Abstract
The prominence of obesity in the clinical population as well as the strong association of cardiovascular risk factors with obesity has prompted the investigation of the adipose tissue and its physiological contribution to cardiovascular health. A notable finding in these investigations was the discovery of the adipocyte-derived hormone leptin. Leptin is secreted from the adipose tissue, increases in linear fashion in the circulation with increased body mass and is implicated in the development of cardiovascular disease in obesity, notably via pro-hypertensive mechanisms. Leptin stimulates the activation of the sympathetic nervous system in male patients and mice, which has been implicated as the pro-hypertensive pathway for leptin in obesity. However, obese premenopausal females do not exhibit increased sympathetic activation in response to hyperleptinemia in obesity, indicating a sex-discrepancy in mechanisms of obesity-associated hypertension. Our lab recently demonstrated that leptin also induces the adrenal production of aldosterone in a direct fashion and that this pathway leads to the hyperaldosteronemia that is characteristic of obesity. We have also published data that indicate that the implications of leptin-induced aldosterone are of particular impact in obese females. Leptin-mediated hypertension and endothelial dysfunction, a significant predictor of hypertension clinically, require activation of the mineralocorticoid receptor in female mice. The clinical potential of this pathway remains under investigation; however, existing data indicate that a sex discrepancy exists in mechanisms of leptin-mediated hypertension between males and females and that leptin-stimulated aldosterone plays a significant role in females.
1. INTRODUCTION
The adipose tissue was believed to be a strictly energy storage depot and connective tissue by physiologists until landmark studies early in the 1990s demonstrated that adipose tissue acts as an endocrine organ by secreting a number of its own hormones. The principal finding along these lines was first published by Friedman et al. who demonstrated that an obesity-associated hormone, termed leptin, originates from the adipose tissue, increases in the circulation of obese patients and is involved in the regulation of appetite and energy expenditure. The functional roles of leptin have since been expanded to include those involved in inflammation, insulin and glucose homeostasis, sympathetic activity and the control of blood pressure. We recently demonstrated that leptin stimulates the adrenal production of the mineralocorticoid hormone aldosterone, whose levels concurrently increase in obese patients. This newly uncovered function of leptin has opened a new field of research in the evaluation of the implications of leptin-induced aldosterone secretion in the development of cardiovascular diseases in obesity. Many studies have linked hyperleptinemia with hypertension in obese patients and have further provided experimental evidence that leptin plays a central mediating role in the increased risk for hypertension that is driven by obesity. However, clinical evidence indicates that the mechanisms via which leptin leads to hypertension in obesity are sex-discrepant, favoring leptin-induced sympathetic activation mechanisms in men and other, less well understood mechanisms in women. However, evidence is emerging that the relationship of leptin and aldosterone in obesity is likely physiologically implicated in obesity-associated hypertension and cardiovascular health in women.
2. LEPTIN AND ALDOSTERONE IN OBESITY, A NOVEL ROLE TO STIMULATE ALDOSTERONE
The secretion of the adipocyte-derived hormone leptin is upregulated very pronouncedly in obesity. Historically, it was observed that a certain strain of mouse lacking the ob gene, and subsequently lacking production of the Ob peptide, is characterized by a heightened appetite and becomes severely obese (Coleman, 1973; Coleman & Hummel, 1973). The group of Jeffrey Friedman identified in a landmark study the ob gene as the source of an appetite-suppression adipocyte-derived Ob peptide (Zhang et al., 1994). This group went on to show that restoration of the Ob peptide, termed “leptin,” in these mice ameliorates the obese phenotype (Campfield, Smith, Guisez, Devos, & Burn, 1995; Halaas et al., 1995; Pelleymounter et al., 1995), opening the field of research into not just the function of leptin but also the establishment of the adipose tissue as an endocrine organ. It has since been shown that human adipose tissue produces a nonmutated form of the ob gene and, further, that serum leptin levels are closely associated with body fat mass (Considine et al., 1996; Hickey et al., 1996). Based on the initial discovery that leptin is a satiety factor secreted by the adipose tissue, the interest in leptin focused initially on appetite, metabolism, and adiposity. However, leptin exerts a number of extrametabolic effects. The long signaling form of the leptin receptor, initially thought to be expressed in the hypothalamus only, is ubiquitously expressed and leptin has been implicated in the control of immune (Lord et al., 1998), reproductive (Anifandis et al., 2005), bone (Ducy et al., 2000) and cardiovascular function (Hall, do Carmo, da Silva, Wang, & Hall, 2015).
2.1. Aldosterone Levels Increase With Adiposity
In association with the hyperleptinemia that ubiquitously presents in obese patients, aldosterone levels are also elevated with increasing adipose mass in patients. Aldosterone is primarily synthesized in and secreted from the outer layer of the adrenal cortex, the zona glomerulosa, although secondary sources of aldosterone have been identified including the adipose tissue itself (Briones et al., 2012; Silva et al., 2015). Many factors are implicated in the regulation of aldosterone secretion. However, the “classical” stimulators of aldosterone, which include increases in circulating adrenocorticotropin hormone (ACTH), angiotensin II (ANGII) or potassium, are unlikely the underlying mechanism for hyperaldosteronism in obese patients. ACTH sensitivity may be decreased in obesity (Roelfsema, Pijl, Keenan, & Veldhuis, 2012) or unaltered (MacCario et al., 2000), despite characteristically elevated ACTH levels in obese patients, for mechanistic explanations that are currently unknown (Prodam et al., 2013). However, the elevated ACTH levels that are commonly observed in obese patients are also associated with “aldosterone-escape” phenomena. Therefore, sustained ACTH release cannot account for elevated aldosterone levels (Mazzocchi et al., 1986). In addition, elevations in plasma potassium, i.e., hyperkalemia, also induce an increase in aldosterone production physiologically in accordance with the actions of aldosterone on mineralocorticoid receptors in the distal tubule of the renal nephron to promote potassium loss (Roelfsema et al., 2012). However, hypokalemia, rather than hyperkalemia, is more prominent in obesity in humans (Sun et al., 2014). Therefore, it is unlikely that plasma potassium plays a role in aldosterone production regulation in obesity. Furthermore, plasma renin activity, and subsequently plasma ANGII levels, is not correlated with hyperaldosteronism in obese patients (Hiramatsu, Yamada, Ichikawa, Izumiyama, & Nagata, 1981; Rocchini, Katch, Grekin, Moorehead, & Anderson, 1986). Weight loss in obese patients also decreases plasma aldosterone without an associative decrease in plasma renin activity (Goodfriend, Kelley, Goodpaster, & Winters, 1999). Therefore, increases in ANGII do not account for elevations in aldosterone levels in obesity. With the classical regulators of aldosterone secretion ruled out in obese patients, another factor(s) increased in obesity must stimulate aldosterone secretion.
2.2. Hyperleptinemia Increases Aldosterone Production in Animals and Humans
Both aldosterone and leptin plasma levels are increased in response to obesity; therefore, a link between the regulation of the secretion of these hormones is not far-fetched. Aldosterone levels in obese patients increase in association with visceral adipose mass (Goodfriend, Egan, & Kelley, 1999; Goodfriend, Kelley, et al., 1999). Increases in leptin not only correlate with aldosterone in obesity but also predict changes in aldosterone in obese hypertensive patients (de Haro Moraes et al., 2013). These hormones were initially linked by the finding that a factor secreted from adipose tissue stimulates aldosterone synthesis in human adrenocortical cells in vitro (Ehrhart-Bornstein et al., 2003). Collectively, these data indicated that leptin was a candidate hormone capable of stimulating increases in circulating aldosterone levels in obesity via inducing aldosterone synthesis in the adrenal gland.
The first published data implying a role for leptin specifically in the control of aldosterone secretion was that showing that leptin infusion in wild-type C57Bl/6 male and female mice induces an increase in plasma aldosterone levels, both in lean and diet-induced obese mice (Belin de Chantemele, Mintz, Rainey, & Stepp, 2011; Huby et al., 2015). An important model of leptin hypersensitivity, the protein tyrosine phosphatase 1b (Ptp1b) knockout mouse, presents with systemic elevations in leptin receptor activation without hyperleptinemia due to the systemic deletion of Ptp1b, an endogenous molecular “brake” on sustained leptin receptor activity. Leptin sensitivity in female Ptp1b mice is associated with elevations in adrenal zona glomerulosa CYP11B2 (aldosterone synthase) expression as well as elevated aldosterone levels compared to control mice (Huby et al., 2015). Furthermore, endogenous hyperleptinemia in Agouti obese female mice, who notably have functional systemic leptin receptor expression, is also associated with an increase in aldosterone levels, an association also found in hyperleptinemic diet-induced obese mice (Huby et al., 2015). Other transgenic models of obesity in rodents include those in which deleted or mutated leptin signaling results in uncontrolled hyperphagia and obesity. The most prominent models utilized at present are the ob/ob leptin-deficient mouse, the db/db leptin-receptor mutated, and the Zucker leptin-receptor mutated rat. In each of these models, neither adrenal CYP11B2 expression nor aldosterone levels are increased compared to their lean wild-type littermates, despite the presence of pronounced obesity, indicating that adequate leptin signaling is required for obesity to induce elevations in aldosterone production (Huby et al., 2015). Administration of a leptin receptor antagonist, Allo-aca, restores plasma aldosterone levels in Ptp1b-deficient leptin sensitive and Agouti obese female mice to those of their wild-type counterparts (Huby, Otvos, & Belin de Chantemele, 2016), indicating a direct relationship of leptin receptor activation and CYP11B2 activity. The pro-hypertensive effects of leptin have been linked to an increase in renin-angiotensin system activity (Hilzendeger et al., 2012); however, administration of losartan, an ANGII type I receptor antagonist, does not alter aldosterone levels in leptin-infused mice (Huby et al., 2015), indicating that leptin-induced aldosterone is not dependent on increases in ANGII-mediated signaling. Furthermore, the functional role of leptin to stimulate activation of the sympathetic nervous system is well documented (Gautron & Elmquist, 2011; Hall et al., 2010); however, α- and β-adrenergic blockade also does not inhibit leptin-stimulated aldosterone secretion (Huby et al., 2015). Increases in aldosterone production observed in obese mice are, therefore, independent of obesity per se, but rather dependent on leptin signaling.
2.3. Leptin Receptor Activation in Adrenal Cells Stimulates Aldosterone Production
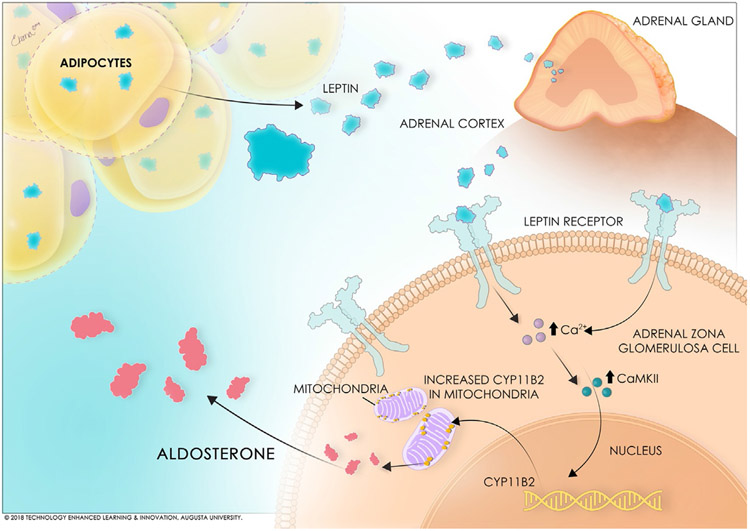
Following these studies indicating an induction of aldosterone production by leptin in in vivo models, the question followed of the cellular and molecular mechanisms whereby leptin stimulates CYP11B2 expression and, subsequently, leads to hyperaldosteronism. Several investigators have demonstrated that functional leptin receptor expression is prominent in adrenal tissues, including in zona glomerulosa cells in which aldosterone is primarily physiologically produced (Glasow et al., 1998; Hoggard et al., 1997; Huby et al., 2015; Malendowicz et al., 2003). Although the proximity of the leptin receptor to the primary CYP11B2 expressing cell type implies a direct relationship of the cell signaling pathway of leptin and this enzyme, our recently published work demonstrated the direct nature of this relationship as is depicted in Fig. 1.
Fig. 1.
Schematic of the stimulation of adrenal aldosterone production by leptin.
We reported that leptin receptors are closely colocalized with CYP11B2 expression in human adrenocortical cells (HAC15 cell line) via fluorescent staining (Huby et al., 2015). Furthermore, leptin administration in the media of HAC15 cells produced a dose-dependent induction of CYP11B2 expression and aldosterone release into the media (Huby et al., 2015), demonstrating an intra-adrenal leptin-derived aldosterone mechanism. Leptin-mediated release of aldosterone by adrenal cells is also likely a calcium-dependent process, which is similar to the mechanism via which ANGII type I receptors stimulate aldosterone production in adrenocortical cells (Huby et al., 2015). We showed that leptin increases calmodulin and calmodulin-dependent protein kinase expressions in HAC15 cells (Huby et al., 2015). Chelation of the intracellular Ca2+ with BAPTA-AM abolishes leptin- as well as ANG II-mediated increases in CYP11B2 promoter activity suggesting that leptin-mediated aldosterone production is Ca2+ maneuverdependent (Huby et al., 2015). This is the first study to investigate leptin receptor-mediated intracellular pathways in adrenal cells that mediate CYP11B2 expression, and therefore prompts further investigation of pathways of calcium-calmodulin signaling and CYP11B2 transcriptional regulation that may also be occurring in adrenal zona glomerulosa cells in response to increased leptin signaling.
Clinical data and characterization of aldosterone-producing disorders indicate that the leptin-aldosterone relationship is linear, i.e., that leptin production is not regulated by aldosterone. It has been observed that congenital adrenal cortical hyperplasia patients, who are characteristically aldosterone deficient, do not have a concurrent reduction in leptin levels (Ariyawatkul, Tepmongkol, Aroonparkmongkol, & Sahakitrungruang, 2017). Furthermore, patients suffering from primary aldosteronism (inappropriate increased aldosterone secretion) are not hyperleptinemic in absence of obesity (Haluzik et al., 2002; Urbanet et al., 2010). Collectively, these data indicate that leptin stimulates adrenal aldosterone production in humans and rodents via upregulation of CYP11B2 expression; however, a feedback mechanism for aldosterone activation to affect leptin production is not implicated.
Although it is tempting to postulate that hyperaldosteronism in obesity is regulated exclusively by increases in the production of leptin by the adipose tissue, it is unlikely that leptin is the only regulator for aldosterone production in obese individuals. Adipose tissue is a highly complex endocrine organ producing a number of adipocyte-derived hormones (adipokines) that include leptin. Other adipokines, such as the immune cytokine interleukin-6, are also increased in obesity and may play a role to stimulate aldosterone secretion (Bastard et al., 1999; Judd et al., 2000; Path, Bornstein, Ehrhart-Bornstein, & Scherbaum, 1997). Bollag et al. demonstrated that very low density lipoproteins (VLDL) stimulate CYP11B2 expression and aldosterone secretion in adrenal cell model systems such as primary cultures of human and bovine adrenal cells and the adrenocortical cell line H295R (Xing et al., 2012). The contributions of these pathways as well as others derived from adipose tissue in obesity remain to be as well elucidated as the pathway currently described for leptin-mediated aldosterone production in adrenal cells. Therefore, further investigation is needed into the individual contributions of various obesity-associated factors to obesity-induced hyperaldosteronism.
3. IMPLICATIONS FOR THE SEX DISCREPANT LEPTIN-ALDOSTERONE RELATIONSHIP IN OBESITY: ROLE FOR REGULATION OF BLOOD PRESSURE IN WOMEN
It is well-accepted that obesity is a significant risk factor for cardiovascular disease. Notably, an increase in body mass index (BMI) elevates the risk for mortality from cardiovascular events such as myocardial infarction or stroke via predisposition to risk factors for such events, notably type II diabetes and hypertension. Importantly, rising rates of hypertension in the clinical population have been directly attributed to rising rates of obesity, with over two-thirds of essential hypertension patients currently presenting with clinical obesity (Henry et al., 2012; Kannel, Zhang, & Garrison, 1990). An interesting complexity to the implications of leptin and aldosterone to the development of cardiovascular disease in obese patients is the sexual discrepancy in both leptin and aldosterone between males and females. Aldosterone and leptin levels are characteristically higher in women compared to men and increased more so per unit of body mass in women as well (Goodfriend, Kelley, et al., 1999). Knowledge of the mechanisms that lead to the sex-discrepancy in the leptin-aldosterone relationship is vital to understanding the physiological implications of these hormones in obese women.
3.1. Adipose Tissue Produces Higher Levels of Leptin in Women
It has long been known that leptin levels are increased in females compared to males, even at comparable BMIs (Considine et al., 1996; Frederich et al., 1995; Hickey et al., 1996; Maffei et al., 1995; Saad et al., 1997). This sexual dimorphism in leptin production is not restricted to certain ages, body compositions or body masses in either rodents (Frederich et al., 1995; Hickey et al., 1996) or humans (Considine et al., 1996; Hickey et al., 1996), implicating sex as a major variable to consider when assessing leptin production. While this sexual dimorphism is well established in the literature, the physiological origin of the differences in leptin production between males and females remains to be fully elucidated. Initially, it was believed that increased leptin in females was attributable to increased propensity for a higher body fat percentage in women compared to men of comparable body mass (Carranza & Earl, 1979; Frederich et al., 1995). However, this concept has been challenged a number of times, with studies showing that correction for body fat percentage indicates a higher production of leptin/gram of fat mass in females compared to males (Frederich et al., 1995; Maffei et al., 1995). In 1995, some light was shed on this phenomenon with Lonnqvist et al. demonstrating that adipose tissues of both lean and obese women intrinsically express the Ob leptin gene at higher levels than males (Lonnqvist, Arner, Nordfors, & Schalling, 1995), giving a physiological explanation for women producing more leptin/gram of adipose tissue than men.
Although differences in adipose-derived leptin production between men and women imply the involvement of sex hormones in leptin production, female sex hormones likely play a minimal role. Havel et al. demonstrated that changes in female sex hormones, namely their depletion as is associated with menopause, are not correlated with changes in leptin production in women (Oliveira, Holloway, Durigon, Collins, & Lal, 1995). This study further showed that premenopausal, postmenopausal, full hysterectomy and hormone replacement therapy status does not predict leptin levels in women (Oliveira et al., 1995), thereby establishing that sex differences in adipocyte leptin production likely are unrelated to female sex hormones.
Emerging evidence suggests that male sex hormones, namely testosterone, may underlie sexual discrepancies in leptin production (Behre, Simoni, & Nieschlag, 1997; Jockenhovel et al., 1997). Studies in transgender individuals have shown that a male to female transition, which is coupled with estrogen supplement and testosterone receptor inhibition, increases body fat and leptin levels whereas, in contrast, a female to male transition characterized by androgen supplementation decreases subcutaneous adipose and reduces leptin levels (Elbers et al., 1997). The primary mechanism via which testosterone decreases leptin production may be via changes in adipose distribution. Testosterone has been shown to repress subcutaneous adipose tissue growth (Elbers et al., 1997; Frederiksen et al., 2012). This role for testosterone may be a significant contributor to the adipose tissue dimorphism that is observed between men and women.
Adipose distribution patterns in women favor subcutaneous adipose deposition (Sasaki & Baba, 1985). Indeed, leptin mRNA expression is higher in subcutaneous fat than in abdominal visceral fat, which is of particular importance given that females have higher subcutaneous adipose mass on average than men (Montague, Prins, Sanders, Digby, & O’Rahilly, 1997; Van Harmelen et al., 1998). Therefore, the contribution of sex adipose tissue deposition and sex hormones (i.e., testosterone) to sex discrepancies in leptin production between males and females is likely multifactorial, as correction for either of these factors eliminates the sex discrepancy in leptin levels (Nagy et al., 1997).
3.2. Obesity Increases the Risk for Hypertension via Leptin-Mediated Mechanisms
Leptin is emerging as a prominent obesity-derived factor mediating the control of blood pressure in obese patients. In the aforementioned models of obesity in rodents in which leptin signaling is nonfunctional, the ob/ob and db/db mouse, hyperphagia and resultant obesity do not present concurrent with hypertension (Belin de Chantemele et al., 2011; Wang, Chandrasekera, & Pippin, 2014). These animal models parallel male and female patients with mutations in human leptin receptor pathways (Greenfield et al., 2009; Ozata, Ozdemir, & Licinio, 1999), whom present as severely obese, but are notably not hypertensive. These data suggest that obesity per se does not increase blood pressure in obesity, but rather that leptin mediates obesity-associated increases in blood pressure. While the implications for hyperleptinemia in the risk of hypertension in obese patients are currently well accepted, the mechanisms that mediate leptin receptor activation and blood pressure elevations are complex, owing at least in part to a distinct sex-discrepancy.
3.3. Leptin Induces Hypertension in Males via Activation of Sympathetic Tone
Increased activation of the sympathetic nervous system is characteristic in obese male patients. Obesity is associated with an increase in muscle sympathetic nerve activity (MSNA), an effect that is mediated by leptin and alleviated with weight loss in males (Hall et al., 2015; Lambert et al., 2007; Straznicky et al., 2011; Tank et al., 2008). Although the neural pathways via which leptin receptor activation leads to an increase in blood pressure are somewhat under debate in the literature at present, it is accepted that leptin induces an activation of sympathetic output from the hypothalamus via activation of leptin receptors in the proopiomelanocortin neurons of the hypothalamic arcuate nucleus, as has been thoroughly reviewed elsewhere (Hall et al., 2015). Although sympathetic drive is most likely implicated in hypertension development in obese males, premenopausal females do not similarly develop increases in sympathetic activation in obesity, an effect that is reversed with menopause (Briant, Charkoudian, & Hart, 2016; Hay, 2016; Lambert et al., 2007). Therefore, the mechanisms for leptin-induced hypertension must involve extra-sympathetic physiological mediators in females.
3.4. Leptin-Mediated Cardiovascular Disease Is Not Mediated by Sympathetic Activation in Women
Despite increased leptin production and evidence pointing to an increased blood pressure sensitivity to leptin in women compared with that in men, neither leptin levels nor obesity is associated with an increase in sympathetic activation in women (Matsumoto, Miyatsuji, Miyawaki, Yanagimoto, & Moritani, 2003; Quilliot, Bohme, Zannad, & Ziegler, 2008). Circulating norepinephrine levels, an index of total sympathetic activation in mice, are decreased in female leptin-sensitive Ptp1b-deficient mice compared to wild-type and are not increased in association with obesity in female Agouti mice. This is in contrast to male obese Agouti mice who do exhibit elevated norepinephrine levels compared to lean mice (Huby, Otvos, & Belin de Chantemele, 2016). Furthermore, the contribution of sympathetic activation to the control of vascular tone is also sex discrepant in obese mice. Obesity in male mice exacerbates blood pressure reductions to ganglionic blockade, indicating a heightened sensitivity to sympathetic vascular tone (Huby et al., 2016). However, female obese Agouti mice have a blunted blood pressure reduction response to ganglionic blockade compared to males (Huby et al., 2016). Hypothalamic leptin receptors are responsible for the appetite regulatory effects of the leptin peptide via activation of the melanocortin 4 receptor (MC4R) which mediates both anorexic and sympathetic actions of leptin. Male MC4R-deficient mice develop obesity and hyperleptinemia but are devoid of hypertension, in contrast to female MC4R deficient rats, whom develop hypertension in association with increased body mass (Maranon et al., 2015). Therefore, the leptin-MC4R sympathetic activation pathway is required for leptin-induced hypertension in male mice, but not in females, raising the question of the female-specific leptin-induced hypertension mediator.
The pathways via which hyperleptinemia leads to hypertension in females are less well understood than those in males; however, strong evidence indicates both that females are more sensitive to leptin-mediated blood pressure increases, and that sympathetic activation is unlikely a major player. In humans, the contribution of leptin to hypertension in women is suggested by studies in Japanese (Fujita & Hata, 2014), Native American (de Simone et al., 2006) and other populations (Sampson et al., 2014) which have demonstrated that hypertension is more strongly associated with changes in BMI, i.e., increases in leptin production, in women than in men. In animal models, evidence for this sex-discrepant leptin-mediated hypertension is apparent in the Ptp1b-deficient leptin-sensitive mouse. Female Ptp1b knockout mice develop hypertension in correlation with increased leptin receptor activation in these mice (Huby et al., 2016). Furthermore, blockade of leptin receptors prevents hypertension in lean female Ptp1b knockout mice implicating, specifically, the leptin receptor pathway in their spontaneous hypertension (Huby et al., 2016). In correlation, hyperleptinemic Agouti obese female mice also develop hypertension in association with increased body mass (Huby et al., 2016). Leptin receptor antagonism ablates increases in blood pressure in Agouti female mice (Huby et al., 2016). Therefore, in both a model of leptin hypersensitivity (Ptp1b knockout) and hyperleptinemia (Agouti obese) female mice are sensitive to leptin-induced elevations in blood pressure; however, until recently, the underlying mechanisms were largely uninvestigated.
3.5. Evidence for Aldosterone-Mineralocorticoid Receptor Activation in the Pathogenesis of Leptin-Mediated Hypertension in Females
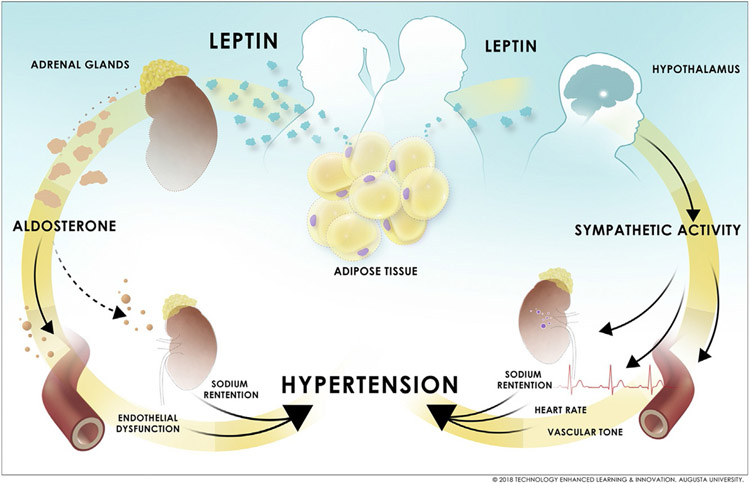
Aldosterone levels are positively associated with hypertension and adiposity, i.e., leptin, in obese women but not in obese men (Kanashiro-Takeuchi, Heidecker, Lamirault, Dharamsi, & Hare, 2009; Vasan et al., 2004). As discussed in the section above, adipose tissue produces leptin more prominently in women; therefore, leptin-stimulated aldosterone production in obese women stimulates an increased induction in aldosterone-mineralocorticoid receptor activation that is specific to females (Fig. 2) (Hamburg et al., 2011). This hypothesis is supported by clinical evidence that indicates that antagonism of aldosterone action via mineralocorticoid receptor blockade is more effective at preventing cardiovascular disease in women compared to men (Kanashiro-Takeuchi et al., 2009). The sex discrepancy in leptin-induced aldosterone production is reproducible in mouse models that have been investigated for the implications of leptin-stimulated aldosterone in the development of hypertension in females: the Ptp1b-deficient leptin sensitive, Agouti obese and diet-induced obese mouse. In each model, leptin hypersensitivity or hyperleptinemia induces an increase in aldosterone level and CYP11B2 expression (Huby et al., 2016). Aldosterone levels are normalized to those of lean females by Allo-aca (leptin receptor antagonist) in female Agouti mice (Huby et al., 2016). Collectively, these data confirm that elevations in aldosterone levels in obese female mice are dependent on hyperleptinemia.
Fig. 2.
Sex-specific mechanisms of leptin-mediated hypertension in obese men and women. © 2018 Technology Enhanced Learning & Innovation, Augusta University.
The functional significance of leptin-induced aldosterone production in females is apparent in studies in rodents in which it has been reported that mineralocorticoid receptor antagonism by spironolactone reduces blood pressure in female Ptp1b-deficient leptin-sensitive mice, while having no effect on blood pressure in males (Huby et al., 2016). Female leptin sensitive Ptp1b-deficient mice and Agouti hyperleptinemic mice present with endothelial dysfunction (impaired vascular relaxation to the endothelium-dependent vasodilator acetylcholine), which is a significant risk factor for hypertension (Dharmashankar & Widlansky, 2010; Hamburg et al., 2011). Mineralocorticoid receptor antagonism restores endothelial function in females of both models to those of controls (Huby et al., 2016). The female propensity for leptin-mediated increases in endothelial dysfunction is implicated in that neither Agouti nor Ptp1b-deficient male mice develop endothelial dysfunction (Huby et al., 2016). In female obese mice that do not produce endogenous leptin, ob/ob mice, no development of endothelial dysfunction is observed; however, restoration of leptin to these mice induces endothelial dysfunction, an effect that is subsequently reversed by spironolactone treatment (Huby et al., 2016). Endothelial dysfunction is a risk factor for hypertension, and accordingly, both Ptp1b female and Agouti obese female mice are hypertensive (Huby et al., 2016). This increase in blood pressure in Agouti mice is prevented by either spironolactone or Allo-aca treatment, indicating that both aldosterone and leptin signaling pathways are required for the increase in blood pressure observed in these female mice (Huby et al., 2016). Collectively, these data imply that leptin-induced hypertension in females may develop via an aldosterone-dependent mechanism that is at minimum associated with, if not caused by, endothelial dysfunction.
4. CONCLUSION AND PERSPECTIVES
It has long been known that the renin-angiotensin aldosterone system is dysregulated in obesity and that a concurrent characteristic rise in aldosterone levels presents alongside hyperleptinemia in obese patients. However, the emergence of a relationship between leptin and aldosterone that extends to an adrenal intracellular relationship is a novel concept only recently investigated. However, emerging data indicate that the regulation of aldosterone levels by leptin is of significant clinical importance, particularly for the ongoing investigation of sex discrepant mechanisms of obesity associated hypertension. The most prevalent and highly utilized therapies for hypertension treatment at present are primarily restricted to angiotensin II inhibition by angiotensin II type I receptor blockers or angiotensin converting enzyme (ACE) inhibitors (Reisin et al., 1997), while secondary therapy options include adrenergic blockade (Wofford, Anderson, Jones, Miller, & Hall, 2001) and thiazide diuretics (James et al., 2014), which are efficacious in many obese patients. However, clinical data indicate that obese women may be more responsive to mineralocorticoid receptor antagonists for treatment of hypertension than obese men (Khosla, Kalaitzidis, & Bakris, 2009), indicating that this pathway not only plays a significant role in vascular tone in women in obesity but also represents a promising therapeutic target. At this time, no clinical report has investigated sex differences in leptin inhibition on blood pressure in the clinical population; however, emerging data indicate a need for such studies.
ACKNOWLEDGMENTS
We would like to thank Lynsey Ekema, MSMI, CMI, Department of Technology Enhanced Learning and Innovation at Augusta University, for assistance in preparing the medical illustration. Financial support and sponsorship: this work was supported by NIH 1R01HL130301-01, AHA 16IRG27770047, 1 F32 HL136191-01A1 and AHA 17POST33410363.
REFERENCES
- Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, et al. (2005). Estradiol and leptin as conditional prognostic IVF markers. Reproduction, 129(4), 531–534. 10.1530/rep.1.00567. [DOI] [PubMed] [Google Scholar]
- Ariyawatkul K, Tepmongkol S, Aroonparkmongkol S, & Sahakitrungruang T (2017). Cardio-metabolic risk factors in youth with classical 21-hydroxylase deficiency. European Journal of Pediatrics, 176(4), 537–545. 10.1007/s00431-017-2875-2. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, & Oberlin F (1999). Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation, 99(16), 2221–2222. [PubMed] [Google Scholar]
- Behre HM, Simoni M, & Nieschlag E (1997). Strong association between serum levels of leptin and testosterone in men. Clinical Endocrinology, 47(2), 237–240. [DOI] [PubMed] [Google Scholar]
- Belin de Chantemele EJ, Mintz JD, Rainey WE, & Stepp DW (2011). Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension, 58(2), 271–279. 10.1161/HYPERTENSIONAHA.110.168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briant LJ, Charkoudian N, & Hart EC (2016). Sympathetic regulation of blood pressure in normotension and hypertension: When sex matters. Experimental Physiology, 101(2), 219–229. 10.1113/EP085368. [DOI] [PubMed] [Google Scholar]
- Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, et al. (2012). Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension, 59(5), 1069–1078. 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, & Burn P (1995). Recombinant mouse OB protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science, 269(5223), 546–549. [DOI] [PubMed] [Google Scholar]
- Carranza A, & Earl PR (1979). Comment on numbers of small and large lymphocytes of goats after thymectomy. Transactions of the American Microscopical Society, 98(4), 595–596. [PubMed] [Google Scholar]
- Coleman DL (1973). Effects of parabiosis of obese with diabetes and normal mice. Diabetologia, 9(4), 294–298. [DOI] [PubMed] [Google Scholar]
- Coleman DL, & Hummel KP (1973). The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia, 9(4), 287–293. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. (1996). Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England Journal of Medicine, 334(5), 292–295. 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- de Haro Moraes C, Figueiredo VN, de Faria AP, Barbaro NR, Sabbatini AR, Quinaglia T, et al. (2013). High-circulating leptin levels are associated with increased blood pressure in uncontrolled resistant hypertension. Journal of Human Hypertension, 27(4), 225–230. 10.1038/jhh.2012.29. [DOI] [PubMed] [Google Scholar]
- de Simone G, Devereux RB, Chinali M, Roman MJ, Best LG, Welty TK, et al. (2006). Risk factors for arterial hypertension in adults with initial optimal blood pressure: The Strong Heart Study. Hypertension, 47(2), 162–167. 10.1161/01.HYP.0000199103.40105.b5. [DOI] [PubMed] [Google Scholar]
- Dharmashankar K, & Widlansky ME (2010). Vascular endothelial function and hypertension: Insights and directions. Current Hypertension Reports, 12(6), 448–455. 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. (2000). Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell, 100(2), 197–207. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. (2003). Human adipocytes secrete mineralocorticoid-releasing factors. Proceedings of the National Academy of Sciences of the United States of America, 100(24), 14211–14216. 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers JM, Asscheman H, Seidell JC, Frolich M, Meinders AE, & Gooren LJ (1997). Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. The Journal of Clinical Endocrinology and Metabolism, 82(10), 3267–3270. 10.1210/jcem.82.10.4284. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, & Flier JS (1995). Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nature Medicine, 1(12), 1311–1314. [DOI] [PubMed] [Google Scholar]
- Frederiksen L, Hojlund K, Hougaard DM, Mosbech TH, Larsen R, Flyvbjerg A, et al. (2012). Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. European Journal of Endocrinology, 166(3), 469–476. 10.1530/EJE-11-0565. [DOI] [PubMed] [Google Scholar]
- Fujita M, & Hata A (2014). Sex and age differences in the effect of obesity on incidence of hypertension in the Japanese population: A large historical cohort study. Journal of the American Society of Hypertension, 8(1), 64–70. 10.1016/j.jash.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Gautron L, & Elmquist JK (2011). Sixteen years and counting: An update on leptin in energy balance. The Journal of Clinical Investigation, 121(6), 2087–2093. 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasow A, Haidan A, Hilbers U, Breidert M, Gillespie J, Scherbaum WA, et al. (1998). Expression of Ob receptor in normal human adrenals: Differential regulation of adrenocortical and adrenomedullary function by leptin. The Journal of Clinical Endocrinology and Metabolism, 83(12), 4459–4466. 10.1210/jcem.83.12.5337. [DOI] [PubMed] [Google Scholar]
- Goodfriend TL, Egan BM, & Kelley DE (1999). Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 60(5–6), 401–405. [DOI] [PubMed] [Google Scholar]
- Goodfriend TL, Kelley DE, Goodpaster BH, & Winters SJ (1999). Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obesity Research, 7(4), 355–362. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, et al. (2009). Modulation of blood pressure by central melanocortinergic pathways. The New England Journal of Medicine, 360(1), 44–52. 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. (1995). Weight-reducing effects of the plasma protein encoded by the obese gene. Science, 269(5223), 543–546. [DOI] [PubMed] [Google Scholar]
- Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, et al. (2010). Obesity-induced hypertension: Role of sympathetic nervous system, leptin, and melanocortins. The Journal of Biological Chemistry, 285(23), 17271–17276. 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, do Carmo JM, da Silva AA, Wang Z, & Hall ME (2015). Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circulation Research, 116(6), 991–1006. 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluzik M, Sindelka G, Widimsky J Jr., Prazny M, Zelinka T, & Skrha J (2002). Serum leptin levels in patients with primary hyperaldosteronism before and after treatment: Relationships to insulin sensitivity. Journal of Human Hypertension, 16(1), 41–45. 10.1038/sj.jhh.1001292. [DOI] [PubMed] [Google Scholar]
- Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, et al. (2011). Relation of brachial and digital measures of vascular function in the community: The Framingham heart study. Hypertension, 57(3), 390–396. 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M (2016). Sex, the brain and hypertension: Brain oestrogen receptors and high blood pressure risk factors. Clinical Science (London, England), 130(1), 9–18. 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- Henry SL, Barzel B, Wood-Bradley RJ, Burke SL, Head GA, & Armitage JA (2012). Developmental origins of obesity-related hypertension. Clinical and Experimental Pharmacology & Physiology, 39(9), 799–806. 10.1111/j.1440-1681.2011.05579.x. [DOI] [PubMed] [Google Scholar]
- Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, et al. (1996). Gender differences in serum leptin levels in humans. Biochemical and Molecular Medicine, 59(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, et al. (2012). A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. American Journal of Physiology. Heart and Circulatory Physiology, 303(2), H197–H206. 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Yamada T, Ichikawa K, Izumiyama T, & Nagata H (1981). Changes in endocrine activities relative to obesity in patients with essential hypertension. Journal of the American Geriatrics Society, 29(1), 25–30. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, & Williams LM (1997). Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochemical and Biophysical Research Communications, 232(2), 383–387. 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. (2015). Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation, 132(22), 2134–2145. 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- Huby AC, Otvos L Jr., & Belin de Chantemele EJ (2016). Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension, 67(5), 1020–1028. 10.1161/HYPERTENSIONAHA.115.06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. (2014). 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA, 311(5), 507–520. 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Jockenhovel F, Blum WF, Vogel E, Englaro P, Muller-Wieland D, Reinwein D, et al. (1997). Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. The Journal of Clinical Endocrinology and Metabolism, 82(8), 2510–2513. 10.1210/jcem.82.8.4174. [DOI] [PubMed] [Google Scholar]
- Judd AM, Call GB, Barney M, McIlmoil CJ, Balls AG, Adams A, et al. (2000). Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Annals of the New York Academy of Sciences, 917, 628–637. [DOI] [PubMed] [Google Scholar]
- Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, & Hare JM (2009). Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clinical and Translational Science, 2(2), 134–142. 10.1111/j.1752-8062.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Zhang T, & Garrison RJ (1990). Is obesity-related hypertension less of a cardiovascular risk? The Framingham study. American Heart Journal, 120(5), 1195–1201. [DOI] [PubMed] [Google Scholar]
- Khosla N, Kalaitzidis R, & Bakris GL (2009). Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. American Journal of Nephrology, 30(5), 418–424. 10.1159/000237742. [DOI] [PubMed] [Google Scholar]
- Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, et al. (2007). Gender differences in sympathetic nervous activity: Influence of body mass and blood pressure. Journal of Hypertension, 25(7), 1411–1419. 10.1097/HJH.0b013e3281053af4. [DOI] [PubMed] [Google Scholar]
- Lonnqvist F, Arner P, Nordfors L, & Schalling M (1995). Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nature Medicine, 1(9), 950–953. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, & Lechler RI (1998). Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature, 394(6696), 897–901. 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- MacCario M, Grottoli S, Divito L, Rossetto R, Tassone F, Ganzaroli C, et al. (2000). Adrenal responsiveness to high, low and very low ACTH 1-24 doses in obesity. Clinical Endocrinology, 53(4), 437–444. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. (1995). Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine, 1(11), 1155–1161. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Neri G, Markowska A, Hochol A, Nussdorfer GG, & Majchrzak M (2003). Effects of leptin and leptin fragments on steroid secretion of freshly dispersed rat adrenocortical cells. The Journal of Steroid Biochemistry and Molecular Biology, 87(4–5), 265–268. [DOI] [PubMed] [Google Scholar]
- Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, et al. (2015). Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 308(8), R708–R713. 10.1152/ajpregu.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Miyatsuji A, Miyawaki T, Yanagimoto Y, & Moritani T (2003). Potential association between endogenous leptin and sympatho-vagal activities in young obese Japanese women. American Journal of Human Biology, 15(1), 8–15. 10.1002/ajhb.10111. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Rebuffat P, Robba C, Gottardo G, & Nussdorfer GG (1986). Short- and long-term effects of ACTH on the adrenal zona glomerulosa of the rat. A coupled stereological and enzymological study. Cell and Tissue Research, 243(2), 303–310. [DOI] [PubMed] [Google Scholar]
- Montague CT, Prins JB, Sanders L, Digby JE, & O’Rahilly S (1997). Depot- and sex-specific differences in human leptin mRNA expression: Implications for the control of regional fat distribution. Diabetes, 46(3), 342–347. [DOI] [PubMed] [Google Scholar]
- Nagy TR, Gower BA, Trowbridge CA, Dezenberg C, Shewchuk RM, & Goran MI (1997). Effects of gender, ethnicity, body composition, and fat distribution on serum leptin concentrations in children. The Journal of Clinical Endocrinology and Metabolism, 82(7), 2148–2152. 10.1210/jcem.82.7.4077. [DOI] [PubMed] [Google Scholar]
- Oliveira DA, Holloway BP, Durigon EL, Collins WE, & Lal AA (1995). Polymerase chain reaction and a liquid-phase, nonisotopic hybridization for species-specific and sensitive detection of malaria infection. The American Journal of Tropical Medicine and Hygiene, 52(2), 139–144. [DOI] [PubMed] [Google Scholar]
- Ozata M, Ozdemir IC, & Licinio J (1999). Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. The Journal of Clinical Endocrinology and Metabolism, 84(10), 3686–3695. 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Path G, Bornstein SR, Ehrhart-Bornstein M, & Scherbaum WA (1997). Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: Expression and effects on steroidogenesis. The Journal of Clinical Endocrinology and Metabolism, 82(7), 2343–2349. 10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. (1995). Effects of the obese gene product on body weight regulation in ob/ob mice. Science, 269(5223), 540–543. [DOI] [PubMed] [Google Scholar]
- Prodam F, Ricotti R, Agarla V, Parlamento S, Genoni G, Balossini C, et al. (2013). High-end normal adrenocorticotropic hormone and cortisol levels are associated with specific cardiovascular risk factors in pediatric obesity: A cross-sectional study. BMC Medicine, 11, 44. 10.1186/1741-7015-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliot D, Bohme P, Zannad F, & Ziegler O (2008). Sympathetic-leptin relationship in obesity: Effect of weight loss. Metabolism, 57(4), 555–562. 10.1016/j.metabol.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Reisin E, Weir MR, Falkner B, Hutchinson HG, Anzalone DA, & Tuck ML (1997). Lisinopril versus hydrochlorothiazide in obese hypertensive patients: A multicenter placebo-controlled trial. Treatment in Obese Patients With Hypertension (TROPHY) Study Group. Hypertension, 30(1 Pt. 1), 140–145. [DOI] [PubMed] [Google Scholar]
- Rocchini AP, Katch VL, Grekin R, Moorehead C, & Anderson J (1986). Role for aldosterone in blood pressure regulation of obese adolescents. The American Journal of Cardiology, 57(8), 613–618. [DOI] [PubMed] [Google Scholar]
- Roelfsema F, Pijl H, Keenan DM, & Veldhuis JD (2012). Diminished adrenal sensitivity and ACTH efficacy in obese premenopausal women. European Journal of Endocrinology, 167(5), 633–642. 10.1530/EJE-12-0592. [DOI] [PubMed] [Google Scholar]
- Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, et al. (1997). Sexual dimorphism in plasma leptin concentration. The Journal of Clinical Endocrinology and Metabolism, 82(2), 579–584. 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- Sampson UK, Edwards TL, Jahangir E, Munro H, Wariboko M, Wassef MG, et al. (2014). Factors associated with the prevalence of hypertension in the southeastern United States: Insights from 69,211 blacks and whites in the Southern Community Cohort Study. Circulation. Cardiovascular Quality and Outcomes, 7(1), 33–54. 10.1161/CIRCOUTCOMES.113.000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki TA, & Baba Y (1985). Chemical-state studies of Zr and Nb surfaces exposed to hydrogen ions. Physical Review B: Condensed Matter, 31(2), 791–797. [DOI] [PubMed] [Google Scholar]
- Silva MA, Cau SB, Lopes RA, Manzato CP, Neves KB, Bruder-Nascimento T, et al. (2015). Mineralocorticoid receptor blockade prevents vascular remodelling in a rodent model of type 2 diabetes mellitus. Clinical Science (London, England), 129(7), 533–545. 10.1042/CS20140758. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Grima MT, Eikelis N, Nestel PJ, Dawood T, Schlaich MP, et al. (2011). The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. The Journal of Clinical Endocrinology and Metabolism, 96(3), E503–E508. 10.1210/jc.2010-2204. [DOI] [PubMed] [Google Scholar]
- Sun K, Su T, Li M, Xu B, Xu M, Lu J, et al. (2014). Serum potassium level is associated with metabolic syndrome: A population-based study. Clinical Nutrition, 33(3), 521–527. 10.1016/j.clnu.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Tank J, Heusser K, Diedrich A, Hering D, Luft FC, Busjahn A, et al. (2008). Influences of gender on the interaction between sympathetic nerve traffic and central adiposity. The Journal of Clinical Endocrinology and Metabolism, 93(12), 4974–4978. 10.1210/jc.2007-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanet R, Pilon C, Calcagno A, Peschechera A, Hubert EL, Giacchetti G, et al. (2010). Analysis of insulin sensitivity in adipose tissue of patients with primary aldosteronism. The Journal of Clinical Endocrinology and Metabolism, 95(8), 4037–4042. 10.1210/jc.2010-0097. [DOI] [PubMed] [Google Scholar]
- Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, et al. (1998). Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes, 47(6), 913–917. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, et al. (2004). Relations of serum aldosterone to cardiac structure: Gender-related differences in the Framingham Heart Study. Hypertension, 43(5), 957–962. 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- Wang B, Chandrasekera PC, & Pippin JJ (2014). Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Current Diabetes Reviews, 10(2), 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford MR, Anderson DC Jr., Brown CA, Jones DW, Miller ME, & Hall JE (2001). Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. American Journal of Hypertension, 14(7 Pt. 1), 694–698. [DOI] [PubMed] [Google Scholar]
- Xing Y, Rainey WE, Apolzan JW, Francone OL, Harris RB, & Bollag WB (2012). Adrenal cell aldosterone production is stimulated by very-low-density lipoprotein (VLDL). Endocrinology, 153(2), 721–731. 10.1210/en.2011-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, & Friedman JM (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505), 425–432. 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]