Abstract
Background
Management of hypopharynx cancer is often extrapolated from larynx cancer. This report analyses treatment patterns and survival limited to hypopharynx cancer using the National Cancer Database (NCDB).
Methods
There are 9314 patients diagnosed with hypopharynx cancer between 2004–2016 in the NCDB. The association between treatment modality and survival was analyzed using Kaplan-Meier survival curves and multivariable Cox regression.
Results
Five-year overall survival ranged from 45% for stage I to 21% for stage IVB. Treatment modality did not influence survival in stage I/II. For stage III/IV, chemoradiation and surgery + adjuvant therapy were equivalent. Surgery yielded improved survival for T4 disease. Human papillomavirus (HPV) positive tumors were present in 21% and were associated with improved hazard ratio of death (0.60, p=<0.0001).
Conclusions
Survival is superior for T4 hypopharynx cancer managed with surgery, while treatment modality does not impact outcomes for other T-stages. HPV-positive tumors are associated with improved survival regardless of treatment.
Keywords: hypopharynx, hypopharyngeal, pyriform sinus, chemoradiation, surgery
INTRODUCTION
Squamous cell carcinoma of the hypopharynx is uncommon, accounting for less than 5% of upper aerodigestive tract primary tumors.1 It is generally associated with poor survival with higher rates of advanced disease and distant spread at diagnosis; however, the natural history of the disease is not well understood.1–4 Due to its low incidence in North America there are limited data addressing hypopharynx cancer and hence the lessons learned from advanced larynx cancer are typically applied directly to advanced hypopharynx cancer. This is not entirely unjustified – the single randomized prospective study evaluating hypopharynx cancer5, 6 employed a similar treatment paradigm to that of the VA larynx study7 and similarly reported equivalent survival for sequential chemotherapy/radiation and primary surgery + adjuvant RT. However, while subsequent trials of different treatment regimens for advanced laryngeal cancer have been conducted there have been no similar follow-ups surrounding hypopharynx cancer. Studies limited to hypopharyngeal cancer suggest a different clinical course compared to that seen with larynx cancer;4 hypopharyngeal cancer may have a somewhat worse outcome than that of the larynx so treatment strategies may not be interchangeable.
Several retrospective single institution studies have suggested high locoregional control with nonsurgical management of T1-2 pyriform sinus cancer8–10 with outcomes similar to those seen with T1-2 cancers of the supraglottic larynx.11 Evaluation of a Canadian cancer registry12 and the Surveillance Epidemiology End Results (SEER) database in the USA13 demonstrated equivalent survival outcomes for patients treated with either resection or radiation for all stages of hypopharynx cancer. However, recent analysis of the NCDB demonstrated a reduced survival associated with non-surgical therapy for T4a larynx cancer14 despite its frequent application. Single institution reports similarly suggest improved outcomes for hypopharynx cancer managed with primary surgery.15 To date there has been no analysis of the NCDB specifically dedicated to hypopharynx cancer. It is unclear how hypopharynx cancer is managed nationwide and whether a particular approach confers a survival advantage. Data from single institution and city level cancer registries have reported 4–29% of hypopharyngeal cancers as HPV associated,16–21 often with a survival benefit.19–21 The NCDB began recording HPV status in 2010. Since the database likely reflects the largest experience with HPV-associated hypopharynx cancer, we evaluated the incidence and survival of the subset of patients treated from 2010–2016 with known HPV status separately.
MATERIALS AND METHODS
The NCDB is a clinical oncology database jointly sponsored by the American College of Surgeons and the American Cancer Society. It includes hospital registry data collected in more than 1,500 Commission on Cancer (CoC)-accredited facilities, and represents more than 70% of newly diagnosed cancer cases nationwide.
Patient Selection
We used the NCDB to find patients with clinical stage I-IVB squamous cell carcinoma of the hypopharynx. We included patients diagnosed between 2004–2016. Patients were identified on the basis of International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes (8070, 8071, 8072, 8074, 8075, 8076, 8083, 8084) and site codes (C129, C130, C132, C138, C139). For the NCDB, clinical staging is coded in accordance with the AJCC Cancer Staging Manual edition in use at the time of diagnosis. Only patients receiving treatment typically undertaken with curative intent were included. Treatment regimens were classified as: surgery, radiation alone (RT) and chemoradiation (CRT). For analyses of locally advanced hypopharyngeal cancer, surgery was further divided into surgery alone and surgery + adjuvant CRT/RT. Patients receiving surgery + chemotherapy (without RT) were excluded in these analyses. For primary surgical therapy procedures considered to be definitive included: local tumor excision, pharyngectomy (partial or total), pharyngectomy with laryngectomy, radical pharyngectomy, surgery NOS. For radiation therapy, only patients receiving external beam radiation or radiation NOS with dose ≥ 60 Gy were included. The primary outcome was overall survival (OS); the NCDB does not collect information on recurrence patterns, cancer-specific survival or salvage therapy. HPV status was available for some patients diagnosed after 2010. It is unknown why some patients were tested and others were not; thus the subset of patients tested may not be representative of the general population of hypopharynx cancer patients. “HPV-positive” status was defined by showing HPV high‐risk type 16, type 18, both types 16 and 18, or another high‐risk type (site‐specific factor codes 20‐60). Patients considered “HPV-negative” were those without high‐risk types, without high‐risk and low‐risk types, or positive only for low‐risk types (site‐specific factor codes 0 and 10).
Statistical methods
Statistical analyses were performed using SAS software version 9.4 and Stata version 15.1. Kaplan-Meier survival analysis and the log-rank test were used to determine the unadjusted association between treatment modality and overall survival. Multivariable analysis was conducted using Cox regression analysis, with an interaction between stage and treatment to allow for different effects of treatments by stage. Overall clinical stage and clinical T-category were considered in separate models. The proportional hazards assumption was assessed via a test of the Schoenfeld residuals. Additional multivariable models were fit in the subset of patients with HPV status known.
Covariates used in adjusted analyses of the entire cohort include: age, gender, race, Charlson/Deyo score, regional income, regional education level, insurance status, year of diagnosis, facility type, tumor grade, and AJCC clinical stage. Age effects were estimated using an increase in 10 years of age. Charlson/Deyo score is recorded as 0 (no comorbid conditions), 1, 2, or 3 (greater than 2). Income was divided into four quartiles and education level captured the percent of adults who did not graduate high school in a patient’s zip code. Insurance status was classified as: uninsured, private, Medicare, Medicaid, other government insurance or unknown. Facility types included community cancer program, comprehensive community cancer program, academic/research program, integrated network cancer program, and other or unknown. In the subgroup with HPV status known, adjusted analyses included HPV status as a covariate in addition to the covariates listed above.
RESULTS
We identified 9314 patients treated with curative intent for clinical stage I-IVB squamous cell carcinoma of the hypopharynx (Table 1). The majority of patients were male (80%) and at diagnosis 1591 (17%) were AJCC clinical stage I-II, 2061 (22%) were stage III, 5662 (61%) were stage IV. Stage I cancer was rare and treated with RT alone or primary surgery with similar frequency (~38%), and with CRT around a quarter of the time. Stage II cancer was most commonly treated with CRT (42%). The most common management technique of advanced stage was primary chemoradiation (67%). When evaluated in terms of larynx preservation, 75% of T3 tumors were managed with RT/CRT and 25% with primary surgery, while 63% of T4 tumors were managed with RT/CRT and 37% with primary surgery. For those managed with resection, adjuvant RT/CRT followed surgery for 46% of T3 patients and 54% of T4 patients. The percentage of patients receiving a given treatment for each AJCC clinical stage and T category is shown in Tables 2 and 3.
TABLE 1.
Patient Characteristics
| Characteristic | Treatment modality | p value | ||
|---|---|---|---|---|
| Surgery (n=2383) | CRT (n=5766) | RT alone (n=1165) | ||
| Age | ||||
| <60 years | 864 (36%) | 2419 (42%) | 271 (23%) | <.0001 |
| ≥60 years | 1519 (64%) | 3347(58%) | 894 (77%) | |
| Gender | ||||
| Male | 1958 (82%) | 4686 (81%) | 852 (73%) | <.0001 |
| Female | 425 (18%) | 1080 (19%) | 313 (27%) | |
| Charlson/Deyo | ||||
| 0 | 1674 (70%) | 4478 (78%) | 857 (73%) | <.0001 |
| 1 | 530 (22%) | 971 (17%) | 210 (18%) | |
| ≥2 | 179 (8%) | 317 (5%) | 98 (8%) | |
| Clinical T | ||||
| 1 | 304 (13%) | 677 (12%) | 279 (24%) | <.0001 |
| 2 | 563 (24%) | 2018 (35%) | 484 (42%) | |
| 3 | 597 (25%) | 1610 (28%) | 207 (18%) | |
| 4 | 857 (36%) | 1297 (22%) | 155 (13%) | |
| unknown | 56 (2%) | 154 (3%) | 37 (3%) | |
| Clinical N | ||||
| 0 | 1061 (44%) | 1193 (21%) | 597 (51%) | <.0001 |
| 1 | 323 (14%) | 1079 (19%) | 168 (14%) | |
| 2 | 863 (36%) | 2963 (51%) | 336 (29%) | |
| 3 | 76 (3%) | 406 (7%) | 32 (3%) | |
| unknown | 60 (3%) | 125 (2%) | 32 (3%) | |
| Overall AJCC stage | ||||
| I | 199 (8%) | 123 (2%) | 191 (16%) | <.0001 |
| II | 333 (14%) | 449 (8%) | 296 (25%) | |
| III | 499 (21%) | 1331 (23%) | 231 (20%) | |
| IV | 50 (2%) | 107 (2%) | 16 (1%) | |
| IVA | 1178 (50%) | 3105 (54%) | 366 (32%) | |
| IVB | 124 (5%) | 651 (11%) | 65 (6%) | |
TABLE 2.
Hazard ratio of death by treatment modality for each AJCC clinical stage
| 1 (n=513) | 2 (1078) | 3 (2061) | 4A (4649) | 4B (840) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | HR | % | HR | % | HR | % | HR | % | HR | |
| Surg | 39 | 1.077 | 31 | 1.082 | 24 | 1.055 | 25 | 1.023 | 15 | 1.214 |
| CRT (ref) | 24 | --- | 42 | --- | 65 | --- | 67 | --- | 77 | --- |
| RT | 37 | 0.802 | 27 | 1.037 | 11 | 1.382* (0.0008) |
8 | 1.393* (<.0001) |
8 | 1.645* (.0011) |
statistically significant with p value in parenthesis
TABLE 3.
Hazard ratio of death by treatment modality for each AJCC clinical T category
| 1 (n=1260) | 2 (3065) | 3 (2414) | 4(2309) | |||||
|---|---|---|---|---|---|---|---|---|
| % | HR | % | HR | % | HR | % | HR | |
| Surg | 24 | 0.995 | 18 | 1.024 | 25 | 1.004 | 37 | 0.864* (0.0108) |
| CRT (ref) | 54 | --- | 66 | --- | 67 | --- | 56 | --- |
| RT | 22 | 0.887 | 16 | 1.013 | 8 | 1.861* (<.0001) |
7 | 1.515* (0.0002) |
statistically significant with p value in parenthesis
Overall survival at one year was 86% for AJCC clinical stage I, 86% for II, 81% for III, 78% for IVA and 68% for IVB. Overall survival at 5 years was 45% for AJCC clinical stage I, 41% for II, 38% for III, 33% for IVA and 21% for IVB (Figure 1). Unadjusted overall survival stratified by treatment modality is shown in Figure 2. A stage-by-stage analysis was performed to assess overall survival as a function of treatment modality while adjusting for known pertinent factors (Table 2). Treatment modality did not significantly influence adjusted survival for either stage I or stage II disease. Radiation alone was consistently associated with worse survival for clinical stages III, IVA, and IVB when compared to CRT. Primary surgery was equivalent to CRT for stage III, IVA, and IVB disease.
FIGURE 1.
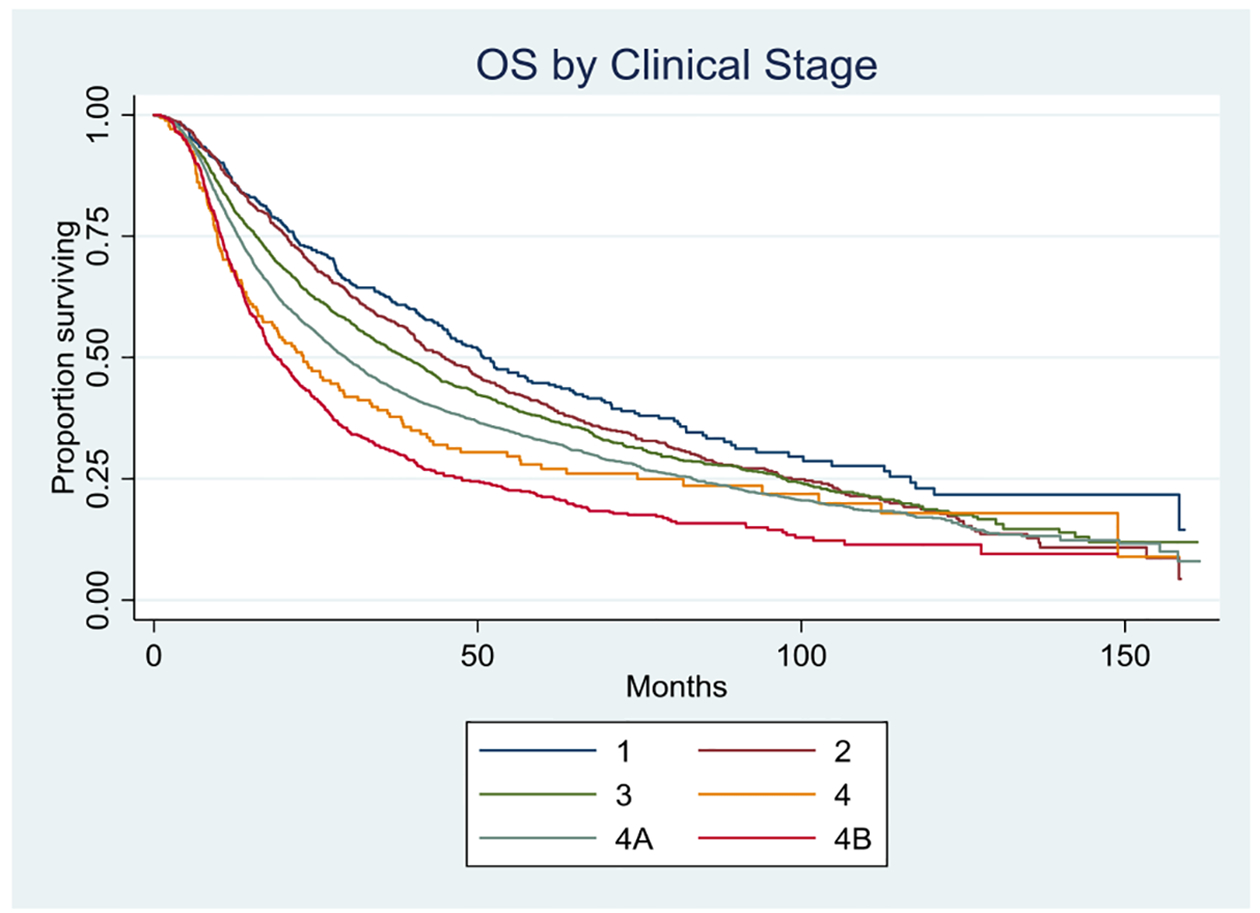
Overall survival by AJCC clinical stage; p=<0.0001
FIGURE 2.
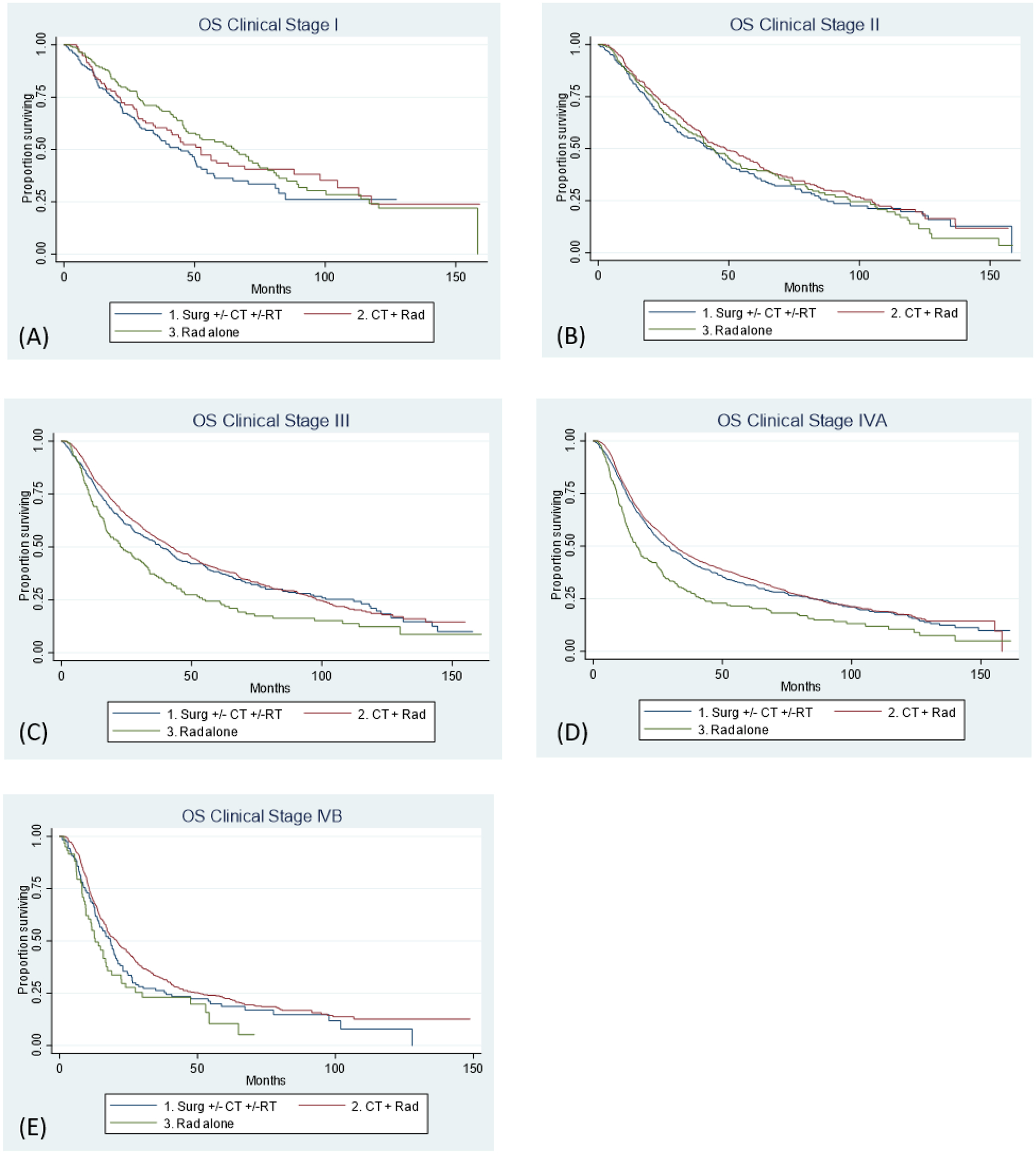
Overall survival by treatment modality for (A) AJCC clinical stage I; p= 0.0877 (B) AJCC clinical stage II; p= 0.2478 (C) AJCC clinical stage III; p=<0.0001 (D) AJCC clinical stage IVA; p=<0.0001 (E) AJCC clinical stage IVB; p=0.0106
Treatment modality did not significantly impact hazard ratio of death for clinical T1-T2. For T3-4 primary tumors, radiation alone was associated with worse OS than CRT (Table 3). For T3 disease, primary surgery and CRT resulted in equivalent outcomes, while for T4 disease outcomes were superior with primary surgery (HR 0.864, p = 0.0108).
For locally advanced disease, analysis of the impact of treatment modality showed HR of death varied over time, with primary surgery patients doing worse than CRT in the early follow up period. Further refinement of the primary surgery group into surgery + adjuvant RT/CRT and surgery alone groups showed that the driver of poor early outcomes was the surgery alone group. For both stages III and IV, surgery + adjuvant therapy and CRT were equivalent, while surgery alone was associated with worse outcomes (HR for surgery alone 1.237, p = 0.05 for stage III and 1.288, p = 0.0002 for stage IV). Similar results were obtained for T3 disease (HR for surgery alone 1.25, p = 0.027). For T4 disease, surgery alone and CRT had equivalent and worse outcomes than surgery +adjuvant therapy (HR 0.78, p = 0.001).
On multivariable analysis, age, gender, race, tumor grade, year of diagnosis, median income, charlson/deyo score, facility type, and insurance status were considered. On MVA, having Charlson/Deyo score >0 and being older were associated with worse outcomes (Table 4). The hazard ratio of death was 1.16, 1.21, and 1.42 for CCI of 1, 2 or 3+ respectively when compared to a CCI of 0 (p = <0.0001, 0.0071, 0.0017, respectively). Private insurance was associated with better outcomes, with no insurance, Medicaid, and Medicare showing worse survival (HR 1.49, 1.40, 1.24, respectively; p = <0.0001 for all three).
TABLE 4.
Results of multivariable analysis
| Characteristic | HR of death | p value |
|---|---|---|
| Age | 1.22 | <.0001 |
| Gender | ||
| Male | 1.039 | 0.2979 |
| Female | ||
| Charlson/Deyo | ||
| 0 | ||
| 1 | 1.157 | <.0001 |
| 2 | 1.214 | 0.0071 |
| 3 | 1.421 | 0.0017 |
| Tumor Grade | ||
| 1 | ||
| 2 | 1.014 | 0.8506 |
| 3 | 0.929 | 0.3187 |
| 4 | 1.065 | 0.7103 |
| Insurance | ||
| Uninsured | 1.488 | <.0001 |
| Private | ||
| Medicaid | 1.397 | <.0001 |
| Medicare | 1.239 | <.0001 |
| Other Government | 1.199 | 0.0624 |
| Facility Type | ||
| Community | 0.976 | 0.7243 |
| Comprehensive Community | 1.012 | 0.8116 |
| Academic/Research | 0.959 | 0.394 |
| Integrated Network Cancer Program |
HPV Status
HPV status for hypopharynx cancer was first entered in 2010 and has since been assigned a field as a matter of course for all patients. From 2010–2016, HPV status was reported for 21% of patients in the cohort (1,985 patients), and found to be positive for 21%. Overall survival was better for HPV-positive status (Figure 3). On MVA, the hazard ratio of death was significantly improved for patients with HPV-positive tumors (0.60, p = <0.0001). More detailed evaluation of the effects of treatment modality and stage were not possible, as dividing the 417 HPV-positive patients into separate stage designation and treatment modality sub-categories resulted in very small strata.
FIGURE 3.
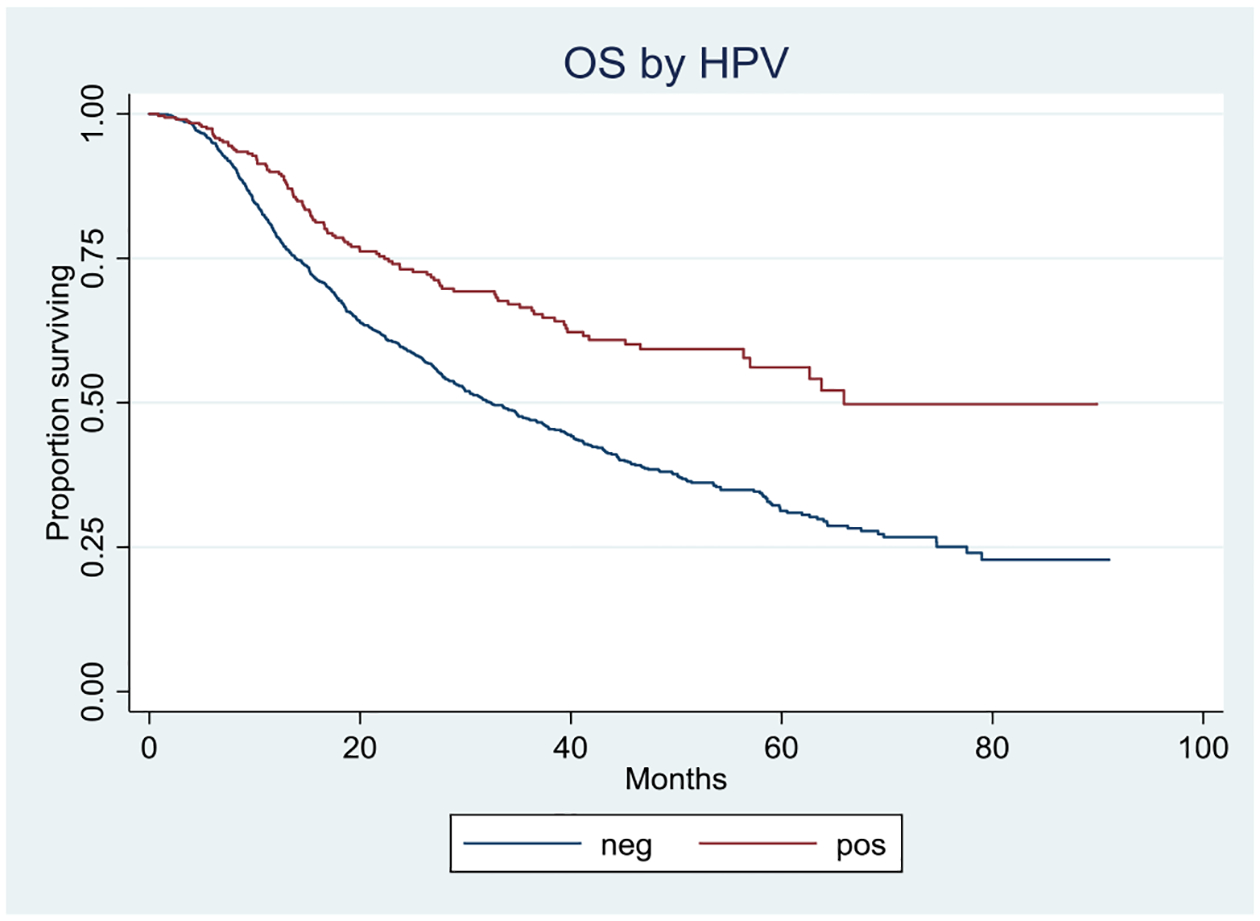
Overall survival by HPV status; p=<0.0001
DISCUSSION
While the rarity of primary squamous cell carcinoma of the hypopharynx limits the ability of single institutions to report outcomes, this NCBD analysis of more than 9000 patients over 12 years provides important information surrounding hypopharynx cancer patterns of care and results. Unfortunately early stage (Stage I/II) hypopharynx cancers are uncommon (17% of those with hypopharynx cancer). The disease is often advanced at diagnosis and typically managed similarly to advanced squamous cell carcinoma of the larynx. However, due to its apparently distinct natural history and patterns of spread we believe that hypopharynx squamous cell carcinoma should be evaluated and considered separately.
Patient characteristics
The hazard ratio of death increases with increasing comorbidity score (HR = 1.16, 1.21, and 1.42 for CCI of 1, 2 or 3 respectively). This has been established in several prior studies of head and neck cancer.22, 23 Insurance status predicts survival and only private insurance was associated with better outcomes. Similar results were seen in an analysis of 1231 head and neck cancer patients.24 Medicaid/uninsured (HR 1.50) and medicare (HR 1.69) patients had significantly lower overall survival compared to patients with private insurance.
Treatment patterns
Close to two-thirds of patients in the United States with hypopharynx cancer are initially managed with primary chemoradiation. This includes 36% of stage I-II hypopharynx cancer patients, who do not have traditional risk factors interpreted as indications for chemoradiation (T3-4 primary disease or nodal disease) and have acceptable reported outcomes for RT alone.8–10 The NCDB does not capture reasons for treatment decisions but patients managed with CRT rather than RT alone were more likely to be younger males with a Charlson comorbidity score of 0. Perhaps due to the prevailing notion that hypopharynx cancer has poor outcomes in general,4 it seems likely that patients thought able to tolerate treatment intensification were approached with CRT without firm indication. Different factors seemed to influence the choice of surgery – the percent of patients managed with resection did not vary with age (~25%) but increased with increasing Charlson comorbidity score. Treatment with an operation depended largely on the category of the hospital administering care. In this series there was no association between facility type and overall survival but 35% of patients received surgery at academic centers while only 13% and 16% of patients at community and comprehensive community cancer centers underwent resection. A similar effect was found in a study comparing treatment patterns in non-teaching to teaching hospitals in the United States.25 From 2000–2010 the proportion of patients submitted to major ablative surgical procedures decreased for non-teaching hospitals, while remaining stable for teaching centers.
Stage I/II disease and the impact of treatment modality on survival
The poor survival of early hypopharynx cancer is noteworthy. More than half of Stage I patients (a majority of whom have a Charlson comorbidity index of 0) are no longer alive within five years of diagnosis. While single institution reports document encouraging relapse free survival (RFS) in early hypopharynx cancer, these reports similarly demonstrate poor survival at 5 years, even for the uncommon T1-2 N0 lesions.8 This is markedly different from the survival seen with T1-2 N0 glottic and supraglottic cancers.26 Consistent with other analyses of early stage hypopharynx cancer27, 28 the current analysis shows no survival advantage from any modality (eg surgery, radiation, or chemoradiation). Chemoradiation is not generally indicated for early stage disease. Indeed, the NCDB suggests that the addition of chemotherapy does not improve survival for limited stage hypopharynx cancer. Conventional methods to intensify radiation do not result in improved outcomes, but the increase in morbidity is certain. Because the NCDB does not report cancer-specific outcomes it is not possible to report RFS. The overall survival of stage I-II hypopharynx cancer is poor by comparison to other head and neck sites.
Advanced disease
Concurrent chemoradiation is the recommended organ preservation therapy for stage III/IV hypopharynx cancer29 and thus not surprisingly RT alone for advanced HPC was associated with an increased risk of death compared to surgery or chemoradiation. Comparison of the two recommended treatment regimens for advanced stage hypopharynx cancer, concurrent CRT and surgery + adjuvant RT/CRT demonstrated equivalence. Surgery without adjuvant radiation was, as expected, associated with worse survival. Surprisingly, only 40% of patients with stage III disease and 58% with stage IV disease received adjuvant RT or CRT. As mentioned above, the NCDB does not capture the rationale behind treatment decisions, thus it is not possible to determine why such a large proportion of patients with locally advanced disease did not receive adjuvant radiation.
Prior literature often suggests equivalently poor outcomes for primary surgery and CRT. Lefebvre et al did not find a significant difference in 5-year (38% v 32.6%,) or 10-year (13.1% v 13.8%) overall survival between induction chemotherapy followed by RT versus primary surgery in a prospective trial of patients with stage II-IV HPC.5 Recent retrospective studies also suggest equivalent overall survival between surgery and CRT for patients with locally advanced hypopharyngeal cancer13, 27, 30, 31 but some series have suggested a benefit to primary surgery. A retrospective analysis of 137 patients with HPC (87% had T3–4 disease) found that resection resulted in a reduction in the risk of death by 48% (p=0.02).15 Improvement in 3-year overall survival for surgically managed patients with stage IVA disease was demonstrated in a small single institution retrospective analysis (75% v 55%, p = 0.04).32
Although this analysis demonstrated equivalence for surgery + adjuvant therapy and CRT for stage III and IV disease, there was a survival benefit to primary surgery + adjuvant RT/CRT over CRT for T4 disease specifically. This is consistent with prior studies. A SEER database analysis33 showed a benefit to surgery over CRT, although median survival with either treatment was poor (25 v 20 months, p<.001). On multivariable Cox regression, a significant survival benefit was only seen for T4 disease as in the current analysis. Similarly, several series have demonstrated improved outcomes with surgical management in T4 larynx cancer both in terms of locoregional control34 and overall survival35, 36.
HPV is the most important prognostic factor in the management of oropharynx cancer.37,38 It is known that there is a subset of non-oropharyngeal primary tumors that are p16 and HPV-ISH positive. p16-positive non-oropharyngeal tumors have been demonstrated to have improved OS when compared to p16-negative tumors.39–41 While NCDB HPV data is limited to patients treated after 2010, this represents a large group of hypopharynx cancer patients (n=1,985). This dataset includes a much higher rate of HPV-positivity than the series from multiple RTOG trials, but the NCDB number is much larger and not subject to protocol entry. Hence the current series is likely a more reliable indicator of the true rate of HPV-positivity among patients with hypopharynx cancer. Although the relative incidence is different, the hazard ratio for OS in this series (HR 0.60, p = <0.0001) is very similar to that of a prior RTOG secondary analysis (HR 0.56, p = 0.01)39 which included oral cavity, hypopharyngeal and laryngeal cancers. (Figure 3). Because most patients in the current study were HPV-negative and the HPV status data was collected in only a minority of patients (with different stages and managed with different modalities), we were unable to determine whether there was an interaction between treatment type and HPV status. However, perhaps one-fifth of hypopharynx cancer patients have HPV-positive disease and survival of those patients is better. The College of American Pathologists recommends staining oropharynx cancer for p16 reflexively, but not other subsites of the upper aerodigestive tract42 – this data suggests that perhaps hypopharynx squamous cell carcinoma should similarly be evaluated for HPV routinely.
CONCLUSIONS
Overall survival for hypopharyngeal cancer is poor, even for early stage disease where adding chemotherapy to radiation does not significantly alter the hazard ratio of death, with similar outcomes for CRT, RT and surgery. For advanced stage, radiation or surgery alone are associated with worse survival but surgery + adjuvant therapy and CRT are equivalent. A statistically significant benefit to surgical management was seen for T4 disease. In this analysis, 21% of patients with hypopharynx cancer are HPV-positive and this appears to be associated with improved survival.
TABLE 5.
Results of multivariable analysis for entire cohort
| Age | 1.22 | <0.0001 |
| Gender | ||
| Male | 1.04 | 0.2979 |
| Female | ||
| Charlson/Deyo | ||
| 0 | ||
| 1 | 1.16 | <0.0001 |
| 2 | 1.21 | 0.0071 |
| 3 (>2) | 1.42 | 0.0017 |
| Tumor grade | ||
| 1 | ||
| 2 | 1.01 | 0.8506 |
| 3 | 0.93 | 0.3187 |
| 4 | 1.07 | 0.7103 |
| Insurance | ||
| Uninsured | 1.49 | <0.0001 |
| Private | ||
| Medicaid | 1.40 | <0.0001 |
| Medicare | 1.24 | <0.0001 |
| Other government | 1.20 | 0.0624 |
| Facility type | ||
| Community | 0.98 | 0.7243 |
| Comprehensive community | 1.01 | 0.8116 |
| Academic/research | 0.96 | 0.394 |
| Integrated network cancer program |
Abbreviation: HR, hazard ratio of death.
TABLE 6.
Patient characteristics for cohort with HPV status known
| HPV negative (n = 1568) | HPV positive (n = 417) | ||
|---|---|---|---|
| Age | |||
| <60 years | 614 (39) | 187 (45) | 0.0354 |
| ≥60 years | 954 (61) | 230 (55) | |
| Gender | |||
| Male | 1265 (81) | 339 (81) | 0.7755 |
| Female | 303 (19) | 78 (19) | |
| Charlson/Deyo | |||
| 0 | 1158 (74) | 333 (80) | 0.0833 |
| 1 | 293 (19) | 61 (15) | |
| ≥2 | 117 (7) | 23 (5) | |
| Clinical T | |||
| 1 | 238 (15) | 74(18) | 0.0044 |
| 2 | 518 (33) | 160(38) | |
| 3 | 398 (26) | 93(22) | |
| 4 | 394 (25) | 79(19) | |
| Unknown | 20 (1) | 11 (3) | |
| Clinical N | |||
| 0 | 500 (32) | 94 (23) | 0.0059 |
| 1 | 241 (15) | 75 (18) | |
| 2 | 753 (48) | 225 (54) | |
| 3 | 67 (5) | 22 (5) | |
| Overall AJCC stage | |||
| I | 94 (6) | 18 (4) | 0.0259 |
| II | 197 (13) | 30 (7) | |
| III | 313 (20) | 86 (21) | |
| IV | 964 (61) | 283 (68) |
Abbreviation: HPV, human papillomavirus.
TABLE 7.
Results of multivariable analysis for subset of patients with known HPV status (no. of patients = 1589)
| Age | 1.26 | <0.0001 |
| Gender | ||
| Male | 1.14 | 0.1626 |
| Female | ||
| HPV status | ||
| Positive | 0.6 | <0.0001 |
| Negative | ||
| Charlson/Deyo | ||
| 0 | ||
| 1 | 1.16 | 0.0836 |
| 2+ | 1.45 | 0.0099 |
| Tumor grade | ||
| 1 | ||
| 2 | 0.72 | 0.0603 |
| 3 | 0.68 | 0.0387 |
| 4 | 0.8 | 0.5652 |
| Insurance | ||
| Uninsured | 1.66 | 0.0063 |
| Private | ||
| Medicaid | 1.44 | 0.0014 |
| Medicare | 1.19 | 0.081 |
| Other government | 0.8 | 0.3512 |
| Facility type | ||
| Community | 1.08 | 0.6382 |
| Comprehensive community | 1.02 | 0.8923 |
| Academic/research | 0.97 | 0.8004 |
| Integrated network cancer program |
Abbreviation: HR, hazard ratio of death.
Footnotes
Meetings: Presented at 2020 Multidisciplinary Head and Neck Cancers Symposium (poster)
REFERENCES
- 1.Cooper JS, Porter K, Mallin K, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31(6):748–58. [DOI] [PubMed] [Google Scholar]
- 2.Berrino F, Gatta G. Variation in survival of patients with head and neck cancer in Europe by the site of origin of the tumours. EUROCARE Working Group. Eur J Cancer. 1998;34(14 Spec No):2154–61. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(9):951–62. [DOI] [PubMed] [Google Scholar]
- 4.Hall SF, Groome PA, Irish J, O’Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. 2008;118(8):1362–71. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre JL, Andry G, Chevalier D, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23(10):2708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(13):890–9. [DOI] [PubMed] [Google Scholar]
- 7.Wolf GT, Fisher SG, Hong WK, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685–90. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall WM, Amdur RJ, Morris CG, Kirwan J, Dziegielewski PT, Werning JW. Primary radiotherapy for squamous cell carcinoma of the pyriform sinus. Eur Arch Otorhinolaryngol. 2016;273(7):1857–62. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard P, Tao Y, Veresezan O, et al. Definitive radiotherapy for squamous cell carcinoma of the pyriform sinus. Radiother Oncol. 2012;105(2):232–7. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima A, Nishiyama K, Morimoto M, et al. Definitive radiotherapy for T1-2 hypopharyngeal cancer: a single-institution experience. Int J Radiat Oncol Biol Phys. 2012;82(2):e129–35. [DOI] [PubMed] [Google Scholar]
- 11.Hinerman RW, Mendenhall WM, Amdur RJ, Stringer SP, Villaret DB, Robbins KT. Carcinoma of the supraglottic larynx: treatment results with radiotherapy alone or with planned neck dissection. Head Neck. 2002;24(5):456–67. [DOI] [PubMed] [Google Scholar]
- 12.Hall SF, Groome PA, Irish J, O’Sullivan B. Radiotherapy or surgery for head and neck squamous cell cancer: establishing the baseline for hypopharyngeal carcinoma? Cancer. 2009;115(24):5711–22. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Lee R. Surgery vs. radiotherapy for locally advanced hypopharyngeal cancer in the contemporary era: A population-based study. Cancer Med. 2018;7(12):5889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover S, Swisher-McClure S, Mitra N, et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int J Radiat Oncol Biol Phys. 2015;92(3):594–601. [DOI] [PubMed] [Google Scholar]
- 15.Tassler AB, Gooding WE, Ferris RL. Hypopharyngeal cancer treatment: Does initial surgery confer survival benefit? Head Neck. 2019;41(7):2167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Z, Hasegawa M, Matayoshi S, et al. Prevalence and clinical features of human papillomavirus in head and neck squamous cell carcinoma in Okinawa, southern Japan. Eur Arch Otorhinolaryngol. 2011;268(11):1625–31. [DOI] [PubMed] [Google Scholar]
- 17.Sethi S, Ali-Fehmi R, Franceschi S, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. International journal of cancer. 2012;131(5):1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann M, Görögh T, Gottschlich S, et al. Human papillomaviruses in head and neck cancer: 8 year-survival-analysis of 73 patients. Cancer letters. 2005;218(2):199–206. [DOI] [PubMed] [Google Scholar]
- 19.Dalianis T, Grün N, Koch J, et al. Human papillomavirus DNA and p16(INK4a) expression in hypopharyngeal cancer and in relation to clinical outcome, in Stockholm, Sweden. Oral oncology. 2015;51(9):857–61. [DOI] [PubMed] [Google Scholar]
- 20.Joo YH, Lee YS, Cho KJ, et al. Characteristics and prognostic implications of high-risk HPV-associated hypopharyngeal cancers. PloS one. 2013;8(11):e78718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendt M, Romanitan M, Näsman A, et al. Presence of human papillomaviruses and p16 expression in hypopharyngeal cancer. Head Neck. 2014;36(1):107–12. [DOI] [PubMed] [Google Scholar]
- 22.Habbous S, Harland LT, La Delfa A, et al. Comorbidity and prognosis in head and neck cancers: Differences by subsite, stage, and human papillomavirus status. Head Neck. 2014;36(6):802–10. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593–602. [DOI] [PubMed] [Google Scholar]
- 24.Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, Taioli E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116(2):476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharyya N, Abemayor E. Patterns of hospital utilization for head and neck cancer care: changing demographics. JAMA Otolaryngol Head Neck Surg. 2015;141(4):307–12; quiz 400. [DOI] [PubMed] [Google Scholar]
- 26.Jones AS, Fish B, Fenton JE, Husband DJ. The treatment of early laryngeal cancers (T1-T2 N0): surgery or irradiation? Head Neck. 2004;26(2):127–35. [DOI] [PubMed] [Google Scholar]
- 27.Jang JY, Kim EH, Cho J, et al. Comparison of Oncological and Functional Outcomes between Initial Surgical versus Non-Surgical Treatments for Hypopharyngeal Cancer. Ann Surg Oncol. 2016;23(6):2054–61. [DOI] [PubMed] [Google Scholar]
- 28.Petersen JF, Timmermans AJ, van Dijk BAC, et al. Trends in treatment, incidence and survival of hypopharynx cancer: a 20-year population-based study in the Netherlands. Eur Arch Otorhinolaryngol. 2018;275(1):181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Network NCC. Head and Neck Cancer (Version 2.2020). [cited 2020]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck_blocks.pdf.
- 30.Kim JW, Kim MS, Kim SH, et al. Definitive Chemoradiotherapy Versus Surgery Followed by Adjuvant Radiotherapy in Resectable Stage III/IV Hypopharyngeal Cancer. Cancer Res Treat. 2016;48(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwae S, Fujii M, Hayashi R, et al. Matched-pair analysis of patients with advanced hypopharyngeal cancer: surgery versus concomitant chemoradiotherapy. Int J Clin Oncol. 2017;22(6):1001–8. [DOI] [PubMed] [Google Scholar]
- 32.Shirai K, Saitoh JI, Musha A, et al. Clinical Outcomes of Definitive and Postoperative Radiotherapy for Stage I-IVB Hypopharyngeal Cancer. Anticancer Res. 2016;36(12):6571–8. [DOI] [PubMed] [Google Scholar]
- 33.Hochfelder CG, McGinn AP, Mehta V, Castellucci E, Kabarriti R, Ow TJ. Treatment sequence and survival in locoregionally advanced hypopharyngeal cancer: A surveillance, epidemiology, and end results-based study. Laryngoscope. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal DI, Mohamed AS, Weber RS, et al. Long-term outcomes after surgical or nonsurgical initial therapy for patients with T4 squamous cell carcinoma of the larynx: A 3-decade survey. Cancer. 2015;121(10):1608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyckhoff G, Plinkert PK, Ramroth H. A change in the study evaluation paradigm reveals that larynx preservation compromises survival in T4 laryngeal cancer patients. BMC cancer. 2017;17(1):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi YS, Park SG, Song EK, et al. Comparison of the therapeutic effects of total laryngectomy and a larynx-preservation approach in patients with T4a laryngeal cancer and thyroid cartilage invasion: A multicenter retrospective review. Head Neck. 2016;38(8):1271–7. [DOI] [PubMed] [Google Scholar]
- 37.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakravarthy A, Henderson S, Thirdborough SM, et al. Human Papillomavirus Drives Tumor Development Throughout the Head and Neck: Improved Prognosis Is Associated With an Immune Response Largely Restricted to the Oropharynx. J Clin Oncol. 2016;34(34):4132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wookey VB, Appiah AK, Kallam A, Ernani V, Smith LM, Ganti AK. HPV Status and Survival in Non-Oropharyngeal Squamous Cell Carcinoma of the Head and Neck. Anticancer Res. 2019;39(4):1907–14. [DOI] [PubMed] [Google Scholar]
- 41.Bates JE, Morris CG, Hitchcock KE, Dziegielewski PT, Mendenhall WM, Amdur RJ. Locally advanced hypopharyngeal and laryngeal cancer: Influence of HPV status. Radiother Oncol. 2019;140:6–9. [DOI] [PubMed] [Google Scholar]
- 42.Fakhry C, Lacchetti C, Rooper LM, et al. Human Papillomavirus Testing in Head and Neck Carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J Clin Oncol. 2018;36(31):3152–61. [DOI] [PubMed] [Google Scholar]


