Abstract
Background and Aim:
Globally, toxoplasmosis is an important zoonotic parasite infection of many warm-blooded animals (including humans). Toxoplasma gondii oocysts are widespread, and their contamination can be primarily attributed to the members of the Felidae family. This study aimed to estimate the prevalence and determine the dense granule antigen 6 (GRA6) genotype of T. gondii among domestic cats in the Phayao Province, Thailand.
Materials and Methods:
A total of 124 fecal samples were collected from owned cats in the Muang district, Phayao, Thailand, from January to December 2020. Fecal samples were tested for the presence of T. gondii DNA using targeted B1 gene polymerase chain reaction (PCR) amplification, and positive samples were subsequently analyzed for their T. gondii genotype through PCR-restriction fragment length polymorphism (RFLP) analysis and sequencing of the GRA6 gene.
Results:
Among the 124 samples, 46 (37.1%) were tested positive for T. gondii. Only 10 positive DNA samples were successfully amplified for the GRA6 marker. Subsequent PCR-RFLP and sequence analyses indicated that all T. gondii isolates from cats in Phayao belonged to GRA6 genotype I.
Conclusion:
Data revealed that toxoplasmosis is remarkably distributed among (studied) domestic cats in Phayao, Thailand. Moreover, the virulent GRA6 allele was found to be circulated among domestic cats in this area. However, no significant correlation was observed between infection rates and different risk factors, which indicated that pet cats of any age, gender, or breed have similar risks of being infected with T. gondii. Our results further suggested that infective oocysts of T. gondii are widely distributed and that environmental contamination with these oocysts will introduce more risks of disease transmission to humans and other animals.
Keywords: domestic cats, genotype, granule antigen 6, Toxoplasma gondii, toxoplasmosis
Introduction
In recent years, pet populations have continuously increased in Thailand and other countries [1–3]. This close contact between humans and domestic animals creates favorable conditions for the transmittance of several zoonotic infectious diseases [4]. Toxoplasmosis, caused by the tissue cyst-forming coccidian Toxoplasma gondii, is a common zoonotic parasite infection associated with pet cat ownership [5]. In addition, T. gondii is considered as an important zoonotic agent that can infect most warm-blooded animals (including humans) [6, 7]. Although toxoplasmosis is generally asymptomatic or mild, it may cause serious infections or even death in human or animal hosts, particularly immunocompromised hosts, including aged, pregnant, or young as well as those suffering from malnutrition [8, 9]. Furthermore, disease severity varies depending on the genotype of T. gondii strains [10, 11]. Genetically, the latter can be classified into three major strain types (genotype or types I, II, and III) that differ in virulence and pathogenicity [12]. Among these strain types, T. gondii genotype I is considered as the most virulent strain.
Many vertebrate animals are involved in the life cycle of T. gondii. For example, a wide range of warm-blooded animals serves as intermediate hosts, whereas cats and other felids serve as the main definitive host of this parasite. Infected domestic cats significantly contribute to the spread of Toxoplasma oocysts throughout the environment [13, 14]. Thus, these felines play an important role in maintaining the sexual reproduction phase of T. gondii life cycle by producing a high number of oocysts in their feces.
Cats could be the main source of toxoplasmosis outbreaks; thus, accurately assessing the occurrence of T. gondii oocysts excreted by domestic cats is important. Moreover, understanding the prevalence of T. gondii among cat populations in the community is essential for effective surveillance, prevention, and control of toxoplasmosis.
Although the detection of T. gondii oocysts in cat fecal matter is conventionally achieved through microscopic examination, this method does not provide information regarding the virulent potential of detected T. gondii strains. Therefore, molecular detection of parasite DNA could be used for screening and typing of T. gondii. Given the absence of epidemiological data regarding feline toxoplasmosis and genotyping in North Thailand, this study aimed to estimate the prevalence and determine the dense granule antigen 6 (GRA6) genotype of T. gondii in domestic cats in Phayao Province.
Materials and Methods
Ethical approval
The study was approved by Animal Ethics Committee, University of Phayao (Approval number: 63 02 04 002).
Study period and location
This study was conducted from January to December 2020. Fecal samples were obtained from cat litter boxes of 124 domestic cats reared in Muang district, Phayao Province (19°11′31″N 99°52′43″E), Thailand. All laboratory examinations were performed at the School of Medical Sciences and the Scientific Instrument and Product Standard Quality Inspection Center, University of Phayao, Phayao, Thailand.
Study design and population
This work was a cross-sectional descriptive study. Only apparently healthy cats were randomly selected for this study. All cats were considered as owned cats. Basic information regarding age (months), sex (male/female), and breed (local/exotic/crossbred) were obtained from their owners. Cats missing any data were excluded from the study.
Fecal DNA extraction
Toxoplasma gondii oocysts were separated from fecal samples (2 g) in accordance with the flotation method of ZnSO4 solution [15]. Flotation materials were harvested and washed 3 times with phosphate-buffered saline, after which DNA was extracted using the QIAamp Stool Mini Kit (following the manufacturer’s protocols). DNA samples were stored at −20°C for subsequent molecular detection and genotyping.
Polymerase chain reaction (PCR) detection of T. gondii
The presence of T. gondii DNA was detected by targeting 115 bp of T. gondii B1 gene using T. gondii species-specific primers (B22: 5′-AACGGGCGAGTAGCACCTGAGGAGA-3′ and B23: 5′-TGGGTCTACGTCGATGGCATGACAAC-3′) [16, 17]. The PCR reaction volume (25 mL) included 5 pmol of each primer and 1 mL of cat fecal DNA. The PCR amplification was performed as follows: 94°C for 5 min, followed by 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min, and a final extension at 72°C for 5 min. DNA of T. gondii was included as a positive control, whereas DNA of related protozoa (i.e., Neospora caninum, Giardia lamblia, and Sarcocystis spp.) and distilled water were included as negative controls. The PCR products were separated and viewed using 2% agarose gel electrophoresis (Nancy-520 pre-stained).
Granule antigen 6 (GRA6) genotyping of T. gondii
All B1 PCR-positive samples were detected by GRA6-nested PCR. A partial fragment of the GRA6 gene was amplified using two specific primer sets: GRA6F1 (5′-ATTTGTGTTTCCGAGCAGGT-3′) and GRA6R1 (5′-GCACCTTCGCTTGTGGTT-3′) for the first PCR and GRA6F2 (5′-TTTCCGAGCAGGTGACCT-3′) and GRA6R2 (5′-TCGCCGAAGAGTTGACATAG-3′) for nested PCR [18].
Each PCR reaction (25 μL) included GoTaq Flexi DNA Polymerase (Promega, Madison, WI, USA), 5 pmol of each primer, and 1 μL of DNA sample. The first PCR round was conducted under the following conditions: 94°C pre-denaturation for 5 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by a final extension at 72°C for 5 min. The nested PCR (with nested primers) was performed using 1 μL of the first round PCR product. The same PCR setup as the first round was used, and a similar program was followed for 40 cycles, except for high-temperature annealing at 58°C. Amplified PCR fragments were analyzed using 1.5% agarose gel electrophoresis.
A total of 344 bp GRA6 PCR products were excised from agarose gel, purified, and subsequently digested in a 20 μL reaction mixture using the restriction enzyme MseI, which were incubated at 65°C for 16 h. The PCR-restriction fragment length polymorphism (RFLP) patterns were visualized using 2% agarose gel electrophoresis. In addition, the PCR product was cloned into a pGEMT vector before DNA sequencing was conducted by Macrogen, Korea.
Sequence analysis
DNA sequences of GRA6 PCR products were assembled and processed using BioEdit version 7.2.5 (Ibis Biosciences, Carlsbad, CA, USA) [19]. Online sequence similarity searches were conducted using blastn (http://blast.ncbi.nlm.nih.gov/) and ToxoDB (https://toxodb.org/toxo/app/workspace/blast/new). In addition, GRA6 sequences were analyzed (in silico) for MseI restriction enzyme digestion using NEBcutter V2.0 (New England Biolabs Inc., USA) at http://nc2.neb.com/NEBcutter2/[20]. T. gondii GRA6 nucleotide sequences obtained in the present study were deposited into the GenBank database (accession number: ON665006-ON665015). Moreover, the phylogenetic tree was built using the maximum-likelihood method in the IQ-tree web server at http://iqtree.cibiv.univie.ac.at/ [21]. The GRA14 sequence of N. caninum [KM241868.1] was included as an outgroup. The final tree was visualized by FigTree 1.4.4 (The University of Edinburgh, Edinburgh, Scotland).
Statistical analysis
The prevalence of T. gondii infection was determined as the proportion of infected cats to the total number of examined cats. A Chi-square test was performed to assess the correlation between the prevalence of infection and variable risk factors. Differences were considered to be statistically significant when p ≤ 0.05.
Results
Domestic cats studied
Of the 124 cats examined, 62 were female and 62 were male. Cats were aged between 2 months and 4 years (with an average age of 11.8 months). They were further allocated into two age groups: <1 year (83) and ≥1 year (41). Based on cat breeds, there were 45 local (Thais), 42 exotic (Persians, Scottish Fold, American Shorthairs, and Himalayan), and 37 crossbred.
Prevalence of T. gondii
DNA examination indicated a high prevalence of T. gondii infection among the 124 cat fecal samples. A 115 bp PCR product was amplified for 46 fecal samples (Figure-1). Positive samples were randomly selected to confirm the presence of T. gondii through B1 PCR product sequencing.
Figure-1.

Representative B1 polymerase chain reaction products indicate the detection of Toxoplasma gondii from domestic cats in Phayao, Thailand. Lane M: 100 bp DNA ladder; lane Tg: Positive control (reference strain T. gondii); lanes 1, 3–4, and 6: Positive T. gondii samples; lanes 2 and 5: Negative samples; lane N: Negative control (Neospora caninum DNA).
The overall rate of T. gondii infection was 37.1% (46/124). In addition, the prevalence of toxoplasmosis was slightly higher (41.0%) in young cats aged under 1 year than in older cats (29.3%) aged over 1 year. Up to 43.5% of female cats were found to be positive for T. gondii infection, whereas the latter only reached 30.7% in male cats. Regarding cat breeds, a higher percentage of infection was recorded for local (44.4%) followed by crossbred (35.1%) and exotics (31.0%). Regarding T. gondii infection, no significant association could be found among age groups, genders, or cat breeds. The prevalence of T. gondii infection based on each parameter is summarized in Table-1.
Table-1.
Prevalence of Toxoplasma gondii infection in domestic cats from Muang District, Phayao, Thailand.
| Parameters | No. of examined | No. of positive | No. of negative | Prevalence (%) | Chi-square | p-value |
|---|---|---|---|---|---|---|
| Age | 1.609 | 0.205 | ||||
| < 1 year | 83 | 34 | 49 | 41.0 | ||
| ≥ 1 year | 41 | 12 | 29 | 29.3 | ||
| Sex | 0.137 | 2.212 | ||||
| Male | 62 | 19 | 43 | 30.7 | ||
| Female | 62 | 27 | 35 | 43.5 | ||
| Breed | 1.782 | 0.41 | ||||
| Local | 45 | 20 | 25 | 44.4 | ||
| Exotic | 42 | 13 | 29 | 31.0 | ||
| Crossbred | 37 | 13 | 24 | 35.1 | ||
| Total | 124 | 46 | 78 | 37.1 |
Genotyping of T. gondii
All PCR-positive samples (n = 46) were subsequently analyzed using GRA6 genotyping. Amplified fragments with an expected size of 344 bp were obtained through nested PCR amplification. Only 10 samples successfully generated GRA6-RFLP profiles. All samples exhibited a type-I genotype-specific pattern displaying two bands of 258 and 86 bp (Figure-2). No unusual or atypical genotypes were detected in the GRA6 locus.
Figure-2.

Polymerase chain reaction (PCR)-restriction fragment length polymorphism patterns for granule antigen 6 (GRA6) Toxoplasma gondii PCR products from domestic cats in Phayao, Thailand. Lane M: DNA markers; lanes 1–4: Undigested PCR product of GRA6 positive samples; lanes 5–8: MseI-digested GRA6 with a type I T. gondii genotype.
Granule antigen 6 sequence analyses
Sequence analysis of GRA6-positive samples (with 98.55–100% sequence homology) was performed on a 344 bp amplicon. For all isolates, the obtained T. gondii sequences showed great similarity to 344 bp GRA6 gene region sequences of T. gondii, which were available on GenBank and ToxoDB databases. Among the 10 GRA6-positive samples, three sequences were identical. Such sequences exhibited the highest similarity (with 100% homology) to that of T. gondii RH-88. Sequence and blast analyses of other positive samples further revealed 99.13–99.71% homology to T. gondii RH-88 (genotype I) compared with the 98.26–99.13% homology to T. gondii ME49 (genotype II) and T. gondii VEG (genotype III).
Analysis of MseI sequence digestion revealed one MseI recognition site (TTAA) at nucleotide position 258/259 within the 344 bp T. gondii GRA6 sequence, which resulted in two fragments (258 and 86 bp). The MseI-RFLP profile for T. gondii GRA6 sequences was consistent with the results obtained in the PCR-RFLP assay.
Phylogenetic analysis was performed to assess the relationship between new T. gondii isolates and each clonal lineage based on their representatives (Figure-3). The new T. gondii isolates from Phayao cats clustered within T. gondii genotype I. Thus, phylogenetic analysis confirmed the findings of RFLP and blastn analyses.
Figure-3.
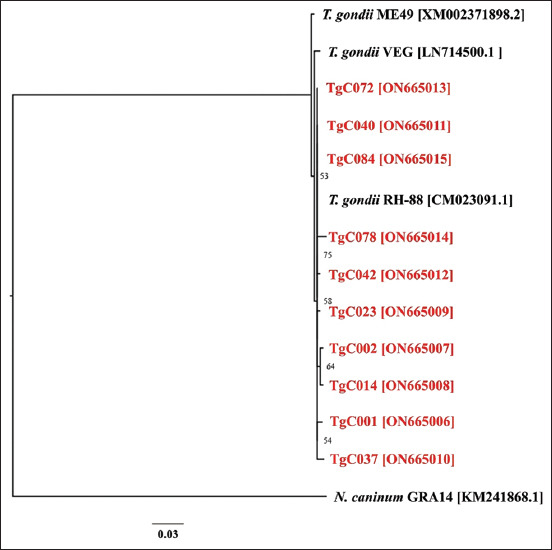
Phylogenetic relationships between 10 new Toxoplasma gondii isolates from domestic cats in Phayao, Thailand, and T. gondii type I-III reference strains based on their granule antigen 6 sequences. The phylogenetic tree was generated with 1000 bootstrap replicates using PhyML. Only bootstrap values of 50% or more are indicated at the nodes. Sequences from the present study are labeled in red letters.
Discussion
The present study revealed a high prevalence of oocyst shedding in cats, in which 37.1% (46/124) of Phayao cats have Toxoplasma DNA in their feces, which might be T. gondii DNA stemming from oocysts. This prevalence was higher than that previously reported in Thailand (4.7%) [22] and neighboring countries such as Malaysia (8.5%) [23] and Indonesia (33.3%) [24]. By contrast, Norhamizah et al. [25] recorded a high incidence of toxoplasmosis; 40.98% of T. gondii oocyst was found in cat feces in Malaysia using modified Kato–Katz and Sheather’s sugar methods. Toxoplasma oocysts were identified by morphological characteristics, but the researchers did not include the molecular identification of T. gondii.
Microscopic examination of fecal oocysts is a low-cost and rapid method for direct examination. It also has limited sensitivity. This method also requires laboratory experience in identifying parasite oocysts as T. gondii oocysts are similar, which are often misdiagnosed with another coccidian [26]. Thus, the prevalence of toxoplasmosis among regions may vary because of geographical variations and selected specimens and methods for parasite detection [27]. In the present study, oocysts in cat feces were initially separated from fecal materials based on the density through flotation before conducting PCR detection. These processes could increase the potential of detecting T. gondii DNA.
In general, T. gondii oocysts are not frequently found during routine stool examinations with only 1% of infected cats reportedly excreting oocysts in a given period [14, 28]. Based on our findings, the high rate of T. gondii-infected cats in this area could contribute to oocyst burden in the environment, although infected cats only excrete oocysts early on after infection [27, 29]. Nevertheless, several oocysts can still be produced daily for 3 weeks after infection [29, 30], and they can survive for several months or even years [31]. These contaminated oocysts can be infectious to humans and other animals, which may cause public health problems [3, 32–34]. Moreover, infected felids in a high Toxoplasma prevalence area can be exposed to oocysts and may be reinfected, resulting in the re-excretion of more oocysts.
In the present study of T. gondii oocyst shedding among cats, the lack of statistical significance regarding age groups, breeds, and gender indicated that these factors were insignificant factors for T. gondii infections in cats. In addition, the role of age in the epidemiology of feline toxoplasmosis among cats in many regions has been determined, showing that the seroprevalence of T. gondii increased with age [35–37]. However, other studies have shown that age had no significant effect on the prevalence of T. gondii oocyst excretion in cats [38, 39]. Thus, the findings of the present study indicated that cats of any age can excrete T. gondii oocysts.
However, the effects of domestic feline breeds or gender on T. gondii oocyst excretion are unclear [40]. Although the current results revealed a higher proportion of oocyst shedding among locally bred cats (44.4%) over exotic (31.0%) or crossbred cats (35.5%), this difference was not significant (p > 0.05). Nevertheless, this result was consistent with the findings of the previous studies [38]. With regard to gender affecting the prevalence of T. gondii oocyst shedding, most previous reports suggested no effect, which was consistent with the current findings [39]. By contrast, Nabi et al. [38] reported a high proportion of infection among male cats.
Several genetic markers (e.g., rhoptry proteins [ROP5,16,18], surface antigen genes [SAG2,3], and dense granule antigens [GRA1-15]) have been utilized for T. gondii strain typing [41]. Among these markers, GRA genes are generally used for genetic characterization and typing of T. gondii isolated from humans, animals, and meat products [42–44]. The GRA6 gene is a polymorphic single-copy gene that may be involved in the pathogenicity and antigenicity of T. gondii [45]. The present study revealed the circulation of the type-I GRA6 allele in fecal samples of domestic cats in Phayao, Thailand. This result was consistent with the detection of type-I T. gondii GRA6 in domestic cats in South Thailand [46] and Okinawa, Japan [47]. Other studies have considered type-II T. gondii as the predominant genotype for feline definitive hosts in China [44] and stray cats in Iran [48]. Furthermore, isolates of T. gondii types I and II have been detected via GRA6 marker PCR-RFLPs in oocyst-contaminated soil samples from North Iran [49].
Conclusion
The present study demonstrated a high occurrence of T. gondii DNA in fecal samples obtained from pet cats in Phayao, Thailand. Analyses of PCR-RFLP patterns and nucleotide sequences confirmed the presence of type-I T. gondii. These results indicated the circulation of type-I T. gondii GRA6 in this area, although such results were based on a single locus. However, data on circulating T. gondii genotypes from different hosts in Thailand are limited; thus, further investigations are necessary. In particular, epidemiological data of other animal species related to the life cycle of T. gondii and analyses of population genetics were required to accurately identify the circulating genotype of T. gondii in Thailand and comprehensively understand the relationship of toxoplasmosis in humans and animals.
Authors’ Contributions
OJ, CS, and KP: Conceived and designed the study. OJ and CP: Collected the samples. OJ, CS, and CP: Performed the experiments. OJ: Conducted molecular work and performed sequence analyses. OJ and KP: Analyzed the data. OJ and CS: Drafted and revised the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This study was funded by the School of Medical Sciences (grant number: MS221002), Phayao, Thailand. We gratefully acknowledge the Department of Protozoology, Faculty of Tropical Medicine, Mahidol University, for providing T. gondii reference sample. We especially thank Ms. Natchaya Chaiwong for helping with specimen collection.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Puranabhandu O. Thailand's Pet Food Market TH2021-0063. Washington DC: UDOAS, Federation of American Scientists; 2021. [Google Scholar]
- 2.Chen J, Xu M, Zhou D, Song H, Wang C, Zhu X. Canine and feline parasitic zoonoses in China. Parasit. Vectors. 2012;5(1):1–8. doi: 10.1186/1756-3305-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torrey E.F, Yolken R.H. Toxoplasma oocysts as a public health problem. Trends Parasitol. 2013;29(8):380–384. doi: 10.1016/j.pt.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Pearce-Duvet J.M. The origin of human pathogens:Evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol. Rev. Camb. Philos. Soc. 2006;81(3):369–382. doi: 10.1017/S1464793106007020. [DOI] [PubMed] [Google Scholar]
- 5.Cong W, Elsheikha H.M, Zhou N, Peng P, Qin S.Y, Meng Q, Qian A. Prevalence of antibodies against Toxoplasma gondii in pets and their owners in Shandong Province, Eastern China. BMC Infect. Dis. 2018;18(1):430–430. doi: 10.1186/s12879-018-3307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009;39(8):877–882. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Tenter A.M, Heckeroth A.R, Weiss L.M. Toxoplasma gondii:From animals to humans. Int. J. Parasitol. 2000;30((12–13)):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey J.P, Jones J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38(11):1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Stull J.W, Stevenson K.B. Zoonotic disease risks for immunocompromised and other high-risk clients and staff:Promoting safe pet ownership and contact. Vet. Clin. North Am. Small Anim. Pract. 2015;45(2):377–392. doi: 10.1016/j.cvsm.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Sibley L.D, Mordue D.G, Su C, Robben P.M, Howe D.K. Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357(1417):81–88. doi: 10.1098/rstb.2001.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao J, Yolken R.H. Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiol.(Oxford, England) 2015;213(4):828–845. doi: 10.1111/apha.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe D.K, Sibley L.D. Toxoplasma gondii comprises three clonal lineages:Correlation of parasite genotype with human disease. J. Infect. Dis. 1995;172(6):1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 13.Jung B.K, Lee S.E, Lim H, Cho J, Kim D.G, Song H, Kim M.J, Shin E.H, Chai J.Y. Toxoplasma gondii B1 gene detection in feces of stray cats around Seoul, Korea and genotype analysis of two laboratory-passaged isolates. Korean J. Parasitol. 2015;53(3):259–263. doi: 10.3347/kjp.2015.53.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu S, Shapiro K, VanWormer E. Dynamics and epidemiology of Toxoplasma gondii oocyst shedding in domestic and wild felids. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14197. DOI:https://doi.org/10.1111/tbed.14197. [DOI] [PubMed] [Google Scholar]
- 15.Foreyt W.J. Veterinary Parasitology Reference Manual. New York: John Wiley and Sons; 2013. [Google Scholar]
- 16.Bretagne S, Costa J.M, Vidaud M, Tran J, Nhieu V, Fleury-Feith J. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J. Infect. Dis. 1993;168(6):1585–1588. doi: 10.1093/infdis/168.6.1585. [DOI] [PubMed] [Google Scholar]
- 17.Hohlfeld P, Daffos F, Costa J.M, Thulliez P, Forestier F, Vidaud M. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N. Engl. J. Med. 1994;331(11):695–699. doi: 10.1056/NEJM199409153311102. [DOI] [PubMed] [Google Scholar]
- 18.Khan A, Su C, German M, Storch G.A, Clifford D.B, Sibley L.D. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of Type I strains. J. Clin. Microbiol. 2005;43(12):5881–5887. doi: 10.1128/JCM.43.12.5881-5887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall T, Biosciences I, Carlsbad C.J.G. BioEdit:An important software for molecular biology. GERF Bull. Biosci. 2011;2(1):60–61. [Google Scholar]
- 20.Vincze T, Posfai J, Roberts R.J. NEBcutter:A program to cleave DNA with restriction enzymes. Nucleic Acids Res. 2003;31(13):3688–3691. doi: 10.1093/nar/gkg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trifinopoulos J, Nguyen L.T, von Haeseler A, Minh B.Q. W-IQ-TREE:A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44((W1)):W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chemoh W, Sawangjaroen N, Nissapatorn V, Sermwittayawong N. Molecular investigation on the occurrence of Toxoplasma gondii oocysts in cat feces using TOX-element and ITS-1 region targets. Vet. J. 2016;215:118–122. doi: 10.1016/j.tvjl.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Wana M.N, Moklas M.A.M, Watanabe M, Unyah N.Z, Abdullahi S.A, Alapid A.A, Nordin N, Basir R, Abd Majid R. Molecular detection and genetic diversity of Toxoplasma gondii oocysts in cat faeces from Klang Valley, Malaysia, Using B1 and REP Genes in 2018. Pathogens (Basel, Switzerland) 2020;9(7):576. doi: 10.3390/pathogens9070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanafiah M, Prastowo J, Hartati S, Aliza D, Nurcahyo R.W. Detection of Toxoplasma gondii copro-prevalence by polymerase chain reaction using repetitive 529 bp gene in feces of pet cats (Felis catus) in Yogyakarta, Indonesia. Vet. World. 2018;11(9):1338–1343. doi: 10.14202/vetworld.2018.1338-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norhamizah A, Norina L, Rashidah C, Norsharina A, Hanafi H, Bahari A.S. Detection of Toxoplasma gondii oocyst in cats using Modified Kato-Katz and Sheather's sugar methods. Malays. J. Vet. Res. 2016;7(2):85–89. [Google Scholar]
- 26.Schares G, Vrhovec M.G, Pantchev N, Herrmann D.C, Conraths F.J. Occurrence of Toxoplasma gondii and Hammondia hammondi oocysts in the faeces of cats from Germany and other European countries. Vet. Parasitol. 2008;152((1–2)):34–45. doi: 10.1016/j.vetpar.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Dubey J.P. Tachyzoite-induced life cycle of Toxoplasma gondii in cats. J Parasitol. 2002;88(4):713–717. doi: 10.1645/0022-3395(2002)088[0713:TILCOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Dubey J.P, Cerqueira-Cézar C.K, Murata F.H.A, Kwok O.C.H, Yang Y.R, Su C. All about toxoplasmosis in cats:The last decade. Vet. Parasitol. 2020;283:109145. doi: 10.1016/j.vetpar.2020.109145. [DOI] [PubMed] [Google Scholar]
- 29.Elmore S.A, Jones J.L, Conrad P.A, Patton S, Lindsay D.S, Dubey J.P. Toxoplasma gondii:Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26(4):190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Lappin M.R. Update on the diagnosis and management of Toxoplasma gondii infection in cats. Top. Companion Anim. Med. 2010;25(3):136–141. doi: 10.1053/j.tcam.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Lélu M, Villena I, Dardé M.L, Aubert D, Geers R, Dupuis E, Marnef F, Poulle M.L, Gotteland C, Dumètre A, Gilot-Fromont E. Quantitative estimation of the viability of Toxoplasma gondii oocysts in soil. Appl. Environ. Microbiol. 2012;78(15):5127–5132. doi: 10.1128/AEM.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumètre A, Le Bras C, Baffet M, Meneceur P, Dubey J.P, Derouin F, Duguet J.P, Joyeux M, Moulin L. Effects of ozone and ultraviolet radiation treatments on the infectivity of Toxoplasma gondii oocysts. Vet. Parasitol. 2008;153((3–4)):209–213. doi: 10.1016/j.vetpar.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Wainwright K.E, Miller M.A, Barr B.C, Gardner I.A, Melli A.C, Essert T, Packham A.E, Truong T, Lagunas-Solar M, Conrad P.A. Chemical inactivation of Toxoplasma gondii oocysts in water. J. Parasitol. 2007;93(4):925–931. doi: 10.1645/GE-1063R.1. [DOI] [PubMed] [Google Scholar]
- 34.Wainwright K.E, Lagunas-Solar M, Miller M.A, Barr B.C, Gardner I.A, Pina C, Melli A.C, Packham A.E, Zeng N, Truong T, Conrad P.A. Physical inactivation of Toxoplasma gondii oocysts in water. Appl. Environ. Microbiol. 2007;73(17):5663–5666. doi: 10.1128/AEM.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bawm S, Phyu A.Z, Chel H.M, Htun L.L, Nakao R, Katakura K. Seroprevalence of Toxoplasma gondii in household cats in Myanmar and molecular identification of parasites using feline faecal oocysts. Food Waterborne Parasitol. 2020;20:00094. doi: 10.1016/j.fawpar.2020.e00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salant H, Spira D.T. A cross-sectional survey of anti-Toxoplasma gondii antibodies in Jerusalem cats. Vet. Parasitol. 2004;124(3):167–177. doi: 10.1016/j.vetpar.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Opsteegh M, Haveman R, Swart A.N, Mensink-Beerepoot M.E, Hofhuis A, Langelaar M.F.M, van der Giessen J.W.B. Seroprevalence and risk factors for Toxoplasma gondii infection in domestic cats in The Netherlands. Prev. Vet. Med. 2012;104(3):317–326. doi: 10.1016/j.prevetmed.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Nabi H, Rashid M.I, Islam S, Bajwa A.A, Gul R, Shehzad W, Akbar H, Ahmad N, Durrani A.Z, Waqas M, Ashraf K. Prevalence of Toxoplasma gondii oocysts through Copro-PCR in cats at pet center (UVAS), Lahore, Pakistan. J. Pak. Med. Assoc. 2018;68(1):115–118. [PubMed] [Google Scholar]
- 39.Tan L, Rani M.A, Sharma R, Hussain S.S, Watanabe M.J.T. Prevalence of Toxoplasma gondii in pet and stray cats in Klang Valley, Malaysia. Trop. Biomed. 2020;37(3):542–550. doi: 10.47665/tb.37.3.542. [DOI] [PubMed] [Google Scholar]
- 40.Must K, Hytönen M.K, Orro T, Lohi H, Jokelainen P. Toxoplasma gondii seroprevalence varies by cat breed. PLoS One. 2017;2(9):0184659. doi: 10.1371/journal.pone.0184659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norouzi M, Tabaei S.J.S, Niyyati M, Saber V, Behniafar H. Genotyping of Toxoplasma gondii strains isolated from patients with ocular toxoplasmosis in Iran. Iran. J. Parasitol. 2016;11(3):316–324. [PMC free article] [PubMed] [Google Scholar]
- 42.Zakimi S, Kyan H, Oshiro M, Sugimoto C, Xuenan X, Fujisaki K. Genetic characterization of GRA6 genes from Toxoplasma gondii from pigs in Okinawa, Japan. J. Vet. Med. Sci. 2006;68(10):1105–1107. doi: 10.1292/jvms.68.1105. [DOI] [PubMed] [Google Scholar]
- 43.Xicoténcatl-García L, Enriquez-Flores S, Correa D. Testing new peptides from Toxoplasma gondii SAG1, GRA6, and GRA7 for serotyping:Better definition using GRA6 in mother/newborns pairs with risk of congenital transmission in Mexico. Front. Cell Infect. Microbiol. 2019;9:368. doi: 10.3389/fcimb.2019.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang Y, Chen J, Meng Y, Zou F, Hu J, Esch G.W. Occurrence and genetic characterization of GRA6 and SAG2 from Toxoplasma gondii oocysts in cat feces, Kunming, China. Southeast Asian J. Trop. Med. Public Health. 2016;47(6):1134–1142. [PubMed] [Google Scholar]
- 45.Fazaeli A, Carter P.E, Darde M.L, Pennington T.H. Molecular typing of Toxoplasma gondii strains by GRA6 gene sequence analysis. Int. J. Parasitol. 2000;30(5):637–642. doi: 10.1016/s0020-7519(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 46.Chemoh W, Sawangjaroen N, Nissapatorn V, Sermwittayawong N. Genotyping of Toxoplasma gondii isolated from cat feces in Songkhla, Southern Thailand. Vet. Parasitol. Reg. Stud. Rep. 2018;13:105–109. doi: 10.1016/j.vprsr.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Kyan H, Takara T, Taira K, Obi T. Toxoplasma gondii antibody prevalence and isolation in free-ranging cats in Okinawa, Japan. J. Vet. Med. Sci. 2021;83(8):1303–1305. doi: 10.1292/jvms.21-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khodaverdi M, Razmi G. Prevalence and genotyping of Toxoplasma gondii in stray cats in Mashhad area, Iran. BMC Vet. Res. 2019;15(1):463. doi: 10.1186/s12917-019-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haghparast-Kenari B, Sarvi S, Sharif M, Ahmadpour E, Hosseini S.A, Daryani A. Isolation and genotypic characterization of Toxoplasma gondii based on GRA6 gene from environmental soil samples in Mazandaran Province, North of Iran. Iran. J. Parasitol. 2020;15(2):158–167. [PMC free article] [PubMed] [Google Scholar]


