed VTE, and bleeding using a defined criteria. Results: Overall, 162 patients met eligibility for analysis. Baseline characteristics were similar between the 2 groups except liver and renal functions. Mortality occurred in 10 (13.3%) patients on apixaban and 23 (26.4%) patients on a heparin-based regimen (P = .059). Confirmed VTE events were not different between the groups (8% vs 13.8%, P = .359), but higher incidence of bleeding occurred in heparin-based group (4% vs 52.9%, P < .001). Conclusion: There were no differences in mortality or confirmed VTE between apixaban and heparin-based regimens except for more bleeding events with the heparins. This study highlights the utility of apixaban in COVID-19.
Keywords: anticoagulants, COVID, disease management, respiratory
Background
Since the onset of the Coronavirus Disease 2019 (COVID-19) pandemic, coagulation abnormalities have been a common finding in affected patients, possibly presenting as disseminated intravascular coagulation (DIC) or other thrombotic complications such as venous thromboembolism (VTE), both with an associated increase in mortality,1-4 COVID-19 increases the risk of thrombotic complications in the venous and arterial vasculature, 1 with the incidence of VTE in COVID-19 patients estimated to be as high as 25% to 27%,2-4 with the intensive care setting estimated to reach 69%. 5
Coagulopathies among COVID-19 patients are associated with elevated levels of fibrinogen, D-dimer, and other acute-phase reactants, 1 and optimal management of anticoagulation is important to improve overall outcomes. Anticoagulation in COVID-19 has been addressed in various guidelines6-8 underscoring the importance of appropriate anticoagulation strategies in COVID-19 patients. Most of these guidelines initially endorsed elevated D-dimer and fibrinogen as key objective markers for thrombotic risk due to their strong correlation with thrombosis and mortality. 3 However, the International Society of Thrombosis and Hemostasis (ISTH), 9 the National Institutes of Health, and American College of Chest Physicians guidelines now recommend thromboprophylaxis with either unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) in all hospitalized non-critically ill COVID-19 patients regardless of VTE risk assessment score unless there are absolute contraindications.3,6-8,10 These guidelines favor the use of heparins against direct oral anticoagulants (DOACs) due to the increased risk of drug-drug interactions with DOACs and the potential declining patient status may worsen bleeding risk. 11 Additionally, DOACs are more difficult to reverse and monitor.
Despite these guideline recommendations, anticoagulation protocols for COVID-19 developed by various institutions also incorporated the use of DOACs in addition to UFH and LMWH to their thromboprophylaxis management strategy. The addition of DOACs was based primarily on their safety and efficacy in other hypercoagulable disease states, the convenience of oral administration as well as lack of required monitoring and less pharmacokinetic (PK) and pharmacodynamic (PD) variability .12,13
Billett et al 14 observed in their study that the DOAC, apixaban, was associated with a reduction in mortality in hospitalized COVID-19 patients when compared to no anticoagulation. Paranjpe et al 15 also suggested that systemic anticoagulation confers a survival benefit in COVID-19 patients regardless of the anticoagulant used, though this study had significant limitations. This conclusion was similar to Kabir 16 who showed early, high-dose anticoagulation in non-critically ill COVID-19 patients improved survival, including with apixaban. Some notable clinical trials on anticoagulation in COVID-19 such as REMAP-CAP, 17 ACTIV4, 18 and ATTACC 19 compared therapeutic heparin-based regimens to the usual care thromboprophylaxis in hospitalized COVID-19 patients without including DOACs as a study arm. Combined results from these trials reported that in non-critically ill patients therapeutic anticoagulation with heparin increased survival to discharge. 20 This leaves the question of the impact of DOACs in non-critically ill COVID-19 patients still unanswered. To build on previous literature and address confounders in previous studies, we examined the safety and efficacy of apixaban compared to therapeutic heparin-based regimens on outcomes in non-critically ill patients hospitalized with COVID-19.
Methods
Study Design
This pilot study was a retrospective cohort study of adult inpatients admitted to the 3 main campuses of Montefiore Medical Center, all located in the Bronx, New York. Data were collected at 2 time periods: the first COVID-19 surge from March 2020 to May 2020, and the second COVID-19 surge from January 2021 to February 2021. Approval for the study was obtained through the institutional review board (IRB #2020-12313).
Patients
Patients ≥18 years of age were included in this study if they were admitted to a general medical floor, if they tested positive for COVID-19 within 72 hours of floor admission, and if they were initiated on either apixaban or a therapeutic heparin-based regimen for at least 72 hours within 48 hours of admission. We excluded patients who within 48 hours of floor admission were either intubated or transferred to the intensive care unit (ICU), identified as palliative care, or had a code status of “do-not-resuscitate/do-not-intubate.” Patients were also excluded if they did not continue their outpatient anticoagulation regimen upon admission or presented with confirmed VTE upon admission. Apixaban was dosed either 5 mg by mouth twice daily or 2.5 mg twice daily based on renal function (Scr > 2.5 mg/dL or CrCl < 30 mL/min) per institutional guideline. The heparin-based regimens were dosed as either low-molecular-weight heparin (LMWH) enoxaparin 1 mg/kg SQ every 12 hours (daily for CrCl < 30 mL/min) or enoxaparin 1.5 mg/kg SQ. Any intravenous infusion of unfractionated heparin (UFH) was administered to a therapeutic aPTT of 46 to 70 following institutional protocol for VTE of 80 units/kg bolus followed by 18 units/kg/h infusion.
Data Sources
All patients eligible for the study were identified in our electronic medical record using Business Universe (SAP BusinessObjects®). Data obtained included index date of admission as well as the type and duration of anticoagulation used. Patients’ baseline demographics, pertinent laboratory markers relevant to coagulopathy, occurrence of bleeding and/or venous thromboembolic (VTE) event, and mortality were obtained through our electronic medical record.
Outcomes
The primary outcome of this study was the difference in all-cause mortality between an apixaban and therapeutic heparin-based regimens from the index date to event occurrence, discharge, or transfer to the ICU. Secondary outcomes assessed were the incidence of venous thromboembolism (VTE) and the incidence of major bleeding between apixaban and heparin-based regimens in hospitalized non-critically ill COVID-19 patients. VTE was determined through either diagnostic imaging (ultrasound and/or CT scan) or by a surrogate indicator (D-dimer and/or fibrinogen elevation) with clinical symptomatology of VTE. Bleeding was determined if the patient was only initiated on pantoprazole IV drip or IV push every 12 hours because of signs and symptoms of gastrointestinal bleeding after anticoagulant initiation, or bleeding defined by the ISTH21,22 criteria as: Fatal bleeding, and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, or pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in hemoglobin level ≥2 g/dL (1.24 mmol/L) or leading to transfusion of ≥2 units of whole blood or red cells.
Statistical Analysis
Collected data were coded and analyzed using OpenEpi® version 3.01. Baseline data on study participants were reported as mean with standard deviation and median with interquartile range for continuous variables, and frequencies and percentages for categorical variables. Differences in continuous variables were assessed using the student t-test and for categorical variables chi-square test or fisher’s exact test.
The primary and secondary outcomes were analyzed using the chi-square test or the Fisher exact test where appropriate. The results of all statistical tests were reported as P-values and odds ratios with confidence intervals as appropriate. A 2-tailed P-value of less than .05 was considered statistically significant.
Results
Baseline Characteristics
During the study period, 162 participants met eligibility criteria and were included in the analysis. This comprised 75 patients on apixaban and 87 on heparin-based regimens. The mean duration of follow up was 42 days. The mean age of the population was 67.9 (±13.8) years with half being male. Rate of acute kidney injury (AKI), AST or ALT> 3 times upper limit of normal, and fibrinogen levels were significantly different among baseline groups. AKI was defined as an increase in serum creatinine by >0.3 mg/dL within 48 hours or an increase by at least 1.5 times from baseline prior to the initiation of anticoagulation in patients. Remdesivir use, steroid use and exposure were not statistically different between the groups (Table 1).
Table 1.
Characteristics of Patients at Baseline.
| Characteristic | Apixaban regimen a (n = 75) | Heparin-based regimen b (n = 87) | P-value |
|---|---|---|---|
| Age, mean (SD), years | 68.4 (13.9) | 67.5 (13.7) | .678 |
| Male, n (%) | 31 (41.3) | 50 (57.5) | .058 |
| Weight (SD), kg | 82.7 (21.5) | 84 (23.3) | .732 |
| Race/ethnicity, n (%) | |||
| White | 7 (9.3) | 19 (21.8) | .114 |
| Black | 29 (38.7) | 23 (26.4) | |
| Hispanic | 25 (33.3) | 27(31) | |
| Others | 14 (18.7) | 18 (20.6) | |
| Comorbidities, n (%) | |||
| History of VTE | 5 (6.7) | 13 (14.9) | .076 |
| Atrial fibrillation | 7 (9.3) | 14 (16.1) | |
| Hypertension | 60 (80) | 68 (78.2) | |
| Cancer | 15 (20) | 8 (9.2) | |
| T2DM | 47 (62.7) | 38 (43.7) | |
| Asthma/COPD | 15 (20) | 14 (16.1) | |
| Immunosuppression | 9 (12) | 5 (5.7) | |
| AKI, n (%) | 21 (28) | 40 (46) | .028 |
| AST or ALT >3 ULN, n (%) | 2 (2.7) | 11 (12.6) | .036 |
| D-dimers, μg/mL, n (%) | |||
| ≤3 | 43 (57.3) | 29 (44.8) | .174 |
| >3 | 29 (38) | 45 (51.7) | |
| Fibrinogen, mean (SD), mg/dL | 675 (203.9) | 604 (189) | .034 |
| Remdesivir use, n (%) | 10 (13.3) | 4 (4.6) | .090 |
| Steroid use, n (%) | 35 (46.7) | 35 (40.2) | .506 |
| Steroid exposure, mean (SD), days | 7 (2.9) | 6 (4) | .068 |
| Anticoagulant use, median (IQR), days | 6 (4,9) | 6 (4,10) | – |
Note. AST = aspartate aminotransferase; ALT = alanine aminotransferase; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; SD = standard deviation; T2DM = type 2 diabetes mellitus; ULN = upper limit of normal; VTE = venous thromboembolism.
Apixaban: Low dose (n = 36), high dose (n = 39).
Heparin-based regimen: UFH (n = 42), LMWH (n = 45).
Primary Outcome
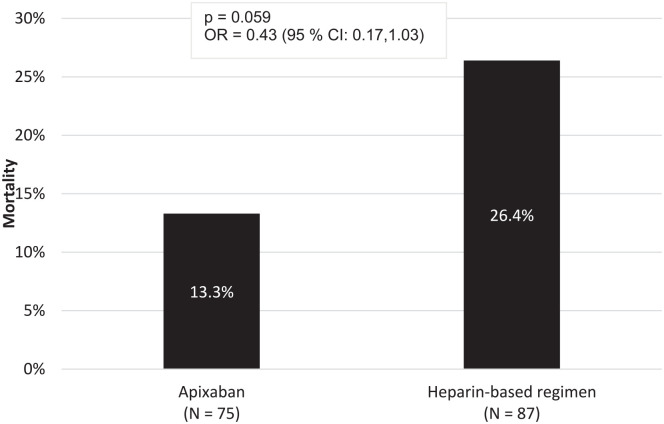
Overall, 33 (20.4%) patients died from the index day of admission till the end of the study. They were made up of 13.3% in the apixaban group and 26.4% in the heparin-based group. Though there was a lower incidence of death in the apixaban group, it was not statistically significant (OR = 0.43, 95% CI: 0.17-1.03, P = .059) (Figure 1). A subgroup analysis comparing the incidence of death among 2.5 mg apixaban, 5 mg apixaban, UFH, and LMWH found no statistically significant difference among the subgroups (χ2 = 4.778, P = .189) (Supplemental Table 2).
Figure 1.
All-cause mortality between apixaban and heparin-based regimen.
Secondary Outcomes
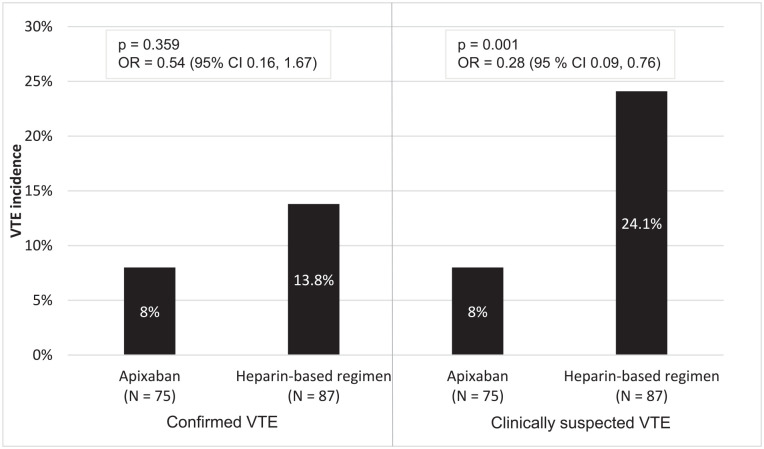
The incidence of confirmed VTE was 8% in the apixaban group compared to 13.8% in the heparin-based group (P = .359). Suspected VTE was 8% in the apixaban group compared to 24.1% in the heparin-based group (P = .001) (Figure 2).
Figure 2.
Confirmed and suspected VTE events between apixaban and heparin-based regimen.
Bleeding occurred in 4% and 52.9% of apixaban and heparin-based regimens respectively (P < .001). Hemoglobin drop ≥2 g/dL in 24 hours after anticoagulant initiation was significantly higher in patients on heparin-based regimen compared to apixaban (P ≤ .001) (Table 2). Hemoglobin drop was then major contributor to overall bleeding rates especially in the heparin-based regimen.
Table 2.
Bleeding Incidence Between Apixaban and Heparin-Based Regimen.
| Characteristics | Apixaban a (N = 75) | Heparin-based regimen b (N = 87) | P-value |
|---|---|---|---|
| Bleeding, n (%) | 3 (4) | 46 (52.9) | <.001 |
| Hemoglobin drop ≥2 g/dL in 24 h, n (%) | 2 (2.7) | 39 (44.8) | <.001 |
| Blood transfusion ≥2 units, n (%) | 0 (0) | 3 (3.4) | .304 |
| Intravenous pantoprazole, n (%) | 1 (1.3) | 4 (4.6) | >.999 |
Apixaban: Low dose (n = 36), high dose (n = 39).
Heparin-based regimen: UFH (n = 42), LMWH (n = 45).
Discussion
Early in the COVID-19 pandemic, DOACs were included as an anticoagulant option in our institution’s COVID-19 management protocols. Apixaban was chosen as the agent of choice in this class because of its better safety profile in patients with impaired renal function and its availability on the hospital formulary.
The primary outcome from this study showed that there was no significant difference in mortality between patients on apixaban and a heparin-based regimen. These results are similar to that of Billet et al 14 which also demonstrated similar in mortality between apixaban and enoxaparin anticoagulation . Our data from Table 1 shows baseline characteristics were similar between the groups except for those admitted with acute kidney injury or AST/ALT ≥ 3 ULN. Significant differences in the agents used in these patients with organ dysfunction reflects their real-world use as the heparins especially UFH as opposed to apixaban are more likely to be selected for therapeutic anticoagulation in patients with renal or hepatic impairment due to risk for excessive anticoagulation with DOACs in these populations and the difficulty of anticoagulant reversal.
Despite the fact that most patients in the apixaban group received remdesivir (Table 1), this agent has been found not to have any impact on overall mortality in COVID-19 patients 23 and as such less likely to affect the results of our primary outcome. Although steroids have been found to reduce overall mortality in COVID-19, 24 steroid use and their exposure time were similar between the groups (Table 1). Thus, the impact of steroids on overall mortality is expected to be similar in both groups. Additionally, data collection was performed at 2 different time periods during the pandemic, during the first COVID surge when standard of care was still evolving with lots of experimental agents being used in COVID-19 management and during the second COVID-19 surge where greater evidence was available to guide care. This ensured that the results of the primary outcome were unduly impacted by standard of care practices in these eras.
For VTE measurement, we chose to report both diagnostic confirmed VTE and clinically suspected VTE to reflect clinical practice. Even though the baseline levels of fibrinogen, a risk factor for developing blood clots, were significantly higher in the apixaban group compared to the heparin-based group (Table 1), confirmed VTE was lower in the apixaban group, though not statistically significant. However, suspected VTE was observed at a significantly higher rate in the heparin-based group than in the apixaban group. Though baseline d-dimer levels were not statistically different between the groups (Table 1), d-dimers greater than 3 μg/mL were seen more often in the patients on heparin-based regimen at baseline underscoring a possible ongoing clotting cascade in the heparin-based group. Additionally, d-dimers and fibrinogen levels were still high at the time of suspected VTE in the heparin-based group (Supplemental Table 3). The aPPT at time of VTE event showed 4 out of 6 on UFH having therapeutic aPPT with the remaining 2 subtherapeutic underscoring the unreliability of aPPT measurements in COVID-19 for UFH. Despite DOACs not currently being recommended as a preferred anticoagulant for the prevention or management of COVID-19 coagulopathy in hospitalized patients, our results did not show any increased risk of VTE with the use of apixaban in non-critically ill hospitalized patients.
Bleeding events were also significantly lower in the apixaban group compared to the heparin-based group, with more significant bleeding in those patients on unfractionated heparin (Supplemental Table 4). A hemoglobin drop greater than 2 mg/dL within 24 hours was significantly higher in the heparin-based regimen group compared to the apixaban group. Blood transfusion ≥2 units or the use of pantoprazole for gastrointestinal bleeding from anticoagulant use were all higher in the heparin-based group. This implied a higher incidence of hemorrhage in the heparin-based group could be due excessive anticoagulation. We also confirmed aPPT at the time of a bleeding event for unfractionated heparin (1/3 therapeutic aPPT, 2/3 supratherapeutic aPPT).
The randomized control trial ACTION compared therapeutic anticoagulation of rivaroxaban or a heparin transitioned to rivaroxaban, versus thromboprophylaxis with a heparin. They found no improvement in clinical outcomes or reduction in death with the therapeutic strategy (0.86 [95% CI: 0.59-1.22], P = .40). 25 Additionally, because the ACTION trial assessed 2 anticoagulation strategies rather than comparing anticoagulants, a crossover was only considered if a patient changed from prophylactic to therapeutic dose (or vice versa) and not between different drugs within the same study group per study protocol. In our study, no patients receiving heparin thromboprophylaxis were included, based on results of prior literature.15,17,18 Also in contrast to our study, compared to rivaroxaban, apixaban has been found to have a lower overall risk of bleeding26,27 and a more recent single-arm study of apixaban use in COVID-19 patients admitted to the ICU found apixaban to be safe and efficacious with no major bleeding events or thrombotic events. 28 As the most preferred anticoagulation approach during hospitalization is still unclear and many off-label and non-evidence-based strategies have been used in institutions to date, our results may provide evidence for further investigation in this clinical setting.
Most similar to our study, Billett et al used logistic regression to show a decrease in mortality with prophylactic use of apixaban (OR = 0.46, P < .001) and enoxaparin (OR = 0.49, P < .001) compared to other anticoagulant strategies. Additionally, therapeutic apixaban was associated with decreased mortality (OR = 0.57, P < .006), similar to the prophylactic regimens. However, these results were taken from the entire cohort including both general medicine and intensive care patients. 14
Our study has several limitations. First being a retrospective study, treatment groups were not allocated randomly creating a selection bias. This bias is seen in the twice as many patients in the heparin group having AKI. Patients with transaminitis were also mostly found in the heparin-based group. Also, as the study took place during 2 different surges of COVID-19, standard treatments and the variants likely changed. We did not have an advantage to monitor the heparin effect with anti-Xa, a more predictable marker of heparin response in COVID-19 patients as aPPT values may be altered from the normal baseline in COVID-19. Some of the patients had comorbidities that required anticoagulation, and this could have an effect on the outcome. We did not account for other medications used by the participants which may have pharmacokinetically or dynamically interacted with the oral anticoagulant or increased the risk of bleeding. This could have impacted the bleeding or VTE incidence in our patients. Additionally, suspected VTE may have been confounded by observation bias from providers. Participants who received various anticoagulants through substitution throughout the hospital stay were also excluded. Therefore, the true impact of anticoagulants when extrapolated to this patient population should be done with caution. Lastly, patients were only followed for the duration of their inpatient stay, limiting our ability to assess any outcomes that occurred post-discharge.
Conclusion
Our study adds to the existing but limited literature in a racially and ethnically diverse population, evaluating optimal anticoagulation strategies in patients with COVID-19 induced coagulopathy. Mortality and VTE events between apixaban and therapeutic heparin-based regimens are not significantly different when used in non-critically ill hospitalized patients. Apixaban, however, appears to have a lower incidence of bleeding compared to heparin-based regimens. More data are needed to better determine this benefit-to-risk profile of apixaban with respect to noncritically ill COVID-19 patients requiring hospitalization.
Supplemental Material
Supplemental material, sj-docx-1-hpx-10.1177_00185787221095764 for Safety and Efficacy Analysis of Apixaban Compared to Heparins in Hospitalized Non-Critically Ill COVID-19 Patients by Daniel Appiah, Nicholas J. Quinn, Emily G. Messing and Keith T. Veltri in Hospital Pharmacy
Footnotes
Authors’ Note: Work by Daniel Appiah was conducted at Montefiore Medical Center, Bronx, NY.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nicholas J. Quinn  https://orcid.org/0000-0002-9703-5845
https://orcid.org/0000-0002-9703-5845
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi: 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi: 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. doi: 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148-150. doi: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743-1746. doi: 10.1111/jth.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. doi: 10.1111/jth.14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The National Institute for Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. 2021. Accessed August 16, 2020. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 8. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72-81. doi: 10.1007/s11239-020-02138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi: 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019. Chest. 2020;158(3):1143-1163. doi: 10.1016/j.chest.2020.05.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872-888. doi: 10.1182/bloodadvances.2020003763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. New Engl J Med. 2019;380(8):711-719. doi: 10.1056/NEJMoa1814468 [DOI] [PubMed] [Google Scholar]
- 13. Cohoon KP, De Sanctis Y, Haskell L, McBane RD, Spiro TE. Rivaroxaban for thromboprophylaxis among patients recently hospitalized for acute infectious diseases: a subgroup analysis of the MAGELLAN study. J Thromb Haemost. 2018;16(7):1278-1287. doi: 10.1111/jth.14146 [DOI] [PubMed] [Google Scholar]
- 14. Billett HH, Reyes-Gil M, Szymanski J, et al. Anticoagulation in COVID-19: Effect of Enoxaparin, heparin, and apixaban on mortality. Thromb Haemost. 2020;120(12):1691-1699. doi: 10.1055/s-0040-1720978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with In-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122-124. doi: 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kabir AA. Anticoagulation is the answer in treating noncritical COVID-19 patients. Open Med. 2021;16(1):1486-1492. doi: 10.1515/med-2021-0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (randomized, embedded, multifactorial adaptive platform trial for community-acquired pneumonia) study. Ann Am Thorac Soc. 2020;17(7):879-891. doi: 10.1513/AnnalsATS.202003-192SD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nct. Anti-thrombotics for adults hospitalized with COVID-19 (ACTIV-4). 2020. Accessed February 12, 2021. https://clinicaltrials.gov/show/NCT04505774
- 19. Houston BL, Lawler PR, Goligher EC, et al. Anti-thrombotic therapy to ameliorate complications of COVID-19 (ATTACC): Study design and methodology for an international, adaptive Bayesian randomized controlled trial. Clin Trials. 2020;17(5):491-500. doi: 10.1177/1740774520943846 [DOI] [PubMed] [Google Scholar]
- 20. Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. New Engl J Med. 2021;385(9):790-802. doi: 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126. doi: 10.1111/jth.13140 [DOI] [PubMed] [Google Scholar]
- 22. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204 [DOI] [PubMed] [Google Scholar]
- 23. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19: final Report. New Engl J Med. 2020;383(19):1813-1826. doi: 10.1056/nejmoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. New Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopes RD, de Barros e Silva PGM, Furtado RHM, et al.; RHM. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253-2263. doi: 10.1016/S0140-6736(21)01203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest. 2016;150(6):1302-1312. doi: 10.1016/j.chest.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 27. Proietti M, Romanazzi I, Romiti GF, Farcomeni A, Lip GYH. Real-world use of apixaban for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2018;49(1):98-106. doi: 10.1161/STROKEAHA.117.018395 [DOI] [PubMed] [Google Scholar]
- 28. Wenzler E, Engineer MH, Yaqoob M, Benken ST. Safety and efficacy of apixaban for therapeutic anticoagulation in critically ill ICU patients with severe COVID-19 respiratory disease. TH Open. 2020;4(4):e376-e382. doi: 10.1055/s-0040-1720962 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hpx-10.1177_00185787221095764 for Safety and Efficacy Analysis of Apixaban Compared to Heparins in Hospitalized Non-Critically Ill COVID-19 Patients by Daniel Appiah, Nicholas J. Quinn, Emily G. Messing and Keith T. Veltri in Hospital Pharmacy