Abstract
We present a new mobile platform to be used in clinical trials aimed at both collecting data and assessing new technologies and treatments for diabetes care. The main components of the platform are a mobile app, that automatically collects data from continuous glucose monitoring sensors and activity trackers, and also allows users to manually log daily events; a cloud database for safe data storage; a web interface, which allows clinicians to monitor patients’ status in real-time. The platform is modular and highly customizable for a multitude of purposes in clinical research. Preliminary tests performed for daily-life data gathering by both clinicians and users are extremely encouraging.
Keywords: mobile platform, telemonitoring, digital therapeutics, wearable sensors, clinical trials
Introduction
Clinical trials are part of clinical research and at the heart of all medical advances. They allow to formally assess the efficacy of new therapies and/or medications to prevent, detect, and treat diseases (including the diabetes pathology), but they are very complex, expensive, and difficult to manage at many levels. For instance, data coming from many different sources, for example, sensors, questionnaires, and patient dairies, have to be collected, integrated and organized in a structured manner to allow to be easily accessed and analyzed. This is commonly solved with a recurrent two-phase approach where, first, patient manually generated data and sensors logs are acquired at the end of the trial, and then annoying time-consuming operations (such as data formatting and entering, removal of duplicates, labelling of events, etc,) are done by one or multiple operators. Another practical challenge arising during the execution of clinical trials is the need of monitoring, possibly in real-time, the patients’ physiological status in order to timely identify potentially dangerous adverse events resulting from the adoption of the therapy and/or medication under assessment. Monitoring patients in real-time is not always possible since it requires the setup of an ad-hoc telemonitoring infrastructure. In turn, this requires clinicians to run multiple periodical visits to retrospectively analyze a large amount of information, which is clearly burdensome for the clinical facilities which host the trial.
In this work, we propose a new platform that solves these problems effectively. The platform, named IMPACT (Integrated Mobile Platform for Automated Clinical Trials), allows to easily collect and structure data by mean of a customizable mobile app. The platform also offers a telemonitoring web interface for clinicians to monitor patients’ status in real-time during diabetes clinical trials. Preliminary clinical test experience with the platform is also presented.
Materials
Platform Structure
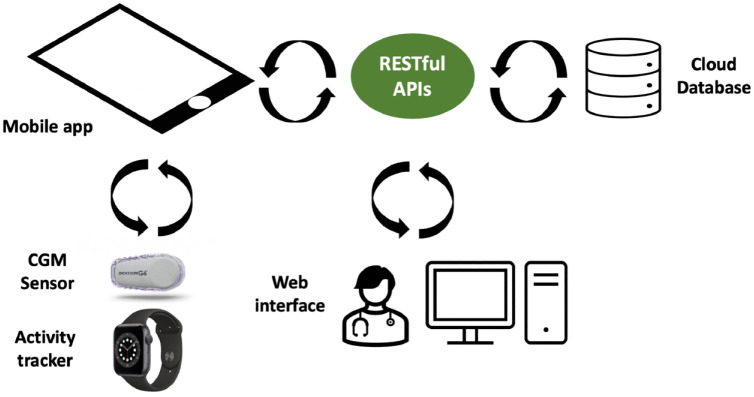
Figure 1 shows an overview of the platform structure. It is composed of 3 fundamental components: a mobile app, which allows to automatically collect sensors data and allows patients to manually insert daily events, a web
Figure 1.
IMPACT platform overview. The mobile app collects data from patients and stores them in the cloud database through ad-hoc RESTful APIs. The cloud database is queried by a web interface to provide real-time monitoring to clinicians.
interface, which enables clinicians to remotely monitor each patient in real-time, and a cloud database, which safely stores data and securely streams them through the platform. In the following sections, details of each component are reported in detail.
Mobile App
The mobile app has been built in Flutter 1 and runs in iOS. It is highly modular and customizable to fit the specific needs of each trial. In particular, it is equipped with several key features. Firstly, it automatically collects glucose measurements from Dexcom G6 (Dexcom Inc., San Diego, CA, USA) continuous glucose monitoring (CGM) sensors, and activity data from Apple Watch (Apple Inc., Cupertino, CA, USA) and Fitbit (Fitbit Inc., San Francisco, CA, USA) smartwatches, that is, heart rate, physical activity, sleep cycles, and steps. It is fully customizable to let patients manually record specific events such as meal intakes, insulin administrations, glucose concentration meter recordings, and symptoms. Moreover, the mobile app allows to set multiple custom alerts and alarms to notify patients when glucose concentration reaches critical levels. This feature can be also leveraged to create personalized notifications that can be used conveniently to remember patients to perform particular tasks peculiar of the trial, such as take some medications at specific hours, or log missing daily events.
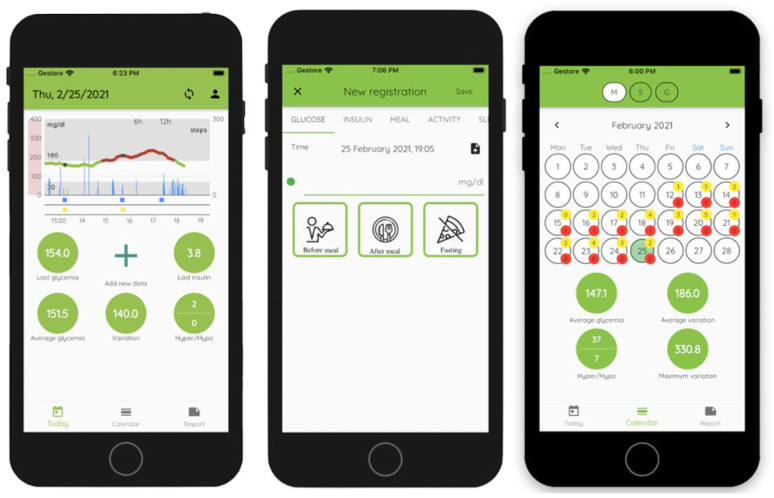
Figure 2 shows a snapshot of 3 app screens. The user interface has been built to be friendly and easy to use. In the home screen, glucose data are clearly shown and colored by glycemic zone. Additionally, multiple, customizable, statistics about glucose concentration in the last 24 hours are highlighted to provide users with immediate feedback about glycemic control. Manual insertion of daily event data is structured to be easy and, at the same time, to guarantee that the information is complete and free from errors. Collected data can be accessed and modified via a calendar screen, which also offers a quick overview of the most critical days. Finally, Ambulatory Glucose Profile (AGP) reports 2 can be easily generated and shared with clinicians.
Figure 2.
Three mobile app screens. To the left, the app home screen, which shows data collected in the last 24 hours and useful glucose control statistics. In the center, the screen to be used to insert new data manually. To the right, the calendar screen, which provide access to already collected data and gives an overview on glucose control for the selected period.
Web Interface and Cloud Database
The web interface of our platform, also built in Flutter, is hosted in the cloud and offers unique functionalities to clinicians to monitor, manage, and enroll patients during the trial. First of all, patients can be monitored in real-time through multiple screens which provide useful insights on their current physiological status. Through the interface, the clinicians can also “blind” specific data categories (eg, glucose readings) to the patients. With this option, all data are still collected by the mobile app, but the blinded categories are not accessible and visible by the patients.
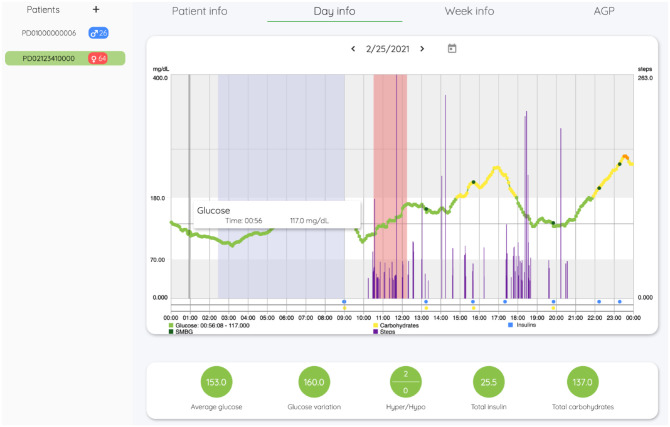
Figure 3 shows the main page of the web interface, where data collected in a subject in the last 24 hours are shown. The user interface and experience are similar to the mobile app’s but tuned according to the clinicians needs. Here, data and events can be easily highlighted and examined remotely any time. Collected data can be examined with 3 degrees of details: daily, weekly, or by generating an AGP report of a specified date range.
Figure 3.
Main page of the IMPACT web interface. On the left panel, each patient can be selected, and new patients can be enrolled in the trial. On the central panel, 4 subpanels allow to access to different information, that is, patient demographics, data collected in a specific day, data collected in specific week, and AGP report. On the bottom panel, statistics about the selected portion of data are provided.
Through the interface, hypoglycemic and hyperglycemic thresholds can be modified patient-by-patient according to their specific needs. Moreover, following the same rationale, clinicians can setup personalized alarms to be triggered when specific conditions hold.
The web interface directly communicates with the cloud database which stores, sends, and synchronizes data through the platform. The database is written in SQL and can be queried through ad-hoc RESTful APIs developed using the Lumen micro-framework. 3 This scheme permits to maximize data safety by guaranteeing that the database can be accessed only through specific secured endpoints. This also allows to increase the scalability of the platform and ensure that all users always deal with up-to-date information. Of note, data storage and processing within the platform complies with the General Data Protection Regulation. 4 In particular, collected data are anonymized, directly accessible by the during clinical trials, and not shared with any other application and/or infrastructure, for example, Apple Health and Google Fit, in order to avoid potential undesired data flows to other systems. Focusing on data access in the case of blinded studies, this is not possible by definition. As such, once the trial is finished and the “blind constraint” is no more necessary, the mobile app unblinds by default all the user-generated the platform data to grant this fundamental access right to the patients.
Results
The platform functionalities have been tested in a small beta session of 20 days involving one individual with type 1 diabetes. Collected data include glucose concentration, automatically gathered from Dexcom G6 CGM sensor, insulin bolus administrations, meal intakes, physical exercise sessions, sleep cycles, step counts, and generic textual notes. This testing session allowed to prove the usability of the platform.
Patient reported that the platform was very intuitive, easy-to-use, and did not add significant burden to its daily T1D management routine. Moreover, the patient indicated its propensity to potentially shift to our platform as default data management tool if available in the future. On the clinical side, the possibility of remotely monitor patients in real-time was highly appreciated and allowed to directly access patient data. if necessary, without the need of setting dedicated visits in the ambulatory. Clinicians reported that even using the web interface of our platform during routine follow-up visits allowed to get a clear picture of patient’s glycemic control and to easily identify the factors influencing patient’s glycemia. Finally, the overall usability appeared good, and all shown statistics allowed both clinician and the patient to improve data readability. The mobile app has been reported to be intuitive and user-friendly.
Conclusion
Through its features, the presented platform has the potential of easing the conduction of clinical trials on diabetes. Indeed, by automatically collecting and structuring data, and enabling remote and real-time patient monitoring, it greatly reduces the burden on both clinicians and clinical facilities. The possibility brought by its modularity and customization opportunities, makes it fit to most every kind of experiments. Indeed, in this paper we focused on the application of our platform to clinical trials involving people affected by T1D, being this the test bed use-case during its development, however, we built it with particular attention in terms of flexibility and modularity which allow to easily adapt it to clinical trials involving both healthy subjects and people affected by other pathologies than diabetes. All these elements together make evident the practical value of this work and hints its potential impact on clinical research.
In this brief manuscript, we focused on describing the technology rather than reporting extensive analyses on the platform performance. In fact, in this work, we limited ourselves at testing it in a small beta session involving just one subject. For this reason, we are planning at setting up a new testing session in the next future, involving multiple subjects, that will allow to extensively obtain quantitative metrics on the platform performance and usage. Future work will also involve the development of the mobile app also in the Android environment, as well as expanding the set of compatible devices for the automatic collection of patient data, such as smart pens for insulin administration and other wearable devices for activity tracking, to further improve the user experience and the platform capabilities. Furthermore, being of enormous interest and importance to the community, especially from the pharmaceutical regulatory point-of-view, we are currently working on making the platform compliant with 21 CFR Part 11 5 by implementing a trackable and securely signed data flow that will allow to fulfill this requirement by recording any user action, identifying the user which generated each data point, and guaranteeing the quality and immutability of the cloud database.
Acknowledgments
None
Footnotes
Abbreviations: (CGM) continuous glucose monitoring, (AGP) ambulatory glucose profile
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Daniela Bruttomesso, and Federico Boscari have received lecture fees from Abbott and Roche. Giacomo Cappon, Luca Cossu, Giovanni Sparacino, and Andrea Facchinetti declare no conflict of interest in connection with the submitted material.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Part of this work was supported by MIUR (Italian Minister for Education) under the initiative “Departments of Excellence” (Law 232/2016).
ORCID iDs: Giacomo Cappon  https://orcid.org/0000-0003-4358-9268
https://orcid.org/0000-0003-4358-9268
Luca Cossu  https://orcid.org/0000-0002-8804-0722
https://orcid.org/0000-0002-8804-0722
Giovanni Sparacino  https://orcid.org/0000-0002-3248-1393
https://orcid.org/0000-0002-3248-1393
References
- 1. Flutter [Internet]. Google; c2017-2021 [cited 20 March 2021]. Available from: https://www.flutter.dev.
- 2. Johnson ML, Martens TW, Criego AB, Carlson AL, Simonson GD, Bergenstal R. Utilizing the Ambulatory Glucose Profile to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther. 2019; Suppl 2: S217-S225. [DOI] [PubMed] [Google Scholar]
- 3. Lumen micro-framework [Internet]. Taylor Otwell; c2016-2021 [cited 20 March 2021]. Available from: https://lumen.laravel.com.
- 4. European Parliament and Council of European Union. Regulation (EU) 2016/679. Accessed March 20, 2021. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN (2016)
- 5. US Food and Drug Administration. 21 CFR part 11. Accessed May 6, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11 (1997).