Abstract
AIM
To assess alterations in growth factors, inflammatory mediators, and cytokines associated with vitreous-retinal diseases in vitreous humor from patients with proliferative diabetic retinopathy (PDR), and to identify potential new treatment targets and strategies.
METHODS
Control vitreous samples were collected from patients with macular hole, epiretinal membranes, or rhegmatogenous retinal detachments, and PDR samples from patients with complications of PDR, who required pars plana vitrectomy. Specimens were stored at −80°C and then investigated by Luminex multi-factor assay. Parametric and nonparametric analyses of demographic characteristics and cytokine expression levels were conducted using SPSS.
RESULTS
There were no significant differences in demographic characteristics between patients with and without PDR. Expression levels of growth factors [platelet-derived growth factor (PDGF)-AA, glial cell line-derived neurotrophic factor (GDNF), and vascular endothelial growth factor A (VEGFA)], inflammatory mediators [interleukin (IL)-8, IL-11, and tumor necrosis factor-α (TNF-α)] and cytokines [chemokine C-X-C ligand (CXCL)10, interferon-γ (IFN-γ), and granulocyte macrophage-colony stimulating factor (GM-CSF)] were significantly elevated in vitreous humor from patients with PDR compared with those in the control group (all P<0.05). Further, VEGFA levels were lower in patients with PDR treated with anti-VEGF injection than those who were not (P<0.05), and there was no difference between the PDR group treated with anti-VEGF and controls (P>0.05).
CONCLUSION
This proof-of-concept study demonstrates the potential for combinational therapeutic strategies to ameliorate diabetic retinopathy progression by targeting growth factors, inflammatory factors, and cytokines, in addition to VEGFA.
Keywords: vitreous, proliferative diabetic retinopathy, growth factors, inflammation, cytokines
INTRODUCTION
Diabetic retinopathy (DR) is a leading cause of blindness, due to the prevalence of diabetes mellitus (DM) worldwide[1]. Breakdown of the blood-retinal barrier and the resulting complex pathophysiology lead to formation of microaneurysms, cotton-wool patches, and nonperfusion, which characterize non-proliferative diabetic retinopathy (NPDR)[2]. When DR progresses and becomes severe, vitreous hemorrhage (VH), fibrovascular membrane formation, and even tractional retinal detachment (TRD) can occur and indicate the development of proliferative diabetic retinopathy (PDR). Current therapies targeting DR with neovascularization or diabetic macular edema mainly focus on intravitreal injection of anti-vascular endothelial growth factor (VEGF) antibody[3]; however, not all patients respond positively. The huge burden of this increasingly severe disease necessitates better understanding of the molecular mechanisms underlying DR as a crucial objective in the struggle to prevent vision loss.
The critical role of growth factors, particularly VEGF-family proteins, in DR pathophysiology is established. Prior studies have focused on VEGF-A, -B, -C, and -D[4], as well as placental growth factor (PlGF)[5]. Research to identify other growth factors that are correlated with DR progression, which may cooperate directly with, or be independent from, VEGF, could inform novel therapeutic interventions. Notably, basic fibroblast growth factor (bFGF) functions in coordination with VEGF to worsen vascular permeability in experimental retinopathy[6]–[7]. To date, novel proangiogenic targets, such as platelet-derived growth factor (PDGF), are subject to ongoing investigation for their potential for use in patients poorly responsive to anti-VEGF therapy and as a means to reduce the adverse effects of long-term VEGF inhibition for treatment of neovascular eye disease[8]. Glial cell line-derived neurotrophic factor (GDNF) suppresses vascular hyperpermeability in retinopathy pathology by decreasing VEGF expression in endothelial cells[9]. Therefore, in addition to VEGFA, we analyzed bFGF, PDGF-AA, and GDNF in vitreous samples in this study.
In addition to assessing the role of growth factors, many studies have focused on the recruitment and important effects of inflammatory mediators in vitreoretinal diseases[10]–[11]. Inflammatory flux can be induced by hyperglycemia and hypoxia under diabetic conditions, thereby inducing oxidative stress, inflammatory storm, and infiltration of inflammatory factors, among other effects, leading to deterioration of DR[12]–[13]. Hence, in developing potential therapeutic targets for future DR treatments, it is fundamental to include an inflammatory mediator profile.
While growth factors and inflammatory mediators are major elements influencing retinal neovascular disease, including DR, other cytokines likely contribute to the initiation and progression of these diseases[14]. Chemokines may function similarly to VEGF or assist VEGF in driving retinal cell proliferation and motility to influence DR development[15]. Therefore, establishing a comprehensive profile of vitreous proteins, including vascular growth factors, inflammatory factors, and chemokines, across a range of DR samples may help to direct future investigations and strategies.
SUBJECTS AND METHODS
Ethical Approval
This study was performed in accordance with the tenets of the Declaration of Helsinki and approved by the local Research Ethics Committee of Shanghai General Hospital. Informed consent was obtained from all individual participants included in the study.
Study Population
This study was a prospective analysis of 31 eyes from 31 patients undergoing pars plana vitrectomy from April 2020 to September 2020 at the National Clinical Ophthalmic Center, Shanghai General Hospital. The inclusion criteria were as follows: all patients were to undergo pars plana vitrectomy by a single surgeon during the above time period for indications including macular hole (MH), epiretinal membranes (ERM), or rhegmatogenous retinal detachment (RRD, control group); or for complications of PDR (VH or TRD, PDR group). Patients with a history of prior vitrectomy, choroidal detachments, retinal vascular occlusion, uveitis, lens dislocation, complicated anterior segment surgery, glaucoma, or trauma were excluded. Patients with PDR who had received treatment with intravitreal anti-VEGF agents were included for comparison with other patients with PDR and untreated eyes. Patient clinical characteristics, including sex, age, eye, pretreatment with ranibizumab (Lucentis, 0.5 mg), fasting blood glucose (FBG), serum creatinine, and blood urea nitrogen (BUN), were extracted from their medical records.
Vitreous Sample Collection, Preparation, Storage, and Use
At the beginning of the surgical procedure, undiluted vitreous samples were collected from patients as previously reported[16]. Briefly, before initiating infusion and starting the vitrectomy, a 25-gauge vitreous cutter was used to obtain an undiluted vitreous sample (approximately 1 mL). A 2-mL syringe was attached to the vitreous cutter to contain the specimen. Once sufficient volume of vitreous fluid was obtained, infusion was initiated and the syringe removed to continue the vitrectomy. Undiluted specimens were injected into cryogenic vials and immediately transferred to liquid nitrogen, followed by storage at –80°C until further analysis.
A multi-factor assay (R&D, USA) was performed on a Luminex instrument according to the manufacturer's protocol. All samples were plated in duplicate. Fifteen biomarkers were analyzed, including the growth factors, VEGFA, bFGF, PDGF-AA, and GDNF; the cytokines and chemokines, interleukin (IL)-6, -7, -8, -11, tumor necrosis factor-α (TNF-α), C-C motif chemokine ligand 2 (CCL2), chemokine C-X-C ligand (CXCL)10, Fractalkine/CX3C chemokine ligand 1 (CX3CL1), CXCL1, interferon-γ (IFN-γ), and granulocyte macrophage-colony stimulating factor (GM-CSF). Results are reported as pg/mL.
Protein-Protein Interactions
Predicted protein-protein interactions were generated following analysis of significantly different molecules input into the STRING database (http://string-db.org). The molecules include VEGFA, bFGF, PDGF-AA, GDNF, IL-6, -7, -8, -11, TNF-α, CCL2, CXCL10, CX3CL1, CXCL1, IFN-γ, and GM-CSF.
Statistical Analysis
Vitreous specimens were divided into two groups: 1) those who had undergone vitrectomy for MH, ERM, or RRD with no history of diabetes mellitus (DM, control group, n=20); 2) eyes with complications of PDR (PDR group, n=11). Data management and analyses were performed using SPSS software (SPSS, Inc., Chicago, IL, USA). All data were assessed for normal distribution, and if distributions were skewed, analyzed using nonparametric tests. Analyses of patient characteristics were conducted by Chi-square, unpaired t-, and Mann-Whitney U tests. Mann-Whitney U tests were used to compare levels of individual biomarkers between groups, as well as between pretreated and untreated eyes within the PDR group. For all tests a P value <0.05 was considered significant.
RESULTS
Clinical Characteristics
Vitreous samples were collected from 31 eyes of 31 patients. The characteristics and distributions of the subjects included in this study are listed in Table 1. A total of 20 eyes without DM, and 11 eyes with PDR were enrolled. There was no significant difference in age (56.85±11.90 vs 51.6±11.5y) or sex (9/11 vs 6/5 male/female) between the cohorts (P=0.26 and P=0.94 respectively). Six patients received intravitreal anti-VEGF therapy three days prior to vitrectomy. FBG was significantly higher in PDR group (8.11±1.382 mmol/L) than the control group (5.6±0.766 mmol/L; P<0.001). Although there was no significant difference in serum creatinine (68.645±23.861 vs 162.155±248.694 µmol/L, P=0.24) or BUN (5.802±1.807 vs 9.416±8.348 mmol/L, P=0.19) levels between the control and PDR groups, levels tended to be elevated in patients with PDR relative to the control group.
Table 1. Clinical characteristics of the subjects included in this study.
| Demographic | Control (n=20) | PDR (n=11) | P |
| Age (y, mean±SD) | 56.85±11.90 | 51.6±11.5 | 0.26 |
| Sex (M:F) | 9:11 | 6:5 | 0.94 |
| Eye type (OD:OS) | 14:6 | 6:5 | 0.36 |
| Pretreatment with anti-VEGF | 0 | 6 | N/A |
| FBG (mmol/L) | 5.6±0.766 | 8.11±1.382 | <0.001a |
| Serum creatinine (µmol/L) | 68.645±23.861 | 162.155±248.694 | 0.24 |
| BUN (mmol/L) | 5.802±1.807 | 9.416±8.348 | 0.19 |
VEGF: Vascular endothelial growth factor; FBG: Fasting blood glucose; BUN: Blood urea nitrogen; PDR: Proliferative diabetic retinopathy; N/A: Not available. OD: Right eye; OS: Left eye. aStatistically significant P value by unpaired t-test.
Protein Expression in Vitreous of Patients with Different Vitreoretinal Diseases
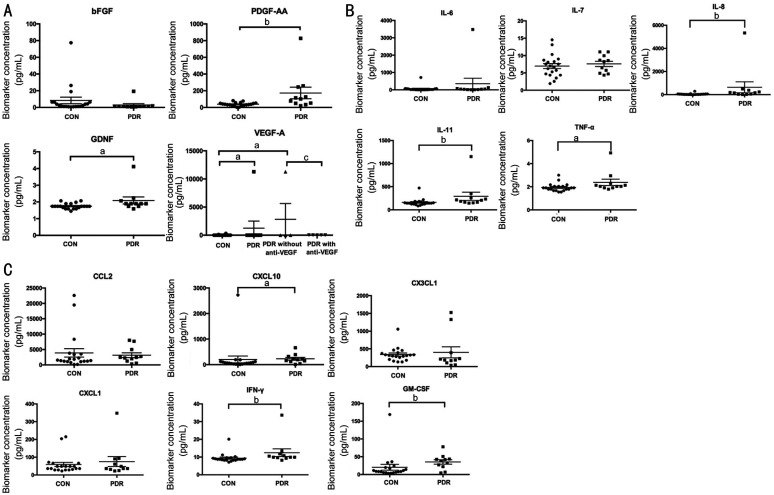
To evaluate the roles of growth factors, inflammatory factors, and cytokines in PDR progression, biomarkers of various pathways were detected in vitreous samples. Among the growth factors, bFGF exhibited no significant difference between control and PDR specimens, while PDGF-AA (∼4.5 fold) and GDNF (∼1.2 fold) levels were clearly higher in PDR vitreous than in samples from control patients. Further, VEGFA was elevated to 38-fold in samples from patients with diabetes relative to controls. As some patients with PDR received anti-VEGF therapy prior to surgery, unsurprisingly, VEGFA was significantly inhibited by intravitreal injection of anti-VEGF agents, and thus its expression was similar to that of control group. In contrast, VEGFA expression in samples from patients with PDR who had not undergone anti-VEGF injection was much higher (∼82.6 fold) than that in control samples (Figure 1A). Of the inflammatory factors examined, vitreous levels of IL-8 (∼14.7 fold), IL-11 (∼1.8 fold), and TNF-α (∼1.2 fold) were clearly higher in patients with PDR than controls, while those of IL-6 and IL-7 did not differ significantly between control and diabetic samples (Figure 1B). Subsequently, cytokines were explored in vitreous samples from patients with diabetic conditions and controls. Levels of CXCL10 (∼1.2 fold), IFN-γ (∼1.3 fold), and GM-CSF (∼1.7 fold) were significantly enhanced in PDR vitreous vs control samples; however, there were no significant differences in levels of CCL2, CX3CL1, and CXCL1 between control and PDR specimens (Figure 1C).
Figure 1. Expression levels of different factors in vitreous humor of control and PDR patients.
A: Altered growth factors including bFGF, PDGF-AA, GDNF, and VEGF-A in vitreous; B: Inflammatory mediators containing IL-6, -7, -8, -11, and TNF-α were detected in vitreous samples; C: Vitreous cytokines including CCL2, CXCL10, CX3CL1, CXCL1, IFN-γ, and GM-CSF were evaluated. aP<0.05, bP<0.01, PDR vs CON; cP<0.05, PDR with anti-VEGF vs PDR without anti-VEGF. CON: Control group; bFGF: Basic fibroblast growth factor; CCL2: C-C motif chemokine ligand 2; CXCL: Chemokine C-X-C ligand; CX3CL1: Fractalkine/CX3C chemokine ligand 1; GM-CSF: Granulocyte macrophage-colony stimulating factor; IL: Interleukin; IFN-γ: Interferon-γ; PDGF: Platelet-derived growth factor; PDR: Proliferative diabetic retinopathy; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor.
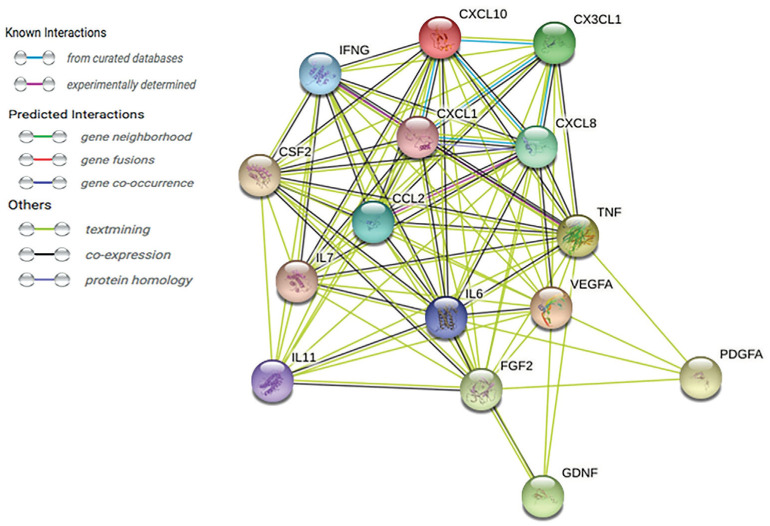
Based on the results of this study, we constructed a complex protein network, based on the STRING database (Figure 2). The STRING database (http://string-db.org) provides comprehensive assessment and integration of protein-protein interactions, including direct (physical) and indirect (functional) associations. This analysis may provide improved understanding of the interactions of growth factors, inflammatory mediators, and cytokines in DR pathogenesis.
Figure 2. A string pathway demonstrating the interdependent relationships of the 15 biomarkers.
DISCUSSION
Identification of Biomarkers in Proliferative Diabetic Retinopathy Vitreous
The identification and quantification of biomarkers related to different functions in vitreous humor from patients with PDR could contribute to comprehensive understanding of DR pathogenesis. Growth factors, inflammatory factors, and cytokines, are potential targets for further investigation and therapeutic intervention. Previous studies of DR have largely focused on aqueous humor or serum, rather than vitreous, as patients with NPDR do not usually require surgically intervention, leading to difficulty in obtaining vitreous[17]–[19]; however, biomarker levels in the aqueous humor reflect the aqueous recycling and topical pathophysiology of diabetic patients, and do not reliably respond to their counterparts in the vitreous; hence, direct evaluation of vitreous proteins is preferable[20]. In this study, we assessed a network of growth factors, inflammatory factors, and cytokines in the vitreous humor of patients with PDR (including VH and TRD), and detected significantly altered levels of 15 chemokines. Unsurprisingly, certain growth factors, inflammatory factors, and cytokines were elevated in the PDR group relative to samples from patients with non-diabetes conditions.
PDGF, GDNF, and VEGF family
In this study, we found that levels of PDGF-AA, GDNF, and VEGFA were significantly increased in vitreous from patients with PDR, consistent with previous observations in fibrous vascular membrane and vitreous humor samples from patients with PDR[15]. PDGF is a strong inducer of angiogenesis, and PDGF-AA is related to proteins involved in neovascularization and fibrosis, such as angiopoietin-1 (Ang-1) and -2 (Ang-2), transforming growth factor β (TGF-β), and PlGF[21]. These findings support a possible role for PDGF as a pivotal force in accelerating pathological angiogenesis, and even fibrosis, in patients with PDR. Notably, clinical trials of intravitreal injection with a combination of a PDGF inhibitor and an anti-VEGF agent in neovascular age-related macular degeneration reported a favorable short-term safety profile[22], indicating that combination therapy targeting PDR is a possibility.
In addition to being a microvascular complication of diabetes, DR can be considered a neurodegenerative disease that causes impaired vision at the onset of diabetes[23]. The neural changes involved in DR can further trigger retinal neuronal survival signaling, including the production of neurotrophins[24]. Neurotrophins, released from glial cells, such as muller cells, are functionally and structurally related to growth factors with crucial roles in the development, maintenance, survival, and repair of the nervous system, and also have critical roles in angiogenesis and fibrosis[25]. Given the vital role of neurotrophins in modulating neuronal function, it is not surprising that neurodegenerative disorders, such as DR, are associated with altered expression of neurotrophins. In the present study, levels of GDNF were significantly enhanced in vitreous humor from patients with PDR, similar to a report from a previous study[26]. Increased understanding of neurotrophin expression and its related signaling in the retina may improve therapeutic approaches for the management of DR.
Furthermore, the expression of VEGFA was evaluated in vitreous humor from patients with PDR pretreated or not with anti-VEGF agent prior to vitrectomy. As expected, administration of anti-VEGF treatment significantly suppressed the enhanced levels of VEGFA induced by PDR. It is established that VEGF is a critical stimulator of neovascularization and closely associated with DR deterioration by disrupting retinal microvascular structure and function[27]. Although administration of anti-VEGF therapy has been widely applied to inhibit neovascularization in retinal diseases, and is not limited to DR, the disadvantages of tolerance development, undesirable responses, and side effects of anti-VEGF therapy mean that invention of novel therapeutic targets to ameliorate DR is urgently required. The findings of this study indicate that combination of PDGF or GDNF inhibitors with anti-VEGF treatment, may help to prevent neovascularization and fibrosis in DR. The detailed mechanism involved and the efficacy of such a combination treatment strategy in DR warrant further study in the future.
Inflammatory Flux in Diabetic Retinopathy
This study also presents evidence of significant increases in inflammatory proteins, including IL-8, IL-11, and TNF-α. IL-8 is a crucial mediator of ocular inflammation and angiogenesis in retinal ischemic disease[28]–[29] and increased IL-8 is correlated with DR progression. Moreover, our observation of IL-11 upregulation in vitreous humor of patients with PDR was consistent with a previous publication, which proposed that IL-11/IL-11Rα signaling and CD163+ M2 macrophages may be involved in angiogenesis in PDR[30]. Moreover, TNF-α is activated and can induced the expression of proinflammatory proteins in DR, contributing to DR progression[31]. During inflammation, inflammatory cells may activate the expression of growth factors, angiogenic cytokines, and proteases which induce formation of new vessels and tissue damage. As discussed by Yoshimura et al[32], it is possible that inflammatory factors also contribute to elevated vascular permeability while, unlike growth factors, they primarily induce proliferative disease, leading to increased expression levels of inflammatory mediators. Thus, inflammation and angiogenesis are interlinked, and it is predictable that inflammation both has a role in the early stages of DR and occurs later in the disease course, alongside neovascularization and edema. The increased levels of inflammatory proteins detected in the present study suggest a role for the use of anti-inflammatory agents or steroids, in conjunction with current anti-VEGF therapy, to treat PDR.
Enrollment of Chemokines in Diabetic Retinopathy
The identification and assessment of chemokines in the vitreous humor of diabetic patients with PDR may provide more comprehensive insights into the pathophysiology of DR. Chemotactic cytokines are potential targets for further investigation and treatment. Patients with PDR (including VH and TRD) had significantly elevated levels of CXCL10, IFN-γ, and GM-CSF, which were associated with early retinal damage and DR development. Previous reports have suggested a crucial role for angiogenic growth factors, neurotrophin, and inflammatory mediators in DR progression, while investigation of chemokines suggested they could be useful for DR prognosis or diagnosis[18],[33]. Chemokines can mediate leukocytic activation and migration, which may induce leukostasis, eventually resulting in capillary occlusion and retinal hypoxia.
CXCL10 was increased in the PDR group relative to controls, suggesting that it has a role in DR progression. CXCL10 recruits leukocytes after binding to its receptor, C-X-C chemokine receptor (CXCR) 3. As previously reported, CXCL10 can regulate retinal inflammation through modulation of endoplasmic reticulum stress-induced NF-κB, RelA, and STAT3 activation in photoreceptor cells treated with advanced glycation end products and high glucose[34]. In addition, CXCL10 secretion can affect outer retinal and choroidal neovascularization induced by ciliary neurotrophic factor (CNTF)[35]. These studies revealed that elevation of chemokine CXCL10 not only induces inflammation, but may also influence neovascularization during DR progression. Further, upregulation of IFN-γ and GM-CSF also contribute to inflammation induction in DR.
The present analysis has several limitations. The sample size was relatively small and analysis of vitreous humor from patients with non-proliferative DR would be beneficial to allow comparison of cytokine levels at the different stages; however, we were unable to obtain such samples from patients who did not require surgery. Further, we did not collect patient aqueous humor or serum samples. Previous studies have demonstrated that circulating cytokines correlate poorly with ocular cytokines, and aqueous humor does not accurately reflect the expression of cytokines in the vitreous. Further studies will focus on interdependent mechanisms in DR pathogenesis involving growth factors, inflammatory mediators, and cytokines.
In summary, expression levels of growth factors (PDGF-AA, GDNF, and VEGFA), inflammatory mediators (IL-8, IL-11, and TNF-α), and cytokines (CXCL10, IFN-γ, and GM-CSF) were significantly elevated in vitreous humor samples from patients with PDR. The results of this comprehensive analysis suggest potential combination therapeutic strategies, based on anti-VEGF, which may ameliorate DR progression. Further investigations are warranted to verify the efficacy and mechanisms involved in combination anti-angiogenic, neuroprotective, and anti-inflammation strategies for treating DR.
Acknowledgments
Authors' contributions: Gong QY, Yu SQ and Qian TW conceived and designed the experiments. Gong QY and Hu GY analyzed and interpreted the data. Gong QY and Qian TW contributed to drafting the article, Xu X supervised the project throughout the process.
Foundations: Supported by the National Natural Science Foundation of China (No.82101132; No.81800878).
Conflicts of Interest: Gong QY, None; Hu GY, None; Yu SQ, None; Qian TW, None; Xu X, None.
REFERENCES
- 1.Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61(1):29–38. doi: 10.1007/s00125-017-4435-8. [DOI] [PubMed] [Google Scholar]
- 2.Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, Simó R. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8(4):337–347. doi: 10.1016/S2213-8587(19)30411-5. [DOI] [PubMed] [Google Scholar]
- 3.Writing Committee for the Diabetic Retinopathy Clinical Research Network. Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137–2146. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen QD, De Falco S, Behar-Cohen F, Lam WC, Li XR, Reichhart N, Ricci F, Pluim J, Li WW. Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol. 2018;96(1):e1–e9. doi: 10.1111/aos.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youngblood H, Robinson R, Sharma A, Sharma S. Proteomic biomarkers of retinal inflammation in diabetic retinopathy. Int J Mol Sci. 2019;20(19):4755. doi: 10.3390/ijms20194755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester JV, Kuffova L, Delibegovic M. The role of inflammation in diabetic retinopathy. Front Immunol. 2020;11:583687. doi: 10.3389/fimmu.2020.583687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FL, Wang PY, Chuang YF, Wang JH, Wong VHY, Bui BV, Liu GS. Gene therapy intervention in neovascular eye disease: a recent update. Mol Ther. 2020;28(10):2120–2138. doi: 10.1016/j.ymthe.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikiori N, Osanai M, Chiba H, Kojima T, Mitamura Y, Ohguro H, Sawada N. Glial cell-derived cytokines attenuate the breakdown of vascular integrity in diabetic retinopathy. Diabetes. 2007;56(5):1333–1340. doi: 10.2337/db06-1431. [DOI] [PubMed] [Google Scholar]
- 10.Dan-Brezis I, Zahavi A, Axer-Siegel R, Nisgav Y, Dahbash M, Weinberger D, Ehrlich R, Livnat T. Inflammation, angiogenesis and coagulation interplay in a variety of retinal diseases. Acta Ophthalmol. 2019 doi: 10.1111/aos.14331. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Shahulhameed S, Vishwakarma S, Chhablani J, Tyagi M, Pappuru RR, Jakati S, Chakrabarti S, Kaur I. A systematic investigation on complement pathway activation in diabetic retinopathy. Front Immunol. 2020;11:154. doi: 10.3389/fimmu.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19(4):942. doi: 10.3390/ijms19040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromberg-White JL, Glazer L, Downer R, Furge K, Boguslawski E, Duesbery NS. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Invest Ophthalmol Vis Sci. 2013;54(10):6472–6480. doi: 10.1167/iovs.13-12518. [DOI] [PubMed] [Google Scholar]
- 15.Zeng YK, Cao D, Yu HH, Hu YY, He M, Yang DW, Zhuang XN, Zhang L. Comprehensive analysis of vitreous humor chemokines in type 2 diabetic patients with and without diabetic retinopathy. Acta Diabetol. 2019;56(7):797–805. doi: 10.1007/s00592-019-01317-6. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo JG, Bula DV, Yang L, Chen DF. Retinal biopsy techniques for the removal of retinal tissue fragments. Ophthalmic Surg Lasers Imaging. 2005;36(1):76–78. [PubMed] [Google Scholar]
- 17.Dong N, Xu B, Wang BS, Chu LQ. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19(4):1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 18.Wu HL, Hwang DK, Song XD, Tao Y. Association between aqueous cytokines and diabetic retinopathy stage. J Ophthalmol. 2017;2017:9402198. doi: 10.1155/2017/9402198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song S, Yu XB, Zhang P, Dai H. Increased levels of cytokines in the aqueous humor correlate with the severity of diabetic retinopathy. J Diabetes Complicat. 2020;34(9):107641. doi: 10.1016/j.jdiacomp.2020.107641. [DOI] [PubMed] [Google Scholar]
- 20.Ecker SM, Hines JC, Pfahler SM, Glaser BM. Aqueous cytokine and growth factor levels do not reliably reflect those levels found in the vitreous. Mol Vis. 2011;17:2856–2863. [PMC free article] [PubMed] [Google Scholar]
- 21.Lei HT, Rheaume MA, Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res. 2010;90(3):376–381. doi: 10.1016/j.exer.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe GJ, Ciulla TA, Ciardella AP, et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: a phase IIb, multicenter, randomized controlled trial. Ophthalmology. 2017;124(2):224–234. doi: 10.1016/j.ophtha.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61(9):1902–1912. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bikbova G, Oshitari T, Baba T, Yamamoto S. Neurotrophic factors for retinal ganglion cell neuropathy - with a special reference to diabetic neuropathy in the retina. Curr Diabetes Rev. 2014;10(3):166–176. doi: 10.2174/1573399810666140508121927. [DOI] [PubMed] [Google Scholar]
- 25.Taylor S, Srinivasan B, Wordinger RJ, Roque RS. Glutamate stimulates neurotrophin expression in cultured Müller cells. Mol Brain Res. 2003;111(1-2):189–197. doi: 10.1016/s0169-328x(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 26.Behl T, Kotwani A. Downregulated brain-derived neurotrophic factor-induced oxidative stress in the pathophysiology of diabetic retinopathy. Can J Diabetes. 2017;41(2):241–246. doi: 10.1016/j.jcjd.2016.08.228. [DOI] [PubMed] [Google Scholar]
- 27.Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015;99:137–148. doi: 10.1016/j.phrs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM. Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm. 2011;19(6):401–412. doi: 10.3109/09273948.2011.618902. [DOI] [PubMed] [Google Scholar]
- 29.Schoenberger SD, Kim SJ, Shah R, Sheng JS, Cherney E. Reduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac, 0.45%, in patients with diabetic retinopathy. JAMA Ophthalmol. 2014;132(1):32–37. doi: 10.1001/jamaophthalmol.2013.6203. [DOI] [PubMed] [Google Scholar]
- 30.Abu El-Asrar AM, Ahmad A, Allegaert E, Siddiquei MM, Gikandi PW, de Hertogh G, Opdenakker G. Interleukin-11 overexpression and M2 macrophage density are associated with angiogenic activity in proliferative diabetic retinopathy. Ocular Immunol Inflamm. 2020;28(4):575–588. doi: 10.1080/09273948.2019.1616772. [DOI] [PubMed] [Google Scholar]
- 31.Rezzola S, Loda A, Corsini M, Semeraro F, Annese T, Presta M, Ribatti D. Angiogenesis-inflammation cross talk in diabetic retinopathy: novel insights from the chick embryo chorioallantoic membrane/human vitreous platform. Front Immunol. 2020;11:581288. doi: 10.3389/fimmu.2020.581288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, Ueno A, Hata Y, Yoshida H, Ishibashi T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4(12):e8158. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semeraro F, Morescalchi F, Cancarini A, Russo A, Rezzola S, Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: therapeutic implications. Diabetes Metab. 2019;45(6):517–527. doi: 10.1016/j.diabet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhu S, Liu H, Sha HB, Qi L, Gao DS, Zhang WB. PERK and XBP1 differentially regulate CXCL10 and CCL2 production. Exp Eye Res. 2017;155:1–14. doi: 10.1016/j.exer.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bucher F, Aguilar E, Marra KV, Rapp J, Arnold J, Diaz-Aguilar S, Lange C, Agostini H, Schlunck G, Stahl A, Friedlander M. CNTF prevents development of outer retinal neovascularization through upregulation of CxCl10. Invest Ophthalmol Vis Sci. 2020;61(10):20. doi: 10.1167/iovs.61.10.20. [DOI] [PMC free article] [PubMed] [Google Scholar]