Abstract
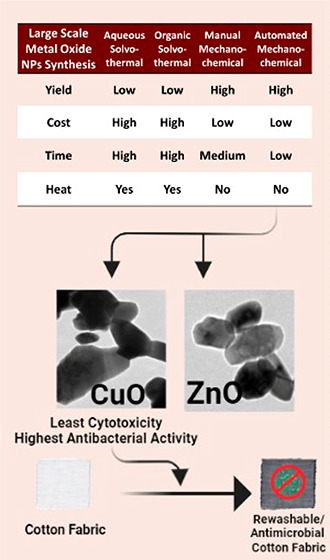
To examine the utilization of metal oxide nanoparticles (NPs) in different commercial products, this work focuses on the determination of cost-effective and scalable synthesis protocols. The solvothermal protocol is well-known as a scalable method but has recently been shown to lack economic feasibility. The mechanochemical method has recently been recognized to be a more economic and environmentally friendly substitute for the solvothermal method. In this study, zinc oxide nanoparticles (ZnO NPs) and copper oxide nanoparticles (CuO NPs) were synthesized using two (aqueous and organic) solvothermal (wet) methods and two (manual and automated) mechanochemical (dry) methods. The four methods were evaluated and compared. The automated mechanochemical method generated a significantly higher yield of ZnO NPs (82%) and CuO NPs (84%) using the least energy and time. However, the prepared ZnO NPs displayed higher cytotoxicity against Vero E6 cells when compared to that of CuO NPs. Because of their low cytotoxicity, CuO NPs synthesized via the automated mechanochemical method were selected for application onto cotton fabrics. Lower cytotoxicity was observed for CuO NPs treated fabrics with an IC50 of 562 mg/mL and ZnO treated fabrics with an IC50 at 23.93 mg/mL when the treated fabrics were tested against L929 fibroblast cells. Additionally, the cotton fabrics retained bactericidal and virucidal effects after four washes. Thus, the current study recommends the automated mechanochemical method as a cost-effective scalable approach for the synthesis of CuO NPs. The application of CuO NPs onto cotton fabrics generated washable antimicrobial face masks.
1. Introduction
Metal oxides are very important components that are included in multiple industries such as the manufacturing of solar cells, disinfectants, sensors, UV-light-emitting devices, UV blockers, pharmaceuticals and cosmetic products, industrial catalysts, antimicrobials, and self-cleaning agents.1,2 However, the efficiency of their bulk compared to their nanoform gives an edge toward metal oxide nanomaterials due to the high surface area to volume ratio among other factors. Such a property alone accelerates and enhances the efficacy of metal oxide nanoparticles (NPs) with the utilization of lower concentrations.3,4 Metal oxide NPs, most commonly zinc oxide (ZnO NPs) and copper oxide (CuO NPs), have attracted much attention due to their low toxicity and antimicrobial properties. Additionally, these nanoparticles show promising antibacterial activity against both Gram-positive and Gram-negative bacteria via reactive oxygen species (ROS) formation, damaging bacterial proteins and DNA and causing bacterial membrane collapse, reducing the probability of developing antimicrobial resistance.5,6
Different techniques have been utilized for the synthesis of metal oxide NPs such as organic and water solvothermal, mechanochemical, and plasma syntheses. Despite the success witnessed with such techniques in the synthesis of different kinds of metal oxide NPs of different sizes and shapes, they are restricted to small-scale synthesis and are difficult to be scaled up to meet different industrial purposes or the yield of which becomes small upon upscaling. In the mechanochemical synthesis, the metal salts and the reducing agent are utilized, in their solid form, without the addition of any kind of liquid for the reaction to proceed.7 Therefore, aggregation issues that can be found in the other techniques are eliminated.7,8 The heat is generated from the reaction of the chemicals, and thus there is no need for external heat for the reaction to proceed.8,9 Afterward, the reaction is washed with water to remove the salt byproduct, and the metal oxide NPs are sintered at high temperatures to remove any impurities and water. The advantage is the control of the particle size of the metal oxide nanoparticles by modifications in time of milling, sintering, and reactant ratios.10
The wet synthesis (also called the solvothermal method) utilizes a co-precipitation approach for the synthesis of the metal oxide NPs via displacement reactions from sodium hydroxide.11−13 In this approach, changes in the concentration of alkali and metal salt can change the outcome of the shape, size, and paramagnetic properties of the metal oxide NPs. Agglomeration is possible due to the nature of the reaction where several nucleations occur due to the high concentration of the metal salt in the solution following the growth phase in which the nanoparticles are formed. The solvothermal synthesis can easily be scalable due to the low cost of the raw materials and other synthesis conditions.12−14 However, other points of view recently emerged; for instance, Nandiyanto et al. showed that the scalability of this method is not too attractive and not profitable.15,16 This study aimed to examine two solvothermal protocols based on the most utilized solvents: water as an aqueous solvent and ethanol as an organic solvent. In addition, two dry synthesis protocols (mechanochemical protocols) were examined as they were suggested recently as efficient scalable protocols for metal oxide nanoparticle production. The mechanochemical synthesis by grinding or milling has been introduced as a more economically favorable and more environmentally friendly alternative approach to traditional chemical preparation in solution. This is because this method is simple, resulting in materials with superior properties, and it involves no or limited liquid and gaseous waste. The latter aspect is now gaining increasing attention, as environmental problems and depleting energy reservoirs are becoming increasingly critical.17,18
Metal oxide NP fixation to a cotton fabric shall result in a medical textile, an emerging type of industrial textile, which can be used as a washable and reusable face mask. Several methods (classified into in situ and ex situ) were previously reported for the fixation of metal oxide NPs to produce antibacterial cotton fabrics.19 For industrial purposes, a well-scalable method of metal oxide production should be established. Thus, through the current work, the solvothermal method and the mechanochemical method will be studied and assessed for the selection of a scalable synthesis procedure for the synthesis of metal oxide NPs. Four approaches will be performed and compared: two mechanochemical (manual mechanochemical (MMC) and automated mechanochemical (AMC)) and two solvothermal methods (organic solvothermal (OST) and aqueous solvothermal (AST)). The NPs will be assessed in terms of the yield of the nanoparticles achieved, the cost effectiveness of the method, and the physicochemical properties of the synthesized NPs as well as their antibacterial efficacy and cytotoxicity. The particles resulting from the recommended approach will be further tested in an actual application to examine their efficacy, safety, and stability. This will be done by applying the nanoparticles synthesized via the most scalable approach to cotton fabrics and characterized for their antibacterial, antiviral activity, safety, and stability after several washing cycles. This will allow the confirmation of efficacy, safety, and stability of the recommended approach for scaling up the synthesis of metal oxide NPs. In addition, to ensure the applicability of the functionalized cotton fabrics as face masks, wettability and air permeability tests will be performed.
2. Results and Discussion
2.1. Characterization of the Prepared Nanoparticles
2.1.1. Influence of Calcination on the Synthesized Crystals
X-ray diffraction (XRD) charts of ZnO NPs prepared in ethanol, before and after calcination, were superimposable except for their intensities. The postcalcination XRD pattern showed a significantly higher intensity which may be attributed to a better crystallization of the samples and a larger average particle size.20 The average particle sizes of the prepared ZnO NPs before and after calcination were calculated to be 5.9 and 83.6 nm, respectively, using the Debye–Scherrer formula. The increase in average particle size after calcination is in harmony with the increase in XRD peaks intensity mentioned above. The XRD patterns are presented in Figure 1. The synthesis of ZnO NPs in ethanol produced nanoparticles with precalcination and postcalcination zeta-potential values of 32.6, and 16.7 mV respectively. The decrease in zeta-potential following calcination may be attributed to the increase in the crystal size, leading to the decrease in total surface area, hence the reduction of the observed zeta-potential. In particular, calcination causes the crystallinity of the nanoparticles to increase. This would lead to an increase in particle size as calculated by the Debye–Scherrer equation. Due to this increase in particle size, the surface charge density would inevitably decrease. This is due to the distribution of charge on a larger surface area creating a weaker hydrodynamic shell around the nanoparticles.21 The zeta-potential distribution charts of ZnO NPs prepared in ethanol with pre- and postcalcination are presented in Figure S1. The remarkably improved crystallization encouraged the retention of this step in the rest of the study.
Figure 1.

X-ray diffraction patterns of the prepared ZnO NPs via the organic solvothermal method pre- and postcalcination.
2.1.2. Fourer Transform Infrared (FTIR) Analysis
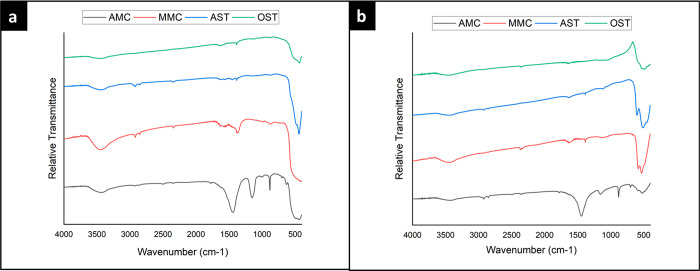
FTIR charts of ZnO NPs prepared with the four different methods, i.e., solvothermal method (in ethanol and water) and mechanochemical method (manual and automated), are shown in Figure 2. The strong peaks appearing between 400 and 600 cm–1 can be attributed to the characteristic absorption band of Zn–O. On the other hand, the ZnO NPs prepared via AMC synthesis displayed three characteristic absorption bands at 881 cm–1 (asymmetric C–O–C stretching), 1152 cm–1 (asymmetric stretching of carboxylate group), and 1439 cm–1 (asymmetric stretching vibration of carboxyl anions). Such bands may be attributed to the remainder of the acrylate group from the Printofix (PTF) (which was used in the mechanochemical synthesis). Likewise, the two peaks displayed by the ZnO NPs prepared via MMC synthesis at 1384 cm–1 (symmetric C–CH3 bending) and 1547 cm–1 (asymmetric stretching vibration of carboxyl anions) may be attributed to the remainder of the acrylate group in Printofix used during the preparation.22,23 In addition, the peaks presented at 3400–3500 cm–1 represent the hydroxyl stretching vibration from the acrylate remaining parts.23 These peaks are only found in the spectra obtained from the NPs prepared by the mechanochemical methods, which involved the use of Printofix.
Figure 2.
FTIR charts of (a) ZnO NPs and (b) CuO NPs prepared using four different methods: AMC, MMC, AST, and OST. The absorption peaks appearing from 800 to 1600 cm–1 and from 3400 to 3500 cm–1 were obtained from the NPs prepared by the mechanochemical methods, which involved the use of Printofix.
Similarly, FTIR charts of the CuO NPs prepared via the four different methods are presented in Figure 2, as well. The observed peaks at 400–600 cm–1 are representative of the stretching vibration of Cu–O bond formation. The remaining peaks can be attributed to the presence of acrylate moieties on the nanoparticles’ surface. The additional absorption bands found in the charts of CuO NPs prepared via mechanochemical synthesis, whether automated or manual synthesis, can be due to the presence of remaining Printofix used in these methods, as in the case of ZnO NPs mechanochemical synthesis.22,23 Likewise, the peaks presented at 3434 cm–1 represent the hydroxyl stretching vibration from the acrylate remaining parts.23 It is suggested to remove the excess Printofix by increasing the calcination temperature and/or time.24
2.1.3. X-ray Diffraction Analysis and Particle Size Characterization
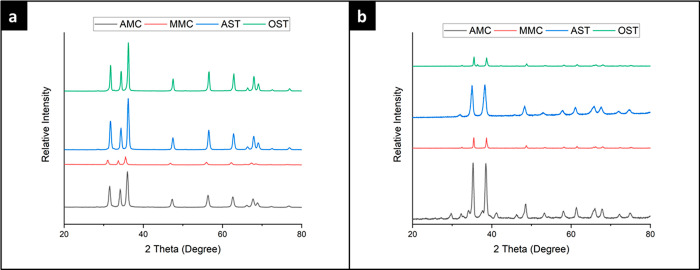
The XRD patterns of the prepared ZnO NPs via four different methods are presented in Figure 3. XRD peaks of ZnO crystals prepared in ethanol were found at 31.7, 34.5, 36.2, 47.6, 56.6, 62.9, 66.5, 68.1, 69.3, 72.6, and 77.1°, and these data are consistent with the JCPDS data of ZnO (JCPDS card no. 36-1451). No excess peaks were observed, indicating the absence of impurities in the samples. Likewise, the XRD patterns of the ZnO crystals prepared in water showed the typical ZnO peaks with no significant difference. Notable line broadening of the diffraction peaks is an indication that the synthesized materials are in the nanometer range. The average particle size of these nanocrystals was found to be 74.2 nm, not too far from the particle size of the nanocrystals prepared in ethanol, 83.6 nm.
Figure 3.
X-ray diffraction patterns of (a) ZnO NPs and (b) CuO NPs prepared using four different methods: AMC, MMC, AST, and OST.
The mechanochemical synthesis in both methods similarly showed the typical ZnO NPs peaks. Both techniques yielded the same characteristic crystalline structure estimating a higher average particle size of ZnO NPs from the automatic dry synthesis, 70 nm, than the average size from manual dry synthesis, 51.7 nm, suggesting a better crystallinity resulting from using the automatic method.20
The XRD patterns of the prepared CuO NPs via four different methods are presented in Figure 3. CuO has 11 main peaks that usually show up in XRD tests. The peaks have appeared clearly without significant broadness. XRD peaks of CuO crystals were found at 29.75, 35.24, 38.51, 48.59, 53.27, 58.1, 61.34, 66.05, 67.88, 72.26, and 74.87°. All of the peaks of CuO NPs can be indexed to the monoclinic crystal system CuO (C2/c space group, JCPDS card no. 45-0937). No characteristic peaks of any impurities were detected, suggesting that high-quality CuO NPs were prepared. The presence of sharp structural peaks in the XRD patterns and crystallite size of less than 100 nm suggested the nanocrystalline nature of CuO NPs.20,25 The crystallite sizes corresponding to the nanoparticles prepared in ethanol and water were found to be 133.8 and 38.5 nm, respectively. The XRD patterns of the synthesized CuO NPs via MMC and AMC synthesis showed the average particle sizes to be 75.1 and 50.5 nm, respectively.
2.1.3.1. Zeta-Potential Analysis
The synthesis of ZnO NPs via the AST method showed a zeta-potential of 10.7 mV, which is not too far from the zeta-potential of the NPs synthesized via the OST method, 16.7 mV. Using the MMC synthesis method, the zeta-potential of ZnO NPs was found to be 5 mV. The considerable decrease in zeta-potential may be explained by the Printofix traces remaining on the NPs’ surface, which can agree with the acrylate group found in the FTIR charts obtained from these NPs. On the other hand, the automated synthesis of the ZnO NPs resulted in a zeta-potential of 31.5 mV. The negative charge of the nanoparticles prepared via the AMC method may be justified by the amount of Printofix remaining on the NPs’ surface. More Printofix remnants led to a highly negative charge, compared to that of the NPs prepared via MMC. This is in line with the FTIR charts obtained from these NPs. The zeta-potential distribution charts of ZnO NPs prepared via four different methods, AMC, MMC, AST, and OST, are presented in Figure S2.
The synthesis of CuO NPs in water produced nanoparticles with a zeta-potential of 12.3 mV, whereas in ethanol, the nanoparticles produced showed a zeta-potential of 8.25 mV. Using the MMC synthesis method, the zeta-potential of CuO NPs was found to be 2.95 mV. For the CuO NPs synthesized via the AMC method, the zeta-potential was −25.0 mV. The decrease in NPs’ charge prepared via mechanochemical methods, especially the automated method, may be justified by the Printofix remnants on the NPs’ surface, and this is in line with the FTIR charts obtained from these NPs. The zeta-potential distribution charts of ZnO NPs prepared via four different methods, AMC, MMC, AST, and OST, are presented in Figure S3.
2.1.4. Yield and Cost Analysis
Generally, yields from the solvothermal methods for both CuO NPs and ZnO NPs were lower than those generated by mechanochemical methods.
As shown in Figure 4, the mechanochemical manual method for CuO NPs has an average percentage yield of 91%, which is significantly higher than that of the ZnO NPs synthesis via OST (79%; p = 0.0033) and AST (41.6%; p = 0.001). Moreover, the AMC method for CuO NPs resulted in an average yield of 84%, which was significantly higher than that of AST (41.6%; p = 0.0118).
Figure 4.

Yield percentage of CuO NPs and ZnO NPs (*p < 0.05, **p < 0.01) prepared using AMC, MMC, AST, and OST.
For ZnO NPs, the mechanochemical manual synthesis produced the highest average yield of 83.16%, whereas the solvothermal organic synthesis displayed an average yield of 47.5% (p = 0.0033). However, for ZnO NPs, there was no significant difference between mechanochemical manual (83.2%) and AST average yields (69.9%; p = 0.325).
The AMC synthesis produced the second-best yield of 82.45%; however, it was selected for scaling up as this method requires less time and effort due to its automated nature compared to the manual method which is both time- and labor-intensive. The actual yield of all synthesis methods, averaged from experimental triplicates, is presented in Table S1 in the Supporting Information.
The solvothermal aqueous and organic methods are the most time-consuming and most labor-intensive (which, in turn, increases the cost of preparation) in comparison to the mechanochemical method. Not only do the solvothermal methods require several steps, but also one of the steps requires NaOH solution to be added into the Zn acetate solution dropwise. The mechanochemical method achieved a higher yield with fewer materials and was less labor-intensive. With a lower industrial cost and higher yield, the mechanochemical method is used further in the study to scale up CuO NPs and ZnO NPs synthesis and for loading into the fabrics for further characterization. Thus, the mechanochemical method is the recommended protocol for the large-scale production of metal oxide NPs. The cost effectiveness analysis is presented in Table 1. Direct costs of the preparation of the nanoparticles using the four different methods are presented along with detailed prices of the materials used in Table S2 in the Supporting Information.
Table 1. Cost Effectiveness of the Production of Metal Oxide NPs.
| solvothermal organic | solvothermal aqueous | automated or manual mechanochemical | |
|---|---|---|---|
| direct cost (USD) | 23650 | 24800 | 22800 |
| solvent | ethanol | water | N/A |
| time | 4 h | 4 h | 5 min |
| heating | 80 °C | 80 °C | N/A |
| sintering | 400 °C for 4 h | 400 °C for 4 h | 400 °C for 4 h |
| steps | 5 | 5 | 2 |
| yield percent (%) (CuO, ZnO) | 79.5%, 47.47% | 41.58%, 69.9% | 87.5%, 82.5%a |
The yield percent of the mechanochemical method is the average of the yields from the manual and automated methods.
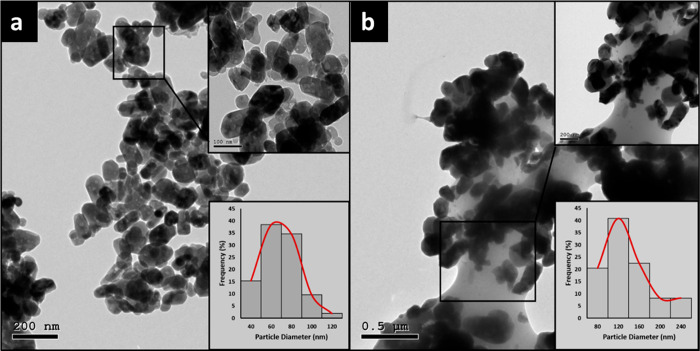
2.1.5. High-Resolution Transmission Electron Microscopy (HRTEM)
HRTEM characterization was performed to determine the morphologies and particle diameter distribution of ZnO and CuO NPs prepared via the AMC method. The morphologies of ZnO and CuO NPs are shown in Figure 5. As presented in Figure 5, the particle size distribution obtained by ImageJ (NIH, Bethesda, MD, USA) was determined, showing the average diameters of ZnO NPs and CuO NPs to be 59.78 and 116.57 nm, respectively.
Figure 5.
HRTEM image and particle diameter (nm) distribution histogram of (a) ZnO NPs and (b) CuO NPs prepared using the AMC method.
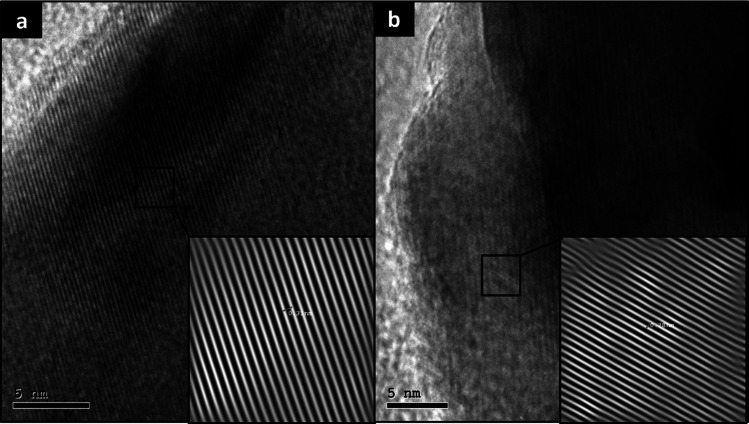
The inverse fast Fourier transmission (IFFT) pattern confirmed that the distances between lattice fringes (i.e., the interplanar distance) were 0.31 and 0.28 nm for ZnO NPs and CuO NPs, respectively. The interplanar distance of ZnO lattice fringes is in accordance with the atomic spacing on the a- and b-axes of ZnO with the wurtzite structure.26 The HRTEM image of CuO NPs showed the interplanar distance also to be in harmony with previous studies, confirming the formation of CuO NPs.27 The magnified lattice fringes and IFFT pattern of ZnO NPs and CuO NPs prepared via the AMC method are presented in Figure 6a and 6b, respectively.
Figure 6.
Magnified lattice fringes and IFFT pattern of (a) ZnO NPs and (b) CuO NPs prepared via the AMC method.
The selected area electron diffraction (SAED) pattern is very important in the study of small crystals and partly disordered material on a scale of a few hundred angstrom units or less.28 As shown in Figure 7, the SAED patterns of ZnO NPs and CuO NPs prepared via the AMC method present diffraction patterns consisting of sharp spots, and thus the NPs’ structures must be ordered.
Figure 7.
SAED patterns of (a) ZnO NPs and (b) CuO NPs prepared using the AMC method; distinct bright rings indicate the preferential orientation of nanocrystals.
2.1.6. Cytotoxicity Evaluation of the Metal Oxide Nanoparticles
The metal oxide NPs were tested against their safety toward normal cells in a dose–response curve graph to determine each metal oxide’s IC50 in calcined (denoted as M1) and noncalcined form (denoted as M2). In general, the calcined form of metal oxides was safer toward normal cells, showing ZnOM1 and CuOM1 to have an IC50 of 51.2 and 133.7 μg/mL, respectively. The noncalcined form of the metal oxides was highly toxic relative to their calcined form with ZnOM2 and CuOM2 having an IC50 of 13.84 and 57.07 μg/mL respectively. Figure 8 shows the IC50 curve of Z1, Z2, C1, and C2 against the viability of Vero E6 cells. This shows the high toxicity of ZnO NPs, as shown by other co-workers,29,30 while the CuO NPs is much safer.31
Figure 8.
Cytotoxicity evaluation of AMC’s calcined and noncalcined metal oxide NPs prepared using AMC, MMC, AST, and OST.
2.2. Characterization of the Treated Cotton Fabrics
According to the previous trials, the automated mechanochemical method produced nanoparticles with a higher yield, with the least energy and time requirements. Moreover, ZnO NPs showed higher cytotoxicity compared to that of the CuO NPs; therefore, CuO NPs synthesized via the mechanochemical method were selected to be further characterized by an actual application which is, in this case, the loading on reusable masks.
2.2.1. Wettability Test
The wettability evaluation for the treated cotton fabric is very important owing to its ability to give us information about the penetration rate of any solutions into a textile sample, which depends on the rate of diffusion. In addition, it is well-known that the fabrics which do not absorb the solution drop within 200 s are described as unwettable fabric.32 The untreated and treated cotton fabrics are porous materials with good water absorption abilities. When a water droplet is placed onto the surface of untreated cotton fabrics, it is immediately absorbed.
Thus, the interaction between the treated cotton fabrics and the surrounding surfaces can indicate the reactivity of the mask. The treatment of cotton fabrics with CuO NPs and ZnO NPs may provoke hydrophilic behaviors in the fabricated composition, which can promote the biological activity of the scaffold toward the biological tissues. Therefore, the contact angle of the scaffolds should be investigated. The wettability property of fiber is detected in the function of contact angle property, and the untreated and treated cotton fabrics with 0.2 w/v of both CuO NPs and ZnO NPs (CuO NPs-1 and ZnO NPs-1) have no contact angle and are close to zero. The contact angle of cotton fabrics increased to about 60 and 58° when treated with 0.4 (w/v) of CuO NPs and ZnO NPs (CuNPs-2 and ZnONPs-2), as illustrated from Figure 9A,C. The fabric treated with CuO NPs and ZnO NPs (CuO NPs-3 and ZnO NPs-3) recorded 70 and 67°, respectively (Figure 9B,D). Thus, the samples of cotton fabrics (CuO NPs-3 and ZnO NPs-3) showed the highest adhesion to the surface and biological effectiveness, too.
Figure 9.
Contact angle of cotton fabrics treated with (A) 0.4 (w/v) CuO NPs, (B) 0.6 (w/v) CuO NPs, (C) 0.4 (w/v) ZnO NPs, and (D) 0.6 (w/v) ZnO NPs.
By and large, from the aforementioned data of the contact angle, the treated cotton fabric can be considered as hydrophilic fabric even after treatment with a high concentration of the prepared nanoparticles (ZnO NPs and CuO NPs) because the obtained contact angle values are below 100°. The lower values of contact angles for the treated cotton fabrics when compared with other materials can be attributed to most of the utilized nanoparticles being able to easily penetrate the pores of the cotton fabric and has no chance to form a hydrophobic film on the surface of the cotton fabrics. This, in turn, reduces the contact angle and maintains the hydrophilicity of the resultant treated fabrics.
2.2.2. Air Permeability Test
Moving to the air permeability evaluation for untreated and treated cotton fabric (Table 2), it is observed that the untreated fabric has the highest value of oxygen transmission rate (OTR) (0.037 cc/m2·day). However, the treated fabrics with nanoparticles of both CuO NPs and ZnO NPs lead to decreasing the value of OTR. However, the results signify that the OTR of the treated fabric samples with both CuO NPs and ZnO NPs (using different concentrations) has no noticeable change when compared with the untreated cotton fabrics.
Table 2. Oxygen Transmission Rate of Untreated and Treated Cotton Fabrics.
| code sample | OTR (cc/m2·day) |
|---|---|
| blank | 0.037 |
| CuO NPs-1 | 0.027 |
| CuO NPs-2 | 0.024 |
| CuO NPs-3 | 0.019 |
| ZnO NPs-1 | 0.023 |
| ZnO NPs-2 | 0.017 |
| ZnO NPs-3 | 0.009 |
2.2.3. Bacterial Permeability Test
Ultimately, it is observed that the treated cotton fabrics with 0.6 (w/v) of CuO NPs and ZnO NPs have a value of around 96.2 and 97.45%, respectively, during the bacterial permeability measurements. These obtained values confirm that the treated cotton fabrics can be used for preventing the infected bacteria to pass through the treated fabrics to the face or human body.
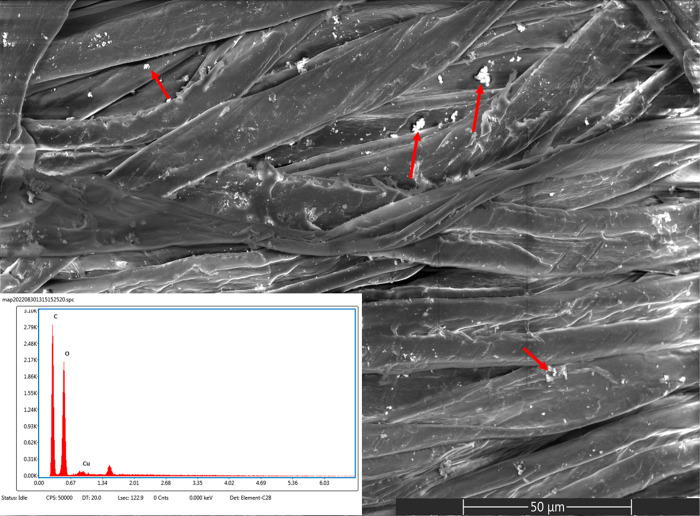
2.2.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX) Study
The SEM examination of a CuO NPs treated cotton fabric demonstrated the attachment of CuO NPs (bright white color spots) to the fibers’ surface of the cotton fabric. The EDX analysis also confirmed the existence of CuO NPs on the cotton fabric surface. The SEM and EDX study results are presented in Figure 10.
Figure 10.
SEM and EDX examination of CuO NPs treated cotton fabric.
2.2.5. MTT Assay
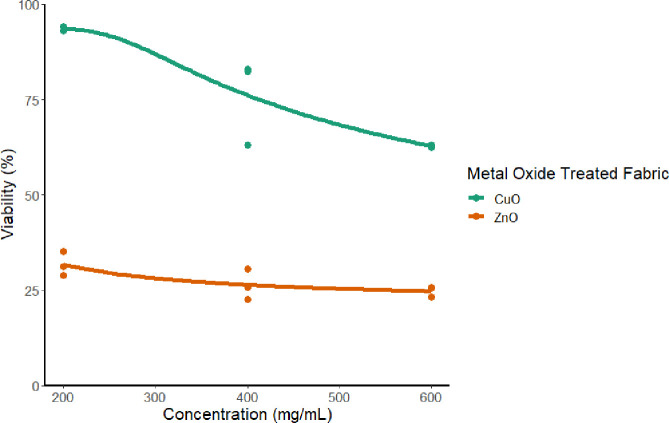
After padding and curing the fabrics with different concentrations of metal oxide nanoparticles, we tested their safety against normal cells. The IC50 values of ZnO-cured fabrics and CuO-cured fabrics were 23.93 and 562 mg/mL, respectively. Cytotoxicity of the metal oxide fabrics at different concentrations of metal oxide nanoparticles is presented in Figure 11. At the same concentration, CuO NPs treated fabrics showed a cytotoxicity lower than that of fabrics treated with ZnO NPs, hence the rest of the study only tested the CuONPs treated fabrics.
Figure 11.
Cytotoxicity of the metal oxide NPs treated cotton fabrics at different concentrations against their viability.
2.2.6. Antibacterial Activity
The scalability of the metal oxide NPs can affect its antibacterial activity; therefore, it is essential to test its antibacterial efficiency as a stand-alone (CuO NPs) and when fixated/attached to textiles such as cotton fabrics. There have been several studies on the antimicrobial activity of CuO NPs, but most of them were based on the diffusion of the CuO NPs to the medium (when the broth dilution method was used) or through agar (when the disk diffusion method was used). However, “killing upon contact” is a different procedure, and our prototype depends on CuO NPs being fixated in the textile; therefore, neither of the methods mentioned can be utilized because CuO NPs cannot diffuse into any media. Thus, we have to opt for another ISO protocol method (ISO 20743:2021) for the antibacterial activity that relies on killing bacteria via a contact method instead of diffusion. The fabrics were soaked in 1.5% of CuO NPs and 1% PTF and then padded and cured. Then the fabrics were washed a few times to determine the efficiency of the PTF adherence of the CuO NPs to the surface of fabrics. They were tested at the zero, second, and fourth washing cycles to test the efficiency of the antibacterial properties of the soaked fabrics. The washed and nonwashed fabrics showed complete antibacterial activity in comparison to the control (Figure 12).
Figure 12.
Antibacterial activity of the washed and nonwashed fabrics: (a) blank fabrics, (b) nonwashed fabrics, (c) two washing cycles, and (d) four washing cycles. Each fabric was tested against S. aureus (on the left-hand side) and E. coli (on the right-hand side).
2.2.7. Antiviral Activity
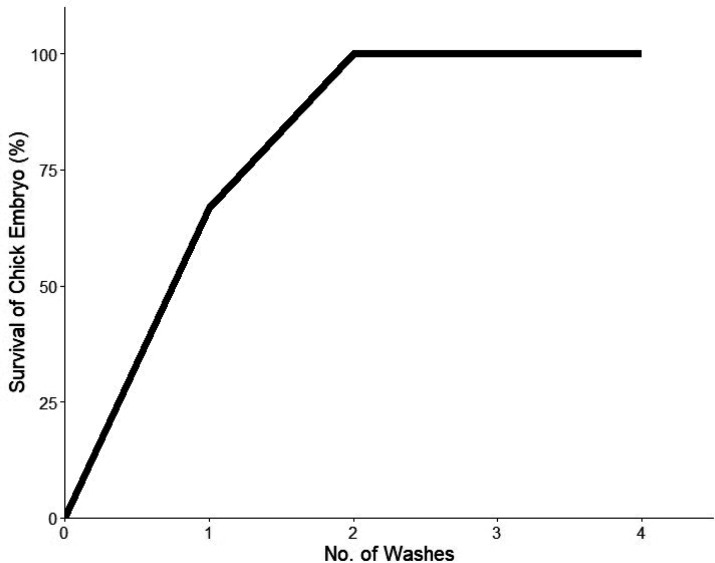
The CuO NPs treated fabrics that had two and four washing cycles showed antiviral effects against infectious bronchitis virus (IBV) and protected the chick embryo with 100% efficiency (n = 3). However, the CuO NPs treated fabrics that were not washed (zero washing cycle) showed 65% survival, possibly due to the toxicity of the CuO NPs toward the chick embryo, as shown in Figure 13. This is due to the excess CuO NPs that are not fixed to the fabric (by PTF) but only adsorbed on the surface of the fabric, due to the postloading treatment. Therefore, when the fabric of the zero washing cycle is reconstituted in PBS, it releases the excess CuO NPs into the medium. This medium is then injected into the chick embryo egg, which showed early toxicity (dwarfing) rather than protection from IBV.
Figure 13.
Binary odds ratio graph showing the number of washes of the fabrics against the survival percentage of the chick embryo. Following the second wash, the fabric protected the chick embryo against IBV.
3. Conclusions
This article has addressed the scalability of metal oxide nanoparticle synthesis by comparing four different well-established methods regarding the quality and quantity of the produced nanoparticles as well as the cost efficiency of the synthesis methods. For that, the synthesis of zinc oxide nanoparticles (ZnO NPs) and copper oxide nanoparticles (CuO NPs) was performed via two solvothermal methods (via organic or aqueous solvent) and two mechanochemical methods (via manual or automated approaches). Characterization of the synthesized nanoparticles has been performed using XRD, infrared spectroscopy, and zeta-potential analysis, showing results comparable to what can be found in the literature. Moreover, statistical Kruskal–Wallis analysis was used to compare the yield percentages of all methods. The automated mechanochemical method was proven to be optimal in terms of cost, efficiency, and time-saving process for the synthesis of the NPs with yields reaching 82.45% for ZnO NPs and 84% for CuO NPs at the pilot scale. Regarding their cytotoxic effects, CuO NPs presented lower cytotoxicity against Vero E6 cells, hence they were the ones selected to apply to cotton fabrics for application as a washable face mask. The resultant mask was proven to exhibit strong antibacterial activity against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) as well as antiviral properties against IBV. The developed mask demonstrated effective antimicrobial activity after four washes, thus proving the stability of the nanoparticles on the fabrics. Furthermore, the loaded fabrics displayed no cytotoxicity on murine L929 normal fibroblast cells. Thus, the automated mechanochemical method is the recommended method for scaling up the production of metal oxide nanoparticles due to the low cost, efficacy, and short time of production, thus introducing it as having strong potential for industrial use. Finally, the mechanochemical method is recommended by the current study as a cost-effective and easily scalable approach to produce CuO NPs that can be, simultaneously, strongly effective, safe, and stable upon loading to washable antimicrobial face masks.
4. Methods
Printofix (MTB EG liquid, based on acrylate), a capping agent, and a binder, were supplied by Clariant (Muttenz, Switzerland). The chemicals used in the preparation of metal oxide NPs were used without further purification: sodium hydroxide pellets (Sigma-Aldrich, St Louis, Missouri, USA), zinc acetate dihydrate (Lobachemie PVT. Ltd., Mumbai, India), zinc nitrate hexahydrate (Oxford Laboratory Reagents, USA), copper acetate monohydrate (Qualikems, Vadodara, Gujarat, India), and ethanol (ChemLab, Belgium). Cotton fabric (100%) in plane-weave was purchased from El-Mehalla Elkobora Co. Egypt. For the antibacterial assay, tryptic soy broth was obtained from Neogen, UK. Agar–Agar was obtained from B&V, Italy.
4.1. Wet Chemistry Protocol (Solvothermal Synthesis)
Metal oxide nanoparticles were synthesized via the well-established solvothermal synthesis method, with some modifications. Briefly, metal sallts and alkali were dissolved separately, followed by dropwise addition of the alkali solution to the metal salt solution. The metal hydroxide precipitates were then separated and washed to remove any remaining alkali before calcination. After that, the metal oxide nanoparticles were washed and dried.33−36
Since calcination is an energy-consuming step, its importance was first assessed. ZnO NPs were synthesized in ethanol and characterized pre- and postcalcination via XRD analysis. In addition, as using water in the synthesis is expected to be less costly, the solvothermal synthesis of ZnO NPs and CuO NPs was also performed in water to assess the cost effectiveness of using it as a solvent.
4.1.1. Synthesis of Nanoparticles via Organic Solvothermal Method
ZnO NPs were prepared in ethanol as previously reported by Shamhari et al., with some modifications. Briefly, 66 mL of 0.1 M zinc acetate dihydrate in ethanol was heated at 70 °C with constant stirring in a covered 1 L Schott bottle for 20 min. Then 33 mL of 0.4 M sodium hydroxide was also dissolved in ethanol separately in a 250 mL Schott bottle. The molar ratio of zinc acetate dihydrate/sodium hydroxide used was 1:2. Sodium hydroxide solution was added to the zinc acetate solution dropwise while being vigorously stirred at 70 °C. Then the mixture was left to stir with heating for 4 h until the reaction was completed. Afterward, the produced precipitate was collected by centrifugation at 12000 rpm for 5 min in a Hermle Z 326 K (Labortechnik GmbH, Wehingen, Germany) centrifuge. Calcination of the obtained precipitate was carried out in a muffle furnace (LT, Nabertherm, Germany) at 400 °C for 4 h. ZnO NPs were then neutralized (using 1% sulfuric acid) and washed with distilled water until neutral pH was reached. The obtained product was then completely dried overnight in an oven (Heratherm OGS100, Thermo Scientific, Germany) at 60 °C.33 The same procedure was adopted for the synthesis of CuO NPs in ethanol. The molar ratio of copper acetate monohydrate/sodium hydroxide used was also 1:2.
4.1.2. Synthesis of Nanoparticles via Aqueous Solvothermal Method
As discussed by Sridhar et al., 66 mL of 0.1 M zinc acetate dihydrate solution was prepared in distilled water and stirred until it was completely dissolved. The reaction begins with the dropwise addition of 33 mL of 0.4 M sodium hydroxide to the zinc acetate dihydrate solution, both being above 65 °C, and a white precipitate can be noticed. The molar ratio of zinc acetate dihydrate/sodium hydroxide used was 1:2. Stirring with heating continued for an additional 4 h. Afterward, the produced precipitate was washed and collected by centrifugation at 12000 rpm for 5 min via a Hermle Z 326 K (Labortechnik GmbH, Wehingen, Germany) centrifuge. Calcination of the obtained precipitate was carried out in a muffle furnace (LT, Nabertherm, Germany) at 400 °C for 4 h. Then the solution was settled and washed first with the addition of 1 mL of sulfuric acid (1% V/V) with distilled water until the pH was neutralized. The obtained product was then completely dried overnight in an oven (Heratherm OGS100, Thermo Scientific, Germany) at 60 °C.34
The same procedure was maintained to prepare CuO NPs in distilled water. The molar ratio of copper acetate monohydrate/sodium hydroxide used was also 1:2.35,36
4.2. Dry Chemistry Protocol (Mechanochemical Synthesis)
The scalability of the mechanochemical synthesis process was evaluated by conducting two different methods (manual and automated) and then calculating their yields and comparing their characterization results. The synthesis, in general, is much more economic, safe, and time-saving than the solvothermal method,37 hence, the optimization and characterization were thoroughly conducted and repeated to reach the optimum protocols and to be able to evaluate the scalability of this process. The mechanism to mechanically activate chemical reactions is by repeatedly causing the shifts of atoms from the equilibrium stable positions via mechanical energy input. Hence, the bond lengths and angles change, and in some cases, the excitation of electron subsystems changes, as well.37 Consequently, new bonds of interest will be formed, as described by Tadjarodi and Roshani.38 Since the metal salts are not used in soluble form, Printofix will be added in the dry chemistry protocol as a capping agent.
4.2.1. Synthesis of Nanoparticles via Manual Mechanochemical Method
Dry synthesis was applied to produce nanoparticles of ZnO and CuO with the aid of a capping agent during the manual milling process of the chemicals at a solid state in a ceramic mortar. First, Printofix, the capping agent, was ground with 0.01 mol of sodium hydroxide for 2 min to initiate the disassembly of the free hydroxyl group in the sodium hydroxide (with a weight ratio of Printofix/sodium hydroxide being 3:1). Then 0.005 mol of zinc acetate dihydrate salt was added (or copper acetate monohydrate in the case of CuO NP synthesis), and the whole mixture was ground for 5 min. The molar ratio of metal salts/sodium hydroxide used was maintained at 1:2. The resulting paste was placed in a ceramic mortar and calcined to 400 °C in the muffle furnace (LT, Nabertherm, Germany) for 4 h. The mortar was left to cool in the air for 60 min, and then the powder was mechanically ground again for 2 min for easier collection in a falcon tube. The washing of NPs started by mixing the powder with distilled water in 50 mL falcon tubes and then centrifugation for 5 min at 12000 rpm via a Hermle Z 326 K (Labortechnik GmbH, Wehingen, Germany) centrifuge. One milliliter of sulfuric acid (1%) was added during the first wash. The number of washing steps depends on the amount of powder being washed. The pH value was measured after each wash to evaluate if there has been any remaining sodium hydroxide. The washed powders were then dried in an oven (Heratherm OGS100, Thermo Scientific, Germany) at 60 °C for 24 h and then ground well to prepare them for the characterization steps.37
4.2.2. Synthesis of Nanoparticles via Automated Mechanochemical Method
The automated synthesis of ZnO NPs and CuO NPs was similar to the above-mentioned manual procedure, with one difference being in the grinding and mixing method, using a grinder machine (Mediatech, AK, USA) instead of a mortar. The molar ratio of metal salts/sodium hydroxide used was maintained to be 1:2.37
4.3. Characterization of the Prepared Nanoparticles
4.3.1. Influence of Calcination on the Synthesized Crystals
The influence of calcination on the synthesized nanoparticles was assessed since it is the most energy-consuming step. For that, a comparison between the ZnO NPs prepared via OST pre- and postcalcination was performed regarding their XRD zeta-potential charts.
4.3.2. FTIR Analysis
Infrared absorption spectra of samples were obtained using FTIR spectroscopy on a Nicolet 380 spectrometer (ThermoScientific Nicolet, Waltham, MA, USA). For FTIR studies, the metal oxide samples were mixed with KBr powder in an approximate ratio of 1:10 and then pressurized into discs. The transmittance mode was adjusted to a frequency range of 400–4000 cm–1.
4.3.3. X-ray Diffraction Analysis (XRD) and Particle Size Evaluation
XRD patterns of the obtained powders were performed using a D8 Discover (Bruker). XRD patterns were obtained using an IV diffractometer operating at 40 kV, 30 mA, with a Cu Kα radiation wavelength of λ = 1.5406 Å. Diffractograms were taken from 20 to 80° in a 2θ scale and 0.03° step size for phase identification. The average particle size of the different prepared nanoparticles was calculated using the Debye–Scherrer equation:
where D is crystallite diameter (nm), K of 0.9 is the Scherrer constant, λ is the X-ray wavelength of Cu K radiation, θ is the Bragg diffraction angle (i.e., peak position, in radians), and β is the full width at half-maximum (fwhm) of the respective diffraction peak, in radians.39
4.3.4. Zeta-Potential Analysis
The zeta-potential values of ZnO NPs synthesized via the OST method pre- and postcalcination were assessed and compared. Then the zeta-potential values of the synthesized ZnO NPs, as well as CuO NPs, via four different methods, AMC, MMC, AST, and OST, were assessed and compared. A small amount of the prepared nanoparticles was dispersed in distilled water and sonicated and then characterized regarding their zeta-potential using a Malvern Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK).
4.3.5. Yield and Cost Analysis
The influence of the synthesis methods, mechanochemical (manual and automated) and solvothermal (aqueous and organic), on the metal oxide nanoparticles yield was evaluated. Ten grams of the metal salt precursors (zinc acetate dihydrate or copper acetate monohydrate) was used to prepare the nanoparticles via the same procedures described above. The theoretical yield of zinc oxide NPs was calculated to be 3.71 g, whereas that of CuO NPs was calculated to be 3.98 g. After synthesis and complete drying of the nanoparticles, the actual yield was weighed and the yield (%) was calculated using the following equation:
An experimental triplicate of all synthesis procedures was performed, and the yield (%) was averaged.
The cost effectiveness analyses of the four synthesis methods were performed in terms of yield of the produced nanoparticles versus the cost of materials, the number of steps utilized to obtain the metal oxide nanoparticles, and time consumption before reaching the washing step.
4.3.6. High-Resolution Transmission Electron Microscopy (HRTEM)
Direct images of the prepared nanoparticles were obtained by HRTEM characterization to observe their morphology and to investigate their particle size distribution. An aliquot of ZnO and CuO NPs, prepared for the AMC protocol, was dispersed in methanol. Then this mixture was added dropwise and dried over a carbon-coated copper 200 mesh grid, imaged, and photographed. HRTEM was performed with a JEOL-JEM 2100 electron microscope (Musashino, Akishima, Tokyo, Japan), operating at 160 kV. Image processing software, ImageJ (NIH, Bethesda, MD, USA), was used for the particle size distribution investigation of the NPs. The particle diameter (nm) histogram was produced from a total of 50 measurements. The inverse fast Fourier transmission pattern and the selected area electron diffraction patterns were obtained using transmitted and one of the diffracted beams.
4.3.7. Cytotoxicity Evaluation of the Metal Oxide Nanoparticles
Vero E6 cells were cultured using Dulbecco’s modified Eagle’s medium (DMEM) high glucose with 10% fetal bovine serum (FBS) supplemented with penicillin–streptomycin, l-glutamine, and sodium pyruvate. Cells were seeded in a 96-well plate and incubated for 24 h at 37 °C and 5% CO2 for attachment. The metal oxide nanoparticles (ZnO NPs and CuO NPs) of calcined and noncalcined samples were autoclaved before suspension in a sterile growth medium. Then serially diluted by concentrations of 500, 250, 125, and 62.5 31.25 μg/mL, each sample was repeated in triplicate. The cells were left for incubation with metal oxide nanoparticles for 48 h. Thirty microliters of MTT (25 mg/mL) was added to the cells and incubated for 3 h; then the medium was removed and replaced with 100 μL of DMSO and left incubating for 10 min before plate reading with Floustar (BMG labtech, Germany) at 570 nm. The dose–response curve was used to calculate the IC50.
4.4. Treatment of Cotton Fabrics with the As-Prepared Nanoparticles (ZnO NPs and CuO NPs)
First, different concentrations (0.2, 0.4, and 0.6% (w/v)) of the as-synthesized nanoparticles of both ZnO and CuO were dispersed in 200 mL of distilled water and kept under mechanical stirring for 15 min. After that, the resultant mixtures were subjected to sonication.
In the pretreatment processes, the fabric was alkaline scoured with NaOH and bleached with hydrogen peroxide. After the pretreatment, fabric samples were intensively rinsed with hot and cold distilled water and dried at 80 °C. After that, the bleached cotton fabrics (small pieces) were used for finishing with the as-prepared ZnO NPs and CuO NPs.
The cotton fabrics were cut into small pieces (15 × 15 cm), and each piece was padded into the solution of ZnO NPs and CuO NPs and kept under shaking in a water bath for 15 min. Then the padded cotton fabrics were submitted for the pad–dry–cure method using two successive cycles at 100% power of 0.2 MPa to equally distribute the nanoparticles into and onto the surface of the fabrics. Ultimately, the wet fabrics were dried in an oven at 80 °C for 2 min and cured at 140 °C for 3 min.40 For illustration, we have three codes, CuO NPs-1, CuO NPs-2, and CuO NPs-3, for the fabric treated with the three different concentrations of CuO NPs: 0.2% (w/v), 0.4% (w/v), and 0.6% (w/v), respectively. On the other hand, the fabrics treated with 0.2% (w/v), 0.4% (w/v), and 0.6% (w/v) of ZnO NPs were labeled as ZnO NPs-1, ZnO NPs-2, and ZnO NPs-3, respectively, as shown in Table 3.
Table 3. List of the Codes for the Treated Cotton Fabrics with Different Concentrations of the Utilized Nanoparticles.
| code sample | treated cotton fabric concentration |
|---|---|
| CuO NPs-1 | 0.2% (w/v) |
| CuO NPs-2 | 0.4% (w/v) |
| CuO NPs-3 | 0.6% (w/v) |
| ZnO NPs-1 | 0.2% (w/v) |
| ZnO NPs-2 | 0.4% (w/v) |
| ZnO NPs-3 | 0.6% (w/v) |
4.5. Characterization of the Treated Cotton Fabrics
4.5.1. Fabric Sample Preparation
Printofix was applied by stirring the fabrics in 1% Printofix solution in distilled water for 10 min, followed by heat fixation of the polymer at 80 °C for 30 min in an oven (Heratherm OGS100, Thermo Scientific, Germany).40
Manual washing of face masks is assumed since frequent washing would be required.41 Fabric washing cycles were carried out in a beaker, according to AATCC test method 107-2009, with some modifications. Four pieces of metal oxide NPs treated cotton fabrics (6 × 6 cm2 each) were stirred for 45 min at 200 rpm and 40 °C, with 300 mL of 0.3% (w/v) detergent in distilled water. Then the detergent was removed by rinsing the metal oxide NPs treated fabrics with distilled water. The fabrics were dried overnight at room temperature.42 The previous steps were repeated for each wash, and an antibacterial activity assessment was performed on samples acquired after each wash cycle.
Afterward, the samples were cut into 0.4 g pieces each. For each group (blank, CuO without PTF, CuO with PTF, CuO with PTF first wash, CuO with PTF second wash, CuO with PTF third wash, and CuO with PTF fourth wash), they were covered in foil and underwent standard autoclaving (121 °C for 15 min). Petri dishes were sterilized by baking in the oven at 180 °C for 2 h.
4.5.1.1. Wettability Test
The wettability of the finished fabrics was assessed using a drop test of water as described in British Standard BS 4774:1974. Contact angles were measured with a deionized water droplet on a Dataphysics OCA 20 (Dataphysics OCA 20, Germany) instrument at room temperature. All contact angles were determined by averaging values measured at 5–6 different points on each sample surface.
4.5.1.2. Air Permeability Test
With the use of an N530 B gas permeability analyzer (China), the oxygen transmission rate was measured. This rate of gas transmission was determined under the standards of ASTM D1434-82(2003) and ISO2556-2001.
4.5.1.3. Bacterial Permeability Test
Measurement of bacterial permeability of masks was performed according to the Egyptian standard specification No. 7803/2014. This Egyptian standard concerns the composition, performance requirements, and test methods of surgical masks to prevent the transmission of infection from the medical team to the patients (and sometimes the opposite) during surgeries, whether when performing surgeries or any other medical place with similar requirements. This test has been done by making a solution or spray of standard bacteria to pass through the mask using a suction pump to pass through the mask and measuring the passed bacteria. The mask passes the test if 95% or more of bacteria were not passed through the mask.
4.5.1.4. Scanning Electron Microscopy and Energy Dispersive X-ray Study (SEM-EDX)
The CuO NPs treated cotton fabrics were fixed on a carbon-painted copper grid and examined via a scanning electron microscope (Thermo Scientific, Japan) setup fitted with EDX. The surface morphology of the CuO NP treated cotton fabrics was inspected via a magnification software suite with SEM. In addition, the existence of CuO NPs on the fabric was confirmed through EDX analysis.
4.5.1.5. MTT Assay
Murine L929 normal fibroblast cells were cultured using DMEM high glucose with 10% FBS supplemented with penicillin–streptomycin, l-glutamine, and sodium pyruvate. Cells were seeded in a 96-well plate and incubated for 24 h at 37 °C and 5% CO2 for attachment. The concentrations of ZnO NPs and CuO NPs used were 200, 400, and 600 mg/mL that were soaked in the fabrics prior to padding and curing. Then the fabrics were autoclaved, and each sample was cut into 5 × 5 mm and fitted into each well. The cells were incubated with fabrics for 48 h and then the fabrics were removed. Thirty microliters of MTT (25 mg/mL) was added to the cells and incubated for 3 h; then the medium was removed and was replaced with 100 μL of DMSO and left for 10 min before plate reading with Omega Fluostar plate reader (BMG labtech, Germany) at 570 nm. The dose–response curve was used to calculate the IC50.
4.5.1.6. Antibacterial Activity
The antibacterial activity of the metal oxide NPs treated fabrics will be tested vis-à-vis its laundering durability by washing the fabrics five times and testing the antibacterial activity of each sample.
4.5.2. Overnight Culture Preparation
Four hundred milliliters of nutrient broth (3.6 g of broth powder in 400 mL of distilled water) and conical flasks covered with aluminum foil were sterilized via a standard autoclave. S. aureus and E. coli colonies from streaks were taken via an inoculum loop and inoculated in a conical flask with 100 mL of nutrient broth and left in the incubator at 37 °C overnight.
4.5.3. Antibacterial Assay for the Fabric Samples
The antibacterial assay for fabric specimens was done according to ISO20743:2021. Briefly, each fabric is spread in a 50 mL falcon tube. Overnight culture was diluted to log 8/mL by reaching a concentration of 0.1 OD using an Omega Fluostar plate reader (BMG labtech, Germany). The dilution was diluted further to log 5/mL, and 200 μL of inoculum was spread on the fabrics and capped. The falcon tubes were left in the incubator for 24 h. The fabrics were washed with 20 mL of 0.9% normal saline inside the falcon tube. Serial dilution was done from the aliquot of the washed normal saline and plated in sterile nutrient agar (1.5% agar–agar and 0.4% nutrient broth). The plated agars were left in the incubator overnight to count the colonies formed.
4.5.3.1. Antiviral Activity
The antiviral assay for the fabrics samples was done according to ISO 21702. Infectious bronchitis virus was inoculated at 200 μL onto the fabric samples inside a 50 mL falcon tube for 30 min at an egg infective dose of 107/mL. Then the virus was reconstituted into 5 mL of PBS and shaken gently for 30 s. Next, 100 μL of the PBS solution was infected in chicken egg embryo and left for 5 days under standard incubation. The dwarfing or curling was observed and tallied for protected or unprotected embryos from the virus. Afterward, the survival percentage was plotted against the number of washes of the cloth (n = 3).
4.6. Statistical Analysis
Statistical analysis was performed using the R Studio program version 4.1.0. A Kruskal–Wallis test was used as well as Scheirer–Ray–Hare test (a nonparametric two-way ANOVA) for yield analysis.43
Acknowledgments
We would like to thank the Science, Technology & Innovation Funding Authority (STDF) for funding the materials used in this study, as well as, part of the performed characterization. This project (No. 43823) was supported by a grant from Emergency Call I.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04692.
Actual yield of the four synthesis methods, averaged from experimental triplicates, for synthesis of both ZnO-NPs and CuO-NPs (Table S1); direct costs of metal oxide nanoparticle preparation using different methods (Table S2); zeta-potential distribution graphs of ZnO NPs prepared in ethanol (a) precalcination and (b) postcalcination (Figure S1); zeta-potential distribution graphs of ZnO NPs prepared via four different methods: (a) AMC, (b) MMC, (c) AST, and (d) OST (Figure S2); zeta-potential distribution graphs of CuO NPs prepared via four different methods: (a) AMC, (b) MMC, (c) AST, and (d) OST (Figure S3) (PDF)
Author Present Address
⊥ Department of Chemistry, Bioscience and Environmental Engineering, University of Stavanger, Stavanger, Rogaland, NO, 4036, Norway
The authors declare no competing financial interest.
Supplementary Material
References
- Mirzaei H.; Darroudi M. Zinc Oxide Nanoparticles: Biological Synthesis and Biomedical Applications. Ceram. Int. 2017, 43 (1), 907–914. 10.1016/j.ceramint.2016.10.051. [DOI] [Google Scholar]
- Chavali M. S.; Nikolova M. P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1 (6), 1–30. 10.1007/s42452-019-0592-3. [DOI] [Google Scholar]
- Chen X.; Selloni A. Introduction: Titanium Dioxide (TiO2) Nanomaterials. Chem. Rev. 2014, 114 (19), 9281–9282. 10.1021/cr500422r. [DOI] [PubMed] [Google Scholar]
- Cheong K. Y.; G. Impellizzeri M. A. F.. Emerging Materials for Energy Conversion and Storage; Elsevier, 2018. [Google Scholar]
- Shehabeldine A. M.; Hashem A. H.; Wassel A. R.; Hasanin M. Antimicrobial and Antiviral Activities of Durable Cotton Fabrics Treated with Nanocomposite Based on Zinc Oxide Nanoparticles, Acyclovir, Nanochitosan, and Clove Oil. Appl. Biochem. Biotechnol. 2022, 194 (2), 783–800. 10.1007/s12010-021-03649-y. [DOI] [PubMed] [Google Scholar]
- Mallakpour S.; Azadi E.; Hussain C. M. The Latest Strategies in the Fight against the COVID-19 Pandemic: The Role of Metal and Metal Oxide Nanoparticles. New J. Chem. 2021, 45 (14), 6167–6179. 10.1039/D1NJ00047K. [DOI] [Google Scholar]
- Xie J.; Cao Y.; Jia D.; Li Y.; Wang Y. Solid-State Synthesis of Y-Doped ZnO Nanoparticles with Selective-Detection Gas-Sensing Performance. Ceram. Int. 2016, 42 (1), 90–96. 10.1016/j.ceramint.2015.07.135. [DOI] [Google Scholar]
- Garcia R. A.; Stevanovic T.; Berthier J.; Njamen G.; Tolnai B.; Achim A. Cellulose, Nanocellulose, and Antimicrobial Materials for the Manufacture of Disposable Face Masks: A Review. BioResources 2021, 16 (2), 4321–4353. 10.15376/biores.16.2.Garcia. [DOI] [Google Scholar]
- Koch C. C. The Synthesis and Structure of Nanocrystalline Materials Produced by Mechanical Attrition: A Review. Nanostructured Mater. 1993, 2 (2), 109–129. 10.1016/0965-9773(93)90016-5. [DOI] [Google Scholar]
- Seyedi M.; Haratian S.; Khaki J. V. Mechanochemical Synthesis of Fe2O3 Nanoparticles. Procedia Mater. Sci. 2015, 11 (2000), 309–313. 10.1016/j.mspro.2015.11.093. [DOI] [Google Scholar]
- Wang J.; Du G. COVID-19 May Transmit through Aerosol. Ir. J. Med. Sci. 2020, 189 (4), 1143–1144. 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corma A.; Iborra S. Optimization of Alkaline Earth Metal Oxide and Hydroxide Catalysts for Base-Catalyzed Reactions. Adv. Catal. 2006, 49, 239–302. 10.1016/S0360-0564(05)49004-5. [DOI] [Google Scholar]
- Stankic S.; Suman S.; Haque F.; Vidic J. Pure and Multi Metal Oxide Nanoparticles: Synthesis, Antibacterial and Cytotoxic Properties. J. Nanobiotechnology 2016, 14 (1), 1–20. 10.1186/s12951-016-0225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar M.; Munisa L.; Saleh R. Co-Precipitation Synthesis and Characterization of Nanocrystalline Zinc Oxide Particles Doped with Cu2+ Ions. Mater. Sci. Appl. 2012, 3 (8), 543–551. 10.4236/msa.2012.38077. [DOI] [Google Scholar]
- Miwa T.; Miya G.; Zhu P.; Dodou D. Economic Evaluation of the Production Magnesium Oxide Nanoparticles via Liquid-Phase Route Economic Evaluation of the Production Magnesium Oxide Nanoparticles via Liquid-Phase Route. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 306, 012011. 10.1088/1757-899X/306/1/012011. [DOI] [Google Scholar]
- F Putra R.; Satari C.; S Sidqi R.; R Putri S.; B D Nandiyanto A. Techno-Economic Analysis on the Production of Copper Oxide Nanoparticles by Green Synthesis Method Using Abultion Indicum Leaf Extract on an Industrial Scale. Int. J. Res. Appl. Technol. 2021, 1 (1), 187–199. 10.34010/injuratech.v1i1.5667. [DOI] [Google Scholar]
- Li R.; Wang J.; He Y.; Dong F.; Bian L.; Li B. Mechanochemical Synthesis of Defective Molybdenum Trioxide, Titanium Dioxide, and Zinc Oxide at Room Temperature. ACS Sustainable Chem. Eng. 2019, 7 (14), 11985–11989. 10.1021/acssuschemeng.9b00374. [DOI] [Google Scholar]
- Amrute A. P.; De Bellis J.; Felderhoff M.; Schuth F. Mechanochemical Synthesis of Catalytic Materials. Chem. Eur. J. 2021, 27, 6819–6847. 10.1002/chem.202004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román L. E.; Gomez E. D.; Solís J. L.; Gómez M. M. Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles. Molecules (Basel, Switzerland) 2020, 25, 5802. 10.3390/molecules25245802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Liu X.; Yang L.; Wang Y.; Zhang Y.; Lang J.; Gao M.; Feng B. Effect of Annealing Temperature on the Structure and Optical Properties of ZnO Nanoparticles. J. Alloys Compd. 2009, 477 (1–2), 632–635. 10.1016/j.jallcom.2008.10.135. [DOI] [Google Scholar]
- Campora S.; Mohsen R.; Passaro D.; Samir H.; Ashraf H.; Al-mofty S. E.; Diab A. A.; El-sherbiny I. M.; Snowden M. J.; Ghersi G. Functionalized Poly(N-Isopropylacrylamide)-Based Microgels in Tumor Targeting and Drug Delivery. Gels 2021, 7, 203. 10.3390/gels7040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S.; Khatri A.; Baig U.; Javeed A.; Ali Rind N. Coloration of Cellulose Nanofibres with Pigments. Coloration Technology 2020, 136, 427–434. 10.1111/cote.12485. [DOI] [Google Scholar]
- Ibrahim N. A.; Eid B. M.; Khalil H. M.; Almetwally A. A. A New Approach for Durable Multifunctional Coating of PET Fabric. Appl. Surf. Sci. 2018, 448, 95–103. 10.1016/j.apsusc.2018.04.022. [DOI] [Google Scholar]
- Liu Z. L.; Deng J. C.; Deng J. J.; Li F. F. Fabrication and Photocatalysis of CuO/ZnO Nano-Composites via a New Method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2008, 150 (2), 99–104. 10.1016/j.mseb.2008.04.002. [DOI] [Google Scholar]
- Ma X. H.; Zeng S. S.; Zou B. K.; Liang X.; Liao J. Y.; Chen C. H. Synthesis of Different CuO Nanostructures by a New Catalytic Template Method as Anode Materials for Lithium-Ion Batteries. RSC Adv. 2015, 5 (71), 57300–57308. 10.1039/C5RA10825J. [DOI] [Google Scholar]
- Baruah S.; Thanachayanont C.; Dutta J. Growth of ZnO Nanowires on Nonwoven Polyethylene Fibers. Sci. Technol. Adv. Mater. 2008, 9, 025009. 10.1088/1468-6996/9/2/025009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H.; Zhu L.; Zheng W.; Zhang J.; Gao F.; Wang Y. Microwave-Assisted Template-Free Synthesis of Butterfly-like CuO through Cu2Cl(OH)3 Precursor and the Electrochemical Sensing Property. Solid State Sci. 2016, 61, 146–154. 10.1016/j.solidstatesciences.2016.09.017. [DOI] [Google Scholar]
- Talam S.; Karumuri S. R.; Gunnam N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. ISRN Nanotechnol. 2012, 2012, 1–6. 10.5402/2012/372505. [DOI] [Google Scholar]
- Yang S. T.; Liu J. H.; Wang J.; Yuan Y.; Cao A.; Wang H.; Liu Y.; Zhao Y. Cytotoxicity of Zinc Oxide Nanoparticles: Importance of Microenvironment. J. Nanosci. Nanotechnol. 2010, 10 (12), 8638–8645. 10.1166/jnn.2010.2491. [DOI] [PubMed] [Google Scholar]
- Namvar F.; Rahman H. S.; Mohamad R.; Azizi S.; Tahir P. M.; Chartrand M. S.; Yeap S. K. Cytotoxic Effects of Biosynthesized Zinc Oxide Nanoparticles on Murine Cell Lines. Evidence-based Complement. Altern. Med. 2015, 2015, 1. 10.1155/2015/593014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafagh M.; Rahmani F.; Delirezh N. CuO Nanoparticles Induce Cytotoxicity and Apoptosis in Human K562 Cancer Cell Line via Mitochondrial Pathway, through Reactive Oxygen Species and P53. Iran. J. Basic Med. Sci. 2015, 18 (10), 993–1000. [PMC free article] [PubMed] [Google Scholar]
- Uddin F.; Lomas M. Wettability of Easy-Care Finished Cotton. Fibres Text. East. Eur. 2010, 81 (4), 56–60. [Google Scholar]
- Shamhari N. M.; Wee B. S.; Chin S. F.; Kok K. Y. Synthesis and Characterization of Zinc Oxide Nanoparticles with Small Particle Size Distribution. Acta Chim. Slov. 2018, 65 (3), 578–585. 10.17344/acsi.2018.4213. [DOI] [PubMed] [Google Scholar]
- Sridar R.; Ramanane U. U.; Rajasimman M. ZnO Nanoparticles - Synthesis, Characterization and Its Application for Phenol Removal from Synthetic and Pharmaceutical Industry Wastewater. Environ. Nanotechnology, Monit. Manag. 2018, 10, 388–393. 10.1016/j.enmm.2018.09.003. [DOI] [Google Scholar]
- Topnani N.; Kushwaha S.; Athar T. Wet Synthesis of Copper Oxide Nanopowder. Int. J. Green Nanotechnol. Mater. Sci. Eng. 2010, 1 (2), M67–M73. 10.1080/19430840903430220. [DOI] [Google Scholar]
- Fukumoto S.; Nakanishi K.; Kanamori K. Direct Preparation and Conversion of Copper Hydroxide-Based Monolithic Xerogels with Hierarchical Pores. New J. Chem. 2015, 39 (9), 6771–6777. 10.1039/C5NJ00479A. [DOI] [Google Scholar]
- Tsuzuki T. Mechanochemical Synthesis of Metal Oxide Nanoparticles. Commun. Chem. 2021, 4 (1), 143. 10.1038/s42004-021-00582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjarodi A.; Roshani R. A Green Synthesis of Copper Oxide Nanoparticles by Mechanochemical Method. Curr. Chem. Lett. 2014, 3 (4), 215–220. 10.5267/j.ccl.2014.7.001. [DOI] [Google Scholar]
- Es’haghi Z.; Mohammadian M.; Hooshmand S. Green and Chemical Synthesis of Zinc Oxide Nanoparticles and Size Evaluation by UV-Vis Spectroscopy. J. Nanomedicine Res. 2018, 7 (1), 52–58. 10.15406/jnmr.2018.07.00175. [DOI] [Google Scholar]
- Hebeish A.; El-Naggar M. E.; Fouda M.G.; Ramadan M. A.; Al-Deyab S. S.; El-Rafie M. H. Highly effective antibacterial textiles containing green synthesized silver nanoparticles. Carbohydr. Polym. 2011, 86, 936–940. 10.1016/j.carbpol.2011.05.048. [DOI] [Google Scholar]
- Allison A. L.; Ambrose-Dempster E.; Bawn M.; Arredondo M. C.; Chau C.; Chandler K.; Dobrijevic D.; Aparasi T. D.; Hailes H. C.; Lettieri P.; Liu C.; Medda F.; Michie S.; Miodownik M.; Munro B.; Purkiss D.; Ward J. M.. The Impact and Effectiveness of the General Public Wearing Masks to Reduce the Spread of Pandemics in the UK: A Multidisciplinary Comparison of Single-Use Masks versus Reusable Face Masks. UCL Open Environ. 2021, 3. 10.14324/111.444/ucloe.000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P.; Zhang Z.; El-Moghazy A. Y.; Wisuthiphaet N.; Nitin N.; Sun G. Daylight-Induced Antibacterial and Antiviral Cotton Cloth for Offensive Personal Protection. ACS Appl. Mater. Interfaces 2020, 12 (44), 49442–49451. 10.1021/acsami.0c15540. [DOI] [PubMed] [Google Scholar]
- Rohlf F. J.; Sokal R. R.. Biometry; Macmillan Learning, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.