Abstract
Coronavirus disease 2019 (COVID-19) has been extensively associated with microvascular and macrovascular thrombosis. Several reports have demonstrated a link between COVID-19 and pulmonary embolism, deep vein thrombosis, myocardial infarction, stroke, and aortic thrombosis. Renal artery thrombosis is of special interest because of its life-threatening consequences, such as acute kidney injury and renal infarction. We present a case of left renal artery thrombosis as a long-term complication of COVID-19. Moreover, we demonstrate the effectiveness of interventional radiology to regain vascularization of the affected kidney.
Keywords: Renal artery thrombosis, COVID-19, Interventional radiology
Introduction
Since December 2019, humankind has been living with the coronavirus disease 2019 (COVID-19) pandemic, which has been an extreme medical burden to healthcare systems worldwide. COVID-19 is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To date, more than 2 million people have died from this disease in Europe [1]. Although COVID-19 predominantly presents with clinical respiratory complications, the course of this disease may extend far beyond the respiratory tract and affect different organs of the body. There is ample evidence in the literature regarding the extrapulmonary (cardiovascular, renal, hepatic, gastrointestinal, and neurological) implications of COVID-19 [2], [3], [4]–5]. Therefore, the high risk of the development of thromboembolic events with COVID-19 has been extensively documented [6], [7]–10].
Thrombotic complications with COVID-19 are major contributors to the high rate of morbidity and mortality associated with this disease, suggesting that thrombosis might significantly contribute to multi-organ failure observed with severe COVID-19 cases [11]. According to a study performed in the United States, the incidence of thrombotic complications is more than 30% for hospitalized patients despite systemic thromboprophylaxis [12]. Both arterial and venous thrombotic events have been encountered with COVID-19 [13]. The exact underlying cause of COVID-19-associated coagulopathy is still not clear; however, several thrombogenic mechanisms have been proposed, such as endothelial cell inflammation and dysfunction, abnormal blood flow dynamics and activated platelets, high concentrations of von Willebrand factor, cytokine storm, cell-free DNA, antiphospholipid antibody syndrome, histones, and viral RNA that collectively cause factor XI activation, macrophage activation syndrome, thrombin generation, fibrin formation, activation of the complement cascade, and dysregulation of the renin-angiotensin system [6,[8], [9]. Microvascular and macrovascular thromboembolic complications have been observed with COVID-19 in the vasculature of the lungs, spleen, brain, gut, kidneys, and periphery [14], [15]–16]. The most common thrombotic conditions that have been associated with COVID-19 include deep vein thrombosis, pulmonary embolism, myocardial infarction, catheter-related thrombosis, and arterial thrombosis [10,17,18]. Although the incidence of venous thrombosis seems to be higher, the arterial thromboembolic complications should be of much concern because of their life-threatening consequences, such as limb loss, stroke, end organ failure, and death. Hence, COVID-19-induced thrombosis in the major arterial vessels should be addressed with caution. Renal artery thrombosis is a rare condition triggered by the COVID-19 hypercoagulable state. Few reports in the literature have demonstrated the direct connection between COVID-19-associated coagulopathy and thrombosis of the renal arteries [19–23]. The majority of reports have presented cases involving the development of renal artery thrombosis during the course of the COVID-19 infectious process. We report a case of renal artery thrombosis as a long-term complication of COVID-19 after full recovery from this infectious disease.
Case report
A man in his fifth decade of life was admitted to the emergency department for abdominal pain that mainly projected to the left lumbar region. His medical history was unremarkable in terms of chronic diseases and other secondary diseases; however, he had recovered from COVID-19 with moderate-to-severe symptoms without hospitalization approximately 2 months previously. The patient had been fully vaccinated with two doses of the Pfizer-BioNTech COVID-19 vaccine. At the time of infection, Kosovo experienced another spike in the COVID-19 pandemic, with approximately 5000 new cases per week that were largely associated with the delta variant. At that particular time point, approximately 50% of the population had been vaccinated with at least one dose. On admission, the patient presented with general discomfort, asthenia, and loss of appetite during the previous 2 days. The clinical examination revealed normal vital signs, blood pressure, body temperature, and a slightly distended abdomen. Routine laboratory test results were within the reference values. The patient was discharged with a diagnosis of abdominal distention. Later that day, his abdominal pain became aggravated, and he returned to the emergency department for medical treatment. At the time of the second admission, his vital signs were normal, his blood pressure was 145/105 mmHg, and he denied any other relevant clinical symptoms. The COVID-19 reverse-transcriptase polymerase chain reaction and rapid antigen test results were negative; however, he had a high titer of SARS-CoV-2 IgG antibodies. It is worth noting that laboratory findings showed an increased activated partial thromboplastin time of 64.2 seconds (normal: 25.1-35.0 seconds). However, the following laboratory test results appeared to be within the reference values: C-reactive protein, 4.3 mg/dL (normal: 0.0-10.0 mg/dL); troponin, 2.3 pg/mL (normal: 0.0-40.0 pg/mL); urea, 29.96 g/dL (normal: 6.0-24.0 g/dL); creatinine, 1.2 mg/dL (normal: 0.7-1.3 mg/dL); D-dimer, 142 ng/mL (normal: 0.0-243.0 ng/mL); and CK-MB, 2.2 ng/mL (normal: 0.0-4.9 ng/mL). The patient described no change in urine production, and the urinalysis results were normal. Abdominal computed tomography angiography showed 70% occlusion at the mid and distal parts of the left renal artery caused by thrombosis, which was associated with ischemic changes in the lower lobe of the left renal parenchyma (Fig. 1; Supplementary Videos 1 and 2). During hospitalization, the patient was rehydrated with intravenous fluid therapy, and his abdominal pain was controlled as needed with appropriate analgesic therapy. When the diagnosis of renal artery thrombosis was made, anticoagulant therapy was initiated (aspirin 100 mg every 24 hours and heparin 25,000 UI/50 mL with an administration speed of 2.1 mL/h).
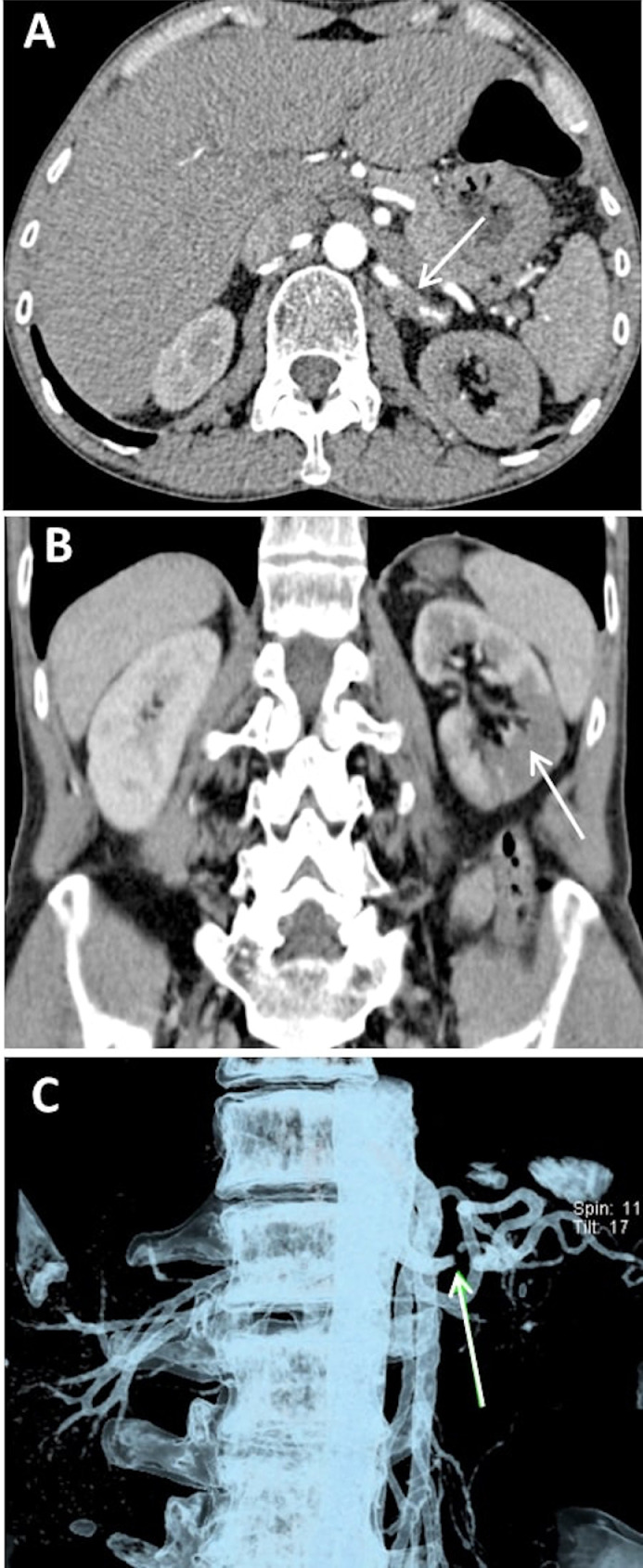
Fig. 1.
Abdominal computed tomography angiography (CTA) scan. (A) Left renal artery with 70% occlusion. The white arrow indicates the occlusion site (axial plane). (B) Ischemic changes in the left kidney (coronal plane). (C) Three-dimensional (3D) visualization using a volume rendering technique (VRT). The white arrow indicates the occlusion site in the left renal artery.
The patient had no history of atrial fibrillation or other heart valvular diseases, as confirmed by a normal electrocardiographic examination. This ruled out the possibility of renal artery thrombosis of cardiogenic origin. Because of the possible risk of thrombosis attributable to acute kidney injury, digital subtraction angiography was performed. After digital subtraction angiography, thrombus aspiration plus intrarenal artery bolus administration of tirofiban (5 mL, 0.25 mg/mL) was performed. Because the thrombus aspiration results were not satisfactory, percutaneous transluminal angioplasty and stent insertion were performed. As a result, complete revascularization was achieved (Fig. 2; Supplementary Videos 3 and 4). The patient was discharged with negative lumbar abdominal pain and normal renal function after 3 days of hospitalization. Oral anticoagulant therapy (aspirin 100 mg every 24 hours and clopidogrel 75 mg every 24 hours) was continued after discharge. At the 2-month follow-up evaluation, the patient was symptom-free and experienced no episodes of recurrent abdominal pain. During examination, normal renal function was observed, and the urine production level was within the reference range. Moreover, the patient reported a normal quality of life and was fully engaged in his activities of daily living.
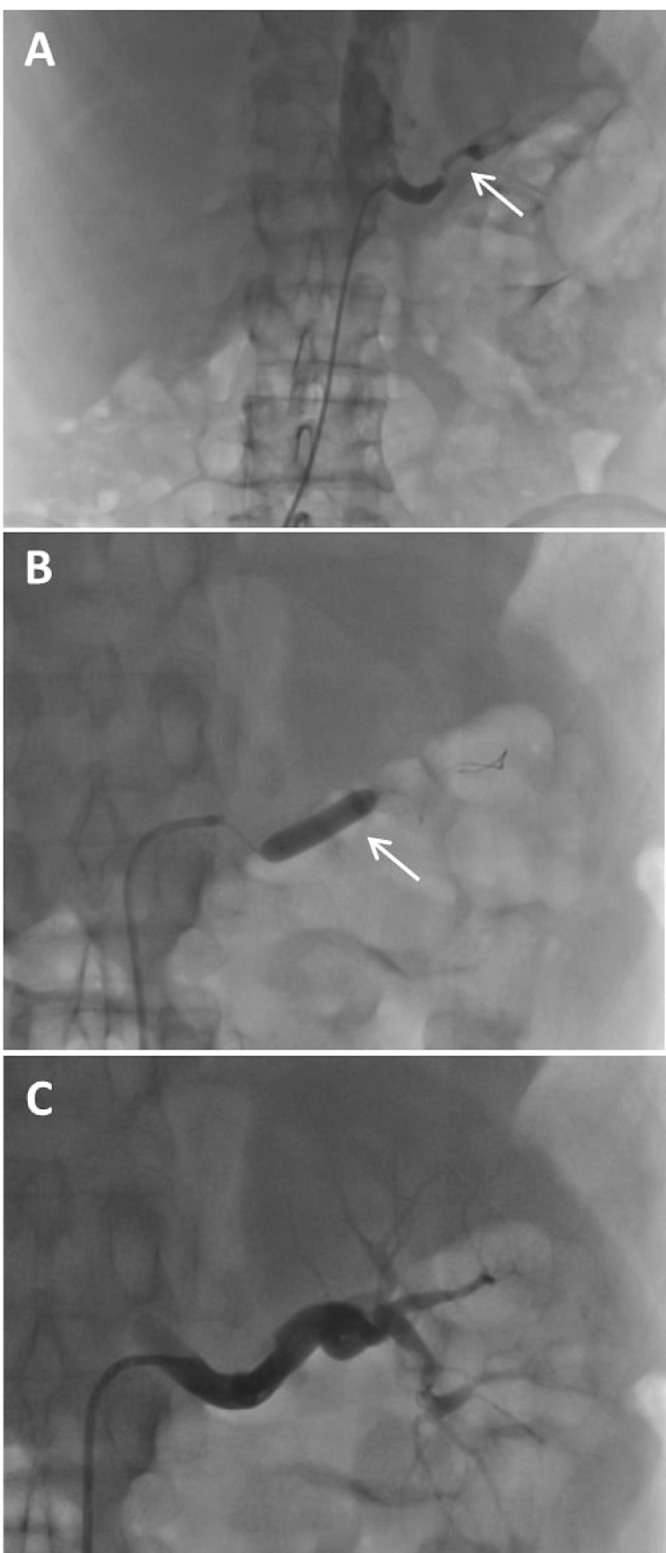
Fig. 2.
Digital subtraction angiography (DSA). (A) The site of occlusion before revascularization (white arrow), (B) percutaneous transluminal angioplasty (PTA) of the left renal artery and stent insertion (white arrow). (C) Image of the left kidney after revascularization.
Discussion
Thromboembolic events are a serious implication of COVID-19 [6–10]. In this context, thrombosis of major blood vessels, such as renal arteries, is of special interest. Although renal artery thrombosis seems rare, several studies have reported its occurrence and association with COVID-19 coagulopathy [19,20,22–24]. Both unilateral and bilateral thromboses have been reported. Moreover, clinical practice has shown that renal artery thrombosis may also occur concomitantly with other thrombotic complications in the body, such as pulmonary embolism and thrombosis in different body zones, such as the heart, aorta, limbs, and brain [21,25,26]. The majority of the aforementioned cases were strongly correlated with COVID-19-induced thrombosis because the diagnosis was demonstrated by reverse-transcriptase polymerase chain reaction test results, chest imaging findings (eg, ground-glass opacities), and a relatively high D-dimer level. In contrast, we present a case of renal artery thrombosis after COVID-19 resolution (the patient had negative SARS-CoV-2 diagnostic test results). Because other possible non-COVID triggers of thrombosis (atrial fibrillation, valvular diseases, endocarditis, atherosclerosis, prosthetic devices) were excluded through medical examination and laboratory test results, the only logical cause of this thrombosis could be the gradual process of precipitating events initiated by COVID-19. Hence, it is most likely that endothelial injury during infection triggered the thrombotic cascade, which later culminated in significant occlusion of the left renal artery and manifested as severe abdominal pain. This is also supported by serological test results, which indicated a high titer of anti-SARS-CoV-2 IgG antibodies. Our findings are in accordance with those of Deshmukh et al., who also reported a case of renal artery thrombosis as a result of COVID-19 [25]. To the best of our knowledge, this is the second report of renal artery thrombosis as a long-term complication of COVID-19. Because our patient was not at risk for bleeding and did not have contraindications to interventional radiology, successful digital subtraction angiography was performed. Renal perfusion was regained and kidney function remained normal at the time of the follow-up evaluation. An interventional procedure (angioplasty) was performed for only one previously reported case [20]; however, no significant success occurred. Our case demonstrates the usefulness of interventional radiology for achieving revascularization in cases that meet the eligibility criteria and in which the benefits outweigh the risks.
Since the beginning of the COVID-19 outbreak, several reports that address the disease from different perspectives have published. Most of these data are related to the acute manifestations of this disease. Therefore, we still have limited knowledge of the long-term consequences of COVID-19. However, the increasing evidence will allow a better understanding of the pulmonary and extrapulmonary health implications after COVID-19 [27]. Our case report of renal artery thrombosis as a complication of COVID-19 contributes to furthering the understanding of this disease.
Conclusions
Although the majority of concerns are associated with the acute manifestations of SARS-CoV-2 infection, our case report shows that COVID-19 may also act as a latent trigger of renal artery thrombosis and can affect kidney function far beyond the acute phase. This should warn clinicians that long-term implications should not be overlooked in after COVID, especially in patients with high titers of anti-SARS-CoV-2 IgG antibodies. Additionally, this case demonstrates the effectiveness of percutaneous transluminal angioplasty and stent insertion for thrombosis of major blood vessels after COVID-19 when there is no risk of bleeding or other contraindications to interventional radiology.
Patient consent
Informed consent was obtained from the patient prior to the publication of this article.
Footnotes
Competing Interests: The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2022.10.028.
Appendix. Supplementary materials
References
- 1.Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/europe/emergencies/situations/covid-19 Accessed July 7, 2022.
- 2.Johnson KD, Harris C, Cain JK, Hummer C, Goyal H, Perisetti A. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front Med. 2020;7:526. doi: 10.3389/fmed.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elrobaa IH, New KJ. COVID-19: pulmonary and extra pulmonary manifestations. Front Public Health. 2021;9:711616 doi: 10.3389/fpubh.2021.711616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis TJ, Qasem A, Abdelli LS, Naser SA. Extra-pulmonary complications in sars-cov-2 infection: a comprehensive multi organ-system review. Microorganisms. 2022;10(1):153. doi: 10.3390/microorganisms10010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai CC, Ko WC, Lee PI, Jean SS, Hsueh PR. Extra-respiratory manifestations of COVID-19. Int J Antimicrob Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker RC. COVID-19 update: COVID-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. Am J Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier M, Faille D, Dossier A, Mageau A, Nicaise Roland P, Ajzenberg N, et al. Arterial thrombotic events in adult inpatients with COVID-19. Mayo Clin Proc. 2021;96(2):295–303. doi: 10.1016/j.mayocp.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etkin Y, Conway AM, Silpe J, Qato K, Carroccio A, Manvar-Singh P, et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA. 2020;324(24):2548–2549. doi: 10.1001/jama.2020.23422. [DOI] [PubMed] [Google Scholar]
- 14.Vulliamy P, Jacob S, Davenport RA. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol. 2020;189:1053–1054. doi: 10.1111/bjh.16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goudarzi E, Yousefimoghaddam F, Ramandi A, Khaheshi I. COVID-19 and peripheral artery thrombosis: a mini review. Curr Prob Cardiol. 2021;47(10):100992. doi: 10.1016/j.cpcardiol.2021.100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Shamy O, Munoz-Casablanca N, Coca S, Sharma S, Lookstein R, Uribarri J. Bilateral renal artery thrombosis in a patient with COVID-19. Kidney Med. 2021;3(1):116–119. doi: 10.1016/j.xkme.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philipponnet C, Aniort J, Chabrot P, Souweine B, Heng AE. Renal artery thrombosis induced by COVID-19. Clin Kidney J. 2020;13(4):713. doi: 10.1093/ckj/sfaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boui M, Hammoune N, Slioui B, Bellasri S, Ben Elhend S, Zouaki Z, et al. Concomitant acute pulmonary embolism, intracardiac thrombus and renal artery thrombosis in COVID-19 patient. Thromb Update. 2021;3 doi: 10.1016/j.tru.2021.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Lin C, Chen Y, Wu X, Lin M, Chen S, et al. Renal artery thrombosis in SARS-CoV-2 infection: a case report. BMC Nephrol. 2022;23(1):1–4. doi: 10.1186/s12882-022-02808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ergün R, Ergün D, Shalabi HS, Shalabi MY, Kanat F, Tulek B, et al. COVID-19 and renal artery thrombosis: a case report. Resp Case Rep. 2022;11(1):25–29. doi: 10.5505/respircase.2022.40374. [DOI] [Google Scholar]
- 24.Acharya S, Anwar S, Siddiqui FS, Shabih S, Manchandani U, Dalezman S. Renal artery thrombosis in COVID-19. IDCases. 2020;22:e00968. doi: 10.1016/j.idcr.2020.e00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshmukh SB, Upadhyay KM, Kulkarni A, Deshpande S, Purohit R, Kulkarni M. Renal artery thrombosis: a post COVID-19 sequel. J Adv Res Med. 2020;7(2):22–24. doi: 10.24321/2349.7181.202009. [DOI] [Google Scholar]
- 26.Kenizou D, Perrin C, Harzallah I, Bresson D, Allimant P, Calcaianu M. Multiple arterial thrombosis in a 78-year-old patient: catastrophic thrombotic syndrome in COVID-19. CJC Open. 2021;3(2):198–200. doi: 10.1016/j.cjco.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarei M, Bose D, Nouri-Vaskeh M, Tajiknia V, Zand R, Ghasemi M. Long-term side effects and lingering symptoms post COVID-19 recovery. Rev Med Virol. 2022;32(3):e2289. doi: 10.1002/rmv.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.