Abstract
Background and Aim:
Ruminant slaughterhouse is one of the food-producing units to meet the protein demand of the people in Central Java. This study aimed to evaluate the implementation of sanitation and hygiene in ruminant slaughterhouses in Central Java based on their veterinary control number(NKV) certification and the microbiological quality of the meat produced.
Materials and Methods:
This study was conducted from September 2021 to December 2021. Thirty-three priority slaughterhouses, representing 33 districts/cities in Central Java Province, were assessed for their hygiene and sanitation practices according to the NKV criteria mandated by The Minister of Agriculture Regulation No. 11/2020 on NKV Certification for Animal Production Unit. Sixty-six meat samples from these slaughterhouses were obtained for microbiological analysis. The total plate count (TPC), counts of Escherichia coli and Staphylococcus aureus, and the presence of Salmonella spp. were determined. The microbiological tests followed the standard national testing procedure according to the Indonesian National Standard 2897:2008 on Method of Analysis for Microbiological Contaminants in Meat, Eggs, Milk, and its derived products.
Results:
The sanitation hygiene assessment of the 33 slaughterhouses showed that seven (21.2%) met the NKV criteria level 3, while the others did not. The average TPC of the meat samples was 1.57 × 105 CFU/g (4.93 log10), the S. aureus count was 7.6 CFU/g, and the E. coli count was 9.2 most probable number/g. Only one sample (1.50%) tested positive for Salmonella spp. A comprehensive assessment comparing the NKV criteria with the level of meat contamination showed that the ruminant slaughterhouses that satisfied the NKV criteria had more meat samples (85.71%), on average, that complied with the Indonesian National Standard for microbial contamination compared with those that did not satisfy the NKV criteria (69.23%). The odds ratio was 2.67.
Conclusion:
Most of the priority ruminant slaughterhouses in Central Java did not meet the NKV standards. The research only looks at the level of hygiene sanitation according to NKV standards in slaughterhouses, the level of contamination produced does not reflect the level of the consumer; therefore, the level of contamination should continue to be investigated at the post-production stage.
Keywords: bacterial contamination, hygiene sanitation, meat, slaughterhouse
Introduction
Meat contains proteins, essential amino acids, fats and fatty acids, carbohydrates, vitamins, and minerals, all of which help in cell maintenance and repair and provide energy for daily activities [1, 2]. This nutritional composition, however, also provides the best medium for the growth of spoilage microbes and foodborne pathogens. The ability of microorganisms to attach to surfaces where meat is stored while being sold often causes meat contamination [3]. Poor meat quality can cause foodborne illnesses, such as those caused by contaminating Escherichia coli O157 H7, Salmonella spp., Campylobacter spp., Yersinia enterocolitica, and Listeria monocytogenes. The level of E. coli contamination is used to assess food microbiological quality and determine whether proper sanitation has been maintained [4]. Escherichia coli is used as an indicator for detecting fecal contamination in drinking water and other matrices [5]. Staphylococcus aureus, a commensal and opportunistic pathogen, causes one of the most harmful types of food poisoning in the world. Staphylococcus aureus can cause a broad spectrum of infections, ranging from superficial skin conditions to severely invasive diseases [6]. Common symptoms of foodborne illnesses include vomiting, diarrhea (with or without blood), fever, abdominal cramping, headache, dehydration, myalgia, and arthralgia [7]. Bacterial contamination of meat can occur in slaughterhouses until it is ready for consumption. In general, contamination occurs when the meat comes into contact with dirty hands, clothing, equipment, or facilities. Keeping the processing areas clean will result in lower chances of microbial cross-contamination [8]. Under optimum conditions, bacterial cells can double every 15–30 min. For most bacteria, individual cells can multiply into more than a million in 5 h [9].
The application of the slaughter process also impacts microbial contamination, affecting meat quality and food safety for consumers [10]. High levels of microbial contamination in meat reduce its shelf life and adversely affect its sensory properties [8]. In Indonesia, the quality assessment of the sanitation and hygiene of slaughterhouses is achieved by evaluation and categorization based on standards set by the government. To recognize the fulfillment of sanitary and hygiene requirements in slaughterhouses, such as good veterinary practices, biosecurity, animal welfare, buildings, facilities and equipment, meat handling, hygiene personnel, sanitary hygiene, and testing by the Accredited External Laboratory, certificates of Veterinary Control Number (NKV) are issued in accordance with The Minister of Agriculture Regulation No.11 of 2020 [11]. Central Java is a province that produces 59.952 tons of beef per year [12], most of which comes from 82 ruminant slaughterhouses (rumah potong hewan ruminansia [RPH-R]) owned by the government in districts and cities that engage in ruminant livestock slaughter to meet the needs of local communities [13]. The impact of sanitation hygiene applications on the microbial contamination of meat produced in RPH-R in Central Java needs to be evaluated.
This study aimed to determine the level of hygiene sanitation applications in government-owned RPH-R in Central Java and the level of microbial contamination, including the total plate count (TPC), the amount of E. coli, S. aureus, and Salmonella spp., in the meat produced from these slaughterhouses.
Materials and Methods
Ethical approval
Ethical approval for animal research was not required as live animals were not used in this study.
Study period and location
This study was conducted from September 2021 to December 2021. Thirty-three priority slaughterhouses, representing 33 districts/cities in Central Java Province, were assessed for their hygiene and sanitation practices.
Selection and NKV assessment of RPH-R
A total of 33 government slaughterhouses that slaughter bovines (cows and/or buffaloes) in Central Java Province were selected for this study, considering those with the largest number of slaughtered cattle or those that became a development priority in the city or regency. The assessment of slaughterhouses used the NKV assessment formula, which includes good veterinary practices, building facilities and equipment, personal hygiene, and slaughter and meat handling processes. The results, based on the number of major and minor mismatched findings in accordance with the NKV regulations, led to the RPH-R being grouped into four categories: level 1 (best), 2, 3, and no criteria (NC).
Meat samples for microbiological testing
A total of 66 meat samples were collected from the 33 slaughterhouses for microbiological testing, two samples per slaughterhouse. The meat samples were obtained after skinning and the carcass was cut before being transported in a vehicle to the Veterinary Public Health Laboratory of the Central Java Provincial Animal Husbandry and Health Office, where procedures following the Indonesian National Standard (SNI) 2897:2008 were undertaken to determine the level of contamination in the meat samples using TPC, and test for E. coli, S. aureus, and Salmonella spp.
Total plate count
The TPC was determined by initially homogenizing 25 g of each meat sample with 225 mL of 0.1% Buffered Peptone Water (BPW), which was diluted 10-fold, for 1–2 min. A serial dilution was performed by adding 1 mL of the previous suspension into 9 mL of BPW. For each dilution, 1 mL of the suspension was placed in a Petri cup, 15–20 mL of plate count agar cooled to 45°C was added, and the cup was incubated at 34–36°C for 24–48 h. The number of colonies for each dilution series, except the Petri dishes containing spreader colonies, was calculated. Plates with 25–250 colonies were selected [14].
Escherichia coli count
The number of E. coli in the samples was estimated using the most probable number (MPN). In the hypothesis test (three series of tubes), up to 25 g of sample was weighed, homogenized in up to 225 mL (1:9) of 0.1% BPW in the stomacher, and diluted within the range of 10–1000-fold. From each dilution, 1 mL of sample was pipetted into three series of lauryl sulfate tryptose broth (LSTB) tubes containing a Durham tube within. The samples were incubated at 35°C for 24–28 h. The presence of emerging gas bubbles in the Durham tube indicated a positive test result. The resending test was not repeated. In the confirmation test, the positive strain was transferred using an inoculation needle from each LSTB tube into an E. coli broth (ECB) tube containing a Durham tube within. The ECB tube was incubated at 45.5°C for 24 h. When it tested negative, the tube was incubated again until 48 h. The amount of E. coli per gram of meat was determined using the MPN table based on the number of ECB tubes that contained gas inside the Durham tube. During isolation and identification, a positive sample from the ECB was scratched on eosin methylene blue agar and incubated at 35°C for 18–24 h. Black/dark E. coli colonies, 2–3 mm in diameter, were observed at the center of the colony with or without shiny metallic green color on the agar [14].
Staphylococcus aureus count
Staphylococcus aureus was detected by inoculating 1 mL of each dilution onto Baird–Parker Agar medium, supplemented with 5% v/v egg yolk tellurite emulsion. The inoculum was flattened over the surface of the medium using a glass rod (hockey stick) and left until it was absorbed. The samples were incubated at 35°C for 45–48 h. Staphylococcus aureus colonies exhibited a round, slippery and smooth, and convex diameter of 2–3 mm, gray to pitch black color, and surrounding dark zones with or without a bright outer zone [14].
Detection of Salmonella spp.
In this test, 25 g sample was weighed and placed in a sterile plastic bag, 225 mL of sterile lactose broth was added, the mixture was homogenized in a stomacher for 120 h, and incubated for 24 ± 2 h at 36°C ± 1°C. Then, 1 mL of the selectively enriched sample from the lactose broth was added to 10 mL of tetrathionate broth in a sterile test tube, up to 20 μL of iodine was added, and the sample was incubated at 36°C ± 1°C for 24 ± 2 h. For isolating Salmonella, selective media samples that had been incubated on each selective add-on medium were placed on a scratched quadrant on xylose lysine deoxycholate agar media and incubated at 36°C ± 1°C for 24 ± 2 h in the reverse position. After incubation, any typical colonies growing on the gelatin media were observed. The Salmonella colonies on this media were pink with or without black in the middle; some appeared large, were shiny black in the middle, or were all black [14].
Relationship between NKV criteria and microbial contamination
The application of NKV and microbial contamination levels was analyzed descriptively by grouping the slaughterhouses based on the level of application of the NKV list. The actual number of RPH-R eligible for NKV certificate levels 1, 2, and 3 or those that were unqualified, and the average microbial contamination detected in the meat samples were noted adjacent to each other. The relationship between the two and its strength was calculated using the Chi-squared test and odds ratios.
Results and Discussion
Level of NKV in RPH-R
Thirty-three priority slaughterhouses from 33 city districts were selected for this study, of which 6 RPH-R had previously obtained level 3 NKV certificates. Based on the regulations of the Ministry of Agriculture, the lowest level of NKV was obtained when the maximum error limit was 37, that is, 19 major error and 18 minor error limits (Figure-1).
Figure-1.

Nonconformity of priority slaughterhouses in cities/regencies.
An RPH-R was considered to meet the NKV criteria if the number of mismatches was below the maximum limit. Seven slaughterhouses, from Magelang, Salatiga City, Surakarta City, Banyumas, Cilacap, Semarang, and Grobogan, satisfied NKV level 3 criteria, while no slaughterhouses were included in the level 1 and 2 categories (Figure-2). The NKV certification changed in the previous year for six locations: Wonogiri, Magelang, Salatiga City, Surakarta City, Boyolali, and Banyumas. In RPH-R that met the level 3 criteria, the changes were consistent with the application of hygiene; however, the sanitation level was still low and needed regular guidance and supervision. This result is in accordance with the regulations that require level 3 slaughterhouses to be monitored and surveyed every 4 months [11].
Figure-2.

Number of slaughterhouses based on the NKV level.
The number of priority slaughterhouses that met the new NKV level 3 criteria showed good but low implementation levels. According to the regulations, these slaughterhouses can only supply meat to one province.
Based on the major and minor mismatched findings, RPH-R in Central Java faces the following problems:
Any animals that arrive are unaccompanied by a Veterinary Certificate or Animal Health Certificate
No physical separation exists between clean and dirty spaces
Light intensity in antemortem and postmortem examination area is <540 lux
Air flows from dirty areas to clean areas
Hand-washing facilities are hand-operated
Temperature in the carcass handling room and meat is higher than 15°C
Meat transport does not prevent contamination
No written program is available for insect control, rodents, and/or other disruptive animals
No laboratory testing is available to examine the effectiveness of sanitation programs.
Given these conditions, slaughterhouses need regular improvement to meet the minimum level of NKV criteria because it guarantees the maintenance of sanitary hygiene requirements. According to Kuntoro et al. [15], low implementation of hygiene sanitation, as indicated by a high number of mismatches on the NKV checklist, is closely related to high levels of microbial contamination in meat. Besides facilitating the supervision and monitoring of animal food products and tracking the problems related to food safety, the NKV certification of a business unit can become its identity [16].
Slaughterhouses that have met the requirements still need regular monitoring of their products because important major findings may still occur in the field. The goal is that business unit that have already earned NKV certificates guarantee the fulfillment of hygiene and sanitation requirements, assuring the safety of their animal products [17].
Microbial contamination levels
TPC is the enumeration of microorganisms that grow in aerobic conditions at moderate temperatures of 20–45°C. This calculation encompasses all pathogens and nonpathogens and is used to determine the hygiene status of food products. The microbiological growth medium, which is a nonselective medium, is used while determining TPC [18]. High levels of microbial contamination can increase damage and decrease the shelf life of meat while potentially carrying pathogenic bacteria that can cause foodborne illnesses [8, 18].
Figure-3 shows that the average TPC of meat from priority slaughterhouses in Central Java was 4.93, which is below the maximum limit set by SNI (1 × 106 CFU/g or 6 log10TPC). The highest contamination value was obtained in meat from Tegal Regency with a log10TPC value of 5.76 while the lowest was recorded in meat from Banyumas Regency with a log10TPC value of 3.90. In this study, 100% of the meat samples exhibited TPC values that met the SNI standards. In contrast, Jacob et al. [19] showed that 63.33% of the meat samples failed to meet the SNI standards in Kupang, while Mufidah et al. [20] attained a value of 33.33% for the same in Probolinggo, East Java. The different levels of microbial contamination are influenced by differences in the hygiene and sanitation applications in each slaughterhouse.
Figure-3.

Total plate count of meat from priority slaughterhouses in each city/regency.
Microorganisms can contaminate meat through blood circulation at the time of slaughter or due to the use of unclean equipment or improper hygiene. Contamination may also occur after slaughtering: during skinning, evisceration, carcass handling, cooling, freezing, thawing, packaging, storage, distribution, and before consumption of the meat [15]. Thus, the difference in procedures and quality of sanitation hygiene in each slaughterhouse will affect the level of contamination.
Escherichia coli contamination requires special attention because a dangerous strain, E. coli O157:H7, which causes food and waterborne diseases, has a high prevalence of 6.3% in one of the Indonesian provinces [21]. Figure-4 shows the level of E. coli contamination observed in this study.
Figure-4.
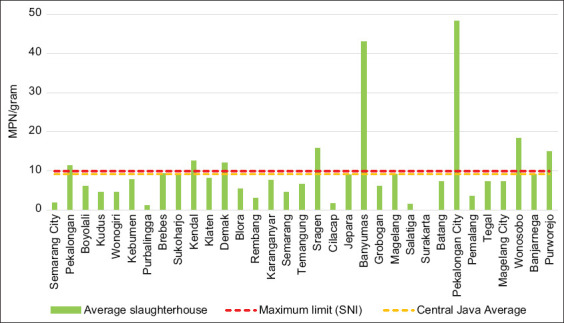
Escherichia coli contamination in meat from priority slaughterhouses in each city/regency.
Of the 66 samples collected, 80.3% (53) were contaminated with E. coli. In contrast, a study conducted in East Java reported an E. coli contamination rate of just 32.5% [18]. A study in Africa reported a similar prevalence of 91.9% (87.2–96.0%) while another study in Malaysia found 55% of the samples contaminated with E. coli [22, 23]. The average contamination rate in all priority slaughterhouses in Central Java was 9.02 MPN/g, which is close to the allowed maximum limit of E. coli contamination (10 MPN/g) [24, 25]. Seven districts (21.21%) and 20.3% of the samples exhibited an average E. coli contamination exceeding the maximum allowed limit. The highest value of E. coli contamination (48.3 MPN/g) was recorded in the meat from Pekalongan City, while the lowest was recorded in the meat from Surakarta City (<3 MPN/g).
In this study, 86% of the meat samples were contaminated with S. aureus. However, the overall contamination rate of 7.65 CFU/g was still below the maximum limit set by SNI (100 CFU/g) [24, 25]. Figure-5 shows the level of S. aureus contamination observed in this study. The previous investigations in East Java, Indonesia, Ethiopia, and Malaysia recorded S. aureus contamination levels of 20%, 22.5%, and 32%, respectively [18, 26, 27]. Humans can be a source of contamination because we act as reservoirs by carrying the enterotoxin-producing S. aureus on our hands or in our nostrils, which are the two main sources of food contamination: Mechanical contact or aerosol droplets [28]. The application of hygienic food production and public education on food safety is the main strategies to prevent Staphylococcal food poisoning [29].
Figure-5.

Staphylococcus aureus contamination in meat from priority slaughterhouses in each city/regency.
Globally, Salmonella spp. cause millions of cases of enteric diseases, and thousands of hospitalizations and deaths every year [30]. In this study, 1.5% of the samples tested positive for Salmonella. Similar studies from other regions have showed a Salmonella prevalence of 3.1%, 0%, and 2.5% [18, 31, 32]. A positive sample result is not in accordance with SNI 7388:2009, which recommends a negative result per 25 g of Salmonella spp. [24, 25]. Salmonella spp. can contaminate meat because they live in the intestines of animals. The use of sanitation systems before the slaughtered meat is cut and handled also influences Salmonella spp. infection [32].
Relationship between NKV criteria and microbial contamination
Table-1 provides comprehensive details regarding the NKV criteria and the fulfillment of SNI for bacterial contamination, which was deemed fulfilled (good) if the mean microbial contamination from TPC, E. coli, and S. aureus tests was under the maximum limit of SNI and the Salmonella spp. test was negative.
Table-1.
Veterinary control number (NKV) criteria and microbial contamination.
| Location of Slaughterhouse | NKV criteria | Maximum contamination limit | Microbial contamination status | |||
|---|---|---|---|---|---|---|
|
| ||||||
| TPC (106 CFU/g) | S. aureus (102 CFU/g) | E coli (101 MPN/g) | Salmonella. Spp. (negative) | |||
| Semarang City | NC | V | V | V | - | Good |
| Pekalongan | NC | V | V | X | - | No Good |
| Boyolali | NC | V | V | V | - | Good |
| Kudus | NC | V | V | V | - | Good |
| Wonogiri | NC | V | V | V | + | No Good |
| Kebumen | NC | V | V | V | - | Good |
| Purbalingga | NC | V | V | V | - | Good |
| Brebes | NC | V | V | V | - | Good |
| Sukoharjo | NC | V | V | V | - | Good |
| Kendal | NC | V | V | X | - | No Good |
| Klaten | NC | V | V | V | - | Good |
| Demak | NC | V | V | X | - | No Good |
| Blora | NC | V | V | V | - | Good |
| Rembang | NC | V | V | V | - | Good |
| Karanganyar | NC | V | V | V | - | Good |
| Semarang | Level 3 | V | V | V | - | Good |
| Temanggung | NC | V | V | V | - | Good |
| Sragen | NC | V | V | X | - | No Good |
| Cilacap | Level 3 | V | V | V | - | Good |
| Jepara | NC | V | V | V | - | Good |
| Banyumas | Level 3 | V | V | X | - | No Good |
| Grobogan | Level 3 | V | V | V | - | Good |
| Magelang | Level 3 | V | V | V | - | Good |
| Salatiga | Level 3 | V | V | V | - | Good |
| Surakarta | Level 3 | V | V | V | - | Good |
| Batang | NC | V | V | V | - | Good |
| Pekalongan City | NC | V | V | X | - | No Good |
| Pemalang | NC | V | V | V | - | Good |
| Tegal | NC | V | V | V | - | Good |
| Magelang City | NC | V | V | V | - | Good |
| Wonosobo | NC | V | V | X | - | No Good |
| Banjarnegara | NC | V | V | V | - | Good |
| Purworejo | NC | V | V | X | - | No Good |
V=under maximum limit SNI,X=upper maximum limit SNI,-= negative Salmonella spp.,+ = positive Salmonella spp.,TPC=Total plate count, Good: if the level of microbial contamination is under the maximum contamination limit according to SNI standard (comply SNI), No Good: if the level of contamination exceeds/upper the maximum contamination limit according to SNI standard (not comply SNI).
Table-2 shows the relationship between the NKV criteria and microbial contamination. Cross-tabulation showed that among the RPH-R samples that were level 3 NKV certified, 85.71% met the maximum SNI limits, while 14.3% exceeded it. Furthermore, among the samples with an NC certification, 69.23% satisfied the SNI limits, while 30.77% did not. A Chi-square value of 0.15 and an odds ratio of 2.67 with a low level of significance (p = 0.7 and p = 0.39) indicate no correlation between NKV criteria and the level of microbiological contamination. The varying microbial contamination levels of fresh meat are influenced by various factors, such as maintenance, transport, cutting and packaging, and the hygiene and processing conditions in slaughterhouses [33].
Table-2.
Cross tabulation of NKV criteria and microbial contamination status.
| No good microbial contamination | Good microbial contamination | Total | |
|---|---|---|---|
| No criteria | 8 | 18 | 26 |
| NKV Level 3 | 1 | 6 | 7 |
| 9 | 24 | 33 |
Chi square : 0.15 (p=0.7) Odd Ratio: 48/18=2.67; P=0.39
To evaluate the findings presented above, sanitation implementation needs to be considered. Sanitation, hygiene, and implementation of good manufacturing practices aim to reduce the number of microbes to a safe and acceptable level [34]. Cleaning and disinfection programs contribute to good environmental conditions and they must be validated based on the regulations of each country [35]. The objectives of cleaning and sanitizing surfaces in contact with food are to remove the food (nutrients) that bacteria can grow on and to kill the bacteria that are already present. Cleaning/sanitizing procedures must be evaluated for their adequacy. Adherence to prescribed written procedures (inspection, swab testing, and direct observation of personnel) should be continuously monitored and records maintained to evaluate long-term compliance [36].
The slaughter and handling of meat at room temperature affects the rate of bacterial growth in all slaughterhouses. Besides cleanliness, keeping meat products cold is the second most important requirement for achieving the desired shelf life. Microorganisms multiply rapidly at high temperatures, and the development of mucus is a visual sign of microbial growth [8]. Temperature and gas composition are the main extrinsic factors that affect microbial growth [37]. Temperatures between 4.4°C and 60°C constitute the danger zone. When potentially harmful foods are left at temperatures in this range, the bacteria in and on those foods will grow rapidly [38]. Thus, the level of contamination can be reduced again if a lower temperature is applied.
Another problem in all slaughterhouses is the nonseparation between “dirty” and “clean” areas, which can lead to cross-contamination of meat products with animal manure, due to workers handling dirty areas, or with products that are unfit for consumption. Rahkio and Korkeala [39] reported associations between the microbiological contamination of air and carcasses and the movements of workers. The layout of the slaughtering line was shown to be important in decreasing airborne contamination. The separation of the clean and unclean parts of the line and that of the weighing area from other clean parts of the line decreases the contamination level. Adjusted air flow is expected to reduce carcass contamination.
This study showed that the extent of microbial contamination of the meat, in terms of the TPC and Staphylococcus count, still met the requirements, but contamination levels are likely to rise during transportation to consumers. Notably, the conditions of the buying and selling facilities in Central Java are inadequate, which is in accordance with Aminullah et al. [40], who stated that, in traditional markets where beef is sold, a temperature above room temperature, crowded populations, and the lack of water to clean equipment, all affect the number of microorganisms on the meat. Thus, when the meat reaches consumers in optimal conditions, rapid bacterial growth occurs. Therefore, the status of microbes in meat to meet consumer requirements is supported by adequate treatments that can inhibit microbial growth. The factors that affect bacterial growth are divided into two groups: Intrinsic and extrinsic. Intrinsic factors include the nutritional value of meat, water content, pH, oxidation–reduction potential, and the absence of obstruction or inhibitory substances. Extrinsic factors comprise temperature, relative humidity, absence of oxygen, and form or condition [41]. The extent of meat damage depends on the initial number of microbes–meat will spoil faster if it has a high number of initial microbes. Therefore, good hygiene during meat processing in slaughterhouses is essential to determine the quality of the final product [38, 42]. The type and number of bacteria depend on early meat contamination and specific storage conditions, which can affect the development of different decay-related microbial populations; thus, directing the type and speed of the decay [43]. Bacterial contamination during the slaughter process is a safety issue that must be considered because it affects saving time in meat production [44].
Thus, the main goal of meat processing is good meat handling, which can suppress bacterial growth factors and guarantee that the meat reaches the consumer in a good condition. Veterinary Control Number certification guides the good handling of meat and guarantees that sanitation hygiene is being implemented.
Conclusion
We found that only 7/33 (21.21%) selected for this study satisfied the NKV criteria. The contamination level was generally low, but seven slaughterhouses offered meat with an average level of E. coli contamination above the maximum limit. In contrast, meat from one slaughterhouse tested positive for Salmonella spp. Rumah potong hewan ruminansia that met the NKV criteria showed, produced more meat with a permissible level of microbial contamination than the RPH-R that did not meet the NKV criteria. Therefore, NKV certification, testifying the application of sanitation hygiene, must be implemented in all slaughterhouses.
Authors’ Contributions
WSN: Conceptualized and designed the study, interpretation and discussed the result, supervised the research process, and revised the manuscript. ED: Sampling coordinator, collected the data and data analysis, and drafted the manuscript. HH: Collected and tested the samples and revised the manuscript. AS: Collected and tested the samples, and revised the manuscript. PR: Collected and tested the samples and revised the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
The authors are thankful to Gadjah Mada University, Indonesia, for funding this research through Rekognisi Tugas Akhir grant 2021 (Contract No. 3143/UN1.P.III/DIT-LIT/PT/2021).
Footnotes
The authors are thankful to Gadjah Mada University, Indonesia, for funding this research through Rekognisi Tugas Akhir grant 2021 (Contract No. 3143/UN1.P.III/DIT-LIT/PT/2021).
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Zerabruk K, Retta N, Muleta D, Tefera A.T. Assessment of microbiological safety and quality of minced meat and meat contact surfaces in selected butcher shops of Addis Ababa, Ethiopia. J. Food Q(2019) 2019:3902690. [Google Scholar]
- 2.Ahmad R.S, Imran A, Hussain M.B, Arshad M.S. Nutritional Composition of Meat. Meat Science and Nutrition. InTech, Vienna. 2018 [Google Scholar]
- 3.Mouafo H.T, Baomog A, Adjele J.J, Sokamte A.T, Mbawala A, Ndjouenkeu R. Microbial profile of fresh beef sold in the markets of Ngaoundéré, Cameroon, and antiadhesive activity of a biosurfactant against selected bacterial pathogens. J. Food Q. 2020;2020:5989428. [Google Scholar]
- 4.Hanum G.A, Kurniawati A, Normaliska R. AQ-11 Analysis Total Plate Count (TPC) Escherichia coli and Salmonella spp. on Frozen Beef Imported through Tanjung Priok Port Hemera Zoa. Proceeding of the 20th Fava Congress and The 15th KIVNAS PDHI. 2018:376–378. [Google Scholar]
- 5.Odonkor S.T, Ampofo J.K. Escherichia coli as an indicator of bacteriological quality of water:An overview. Microbiol. Res. 2013;4(1):5–11. [Google Scholar]
- 6.Kadariya J, Smith T.C, Thapaliya D. Staphylococcus aureus and staphylococcal foodborne disease:An ongoing challenge in public health. Biomed. Res. Int. 2014;2014:827965. doi: 10.1155/2014/827965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Switaj T.L, Winter K.J, Christensen S.R. Diagnosis and management of foodborne illness. Am. Fam. Physician. 2015;92(5):358–365. [PubMed] [Google Scholar]
- 8.FAO (1991) Guidelines for Slaughtering. Meat Cutting and Further Processing. Food and Agriculture Organization of the United Nations Rome. Available from: https://www.fao.org/3/t0279e/T0279E00.htm#TOC . Retrived on 20-05-2021.
- 9.Musa M, Khalid K, Anuar J. Assessment of food temperature and food holding time among cafeterias in UiTM Dungun Terengganu. J. Appl. Environ. Biol. Sci. 2015;5((6S)):21–27. [Google Scholar]
- 10.Lobwaer A, Pahlevi C, Dewi A.R.S. Strategy of ruminansia animal household management (RPH-R) Mopah in improving real regional revenue (PAD) Regency of Merauke. Hasanuddin J. Appl. Bus. Entrepreneurship. 2019;2(2):62–70. [Google Scholar]
- 11.Ministry of Agriculture Republic Indonesia. Regulation of the Minister of Agriculture of the Republic Indonesia No.11 of 2020 about Veterinary Control Number for Animal Production Unit Ministry of Agriculture Republic Indonesia, Indonesia. 2020 [Google Scholar]
- 12.Directorate General of Livestock and Animal Health Resources. Livestock and animal Health statistics Department of Agriculture, Republic of Indonesia, Jakarta. 2021. Available from: http://ditjenpkh.pertanian.go.id/userfiles/file/Buku_Statistik_2021.pdf?time=1633686831406 . Retrieved on 19-02-2022.
- 13.Department of Animal Husbandry and Animal Health of Central Java Province. Livestock statistics of Central Java Province Department of Animal Husbandry and Animal Health, Central Java Province. 2021 [Google Scholar]
- 14.The National Standardization Agency of Indonesia. Indonesian National Standard Number 2987:2008 About the Microbial Contamination Test on Meat, Eggs, and Milk and Processed Product. Indonesia, Jakarta, Indonesia: The National Standardization Agency of; 2008. [Google Scholar]
- 15.Kuntoro B, Maheswari R.R.A, Nuraini H. Mutu fisik dan mikrobiologi daging sapi asal rumah potong hewan (RPH) Kota Pekanbaru. J. Peternakan. 2013;10(1):1–8. [Google Scholar]
- 16.Lestariningsih L, Nada M.S, Yasin M.Y, Ropida S, Abidin M.K. Peranan nomor kontrol veteriner terhadap jaminan mutu keamanan produk hasil peternakan. Briliant J. Riset Konseptual. 2020;5(1):180–188. [Google Scholar]
- 17.Directorate General.of Livestock and Animal Health Resources. Kementan Keluarkan Peraturan Soal NKV. Ministry of Agriculture, Republic of Indonesia, Jakarta. 2020. Available from: https://www.ditjenpkh.pertanian.go.id/kementan-keluarkan-peraturan-soal-nkv . Retrived on 20-05-2021.
- 18.Soepranianondo K, Wardhana D.K. Budiarto Diyantoro. Analysis of bacterial contamination and antibiotic residue of beef meat from city slaughterhouses in East Java Province, Indonesia. Vet. World. 2019;12(2):243–248. doi: 10.14202/vetworld.2019.243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob J.M, Hau E.E.R, Rumlaklak Y.Y. Gambaran total plate count (TPC) pada daging sapi yang diambil di rumah potong hewan (RPH) Kota Kupang. J. Politanku. 2018;23(1):483–487. [Google Scholar]
- 20.Mufidah N, Kalsum U, Ali U. Studi manajemen penanganan sapi antemortem dan postmortem serta kelayakan daging sapi konsumsi di beberapa rumah potong hewan (RPH) Kabupaten Probolinggo. JIPTP. 2021;2(1):23–32. [Google Scholar]
- 21.Suardana I.W, Widiasih D.A, Nugroho W.S, Wibowo M.H, Suyasa I.N. Frequency and risk-factors analysis of Escherichia coli O157:H7 in Bali-cattle. Acta Trop. 2017;172:223–228. doi: 10.1016/j.actatropica.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Zelalem A, Abegaz K, Kebede A, Terefe Y, Vipham J.L. Investigation on Salmonella enterica, Escherichia coli, and coliforms in beef from Ethiopian abattoirs:A potential risk of meat safety. Food Sci. Nutr. 2022;10(6):1714–1724. doi: 10.1002/fsn3.2752. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Chong E.S, Bidin Z, Bakar N, Zulfakar S.S. Bacterial contamination on beef carcass at selected abattoirs located in Selangor, Malaysia. Malays. Appl. Biol. 2017;46(1):37–43. [Google Scholar]
- 24.The National Standarization Agency of Indonesia (Badan Standarisasi Nasional-BSN) Indonesian National Standard Number 3932:2008 About Beef Carcass Quality. Indonesia, Jakarta, Indonesia: The National Standardization Agency of; 2008. [Google Scholar]
- 25.The National.Standardization Agency of Indonesia (Badan Standarisasi Nasional-BSN) SNI 7388:2009. Jakarta, Indonesia: The National Standardization Agency of Indonesia (Badan Standarisasi Nasional-BSN); 2009. Maximum Limit of Microbial Contamination in Food. [Google Scholar]
- 26.Bersisa A, Tulu D, Negera C. Investigation of bacteriological quality of meat from abattoir and butcher shops in Bishoftu, Central Ethiopia. Int. J. Microbiol. 2019;2019:6416803. doi: 10.1155/2019/6416803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fauzi F, Arshad M.M, Ruhil H.H, Al-Sutan I.I. Prevalence of Pathogenic Food-borne Bacteria from Beef in Retail Stalls in Kelantan. In:Proceedings of International Seminar on Livestock Production and Veterinary Technology. 2016 [Google Scholar]
- 28.Shawish R.R, Al-Humam N.A. Contamination of beef products with staphylococcal classical enterotoxins in Egypt and Saudi Arabia. GMS Hyg. Infect. Control. 2016;2016(11):1–8. doi: 10.3205/dgkh000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pal M, Ketchakmadze D, Durglishvili N, Ketchakmadze K. Staphylococcus aureus:A major pathogen of food poisoning:A rare research report. Nutr. Food Process. 2022;5(1):1–3. [Google Scholar]
- 30.Hur J, Jawale C, Lee J.H. Antimicrobial resistance of Salmonella isolated from food animals:A review. Food Res. Int. 2012;45(2):819–830. [Google Scholar]
- 31.Indriyani D.P, Tyasningsih W, Praja R.N. Isolasi dan identifikasi salmonella pada daging sapi di Rumah Potong Hewan Banyuwangi. J. Medik Vet. 2019;2(2):83–88. [Google Scholar]
- 32.Rustam R, Tamal M.A, Ariansyah J. Existence of Salmonella sppin Beef Meat in Sangatta, Kutai Timur District. Hasanuddin J. Anim. Sci. (HAJAS) 2019;1(2):10–14. [Google Scholar]
- 33.Biswas A.K, Kondaiah N, Anjaneyulu A.S.R, Mandal P.K. Causes, concerns, consequences and control of microbial contaminants in meat-a review. Int. J. Meat Sci. 2011;1(1):27–35. [Google Scholar]
- 34.Abebe E, Gugsa G, Ahmed M. Review on major foodborne zoonotic bacterial pathogens. J. Trop. Med. 2020;2020(3):1–19. doi: 10.1155/2020/4674235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ageria D.A, Libonatti C, Civit D. Cleaning and disinfection programmes in food establishments:A literature review on verification procedures. J. Appl. Microbiol. 2020;131(1):23–35. doi: 10.1111/jam.14962. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt R.H. Basic Elements of Equipment Cleaning and Sanitizing in Food Processing and Handling Operations. University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS. 1997. Available from: https://www.edis.ifas.ufl.edu/pdf/FS/FS07700.pdf . Retrived on 25-02-2021.
- 37.Montville T.J, Matthews K.R, Doyle M.P, Beuchat L.R, Montville T.J. 4th ed. Washington DC: ASM Press; 2012. Physiology, Growth, and Inhibition of Microbes in Foods Food Microbiology Fundamentals and Frontiers; p. 3.p. 18. [Google Scholar]
- 38.Godwin S.L, Chen F.C, Stone R. Avoiding the Food “Danger Zone”when it is Hot Outside. Extension Publications. 2012;20 Available from: https://digitalscholarship.tnstate.edu/extension/20 . Retrived on 25-02-2021. [Google Scholar]
- 39.Rahkio T.M, Korkeala H.J. Airborne bacteria and carcass contamination in slaughterhouses. J. Food Protection. 1997;60(1):38–42. doi: 10.4315/0362-028x-60.1.38. [DOI] [PubMed] [Google Scholar]
- 40.Aminullah Z, Lokapirnasari W.P, Harijani N, Effendi M.H, Tyasningsih W. Total plate count of beef meat at traditional markets in south of Surabaya, Indonesia. Ecol. Environ. Conserv. 2020;26((November Suppl. Issue)):S291, S294. [Google Scholar]
- 41.Soeparno. Ilmu Dan Teknologi Daging. Yogyakarta: Edisi kedua. Gadjah Mada University Press; 2015. [Google Scholar]
- 42.Diyantoro Wardhana D.K. Risk factors for bacterial contamination of bovine meat during slaughter in ten Indonesian abattoirs. Vet. Med. Int. 2019;2019(3):1–6. doi: 10.1155/2019/2707064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doulgeraki A.I, Ercolini D, Villani F, Nychas G.J.E. Spoilage microbiota associated with the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012;157(2):130–141. doi: 10.1016/j.ijfoodmicro.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Bakhtiary F, Sayevand H.R, Remely M, Hippe B, Hosseini H, Haslberger A.G. Evaluation of bacterial contamination sources in meat production line. J. Food Q. 2016;39(6):750–756. [Google Scholar]


