Abstract
Peptides are very diverse molecules that can participate in a wide variety of biological processes. In this way, peptides are attractive for doping, since these molecules can activate or trigger biological processes that can improve the sports performance of athletes. Peptide molecules are found in the official World Anti-Doping Agency lists, mainly in sections S2, S4, and S5. In most cases, these molecules have a very short half-life in the body and/or are identical to natural molecules in the body, making it difficult to analyze them as performance-enhancing drugs. This article reviews the role of peptides in doping, with special emphasis on the peptides used as reference materials, the pretreatment of samples in biological matrices, the instrumentation, and the validation of analytical methodologies for the analysis of peptides used in doping. The growing need to characterize and quantify these molecules, especially in complex biological matrices, has generated the need to search for robust strategies that allow for obtaining sensitive and conclusive results. In this sense, strategies such as solid phase peptide synthesis (SPPS), seeking to obtain specific peptides, metabolites, or isotopically labeled analogs, is a key tool for adequate quantification of different peptide molecules in biological matrices. This, together with the use of optimal methodologies for sample pretreatment (e.g., SPE or protein precipitation), and for subsequent analysis by high-resolution techniques (mainly hyphenated LC-HRMS techniques), have become the preferred instrumentation to meet the analytical challenge involved in the analysis of peptides in complex matrices.
1. Introduction
Doping in sports is an unacceptable practice that has become increasingly common among elite athletes and even those who practice sports in a noncompetitive manner.1 They use an expanding range of substances that help them improve their performance during competition in order to achieve sporting success, enjoy the economic benefits derived therefrom, or increase their muscle mass solely for aesthetic purposes, as in the case of bodybuilding.2 These substances range from structurally simple molecules to more complex ones such as peptides, proteins, and even gene therapy or physical methods like blood doping.3−5
Historically, the use of doping substances dates from ancient times. In Mesopotamia and ancient Egypt, poppy and opiate derivatives were consumed for their stimulant effects.6 Furthermore, several cultures practiced organotherapy, which consisted in eating human or animal organs to cure diseases, increase vitality, or improve performance.7 For example, the Indians and Egyptians ate testicles to cure impotence and strengthen virility, and the Aztecs ate hearts to gain courage and strength.6,8 Athletes during those times sought to improve their performance in competition through diets unusually high in protein or through the consumption of stimulant substances such as brandy, wine, hallucinogenic mushrooms, and other stimulants that were usually extracted from natural sources, which helped them to overcome fatigue and thus improve their performance.7
Doping in sports grew alongside the development of modern medicine in the 19th century, when scientific advances, especially in pharmacology, allowed the optimization of the use of naturally occurring stimulants, especially alkaloids, such as caffeine, morphine, cocaine, and strychnine.6 In sports requiring high endurance and aerobic capacity, such as cycling, swimming, or running, the use of these substances by competitors to outdo their opponents became very common.7
Because the substances used in doping can affect the health of athletes and even have fatal consequences, and to promote fair competition in sports, nowadays antidoping tests have been introduced in almost all sports. The first efforts to ban doping were made by the International Athletics Federation (FIA) by prohibiting the use of stimulant substances in 1923.7 However, it is generally considered that the fight against doping as it is known today began in 1961 at the initiative of the International Olympic Committee (IOC) when it created a Medical Commission following the death of the Danish cyclist Knud Enemark Jensen at the Olympic Games (OG) in Rome, who collapsed during the team competition and died before the eyes of the whole world. The cause of his death, although not known exactly, has been attributed to the use of a vasodilator or an amphetamine used for doping purposes.9
Today, the World Anti-Doping Agency (WADA), created on November 10, 1999, coordinates international efforts against the use of doping agents in sports. These efforts have made it more difficult for athletes to use doping substances, mainly due to the progress in analytical sciences for detecting their use, the extensive testing program both in and out of competition, and the novel implementation of the athletes’ biological passport, which is based on the personalized monitoring of doping biomarkers throughout the athletes’ career, which constitutes a new paradigm in the antidoping fight.10,11
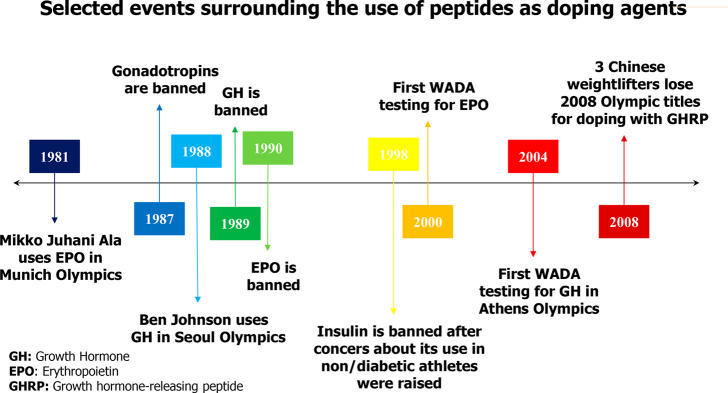
Despite all the above, the fight against doping in sports continues to be a major challenge, due to the rapid emergence of new doping substances, since the analytical methods available to detect their use must be more and more sophisticated to achieve their purpose. Such is the case with structurally complex peptide-type molecules since they behave in the same way as their endogenous analogues and have short half-life times. In addition, the lack of reference standards of these molecules makes the development of new analytical methods a major challenge.12Figure 1 summarizes the most important milestones of the history of peptides as doping agents.
Figure 1.
Timeline of selected events related to the use of peptides as doping agents.
In this review, we explore the role of peptides in doping, namely: (i) peptides used for doping purposes, (ii) peptides as reference standards, in-house standards, or biomarkers to design, develop, and validate analytical methods, (iii) pretreatment of samples containing peptides, and (iv) analytical methods used for peptide detection.
2. Peptides in Doping
Sports affect people’s physical and mental health and foster fundamental values in life in society, such as teamwork, respect for the rules of the game, solidarity, and concentration.13 Biologically active peptides have become a threat to clean, honest sports.14 This problem is worsening due to the role that the pharmaceutical industry has played in the design and development of peptide analogs that mimic natural protein hormones. In the same vein, the production and commercialization of peptides used for the diagnosis and treatment of various diseases has grown steadily since the second half of the 20th century, reaching more than 100 peptide drugs approved just for the year 2020.15
Small peptides and peptide hormones are classified in the official WADA lists into 3 sections: section S2, peptide hormones, growth factors, related substances, and mimetics; section S4, hormone and metabolic modulators, and section S5, diuretics and masking agents.16 In accordance with the data reported by the WADA Anti-Doping Testing Figures in 2020, peptide hormones were responsible for 48 (3% of total findings) adverse analytical findings (AAF), hormones and metabolic modulators for 127 AAF (8%), and diuretics for 213 AAF (14%).17 Some examples of peptides in these sections are listed in Table 1.
Table 1. WADA Official Classification of Peptides Used in Doping.
| Category and group of prohibited substances and methods | Relevant peptides and protein derivatives |
|---|---|
| S2. peptide hormones, growth factors, related substances, and mimetics | erythropoietin (EPO) and erythropoiesis-stimulating agents (ESAs) |
| chorionic gonadotropin (CG) and luteinizing hormone (LH) and their releasing factors (leuprorelin, goserelin, buserelin, deslorelin, gonadorelin, nafarelin, triptorelin, etc.) | |
| growth hormone (GH), its fragments and releasing factors (sermorelin, GHRH, GHRPs, etc.) | |
| growth factors (GFs) and growth factor modulators (IGF-1, GnRH, FGFs, HGF, MGFs, PDGF, and VEGF) | |
| S4. hormone and metabolic modulators | insulins and insulin-mimetics |
| S5. diuretics and masking agents | vasopressin and vasopressin analogues (desmopressin, felypressin, terlipressin, and lypressin) |
2.1. Peptide Hormones, Growth Factors, Related Substances, And Mimetics
The use and analysis of peptides and proteins has become a fundamental aspect of study for doping control laboratories, especially because of their ability to improve the physical performance of athletes18 and because of the complexity of detecting these molecules, which has generated the need to develop more sensitive and sophisticated analytical methods.12 For these reasons, this group of substances is prohibited by the official WADA lists, where molecules such as erythropoietin, insulin-like growth factor-I, growth hormone, and gonadotropin, among others, are singled out.
Erythropoietin (EPO) is a hormone produced naturally by the body19 that induces erythropoiesis and promotes the proliferation of oxygen-releasing erythrocytes.20 Since recombinant human erythropoietin (rHuEPO) became available in the 1980s,3 its use in sports has increased considerably, leading to its ban in sports in official listings in the early 1990s.19,20 The use of EPO and ESAs has been reported in different sports, such as the well-known cases in cycling,21 athletics,22 and more recently mixed martial arts (MMA).23
Growth hormone (GH) is a polypeptide hormone secreted by the pituitary gland.24 The use of GH has been reported since the 1980s to increase the performance of athletes,25 mainly due to its anabolic26 and lipolytic effects,27 i.e., it increases the body mass of athletes and also decreases their fatty mass.28 As has been reported, the anabolic activity of GH is mediated by the generation of insulin-like growth factor-I (IGF-I), which has established it as another important molecule in the field of doping control.29 Human chorionic gonadotropin (hCG) is a substance prohibited only in men, used to stimulate testosterone secretion, causing anabolic effects in muscle tissue by enhancing the muscular and skeletal mass.30
The difficulty for the detection of peptide hormones as doping agents is that they are triggered by the nature of the molecule administered. These peptides have a short half-life in blood, which considerably shortens the time window for their detection in body fluids. In addition, hormones such as hGH and hCG can exist as a mix of multiple isoforms or degradation fragments that differ in length, sequence, rate of clearance and excretion, and biological potency. hGH is secreted in such minute quantities in urine that the development of antidoping tests for hGH in the urine matrix is not viable, notwithstanding the use of novel analyte concentration techniques.12
Similarly, the use of GH secretagogues (GHSs) (e.g., ghrelin), growth hormone-releasing hormone (GHRH) [e.g., sermorelin or GHRH (1–29)] and GH-releasing peptides (GHRPs) is also prohibited by WADA.31 GHRPs are a group of small synthetic peptides that have been shown to exhibit the effect of releasing growth hormone in animals and humans32 and that also function as cytoprotective and cardioprotective agents.33
Human gonadotropins, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and human chorionic gonadotropin (hCG) are hormonal glycoproteins that are responsible for regulating the function of the sexual organs in humans and play a fundamental role in normal growth, sexual development, and reproduction.34,35 Currently, there are several synthetic gonadotropin-releasing hormones (GnRH) on the market, such as leuprorelin, goserelin, buserelin, deslorelin, gonadorelin, nafarelin, and triptorelin, among others. These GnRH analogues stimulate the secretion of LH and testosterone and have the possibility of exerting effects such as doping and/or masking agents, which is why they are currently prohibited for use in sports.36
2.2. Hormone and Metabolic Modulators
Hormones and metabolic modulators are among the most potent performance-enhancing drugs used illicitly in doping.37 Insulin and its analogues are increasingly used as doping agents1 and have been part of the list of prohibited substances since 1999,38 mainly due to their property of altering metabolic processes, promoting muscle growth and energy supply.38,39
Insulin is a peptide hormone secreted by the β cells of the pancreatic islets of Langerhans. Insulin exerts its function of regulating glucose homeostasis by facilitating the uptake of cellular glucose in the liver, fatty tissues, and skeletal muscles.40 Insulin has been reported to have properties that promote enhanced athletic performance through the promotion of amino acid uptake, protein anabolism, increased glucose uptake, and muscle glycogen storage.24,26 Furthermore, due to the short half-life of insulins (especially the short-acting insulins), they are often very difficult to detect or to differentiate from the athlete’s own insulin, which has made insulin a potential drug of abuse in the sport community.28
The misuse of insulins in strength and endurance sports has been previously reported in various sports, especially in bodybuilding, cycling, and weightlifting, among others.25,37,41 The main and most common side effect that is generated using insulin is hypoglycemia, which, in the case of inadequate therapeutic management or lack of professional medical assistance, can potentially lead to coma or even death among athletes.42
There are very few cases of insulin abuse reported in the literature; however, the problem of insulin abuse may be much more widespread. One study revealed that at least 10% of a physician’s 450 regular patients admitted to using insulin and that most of them obtained insulin from diabetic friends. The short-acting insulin has a half-life of about 4 min in the human body; it vanishes rapidly and is very difficult to detect. Even when detected, it is impossible to distinguish it from the athlete’s own insulin.43
2.3. Diuretics and Masking Agents
Diuretics and masking agents are compounds that are taken with the express purpose of hiding the presence of specific illegal drugs that are screened during athletic drug testing.44 The peptide that should be emphasized in this group of substances is desmopressin (N-deamino-8-d-arginine-vasopressin or dDAVP), which was first included in the WADA List of Prohibited Substances and Methods in 2011.45
Desmopressin is a synthetic analogue of vasopressin with increased antidiuretic activity and decreased pressor activity.45 It works by binding to V2 receptors and by limiting the amount of water that is eliminated in urine, increasing water reabsorption. It is indicated for the therapeutic management, prevention, and control of polyuria, polydipsia, and dehydration in patients with diabetes insipidus and in primary nocturnal enuresis.45 Desmopressin has been studied as a masking agent for blood doping in sports, where it generates a hemodilution effect, significantly decreasing the hematological values measured by the antidoping authorities to detect blood doping.45,46
Vasopressin (a nine-amino-acid peptide), also known as arginine-vasopressin (AVP) or antidiuretic hormone (ADH), is a natural hormone with potent vasoconstrictive effects.47 Vasopressin is synthesized in the hypothalamus and has the property of increasing blood pressure through vasoconstriction (due to activation of V1a receptors). Also, vasopressin activates V2, V1b, and oxytocin receptors, which explains its antidiuretic and procoagulant activity.47,48
Other diuretics relevant to the study of antidoping substances are felypressin (phenylalanine-lys-vasopressin), terlipressin (trigly-8-lys-vasopressin), and lypressin (8-lys-vasopressin), which have been included in screening studies to detect peptide hormones in human urine.49 It is important to point out that WADA states that local administration of felypressin in connection with local anesthesia during a dental treatment is not prohibited.16
3. Peptide Synthesis and Reference Materials: Obtention and Characterization
A pure reference material, or an appropriately certified solution of the pure material, is used in development of analytical methods, methodology validation, and the detection and analysis of an analyte.50,51 The reference material in routine analysis is used to prepare working calibration solutions and are crucial in the measurement process.52
A certified pure reference material will have the identity of the substance appropriately confirmed and will have a purity value with a measurement uncertainty to clearly define the mass fraction of the substance that is present. Currently, there are many reference materials used in antidoping analysis.52 Unfortunately, this is not the case for peptide-type doping agents, which constitutes a major challenge for the modern antidoping agencies.52 For this reason, it is necessary to develop peptide reference materials that have the required quality for reliable identification and quantification of peptides used in doping.
Although nowadays peptides of all types are commercially available, their quality can vary greatly, because the production, purification, and characterization methods are different for each manufacturer, which makes their traceability difficult.53 In this regard, it is necessary to ensure the quality of reference materials through the standardization of their production, purification, and characterization. This topic is another analytical challenge by itself, especially since the quantification process requires not only establishing the whole content of the peptide in the sample (using, e.g., amino acid analysis, with TLC, HPLC, GC-MS, LC–MS, CE, or RMN methods) but also the content of the counterion (using either LC or GC methodologies), water/humidity content (using Karl Fischer), and impurities tests for the evaluation of residual solvents (such as ACN, DMF, DCM through GC-HS-MS), heavy metals, and peptide-related and nonrelated impurities.
The use of internal standards (ISs) is well established and recommended for doping control assays by WADA. Especially when the sample preparation comprises various steps with the potential to deplete the target analyte, ISs offer a way to control the reliability of the results. ISs compensate for any loss of the target analyte during sample pretreatment to the exact same extent and provide clear mass spectrometric differences. These characteristics are valid for isotope-labeled ISs, with one or more amino acids being replaced by their isotopically labeled analogues (e.g., D8-Synacthen).54
For quantitative analysis, isotopically labeled analogues are used to ensure reliable results. In qualitative analyses, analogue peptides with one or more amino acid substitutions can be used as ISs. This strategy consists of the use of peptides that have some differences in their physicochemical properties from the prohibited compounds but whose specific changes do not affect the recovery percentage during the pretreatment process, which allows an adequate interpretation of the result of the analysis.54
Thomas et al. used internal standards commercially available to analyze 12 prohibited peptides. The internal standards were bovine insulin, D8-Synacthen, Des-pGlu-LH-RH, acetyl-(Tyr1, d-Arg2)-GRF (1–29), and R3-IGF-1. A mixture of these ISs was spiked with 500 pg/mL into each specimen (blood or urine) as the first sample preparation.54 Cuervo et al. used the GHRP-2 deamidated labeled in lysine (13C6 and 15N2, β-Nal = β-naphthylalanine) as an IS for the analysis of 17 substances: GHRP-1, GHRP-2, GHRP-4, GHRP-5, GHRP-6, hexarelin, anamorelin, ipamorelin, alexamorelin, LHRH, leuprolide, buserelin, triptorelin, desmopressin, lypressin, deamidated GHRP-2, and deamidated GHRP-4.55 Zvereva et al. used the synthetic peptides GHRP-1, GHRP-2, GHRP-4, GHRP-5, GHRP-6, alexamorelin, hexarelin, ipamorelin, N-acetyl-LKKTETQ (TB-500), and desmopressin to study the metabolism of in vitro models.56
Coppieters et al. used a stock standard solution containing 50 peptides, which included the synthetic peptides ibutamoren, bovine insulin, tabimorelin, LHRH, terlipressin, TB-500 and its metabolites (which were synthesized in-house), and additionally a mixture containing ISs such as [deaminoCys1, Val4, d-Arg8]-vasopressin and 13C, 15N GHRP-2 (1–3) for the analysis of these peptides in urine samples.57 Thomas et al. reported a method for identifying small peptides (<2 kDa) using five ISs (lys8-vasopressin, 2H-labeled GHRP-5, 2H-labeled GHRP-6, and 2H-labeled GHRP-4) to control the matrix effects over the chromatographic run.58 Other studies have used a variety of ISs, such as 2H3-d-Ala-D-(β-naphthyl)-Ala-Ala-OH, bovine insulin, 13C615N4-labeled T1 peptide of IGF-1, etc.59
Solid-phase peptide synthesis (SPPS) allows obtaining small peptides (less than 20 amino acids) in a relatively easy, economical, and environmentally friendly manner,60 making it a powerful tool for obtaining in-house reference materials. Furthermore, there are purification methods based on LC or reverse-phase solid-phase extraction (RP-SPE) that yield peptides with chromatographic purities greater than 95%,61 allowing them to be used as reference materials, as long as an adequate characterization via HPLC and LC–MS is performed, to confirm their identity and ensure that there are no impurities that coelute with the peptide of interest.53 Peptides GHRP-4, GHRP-5, GHRP-6, desmopressin, vasopressin, and leuprolide were synthesized via SPPS using the Fmoc/tBu strategy (SPPS-Fmoc/tBu) to get in-house reference materials. The synthesis proceeded in a good manner, obtaining peptides with high purity and yields, indicating that this methodology is viable for obtaining in-house reference standards to develop analytical techniques focused on the detection of peptides used in doping.62,63
A crucial aspect in obtaining reference standards is the unequivocal identification and characterization of each of the peptides. In this sense, the construction of databases and libraries of mass spectra has been reported to be a fundamental tool for the identification of peptides in proteomic studies,64 and in the same way, this tool could be fundamental for the study of peptides used in doping.
Although research in this field is in its early stages, important advances have been made recently, such as the work reported by Plachká et al., who built a library of 192 doping agents, reporting retention times, TWCCSN2 values, and m/z ratios.65 The database was built with molecules from different classes and related sections in the WADA listings, but agents such as anamorelin stand out as peptide molecules in the library. To the best of our knowledge, this is the first reported large library that is built exclusively for doping agents that involves parameters such as retention times and m/z ratios. Therefore, the continuous development of these libraries, and the joint work with databases already developed, will be essential to advance in the study of different peptides in doping, and thus, obtaining in-house reference materials could be key for facilitating these investigations.
4. Sample Preparation
In doping control analysis for small molecules, such as peptides, the sample pretreatment is relevant, since these molecules are susceptible to oxidation, enzymatic proteolysis, and degradation. Therefore, the storage of blood and urine samples is of vital importance for carrying out an adequate analysis. Freezing at −20 °C is adequate and avoids peptide degradation.54
The analysis of peptide hormones and small peptides in biological matrices is an area of relevance in the study of doping.66 The first step in the analysis of a sample in a doping control laboratory is to carry out a screening procedure, where an analytical methodology is used (mainly using chromatographic techniques coupled with mass spectrometry) to detect the suspected substance in a complex sample.58 Subsequently, after confirming the presence of the prohibited substance in the sample, it is necessary to carry out a confirmatory analysis (where the WADA likewise recommends the use of mass-coupled chromatographic techniques).67
The detection and quantification of peptide hormones has been shown to be a challenge for antidoping laboratories, mainly due to the heterogeneity of molecules and groups of substances that involve peptides and proteins.68 In this vein, the analytical techniques used for its detection have had to evolve toward more robust, reliable, and sensitive methods, and this evolution has also been reflected in the sample preparation processes.
Initially, GC-MS analysis required extraction and derivatization processes, as has been reported for the analysis of diuretics in doping.44 At present, the development of more robust methods based on LC–MS has become a standard analytical technique in doping control and toxicology laboratories,69 which has allowed for the analysis of multiple analytes and groups of substances in the same sample and has also expanded the possibilities for sample preparation, even allowing the detection of substances without the need for sample preparation, only using “dilute-and-shoot”-LC–MS (DS-LC–MS), which has led to its use in antidoping, among other disciplines.69−71
Doping control tests are generally carried out using urine as a biological matrix, although research work has also been reported using matrices such as blood, plasma, serum, and hair, among others.72,73 The methods used to extract peptide hormones from these biological matrices involve the same traditional methods used for the quantification of peptides and proteins, mainly solid-phase extraction (SPE), liquid–liquid extraction (LLE), protein precipitation (PP), and immunoaffinity purification (IAP), as reported in previous articles.32,74,75
SPE is the methodology most used in sample pretreatment, and it involves various separation mechanisms such as reverse, normal, ion-exchange, etc. The cartridge matrix containing the stationary phase allows analyte retention after the analyte is able to be eluted with an isocratic or gradient elution, in a way similar to HPLC chromatography. Mixed-mode SPE involves reversed-phase and ion-exchange chromatography mechanisms and is an effective way to extract peptides from plasma and urine. There are four major mixed-mode IE-RPSPE sorbents: strong cation exchange, weak cation exchange, strong anion exchange, and weak anion exchange.32 SPE is used as an enrichment methodology and/or prepurification step. In this context, Insuasty Cepeda et al. developed an RP-SPE methodology that allows for obtaining peptides with purities higher than 95%, demonstrating that this technique could be used to purify short peptides.61
Another technique used in the preparation of peptide samples in biological matrices is enzymatic digestion, which can be carried out before or after the other pretreatment methods. Enzymatic digestion involves the use of proteolytic enzymes (such as trypsin, chymotrypsin, etc.), which seeks to produce shorter peptide fragments that can facilitate the analysis and detection of peptides and proteins (peptide fingerprint).76 This method for preparing peptide samples was developed mainly for mass spectrometry-based proteomic studies;77 however, its use has been extended to the characterization of these molecules in antidoping studies. The use of enzymatic digestion is often preferred in bottom-up analysis, while for shorter peptides, top-down approaches are often used (which typically do not require protease digestion), followed by LC–MS/MS analysis.76,78Table 2 summarizes some of the different approaches and conditions of pretreatment techniques used for the analysis of peptides in doping.
Table 2. Overview of Sample Pre-Treatment in Analysis of Peptides Used in Doping.
| Method | Analyte | Pretreatment conditions |
|---|---|---|
| RP-SPE | porcine insulin, novolog, apidra, lantus DesB(30–32) metabolite, humalog and human insulin, GH-RH(1–29), LH-RH, synacthen, CJC-1295, LongR3-IGF-1, and IFG-1 | preconditioned solution: ACN (2 mL), water (2 mL); load solution: 1–10 mL of urine fortified with ISs (500 pg/mL) and 10% of ACN); rinsing solution: water; eluting solution: ACN/water (80:20, v/v)54 |
| GHRPs, desmopressin, vasopressin, felypressin, terlipressin, leuprolide, LHRH, ibutamoren, anamorelin | preconditioned solution: MeOH (1 mL), water (1 mL); load solution: 7 mL of urine, 40 μL of IS (100 ng/mL), pH 6.5–7.5, 100 μL of PBS; rinsing solution: water (1 mL), 20% ACN in water (1 mL); eluting solution: 1 mL of 95/5 (75% ACN in water/FA)55 | |
| GHRPs, desmopressin, vasopressin, and leuprolide | preconditioned solution: MeOH (12 mL, 3×), water (12 mL, 3×); load solution: peptide (50 mg/mL) in water with 0.5% TFA; rinsing solution: water with 0.5% TFA (12 mL); eluting solution: gradient of 5–50% ACN with 0.5% TFA62,63 | |
| RP-SPE/IE-SPE | GHRPs and opioid peptides | preconditioned solution: MeOH (2 mL), water (2 mL); load solution: plasma containing 10 ng mL–1 each of the peptides; rinsing solution: ACN/water; eluting solution: ACN (10–90%)/ water with 2% FA32 |
| GHRPs, anamorelin, leuprolide, LHRH, triptorelin, [deamino-Cys1-Val4-d-Arg8]-vasopressin (ISTD) | preconditioned solution: MeOH (1 mL), water (1 mL); load solution: 1 mL of urine; rinsing solution: 1 mL 5% of ammonium hydroxide and 1 mL 20% ACN; eluting solution: 0.5 mL of NH3/FA 10%/MeOH 8/12/80 v/v50 | |
| GHRPs, anamorelin, and [deamino-Cys1-Val4-d-Arg8]-vasopressin (ISTD) | preconditioned solution: MeOH (1 mL), water (1 mL); load solution: 2 mL of sample (HRPs), 40 μL IS (2 μg/mL diamino-Cys1Val4d-Arg8-vasopressin), 200 μL 0.8 M PBS, pH 7; rinsing solution: 1 mL water and 0.5 mL of MeOH; eluting solution: ACN/water with 2% FA 1:3 v/v79 | |
| microextraction RP-SPE | GHRPs, desmopressin, vasopressin, felypressin, terlipressin, leuprolide, LHRH, ibutamoren, and anamorelin | preconditioned solution: MeOH (0.2 mL), water (0.2 mL); load solution: 0,75 mL of urine, 15 μL of IS (100 ng/mL), pH 6.5–7.5, 50 μL of PBS; rinsing solution: water (0.2 mL), 20% ACN in water (0.2 mL); eluting solution: 25 μL of 95/5 (75% ACN in water/FA) (2×)55 |
| enzymatic digestion | GHRPs, TB-500 and desmopressin | incubation: 24 h at 37 °C; enzyme/protein ratio: 1:20, 1:50, and 1:100 (w/w); peptide concentration: 0.2 mg/mL; buffer solutions: 50 mM Tris-HCl, pH 7.0 (rhAPN); 50 mM MES, pH 6.0, 5 mM CaCl2 (rhCPM); 0.2 M Na2HPO4/0.2 M NaH2PO4 2/7 (v/v) (Leu-AP); 50 mm Tris-HCl, pH 7.65, 0.1 M NaCl (rhCPB and CPB); 25 mM Tris-HCl, pH 8.85, 1 mM EDTA (endopeptidase Lys C)56 |
| GHRPs, sermorelin, IGF-1, hCG | incubation: 37 °C for 16 h; enzyme/protein ratio: 1:200 (w/w); peptide concentration: 0.2 mg/mL; buffer solutions ammonium bicarbonate, 100 μL of 50 mM acetic acid, pH 8 (2.5 μg sequencing grade trypsin)80 | |
| liquid–liquid extraction | GHRPs, anamorelin, and [deamino-Cys1-Val4-d-Arg8]-vasopressin (ISTD) | sample solution: 2 mL of sample, 200 μL IS (2 μg/mL diamino- Cys1Val4d-Arg8-vasopressin), 200 μL ACN, 200 μL carbonate buffer, pH 12; extraction solution: ethyl acetate, shaking, 30 min79 |
| immunoaffinity | porcine insulin, novolog, apidra, lantus DesB(30–32) metabolite, humalog and human insulin, synacthen, LH-RH, GH-RH(1–29), CJC-1295, longR3-IGF-1 and IFG-1 | samples dissolved in PBS, pH 7.4, and mixed with primary antibodies (1–3 μg of IgG), paramagnetic beads solution (40 μL of antimouse, 40 μL of antirabbit and 20 μL of protein A suspension), incubation for 1–3 h at 22 °C54 |
5. Liquid Chromatography (LC) Analysis
The chromatographic separation of small peptides is possible with HILIC,81 cation-exchange,82 size exclusion, and supercritical fluid chromatography,83 and even comprehensive RP-HPLC–MS multianalyte detection methods have been applied on the nano, micro, and analytical scale; however, applications based on the use of nanoliquid chromatography yielded the lowest LODs, but due to longer run times and difficulties with the robust operation of such instruments, it did not gain popularity for routine small peptide analysis.
Recently reversed-phase HPLC (RP-HPLC), has become the standard platform for peptide analysis and bottom-up LC–MS proteomics, based upon the hydrophobic characters of sorbent, analyte, and organic solvent content, as well as the presence of various mobile phase additives. Analytical columns (50–150 mm) are preferred with varying particle sizes (1.7–5 μm) and column chemistry.84
Cuervo et al. found that C18 chemistry was superior to C8 columns in terms of peptide peak areas and peak shape.55 The same research group and Mazzarino et al found that the use of fused core particles was found to be superior compared to the classical particles.49 Improved chromatography was achieved for several GHRPs by applying an acetylation step, which rendered the peptides more hydrophobic and reduced their charge.85 Various mobile phase compositions were tested by different laboratories. However, an aqueous solvent that may contain formic acid (AF) (0.1–1%) and acetonitrile (ACN) fortified with formic acid (0.1–1%) became the prevailing one.
Electrospray ionization mass spectrometry (ESI-MS) has become the premier analytical platform for the MS analysis of proteins and peptides. The hallmark of ESI-MS for proteins and other large biomolecules, multiple charging, extends the effective mass range of the analyzer in direct proportion to the number of charges per ion. Modifying mobile phase compositions by adding solvents such as DMSO is trending for supercharging proteins.84 In the context of peptide ESI, the benefits of adding DMSO to improve ionization efficiency have also long been described in the field of proteomics.86 Compared to their lower-charged counterparts, more highly charged proteins and peptides dissociate more efficiently, providing higher sequence coverage in tandem MS (MS/MS). Its application for the routine measurements of small peptides was evaluated by Judák et al.87
For Görgens et al., several assays enabling the detection of peptidic drugs and drug candidates (<2 kDa) prohibited in sports have been developed for doping control purposes. While the first analytical methods were based on sophisticated and laborious sample preparation procedures, e.g., SPE, more recent publications demonstrated the fitness-for-purpose of simplified approaches, e.g., the dilute-and-inject approach. This last strategy has shown that the omission of the SPE-based sample preparation is possible with conventional one-dimensional LC systems but has also aided others to lower the LODs of already established multidimensional direct urinary injection methods.88 According to the results observed by various research groups, depending on the experimental setup applied, ionization can be enhanced, resulting in a higher signal intensity, which seems to vary among instruments.86
Atmospheric pressure chemical ionization (APCI)89 was used for the ionization of smaller peptides/AAs, however, electrospray ionization (ESI) is considered a more ideal atmospheric pressure ionization source for biomolecule analysis. Under typical ESI conditions, small peptides form singly-, doubly-, and triply charged gas-phase ions. It has been demonstrated that mobile phase additives may influence charge state distribution; in the case of DMSO, shifts toward lower charge states were observed.87,90,91 For Hahne et al., this is possibly caused by the high gas-phase basicity of DMSO;86 otherwise, Nshanian et al. found that changes in solvent composition might simply cause charges to be allocated differently when transitioning from droplets to gas-phase ions.84
6. Mass Spectrometry (MS) Analysis
For Thomas et al., the determination of peptides by means of mass spectrometry after liquid chromatographic separation is the preferred technique for complex analytical assays. Highly reproducible, specific, and sensitive results are obtained with hyphenated UHPLC separation and high-resolution mass spectrometry for small peptides and their metabolites in antidoping laboratories.92,93
Multiple reaction monitoring (MRM) is a powerful method for the sensitive detection of target analytes using low-resolution triple quadrupole (QqQ) mass spectrometry. However, for multitarget approaches dealing with hundreds of analytes, targeted MS/MS techniques are often limited by the attended cycle time of the instruments, and therefore, the identification of sensitive and selective multiple reaction monitoring (MRM) transitions is required for low concentration detection.49
The uses of high-resolution mass spectrometry (HRMS), either by time-of-flight (TOF)55 or Orbitrap instrumentation,87,88 has increased in antidoping laboratories. The fact that each molecule has its own unique exact mass allows us to elucidate chemical formulas from unknown compounds as well as confirm a known compound with great certainty.94 HRMS especially became popular in small peptideanalysis due to features such as the possibility of extracting exact mass peptide isotopes to use them as specific confirming ions,90 the use of full scan acquisition, which allows searching for initially untargeted substances by retrospective data evaluation, data-dependent analysis (DDA), which combines targeted and nontargeted analysis, and the possibility to incorporate several small peptides into comprehensive multiclass analyte methods.95,96Table 3 lists the classification, sequence and confirmatory ion for the most relevant peptides used in doping.
Table 3. WADA Classification, Sequence, and HRMS-Related Characteristics of the Main Small Peptides Used in Doping.
| Compound | WADA list | Sequence | Charge state/target ion (confirming ion) [m/z] |
|---|---|---|---|
| alarelin | S2 | pGlua-HWSYdALRP-NH(Et) | 2+/584.3065 (584.8080) |
| alexamorelin | S2 | AH-dMrpa-AWdFK-NH2 | 2+/479.7560 (480.2574) |
| buserelin | S2 | pGlua-HWSY-dStBu-LRP-NH(Et) | 2+/620.3353 (620.8367) |
| deslorelin | S2 | pGlua-HWSYdWLRP-NH(Et) | 2+/641.8276 (642.3291) |
| desmopressin | S5 | Mpaa-YFQNCPdRG-NH2 (disulfide Bridge Mpa1-Cys6) | 1+/1069.4342 (1070.4370) |
| felypressin | S5 | CFFQNCPKG-NH2 (disulfide Bridge Cys1-Cys6) | 2+/520.7257 (521.2271) |
| fertirelin | S2 | pGlua-HWSYGLRP-NH(Et) | 2+/577.2987 (577.8001) |
| GHRP-1 | S2 | AH-2Nala-AWdFK-NH2 | 2+/478.2505 (478.7520) |
| GHRP-1 (3–6)b | S2 | dNala-AWdF-OH | 1+/620.2883 (621.2913) |
| GHRP-2 | S2 | dA-dNala-AWdFK-NH2 | 2+/409.7210 (410.7240) |
| GHRP-2 (1–3)b | S2 | dA-dNala-A-OH | 1+/358.1761 (359.1792) |
| GHRP-3 | S2 | Aiba-dWdPdIR-NH2 | 1+/655.4038 (656.4067) |
| GHRP-4 | S2 | dWAWdF-NH2 | 1+/608.2980 (609.3010) |
| GHRP-5 | S2 | YdWAWdF-NH2 | 1/+771.3613 (772.3643) |
| GHRP-6 | S2 | HdWAWdFK-NH2 | 1/+437.2296 (437.7312) |
| GHRP-6 (2–5)b | S2 | dWAWdF-OH | 1/+609.2820 (610.2850) |
| goserelin | S2 | pGlua-HWSY-dStBua-LRP-AzaGlya-NH2 | 2+/635.3280 (635.8294) |
| hexarelin | S2 | H-dMrpa-AWdFK-NH2 | 2+/444.2374 (444.7388) |
| hexarelin (1–3)b | S2 | H-dMrpa-A-OH | 1+/427.2088 (428.2117) |
| histrelin | S2 | pGlua-HWSY-dHBzla-LRP-NH(Et) | 2+/662.3409 (662.8423) |
| ipamorelin | S2 | Aiba-H-dNal-dFK-NH2 | 2+/356.7001 (357.2016) |
| ipamorelin (1–4)b | S2 | Aiba-H-dNal-dF-OH | 1+/585.2820 (586.2850) |
| leuprolide | S2 | pGlua-HWSYdLLRP-NH(Et) | 2+/605.3300 (605.8314) |
| leuprolide (5–9)b | S2 | YdLLRP-NH(Et) | 2+/344.7289 (345.2303) |
| LHRH | S2 | pGlua-HWSYGLRPG-NH2 | 2+/591.7938 (592.2953) |
| LHRH (2–10)b | S2 | HWSYGLRPG-NH2 | 2+/536.2778 (536.7792) |
| nafarelin | S2 | pGlua-HWSY-dNala-LRPG-NH2 | 2+/661.8251 (662.8279) |
| nafarelin (5–10)b | S2 | Y-dNala-LRPG-NH2 | 2+/401.2239 (401.7253) |
| peforelin | S2 | pGlua-HWSHDWKPG-NH2 | 2+/630.2889 (630.7903) |
| TB500 | S2 | Ac-LKKTETQ-OH | 2+/445.2531 (445.7546) |
| triptorelin | S2 | pGlua-HWSYdWLRPG-NH2 | 2+/656.3227 (656.8241) |
Abbreviations: Aib, aminoisobutyric acid; AzaGly, azaglycine; HBzl, benzylhistidine; Mpa, mercaptopropionic acid; Mrp, methyltryptophane; Nal, 2-naphthyl-alanine; pGlu, pyroglutamic acid; StBu, tercbutylserine.
Metabolite.
7. Validation
The validation of analytical methods is a fundamental aspect of establishing reference methods and determining with sufficient evidence that these methodologies have the capacity to generate reliable, reproducible, and accurate results. Analytical methods must be validated considering performance parameters such as selectivity, specificity, accuracy, precision, linearity range, limit of detection (LOD), limit of quantification (LOQ), and robustness, among others.
For doping control laboratories, it is of utmost importance to ensure the quality and reliability of the analytical method, since it is based on this that decisions of a sporting, competitive, and even legal nature are made. To set standards for laboratory antidoping procedures around the world, WADA has developed the International Standard for Laboratories (ISL), which states that confirmation methods for nonthreshold substances [a substance included in the Prohibited List for which the identification constitutes an adverse analytical finding (AAF)] must be validated.
Several guidelines and protocols for the validation of methodologies have been generated at an international level, among which are those developed by the Food and Drug Administration (FDA), World Health Organization (WHO), European Medicines Agency (EMA), International Council on Harmonization (ICH), current good manufacturing practice (cGMP), International Organization for Standardization (ISO), and Association of Analytical Chemists (AOAC), among others.
However, regarding peptide analysis and validation, no specific validation guide has been established for this group of substances so far. For this reason, different investigations of peptides in doping have been published where the validation process has been carried out using as guidelines the official WADA documents (ISL, TD IDCR, and TD MRPL), Eurachem guidelines, ICH Technical Requirements, the FDA’s Guidelines for the Validation of Analytical Methods, ISO/IEC standards, and small molecule validation documents.
Lange et al. used dried blood spot (DBS) sample preparation, followed by SPE for the detection of 46 low-molecular-weight peptide molecules (<2 kDa) by LC-HRMS.90 The method was validated using the official WADA guidelines for the validation of initial testing procedures (ITPs) for nonthreshold substances, determining the LOD (between 0.5 and 20 ng/mL), linearity (ranging from coefficients of correlation r between 0.9862 and 0.9999), precision (at 20, 50, and 100 ng/mL), recovery at 20 ng/mL, carryover after 100 ng/mL, and the matrix effect.90
In addition, Min et al. developed and validated an LC–MS/MS-based method for the simultaneous analysis of seven different GHRPs (alexamorelin, GHRP-1, -2, -4, -5, -6, and hexarelin) and three GHRSs (anamorelin, ibutamoren, and ipamorelin) in human urine.97 For this purpose, they followed the WADA and ISO/IEC17025 guidelines. They validated the selectivity, linearity (ranging from coefficients of correlation r between 0.9984 to 1), matrix effect (50.0%–141.2%), recovery (10.4%–100.8%), intra- and interday precision (CV < 20% for intraday and CV < 25% for interday precision), and LOD (for screening 0.05–0.5 ng/mL and for confirmation 0.05–1 ng/mL), thus demonstrating that the validated method can be useful for the simultaneous qualitative determination of these peptides.97
These same guidelines were used by Seo et al. for the validation of a method for the quantification of insulin growth factor I (IGF-I), a biomarker for GH misuse, in human serum samples using LC-HRMS following a rapid sample preparation method.98 In this regard, they validated the linearity (correlation r 0.9994), selectivity, intra- and interday precision (CV < 2% and CV < 6% respectively), recovery (>95%), accuracy (>99%), LOQ (20 ng/mL), and LOD (15 ng/mL), and compared the method with those usually applied for detecting IGF-I, finding that their method was faster and cheaper and required a low sample volume.98
The use of Eurachem validation guidelines was reported to determine the LOD, selectivity, and specificity of desmopressin in 10 human plasma samples and in 10 urine samples.99 For human plasma samples, the LOD was established at 50 pg/mL, and additionally it was found that the method exhibited good selectivity, since an adequate separation was achieved between desmopressin, the ISs, and the carrier peptide. Finally, for the urine samples, an LOD of 25 pg/mL was found, likewise achieving a good separation between desmopressin, the ISs, and the carrier peptide.99
Leuprolide was used to develop and validate a quantification methodology in human plasma based on LC–MS. The validation was carried out in accordance with the ICH guidelines (Technical Requirements for Registration of Pharmaceuticals for Human Use, 2005). The assay exhibited a linear dynamic range of 0.0500–40 ng/mL for each analyte, with a lower limit of quantification (LLOQ) of 0.0500 ng/mL. The method presented a linear dynamic range of 0.0500–40 ng/mL, with a lower limit of quantification (LLOQ) of 0.0500 ng/mL. Additionally, the specificity, selectivity, precision, accuracy, extraction recovery, and matrix effect were established.100
Tables 4a and 4b summarize the experimental conditions (pretreatment of the samples, worked matrix, analytical methodology, and equipment configuration), and the established validation parameters (LOD, LOQ, linear range, % recovery, matrix effect, among others), respectively.
Table 4a. Experimental Conditions of Methodologies to Analyze Peptides Used in Doping.
| Analyte | Guide | Experimental conditions | Matrix/pretreatment procedure |
|---|---|---|---|
| GHRPs and GHSs | WADA ISO/IEC 17025 | RP-HPLC, Kinetex C18 column (100 mm × 2.1 mm, 2.6 μm), mobile phase A (0.1% FA in water), B (0.1% FA in ACN), temperature oven 35 °C, flow rate 0.5 mL/min; 5/95/95/5/5% B in 0.5/5.5/6.5/8.0 min; LC–MS/MS conditions: ESI-triple quadrupole, ion spray voltages: 4.5 kV in positive mode; capillary and vaporizer temperatures: 320 °C, sheath and auxiliary gas flow rate: 60 arbitrary units, respectively97 | matrix: urine; pretreatment sample: IE/RP-SPE97 |
| mixture of peptides used in doping | ISO-17025 | RP-HPLC, ACQUITY UPLC CSH C18 column (100 × 1 mm2, 1.7 μm), 210 nm, mobile phase A (0.1% FA in water), B (0.1% FA in ACN), temperature oven: 35 °C, flow rate: 0.3 mL/min; 2/2/24/30/50/99/99/2/2% B in 1/2/13/21/23/24/26/27/32 min; screening: UPLC-DAD, ESI-IT, mode positive, spray voltage: 4.5 kV, end plate voltage: 500 V; nebulizer 2 bar, desolvation gas temperature: 250 °C at a flow rate: 10 L/min101 | matrix: water; pretreatment sample: centrifugation101 |
| mixture of peptides used in doping | WADA | UHPLC, HF-X Hybrid Quadrupole-Orbitrap, A Poroshell 120 EC-C8 analytical column (3 × 50 mm, 2.7 μm), mobile phase A (0.1% FA in water), B (0.1% FA, 1% DMSO in ACN), temperature column: 30 °C, flow rate: 0.35 mL/min; 1/40/90/90/1% B in 1/11/11.5/13/16 min; mode positive, ionization voltage: 3.3 kV90 | matrix: blood pretreatment sample: centrifugation90 |
| mixture of peptides used in doping | WADA-ISO/IEC 17025 | UHPLC, Agilent Poroshell 120 ECC18 column (50 × 2.1 mm, 2.7 μm), mobile phase A (0.2% FA in water), B (0.2% FA in ACN), temperature column: 40 °C, flow rate 0.4 mL/min; 1/1/60/100/1/1% B in 0/1/7/7.1/9.1/9.2/11 min; injection volume: 10 μL, Q-TOF parameters: positive mode, drying and nebulizing gas: nitrogen; drying gas flow: 12 L/min, 250 °C, nebulizer gas pressure: 40 psi, sheath gas flow: 8 L/min, 350 °C; capillary and nozzle voltages were 4000 and 1000 V, respectively55 | matrix: urine; pretreatment sample: RP-SPE55 |
| GHRHs | N.R. | UHPLC, Waters Symmetry trapping column C18 (180 μm × 20 mm, 5 μm), mobile phase A (0.1% FA in water), B (0.1% FA in ACN), flow rate: 350 nL/min; 1/60/80/1/1% B in 0/20/22/23/31 min; Q-Orbitrap parameters: positive mode, transfer capillary temperature: 150 °C, spray voltage: 2 kV102 | matrix: plasma; pretreatment sample: immunoaffinity-SPE102 |
| GHRP-6 | FDA | Nano-HPLC-Q-TOF, monolithic nanocolumn (200 μm × 5 cm) and a trap column (200 μm × 5 mm) from LC Packings, mobile phase A (0.1% FA in water), B (0.1% FA in ACN/water 80/20 v/v), flow rate: 0.8 μL/min; 50/100/100/10% B in 0/2/5/7 min; capillary voltage: 2.5 kV; cone voltage: 40 V103 | matrix: human plasma; pretreatment sample: precipitation, centrifugation, and desalting103 |
| IGF-1 | WADA-ISO/IEC 17025 | UHPLC-Q-Exactive, Kinetex C18 column (100 × 2.1 mm, 2.6 μm), mobile phase A (0.1% FA in water), B (0.1% FA in MeOH), temperature column: 40 °C, flow rate: 300 μL/min; gradient elution 2/2/95/5/5% B in 0/0.5/8.5/10/12.5 min, mode positive, ion spray voltage: 4.5 kV, capillary heaters temperature: 320 °C, probe heaters: 438 °C, sheath gas flow rate: 51 arbitrary units, auxiliary gas flow rate: 14 arbitrary units98 | matrix: mouse serum; pretreatment sample: trypsin digestion, IS 15N-IGF-I98 |
| Leuprolide | ICH | HPLC, HILIC column (150 mm × 2.1 mm, 6 μm), mobile phase [25 mL CH3COONH4 (0.2 M), 320 mL acetic acid (0.522 M) pH 3], 655 mL water/ACN with 1% FA 25/75, v/v, isocratic elution, flow rate: 1.0 mL/min, temperature column: 25 °C; API-triple quadrupole, mode positive; sheath, auxiliary, and ion sweep gas: nitrogen; ion spray voltage: 5.5 kV, capillary temperature: 750 °C100 | matrix: human plasma or serum; pretreatment sample: RP-SPE100 |
| desmopressin and vasopressin | N.R. | RP-HPLC, Pyramid C18 column (50 mm × 2.1 mm, 1.9 μm), mobile phase A (0.1% FA in water), B (ACN), gradient elution: 0/100/100/0/0% B, in 0/8/8.5/9/15 min; flow rate: 0.3 mL/min triple quadrupole-TOF, mode positive at 500 °C; collision and auxiliary/sheath gas nitrogen104 | matrix: urine; pretreatment sample: EI-RP-SPE104 |
Table 4b. Validation of Methodologies to Analyze Peptides Used in Doping.
| Analyte | Guide | Validation parameters | |
|---|---|---|---|
| GHRPs and GHSs | WADA-ISO/IEC 17025 | selectivity, matrix effects, recovery, linearity, intra- and interassay precisions, and LOD | linearity (r = 0.9984–1) |
| matrix effect = 50.0%–141.2% | |||
| recovery = 10.4%–100.8% | |||
| intra- and interday precision (CV < 20% and CV < 25%, respectively) | |||
| LOD (for screening 0.05–0.5 ng/mL; for confirmation 0.05–1 ng/mL)97 | |||
| mixture of peptides used in doping | ISO-17025 | selectivity, linearity, precision, accuracy, LOQ, and LOD | linearity (r > 0.999) |
| LOD (μg/mL): GHRP-2 = 0.05; GHRP-6 = 0.05; leuprolide = 0.1; buserelin = 0.1; sermorelin = 2.5 | |||
| LOQ (μg/mL): GHRP-2 = 5; GHRP-6 = 5; Ipamorelin = 5; sermorelin = 40101 | |||
| mixture of peptides used in doping | WADA | selectivity, matrix effects, recovery, linearity, intra- and interassay precisions, carry over, and LOD | linearity (r = 0.9862–0.9999) |
| matrix effect = 33%–156% | |||
| LOD (ng/mL): 0.5–20 | |||
| carryover (after 100 ng/mL) = 0%–9.9% | |||
| recovery (at 20 ng/mL) = 8.0%–69.6%90 | |||
| mixture of peptides used in doping | WADA-ISO/IEC 17025 | selectivity, recovery, matrix effect, precision, sensitivity (limit of detection), cross contamination, carryover, robustness, and stability | LOD (ng/mL): 0.1–1 |
| matrix effect = 12%–80% | |||
| recovery SPE = 17%–95%55 | |||
| GHRHs | N.R. | specificity, linearity, recovery, LLOD, imprecision, and ion suppression/enhancement effects | lower limit of detection (LLOD) (pg/mL) = sermorelin < 50; CJC-1295 |
| recovery = sermorelin 23%; CJC-1295 19%102 | |||
| GHRP-6 | FDA | sensitivity (LOQ, carry over), calibration range and response, accuracy and precision, and sample stability | lower limit of quantification (LLOQ) = 5 ng/mL |
| linearity 5–50 ng/mL (r = 0.988)103 | |||
| IGF-1 | WADA-ISO/IEC 17025 | linearity, selectivity, carry over, LOD, LOQ, intra and interday precision, recovery, and accuracy | linearity (r = 0.9994) |
| LOD = 15 ng/mL | |||
| LOQ = 20 ng/mL | |||
| recovery > 95% | |||
| carryover < 0.03%98 | |||
| leuprolide | ICH | specificity, linearity, LLOQ, selectivity, precision, accuracy, extraction recovery, matrix effect, and stability | linear dynamic range = 0.0500–40 ng/mL |
| lower limit of quantification (LLOQ) = 0.0500 ng/mL100 | |||
| desmopressin and vasopressin | N.R. | specificity, recovery, linearity, precision (intra/inter-day), LOD, LOQ, ion suppression, robustness, accuracy, and stability | linearity (r > 0.99) |
| LOD = 20 pg/mL | |||
| LOQ = 50 pg/mL | |||
| recovery = 81%–103%104 | |||
Finally, several studies in which the analytical methodology has been validated have challenged the developed method in different ways, to guarantee optimal results in real-life tests (in addition to the tests in which the matrix is fortified with the peptide or the peptides of interest). In this sense, the application of these methods has been reported against biological samples (such as human urine, serum, and plasma), in which some of these peptides have been previously administered (with their respective informed consent).
Lang et al. conducted a study in human serum, which involved the subcutaneous administration of the peptides GHRP-2 and GHRP-6. The evaluation of the levels of both peptides in serum was carried out at three different times, finding that the peptides were at detectable levels up to 90 min postadministration.90 GHRP-6 peptide was also analyzed in a phase 1 clinical trial by Gil et al., where they were able to evaluate the peptide in human plasma, above the LLOQ, up to 12 h after administration.103
Knoop et al. carried out the subcutaneous administration of a single dose of sermorelin, finding that at none of the evaluated times the whole peptide could be found, but at 30 min it was possible to identify one of its metabolites [sermorelin (3–29)]. Additionally, the authors report that the results were comparable for plasma and serum samples, which confirms the possibility of using both biological matrices in doping control studies.102
Thomas et al. performed an excretion study in urine samples. The authors used two different routes of administration (oral and intranasal), seeking to compare the bioavailability profiles for both routes of administration and, additionally, establishing a detection range for Desmopressin of up to 20 h, with maximum concentrations recorded between 3 h and 7 h.104
The use of samples from athletes has also been reported, although to a lesser extent compared to clinical trials or pharmacokinetic studies. Seo et al. reported the use of 209 serum samples collected from athletes to establish and compare IGF-1 concentration levels. Although the method was successfully validated and compared with current methodologies, the authors report that a limitation in these assays involved the lack of samples with high concentrations of IGF-1.98
8. Conclusions
The analysis of prohibited substances used to improve athletic performance is an analytical challenge that requires the continuous optimization of methodologies in terms of sensitivity, precision, accuracy, recovery rate, decrease in the number of false positives, and analysis time, among others. The role of peptides in research focused on antidoping presents extensive challenges for the future, mainly because it is necessary to obtain reference materials that allow the continuous development of analytical methodologies for their adequate detection. Although many efforts have been made in this field, the chemical synthesis of peptides has been a relatively unexplored area. Therefore, we believe that solid-phase peptide synthesis (SPPS) using the Fmoc/tBu strategy can be an important alternative for obtaining in-house reference materials with high percentages of yield and purity, which could be an additional step in the fight against doping, and that it would be suitable for the routine analysis of peptides with possible use as a doping agent.
The detection and/or quantification of synthetic peptides used in doping poses an additional analytical challenge due to factors such as emergence of new peptides, very short lifetimes, and low concentrations in the body, and they can mimic naturally produced molecules, trigger biological processes indirectly, etc. Although significant progress has been made in the pretreatment of samples and the instrumentation, which has allowed the development of more sensitive, precise, and accurate analytical methods, there are still some aspects that need improvement, such as detection and quantification of peptides with low concentrations in complex matrices and identification and characterization of biomarkers, especially for peptides with short half-lives, to obtain reference materials, especially those synthesized at home, in order to create strategies for the rapid detection of peptides, which are continually entering the doping market, among others.
Acknowledgments
This research was funded by Ministerio del Deporte (MinDeportes) Project: “Implementación de técnicas analíticas basadas en la espectrometría de masas para la identificación de moléculas de tipo proteico relacionadas con el dopaje.” Hermes Code: 51286.
Author Contributions
N.A.G.-G. and N.M.G.-L. contributed equally to this paper and are considered as first authors. The manuscript was written through the contribution of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Birzniece V. Doping in Sport: Effects, Harm and Misconceptions. Intern Med. J. 2015, 45 (3), 239–248. 10.1111/imj.12629. [DOI] [PubMed] [Google Scholar]
- Morente-Sánchez J.; Zabala M. Doping in Sport: A Review of Elite Athletes’ Attitudes, Beliefs, and Knowledge. Sports Medicine 2013, 43 (6), 395–411. 10.1007/s40279-013-0037-x. [DOI] [PubMed] [Google Scholar]
- Hackney A. C.Peptide-Protein Hormones. In Doping, Performance Enhancing Drugs, and Hormones in Sport; Elsevier, 2018; pp 49–63, 10.1016/b978-0-12-813442-9.00005-5. [DOI] [Google Scholar]
- Neuberger E. W. I.; Simon P.. Gene and Cell Doping: The New Frontier - Beyond Myth or Reality. In Acute Topics in Anti-Doping; Rabin O.; Pitsiladis Y., Eds.; Karger: Basel, 2017; Vol. 62, pp 91–106, 10.1159/000465456. [DOI] [PubMed] [Google Scholar]
- Atkinson T. S.; Kahn M. J. Blood Doping: Then and Now. A Narrative Review of the History, Science and Efficacy of Blood Doping in Elite Sport. Blood Rev. 2020, 39, 100632. 10.1016/j.blre.2019.100632. [DOI] [PubMed] [Google Scholar]
- Conti A. A. Doping in Sports in Ancient and Recent Times. Med. Secoli. 2010, 22, 181–190. [PubMed] [Google Scholar]
- Yesalis C. E.; Bahrke M. S. History of Doping in Sport. International Sports Studies 2002, 24 (1), 42–76. [Google Scholar]
- Müller R.History of Doping and Doping Control. In Handbook of Experimental Pharmacology; Thieme D.; Hemmersbach P., Eds.; Springer-Verlag: Berlin, 2010; pp 1–24. [DOI] [PubMed] [Google Scholar]
- Ljungqvist A.Brief History of Anti-Doping. In Acute Topics in Anti-Doping; Rabin O.; Pitsiladis Y., Eds.; Karger: Basel, 2017; Vol. 62, pp 1–10, 10.1159/000460680. [DOI] [PubMed] [Google Scholar]
- Willick S. E.; Miller G. D.; Eichner D. The Anti-Doping Movement. PM and R 2016, 8 (3), S125–S132. 10.1016/j.pmrj.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Robinson N.; Sottas P. E.; Schumacher Y. O.. The Athlete Biological Passport: How to Personalize Anti-Doping Testing across an Athlete’s Career? In Acute Topics in Anti-Doping; Rabin O.; Pitsiladis Y., Eds.; Karger: Basel, 2017; Vol. 62, pp 107–118, 10.1159/000460722. [DOI] [PubMed] [Google Scholar]
- Barroso O.; Handelsman D. J.; Strasburger C.; Thevis M. Analytical Challenges in the Detection of Peptide Hormones for Anti-Doping Purposes. Bioanalysis 2012, 4 (13), 1577–1590. 10.4155/bio.12.128. [DOI] [PubMed] [Google Scholar]
- Aith F. M. A. Anti-Doping Control and Public Health: Limits to the Exposure of Human Health to Risk in the Name of Sporting Glory. Rev. Saude Publica 2013, 47 (5), 1015–1018. 10.1590/S0034-8910.2013047005129. [DOI] [PubMed] [Google Scholar]
- Guan F.; Fay S.; Li X.; You Y.; Robinson M. A. Identification of Ex Vivo Catabolites of Peptides with Doping Potential in Equine Plasma by HILIC-HRMS. Drug Test Anal 2020, 12 (6), 771–784. 10.1002/dta.2781. [DOI] [PubMed] [Google Scholar]
- D’Aloisio V.; Dognini P.; Hutcheon G. A.; Coxon C. R. PepTherDia: Database and Structural Composition Analysis of Approved Peptide Therapeutics and Diagnostics. Drug Discov Today 2021, 26 (6), 1409–1419. 10.1016/j.drudis.2021.02.019. [DOI] [PubMed] [Google Scholar]
- World Anti-Doping Agency . World Anti-Doping Code: International Standard Prohibited List 2021. 2021; pp 1–24. [Google Scholar]
- World Anti-Doping Agency . 2020 Anti-Doping Testing Figures. 2020; pp 1–26. [Google Scholar]
- Thevis M.; Schänzer W. Identification and Characterization of Peptides and Proteins in Doping Control Analysis. Curr. Proteomics 2005, 2, 191–208. 10.2174/157016405774641147. [DOI] [Google Scholar]
- Robinson N.; Giraud S.; Saudan C.; Baume N.; Avois L.; Mangin P.; Saugy M. Erythropoietin and Blood Doping. Br. J. Sports Med. 2006, 40, i30–i34. 10.1136/bjsm.2006.027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K. V.; Diep D.; Chen K. J. Q.; Huang L.; Gulenko O. Effect of Erythropoietin on Athletic Performance: A Systematic Review and Meta-Analysis. BMJ. Open Sport Exerc Med. 2020, 6 (1), e000716. 10.1136/bmjsem-2019-000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P.; ten Have C.; Lauchs M. A Case Study Analysis of a Sophisticated Sports Doping Network: Lance Armstrong and the USPS Team. Int. J. Law Crime Justice 2016, 46, 57–68. 10.1016/j.ijlcj.2016.03.001. [DOI] [Google Scholar]
- Citartan M.; Gopinath S. C. B.; Chen Y.; Lakshmipriya T.; Tang T. H. Monitoring Recombinant Human Erythropoietin Abuse among Athletes. Biosens Bioelectron 2015, 63, 86–98. 10.1016/j.bios.2014.06.068. [DOI] [PubMed] [Google Scholar]
- Woolf J. R.; Yoon H.; Perkari K. Fighting and Doping: Professional Mixed Martial Artists Experience and Exposure to Performance-Enhancing Substances and Supplements. Perform Enhanc Health 2021, 9 (1), 100190. 10.1016/j.peh.2021.100190. [DOI] [Google Scholar]
- Sonksen P. H. Hormones and Sport: Insulin, Growth Hormone and Sport. Journal of Endocrinology 2001, 170, 13–25. 10.1677/joe.0.1700013. [DOI] [PubMed] [Google Scholar]
- Holt R. I. G.; Erotokritou-Mulligan I.; Sönksen P. H. The History of Doping and Growth Hormone Abuse in Sport. Growth Hormone and IGF Research 2009, 19 (4), 320–326. 10.1016/j.ghir.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Anderson L. J.; Tamayose J. M.; Garcia J. M. Use of Growth Hormone, IGF-I, and Insulin for Anabolic Purpose: Pharmacological Basis, Methods of Detection, and Adverse Effects. Mol. Cell. Endocrinol. 2018, 464, 65–74. 10.1016/j.mce.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristina Parr M.; Müller-Schöll A. Pharmacology of Doping Agents—Mechanisms Promoting Muscle Hypertrophy. AIMS Mol. Sci. 2018, 5 (2), 145. 10.3934/molsci.2018.2.131. [DOI] [Google Scholar]
- Holt R. I. G.; Sönksen P. H. Growth Hormone, IGF-I and Insulin and Their Abuse in Sport. Br. J. Pharmacol. 2008, 154 (3), 542–556. 10.1038/bjp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha N.; Cowan D. A.; Sönksen P. H.; Holt R. I. G. Insulin-like Growth Factor-I (IGF-I) Misuse in Athletes and Potential Methods for Detection. Anal Bioanal Chem. 2013, 405 (30), 9669–9683. 10.1007/s00216-013-7229-y. [DOI] [PubMed] [Google Scholar]
- Laudo C.; Puigdevall V.; Rio M. J. d.; Velasco A. Hormonas Utilizadas Como Agentes Ergogénicos: Situación Actual Del Problema. An Sist Sanit Navar 2006, 29 (2), 207–218. 10.4321/S1137-66272006000300005. [DOI] [PubMed] [Google Scholar]
- Rogol A. D.; Miller G. D.; Eichner D. Growth Hormone, Growth Hormone Secretagogues, and Insulin-like Growth Factor-1 in Sports: Prohibited Status, Therapeutic Use Exemptions, and Analytical Detectability. Curr. Opin Endocr Metab Res. 2019, 9, 8–13. 10.1016/j.coemr.2019.05.003. [DOI] [Google Scholar]
- Guan F.; Robinson M. A. Comprehensive Solid-Phase Extraction of Multitudinous Bioactive Peptides from Equine Plasma and Urine for Doping Detection. Anal. Chim. Acta 2017, 985, 79–90. 10.1016/j.aca.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Berlanga-Acosta J.; Abreu-Cruz A.; Barco Herrera D. G.-d.; Mendoza-Mari Y.; Rodriguez-Ulloa A.; Garcia-Ojalvo A.; Falcon-Cama V.; Hernandez-Bernal F.; Beichen Q.; Guillen-Nieto G. Synthetic Growth Hormone-Releasing Peptides (GHRPS): A Historical Appraisal of the Evidences Supporting Their Cytoprotective Effects. Clin Med. Insights Cardiol 2017, 11, 1–9. 10.1177/1179546817694558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra D.; Humaidan P. A Review of Luteinising Hormone and Human Chorionic Gonadotropin When Used in Assisted Reproductive Technology. Reproductive Biology and Endocrinology 2014, 12 (1), 95. 10.1186/1477-7827-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases . Gonadotropins. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; U.S. National Library of Medicine, 2012; pp 1–12. [PubMed] [Google Scholar]
- Handelsman D. J.; Idan A.; Grainger J.; Goebel C.; Turner L.; Conway A. J. Detection and Effects on Serum and Urine Steroid and LH of Repeated GnRH Analog (Leuprolide) Stimulation. Journal of Steroid Biochemistry and Molecular Biology 2014, 141, 113–120. 10.1016/j.jsbmb.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Handelsman D. J.Performance Enhancing Hormones in Sports Doping. In Endocrinology: Adult and Pediatric; Elsevier Inc., 2015; Vol. 1–2, pp 441–454, 10.1016/B978-0-323-18907-1.00024-X. [DOI] [Google Scholar]
- Thevis M.; Thomas A.; Schänzer W.. Insulin. In Handbook of Experimental Pharmacology; Thieme D.; Hemmersbach P., Eds.; Springer-Verlag: Berlin, 2010; Vol. 195, pp 209–226, 10.1007/978-3-540-79088-4_10. [DOI] [PubMed] [Google Scholar]
- Graham M. R.; Baker J. S.; Davies B.. Peptide Hormones, Metformin and New-Wave Practices and Research Therapies. In Chemically Modified Bodies: The Use of Diverse Substances for Appearance Enhancement; Hall M., Ed.; Palgrave Macmillan, 2016; pp 201–229, 10.1057/978-1-137-53535-1_11. [DOI] [Google Scholar]
- Benni J. M.; Patil P. A. Non-Diabetic Clinical Applications of Insulin. J. Basic Clin Physiol Pharmacol 2016, 27 (5), 445–456. 10.1515/jbcpp-2015-0101. [DOI] [PubMed] [Google Scholar]
- Evans P. J.; Lynch R. M. Insulin as a Drug of Abuse in Body Building. Br J. Sports Med. 2003, 37 (4), 356–357. 10.1136/bjsm.37.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidet M.; Abdel Wahab A.; Ebadi V.; Cogne Y.; Chollet-Xemard C.; Khellaf M. Severe Hypoglycemia Due to Cryptic Insulin Use in a Bodybuilder. Journal of Emergency Medicine 2019, 56 (3), 279–281. 10.1016/j.jemermed.2018.10.030. [DOI] [PubMed] [Google Scholar]
- Saugy M.; Robinson N.; Saudan C.; Baume N.; Avois L.; Mangin P. Human Growth Hormone Doping in Sport. Br. J. Sports Med. 2006, 40, i35–i39. 10.1136/bjsm.2006.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwallader A. B.; de La Torre X.; Tieri A.; Botrè F. The Abuse of Diuretics as Performance-Enhancing Drugs and Masking Agents in Sport Doping: Pharmacology, Toxicology and Analysis. Br. J. Pharmacol. 2010, 161 (1), 1–16. 10.1111/j.1476-5381.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Gomar F.; Martinez-Bello V. E.; Derbré F.; García-López E.; García-Valles R.; Brioche T.; Ferrando B.; Ibañez-Sania S.; Pareja-Galeano H.; Gomez-Cabrera M. C.; Viña J. Rapid Hemodilution Induced by Desmopressin after Erythropoietin Administration in Humans. Journal of Human Sport and Exercise 2011, 6 (2), 315–322. 10.4100/jhse.2011.62.12. [DOI] [Google Scholar]
- Sanchis-Gomar F.; Martinez-Bello V. E.; Nascimento A. L.; Perez-Quilis C.; Garcia-Gimenez J. L.; Viña J.; Gomez-Cabrera M. C. Desmopresssin and Hemodilution: Implications in Doping. Int. J. Sports Med. 2010, 31 (1), 5–9. 10.1055/s-0029-1239500. [DOI] [PubMed] [Google Scholar]
- Demiselle J.; Fage N.; Radermacher P.; Asfar P. Vasopressin and Its Analogues in Shock States: A Review. Ann. Intensive Care 2020, 10 (1), 1–7. 10.1186/s13613-020-0628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaadi A. S.; Sushko K.; Bui V.; van den Anker J.; Razak A.; Samiee-Zafarghandy S. Efficacy and Safety of Vasopressin and Terlipressin in Preterm Neonates: A Protocol for a Systematic Review. BMJ. Paediatr Open 2021, 5 (1), e001067. 10.1136/bmjpo-2021-001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarino M.; Calvaresi V.; de la Torre X.; Parrotta G.; Sebastianelli C.; Botrè F. Development and Validation of a Liquid Chromatography-Mass Spectrometry Procedure after Solid-Phase Extraction for Detection of 19 Doping Peptides in Human Urine. Forensic Toxicol 2015, 33 (2), 321–337. 10.1007/s11419-015-0279-4. [DOI] [Google Scholar]
- Shen L.; Zhang L.; Yang X.; He C.; Zhou X.; Xu Y.; Yan K. Simultaneous Detection of Sixteen WADA Prohibited GHRPs, GHS, GnRHs and Eight Metabolites in Human Urine by HPLC-MS/MS. Recent Advances in Doping Analysis 2016, 24, 12–19. [Google Scholar]
- Schwenke D. Application of Fluorescence Labeled Proteins as Internal Standards for Sarcosyl-PAGE and Western Blot to Improved Detection of ESAs. Recent Advances in Doping Analysis 2016, 24, 26–31. [Google Scholar]
- MacKay L. G.; Kazlauskas R. The Importance of Reference Materials in Doping-Control Analysis. Anal Bioanal Chem. 2011, 401 (2), 483–492. 10.1007/s00216-011-5049-5. [DOI] [PubMed] [Google Scholar]
- Hoofnagle A. N.; Whiteaker J. R.; Carr S. A.; Kuhn E.; Liu T.; Massoni S. A.; Thomas S. N.; Reid Townsend R.; Zimmerman L. J.; Boja E.; Chen J.; Crimmins D. L.; Davies S. R.; Gao Y.; Hiltke T. R.; Ketchum K. A.; Kinsinger C. R.; Mesri M.; Meyer M. R.; Qian W. J.; Schoenherr R. M.; Scott M. G.; Shi T.; Whiteley G. R.; Wrobel J. A.; Wu C.; Ackermann B. L.; Aebersold R.; Barnidge D. R.; Bunk D. M.; Clarke N.; Fishman J. B.; Grant R. P.; Kusebauch U.; Kushnir M. M.; Lowenthal M. S.; Moritz R. L.; Neubert H.; Patterson S. D.; Rockwood A. L.; Rogers J.; Singh R. J.; van Eyk J. E.; Wong S. H.; Zhang S.; Chan D. W.; Chen X.; Ellis M. J.; Liebler D. C.; Rodland K. D.; Rodriguez H.; Smith R. D.; Zhang Z.; Zhang H.; Paulovich A. G. Recommendations for the Generation, Quantification, Storage, and Handling of Peptides Used for Mass Spectrometry-Based Assays. Clin Chem. 2016, 62 (1), 48–69. 10.1373/clinchem.2015.250563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.; Schänzer W.; Delahaut P.; Thevis M. Immunoaffinity Purification of Peptide Hormones Prior to Liquid Chromatography-Mass Spectrometry in Doping Controls. Methods 2012, 56 (2), 230–235. 10.1016/j.ymeth.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Cuervo D.; Loli C.; Fernandez-Alvarez M.; Munoz G.; Carreras D. Determination of Doping Peptides via Solid-Phase Microelution and Accurate-Mass Quadrupole Time-of-Flight LC-MS. Journal of Chromatography B 2017, 1065–1066, 134–144. 10.1016/j.jchromb.2017.08.044. [DOI] [PubMed] [Google Scholar]
- Zvereva I.; Semenistaya E.; Krotov G.; Rodchenkov G. Comparison of Various in Vitro Model Systems of the Metabolism of Synthetic Doping Peptides: Proteolytic Enzymes, Human Blood Serum, Liver and Kidney Microsomes and Liver S9 Fraction. J. Proteomics 2016, 149, 85–97. 10.1016/j.jprot.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Coppieters G.; Deventer K.; van Eenoo P.; Judák P. Combining Direct Urinary Injection with Automated Filtration and Nanoflow LC-MS for the Confirmatory Analysis of Doping-Relevant Small Peptide Hormones. Journal of Chromatography B 2021, 1179, 122842. 10.1016/j.jchromb.2021.122842. [DOI] [PubMed] [Google Scholar]
- Thomas A.; Görgens C.; Guddat S.; Thieme D.; Dellanna F.; Schänzer W.; Thevis M. Simplifying and Expanding the Screening for Peptides < 2 KDa by Direct Urine Injection, Liquid Chromatography, and Ion Mobility Mass Spectrometry. J. Sep Sci. 2016, 39 (2), 333–341. 10.1002/jssc.201501060. [DOI] [PubMed] [Google Scholar]
- Thevis M.; Thomas A.; Schänzer W. Doping Control Analysis of Selected Peptide Hormones Using LC-MS(/MS). Forensic Sci. Int. 2011, 213 (1–3), 35–41. 10.1016/j.forsciint.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Jaradat D. M. M. Thirteen Decades of Peptide Synthesis: Key Developments in Solid Phase Peptide Synthesis and Amide Bond Formation Utilized in Peptide Ligation. Amino Acids 2018, 50 (1), 39–68. 10.1007/s00726-017-2516-0. [DOI] [PubMed] [Google Scholar]
- Insuasty Cepeda D. S.; Pineda Castañeda H. M.; Rodríguez Mayor A. V.; García Castañeda J. E.; Maldonado Villamil M.; Fierro Medina R.; Rivera Monroy Z. J. Synthetic Peptide Purification via Solid-Phase Extraction with Gradient Elution: A Simple, Economical, Fast, and Efficient Methodology. Molecules 2019, 24 (7), 1215. 10.3390/molecules24071215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata Velásquez J. D.; Garcia Castañeda J. E.. Síntesis, Purificación y Caracterización de Cuatro Péptidos Secretagogos de La Hormona de Crecimiento Utilizados En Dopaje; Universidad Nacional de Colombia, Bogota, Colombia, 2021. [Google Scholar]
- Ramírez Celis D. A.; García Castañeda J. E.. Síntesis, Purificación y Caracterización de Leuprolide y Los Precursores de Desmopresina y Vasopresina; Universidad Nacional de Colombia, Bogota, Colombia, 2022. [Google Scholar]
- Deutsch E. W.; Perez-Riverol Y.; Chalkley R. J.; Wilhelm M.; Tate S.; Sachsenberg T.; Walzer M.; Käll L.; Delanghe B.; Böcker S.; Schymanski E. L.; Wilmes P.; Dorfer V.; Kuster B.; Volders P. J.; Jehmlich N.; Vissers J. P. C.; Wolan D. W.; Wang A. Y.; Mendoza L.; Shofstahl J.; Dowsey A. W.; Griss J.; Salek R. M.; Neumann S.; Binz P. A.; Lam H.; Vizcaíno J. A.; Bandeira N.; Röst H. Expanding the Use of Spectral Libraries in Proteomics. J. Proteome Res. 2018, 17 (12), 4051–4060. 10.1021/acs.jproteome.8b00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachká K.; Pezzatti J.; Musenga A.; Nicoli R.; Kuuranne T.; Rudaz S.; Nováková L.; Guillarme D. Ion Mobility-High Resolution Mass Spectrometry in Anti-Doping Analysis. Part I: Implementation of a Screening Method with the Assessment of a Library of Substances Prohibited in Sports. Anal. Chim. Acta 2021, 1152, 338257. 10.1016/j.aca.2021.338257. [DOI] [PubMed] [Google Scholar]
- Judák P.; Esposito S.; Coppieters G.; van Eenoo P.; Deventer K. Doping Control Analysis of Small Peptides: A Decade of Progress. Journal of Chromatography B 2021, 1173, 122551. 10.1016/j.jchromb.2021.122551. [DOI] [PubMed] [Google Scholar]
- World Anti-Doping Code International Standard Laboratories (ISL) 2021; World Anti-Doping Agency: Montreal, Quebec, Canada, 2021; pp 1–160. [Google Scholar]
- Schamasch P.; Rabin O. Challenges and Perspectives in Anti-Doping Testing. Bioanalysis 2012, 4 (13), 1691–1701. 10.4155/bio.12.145. [DOI] [PubMed] [Google Scholar]
- Deventer K.; Pozo O. J.; Verstraete A. G.; van Eenoo P. Dilute-and-Shoot-Liquid Chromatography-Mass Spectrometry for Urine Analysis in Doping Control and Analytical Toxicology. Trends in Analytical Chemistry 2014, 55, 1–13. 10.1016/j.trac.2013.10.012. [DOI] [Google Scholar]
- Greer B.; Chevallier O.; Quinn B.; Botana L. M.; Elliott C. T. Redefining Dilute and Shoot: The Evolution of the Technique and Its Application in the Analysis of Foods and Biological Matrices by Liquid Chromatography Mass Spectrometry. Trends in Analytical Chemistry 2021, 141, 116284. 10.1016/j.trac.2021.116284. [DOI] [Google Scholar]
- de Oliveira Arias J. L.; Schneider A.; Batista-Andrade J. A.; Vieira A. A.; Gehrke V. R.; Camargo E. R.; Caldas S. S.; Primel E. G. Evaluation of Dilute-and-Shoot and Solid-Phase Extraction Methods for the Determination of: S -Metolachlor and Metolachlor-OA in Runoff Water Samples by Liquid Chromatography Tandem Mass Spectrometry. Analytical Methods 2017, 9 (39), 5777–5783. 10.1039/C7AY01698K. [DOI] [Google Scholar]
- Thevis M.; Geyer H.; Tretzel L.; Schänzer W. Sports Drug Testing Using Complementary Matrices: Advantages and Limitations. J. Pharm. Biomed Anal 2016, 130, 220–230. 10.1016/j.jpba.2016.03.055. [DOI] [PubMed] [Google Scholar]
- Anizan S.; Huestis M. A. The Potential Role of Oral Fluid in Antidoping Testing. Clin Chem. 2014, 60 (2), 307–322. 10.1373/clinchem.2013.209676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek I.; Blokland M.; Nessen M. A.; Sterk S. Current Trends in Mass Spectrometry of Peptides and Proteins: Application to Veterinary and Sports-Doping Control. Mass Spectrom Rev. 2015, 34 (6), 571–594. 10.1002/mas.21419. [DOI] [PubMed] [Google Scholar]
- Katsila T.; Siskos A. P.; Tamvakopoulos C. Peptide and Protein Drugs: The Study of Their Metabolism and Catabolism by Mass Spectrometry. Mass Spectrom Rev. 2012, 31 (1), 110–133. 10.1002/mas.20340. [DOI] [PubMed] [Google Scholar]
- Finoulst I.; Pinkse M.; van Dongen W.; Verhaert P. Sample Preparation Techniques for the Untargeted LC-MS-Based Discovery of Peptides in Complex Biological Matrices. J. Biomed Biotechnol 2011, 2011, 1–14. 10.1155/2011/245291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissel S.; Frederiksen S. J.; Bunkenborg J.; Højrup P. Enhanced Trypsin on a Budget: Stabilization, Purification and High-Temperature Application of Inexpensive Commercial Trypsin for Proteomics Applications. PLoS One 2019, 14 (6), e0218374. 10.1371/journal.pone.0218374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas B.; López-Ferrer D.; Ramos-Fernández A.; Camafeita E.; Calvo E. Mass Spectrometry Technologies for Proteomics. Brief Funct Genomic Proteomic 2006, 4 (4), 295–320. 10.1093/bfgp/eli002. [DOI] [PubMed] [Google Scholar]
- Colev J.; Berghes B.; Pop V.; Zorio M.; Tarcomnicu I. Liquid-Liquid Extraction as a Simple Alternative for the Analysis of Some of the Growth Hormone Releasing Peptides in Urine. Recent Advances in Doping Analysis 2016, 24, 83–87. [Google Scholar]
- Andersen Hartvig R.; Bjerre Holm N.; Weihe Dalsgaard P.; Ask Reitzel L.; Breum Müller I.; Linnet K. Identification of Peptide and Protein Doping Related Drug Compounds Confiscated in Denmark between 2007–2013. Scandinavian Journal of Forensic Science 2014, 20 (2), 42–49. 10.2478/sjfs-2014-0003. [DOI] [Google Scholar]
- le Maux S.; Nongonierma A. B.; Fitzgerald R. J. Improved Short Peptide Identification Using HILIC-MS/MS: Retention Time Prediction Model Based on the Impact of Amino Acid Position in the Peptide Sequence. Food Chem. 2015, 173, 847–854. 10.1016/j.foodchem.2014.10.104. [DOI] [PubMed] [Google Scholar]
- Harscoat-Schiavo C.; Raminosoa F.; Ronat-Heit E.; Vanderesse R.; Marc I. Modeling the Separation of Small Peptides by Cation-Exchange Chromatography. J. Sep Sci. 2010, 33 (16), 2447–2457. 10.1002/jssc.201000112. [DOI] [PubMed] [Google Scholar]
- Tognarelli D.; Tsukamoto A.; Caldwell J.; Caldwell W. Rapid Peptide Separation by Supercritical Fluid Chromatography. Bioanalysis 2010, 2 (1), 5–7. 10.4155/bio.09.165. [DOI] [PubMed] [Google Scholar]
- Nshanian M.; Lakshmanan R.; Chen H.; Loo R. R. O.; Loo J. A. Enhancing Sensitivity of Liquid Chromatography-Mass Spectrometry of Peptides and Proteins Using Supercharging Agents. Int. J. Mass Spectrom. 2018, 427, 157–164. 10.1016/j.ijms.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms M.; Hall N.; Levina V.; Vine J.; Steel R. A High-Throughput LC-MS/MS Screen for GHRP in Equine and Human Urine, Featuring Peptide Derivatization for Improved Chromatography. Drug Test Anal 2014, 6 (10), 985–995. 10.1002/dta.1624. [DOI] [PubMed] [Google Scholar]
- Hahne H.; Pachl F.; Ruprecht B.; Maier S. K.; Klaeger S.; Helm D.; Médard G.; Wilm M.; Lemeer S.; Kuster B. DMSO Enhances Electrospray Response, Boosting Sensitivity of Proteomic Experiments. Nat. Methods 2013, 10 (10), 989–991. 10.1038/nmeth.2610. [DOI] [PubMed] [Google Scholar]
- Judák P.; Grainger J.; Goebel C.; van Eenoo P.; Deventer K. DMSO Assisted Electrospray Ionization for the Detection of Small Peptide Hormones in Urine by Dilute-and-Shoot-Liquid-Chromatography-High Resolution Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28 (8), 1657–1665. 10.1007/s13361-017-1670-7. [DOI] [PubMed] [Google Scholar]
- Görgens C.; Guddat S.; Thomas A.; Thevis M. Recent Improvements in Sports Drug Testing Concerning the Initial Testing for Peptidic Drugs (< 2 KDa) - Sample Preparation, Mass Spectrometric Detection, and Data Review. Drug Test Anal 2018, 10 (11–12), 1755–1760. 10.1002/dta.2503. [DOI] [PubMed] [Google Scholar]
- Desai M. J.; Armstrong D. W. Analysis of Native Amino Acid and Peptide Enantiomers by High-Performance Liquid Chromatography/Atmospheric Pressure Chemical Ionization Mass Spectrometry. Journal of Mass Spectrometry 2004, 39 (2), 177–187. 10.1002/jms.571. [DOI] [PubMed] [Google Scholar]
- Lange T.; Thomas A.; Walpurgis K.; Thevis M. Fully Automated Dried Blood Spot Sample Preparation Enables the Detection of Lower Molecular Mass Peptide and Non-Peptide Doping Agents by Means of LC-HRMS. Anal Bioanal Chem. 2020, 412 (15), 3765–3777. 10.1007/s00216-020-02634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judák P.; van Eenoo P.; Deventer K. Urinary Matrix Effects in Electrospray Ionization Mass Spectrometry in the Presence of DMSO. Journal of Mass Spectrometry 2018, 53 (10), 1018–1021. 10.1002/jms.4255. [DOI] [PubMed] [Google Scholar]
- Thomas A.; Höppner S.; Geyer H.; Schänzer W.; Petrou M.; Kwiatkowska D.; Pokrywka A.; Thevis M. Determination of Growth Hormone Releasing Peptides (GHRP) and Their Major Metabolites in Human Urine for Doping Controls by Means of Liquid Chromatography Mass Spectrometry. Anal Bioanal Chem. 2011, 401 (2), 507–516. 10.1007/s00216-011-4702-3. [DOI] [PubMed] [Google Scholar]
- Thomas A.; Walpurgis K.; Krug O.; Schänzer W.; Thevis M. Determination of Prohibited, Small Peptides in Urine for Sports Drug Testing by Means of Nano-Liquid Chromatography/Benchtop Quadrupole Orbitrap Tandem-Mass Spectrometry. J. Chromatogr A 2012, 1259, 251–257. 10.1016/j.chroma.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Sleno L. The Use of Mass Defect in Modern Mass Spectrometry. Journal of Mass Spectrometry 2012, 47 (2), 226–236. 10.1002/jms.2953. [DOI] [PubMed] [Google Scholar]
- Görgens C.; Guddat S.; Thomas A.; Wachsmuth P.; Orlovius A. K.; Sigmund G.; Thevis M.; Schänzer W. Simplifying and Expanding Analytical Capabilities for Various Classes of Doping Agents by Means of Direct Urine Injection High Performance Liquid Chromatography High Resolution/High Accuracy Mass Spectrometry. J. Pharm. Biomed Anal 2016, 131, 482–496. 10.1016/j.jpba.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Sardela V. F.; Martucci M. E. P.; de Araújo A. L. D.; Leal E. C.; Oliveira D. S.; Carneiro G. R. A.; Deventer K.; van Eenoo P.; Pereira H. M. G.; Aquino Neto F. R. Comprehensive Analysis by Liquid Chromatography Q-Orbitrap Mass Spectrometry: Fast Screening of Peptides and Organic Molecules. Journal of Mass Spectrometry 2018, 53 (6), 476–503. 10.1002/jms.4077. [DOI] [PubMed] [Google Scholar]
- Min H.; Han B.; Sung C.; Park J. H.; Lee K. M.; Kim H. J.; Kim K. H.; Son J.; Kwon O. S.; Lee J. LC-MS/MS Method for Simultaneous Analysis of Growth Hormone-Releasing Peptides and Secretagogues in Human Urine. Mass Spectrometry Letters 2016, 7 (3), 55–63. 10.5478/MSL.2016.7.3.55. [DOI] [Google Scholar]
- Seo Y.; Park J.; Kim M.; Sung C.; Kwon O. S.; Lee H. J.; Min H. Optimization, Validation, and Comparison of a Rapid Method for the Quantification of Insulin-like Growth Factor 1 in Serum Using Liquid Chromatography-High-Resolution Mass Spectrometry. Drug Test Anal 2021, 13 (2), 451–459. 10.1002/dta.2944. [DOI] [PubMed] [Google Scholar]
- Esposito S.; Deventer K.; T’Sjoen G.; Vantilborgh A.; van Eenoo P. Doping Control Analysis of Desmopressin in Human Urine by LC-ESI-MS/MS after Urine Delipidation. Biomedical Chromatography 2013, 27 (2), 240–245. 10.1002/bmc.2782. [DOI] [PubMed] [Google Scholar]
- Skiba M.; Fatmi S.; Elkasri N.; Karrout Y.; Lahiani-Skiba M. Development, Validation and Method Stability Study of a LC-MS Method to Quantify Leuprolide (Hormone Analog) in Human Plasma. Journal of Chromatography B 2020, 1160, 122345. 10.1016/j.jchromb.2020.122345. [DOI] [PubMed] [Google Scholar]
- Vanhee C.; Janvier S.; Desmedt B.; Moens G.; Deconinck E.; de Beer J. O.; Courselle P. Analysis of Illegal Peptide Biopharmaceuticals Frequently Encountered by Controlling Agencies. Talanta 2015, 142, 1–10. 10.1016/j.talanta.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Knoop A.; Thomas A.; Fichant E.; Delahaut P.; Schänzer W.; Thevis M. Qualitative Identification of Growth Hormone-Releasing Hormones in Human Plasma by Means of Immunoaffinity Purification and LC-HRMS/MS. Anal Bioanal Chem. 2016, 408 (12), 3145–3153. 10.1007/s00216-016-9377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J.; Cabrales A.; Reyes O.; Morera V.; Betancourt L.; Sánchez A.; García G.; Moya G.; Padrón G.; Besada V.; González L. J. Development and Validation of a Bioanalytical LC-MS Method for the Quantification of GHRP-6 in Human Plasma. J. Pharm. Biomed Anal 2012, 60, 19–25. 10.1016/j.jpba.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Thomas A.; Solymos E.; Schänzer W.; Baume N.; Saugy M.; Dellanna F.; Thevis M. Determination of Vasopressin and Desmopressin in Urine by Means of Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry for Doping Control Purposes. Anal. Chim. Acta 2011, 707 (1–2), 107–113. 10.1016/j.aca.2011.09.027. [DOI] [PubMed] [Google Scholar]