Abstract
Saccharomyces boulardii is a nonpathogenic yeast used in the treatment of Clostridium difficile diarrhea and colitis. We have reported that S. boulardii inhibits C. difficile toxin A enteritis in rats by releasing a 54-kDa protease which digests the toxin A molecule and its brush border membrane (BBM) receptor (I. Castagliuolo, J. T. LaMont, S. T. Nikulasson, and C. Pothoulakis, Infect. Immun. 64:5225–5232, 1996). The aim of this study was to further evaluate the role of S. boulardii protease in preventing C. difficile toxin A enteritis in rat ileum and determine whether it protects human colonic mucosa from C. difficile toxins. A polyclonal rabbit antiserum raised against purified S. boulardii serine protease inhibited by 73% the proteolytic activity present in S. boulardii conditioned medium in vitro. The anti-protease immunoglobulin G (IgG) prevented the action of S. boulardii on toxin A-induced intestinal secretion and mucosal permeability to [3H]mannitol in rat ileal loops, while control rabbit IgG had no effect. The anti-protease IgG also prevented the effects of S. boulardii protease on digestion of toxins A and B and on binding of [3H]toxin A and [3H]toxin B to purified human colonic BBM. Purified S. boulardii protease reversed toxin A- and toxin B-induced inhibition of protein synthesis in human colonic (HT-29) cells. Furthermore, toxin A- and B-induced drops in transepithelial resistance in human colonic mucosa mounted in Ussing chambers were reversed by 60 and 68%, respectively, by preexposing the toxins to S. boulardii protease. We conclude that the protective effects of S. boulardii on C. difficile-induced inflammatory diarrhea in humans are due, at least in part, to proteolytic digestion of toxin A and B molecules by a secreted protease.
Clostridium difficile is the causative agent of antibiotic-associated colitis in humans and animals (1, 2). Following antibiotic intake by animals and humans, C. difficile colonizes the intestine and releases two potent protein exotoxins, toxin A and toxin B, which mediate diarrhea and colitis caused by this microbe (16, 20, 24). Although both toxins A and B are potent cytotoxins (20, 27, 15, 32) and induce release of inflammatory mediators from immune cells in vitro (19), only toxin A possesses enterotoxic effects in rodent intestine (38). Injection of toxin A into rat intestinal loops causes fluid secretion, increased mucosal permeability, mucosal damage (7, 17, 38), and release of inflammatory mediators from lamina propria immune cells (8, 9). However, a recent in vitro study showed that toxin B and to a lesser extent toxin A are able to cause tissue damage and electrophysiologic changes in normal human colon in vitro (32), suggesting that both C. difficile toxins are involved in the pathophysiology of human colitis.
Saccharomyces boulardii, a nonpathogenic yeast, is effective in the prevention and treatment of many forms of diarrhea in humans, especially antibiotic-associated diarrhea and colitis (6, 25, 36). Recent studies showed that S. boulardii administration significantly reduced the frequency of diarrhea in patients administered antibiotic therapy and that in combination with vancomycin or metronidazole it reduced the number of relapses of C. difficile infection (26). The mechanism by which S. boulardii mediates its protective intestinal effects has been investigated (10, 11, 12, 13, 22, 37). We previously reported that oral administration of S. boulardii to rats diminished ileal fluid secretion and mucosal damage in response to intraluminal administration of purified toxin A (29). Subsequently, we reported that these protective effects of S. boulardii in rat ileum appeared to be mediated by a 54-kDa serine protease which cleaves toxin A and its intestinal receptor (7).
The present study was undertaken to further elucidate the role of the 54-kDa S. boulardii protease in toxin A-mediated enteritis in rat ileum with a polyclonal antibody directed against the purified S. boulardii protease. We also determined whether this protease has a role in protecting the human colon from the effects of C. difficile toxins A and B. We demonstrate here that toxin A- and B-induced electrophysiologic and cytotoxic effects in human colon are also markedly attenuated by preincubating the C. difficile toxins A and B with purified S. boulardii protease prior to addition to human colonic mucosa.
MATERIALS AND METHODS
Male Wistar rats weighing 200 to 250 g were obtained from Charles River Breeding Laboratories (Wilmington, Mass.). Before the experiments rats were fasted overnight but had free access to water. New Zealand White rabbits used to generate antiserum against S. boulardii protease were obtained from Hare-Marland Laboratories (Hewit, N.J.). Pentobarbital sodium (Nembutal; 50 μg/ml) was obtained from Abbott (North Chicago, Ill.). Sabouraud dextrose broth for culturing S. boulardii was obtained from Difco (Detroit, Mich.). A bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.) was used for measuring protein concentrations. The Bolton-Hunter reagent for toxin labeling (N-succinimidyl [2,3-3H]propionate, 80 Ci/mmol) was purchased from Amersham International (Amersham, England). Fetal calf serum and other tissue culture supplies were obtained from Sigma Diagnostics (St. Louis, Mo.).
Toxin A and B purification and radiolabeling.
Toxins A and B were purified from culture supernatants of C. difficile VPI strain 10463 (American Type Culture Collection, Rockville, Md.) as previously described (7–9, 27). Toxin A and toxin B were radiolabeled with tritium with the Bolton-Hunter reagent as previously described by us (30). Both tritiated toxins retained their cytotoxic activity against rabbit lung R9ab fibroblasts (30). Purity of unlabeled and labeled toxins was assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described by Laemmli (18). Purified toxin A and toxin B preparations contained single protein bands at ∼300 and 270 kDa, respectively.
Purification of S. boulardii protease.
S. boulardii was provided from Biocodex Laboratories (Montrouge, France) as a lyophilized powder. S. boulardii was reconstituted in Sabouraud dextrose broth (1 g in 10 ml of medium) and cultured at 37°C as previously described (7, 29). S. boulardii conditioned medium was obtained after 48 h by centrifuging the yeast culture (1,000 × g for 10 min at 4°C) and filtering the supernatant through a 0.2-μm-pore-size filter. S. boulardii protease was purified as previously described by us (7). Briefly, S. boulardii conditioned medium was concentrated fivefold on a Amicon PM-50 filter (Gelmans Scientific), size fractionated on a G-50 gel filtration column (Sigma), and finally purified on a Octyl-Sepharose CL-4B column (Pharmacia Biotech, Uppsala, Sweden).
Preparation of polyclonal antibodies against purified S. boulardii serine protease.
S. boulardii protease, purified as described above, was used to immunize adult male rabbits. Ten micrograms of the antigen in 10 mM Tris buffer was mixed 1:1 (vol/vol) with adjuvant (Ribi ImmunoChem Research, Inc., Hamilton, Mont.) in distilled water according to the manufacturer’s recommendations. Animals were injected subcutaneously (250 μl per thigh) with the protease-adjuvant mixture every 2 weeks for a period of 4 weeks and then every month for 2 months. One rabbit was immunized with vehicle plus adjuvant alone (control). Sera were collected 7 days after the last two injections, and the immunoglobulin G (IgG) fractions were purified on a protein A-Sepharose column (Pharmacia Biotech). Titers of anti-S. boulardii protease antibodies were determined in vitro by enzyme-linked immunosorbent assay. Fifty microliters of either S. boulardii conditioned medium (50 to 1 μg/well), purified S. boulardii protease (1 to 10 μg/well), or trypsin from bovine pancreas (10 μg/well; Sigma), which served as a protease control, was dissolved in carbonate buffer (pH 8.0), added onto 96-well plates, and incubated for 16 h at 4°C. Wells were then washed four times with 1× phosphate-buffered saline (PBS) containing 0.25% Tween 20 (PBS-T), blocked (2 h at 37°C) in PBS-T containing 2% bovine serum albumin, and then incubated (1 h at 37°C) with anti-S. boulardii protease IgG or control rabbit IgG. After the plates were washed four times with PBS-T, goat anti-rabbit IgG conjugated to horseradish peroxidase (1:5,000) was added and the mixture was incubated for 1 h at 37°C. Wells were then washed six times, and 50 μl of peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was added to each well. The reaction was stopped after 5 to 10 min by addition of PHO3 (1 M), and absorbance at 450 nm was then recorded.
Effect of anti-S. boulardii protease IgG on the proteolytic activity of S. boulardii conditioned medium.
S. boulardii conditioned medium (50 μg) was incubated (22°C) with anti-S. boulardii protease or control rabbit IgG. After 1 h of incubation, the proteolytic activity present in the conditioned medium was determined by the method of Roth et al. (34) as previously described by us (7). Briefly, the mixtures were incubated (37°C) with the nonspecific protease substrate [methyl-14C]methemoglobin (1 μg, 50,000 dpm) in 1 ml of 0.2 M acetate buffer (pH 3.8) in a shaking water bath. After 20 min, the reaction was terminated by adding 0.2 ml of 50% trichloroacetic acid (TCA) and cooling the samples on ice. The reaction mixtures were then centrifuged (700 × g for 30 min at 4°C), and 0.25 ml of the supernatant containing small methemoglobin peptides released by proteolytic digestion was assayed for radioactivity by scintillation counting.
Effect of anti-S. boulardii protease IgG on S. boulardii-mediated protection of C. difficile toxin A enteritis.
To further ascertain the role of S. boulardii serine protease in toxin A enteritis, we studied the effect of anti-S. boulardii protease IgG on the protective effects of S. boulardii in toxin A-induced enteritis in rats. S. boulardii conditioned medium (3.0 mg in 200 μl) was incubated (22°C for 1 h) with anti-S. boulardii protease IgG, control rabbit IgG (1:1,000 for both), or saline alone. At the end of the incubation period 5 μg of toxin A in 50 mM Tris buffer or buffer alone was added and incubated for an additional hour at 37°C. A midline abdominal incision was performed on anesthetized rats as previously described, and two 5-cm-long closed ileal loops were formed (7, 29). Renal excretion of [3H]mannitol was prevented by closing the renal pedicles with silk, and [3H]mannitol (10 μCi) was injected intravenously. Each ileal loop was then injected (400 μl) with one of the toxin A-S. boulardii mixtures described above, the abdominal incision was sutured, and animals were kept under light anesthesia while the body temperature was maintained at 37°C by a heating pad. After 4 h animals were sacrificed by an intraperitoneal bolus of pentobarbital and the loops were removed. Intestinal permeability was determined as blood-to-lumen excretion of [3H]mannitol, and results were expressed as disintegration of [3H]mannitol per minute per centimeter of loop as previously described (7, 8, 29, 38). Intestinal fluid secretion was measured as the ratio of loop weight (milligrams) to length (centimeter) as previously described (7, 8, 38). The study was approved by the Beth Israel and Deaconess Medical Center Institutional Animal Care and Use Committee.
Effect of anti-S. boulardii protease IgG on S. boulardii-mediated proteolytic activity against purified C. difficile toxins A and B.
We previously reported that S. boulardii protease digests toxin A in vitro (7). However, since both toxin A and toxin B damage human colonic mucosae in vitro (32), we examined whether S. boulardii protease also digests toxin B. The effect of S. boulardii protease on C. difficile toxins A and B was determined by a modification (7) of the method of Roth et al. (34). Tritiated toxin A or B (0.1 μg containing 40,000 dpm) was incubated for 30 min at 37°C with or without the S. boulardii conditioned medium (0.1 mg/ml) or purified protease (0.1 μg/ml) in 1 ml of 50 mM Tris buffer (pH 7.4). The reaction was halted by adding 0.2 ml of 50% TCA, and samples were placed on ice for 30 min. Samples were then centrifuged (700 × g for 30 min at 4°C), and 0.25-ml aliquots of the supernatant were measured for radioactivity content by scintillation counting. In similar experiments, the effect of S. boulardii protease IgG on digestion of toxins was assessed by preincubating S. boulardii conditioned medium with the rabbit polyclonal anti-S. boulardii protease IgG as described above.
Effect of anti-S. boulardii protease IgG on S. boulardii-mediated inhibition of the ability of 3H-toxin A and 3H-toxin B to bind to human colonic BBM.
Human colonic brush border membranes (BBM) were prepared from normal colonic segments obtained from surgical resections as previously reported by us (28). Binding of 3H-toxin A and 3H-toxin B to purified human colonic BBM was measured as described previously (28). S. boulardii conditioned medium (100 μg) or medium alone (control) was incubated with anti-S. boulardii protease IgG, control rabbit IgG, or vehicle alone (1 h at 22°C). 3H-toxin A or 3H-toxin B (0.1 μg in 10 μl containing 40,000 dpm) was added and further incubated for 60 min at 37°C. At the end of the incubation the 3H-toxin A– and 3H-toxin B–anti-S. boulardii protease IgG mixtures were added to human colonic BBM (50 μg/tube) and incubated (1 h at 22°C) in a final volume of 200 μl of 50 mM Tris buffer (pH 7.4). After incubation, BBM were washed twice with 1 ml of ice-cold 50 mM Tris buffer (pH 7.4) to remove unbound toxin. Pellets were dissolved in 0.2 ml 10% sodium dodecyl sulfate, and BBM-associated radioactivity was determined by scintillation counting. Specific 3H-toxin A and 3H-toxin B binding was calculated as described previously (7, 28, 30). Background radioactivity in tubes containing 3H-toxin A or 3H-toxin B but no membranes was subtracted.
Effect of S. boulardii protease on toxin A- and B-induced inhibition of protein synthesis in HT-29 cell monolayers.
The effect of purified S. boulardii protease on C. difficile toxin A- and B-mediated inhibition of protein synthesis in human colonic adenocarcinoma (HT-29) cells was determined by a modification of the method described by McClane and McDonell (23) and previously described by us (31). HT-29 cells (American Type Culture Collection) were grown in 250-ml Falcon tissue culture flasks (Becton Dickinson, Lincoln Park, N.J.) and seeded (105 cells/ml) onto 12-well tissue culture plates for 24 h in Dulbecco modified Eagle medium containing 10% heat inactivated fetal calf serum. Purified toxin A or B (1 μg/ml) was first incubated (1 h at 37°C) with either purified S. boulardii conditioned medium (100 μg/ml) or buffer alone. These mixtures were then added to the HT-29 monolayers and incubated for 4 h at 37°C. [3H]leucine (3 μCi/ml) was then added to the culture media, and the mixtures were incubated for an additional 16 h. At the end of the incubation period culture media and cells were collected separately and transferred to glass tubes, and proteins were precipitated by addition of 10% TCA (18 h at 4°C). The mixtures were centrifuged (2,000 × g at 4°C for 30 min), and pellets were washed twice with 10% TCA and finally resuspended in 0.5 ml of 0.3 M NaOH. Radioactivity was counted in 0.2-ml aliquots to determine incorporation of [3H]leucine as an indicator of cellular protein synthesis (31).
Effect of S. boulardii protease on toxin A- and B-induced resistance changes of human colonic mucosae in Ussing chambers.
Human colonic tissues were obtained from colonic resections. A total of 20 tumor-free specimens of human left-sided colon were used in these experiments. Human and rabbit colonic specimens were opened longitudinally, the muscle layers were dissected under the microscope, and up to six colonic mucosal preparations from each specimen (1.0-cm2 surface area) were mounted in Ussing chambers (DCTSYS; Precision Instrument Design, San Diego, Calif.) (32). Luminal and serosal sides were bathed at 37°C with 7 ml of nutrient buffer as previously described (32) and left to equilibrate for 1 h. At the end of the equilibration period the buffer on the apical side was replaced with either fresh nutrient buffer alone (control) or buffer containing toxin A or B (10 μg/ml). In some experiments, purified toxin A (32 nM), toxin B (3 nM), or buffer alone was incubated (1 h, 37°C) with S. boulardii protease (1 μg/ml) prior to placement in the Ussing chambers. Changes in electrical resistance (ohms per square centimeter) were recorded every 30 min over a 4-h period as previously described (32). The protocol for use of human tissues was approved by the Ethics Committee of Beth Israel Deaconess Medical Center.
RESULTS
Anti-S. boulardii protease IgG inhibits proteolytic activity in S. boulardii conditioned medium.
We previously reported that conditioned media of S. boulardii cultures possess proteolytic activity against [14C]methemoglobin substrate (7). We first determined the effect of the anti-S. boulardii protease antiserum on the proteolytic activity present in the S. boulardii conditioned medium. Incubation of S. boulardii conditioned medium with [14C]methemoglobin caused a significant increase in radioactivity released in the TCA supernatant after a 20-min incubation (Fig. 1). Preincubation of S. boulardii conditioned medium with a 10−3 dilution of anti-S. boulardii protease IgG inhibited proteolytic activity by 73%, whereas similar dilution of control rabbit IgG had no significant inhibitory effect (Fig. 1). Addition of a 2 × 10−4 dilution of the anti-S. boulardii protease IgG inhibited proteolytic activity of S. boulardii conditioned medium by 49%, whereas a 10−4 dilution had no significant inhibitory effect (Fig. 1), indicating that the effect of anti-S. boulardii protease is dose dependent.
FIG. 1.
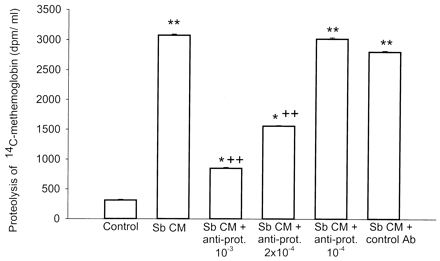
Effect of anti-S. boulardii protease IgG on S. boulardii protease activity against [14C]methemoglobin. S. boulardii conditioned medium (CM) (50 μg) or medium alone was incubated (1 h at 22°C) with different dilutions of rabbit anti-S. boulardii protease polyclonal IgG or with control rabbit IgG. To determine the proteolytic activity in the S. boulardii CM, the mixtures were incubated at 37°C with [methyl-14C]methemoglobin. After 20 min the reactions were terminated by adding TCA, and samples were centrifuged. To determine the proteolytic activity, the radioactivity released in the supernatant was measured by scintillation counting. Results are the means ± standard errors of the means of results from three to six separate experiments, each with duplicate determinations. ∗∗, P < 0.01, and ∗, P < 0.05 versus values for respective controls; ++, P < 0.01 versus values for S. boulardii CM alone. Ab, antibody; prot., protease; Sb, S. boulardii.
Anti-S. boulardii protease IgG reverses the protective effects of S. boulardii in toxin A-mediated enteritis.
We next determined the action of anti-S. boulardii protease IgG on the protective effects of S. boulardii conditioned medium in toxin A-induced enteritis in rat ileum in vivo. As expected (7, 8, 29, 38), injection of purified toxin A into rat ileal loops increased fluid secretion and mucosal permeability to mannitol after 4 h of exposure compared to levels produced in buffer-injected loops (Fig. 2). As previously reported (7, 29), preincubation of toxin A with S. boulardii conditioned medium for 30 min in vitro significantly inhibited toxin A-induced ileal secretion and mucosal permeability (Fig. 2). However, the protective effect of S. boulardii conditioned medium on toxin A-induced ileal responses was significantly reversed when S. boulardii conditioned medium was preincubated with the rabbit polyclonal IgG directed against the 54-kDa S. boulardii protease (Fig. 2). Preincubation of S. boulardii conditioned medium with control rabbit IgG had no effect on the inhibitory effects of S. boulardii in toxin A-mediated fluid secretion and mannitol permeability (Fig. 2).
FIG. 2.
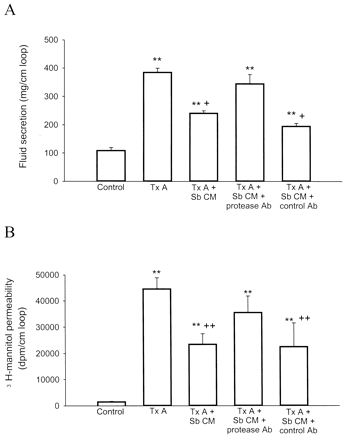
Anti-S. boulardii protease IgG reverses the protective effects of S. boulardii in toxin A-mediated enteritis. Rat ileal loops were injected with 5 μg of toxin A or toxin A preincubated (1 h at 37°C) with S. boulardii conditioned medium (3.0 mg in 200 μl). In other experiments S. boulardii conditioned medium was incubated (22°C for 1 h) with anti-S. boulardii protease IgG or control rabbit IgG (dilution, 1:1,000 for both) before ileal toxin A administration as described above. After 4 h animals were sacrificed and secretion of fluid was measured as the ratio of loop weight (micrograms) to length (centimeters) (A). 3[H]mannitol permeability was expressed as disintegration of [3H]mannitol per minute per centimeter of loop (B). Results are expressed as means ± standard errors of the means of results for each experimental condition (n = 6 to 10). ∗∗, P < 0.01 versus values for the control; +, P < 0.05, and ++, P < 0.01 versus values for toxin A alone. CM, S. boulardii conditioned medium; Tx A, toxin A; Ab, antibody; Sb, S. boulardii.
S. boulardii-mediated proteolysis of C. difficile toxin A and toxin B is inhibited by anti-S. boulardii protease IgG.
We previously reported that the 54-kDa serine protease from S. boulardii digests toxin A in vitro (7). Riegler et al. (32) recently reported that both toxin A and toxin B diminished mucosal resistance and caused epithelial cell damage in human colonic mucosa in vitro. Accordingly, we sought to determine whether S. boulardii protease could also digest C. difficile toxin B. As shown in Table 1, both S. boulardii conditioned medium and its purified protease digested 3H-toxin A and 3H-toxin B. In order to confirm that the effects of S. boulardii protease on toxins A and B were due to proteolysis of the toxin molecules, the nonspecific protease inhibitor α2-macroglobulin was incubated with purified protease and their proteolytic activity on tritiated toxins A and B was determined. Our results show that preincubation with α2-macroglobulin completely prevented digestion of both toxins by S. boulardii protease (Table 1). In addition, preincubation of S. boulardii conditioned medium with anti-S. boulardii protease IgG inhibited digestion of both 3H-toxin A and 3H-toxin B by 40 and 63%, respectively (P < 0.01; Table 1).
TABLE 1.
Anti-S. boulardii protease IgG inhibits S. boulardii-mediated proteolytic activity against purified C. difficile toxins A and Ba
| Tested mixture | Toxin digestion (dpm/ml) |
|---|---|
| 3H-toxin A + buffer | 3,200 ± 500 |
| 3H-toxin A + S. boulardii protease | 6,760 ± 810** |
| 3H-toxin A + S. boulardii protease + α2-macroglobulin | 2,800 ± 350++ |
| 3H-toxin A + S. boulardii CM | 6,690 ± 440** |
| 3H-toxin A + S. boulardii CM + anti-protease IgG | 4,100 ± 550++ |
| 3H-toxin A + S. boulardii CM + control IgG | 7,200 ± 780** |
| 3H-toxin B + buffer (control) | 2,750 + 140 |
| 3H-toxin B + S. boulardii protease | 5,330 ± 200** |
| 3H-toxin B + S. boulardii protease + α2-macroglobulin | 3,250 ± 490++ |
| 3H-toxin B + S. boulardii CM | 5,590 ± 520** |
| 3H-toxin B + S. boulardii CM + anti-protease IgG | 1,970 ± 90++ |
| 3H-toxin B + S. boulardii protease + control IgG | 4,930 ± 210** |
Results are the means ± standard errors of the means of results of four separate experiments, each with triplicate determinations. S. boulardii conditioned medium (CM; 0.1 mg/ml) or purified protease (0.1 μg/ml) was incubated (30 min at 37°C) with either tritiated toxin A or tritiated toxin B (0.1 μg containing 40,000 dpm). The reaction was stopped by adding TCA, and the mixture was placed on ice for 30 min and then centrifuged (700 × g for 30 min at 4°C). The radioactivity content of the supernatant was measured by scintillation counting. In similar experiments, the effect of anti-S. boulardii protease IgG on digestion of toxins was assessed by preincubating S. boulardii conditioned medium with the rabbit polyclonal anti-S. boulardii protease IgG as described above. **, P < 0.01 versus values for corresponding controls; ++, P < 0.01 versus values for S. boulardii protease or S. boulardii conditioned medium alone.
Anti-S. boulardii protease IgG prevents the action of S. boulardii on the ability of 3H-toxin A and 3H-toxin B to bind to human colonic BBM.
We previously reported that exposure of 3H-toxin A to S. boulardii protease results in diminished 3H-toxin A binding to its rat BBM receptor(s) (7). Since previous results indicated the presence of functional toxin A and toxin B receptors on human colon (32), we tested the effect of S. boulardii protease on toxin A and B binding to human colonic BBM receptors. As expected 3H-toxin A and 3H-toxin B bound to human colonic BBM, and approximately 60% of the binding was inhibited by a 1,000-fold excess of unlabeled toxins (data not shown). Incubation of 3H-toxin A or B with purified S. boulardii protease significantly reduced 3H-toxin A and B binding to human colonic BBM by 60 and 58%, respectively (Table 2). A less substantial inhibition was observed when BBM were first incubated with purified protease before addition of radiolabeled toxins A and B (data not shown), suggesting that the S. boulardii protease may also digest the human colonic toxin A and B receptors.
TABLE 2.
Anti-S. boulardii protease IgG prevents S. boulardii-mediated inhibition of 3H-toxin A and 3H-toxin B binding to human colonic BBMa
| Tested mixture | dpm bound per 50 μg of BBM protein |
|---|---|
| 3H-toxin A + buffer (control) | 140 ± 40 |
| BBM + 3H-toxin A | 8,730 ± 200** |
| BBM + 3H-toxin A + S. boulardii CM | 3,370 ± 350++ |
| BBM + 3H-toxin A + S. boulardii CM + anti-protease IgG | 7,750 ± 380** |
| BBM + 3H-toxin A + S. boulardii CM + control IgG | 3,500 ± 250**++ |
| 3H-toxin B + buffer (control) | 220 ± 70 |
| BBM + 3H-toxin B | 8,850 ± 310** |
| BBM + 3H-toxin B + S. boulardii CM | 3,880 ± 420++ |
| BBM + 3H-toxin B + S. boulardii CM + anti-protease IgG | 9,150 ± 390** |
| BBM + 3H-toxin B + S. boulardii CM + control IgG | 4,200 ± 240++** |
Results are the means ± standard errors of the means of three separate experiments performed for each experimental approach, with duplicate determinations made for each. [3H]toxin A or [3H]toxin B (0.1 μg containing 40,000 dpm) was first incubated (30 min at 37°C) with S. boulardii conditioned medium (CM; 100 μg). The mixture was then added to purified human colonic BBM (50 μg/0.2 ml), and [3H]toxin A- or B-specific binding to BBM was measured as described in Materials and Methods. **, P < 0.01 versus values for the corresponding controls; ++, P < 0.01 versus values for [3H]toxin binding in the presence of S. boulardii conditioned medium.
S. boulardii protease prevents toxin A- and B-induced inhibition of protein synthesis in HT-29 cells.
Since previous studies from our laboratory indicated that toxin B inhibited protein synthesis in human fibroblasts and hamster cecal explants (31), we next determined the effect of purified S. boulardii protease on toxin A- and B-induced inhibition of protein synthesis in a human colonic adenocarcinoma (HT-29) cells. Our results showed that incubation of HT-29 cells with 1 μg of toxin A or B per ml resulted in a significant inhibition of protein synthesis after 16 h of incubation (Fig. 3). However, preexposure of toxin A and toxin B to the S. boulardii protease significantly reduced inhibition of protein synthesis caused by the toxins (Fig. 3). In contrast, protein synthesis in HT-29 cells incubated with the S. boulardii protease alone was no different from that of buffer-exposed cells (Fig. 3).
FIG. 3.
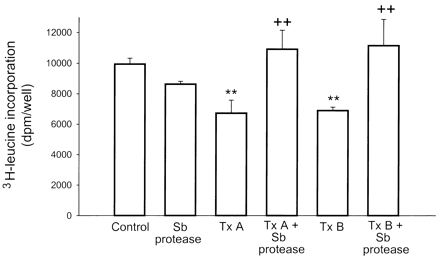
S. boulardii protease prevents toxin A- and B-mediated inhibition of protein synthesis in colonic epithelial (HT-29) cells. Purified toxin A or B (1 μg/ml) was incubated (1 h at 37°C) with either purified S. boulardii conditioned medium (100 μg/ml) or buffer alone before addition to cultured HT-29 cells (106 cells per well). After 4 h [3H]leucine (3 μCi/ml) was added to the culture media and the mixtures were incubated for an additional 16 h. At the end of the incubation period, culture media and cells were collected separately and proteins were precipitated by addition of TCA. Radioactivity contents in the precipitated proteins were measured as an indicator of protein synthesis and expressed as disintegrations per minute per well. Results are expressed as means ± standard errors of the means of results for each group; four to six wells were tested for each experimental condition, and duplicate determinations were made for each. ∗∗, P < 0.01 versus values for the control; ++, P < 0.01 versus values for toxin A or B alone. Tx, toxin; Sb, S. boulardii.
S. boulardii protease inhibits toxin A- and toxin B-mediated reduction in tissue resistance of human colonic mucosa mounted in Ussing chambers.
We recently reported that toxin A and B reduced tissue resistance in human colon in vitro (32). We next tested the ability of S. boulardii protease to inhibit this toxin-mediated colonic effect. Normal colonic mucosa incubated with either buffer alone or buffer containing S. boulardii protease for 3.5 h showed stable electrophysiological values (Fig. 4). Exposure to either toxin A or toxin B caused a significant drop in colonic transepithelial resistance during the 3.5-h incubation period. Preincubation of toxin A and B with S. boulardii protease prevented the actions of the toxins on transepithelial resistance (Fig. 4).
FIG. 4.
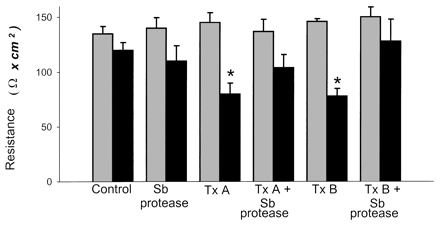
S. boulardii protease prevents toxin A- and B-mediated reduction of colonic resistance. Human colonic mucosal sheets were placed in Ussing chambers and incubated (3.5 h at 37°C) with either buffer alone or buffer containing 32 nM toxin A, 3 nM toxin B, or purified S. boulardii protease (1 μg/ml). Where indicated, toxin A or toxin B was preincubated (1 h at 37°C) with S. boulardii protease prior to placement in the Ussing chambers. Potential differences and short-circuit currents were recorded every 30 min to calculate changes in resistance (ohms per square centimeter). Results are expressed as means ± standard errors of the means of results for each group. Resistance baseline values 10 min before application of toxins (light gray bars) and 3.5 h after exposure to toxins (dark gray bars) are shown. Six to eight chambers were tested for each experimental condition. ∗, P < 0.01 versus the 10-min values. Sb, S. boulardii; Tx, toxin.
DISCUSSION
Our previous studies suggested that a 54-kDa serine protease purified from conditioned medium of S. boulardii cultures mediates the inhibitory action of the yeast on C. difficile toxin A enteritis in rats by digesting the toxin A molecule and by inhibiting toxin A binding to its surface BBM enterocyte receptor (7, 29). Since both C. difficile toxins A and B exert direct effects on human colonic cell lines and native human colonic mucosa (3, 21), we studied here the action of the S. boulardii protease on the toxin B molecule and on toxin A- and B-mediated effects in human colonocytes in vitro. We report that the 54-kDa S. boulardii protease, in addition to cleaving toxin A, possesses enzymatic activity against C. difficile toxin B (Fig. 1). Moreover, the S. boulardii protease diminishes the ability of toxins A and B to bind to human colonic BBM (Table 2) and inhibits the effects of both toxins on colonic epithelial cells and native human colonic mucosa in vitro (Fig. 3 and 4). This study is the first demonstration that the S. boulardii protease inhibits the action of both C. difficile toxins in human colon and that it may be relevant to the mechanism by which this nonpathogenic yeast exerts its beneficial effects in human C. difficile colitis. To our knowledge this is a unique mechanism of action for a biotherapeutic agent.
Our results indicate that a polyclonal antibody directed against the 54-kDa S. boulardii protease reversed the proteolytic activity of S. boulardii conditioned medium against toxins A and B (Fig. 1) and the inhibition of radiolabeled toxin A and B binding to human colonocyte BBM mediated by S. boulardii (Table 2). Most importantly, the anti-S. boulardii protease antibody also almost completely reversed the inhibitory effect of S. boulardii conditioned medium on fluid secretion and mucosa permeability observed after administration of toxin A in rat ileum in vivo (Fig. 2). These results confirm and extend our earlier observations that the S. boulardii protease mediates a large part of the yeast’s effects against C. difficile (7, 29).
We recently demonstrated that only luminal, and not basolateral, administration of C. difficile toxins induces dose-dependent damage of surface, but not crypt, colonocytes (32, 33) and that functional toxin A receptors are localized in the apical membrane (32). Furthermore, toxin-mediated damage is accompanied by a reduction in transepithelial resistance (15, 32). In keeping with these observations, results in the present study demonstrate that toxins A and B induce a reduction of epithelial barrier integrity (Fig. 4) probably via binding to specific receptors on surface colonocytes. Indeed, using tritiated toxins A and B we demonstrated here the presence of specific receptors for both toxins on human colonic BBM (Table 2). In addition, incubation of toxins A and B with S. boulardii conditioned medium and purified S. boulardii protease reversed a toxin-mediated drop in tissue resistance (Fig. 4) and reduced toxin receptor binding to human BBM (Table 2). Since both S. boulardii and its 54-kDa protease digest toxin A and toxin B, we conclude that the protective effects of S. boulardii on toxin A- and B-induced colonic responses are mediated by proteolytic cleavage of the toxins.
Although results in this and our previous studies (7, 29) point to a major role for the S. boulardii serine protease in the protective effects of the yeast in C. difficile colitis, several other proposed mechanisms may also account for the protective effects of S. boulardii in this infection. These include factors produced by S. boulardii in vivo which may inhibit C. difficile growth and cause reduced toxin production (10, 14). In addition, the ability of S. boulardii to stimulate host mucosal disaccharidase activity (4) and enhance the intestinal mucosal immune response (5) may also be involved in the mechanism by which S. boulardii reduces the recurrence of C. difficile colitis.
ACKNOWLEDGMENTS
This work was supported by a grant from Biocodex Laboratories, Montrouge, France, and by National Institutes of Health grant DK34583.
REFERENCES
- 1.Bartlett J G, Chang T W, Gurwith M, Gorbach S L, Onderdonk A B. Antibiotic associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett J G, Onderdonk A B, Cisneros R L, Kasper D L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1978;136:701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- 3.Branka J E, Vallette G, Jarry A, Bou-Hanna C, Lemarre P, Van P N, Laboisse C L. Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology. 1997;112:1887–1894. doi: 10.1053/gast.1997.v112.pm9178681. [DOI] [PubMed] [Google Scholar]
- 4.Buts J-P, Bernasconi P, Craynest M, Maldague P, DeMeyer R. Response of human and rat intestinal mucosa to oral administration of Saccharomyces boulardii. Pediatr Res. 1986;20:192–196. doi: 10.1203/00006450-198602000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Buts J-P, Bernasconi P, Vaerman J-P, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Digest Dis Sci. 1990;35:251–256. doi: 10.1007/BF01536771. [DOI] [PubMed] [Google Scholar]
- 6.Buts J-P, Corthier G, Delmee M. Saccharomyces boulardii for Clostridium difficile-associated enteropathies in infants. J Pediatr Gastroenterol Nutr. 1993;16:419–425. doi: 10.1097/00005176-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Castagliuolo I, LaMont J T, Nikulasson S T, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64:5225–5232. doi: 10.1128/iai.64.12.5225-5232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castagliuolo I, Kelly C P, Qiu B S, Nikulasson S T, LaMont J T, Pothoulakis C. IL-11 inhibits Clostridium difficile toxin A enterotoxicity in rat ileum. Am J Physiol. 1997;273:G333–G341. doi: 10.1152/ajpgi.1997.273.2.G333. [DOI] [PubMed] [Google Scholar]
- 9.Castagliuolo I, Keates A C, Qiu B S, Kelly C P, Nikulasson S T, Leeman S E, Pothoulakis C. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci USA. 1997;94:4788–4793. doi: 10.1073/pnas.94.9.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castex F, Corthier G, Jouvert S, Elmer G W, Guibal J, Lucas F, Bastidel M. Prevention of pseudomembranous cecitis by Saccharomyces boulardii: topographical histology of the mucosa, bacterial counts and analysis of toxin production. Microecol Ther. 1989;19:241–250. [Google Scholar]
- 11.Castex F, Corthier G, Jouvert S, Elmer G W, Lucas F, Bastide M. Prevention of Clostridium difficile-induced experimental pseudomembranous colitis by Saccharomyces boulardii: a scanning electron microscopic study. J Gen Microbiol. 1990;136:1085–1089. doi: 10.1099/00221287-136-6-1085. [DOI] [PubMed] [Google Scholar]
- 12.Corthier G, Dubos F, Ducluzeau R. Prevention of Clostridium difficile mortality in gnotobiotic mice by Saccharomyces boulardii. Can J Microbiol. 1986;32:894–896. doi: 10.1139/m86-164. [DOI] [PubMed] [Google Scholar]
- 13.Elmer G W, Corthier G. Modulation of Clostridium difficile induced mortality as a function of the dose and the viability of the Saccharomyces boulardii used as a preventive agent in gnotobiotic mice. Can J Microbiol. 1990;37:315–317. doi: 10.1139/m91-049. [DOI] [PubMed] [Google Scholar]
- 14.Elmer G W, McFarland L V. Suppression by Saccharomyces boulardii of toxigenic Clostridium difficile overgrowth after vancomycin treatment in hamsters. Antimicrob Agents Chemother. 1987;31:129–131. doi: 10.1128/aac.31.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht G, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human epithelial monolayers. J Clin Investig. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 17.Kelly C P, Becker S, Linevsky J K, Joshi M A, O’Keane J C, Dickey B F, LaMont J T, Pothoulakis C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Investig. 1994;93:1257–1265. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Linevsky J K, Pothoulakis C, Keates S, Warny M, Keates A C, LaMont J T, Kelly C P. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am J Physiol. 1997;273:G1333–G1340. doi: 10.1152/ajpgi.1997.273.6.G1333. [DOI] [PubMed] [Google Scholar]
- 20.Lyerly D M, Kriven H C, Wilkins T D. Clostridium difficile toxins. Clin Microbial Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahida Y R, Makh S, Hyde S, Gray T, Borriello S P. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–385. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massot J, Sanchez O, Astoin R, Parodi A L. Bacterio-pharmacological activity of Saccharomyces boulardii in clindamycin-induced colitis in the hamster. Arzneim-Forsch. 1984;34:794–797. [PubMed] [Google Scholar]
- 23.McClane B A, McDonell J L. The effect of Clostridium perfringens enterotoxins on morphology, viability and macromolecular synthesis in Vero cells. J Cell Physiol. 1979;99:191–200. doi: 10.1002/jcp.1040990205. [DOI] [PubMed] [Google Scholar]
- 24.McFarland L V, Mulligan M E, Kwok R Y, Stamm W E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1988;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 25.McFarland L V, Surawicz C M, Elmer G W, Moyer K A, Melcher S A, Greenberg R, Bowen K. Multivariate analysis of the clinical efficacy of a biotherapeutic agent, Saccharomyces boulardii, for the prevention of antibiotic-associated diarrhea. Am J Epidemiol. 1993;138:649. . (Abstract.) [Google Scholar]
- 26.McFarland L V, Surawicz C M, Greenberg R N, Fekety R, Elmer G W, Moyer K, Melcher S A, Bowen K E, Cox J, Noorani Z, Hamilton G, Rubin M, Greenwald D. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- 27.Pothoulakis C, Barone L M, Ely R, Faris B, Clark M E, Franzblau C, LaMont J T. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986;261:1316–1321. [PubMed] [Google Scholar]
- 28.Pothoulakis C, Galili U, Shen C, Castagliuolo C, Kelly C P, Nikulasson S, Dudeja P, Brasitus T A, LaMont J T. Human anti-Gal binds to the same receptor and mimics the effects of C. difficile toxin A in rat colon. Gastroenterology. 1996;110:1704–1712. doi: 10.1053/gast.1996.v110.pm8964394. [DOI] [PubMed] [Google Scholar]
- 29.Pothoulakis C, Kelly C P, Joshi M A, Gao N, O’Keane C J, Castagliuolo I, LaMont J T. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 30.Pothoulakis C, LaMont J T, Eglow R, Gao N, Rubbins J B, Theoharides T C, Dickey B F. Characterization of rabbit ileal receptors for Clostridium difficile toxin A. Evidence for a receptor-coupled G protein. J Clin Investig. 1991;88:119–125. doi: 10.1172/JCI115267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pothoulakis C, Triadafilopoulos G, Clark M, LaMont J T. Clostridium difficile cytotoxins inhibit protein synthesis in fibroblasts and intestinal mucosa. Gastroenterology. 1986;91:1147–1153. doi: 10.1016/s0016-5085(86)80010-5. [DOI] [PubMed] [Google Scholar]
- 32.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacheri J, Bischof G, Consentini E, Feil W, Schiessel R, LaMont J T, Wenzl E. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riegler M, Sedivy R, Sogukoglu T, Sears C, Castagliuolo I, Pothoulakis C, Consentini E, Bischof G, Teleky B, Feil W, Hamilton G, LaMont J T, Wenzl E. Differential effects of Bacteroides fragilis toxin (BFT)-2 and Clostridium difficile toxin A. Gastroenterology. 1997;112:A1072. . (Abstract.) [Google Scholar]
- 34.Roth J S, Losty T, Wierbicki E. Assay of proteolytic activity using a 14C-labelled hemoglobin. Anal Biochem. 1971;42:214–221. doi: 10.1016/0003-2697(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan N M, Pellet S, Wilkins T D. Purification and characterization of toxins A and B from Clostridium difficile. Infect Immun. 1982;35:1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surawicz C M, Elmer G W, Speelman P, McFarland L V, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 37.Toothaker R D, Elmer G W. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob Agents Chemother. 1984;26:552–556. doi: 10.1128/aac.26.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Triadafilopoulos G, Pothoulakis C, O’Brien M, LaMont J T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]


