Abstract
BACKGROUND
The presence of liver metastasis (LM) is an independent prognostic factor for shorter survival in non-small cell lung cancer (NSCLC) patients. The median overall survival of patients with involvement of the liver is less than 5 mo. At present, identifying prognostic factors and constructing survival prediction nomogram for NSCLC patients with LM (NSCLC-LM) are highly desirable.
AIM
To build a forecasting model to predict the survival time of NSCLC-LM patients.
METHODS
Data on NSCLC-LM patients were collected from the Surveillance, Epidemiology, and End Results database between 2010 and 2018. Joinpoint analysis was used to estimate the incidence trend of NSCLC-LM. Kaplan-Meier curves were constructed to assess survival time. Cox regression was applied to select the independent prognostic predictors of cancer-specific survival (CSS). A nomogram was established and its prognostic performance was evaluated.
RESULTS
The age-adjusted incidence of NSCLC-LM increased from 22.7 per 1000000 in 2010 to 25.2 in 2013, and then declined to 22.1 in 2018. According to the multivariable Cox regression analysis of the training set, age, marital status, sex, race, histological type, T stage, metastatic pattern, and whether the patient received chemotherapy or not were identified as independent prognostic factors for CSS (P < 0.05) and were further used to construct a nomogram. The C-indices of the training and validation sets were 0.726 and 0.722, respectively. The results of decision curve analyses (DCAs) and calibration curves showed that the nomogram was well-discriminated and had great clinical utility.
CONCLUSION
We designed a nomogram model and further constructed a novel risk classification system based on easily accessible clinical factors which demonstrated excellent performance to predict the individual CSS of NSCLC-LM patients.
Keywords: Non-small cell lung cancer, Liver metastasis, Nomogram, Risk classification system
Core Tip: Metastatic disease to distant organs from non-small cell lung cancer (NSCLC) is the main reason for poor survival. The liver is one of the most commonly involved extra-pulmonary sites of metastasis in NSCLC patients. The presence of liver metastasis (LM) is an independent prognostic factor for shorter survival in NSCLC patients. The median overall survival of patients with involvement of the liver is less than 5 mo. At present, identifying prognostic factors and constructing survival prediction nomogram for NSCLC patients with LM (NSCLC-LM) are highly desirable. We aimed to identify independent predictors and further build a novel risk stratification system to predict cancer-specific survival (CSS) of NSCLC-LM patients. To the best of our knowledge, our study was the first Surveillance, Epidemiology, and End Results (SEER)-based study to determine prognostic factors affecting CSS in NSCLC patients with liver involvement.
INTRODUCTION
Lung cancer is the leading cause of cancer morbidity and mortality worldwide, with more than 2.0 million new cases diagnosed (more than 10% of the diagnosed cancers) and approximately 1.8 million deaths (close to 20% of the cancer-related deaths) in 2020[1]. Approximately 85% of all lung cancers belong to the non-small cell lung cancer (NSCLC) type[2]. Patients presenting with distant organ involvement account for 40% to 65% of the diagnosed NSCLC cases[3]. Their five-year survival rate is as low as 5%[4,5]. Bone, brain, liver, and the adrenal glands are commonly affected extrapulmonary sites of metastasis in NSCLC patients[3]. Liver involvement accounts for 17% of the NSCLC cases with distant organ metastases and most of those patients have involvement of another extra-hepatic organ[3,6,7]. Recent reports have shown an association between specific organ metastases of NSCLC and survival time, and the presence of liver metastasis (LM) is associated with the worst prognosis. The median overall survival is approximately 4 mo and only a few patients have long lifespans[6,8]. A growing number of researchers have realized that the individual survival disparities are caused by differences in clinicopathological characteristics, such as marital status, age, sex, tumor site, tumor stage, and treatment methods. However, studies focusing on how to stratify the prognosis of NSCLC patients with liver involvement are relatively rare. Therefore, development of a risk classification system with technical feasibility and easy accessibility to predict the survival of NSCLC patients with LM (NSCLC-LM) is highly desirable.
Nomogram has been regarded as a practical and convenient clinical tool to estimate an individual’s clinical outcome by utilizing several clinicopathological variables[9]. A nomographic chart transforms complex patient information into a visual graph, which is characterized by its excellent predictive accuracy and definite reliability when generally applied to decision-making by clinicians.
The purpose of our retrospective study was to determine the demographic information and clinicopathological characteristics correlating with the prognosis of NSCLC-LM and further construct and validate a nomogram predictive model using the SEER database. Then, the novel risk classification system based on the nomogram scores was refined.
MATERIALS AND METHODS
Data source and study design
The SEER database established by the National Cancer Institute is recognized as one of the most authoritative sources of follow-up data for cancer patients in the world. The clinicopathological information of millions of patients in 18 cancer registries of the United States has been recorded in detail over the past 40 years.
We identified and collected the data of all the NSCLC-LM cases diagnosed between 2010 and 2018. The selection criterion was primary and microscopically confirmed NSCLC-LM. Patients with multiple primary cancers and incomplete data regarding distant metastatic sites or survival were excluded. Clinical variables for each case included age (< 65 and ≥ 65 years old), marital status (married and unmarried), sex (female and male), race (black, white, and other), primary site (upper lobe, middle lobe, lower lobe, main bronchus, and other), histological type (adenocarcinoma, squamous cell carcinoma, and other NSCLCs), T stage (T1, T2, T3, and T4), N stage (N0, N1, N2, and N3), metastatic pattern (liver only; liver and bone; liver and brain; liver, bone, and brain), radiotherapy (yes and no/unknown), and chemotherapy (yes and no/unknown). The primary endpoint of the study was cancer-specific survival (CSS) which was defined as the length of time from the initial diagnosis of NSCLC-LM to the time of death from NSCLC-LM.
Statistical analysis
All cases were randomly divided into a training cohort and a validation cohort at a 7:3 ratio. The training cohort was used to establish the nomogram model, and the validation cohort was used to test the model. Categorical variables are shown as frequencies and proportions. The training and validation sets were compared using the chi-squared test.
The independent prognostic variables were first analyzed using the Kaplan-Meier method and the log-rank test. Significant variables screened by univariate analysis were then entered into a multivariate regression analysis, yielding hazard ratios (HR). Finally, those independent predictors were used to construct a nomogram for predicting 3-, 6-, and 12-mo CSS. The predictive ability of the nomogram was assessed by the concordance index (C-index), calibration curves, and decision curve analyses (DCAs)[10,11]. DCAs were constructed to estimate the clinical applicability of the survival prediction model[12]. In addition, a novel risk stratification system was introduced based on an individual’s nomogram scores and was used to divide the training cohort into three risk groups with similar numbers of cases in the low-, intermediate-, and high-risk groups. Joinpoint analysis was used to estimate the incidence trend of NSCLC-LM.
All statistical analyses were performed using SPSS 24.0 (IBM Corporation, Armonk, NY, United States) and R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). All data were extracted using SEER*Stat Software version 8.3.9 (Information Management Services, Inc., Calverton, MD, United States). Two-sided P values of < 0.05 were considered statistically significant.
RESULTS
Incidence and survival of NSCLC-LM
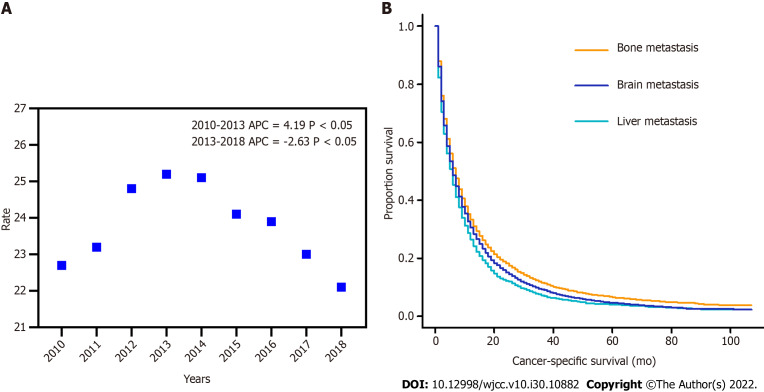
Overall, the incidence rate of NSCLC-LM could be divided into two stages (stage 1: 2010-2013 and stage 2: 2013-2018). The age-adjusted incidence (AAI) of NSCLC-LM increased from 22.7 cases per 1000000 in 2010 to 25.2 cases in 2013, and then declined to 22.1 cases in 2018. The annual percentage change (APC) was 4.19% and -2.63% (P < 0.05), respectively (Figure 1A). The median CSS of patients with NSCLC-LM (12 mo) was significantly shorter than patients with bone (16 mo) or brain (14 mo) metastasis (P < 0.001) (Figure 1B).
Figure 1.
Incidence and survival of non-small cell lung cancer-liver metastasis. A: The age-adjusted incidence of non-small cell lung cancer patients with liver metastasis between 2010 and 2018; B: Kaplan-Meier curves showing cancer-specific survival of patients with liver, bone, and brain metastasis.
Patient characteristics
Overall, a total of 4475 NSCLC-LM patients meeting the eligibility criteria were divided into the training (3135, 70%) and validation cohorts (1340, 30%). Table 1 shows the demographic and clinicopathological characteristics of the NSCLC-LM patients. In all cohorts, the median age at diagnosis was 67 years (range: 23-96 years). More than half of the patients were married (2463, 55.0%) and male (2537, 56.7%). The majority of patients in all sets were white (3517, 78.6%) and had adenocarcinomas (2967, 66.3%). The most common primary site of NSCLC was the upper lobe (2452, 54.8%). In terms of metastatic patterns, more than one-third of the cases presented with liver and bone metastases (1701, 38.0%) and liver involvement only (1598, 35.7%). The majority of patients (2417, 54.0%) received chemotherapy and 1871 patients (41.8%) received radiotherapy. The median age of the training cohort was 67 years (range: 23-96 years) and the median survival was 3 mo [95% confidence interval (CI): 2.8-3.2]. The 3-, 6-, and 12-mo CSS rates were 48.8%, 32.7%, and 17.0%, respectively. The median age of the validation cohort was 66 years (range: 32-93 years) and the median survival was 3 mo (95%CI: 2.6-3.4). The 3-, 6-, and 12-mo CSS rates were 48.0%, 32.1%, and 16.9%, respectively. The demographic and clinicopathological characteristics of the patients were not significantly different between the training and validation cohorts.
Table 1.
Baseline characteristics of the overall, training, and validation cohorts
|
Characteristic
|
Overall cohort (n = 4475)
|
Training cohort (n = 3135)
|
Validation cohort (n = 1340)
|
P
value
|
| Age, yr | 0.839 | |||
| Median (range) | 67 (23-96) | 67 (23-96) | 66 (32-93) | |
| < 65 | 1930 (43.1) | 1349 (43.0) | 581 (43.4) | |
| ≥ 65 | 2545 (56.9) | 1786 (57.0) | 759 (56.6) | |
| Marital status | 0.413 | |||
| Married | 2463 (55.0) | 1713 (54.6) | 750 (56.0) | |
| Unmarried | 2012 (45.0) | 1422 (45.4) | 590 (44.0) | |
| Sex | 0.808 | |||
| Female | 1938 (43.3) | 1354 (43.2) | 584 (43.6) | |
| Male | 2537 (56.7) | 1781 (56.8) | 756 (56.4) | |
| Race | 0.263 | |||
| Black | 566 (12.6) | 413 (13.2) | 153 (11.4) | |
| White | 3517 (78.6) | 2451 (78.2) | 1066 (79.6) | |
| Other1 | 392 (8.8) | 271 (8.6) | 121 (9.0) | |
| Primary site | 0.922 | |||
| UL | 2452 (54.8) | 1725 (55.0) | 727 (54.3) | |
| ML | 198 (4.4) | 133 (4.2) | 65 (4.9) | |
| LL | 1219 (27.2) | 853 (27.2) | 366 (27.3) | |
| MB | 240 (5.4) | 167 (5.3) | 73 (5.4) | |
| Other | 366 (8.2) | 257 (8.2) | 109 (8.1) | |
| Histological type | 0.786 | |||
| ADC | 2967 (66.3) | 2085 (66.5) | 882 (65.8) | |
| SCC | 1169 (26.1) | 810 (25.8) | 359 (26.8) | |
| Other NSCLCs | 339 (7.6) | 240 (7.7) | 99 (7.4) | |
| T stage | 0.910 | |||
| T1 | 507 (11.3) | 362 (11.5) | 145 (10.8) | |
| T2 | 1197 (26.7) | 839 (26.8) | 358 (26.7) | |
| T3 | 1204 (26.9) | 839 (26.8) | 365 (27.2) | |
| T4 | 1567 (35.0) | 1095 (34.9) | 472 (35.2) | |
| N stage | 0.170 | |||
| N0 | 831 (18.6) | 596 (19.0) | 235 (17.5) | |
| N1 | 358 (8.0) | 239 (7.6) | 119 (8.9) | |
| N2 | 2312 (51.7) | 1601 (51.1) | 711 (53.1) | |
| N3 | 974 (21.8) | 699 (22.3) | 275 (20.5) | |
| Metastatic pattern | 0.166 | |||
| Liver only | 1598 (35.7) | 1117 (35.6) | 481 (35.9) | |
| Liver + bone | 1701 (38.0) | 1166 (37.2) | 535 (39.9) | |
| Liver + brain | 415 (9.3) | 301 (9.6) | 114 (8.5) | |
| Liver + bone + brain | 761 (17.0) | 551 (17.6) | 210 (15.7) | |
| Radiotherapy | 0.497 | |||
| Yes | 1871 (41.8) | 1321 (42.1) | 550 (41.0) | |
| No/unknown | 2604 (58.2) | 1814 (57.9) | 790 (59.0) | |
| Chemotherapy | 0.287 | |||
| Yes | 2417 (54.0) | 1677 (53.5) | 740 (55.2) | |
| No/unknown | 2058 (46.0) | 1458 (46.5) | 600 (44.8) |
Other only includes American Indian/Alaskan Native and Asian/Pacific Islander.
UL: Upper lobe; ML: Middle lobe; LL: Lower lobe; MB: Main bronchus; ADC: Adenocarcinoma; SCC: Squamous cell carcinoma; NSCLC: Non-small cell lung cancer.
Independent predictors of NSCLC-LM
The Cox proportional hazards regression model was performed in the training cohort to identify independent prognostic factors of CSS. Age, sex, race, pathological type, T stage, metastatic pattern, and chemotherapy were significantly associated with CSS in univariate analysis, and all of those variables were further proved to be independent predictors of CSS by multivariate analysis (Table 2).
Table 2.
Univariate and multivariate analyses of cancer-specific survival in the training cohort
| Characteristic |
Univariate analysis
|
|
|
Multivariate analysis
|
|
|
|
HR
|
95%CI of HR
|
P
value
|
HR
|
95%CI of HR
|
P
value
|
|
| Age, yr | ||||||
| ≥ 65 vs < 65 | 1.312 | 1.221-1.411 | < 0.001 | 1.236 | 1.147-1.332 | < 0.001 |
| Marital status | ||||||
| Unmarried vs Married | 1.245 | 1.158-1.338 | < 0.001 | 1.103 | 1.023-1.189 | 0.011 |
| Sex | ||||||
| Male vs Female | 1.219 | 1.134-1.311 | < 0.001 | 1.196 | 1.109-1.289 | < 0.001 |
| Race | ||||||
| White vs Black | 1.128 | 1.043-1.221 | 0.003 | 1.061 | 0.953-1.182 | 0.281 |
| Other1 vs Black | 0.781 | 0.714-0.855 | < 0.001 | 0.718 | 0.610-0.844 | < 0.001 |
| Pathological type | ||||||
| ADC vs SCC | 0.814 | 0.749-0.884 | < 0.001 | 0.905 | 0.829-0.988 | 0.026 |
| Other NSCLCs vs SCC | 0.786 | 0.677-0.913 | 0.002 | 0.880 | 0.756-1.025 | 0.101 |
| T stage | ||||||
| T2 vs T1 | 1.195 | 1.052-1.357 | 0.006 | 1.179 | 1.037-1.340 | 0.012 |
| T3 vs T1 | 1.277 | 1.124-1.451 | < 0.001 | 1.226 | 1.078-1.395 | 0.002 |
| T4 vs T1 | 1.358 | 1.201-1.536 | < 0.001 | 1.308 | 1.155-1.482 | < 0.001 |
| N stage | ||||||
| N1 vs N0 | 1.050 | 0.901-1.225 | 0.530 | - | - | - |
| N2 vs N0 | 1.019 | 0.925-1.122 | 0.709 | - | - | - |
| N3 vs N0 | 0.988 | 0.883-1.105 | 0.831 | - | - | - |
| Metastatic pattern | ||||||
| Liver + bone vs Liver only | 1.181 | 1.086-1.285 | < 0.001 | 1.281 | 1.176-1.396 | < 0.001 |
| Liver + brain vs Liver only | 1.328 | 1.166-1.512 | < 0.001 | 1.541 | 1.351-1.758 | < 0.001 |
| Liver + bone+ brain vs liver only | 1.244 | 1.121-1.381 | < 0.001 | 1.562 | 1.400-1.742 | < 0.001 |
| Radiotherapy | ||||||
| Yes vs No/unknown | 0.967 | 0.900-1.040 | 0.364 | - | - | - |
| Chemotherapy | ||||||
| Yes vs No/unknown | 0.346 | 0.321-0.373 | < 0.001 | 0.352 | 0.326-0.381 | < 0.001 |
Other only includes American Indian/Alaskan Native and Asian/Pacific Islander.
HR: Hazard ratio; CI: Confidence interval; ADC: Adenocarcinoma; SCC: Squamous cell carcinoma; NSCLC: Non-small cell lung cancer.
Nomogram construction and validation
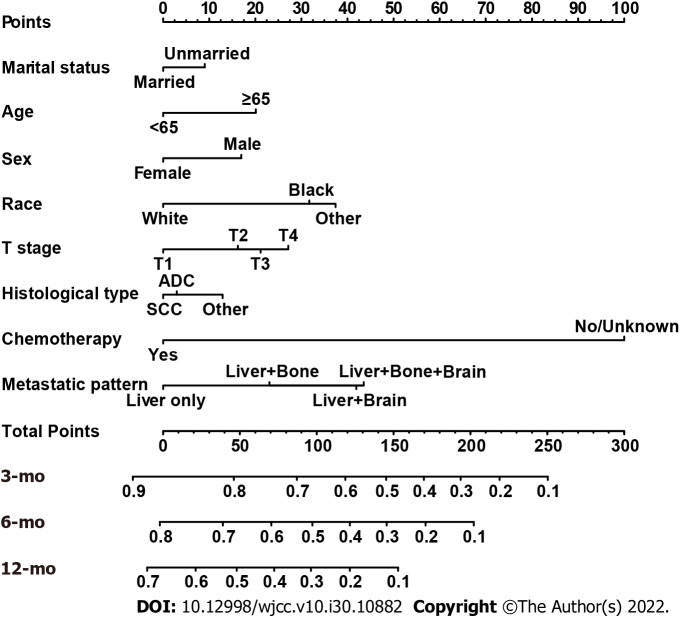
Based on the independent variables above, a nomogram was constructed to predict 3-, 6-, and 12-mo CSS (Figure 2). The nomogram also generated corresponding scores for each predictive variable (Table 3). The probability of an individual’s CSS can be easily calculated by adding the corresponding nomogram points for the patient. The forecasting model also indicated that chemotherapy made the greatest contribution to a patient’s CSS. For each predictor, a vertical line is drawn downward to determine the nomogram points, and the points are added together to obtain the patient’s total nomogram points. A vertical line is drawn from the location of the total point axis down to the survival axes. The number on this line indicates the predicted 3-, 6-, and 12-mo CSS. For example, a 65-year-old (score of 20), married (score of 0), black woman (score of 31.7) had squamous-cell lung carcinoma (score of 12.9). The tumor size was 2 cm (T1 stage, score of 0) but had metastasized to the liver (score of 0). She received chemotherapy (score of 0). The total nomogram score of this patient was 64.6, and a line was drawn down to the survival axes to determine the 3- (78%), 6- (64%), and 12-mo (48%) CSS probabilities.
Figure 2.
Nomogram for predicting 3-, 6-, and 12-mo cancer-specific survival of patients presenting with liver metastases at the initial diagnosis of non-small cell lung cancer.
Table 3.
Corresponding nomogram scores of each variable
|
Characteristic
|
Nomogram score
|
| Age, yr | |
| < 65 | 0 |
| ≥ 65 | 20.09207 |
| Marital status | |
| Married | 0 |
| Unmarried | 9.018027 |
| Sex | |
| Female | 0 |
| Male | 16.9568 |
| Race | |
| Black | 31.70234 |
| White | 37.39893 |
| Other1 | 0 |
| Histological type | |
| ADC | 2.971477 |
| SCC | 12.914637 |
| Other NSCLCs | 0 |
| T stage | |
| T1 | 0 |
| T2 | 16.23500 |
| T3 | 21.12835 |
| T4 | 27.17024 |
| Metastatic pattern | |
| Liver only | 0 |
| Liver + bone | 23.05159 |
| Liver + brain | 41.91404 |
| Liver + bone + brain | 43.49717 |
| Chemotherapy | |
| Yes | 0 |
| No/unknown | 100 |
Other only includes American Indian/Alaskan Native and Asian/Pacific Islander.
ADC: Adenocarcinoma; SCC: Squamous cell carcinoma.
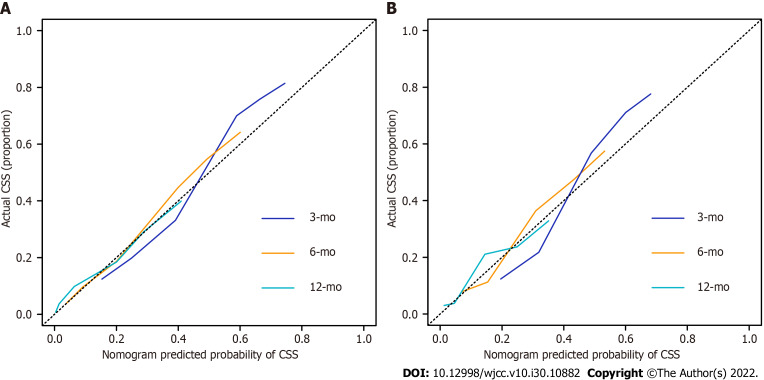
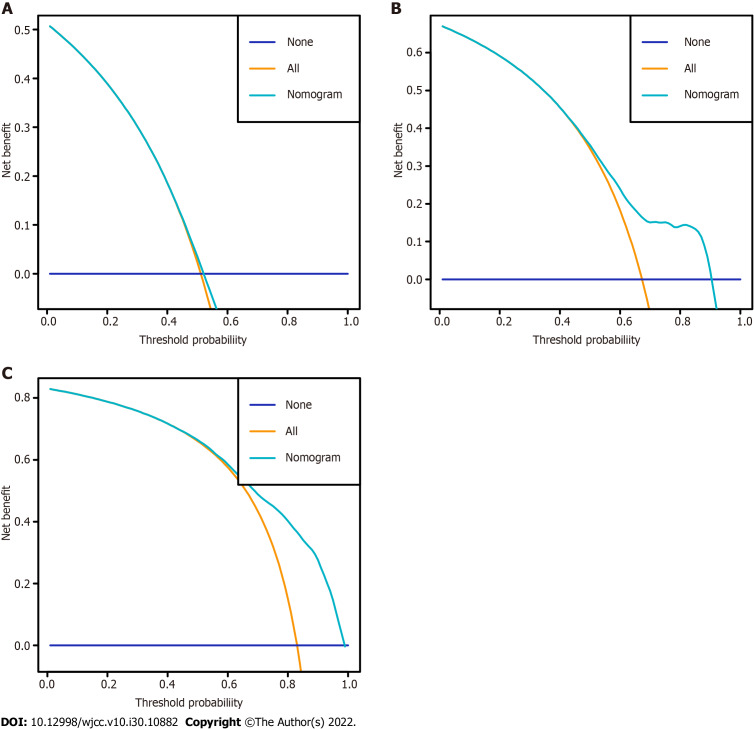
The nomogram was validated internally and externally in the training and validation cohorts, respectively. The C-indices were 0.726 (95%CI: 0.708-0.744) and 0.722 (95%CI: 0.692-0.751), respectively. Both the internal (Figure 3A) and external (Figure 3B) calibration curves showed a good correlation between the actual and predicted probabilities of 3-, 6-, and 12-mo CSS. In addition, DCAs demonstrated that the predictive model yielded preferable net benefits among an extremely wide field of threshold probabilities, suggesting the substantial clinical benefit of the formulated model (Figure 4).
Figure 3.
Calibration curves for predictions of cancer-specific survival at 3, 6, and 12 mo. A: Training cohort; B: Validation cohort. CSS: Cancer-specific survival.
Figure 4.
Decision curves of the nomogram for predicting cancer-specific survival at 3 mo, 6 mo, and 12 mo in the training cohort. A: 3 mo; B: 6 mo; C: 12 mo.
Novel risk classification system construction
A novel risk classification system for CSS was also established to classify all patients based on their individual total scores generated by the formulated nomogram scale. The system classified the patients into three risk groups of nearly equal numbers. According to the risk stratification system, the patients in the training cohort were divided into low-risk (1007/3136, 32.1%; score 0-119.9), intermediate-risk (1102/3136, 35.1%; score 120.0-199.9), and high-risk groups (1026/3136, 32.7%; score 200.0-267.0). In the training cohort, the median CSS rate of patients in the low-, intermediate-, and high-risk groups was 9.0 mo (95%CI: 8.3-9.7), 4.0 mo (95%CI: 3.7-4.3), and 1.0 mo (95%CI: 0.9-1.1), respectively (Figure 5).
Figure 5.
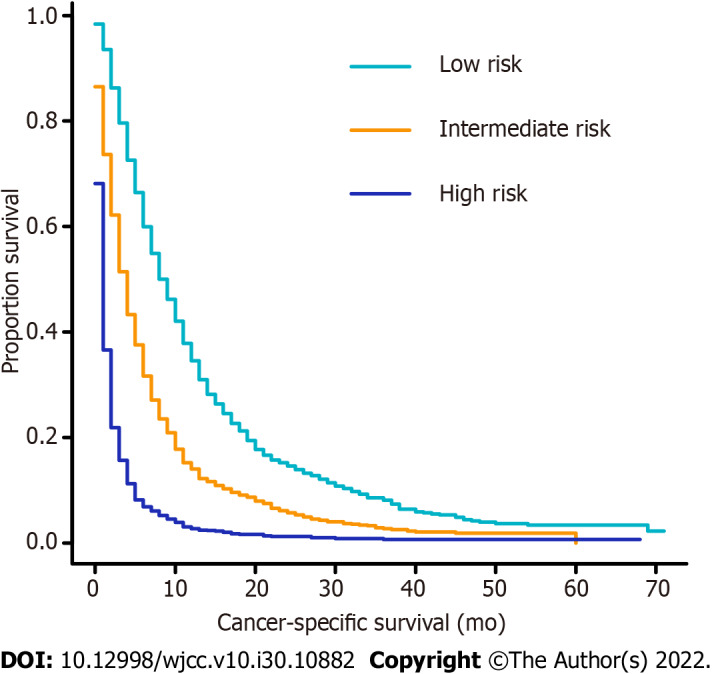
Kaplan–Meier curves of cancer-specific survival for patients in the training cohort according to the novel risk stratification. Low risk vs intermediate risk, P < 0.001; low risk vs high risk, P < 0.001; intermediate risk vs high risk, P < 0.001.
DISCUSSION
Distant metastasis, particularly to the liver, is an important negative prognostic factor for NSCLC patients and leads to a reduction in health-related quality of life and a significant increase in cancer-related mortality. Despite recent advances in various treatment strategies, such as tyrosine kinase inhibitors and immune checkpoint blockade therapy, to prolong patient survival compared to traditional chemotherapy, NSCLC patients who present with LM are still regarded as belonging to a special subgroup whose odds of survival is slim beyond the first 8 mo after diagnosis[6,13-16]. Therefore, investigations into the prognostic factors associated with survival and the construction of feasible risk stratifications for NSCLC-LM is critical for treatment selection and prognosis assessment.
The nomogram model is a statistical tool which consists of several significant variables to estimate an individual’s prognosis in the form of a graphic display. Several nomograms for NSCLC have already been formulated in previous studies[17,18]. As far as we know, this is the first survival prediction nomogram developed using the data from an extensive number of NSCLC-LM from a large contemporary population-based cohort database. All predictors integrated into the model were common patient clinical variables which are readily available to clinicians. This nomogram also stratified patients into different risk subgroups, which might be meaningful and informative for individual treatments. Furthermore, these survival prediction models may be helpful for designers of clinical trials to formulate appropriate inclusion and exclusion criteria and arrange reasonable patient groups. In addition, the SEER database includes 18 registries and covers 28% of the US population, which makes our results more representative and more generalizable than other single-center studies.
In this retrospective study, we primarily explored independent prognostic factors for the CSS of NSCLC-LM patients. Several clinicopathological features, including age, sex, race, pathological type, T stage, metastatic pattern, and chemotherapy were identified that could independently affect the CSS. First, this retrospective study showed that the metastatic pattern was an important prognostic factor which independently affected the CSS of NSCLC-LM patients. In our study, the most common metastatic pattern was liver and bone, followed by liver only, liver, bone, and brain, and liver and brain. The metastatic pattern of liver, bone, and brain had a significantly higher HR for death compared to other clinicopathological variables by multivariate regression analysis (HR = 1.562, 95%CI: 1.400-1.742, P < 0.001). Several studies have explored the association between the number of metastatic sites and the prognosis of NSCLC patients and reported that multiple organ involvement was significantly associated with poorer survival[5,19]. Therefore, we further divided multiple organ involvement into different metastatic patterns, and then investigated the effect of the metastatic patterns on survival time.
This study also showed that patients of younger ages (< 65 years old) had survival advantages over older patients, which was similar to previous studies. Wu et al[20] also found that age ≥ 65 years was an independent factor affecting the survival of stage I-IIIA NSCLC patients. Two other studies explored the outcomes of immunotherapy for different age groups and found that older patients had a significantly higher HR for death than younger patients[21,22]. Subramanian et al[23] attributed the age-related prognostic differences to differences in the clinical characteristics, including sex, pathology, and diagnosis stage, between the younger and older patients. The organ function of the younger patients was in a relatively balanced state, which was significantly different from that of the elderly patients. This also made a significant contribution to the different prognoses[24,25]. As for gender, this study revealed that female gender was a favorable prognostic factor in NSCLC, which was consistent with the results of many previous studies[26-28]. Yoshida et al[26] explained that women were prone to early-stage disease, contributing to longer survival times compared to men. Similarly, Hanagiri et al[28] also reported that 69% of the female patients were found to have NSCLC through medical tests for other diseases and 45% of the male patients were diagnosed with lung cancer-related symptoms, so there were more late male NSCLC patients than female. Interestingly, a previous study suggested that most lung adenocarcinomas occurred in the non-smoking population, whereas most of the women were non-smokers. Furthermore, the survival advantage of women with NSCLC disappeared after adjustment for the confounding factor of smoking. Thus, the main factors affecting the prognosis of lung cancer were smoking and pathological types, rather than sex[29]. We also explored the effect of marital status on the CSS of NSCLC-LM patients and found that unmarried patients were at significantly higher risk for poorer prognoses. Wu et al[30] retrospectively analyzed more than 70000 NSCLC patients and concluded that unmarried patients had shorter survival time compared to married patients, regardless of the stage of the disease. The living conditions of married patients are more stable and the interaction with and caretaking of a spouse play important roles in the physiology and psychology of patients with cancer. Most married patients receive care and support from their spouses in life and have high compliance with medical treatment and regular examinations. As for the treatment, our study revealed that the CSS was better in the chemotherapy cohort than in the non-chemotherapy cohort. We believe that this is because chemotherapy and radiotherapy were the cornerstone of lung cancer treatment strategy for advanced NSCLC before the era of molecular targeted therapy and immunotherapy and only three therapeutic measures, including surgery, chemotherapy, and radiotherapy, are included in the SEER database. However, we found that radiotherapy did not improve the survival of NSCLC-LM. This may be because most of the patients in our study had multiple organ metastases. Previous studies showed that extrahepatic metastasis, as well as the size and number of liver lesions, significantly affected the outcome of radiotherapy in NSCLC-LM[31]. Considering the stage, we found that higher T stages were significantly associated with poorer survival, whereas the N stage did not affect the prognosis, which was consistent with two previous studies[6,32]. Those studies reported that lymph node involvement was associated with a poor prognosis for patients with lung cancer, but that effect was not seen in the prognosis of NSCLC-LM, a finding not explained by clinical experience[6,32]. Multivariate analysis also demonstrated that race and histological type were independent predictors, which was also consistent with the results reported by previous studies[33-35]. Deng et al[36] demonstrated that the survival of adenocarcinoma patients was superior to that of patients with other histological types in metastatic NSCLC patients, but that study did not directly compare the survival rates of squamous cell carcinoma and adenocarcinoma patients. Wang et al[33] found that squamous cell carcinoma was a risk factor for poor prognosis compared to adenocarcinoma in localized and regional metastatic NSCLC patients, but no significant difference was found in patients with distant metastases. Interestingly, the present study showed that squamous cell carcinoma was the worst prognostic factor among histological types in NSCLC-LM. With regard to race, Lathan et al[37] reported that black patients who underwent surgical staging, but did not receive resection, had a better prognosis than their white counterparts. In our study, white patients had an inferior prognosis to black patients among those with liver involvement. The primary cause of such obvious differences between our study and those of others might be differences in the inclusion criteria and sample size. That is, our study indicated that the clinicopathological characteristics had different influences on the prognosis of patients with liver metastasis. All the risk factor information is easily available and deserves close attention in clinical work. Moreover, more active treatment measures should be provided to patients with these risk factors. In addition, several clinicopathological characteristics were poor indicators for patients with hepatic metastasis from NSCLC. These characteristics might be good prognostic indicators for patients with other organ involvement, which suggests that clinicians should personally evaluate the potential survival of each of their NSCLC patients.
Based on the risk factors extracted by the Cox proportional hazards model, a prognostic nomogram for predicting 3-, 6-, and 12-mo CSS in NSCLC-LM was constructed. High C-indices in the internal and external validation demonstrated perfect discrimination ability of the new model and the calibration plots revealed that the nomogram prediction agreed well with observed rates. In addition, the DCAs showed good clinical applicability of the model. All these factors ensured that the formulated model could conveniently and accurately predict patient prognosis, facilitating the formulation of effective treatment strategies or interventions. Finally, using the corresponding nomogram scores, a novel risk classification system was formulated to stratify all patients into low-, intermediate-, and high-risk groups. High-risk patient populations should receive closer attention and more rigorous follow-up in order to monitor carefully for any progression or reoccurrence and adjust treatment plan in a timely manner as changes in their condition occur. In addition, by identifying high-risk patients, palliative care, like spiritual guidance or psychological support, can be given sooner and their participation in clinical trials of anti-cancer drugs can be encouraged.
Our study was innovative and superior to other risk models and generated a reliable tool that clinicians can easily use by gathering and entering clinicopathological variables to assign patient risk and predict survival. Nonetheless, several limitations deserve attention and need to be improved. One of the main limitations resulted from the SEER dataset itself. For example, the specific clinical information of certain patients, such as chemotherapy and radiation therapy, was not always given clearly and was labeled as “no/unknown”. This did not reflect the real situation and reliable conclusions could not be drawn after statistical analysis. In addition, the absence of therapeutic regimens, including molecular targeted therapy and immunotherapy, could reduce the long-term significance of the prognostic model. Besides, the types of data accessible through the SEER program were limited. For example, there is a lack of data on changes in tumor size during the course of the disease, type of gene mutations, performance status, and the sequence of treatments. These factors might have a major impact on the prognosis of NSCLC-LM patients. Furthermore, as a retrospective study, inherent selection biases were inevitable. Finally, the constructed nomogram was validated in a subgroup of patients that met the inclusion criteria, but the strictest external validation based an independent large sample size needs to be performed.
CONCLUSION
A nomogram was constructed using several key variables and was validated as a convenient and reliable instrument for survival prediction in NSCLC-LM. Based on the prognostic nomogram, a novel risk classification system was developed and effectively identified a high-risk population, which could aid in guiding treatment strategies and prognostic evaluation for clinicians.
ARTICLE HIGHLIGHTS
Research background
The risk factors affecting the cancer-specific survival (CSS) of non-small cell lung cancer (NSCLC) patients with liver metastasis (LM) (NSCLC-LM) are not well known.
Research motivation
A nomographic chart transforms complex patient information into a visual graph, which is characterized by its excellent predictive accuracy and definite reliability when generally applied to decision-making by clinicians.
Research objectives
To build a forecasting model to predict the survival time of NSCLC-LM patients.
Research methods
Joinpoint analysis was used to estimate the incidence trend of NSCLC-LM. Cox regression was applied to identify the independent prognostic predictors of CSS. A survival prediction model was constructed for predicting 3-, 6-, and 12-mo CSS. The predictive ability of the nomogram was estimated using calibration curves and decision curve analyses (DCAs).
Research results
Clinical variables including age, marital status, sex, race, histological type, T stage, metastatic pattern, and whether the patient received chemotherapy or were identified as independent prognostic factors for CSS (P < 0.05) and were further used to construct a nomogram. The results of DCAs and calibration curves showed that the nomogram was well-discriminated and had great clinical utility.
Research conclusions
A convenient and credible nomogram model was constructed, which could aid in guiding treatment strategies and prognostic evaluation for clinicians.
Research perspectives
Our study may serve as a reference for clinicians to identify high-risk populations for providing individualized therapy.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of the Jilin Province Tumor Hospital.
Conflict-of-interest statement: We have no financial relationships to disclose for this article.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 19, 2022
First decision: July 13, 2022
Article in press: September 16, 2022
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Diez-Alonso M, Spain; Doval D, India S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Zhao S
Contributor Information
Jun-Feng Wang, The First Department of Thoracic Oncology, Jilin Province Tumor Hospital, Changchun 130021, Jilin Province, China.
Hong-Di Lu, The First Department of Thoracic Oncology, Jilin Province Tumor Hospital, Changchun 130021, Jilin Province, China.
Ying Wang, The First Department of Thoracic Oncology, Jilin Province Tumor Hospital, Changchun 130021, Jilin Province, China.
Rui Zhang, The First Department of Thoracic Oncology, Jilin Province Tumor Hospital, Changchun 130021, Jilin Province, China.
Xiang Li, Big Data Center for Clinical Research, Jilin Province Tumor Hospital, Changchun 130021, Jilin Province, China.
Sheng Wang, The First Department of Thoracic Oncology, Jilin Province Tumor Hospital, Changchun 130021, Jilin Province, China. wangsh2334@163.com.
Data sharing statement
No additional data are available.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Stoltzfus KC, Lehrer EJ, Horn SR, Siva S, Trifiletti DM, Meng MB, Verma V, Louie AV, Zaorsky NG. The Epidemiology of Lung Metastases. Front Med (Lausanne) 2021;8:723396. doi: 10.3389/fmed.2021.723396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 5.Chao C, Qian Y, Li X, Sang C, Wang B, Zhang XY. Surgical Survival Benefits With Different Metastatic Patterns for Stage IV Extrathoracic Metastatic Non-Small Cell Lung Cancer: A SEER-Based Study. Technol Cancer Res Treat. 2021;20:15330338211033064. doi: 10.1177/15330338211033064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng SE, Chiou YY, Lee YC, Perng RP, Jacqueline WP, Chen YM. Number of liver metastatic nodules affects treatment options for pulmonary adenocarcinoma patients with liver metastases. Lung Cancer. 2014;86:225–230. doi: 10.1016/j.lungcan.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Kagohashi K, Satoh H, Ishikawa H, Ohtsuka M, Sekizawa K. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol. 2003;20:25–28. doi: 10.1385/MO:20:1:25. [DOI] [PubMed] [Google Scholar]
- 8.Choi MG, Choi CM, Lee DH, Kim SW, Yoon S, Kim WS, Ji W, Lee JC. Different prognostic implications of hepatic metastasis according to front-line treatment in non-small cell lung cancer: a real-world retrospective study. Transl Lung Cancer Res. 2021;10:2551–2561. doi: 10.21037/tlcr-21-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 12.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D, Waterhouse D, Domine M, Garassino M, Chow LQM, Blumenschein G Jr, Barlesi F, Coudert B, Gainor J, Arrieta O, Brahmer J, Butts C, Steins M, Geese WJ, Li A, Healey D, Crinò L. Nivolumab vs docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29:959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- 14.Jiang T, Chu Q, Wang H, Zhou F, Gao G, Chen X, Li X, Zhao C, Xu Q, Li W, Wu F, Xiong A, Zhao J, Xu Y, Su C, Ren S, Zhou C, Hirsch FR. EGFR-TKIs plus local therapy demonstrated survival benefit than EGFR-TKIs alone in EGFR-mutant NSCLC patients with oligometastatic or oligoprogressive liver metastases. Int J Cancer. 2019;144:2605–2612. doi: 10.1002/ijc.31962. [DOI] [PubMed] [Google Scholar]
- 15.Tang C, Liao Z, Hess K, Chance WW, Zhuang Y, Jensen G, Xu T, Komaki R, Gomez DR. Prognosis and predictors of site of first metastasis after definitive radiation therapy for non-small cell lung cancer. Acta Oncol. 2016;55:1022–1028. doi: 10.3109/0284186X.2016.1154602. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Nelson RA, Bogardus A, Grannis FW Jr. Five-year lung cancer survival: which advanced stage nonsmall cell lung cancer patients attain long-term survival? Cancer. 2010;116:1518–1525. doi: 10.1002/cncr.24871. [DOI] [PubMed] [Google Scholar]
- 17.Botticelli A, Salati M, Di Pietro FR, Strigari L, Cerbelli B, Zizzari IG, Giusti R, Mazzotta M, Mazzuca F, Roberto M, Vici P, Pizzuti L, Nuti M, Marchetti P. A nomogram to predict survival in non-small cell lung cancer patients treated with nivolumab. J Transl Med. 2019;17:99. doi: 10.1186/s12967-019-1847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou C, Shan C, Lai M, Zhou Z, Zhen J, Deng G, Li H, Li J, Ren C, Wang J, Lu M, Zhang L, Wu T, Zhu D, Kong FS, Chen L, Cai L, Wen L. Individualized Nomogram for Predicting Survival in Patients with Brain Metastases After Stereotactic Radiosurgery Utilizing Driver Gene Mutations and Volumetric Surrogates. Front Oncol. 2021;11:659538. doi: 10.3389/fonc.2021.659538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Zhang Y, Sun X, Gusdon AM, Song N, Chen L, Jiang G, Huang Y. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol. 2018;144:1835–1842. doi: 10.1007/s00432-018-2702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Zhou L, Huang L, Gu J, Li S, Liu B, Feng J, Zhou Y. Nomogram integrating gene expression signatures with clinicopathological features to predict survival in operable NSCLC: a pooled analysis of 2164 patients. J Exp Clin Cancer Res. 2017;36:4. doi: 10.1186/s13046-016-0477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Ju Q, Qian B, Zhang F, Shi H. The effectiveness of PD-1 inhibitors in non-small cell lung cancer (NSCLC) patients of different ages. Oncotarget. 2018;9:7942–7948. doi: 10.18632/oncotarget.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenstein MRL, Nipp RD, Muzikansky A, Goodwin K, Anderson D, Newcomb RA, Gainor JF. Impact of Age on Outcomes with Immunotherapy in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol. 2019;14:547–552. doi: 10.1016/j.jtho.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian J, Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Piccirillo J, Govindan R. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol. 2010;5:23–28. doi: 10.1097/JTO.0b013e3181c41e8d. [DOI] [PubMed] [Google Scholar]
- 24.Viñal D, Martínez D, Higuera O, de Castro J. Genomic profiling in non-small-cell lung cancer in young patients. A systematic review. ESMO Open. 2021;6:100045. doi: 10.1016/j.esmoop.2020.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant AS, Cerfolio RJ. Differences in outcomes between younger and older patients with non-small cell lung cancer. Ann Thorac Surg. 2008;85:1735–1739; discussion 1739. doi: 10.1016/j.athoracsur.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida Y, Murayama T, Sato Y, Suzuki Y, Saito H, Nomura Y. Gender Differences in Long-Term Survival after Surgery for Non-Small Cell Lung Cancer. Thorac Cardiovasc Surg. 2016;64:507–514. doi: 10.1055/s-0035-1558995. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai H, Asamura H, Goya T, Eguchi K, Nakanishi Y, Sawabata N, Okumura M, Miyaoka E, Fujii Y Japanese Joint Committee for Lung Cancer Registration. Survival differences by gender for resected non-small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol. 2010;5:1594–1601. doi: 10.1097/JTO.0b013e3181f1923b. [DOI] [PubMed] [Google Scholar]
- 28.Hanagiri T, Sugio K, Uramoto H, So T, Ichiki Y, Sugaya M, Ono K, Yasuda M, Nozoe T, Yasumoto K. Gender difference as a prognostic factor in patients undergoing resection of non-small cell lung cancer. Surg Today. 2007;37:546–551. doi: 10.1007/s00595-006-3453-9. [DOI] [PubMed] [Google Scholar]
- 29.Hsu LH, Chu NM, Liu CC, Tsai SY, You DL, Ko JS, Lu MC, Feng AC. Sex-associated differences in non-small cell lung cancer in the new era: is gender an independent prognostic factor? Lung Cancer. 2009;66:262–267. doi: 10.1016/j.lungcan.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. 2017;8:103518–103534. doi: 10.18632/oncotarget.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawood O, Mahadevan A, Goodman KA. Stereotactic body radiation therapy for liver metastases. Eur J Cancer. 2009;45:2947–2959. doi: 10.1016/j.ejca.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Jiang T, Cheng R, Zhang G, Su C, Zhao C, Li X, Zhang J, Wu F, Chen X, Gao G, Li W, Cai W, Zhou F, Zhao J, Xiong A, Ren S, Zhou C. Characterization of Liver Metastasis and Its Effect on Targeted Therapy in EGFR-mutant NSCLC: A Multicenter Study. Clin Lung Cancer. 2017;18:631–639.e2. doi: 10.1016/j.cllc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Lian B, Ye L, Hu J, Song Y. Clinicopathological characteristics and survival outcomes in adenosquamous carcinoma of the lung: a population-based study from the SEER database. Oncotarget. 2018;9:8133–8146. doi: 10.18632/oncotarget.23550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang R, Li P, Li Q, Qiao Y, Xu T, Ruan P, Song Q, Fu Z. Radiotherapy improves the survival of patients with stage IV NSCLC: A propensity score matched analysis of the SEER database. Cancer Med. 2018;7:5015–5026. doi: 10.1002/cam4.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan P, Cao JL, Rustam A, Zhang C, Yuan XS, Bao FC, Lv W, Hu J. Time-to-Progression of NSCLC from Early to Advanced Stages: An Analysis of data from SEER Registry and a Single Institute. Sci Rep. 2016;6:28477. doi: 10.1038/srep28477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng J, Ren Z, Wen J, Wang B, Hou X, Xue Z, Chu X. Construction of a nomogram predicting the overall survival of patients with distantly metastatic non-small-cell lung cancer. Cancer Manag Res. 2018;10:6143–6156. doi: 10.2147/CMAR.S183878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.