Abstract
Purpose:
The purpose of this study was to analyze the impact of virtual group appointments (VGA) on self-reported health-related outcomes and care activities for young adults (YA) with type 1 diabetes (T1D).
Methods:
Fifty-three YA (ages 18-25 years) with T1D participated in a randomized controlled trial (RCT) of the Colorado Young Adults with T1D (CoYoT1) Clinic intervention, encompassing telehealth (TH) with or without VGA. Both new patients (n = 32) and those who participated in a pilot phase (n = 26) were randomized to CoYoT1 Clinic (TH+VGA; n = 23) or TH-only (n = 35) and followed for 1 year. YA completed the Diabetes Distress Scale (DDS), Diabetes Strengths and Resilience (D-STAR), Self-Efficacy in Diabetes (SED), Self-Management of Type 1 Diabetes in Adolescence (SMOD-A), Center for Epidemiologic Studies Depression (CES-D), and EuroQol (EQ-5D) scales at baseline and study end.
Results:
YA were 67% female, 84% white, 10% Latinx, and the mean age was 20.4 years old. At study end, participants in CoYoT1 Clinic reported significantly reduced diabetes distress compared to those in TH-only, who reported increased levels [Effect Size (ES) = −0.40, P = .02]. Specifically, CoYoT1 Clinic participants reported relative reductions in Physician (ES = −2.87, P = .02) and Regimen-related distress (ES = −0.35, P = .01). In addition, participants in CoYoT1 Clinic reported improved self-management of T1D-related problem solving (ES = 0.47, P = .051) and communication with care providers (ES = 0.39, P = .07).
Conclusions:
Virtual group attendance in CoYoT1 Clinic was associated with significant improvements in diabetes-related distress. Long-term exposure to VGA should be investigated in YA with T1D and other pediatric chronic conditions.
Keywords: group appointments, shared medical appointments, telehealth, type 1 diabetes, virtual care, young adults
Introduction
Children and youth with chronic medical conditions are more likely to experience poor psychosocial health, including increased depressive1,2 and anxious 3 symptoms, impaired cognitive and social functioning, 4 and lower overall health-related quality of life (HRQOL) 5 compared to their peers without chronic medical conditions. By the time children and youth with chronic medical conditions reach young adulthood, they are less likely to graduate from high school or have a job; are more likely to lose health insurance coverage; and are more likely to receive Social Security, disability, or other federal assistance than their peers without chronic medical conditions.6,7
For young adults (YA) with type 1 diabetes (T1D), the transition from pediatric to adult care is often associated with higher blood glucose levels, as patients manage intertwined physiological and psychosocial changes. 8 Fewer than one-third of YA with T1D meet self-care recommendations set forth by the American Diabetes Association, and only one in 7 meets the American Diabetes Association goal of attaining glycated hemoglobin (HbA1c) levels less than 7%.9,10 Lower engagement in self-care, elevated HbA1c, and other factors increase their risk for developing diabetes complications, including retinopathy, renal disease, neuropathy, diabetic ketoacidosis, and disordered eating behaviors.7,10-13 Given the challenges YA with T1D face in maintaining optimal physical and mental health, care models for this population must be adapted to improve patient engagement.
Two treatment modalities—telehealth (TH; or virtual care) and group (shared medical) appointments—have demonstrated promise for YA with T1D. Pediatric patients in TH maintain or improve blood glucose levels, increase frequency of follow-up care attendance, and report greater care satisfaction. 14 They also report missing school or work less frequently to attend appointments. 15 Similarly, participants in group appointments maintain or improve blood glucose levels, and report reduced diabetes burden, 16 improved HRQOL, 17 and greater care satisfaction.18,19 The benefits of group appointments have been demonstrated,16-19 but evidence of positive effects following random assignment (as opposed to self-selection into the intervention) is scarce. One study, of the Team Clinic in-person group appointment model in adolescents, shows positive psychosocial benefits in the form of reduced familial conflict and improved mood, 20 but the effects of this type of intervention delivered virtually to YA patients are unknown.
Given the potential of these 2 approaches, an experimental care model combining these modalities, called CoYoT1 (Colorado Young Adults with T1D) Clinic, was developed. CoYoT1 Clinic delivers both individual TH provider visits and virtual group appointments (VGA) for YA with T1D. In a pilot phase, self-selection to CoYoT1 Clinic participation was found to be a feasible and acceptable care model for YA with T1D, with high engagement, retention, and satisfaction. 21 In further analysis, CoYoT1 Clinic participation was also shown to be associated with improved diabetes self-efficacy and communication, and lower distress, compared to participants in usual care. 22 Given the various challenges that implementing VGA poses, including coordination, replication, and insurance billing, this follow-up study was designed to assess whether the inclusion of VGA in the CoYoT1 Clinic care model was critical to the outcomes observed in the pilot phase. The current randomized controlled trial of CoYoT1 Clinic examines whether random assignment to VGA participation is associated with greater improvements in health-related outcomes, including HbA1c, psychological health, and self-care.
Methods
Participants and Study Design
This 12-month randomized controlled trial examined the efficacy of TH-only versus CoYoT1 Clinic (TH+VGA), a virtual adaptation of the Team Clinic group appointment model.18,19 TH-only consisted of 3 visits via TH and 1 in-person visit over 12 months. CoYoT1 Clinic added VGA to TH visits over the same treatment period. Some participants were previously exposed to VGA and TH during a pilot phase assessing the feasibility of CoYoT1 Clinic. TH and VGA visits were conducted using an Internet-connected device and VidyoTM (Vidyo, Inc., Hackensack, NJ), a HIPAA-compliant encrypted videoconferencing platform.
YA with T1D (ages 18-25 years) were recruited from the Barbara Davis Center, a freestanding diabetes center specializing in research and clinical care. Eligible YA were approached in clinic or contacted via phone/email, and those interested provided written informed consent if they were consented in-person or verbal informed consent if they were consented virtually (Vidyo or phone). Eligible participants had to have reliable Internet access and be physically located in Colorado for all scheduled appointments. Participants were excluded if they were newly diagnosed with T1D (<6 months since diagnosis), did not speak English, or had documented severe behavioral or psychological disorders that would preclude VGA participation (by chart review and/or provider recommendation). At baseline and end of study, participants were asked to complete validated questionnaires via REDCap and received $20 and $40 for the completion of the baseline and end of study questionnaires, respectively, with the option to donate their monetary incentives to charity. All study activities were approved by the Institutional Review Board at the University of Colorado, Denver.
CoYoT1 clinic and VGA design
For each visit, CoYoT1 Clinic offered TH with a diabetes specialist (eg, endocrinologist or nurse practitioner) trained in patient-centered care practices, as well as VGA. VGA were designed to address the developmental and psychosocial needs of YA with T1D with the goal of enhancing peer support. All VGA were facilitated by a peer leader and scheduled near, but independent of, individual TH clinic visits. VGA included 4 to 6 YA and one facilitator and lasted approximately 30 minutes each. Four VGA were offered over the duration of the study. Sessions began with an introductory icebreaker, and then transitioned into a patient-driven discussion. Topics included stress management, building a support system, alcohol or drug use, and diabetes burnout. Participants were encouraged to interact with each other by sharing stories and asking questions about diabetes-related topics. Although the facilitator was present, VGA were designed to be patient-driven discussions. Further details from the pilot phase have previously been reported.21,22
Participant-Reported Outcomes and Other Data
Demographic and clinical data, including insulin regimen and continuous glucose monitor use were collected through chart review. At baseline and study end, participants were asked to complete several general and T1D-specific measures, including:
The Diabetes Distress Scale (DDS) 23 assesses emotional, regimen-related, physician-related, and interpersonal distress related to living with T1D. Seventeen items across 4 subscales are rated on a 6-point Likert scale, where higher scores indicate greater distress. Responses are averaged across the measures and within subscales, where scores ≥3 indicate high distress.
The Diabetes Strengths and Resilience Scale (DSTAR-Teen) 24 measures one’s perceptions of competence in T1D self-care and ability to adapt to challenges. Twelve items assessing 2 subscales—confidence and management—are rated on a 5-point Likert scale and totaled; higher scores indicate greater strength.
The Self-Efficacy for Diabetes Scale (SED) 25 includes 35 items that measure one’s own belief in their ability to handle challenges related to T1D, including diabetes-specific, medical, and general situations, on a 6-point Likert scale, where higher scores indicate greater self-perceived ability. Total scale scores are reported.
The Self-Management of Type 1 Diabetes in Adolescence Scale (SMOD-A) 26 assesses 5 dimensions of T1D care, including collaboration with parents (13 items), regimen and care activities (15 items), problem-solving (7 items), communicating about symptoms and care with providers (10 items), and goal-setting (7 items). Items are rated on a 4-point scale and summed within each dimension.
The Center for Epidemiologic Studies Depression Scale (CES-D) 27 utilizes 20 items (0-3 scale) to assess general depressive symptoms, where higher scores indicate greater symptoms; total CES-D scores ≥16 suggest clinically significant levels of depressive symptoms, warranting further assessment by a mental health professional.
The EuroQol-5L (EQ-5D) 28 assesses 5 dimensions of HRQOL—mobility, self-care, usual activities, pain/discomfort, and anxiety/depression—using 5 items (1-5 scale), where higher scores indicate worse functioning.
Statistical Analysis
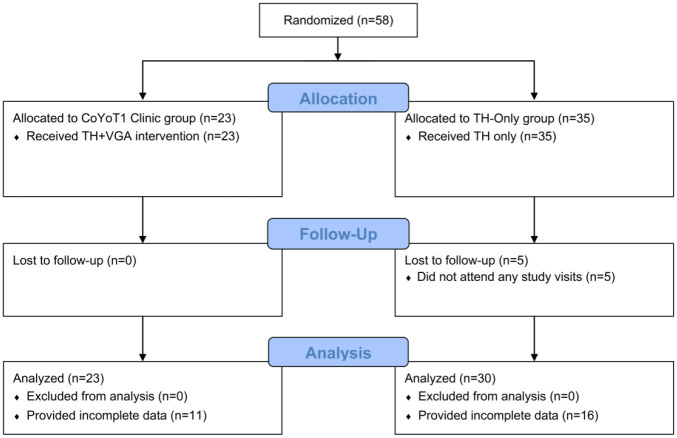
This study was designed to detect an effect size (ES) around ES = 0.75, requiring data from 60 participants among the TH-only and CoYoT1 Clinic groups, assuming a Type I error rate of 0.05 and Type II error rate of 0.20 (ie, 80% power). This ES value corresponds to a 1% difference in HbA1C change, or a 3/4 standard deviation difference between changes in patient-reported measures. Fifty-eight participants were randomized, 53 provided at least some baseline data for analysis, and 26 provided complete follow-up data (Figure 1).
Figure 1.
Schema of the study analysis.
Baseline demographic and clinical data were examined using Fisher’s exact tests or independent samples t-test, as appropriate. HRQOL utility scores were calculated from raw EQ-5D data using a method described by Pickard et al., 29 where EQ-5D responses are assigned US population-normed parameter weights, summed, and subtracted from one. Changes in health-related outcomes from baseline to study end were compared between groups using linear mixed-effects regression models adjusted for age, sex, and baseline values for each outcome; fit via restricted maximum likelihood; and modified for small sample inference using the degrees of freedom calculation method suggested by Kenward and Roger. 30 For binary outcomes (continuous glucose monitor and insulin pump use), mixed effects logistic regression models with similar adjustments were used. A modified intent-to-treat analysis approach was used to include all available data from participants, including those with missing follow-up data, without imputation of missing values. Per-protocol analyses on the subset of participants with complete data who attended most sessions produced comparable significant results with reduced power, so they are not included here. Pilot phase participation was also included as a covariate to account for differences at baseline attributable to any pre-study treatment effects. In cases where significant RCT treatment effects were observed, sub-analyses were conducted with additional interaction terms to examine whether treatment effects were attributable to VGA exposure during the pilot phase; linear mixed effects models with the same specifications as above were utilized. Because the study was powered to detect only a single effect on a health-related outcome, multiple test correction was not employed across outcomes. All model terms were considered significant at P values less than .05. Standardized change scores are reported as Cohen’s dz in figures and tables; SE was calculated for figures using a formula proposed by Hedge and Olkin. 31 Effect sizes for binary outcomes are reported as odds ratios. All analyses were conducted using Stata/SE 14.2 (StataCorp LLC, College Station, TX, USA).
Results
Participant Characteristics and Visit Attendance
Fifty-eight participants were randomized to receive either TH-only (n = 35) or CoYoT1 Clinic (n = 23). As shown in Table 1, participants were 67% female, 84% White, and 10% Latinx. Eighty-one percent of participants (n = 47) had private insurance. At baseline, participants had a mean age of 20.4 (±2.0) years and T1D duration of 10.1 (±5.1) years. Most participants reported insulin pump use (79.6%), but only some (24.5%) reported continuous glucose monitor use; baseline pump (P = .30) and glucose monitor (P = .81) usage did not vary significantly between study groups. New and pilot phase participants were equally represented in analyzed data (P = .87). Nearly half (n = 11) of CoYoT1 Clinic participants attended all VGA. The timing of completed labs and questionnaires relative to study visits was similar between study groups (P = .79).
Table 1.
Demographic and Clinical Data by Study Group.
| Variables | TH-only (n = 35) | CoYoT1 clinic (n = 23) | Total (N = 58) | P Value |
|---|---|---|---|---|
| Demographic data – n (%) | ||||
| Sex, n (%) | ||||
| Female | 25 (71%) | 14 (61%) | 39 (67%) | .40 |
| Male | 10 (29%) | 9 (39%) | 19 (33%) | |
| Race, (%) | ||||
| African-American | 0 | 1 (5%) | 1 (2%) | .68 |
| White | 31 (89%) | 18 (82%) | 49 (84%) | |
| Multi-racial | 2 (6%) | 1 (5%) | 3 (5%) | |
| Unknown | 2 (6%) | 3 (9%) | 5 (9%) | |
| Ethnicity, n (%) | ||||
| Latinx | 4 (11%) | 2 (9%) | 6 (10%) | 1.00 |
| Non-Latinx | 31 (89%) | 21 (91%) | 52 (90%) | |
| Insurance, n (%) | ||||
| Private | 27 (77%) | 20 (87%) | 47 (81%) | .84 |
| Public (Medicaid) | 6 (17%) | 2 (9%) | 8 (14%) | |
| Military (Tricare) | 1 (3%) | 0 | 1 (2%) | |
| Unknown | 1 (3%) | 1 (4%) | 2 (3%) | |
| Clinical data – mean (SD) | ||||
| Age at enrollment (years) | 20.43 (1.94) | 20.52 (2.06) | 20.47 (1.98) | .86 |
| T1D duration (years) | 8.93 (5.22) | 11.00 (4.84) | 10.08 (5.05) | .21 |
| Study group assignment – n (%) | ||||
| New participant | 19 (54%) | 13 (57%) | 32 (55%) | .87 |
| Pilot phase participant | 16 (46%) | 10 (43%) | 26 (45%) | |
| Attendance – mean (SD) | ||||
| TH care visits | 1.77 (1.90) | 3.17 (1.07) | 2.33 (1.75) | .002 |
| VGA sessions | 3.00 (1.28) | |||
Changes in Health-Related Outcomes
Diabetes-specific measures
In general, participants assigned to CoYoT1 Clinic reported reduced diabetes distress and improved management of T1D self-care activities compared to those participants assigned to TH-only (Table 2).
Table 2.
Adjusted Mean Changes in Health-Related Outcomes over Study by Treatment Group.
| Measure/subscale mean (SD) | TH-only (n = 35) | CoYoT1 clinic (n = 23) | Treatment effect | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Study end | Δ | Baseline | Study end | Δ | Effect size | P Value | |
| DDS | n = 26 | n = 16 | n = 18 | n = 16 | ||||
| Average score | 1.94 (0.88) | 2.14 (0.80) | 0.19 | 1.95 (0.85) | 1.79 (0.71) | −0.16 | −0.40 | .02 |
| Emotional distress | 2.35 (1.43) | 2.46 (1.30) | 0.10 | 2.37 (1.36) | 2.19 (1.14) | −0.17 | −0.20 | .20 |
| Physician distress | 1.02 (0.12) | 1.38 (0.09) | 0.36 | 1.02 (0.14) | 1.02 (0.09) | 0.00 | −2.87 | .02 |
| Regimen distress | 2.45 (1.36) | 2.62 (1.15) | 0.17 | 2.46 (1.26) | 2.17 (1.00) | −0.29 | −0.35 | .01 |
| Interpersonal distress | 1.66 (0.84) | 1.81 (0.84) | 0.15 | 1.65 (0.81) | 1.51 (0.71) | −0.14 | −0.35 | .15 |
| DSTAR-teen | n = 26 | n = 16 | n = 18 | n = 16 | ||||
| Total score | 48.45 (8.76) | 46.33 (7.23) | −2.11 | 48.68 (4.54) | 47.13 (3.71) | −1.56 | 0.07 | .75 |
| Confidence | 25.14 (4.22) | 23.73 (3.91) | −1.41 | 25.22 (2.52) | 24.67 (2.17) | −0.54 | 0.22 | .45 |
| Management | 23.26 (5.13) | 22.78 (3.66) | −0.48 | 23.32 (3.37) | 22.21 (2.56) | −1.10 | −0.14 | .51 |
| SED | n = 24 | n = 14 | n = 16 | n = 12 | ||||
| 142.34 (24.85) | 139.33 (23.62) | −3.01 | 141.58 (13.62) | 136.19 (13.76) | −5.39 | −0.10 | .74 | |
| SMOD-A | n = 26 | n = 16 | n = 18 | n = 16 | ||||
| Collaboration | 8.99 (5.43) | 9.70 (5.04) | 0.71 | 8.99 (4.57) | 8.62 (4.26) | −0.37 | −0.21 | .46 |
| Care activities | 36.17 (6.67) | 35.70 (4.70) | −0.46 | 36.13 (8.16) | 37.03 (5.90) | 0.90 | 0.19 | .27 |
| Problem solving | 18.04 (3.44) | 17.51 (1.69) | −0.53 | 18.00 (2.70) | 18.98 (1.39) | 0.98 | 0.47 | .051 |
| Communication | 19.95 (5.31) | 20.47 (3.20) | 0.52 | 19.95 (5.20) | 22.60 (3.24) | 2.65 | 0.39 | .07 |
| Goal setting | 16.89 (5.04) | 16.27 (3.72) | −0.61 | 16.96 (2.56) | 17.44 (1.89) | 0.48 | 0.25 | .24 |
| CES-D | n = 25 | n = 16 | n = 18 | n = 15 | ||||
| 17.08 (12.52) | 17.86 (12.39) | 0.78 | 16.97 (7.88) | 16.81 (7.30) | −0.15 | −0.08 | .71 | |
| EQ-5D utility score | n = 27 | n = 18 | n = 18 | n = 16 | ||||
| 0.76 (1.19) | 0.76 (0.60) | 0.01 | 0.75 (1.44) | 0.81 (0.60) | 0.07 | 0.05 | .88 | |
| HbA1c (%) | n = 18 | n = 14 | n = 22 | n = 14 | ||||
| 8.61 (1.34) | 9.15 (0.93) | 0.54 | 8.62 (1.37) | 9.51 (1.18) | 0.88 | 0.25 | .60 | |
| Continuous glucose monitor use, % Yes (SE) | n = 26 | n = 20 | n = 23 | n = 17 | ||||
| 24.01 (7.87) | 22.22 (8.34) | −1.79 | 25.96 (8.71) | 33.32 (10.52) | 7.36 | 2.52 | .53 | |
| Insulin pump use, % Yes (SE) | n = 26 | n = 20 | n = 23 | n = 18 | ||||
| 71.88 (8.42) | 74.88 (9.22) | 3.00 | 87.92 (6.42) | 94.30 (5.42) | 6.38 | 2.00 | .63 | |
All estimated marginal means have been adjusted for age, sex, pilot phase participation, and baseline values of outcomes. Standard deviations were estimated using individual predicted scores from regression models. For changes in Continuous Glucose Monitor and Insulin Pump Use, standard errors of marginal mean percentages are reported, and odds ratios are reported in lieu of Cohen’s dz Effect Sizes. EQ-5D subscale scores are weighted utility parameters based on US population norms reported in Pickard et al. 29
DDS
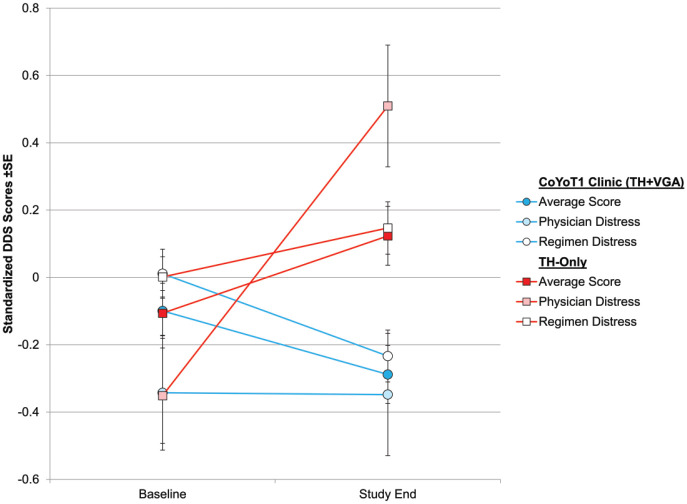
At the end of the study, CoYoT1 Clinic participants reported reductions in T1D-related distress on average, while participants in the TH-only group reported increases in distress over the same period (ES = −0.40, P = .02). Specifically, participants in CoYoT1 Clinic reported reductions in distress related to regimen adherence, while those in TH-only reported increases (ES = −0.35, P = .01; see Figure 2). CoYoT1 Clinic participants reported no changes in distress related to physician interactions, while those in TH-only reported marked increases in physician distress (ES = −2.87, P = 0.02; Figure 2). CoYoT1 Clinic participants also reported reductions in emotional and interpersonal distress relative to those in TH-only, but these were not statistically significant.
Figure 2.
Changes in diabetes distress (DDS) over study year.
DSTAR-teen
CoYoT1 Clinic participation was not associated with significant changes in self-confidence or self-perceived ability to manage T1D symptoms, as assessed by the DSTAR-Teen (ES = 0.07, P = .75).
SED
Compared to TH-only participants, CoYoT1 Clinic participants did not report any significant changes in self-efficacy over the course of the study (ES = −0.10, P = .74).
SMOD-A
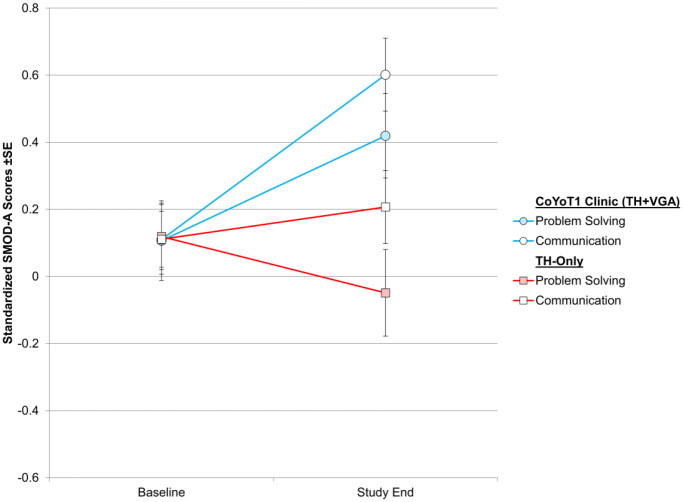
CoYoT1 Clinic participants reported non-significant improvements in problem solving abilities (ES = 0.47, P = .051) and communication with care providers about symptoms and care (ES = 0.39, P = .07), compared to those in TH-only, who reported smaller changes in these areas (see Figure 3).
Figure 3.
Changes in self-management of T1D Care (SMOD-A) over study year.
General HRQOL and depression
No significant changes in HRQOL were observed over the course of the study, as measured by the EQ-5D-based utility score (ES = 0.05, P = .89; see Table 2). Similarly, no changes in depressive symptoms were observed, as measured by the CES-D (ES = −0.08, P = .71).
Hemoglobin A1c and diabetes device use
HbA1c increased over the course of the study in both CoYoT1 Clinic and TH-only participants, and these changes did not significantly differ between study groups (ES = 0.25, P = .60). Similarly, any changes in continuous glucose monitor use (OR = 2.52, P = .53) and insulin pump use (OR = 2.00, P = .63) did not significantly differ between study groups.
Sub-Analyses by Pilot Phase Participation
CoYoT1 Clinic participants reported reductions in diabetes-related distress and self-management of T1D care activities, as compared to TH-only participants. Since a subset of participants was previously exposed to VGA during the pilot phase, these results were examined further while accounting for this previous exposure.
In a model examining average changes in diabetes distress, all participants in CoYoT1 Clinic showed similar reductions in DDS average scores (Δ = −0.44, ES = −0.51, P = 0.03), and previous group exposure provided no significantly greater benefit or impediment over the current study period (Δ = 0.16, ES = 0.23, P = .55). DDS subscales showed similar patterns.
In models assessing changes in SMOD-A subscales, different patterns were observed. Changes in problem solving were similar to those reported for distress, as improvements in CoYoT1 Clinic participants were not significantly different between new participants and those previously exposed to the intervention (Δ = 1.27, ES = 0.39, P = .41). In contrast, relative benefits in communicating with care providers seen in the CoYoT1 Clinic group were primarily attributable to those with previous exposure, who showed large improvements compared to those in TH-only with previous VGA exposure (Δ = 4.29, ES = 0.77, P = .049). Participants new to CoYoT1 Clinic showed no greater changes over the study than new participants in the TH-only group (Δ = −0.31, ES = −0.06, P = .85).
Discussion
YA patients with chronic conditions often encounter feelings of stigma and isolation that traditional healthcare settings may not be fully equipped to combat. Many patients seek out emotional and technical support, advocacy, and humor through disease-specific social media, blogs, and other online resources.32,33 Integrating peer support into the healthcare system is another strategy to address stigma and isolation that may enhance patient health and well-being. This study is one of the first to demonstrate that random assignment to VGA (CoYoT1 Clinic) is associated with a substantial reduction of the psychosocial burden experienced by YA with T1D.
Diabetes-related distress, including worry about completing diabetes self-care tasks and feeling unsupported by family and care providers, is associated with poorer symptom management, resulting in higher HbA1c and more frequently missed insulin boluses. 34 In the current study, diabetes-related distress increased in TH-only participants, suggesting that the peer interactions for CoYoT1 Clinic participants during VGA may address some need for social support not met in usual care.
CoYoT1 Clinic was associated with non-significant improvements in problem solving abilities and communication with care providers about symptoms and care relative to TH-only participants, as measured by the SMOD-A. Although these findings were non-significant, they should be evaluated in a larger sample size due to the large effect size. Self-regulated, effective symptom management is likely to follow reductions in psychosocial barriers (eg, negative attitudes towards diabetes) and increased social support. 35 As YA patients encounter new challenges, VGA may serve as a valuable resource for solving challenges with diabetes and establishing more independence through improved communication with care providers.
Consistent with previous research on the CoYoT1 Clinic,20,22 many YA reported elevated depressive symptoms at baseline and maintained these levels throughout the study. Elevated CES-D scores exceeded 16, which epidemiologic data suggests is a cutoff for participants who may meet the diagnostic criteria for depression. 27 However, formal diagnoses were not made by mental health professionals. This finding is not unexpected considering the VGA intervention was not designed to treat symptoms of depression (eg, via cognitive behavioral therapy or anti-depressant medication), and YA with T1D often struggle with a wide range of psychosocial stressors. Because elevated depressive symptoms increase the likelihood of poorer health outcomes, screening and addressing depressive symptoms is especially critical for these patients.
Although there was no statistically significant difference in HbA1c between groups, this finding must be viewed in the context of the study duration. Previous research relates improvements in diabetes-related distress to reductions in HbA1c. 34 Importantly, improving psychosocial outcomes is co-equal in importance to patients as reducing HbA1c. Improvements in diabetes-related distress observed in the current study may thus represent steps towards improved self-care and blood glucose levels over time. For instance, group appointments have been shown to increase diabetes-related technology uptake.21,36 While non-significant, the over twofold increases in the uptake of insulin pump and continuous glucose monitor use in the current study suggest that future studies should study differences in technology uptake in a larger sample size or over a greater duration of time. Notably, reductions in diabetes related distress were not associated with any changes in HbA1c observed in the current study. Participants whose HbA1c improved were equally distributed across both study groups, but those who reported reduced distress were more commonly assigned to VGA.
Given that some participants in both groups had previous exposure to VGA in a pilot phase, sub-analyses were conducted to elucidate the contribution of prior exposure to significant findings. For diabetes distress, this analysis affirms that randomization to VGA improves diabetes-related distress regardless of prior exposure. New participants randomized to CoYoT1 Clinic contributed equally to the significant group-level reduction in diabetes-related distress, relative to TH-only participants. A similar pattern was observed for changes in T1D-related problem solving abilities, where all CoYoT1 Clinic participants showed similar gains.
Conversely, improvements in communicating with care providers about T1D were seen in all participants on average, except those previously exposed to VGA randomized to TH-only in this study. The loss of virtual groups may have led to participants experiencing changes in the patient-provider relationship or required these participants to adjust learned communication strategies over the study, resulting in lower scores. Those participants in CoYoT1 Clinic during both the pilot phase and RCT showed the largest gains, while all new participants showed similar, smaller gains. Improvements reported by new participants in TH-only and CoYoT1 Clinic may be attributable to the novelty of virtual care appointment, which presented a new structure for talking to care providers (over video conference). Because of these inconsistent results, an RCT with a longer duration of follow-up is needed to investigate whether changes in communication are attributable to TH, VGA, or both.
Limitations and Future Directions
CoYoT1 Clinic participants were not blinded and had more contact with their healthcare team due to VGA. As a result, it is possible that the positive effects of VGA could be due in part to an attention effect from more frequent interactions with their healthcare team, irrespective of the VGA content. Future studies may include a virtual group control condition, where groups convene, but do not discuss diabetes-specific content. However, this approach may be challenging to implement in a real-world clinical setting.
Study participants were predominately white, non-Hispanic, and carried private insurance, limiting study generalizability to different demographic groups. Racial, ethnic, and socioeconomically diverse adolescents and YA have shown equal interest and ability to participate in VGA. 37 An adapted CoYoT1 Clinic model 38 focused on racial, ethnic, and socioeconomically diverse adolescents and YA is currently being evaluated. The benefits of TH alone are also being examined. Given the sociocultural upheaval associated with the COVID-19 pandemic, supportive virtual care will be critical in serving at-risk patients. Future research will examine strategies to facilitate engagement among individuals at highest risk for poor health outcomes, such as those with elevated depressive symptoms, as well as factors associated with positive outcomes, to further develop and refine the intervention.
Conclusions
With a focus on VGA, the CoYoT1 Clinic model addresses many of the psychosocial burdens associated with T1D. Randomization to VGA (CoYoT1 Clinic) was associated with reduced diabetes-related distress. These results support the integration of VGA into chronic disease care models for YA.
Acknowledgments
The authors thank The Leona M. and Harry B. Helmsley Charitable Trust (2015-PG-T1D059) for funding. They also thank all the health care providers and young adults who participated in the research. This study was presented in poster form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
Abbreviations: CES-D, Center for epidemiologic studies depression; CoYoT1, Colorado Young adults with T1D; DDS, diabetes distress scale; D-STAR, diabetes strengths and resilience; EQ-5D, EuroQol
ES, effect size; HbA1c, glycated hemoglobin; HRQOL, health-related quality of life; RCT, randomized controlled trial; SED, self-efficacy in diabetes; SMOD-A, self-management of type 1 diabetes in adolescence; T1D, type 1 diabetes; TH, telehealth; VGA, virtual group appointments; YA, young adults.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding from The Leona M. and Harry B. Helmsley Charitable Trust (2015-PG-T1D059).
ORCID iDs: Daniel I. Bisno  https://orcid.org/0000-0003-4440-7366
https://orcid.org/0000-0003-4440-7366
Mark W. Reid  https://orcid.org/0000-0002-1942-225X
https://orcid.org/0000-0002-1942-225X
Jennifer K. Raymond  https://orcid.org/0000-0003-1866-4932
https://orcid.org/0000-0003-1866-4932
References
- 1. Pinquart M, Shn Y. Depressive symptoms in children and adolescents with chronic illness: an updated meta-analysis. J Pediatr Psychol. 2011;36:375-384. [DOI] [PubMed] [Google Scholar]
- 2. Ferro MA, Gorter JW, Boyle MH. Trajectories of depressive symptoms during the transition to young adulthood: the role of chronic illness. J Affect Disord. 2015;174:594-601. [DOI] [PubMed] [Google Scholar]
- 3. Brady AM, Deighton J, Stansfeld S. Psychiatric outcomes associated with chronic illness in adolescence: a systematic review. J Adolesc. 2017;59:112-123. [DOI] [PubMed] [Google Scholar]
- 4. Suris JC, Michaud PA, Viner R. The adolescent with a chronic condition. Part 1: developmental issues. Arch Dis Child. 2004;89:938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQLTM 4.0 generic core scales. Health Qual Life Outcomes. 2007;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maslow GR, Haydon AA, Ford CA, Halpern CT. Young adult outcomes of children growing up with chronic illness: an analysis of the national longitudinal study of adolescent health. Arch Pediatr Adolesc Med. 2011;165:256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaffney A, Christopher A, Katz A, et al. The incidence of diabetic ketoacidosis during “emerging adulthood” in the USA and Canada: a population-based study. J Gen Intern Med. 2019;34:1244-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 2013;131:1062-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hendricks MC, Monaghan M, Soutor S, et al. A descriptive analysis of self-care behaviors in emerging adults with type 1 diabetes. Diabetes Educ. 2013;39:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971-978. [DOI] [PubMed] [Google Scholar]
- 11. Bryden KS, Dunger DB, Mayou RA, et al. Poor prognosis of young adults with type 1 diabetes: a longitudinal study. Diabetes Care. 2003;26:1052-1057. [DOI] [PubMed] [Google Scholar]
- 12. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nip ASY, Reboussin BA, Dabelea D, et al. Disordered eating behaviors in youth and young adults with type 1 and type 2 diabetes receiving insulin therapy: the SEARCH for diabetes in youth study. Diabetes Care. 2019;42:859-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crossen S, Glaser N, Sauers-Ford H, et al. Home-based video visits for pediatric patients with poorly controlled type 1 diabetes. J Telemed Telecare. 2020;26:349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wood CL, Clements SA, McFann K, et al. Use of telemedicine to improve adherence to American Diabetes Association standards in pediatric type 1 diabetes. Diabetes Technol Ther 2016;18:7-14. [DOI] [PubMed] [Google Scholar]
- 16. Markowitz JT, Laffel LMB. Short report: education and psychological aspects transition in care: support group for young adults with type 1 diabetes. Diabetes Med. 2012;29:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Floyd BD, Block JM, Buckingham BB, et al. Stabilization of glycemic control and improved quality of life using a shared medical appointment model in adolescents with type 1 diabetes in suboptimal control. Pediatr Diabetes. 2017;18:204-212. [DOI] [PubMed] [Google Scholar]
- 18. Raymond JK, Shea JJ, Berget C, et al. A novel approach to adolescents with type 1 diabetes: the team clinic model. Diabetes Spectr. 2015;28:68-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClain MR, Klingensmith GJ, Anderson B, et al. Team clinic: group approach to care of early adolescents with type 1 diabetes. Diabetes Spectr. 2018;31:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majidi S, Reid MW, Fogel J, et al. Psychosocial outcomes in young adolescents with type 1 diabetes participating in shared medical appointments. Pediatr Diabetes. Published online April 19, 2021. doi: 10.1111/pedi.13212 [DOI] [PubMed] [Google Scholar]
- 21. Reid MW, Krishnan S, Berget C, et al. CoYoT1 clinic: home telemedicine increases young adult engagement in diabetes care. Diabetes Technol Ther. 2018;20:370-379. [DOI] [PubMed] [Google Scholar]
- 22. Bakhach M, Reid MW, Pyatak EA, et al. Home telemedicine (CoYoT1 Clinic): a novel approach to improve psychosocial outcomes in young adults with diabetes. Diabetes Educ. 2019;45:420-430. [DOI] [PubMed] [Google Scholar]
- 23. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631. [DOI] [PubMed] [Google Scholar]
- 24. Hilliard ME, Iturralde E, Weissberg-Benchell J, Hood KK. The diabetes strengths and resilience measure for adolescents with type 1 diabetes (DSTAR-Teen): validation of a new, brief self-report measure. J Pediatr Psychol. 2017;42:995-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grossman HY, Brink S, Hauser ST. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabetes Care. 1987;10:324-329. [DOI] [PubMed] [Google Scholar]
- 26. Schilling LS, Dixon JK, Knafl KA, et al. A new self-report measure of self-management of type 1 diabetes for adolescents. Nurse Res. 2009;58:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385-401. [Google Scholar]
- 28. Craig BM, Pickard AS, Lubetkin EI. More common, but less severe: differences between EQ-5D 3-level, youth, and 5-level versions. J Clin Epidemiol. 2014;67:93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22:931-941. [DOI] [PubMed] [Google Scholar]
- 30. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983-997. [PubMed] [Google Scholar]
- 31. Hedge LV, Olkin I. Statistical Methods for Meta-analysis. Academic Press Inc; 2014:86. [Google Scholar]
- 32. Gavrila V, Garrity A, Hirschfeld E, et al. Peer support through a diabetes social media community. J Diabetes Sci Technol 2019;13:493-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hilliard ME, Sparling KM, Hitchcock J, et al. The emerging diabetes online community. Curr Diabetes Rev 2015;11:261-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hessler DM, Fisher L, Polonsky WH, et al. Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med. 2017;34:1228-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nam S, Chesla C, Stotts NA, et al. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93:1-9. [DOI] [PubMed] [Google Scholar]
- 36. Pascual AB, Pyle L, Nieto J, et al. Novel, culturally sensitive, shared medical appointment model for Hispanic pediatric type 1 diabetes patients. Pediatr Diabetes. 2019;20:468-473. [DOI] [PubMed] [Google Scholar]
- 37. Flores Garcia J, Reid MW, Raymond J. Feasibility of shared telemedicine appointments for low SES adolescents and young adults with T1D. Diabetes. 2018;67(suppl 1):1325-P. [Google Scholar]
- 38. Raymond JK, Reid MW, Fox S, et al. Adapting home telehealth group appointment model (CoYoT1 clinic) for a low SES, publicly insured, minority young adult population with type 1 diabetes. Contemp Clin Trials. 2020;88:105896. doi: 10.1016/j.cct.2019.105896 [DOI] [PubMed] [Google Scholar]