Abstract
Nearly 10% of premature and critically ill infants receive fresh-frozen plasma (FFP) transfusions to reduce their high risk of bleeding. The authors have only limited data to identify relevant clinical predictors of bleeding and to evaluate the efficacy of FFP administration. There is still no consensus on the optimal use of FFP in infants who have abnormal coagulation parameters but are not having active bleeding. The aims of this review are to present current evidence derived from clinical studies focused on the use of FFP in neonatology and then use these data to propose best practice recommendations for the safety of neonates receiving FFP.
Keywords: Coagulation tests, Disseminated intravascular coagulation, Fresh-frozen plasma, Hemorrhages, Plasmapheresis, Relative risk, Whole-blood donor units
Introduction
Up to 10% of all premature and critically ill infants receive FFP transfusions.1 These patients comprise a fragile population at risk of hemorrhagic complications, but the data to identify clinical predictors of bleeding and to evaluate the efficacy of FFP administration are limited.2 Consequently, despite continued efforts to develop clinical guidelines on FFP administration in neonates, the recommendations remain under-supported with low-quality evidence, and there is a lack of consensus on its optimal use.3,4 Not surprisingly, FFP continues to be administered to asymptomatic neonates who do not have any clinical evidence of bleeding. In many instances, FFP might be administered just to correct “prolonged” values in coagulation tests that might actually be developmentally appropriate. A large proportion of FFP transfusions in these infants cannot be easily justified based on risk or prior confirmed hemorrhage 5,6
To optimize FFP transfusions in neonates, we need better understanding of the developmental changes in various physiological markers of hemostasis and the interpretation of coagulation tests to detect coagulopathy. The levels of most coagulation proteins in premature infants change with gestational development, resulting in wide ranges of standard coagulation screening tests such as the prothrombin time (PT) and activated plasma thromboplastin time (APTT, Fig. 1).7 Furthermore, the risk of bleeding may not be proportionate to these deviations. There is a need for reference ranges appropriate for accurate/corrected gestational age to evaluate/interpret these maturational changes and coagulation tests in newborns.2,8–11 Figure 2 shows the changes in fibrinogen levels and the change in PT/aPTT seen with development.
Fig. 1:
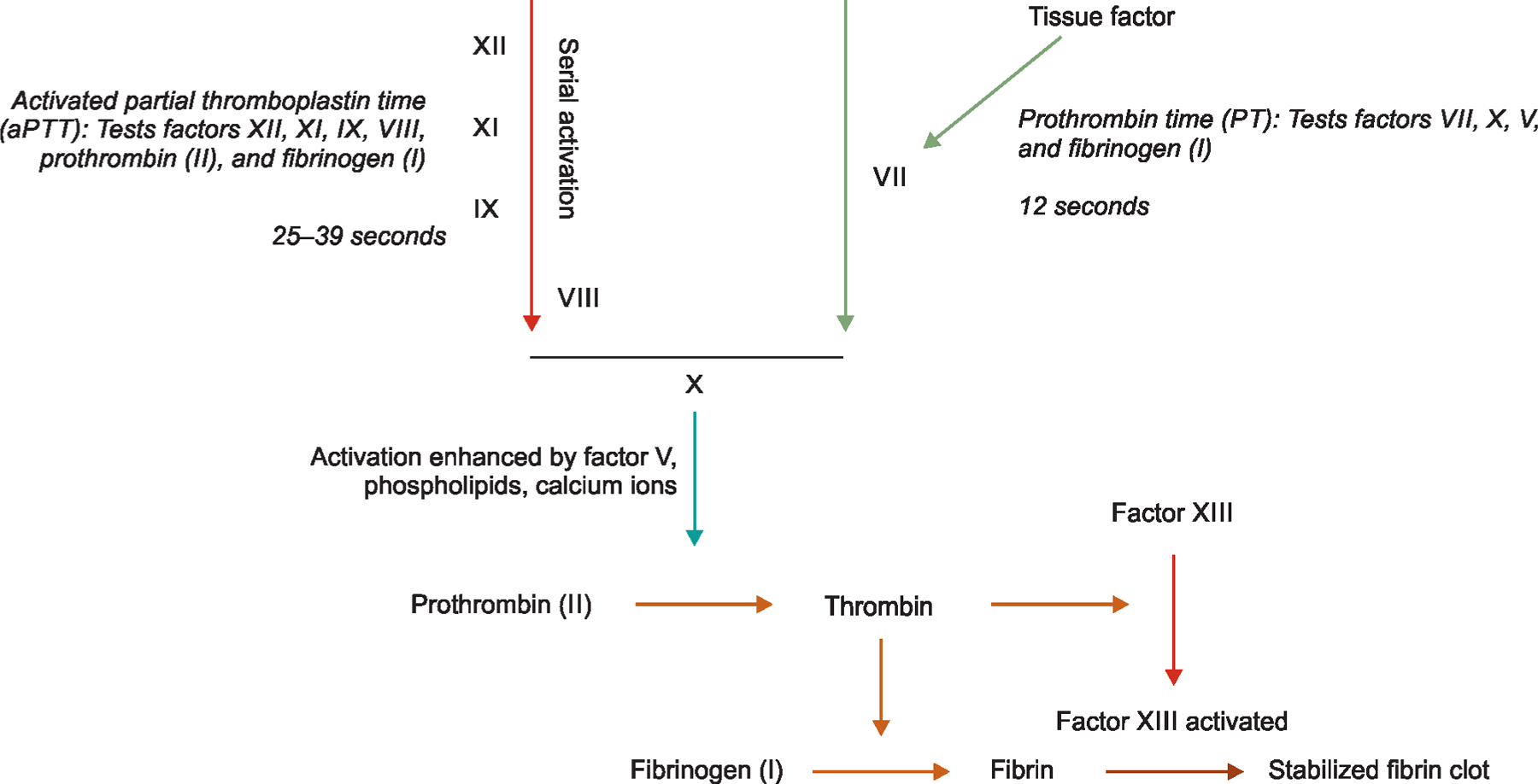
Coagulation cascades and the traditionally used tests for evaluation in critically ill infants
Figs 2A to C:
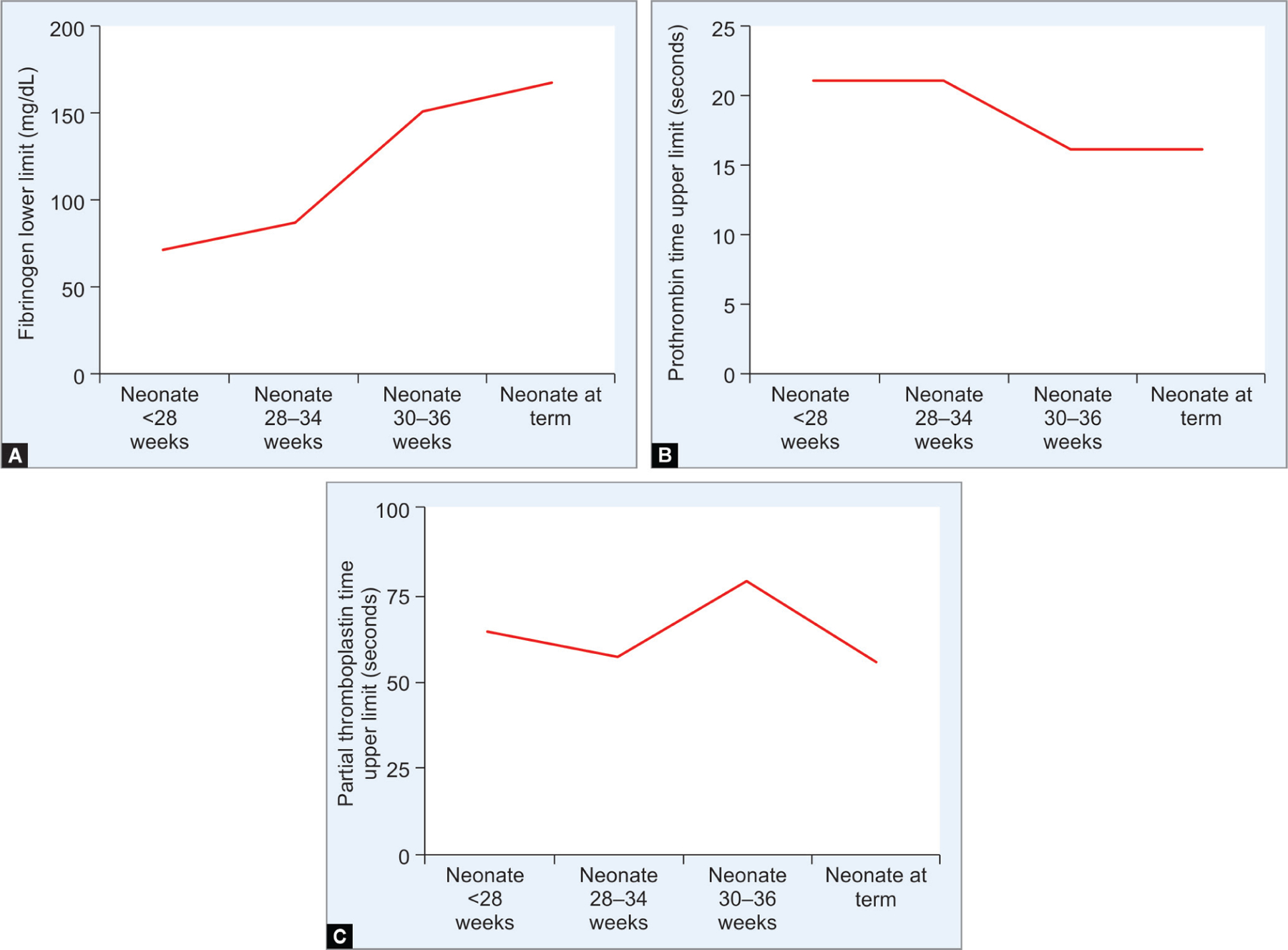
Line diagram in panel (A) show low plasma fibrinogen levels in extremely premature infants that rise toward term gestation. Panels (B and C) show the high degree of variability in prothrombin and PTTs across gestational maturation (adapted from Girelli et al. Blood Transfus 2015;13:484–497)
This review presents current evidence on the use of FFP in neonatology and a set of best practice recommendations for the safety of neonates receiving FFP. We have included evidence from an extensive literature search in databases PubMed, EMBASE, and Scopus. To avoid bias in identification of studies, keywords were short-listed prior to the actual search from anecdotal personal experience and from PubMed’s Medical Subject Heading (MeSH) thesaurus. For primary evaluation, we modified the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to assess the quality of evidence and strength of recommendations (Table 1). In addition, we also used the conventional grading systems of recommendations and the levels of evidence defined by the Oxford Centre for Evidence-based Medicine (Supplemental tables 1–2).12,13
Table 1:
Criteria for assigning grade of evidence and strength of recommendations
| Quality of evidence | Type of clinical study | Consistency of results |
|---|---|---|
| A = High | Randomized trial without important limitations | Considerable confidence in the estimate of effect |
| B = Moderate | Randomized trial with important limitations or exceptionally observational studies with strong evidence | Further research likely to have impact on the confidence in estimate, may change estimate |
| C = Low | Observational studies or case series | Further research is very likely to have impact on confidence, likely to change the estimate |
| Strength of recommendations | Balance between benefits and harms | |
| 1 = Strong | Certainty of imbalance | |
| 2 = Weak | Uncertainty of imbalance |
The grading scheme classifies the quality of evidence as high (grade A), moderate (grade B), or low (grade C) according to the study design and to the consistency of results. The strength of recommendations was further classified as either strong (1) or weak (2) according to the balance between desirable and undesirable outcomes. Strong recommendations (1) are made when there is confidence that the benefits either do or do not outweigh the harm and costs of treatment. Where the magnitude of benefit or not is less certain, a weaker (1) recommendation is made. Grade I recommendations can be applied uniformly to most patients, whereas grade II recommendations require a more individualized application
Preparation, Characteristic, and Storage of FFP
Fresh-frozen plasma is prepared by freezing plasma separated from single whole-blood donor units or during plasmapheresis.1 It is usually separated by centrifugation at 1000–2000gm at 4°C or is isolated during plasmapheresis.14 There are newer techniques where whole blood is passed through semipermeable membranes and do not require centrifugation. These bags are usually stored frozen at 30°C. When needed for clinical use, FFP is thawed by placing the bags in a water bath at a temperature of 30–37°C for 20–30 minutes or in an FDA-approved device for 1–6 minutes.15 After thawing, plasma should be used within 1–6 hours, and if not, then it was stored at 1–6°C. If not frozen, the product should be discarded after 24 hours of preparation. Ideally, FFP should not be frozen once it has been thawed.
The plasma used must be compatible with the recipient in terms of the ABO blood groups (Table 2).8 A standard dose of 10–20 mL/kg administered intravenously over 60 minutes (grade of evidence C, strength of recommendation typically raises circulating levels of the coagulation factors by approximately 20%, which is more than the 10% rise that is usually needed to correct hemostasis.16 Prior to FFP administration, standard precautions that include matching of blood types to confirm ABO compatibility need to be undertaken. The bags should be carefully inspected to ensure that the product is not expired and also for any leaks. The product should not contain any blood clots.
Table 2:
Choice of donors by blood groups for FFP transfusion therapy
| ABO phenotype of the recipient | ABO phenotype of units to transfuse (in order of preference) |
|---|---|
| O | O, A, B, AB |
| A | A, AB |
| B | B, AB |
| AB | AB |
Fresh-frozen plasma preparations should be leukodepleted (white blood cells <1 × 106/unit), preferably at the time of collection (prestorage, grade of evidence C, strength of recommendation 1). These measures may prevent nonhemolytic febrile reaction, reduce the risk of alloimmunization, and lower the risk of transmission of viral infection such as cytomegalovirus (grade of evidence B, strength of recommendation 1).17
Fresh-frozen plasma contains higher levels of soluble coagulation factors than whole blood.18 It contains 400–900 mg/unit of fibrinogen, which is much higher than normal whole blood levels of 200–400 mg/dL; there are also high levels of factors VIII, II, VII, V, IX, X, XI, XII, and XIII (Table 3, data from our hospital laboratory).19,20 There are also high levels of albumin, immunoglobulins, and of naturally occurring anticoagulants such as proteins C and S, antithrombin, and the tissue plasminogen activator inhibitor.21,22 Maintenance of the frozen state is the most important for factor VII.13 The activity of factors V and VIII also declines, although at a slightly slower rate.23 These details are important for the choice of therapeutic measures to prevent/treat hemorrhages in a critically ill infant; other preparations such as cryoprecipitate are richer in fibrinogen, factor VIII and von Willebrand factor but may not be as effective volume expanders.13,14,18
Table 3:
Coagulation factors in FFP
| Coagulation factor | Plasma concentration required for hemostasis (units/mL). Transfusion of 10 mL/kg typically contains amounts of coagulation factors to achieve hemostasis. Factor levels in donor plasma can be assumed to be approximately 1 U/mL |
|---|---|
| I (fibrinogen) | 100–160 mg/dL |
| II (prothrombin) | 0.5 |
| V | 0.1–0.3 |
| VII | 0.05–0.25 |
| VIII | 0.1–0.45 |
| IX | 0.1–0.4 |
| X | 0.1–0.25 |
| XI | 0.15–0.35 |
| XIII | 0.1–0.6 |
| vWF (von Willebrand Factor) | 0.25–0.6 |
Some centers have applied viral inactivation methods to FFP.24 Solvent/detergent treatment of plasma pooled from about 1000 units allows a high degree of standardization, shows known concentration and activity of key bioactive proteins, has reduced immunological risks related to antibodies and leukocytes, and eliminates pathogens such as hepatitis A and parvovirus.24–26 Treatment of FFP with methylene blue, a phenothiazine dye, can also be virucidal.27 There is more biological variability as it is derived by addition of an inactivation method to single units of plasma. Viral inactivation methods may reduce the concentrations of some clotting factors and inhibitors of coagulation.27
Indications for FFP Administration in Infants
The use of FFP in newborn infants have been reported across different clinical setting. Fresh-frozen plasma corrects coagulopathy by replacing deficient/defective clotting factors,28and it is usually used in active bleeding as replacement therapy in conditions associated with coagulopathy. We have summarized the current information in favor of FFP administration in various clinical situations:
• Volume expansion in very preterm infant
Premature ill infants are at high risk of bleeding, particularly intraventricular, and are frequently treated prophylactically with FFP administrations.19,29 Four studies have investigated prophylactic administration of FFP transfusions in pre-term neonates to decrease the incidence of IVH: One showed FFP to reduce IVH, but the other three reported no change in similar IVH rates,13,30–32 and a meta-analysis found no differences in grade of IVH or mortality.33 We suggest that the routine use of FFP should be avoided in preterm infants in the absence of active bleeding (grade of evidence A, strength of recommendation 1).
• Cardiac surgery/cardiopulmonary bypass
About a fifth of all infants with congenital heart defects (CHD, 2–3/1000 births) may require surgery.34 Up to 40% of infants with surgical CHD may show severe complications in the postoperative period,35 with mediastinal blood losses as high as 100 mL/kg. Hypofibrinogenemia and inadequate reversal of heparin effects may contribute to perioperative bleeding. Fresh-frozen plasma, platelets, cryoprecipitate, RBCs, and antifibrinolytic drugs are often administered to prevent/stop bleeding; the concerns are higher because surgical re-exploration of the chest in infants with severe bleeding was successful in controlling the hemorrhage in less than half of all the cases.35 Infants with excessive bleeding may exhibit hypofibrinogenemia at the end of CPB and are frequently treated with FFP/cryoprecipitate;36 hypofibrinogenemia is known to alter clot formation.8 Bleeding risk is increased with concurrent thrombocytopenia, and hence platelet transfusion is appropriate in these settings.37 The contribution of fibrinolysis to bleeding during CPB is still unclear.38 Ongoing fibrinolysis seems to exacerbate bleeding; antifibrinolytics may help reduce perioperative bleeding after cardiac surgery in some, but not in all cases.
During CPB complex coagulopathy occurs and the predictive value of routine tests such as PT, partial thromboplastin time (PTT), and platelet counts may not always be useful.39 The importance of FFP administration has been investigated in several trials that have compared the effectiveness of giving FFP before and after undergoing CPB.40 Studies that evaluated FFP usage in infants, as part of the priming prior to the initiation of CPB, did not reduce blood loss or the need for transfusions in all cases.41
Current guidelines from the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis42 suggest the addition of fresh frozen plasma to the CPB prime in neonates undergoing cardiac surgery (grade of evidence B, strength of recommendation 1).
• Surgery and trauma
Surgical intervention and/or trauma frequently leads to hemorrhages.43 Blood loss and metabolic changes such as acidosis and hypothermia can also accentuate bleeding.44 Inadequate volume resuscitation and low tissue perfusion can increase the tissue expression of procoagulant mediators and lead to disseminated intravascular coagulation (DIC), lactic acidosis, and further disrupt vascular integrity and perfusion to set up a feed-forward loop.45 Newborns and small infants do not compensate for hypovolemia in all conditions as well as adults; 10% loss of intravascular volume can disrupt vasomotor balance, peripheral tissue perfusion, and homeostasis in cardiac output. Hypothermia enhances many of these effects. Respiratory compromise can further accentuate these changes.
We need to carefully evaluate our strategies to minimize transfusions in elective surgical procedures. The benefits of FFP transfusions are not clear.46 The use of prophylactic FFP prior to surgical procedures in infants who have abnormal clotting tests, but no active bleeding, is not supported in clinical studies. A detailed family history of bleeding, therapeutic history, and data on risk of bleeding in various surgical or other invasive procedures are more important than the results of in vitro clotting tests when assessing the risk of clinically significant bleeding. For infants who have abnormal clotting tests and other factors that indicate a significant bleeding risk during a procedure, FFP administration can be considered, although this is not evidence-based (grade of evidence C, strength of recommendation 2).33
• Congenital factor deficiencies
Congenital coagulation disorders are inherited conditions characterized by abnormally low levels of coagulation factors.47 Hemophilia A and B, and von Willebrand disease, comprise nearly 80–85% of all cases with inherited bleeding disorders.48 Hemophilia A and B can rarely present with bleeding symptoms in newborn period, as can homozygous deficiency of coagulation factors II, V, VII, X, and XIII.47 Some of these inherited coagulation factor deficiencies may present in the neonatal period with intracranial and/or scalp hemorrhages, although most infants with these conditions do not have bleeding except with invasive procedures.49 The other 15% result from deficiencies of fibrinogen, prothrombin, factors V, and combined factors V/VIII, VII, X, XI, and XIII.50 These conditions can vary in severity from just a few cutaneous bleeds to potentially life-threatening hemorrhages.
The first line of treatment of inherited bleeding disorders is replacement of the deficient factor, using specific plasma-derived or recombinant products.51 Single coagulation factors are available for the majority of deficient factors except for factors II,V, and XI.52 For factor II deficiency, prothrombin complex concentrates (PCCs) can be used; these contain specific coagulation factors obtained from pooled normal plasma, namely factors II, IX, X, and VII in varying amounts depending on the product.
Fresh-frozen plasma can be administered in newborns and young infants with active bleeding during the initial hospital stay until the specific disorder has been identified, when specific products are not available or in resource limited countries (grade of evidence C, strength of recommendation 1). Large doses of FFP are required in severe hemophilia and 15–25 mL/kg may be required to raise the FVIII/FIX concentration to hemostatic levels (grade of evidence C, strength of recommendation 1).
• Disseminated intravascular coagulation
Disseminated intravascular coagulation can occur in neonates with severe perinatal asphyxia, sepsis, shock, severe hypothermia, or necrotizing enterocolitis. These infants are usually critically ill and may bleed from multiple sites. Laboratory tests may show low platelet counts; prolonged PT and PTT; and decreased fibrinogen. Fibrin degradation products, such as d-dimers, can be elevated. The primary condition needs to be treated aggressively; the bleeding disorder can be controlled using FFP (grade of evidence C, strength of recommendation 1; other products such as cryoprecipitate and platelet concentrates may also be necessary. FFP/other blood products can bring temporizing relief while the primary condition is treated.53 There may not be any strong, clinically relevant differences in the coagulation tests, outcomes of DIC, or in mortality.
• Vitamin K deficiency
Neonates express coagulation factors II, VII, IX, and X, which are vitamin K-dependent, at only about half the levels seen in adults.54 Premature infants have even lower levels. Deficiency of vitamin K presents as hemorrhagic disease of the newborn (HDN) and can be associated with life-threatening intracranial hemorrhages.55 There are two presentations:
Classical HDN occurs in the first week after birth due to a transient deficiency of vitamin K-dependent factors, and it is characterized by cutaneous bruising and/or intestinal hemorrhages in neonates. In infants born to mothers treated with drugs that suppress vitamin K metabolism, HDN can appear within the first 24 hours after birth. Routine administration of vitamin K soon after birth is effective in preventing classical HDN.56
Late HDN appears between 2 and 8 weeks after birth and is usually associated with vitamin K malabsorption. Vitamin K supplementation is effective within a few hours.57 In addition to vitamin K, FFP can also help control severe bleeding or intracranial hemorrhage.57
Neonates who present with acute bleeding due to vitamin K deficiency should be treated upon suspicion with intravenous vitamin K, which will reverse the coagulopathy.58 However, because vitamin K typically takes longer to show therapeutic effect, FFP should also be administered immediately to reduce the risk of devastating intracranial hemorrhages (grade of evidence C, strength of recommendation 1).
• Liver disease
Infants with acute or chronic liver disease may manifest with coagulopathy. Acute liver failure in neonates can occur due to birth asphyxia, sepsis, viral infections, and metabolic diseases. There may be decreased synthesis of pro- and anticoagulant factors, hypo- and dysfibrinogenemia, thrombocytopenia and platelet dysfunction, reduction of gamma-carboxylation of vitamin K-dependent proteins, and hyperfibrinolysis. To correct the coagulopathy, these infants require FFP and cryoprecipitate transfusions in addition to the treatment of the primary liver disease (grade of evidence C, strength of recommendation 2).59
• Extracorporeal membrane oxygenation (ECMO)
Extracorporeal membrane oxygenation is used to provide life support in infants with severe cardiorespiratory failure. These infants may already have coagulopathy due to sepsis, shock, or profound hypoxia, which may increase the risk of intracranial hemorrhage. The foreign surface of the ECMO circuit can activate the coagulation cascade; heparinization can prevent clot formation, but this also increases the risk of bleeding.60 FFP support may be needed in infants after surgery and in those with sepsis. Some infants who have been on prolonged ECMO and need frequent transfusions, FFP has been used to treat possible microhemorrhages.61 However, in a clinical study, scheduled FFP administrations did not increase circuit life or reduce the need for blood product transfusions.62 Patients in the intervention group had similar hemorrhagic and thrombotic complications as the control group.
As limited data are available on the transfusion practice of neonatal ECMO patients, the recommendations are based on expert opinion provided by different ECMO centers.63
• Exchange transfusion
Infants with severe unconjugated hyperbilirubinemia may need exchange transfusion to prevent bilirubin encephalopathy/kernicterus. Many centers use blood reconstituted from FFP and packed erythrocytes for exchange transfusions, although the evidence of benefit from the use of reconstituted blood is limited.64
Partial exchange transfusion (PET) is traditionally used as the method to lower the hematocrit and treat hyperviscosity in neonatal polycythemia. Three randomized clinical trials conducted to compare the efficacy of crystalloid solutions versus plasma used for PET to treat neonatal polycythemia showed variable results.65–67 Two meta-analyses, which included all these three studies, showed no significant difference in the reduction of post-exchange hematocrit.68–70 Fresh-frozen plasma should not be used to treat polycythemia unless there is a coexistent coagulopathy (grade of evidence A, strength of recommendation 1).
Adverse Effects
Fresh-frozen plasma can have several adverse effects:71
Immunological reactions, including early events due to innate immune reactions or delayed events such as alloimmunization to various circulatory antigens.
Nonimmunologic complications seen with blood transfusions such as infections, circulatory overload, and metabolic derangements can also be seen with FFP administration. FFP lacks leukocytes, and therefore the transmission of cytomegalovirus is less frequent. However, human immunodeficiency virus (HIV) and hepatitis can be transmitted.72 Circulatory overload can be seen in infants with pre-existing pulmonary edema or congestive heart failure. Metabolic complications like citrate toxicity and consequent hypocalcemia are rare but have been noted.
Conclusions
Administration of FFP is a frequent intervention in premature and critically-ill infants. Current evidence suggests that FFP should be used primarily in neonates with active bleeding and associated coagulopathy, or for deficiency of congenital factors for which no specific concentrated coagulation factors are available. In the setting of neonatal acquired coagulopathy, evaluation and interpretation of the standard coagulation test (PT, APTT) should be based on reference ranges appropriate for gestational and post-natal age. More information from controlled studies is needed to accurately identify infants who are truly at risk for bleeding complications and who actually need FFP transfusions.
Supplementary Material
KEY POINTS.
Fresh-frozen plasma is prepared by freezing plasma obtained either from single whole-blood donor units or through plasmapheresis; it contains high levels of soluble pro-coagulation factors, natural anticoagulants, and the tissue plasminogen activator inhibitor.
Premature and critically ill infants frequently receive FFP transfusions due to high risk of bleeding; clinical studies show records of such treatment in up to 10% of these infants.
Infants longer clotting times than adults which may be developmentally appropriate, and the risk of bleeding may not be proportionate to these deviations.
Current evidence suggest that the use of FFP should be directed primarily to neonates with active bleeding and associated coagulopathy.
Further information from controlled studies is needed to accurately identify neonates who are truly at risk for bleeding complications requiring FFP administration.
Source of support:
This study was supported by the NIH (grant number R01HL133022).
Footnotes
Conflicts of interest: None
Supplementary Material
The supplementary tables are available online on the website of www.newbornjournal.org.
References
- 1.Dzik W, Rao A. Why do physicians request fresh frozen plasma? Transfusion 2004;44(9):1393–1394. DOI: 10.1111/j.0041-1132.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 2.Neary E, McCallion N, Kevane B, et al. Coagulation indices in very preterm infants from cord blood and postnatal samples. J Thromb Haemost 2015;13(11):2021–2030. DOI: 10.1111/jth.13130. [DOI] [PubMed] [Google Scholar]
- 3.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Evidence Levels of Evidence (Introductory Document). Oxford Centre for Evidence-Based Medicine 2022. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/levels-of-evidence-introductory-document.
- 4.Girelli G, Antoncecchi S, Casadei AM, et al. Recommendations for transfusion therapy in neonatology. Blood Transfus 2015;13(3): 484–497. DOI: 10.2450/2015.0113-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross SJ, Filston HC, Anderson JC. Controlled study of treatment for disseminated intravascular coagulation in the neonate. J Pediatr 1982;100(3):445–448. DOI: 10.1016/s0022-3476(82)80457-5. [DOI] [PubMed] [Google Scholar]
- 6.Neary E, Ni Ainle F, El‐Khuffash A, et al. Plasma transfusion to prevent intraventricular haemorrhage in very preterm infants. Cochrane Database Syst Rev 2016;9:CD012341. DOI: 10.1002/14651858.CD012341. [DOI] [Google Scholar]
- 7.Pal S, Curley A, Stanworth SJ. Interpretation of clotting tests in the neonate. Arch Dis Child Fetal Neonatal Ed 2015;100(3):F270–274. DOI: 10.1136/archdischild-2014-306196. [DOI] [PubMed] [Google Scholar]
- 8.Wallis JP, Dzik S. Is fresh frozen plasma overtransfused in the United States? Transfusion 2004;44(11):1674–165. DOI: 10.1111/j.00411132.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- 9.Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood 1987;70(1):165–172. PMID: 3593964. [PubMed] [Google Scholar]
- 10.Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the healthy premature infant. Blood 1988; 72(5):1651–1657. PMID: 3179444. [PubMed] [Google Scholar]
- 11.Christensen RD, Baer VL, Lambert DK, Henry E, Ilstrup SJ, Bennett ST. Reference intervals for common coagulation tests of preterm infants (CME). Transfusion 2014;54(3):627–632:quiz 626. DOI: 10.1111/trf.12322. [DOI] [PubMed] [Google Scholar]
- 12.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. DOI: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A randomized trial comparing the effect of prophylactic intravenous fresh frozen plasma, gelatin or glucose on early mortality and morbidity in preterm babies. The Northern Neonatal Nursing Initiative [NNNI] Trial Group. Eur J Pediatr 1996;155(7):580–588. DOI: 10.1007/BF01957909. [DOI] [PubMed] [Google Scholar]
- 14.O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryoupernatant. Br J Haematol 2004;126(1):11–28. DOI: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 15.Westphal RG, Tindle B, Howard PL, Golden EA, Page GA. Rapid thawing of fresh frozen plasma. Am J Clin Pathol 1982;78(2):220–222. DOI: 10.1093/ajcp/78.2.220. [DOI] [PubMed] [Google Scholar]
- 16.Gibson BE, Todd A, Roberts I, et al. Transfusion guidelines for neonates and older children. Br J Haematol 2004;124(4):433–453. DOI: 10.1111/j.1365-2141.2004.04815.x. [DOI] [PubMed] [Google Scholar]
- 17.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus 2009;7(2): 132–150. DOI: 10.2450/2009.0005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair PM, Rendo MJ, Reddoch-Cardenas KM, Burris JK, Meledeo MA, Cap AP. Recent advances in use of fresh frozen plasma, cryoprecipitate, immunoglobulins, and clotting factors for transfusion support in patients with hematologic disease. Semin Hematol 2020;57(2):73–82. DOI: 10.1053/j.seminhematol.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neary E, Okafor I, Al-Awaysheh F, et al. Laboratory coagulation parameters in extremely premature infants born earlier than 27 gestational weeks upon admission to a neonatal intensive care unit. Neonatology 2013;104(3):222–227. DOI: 10.1159/000353366. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga S, Takai Y, Nakamura E, et al. The clinical efficacy of fibrinogen concentrate in massive obstetric haemorrhage with hypofibrinogenaemia. Sci Rep 2017;7:46749. DOI: 10.1038/srep46749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pescatore SL. Clinical management of protein C deficiency. Expert Opin Pharmacother 2001;2(3):431–439. DOI: 10.1517/14656566.2.3.431. [DOI] [PubMed] [Google Scholar]
- 22.Nascimento B, Callum J, Rubenfeld G, et al. Clinical review: fresh frozen plasma in massive bleedings - more questions than answers. Crit Care 2010;14(1):202. DOI: 10.1186/cc8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dogra M, Sidhu M, Vasudev R, Dogra A. Comparative analysis of activity of coagulation factors V and VIII and level of fibrinogen in fresh frozen plasma and frozen plasma. Asian J Transfus Sci 2015; 9(1):6–8. DOI: 10.4103/0973-6247.150936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horowitz B, Wiebe ME, Lippin A, et al. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion 1985;25(6):516–522. DOI: 10.1046/j.1537-2995.1985.25686071422.x. [DOI] [PubMed] [Google Scholar]
- 25.Liumbruno GM, Franchini M. Solvent/detergent plasma: pharma-ceutical characteristics and clinical experience. J Thromb Thrombolysis 2015;39(1):118–128. DOI: 10.1007/s11239-014-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dichtelmuller HO, Biesert L, Fabbrizzi F, et al. Robustness of solvent/detergent treatment of plasma derivatives: A data collection from plasma protein therapeutics association member companies. Transfusion 2009;49(9):1931–1943. DOI: 10.1111/j.15372995.2009.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Rubia J, Arriaga F, Linares D, et al. Role of methylene blue-treated or fresh-frozen plasma in the response to plasma exchange in patients with thrombotic thrombocytopenic purpura. Br J Haematol 2001;114(3):721–723. DOI: 10.1046/j.1365-2141.2001.02991.x. [DOI] [PubMed] [Google Scholar]
- 28.Shabanian R, Dehestani A, Dadkhah M, et al. Fresh frozen plasma prime and the level of gammaglobulin after pediatric cardiopulmonary bypass. Am J Clin Exp Immunol 2020;9(5):91–100. PMID: 33489477. [PMC free article] [PubMed] [Google Scholar]
- 29.Houben NAM, Heeger LE, Stanworth SJ, et al. Changes in the use of fresh-frozen plasma transfusions in preterm neonates: A single center experience. J Clin Med 2020;9(11):3789. DOI: 10.3390/jcm9113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hambleton G, Appleyard WJ. Controlled trial of fresh frozen plasma in asphyxiated low birthweight infants. Arch Dis Child 1973;48(1):31–35. DOI: 10.1136/adc.48.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beverley DW, Pitts-Tucker TJ, Congdon PJ, Arthur RJ, Tate G. Prevention of intraventricular haemorrhage by fresh frozen plasma. Arch Dis Child 1985;60(8):710–713. DOI: 10.1136/adc.60.8.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekblad H, Kero P, Shaffer SG et al. Extracellular volume in preterm infants: influence of gestational age and colloids. Early Hum Dev 1991;27(1–2):1–7. DOI: 10.1016/0378-3782(91)90022-u. [DOI] [PubMed] [Google Scholar]
- 33.Osborn DA, Evans N. Early volume expansion for prevention of morbidity and mortality in very preterm infants. Cochrane Database Syst Rev 2004;(2):CD002055. DOI: 10.1002/14651858.CD002055.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kempny A, Dimopoulos K, Uebing A, et al. Outcome of cardiac surgery in patients with congenital heart disease in England between 1997 and 2015. PLoS One 2017;12(6):e0178963. DOI: 10.1371/journal.pone.0178963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirzaei M, Mirzaei S, Sepahvand E, et al. Evaluation of complications of heart surgery in children with congenital heart disease at Dena Hospital of Shiraz. Glob J Health Sci 2015;8(5):33–38. DOI: 10.5539/gjhs.v8n5p33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erdoes G, Gerster G, Colucci G, et al. Prediction of post-weaning fibrinogen status during cardiopulmonary bypass: An observational study in 110 patients. PLoS ONE 2015;10(5):e0126692. DOI: 10.1371/journal.pone.0126692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson SJ, Karam O, Birch R, et al. Transfusion practices in pediatric cardiac surgery requiring cardiopulmonary bypass: A secondary analysis of a clinical database. Pediatr Crit Care Med 2021;22(11): 978–987. DOI: 10.1097/PCC.0000000000002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray MJ, Marsh NA, Hawson GA. Relationship of fibrinolysis and platelet function to bleeding after cardiopulmonary bypass. Blood Coagul Fibrinolysis 1994;5(5):679–685. DOI: 10.1097/00001721-19941000000002. [DOI] [PubMed] [Google Scholar]
- 39.Hofer J, Fries D, Solomon C, et al. A snapshot of coagulopathy after cardiopulmonary bypass. Clin Appl Thromb Hemost 2016;22(6): 505–511. DOI: 10.1177/1076029616651146. [DOI] [PubMed] [Google Scholar]
- 40.Desborough M, Sandu R, Brunskill SJ, et al. Fresh frozen plasma for cardiovascular surgery. Cochrane Database Syst Rev 2015;(7):CD007614. DOI: 10.1002/14651858.CD007614.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JW, Yoo YC, Park HK, et al. Fresh frozen plasma in pump priming for congenital heart surgery: Evaluation of effects on postoperative coagulation profiles using a fibrinogen assay and rotational thromboelastometry. Yonsei Med J 2013;54(3):752–762. DOI: 10.3349/ymj.2013.54.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faraoni D, Meier J, New HV, et al. Patient blood management for neonates and children undergoing cardiac surgery: 2019 NATA Guidelines. J Cardiothorac Vasc Anesth 2019;33(12):3249–3263. DOI: 10.1053/j.jvca.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon’s perspective. Hematol Am Soc Hematol Educ Program 2013;2013:656–659. DOI: 10.1182/asheducation-2013.1.656 [DOI] [PubMed] [Google Scholar]
- 44.Patil V, Shetmahajan M. Massive transfusion and massive transfusion protocol. Indian J Anaesth 2014;58(5):590–595. DOI: 10.4103/0019-5049.144662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004;109(22):2698–2704. DOI: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 46.Kaur P, Basu S, Kaur G, et al. Transfusion protocol in trauma. J Emerg Trauma Shock 2011;4(1):103–108. DOI: 10.4103/0974-2700.76844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mumford AD, Ackroyd S, Alikhan R, et al. Guideline for the diagnosis and management of the rare coagulation disorders: A United Kingdom Haemophilia Centre Doctors’ Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol 2014;167(3):304–326. DOI: 10.1111/bjh.13058. [DOI] [PubMed] [Google Scholar]
- 48.Castaman G, Linari S. Diagnosis and treatment of von Willebrand disease and rare bleeding disorders. J Clin Med 2017;6(4): 45. DOI: 10.3390/jcm6040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bray GL, Luban NL. Hemophilia presenting with intracranial hemorrhage. An approach to the infant with intracranial bleeding and coagulopathy. Am J Dis Child 1987;141(11):1215–1217. DOI: 10.1001/archpedi.1987.04460110085030. [DOI] [PubMed] [Google Scholar]
- 50.Peyvandi F, Menegatti M. Treatment of rare factor deficiencies in 2016. Hematology Am Soc Hematol Educ Program 2016;2016(1):663–669. DOI: 10.1182/asheducation-2016.1.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalmers E, Williams M, Brennand J, et al. Guideline on the management of haemophilia in the fetus and neonate. Br J Haematol 2011; 154(2):208–215. DOI: 10.1111/j.1365-2141.2010.08545.x. [DOI] [PubMed] [Google Scholar]
- 52.Jain S, Acharya SS. Management of rare coagulation disorders in 2018. Transfus Apher Sci 2018;57(6):705–712. DOI: 10.1016/j.transci.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Go H, Ohto H, Nollet KE, et al. Risk factors and treatments for disseminated intravascular coagulation in neonates. Ital J Pediatr 2020;46(1):54. DOI: 10.1186/s13052-020-0815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pichler E, Pichler L. The neonatal coagulation system and the vitamin K deficiency bleeding – a mini review. Wien Med Wochenschr 2008; 158(13–14):385–395. DOI: 10.1007/s10354-008-0538-7 [DOI] [PubMed] [Google Scholar]
- 55.Rai RK, Luo J, Tulchinsky TH. Vitamin K supplementation to prevent hemorrhagic morbidity and mortality of newborns in India and China. World J Pediatr 2017;13(1):15–19. DOI: 10.1007/s12519-016-0062-6. [DOI] [PubMed] [Google Scholar]
- 56.Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol 2016;36(1):S29–35. DOI: 10.1038/jp.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bor O, Akgun N, Yakut A, et al. Late hemorrhagic disease of the newborn. Pediatr Int 2000;42(1):64–66. DOI: 10.1046/j.1442-200x.2000.01173.x. [DOI] [PubMed] [Google Scholar]
- 58.Shearer MJ. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev 2009;23(2):49–59. DOI: 10.1016/j.blre.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Shanmugam NP, Bansal S, Greenough A, et al. Neonatal liver failure: aetiologies and management—state of the art. Eur J Pediatr May 2011;170(5):573–581. DOI: 10.1007/s00431-010-1309-1. [DOI] [PubMed] [Google Scholar]
- 60.Cashen K, Meert K, Dalton H. Anticoagulation in neonatal ECMO: An enigma despite a lot of effort! Front Pediatr 2019;7:366. DOI: 10.3389/fped.2019.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chlebowski MM, Baltagi S, Carlson M, et al. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care 2020;24(1):19. DOI: 10.1186/s13054-020-2726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McMichael ABV, Zimmerman KO, Kumar KR, et al. Evaluation of effect of scheduled fresh frozen plasma on ECMO circuit life: A randomized pilot trial. Transfusion 2021;61(1):42–51. DOI: 10.1111/trf.16164. [DOI] [PubMed] [Google Scholar]
- 63.Yuan S, Tsukahara E, De La Cruz K, et al. How we provide transfusion support for neonatal and pediatric patients on extracorporeal membrane oxygenation. Transfusion 2013;53(6):1157–1665. DOI: 10.1111/j.1537-2995.2012.03688.x. [DOI] [PubMed] [Google Scholar]
- 64.Thayyil S, Milligan DW. Single versus double volume exchange transfusion in jaundiced newborn infants. Cochrane Database Syst Rev 2006;(4):CD004592. DOI: 10.1002/14651858.CD004592.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Deorari AK, Paul VK, Shreshta L, et al. Symptomatic neonatal polycythemia: comparison of partial exchange transfusion with saline versus plasma. Indian Pediatr 1995;32(11):1167–1171. [PubMed] [Google Scholar]
- 66.Roithmaier A, Arlettaz R, Bauer K, et al. Randomized controlled trial of Ringer solution versus serum for partial exchange transfusion in neonatal polycythaemia. Eur J Pediatr Jan 1995;154(1):53–56. DOI: 10.1007/BF01972973. [DOI] [PubMed] [Google Scholar]
- 67.Krishnan L, Rahim A. Neonatal polycythemia. Indian J Pediatr 1997; 64(4):541–546. DOI: 10.1007/BF02737765. [DOI] [PubMed] [Google Scholar]
- 68.Dempsey EM, Barrington K. Short and long term outcomes following partial exchange transfusion in the polycythaemic newborn: a systematic review. Arch Dis Child Fetal Neonatal Ed 2006;91(1):F2–6. DOI: 10.1136/adc.2004.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Waal KA, Baerts W, Offringa M. Systematic review of the optimal fluid for dilutional exchange transfusion in neonatal polycythaemia. Arch Dis Child Fetal Neonatal Ed 2006;91(1):F7–F10. DOI: 10.1136/adc.2004.063925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dempsey EM, Barrington K. Crystalloid or colloid for partial exchange transfusion in neonatal polycythemia: a systematic review and meta-analysis. Acta Paediatr 2005;94(11):1650–1655. DOI: 10.1080/08035250500192748. [DOI] [PubMed] [Google Scholar]
- 71.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion 2012;52 Suppl 1:65S–79S. DOI: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood 2019;133(17):1854–1864. DOI: 10.1182/blood-2018-11-833996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


