Abstract
Background:
The delivery and administration of insulin has undergone many changes over the years. This research examines U.S. trends in insulin use among people with type 1 diabetes (T1D) or type 2 diabetes (T2D) in the U.S. from 2009 to 2018.
Methods:
The IBM® MarketScan® Commercial and Medicare databases were used to identify trends in insulin use over 10 years. The study included people with T1D or T2D who filled a prescription for insulin in any calendar year from 2009 to 2018. The analyses examined insulin regimen and delivery and the use of glucose monitoring systems. Generalized estimating equations were used to test whether trends were statistically significant.
Results:
Individuals with T1D were most commonly prescribed a basal and bolus insulin regimen or short/rapid insulin only, while for people with T2D the use of basal-only insulin increased significantly over the study period. In both groups there was a significant decline in the use of premix insulin from 2009 to 2018. Insulin pump use increased for individuals with T1D, while disposable pen use increased for people in both cohorts. In both cohorts, there was a statistically significant increase in the use of continuous glucose monitoring, although this increase was more pronounced and occurred earlier among individuals with T1D.
Conclusions:
Results indicate significant changes in insulin regimens and delivery and glucose monitoring from 2009 to 2018. These findings suggest that insulin prescribing continues to change in response to the development of new therapeutics, advances in insulin delivery technology, and glucose monitoring systems.
Keywords: glucose monitoring, insulin, insulin devices, type 1 diabetes, type 2 diabetes
Introduction
In the United States in 2018, 34.2 million individuals (10.5% of the population) had diabetes. 1 The majority (30.8-32.5 million) of these individuals had type 2 (T2D), while 1.7 to 3.4 million had type 1 diabetes (T1D). 1 In the treatment of T2D, insulin may be used to augment the glucose-lowering effect of other agents or to mimic the production of insulin in a healthy body. 2 In the treatment of T1D, exogenous insulin therapy is essential for survival and the prevention of complications. 3 For people with either diabetes type, different types of insulin, delivery methods, strengths, and regimens are available to meet individual requirements.
Over time, numerous types of insulin with different pharmacokinetic and pharmacodynamic characteristics have been introduced to better mimic physiologic insulin delivery. In the past decade, innovations in insulins include the development of biosimilar long and rapid-acting U-100 analogs, ultra-rapid insulins, concentrated (U-200) rapid acting insulins, and long-acting and concentrated analogs (U-300 glargine, U-100 and U-200 degludec). 4 In general, these newer insulins increase the number of treatment options available to individuals and can help more accurately mimic the body’s insulin response. In addition, the use of concentrated insulins may result in a decrease in the number of daily injections required and, in some cases, can provide a longer duration of action. 5
In addition to the newer types of insulin, insulin delivery methods have also advanced in recent years. For instance, insulin pens have new safety features and enhancements that reduce physical effort and increase injection accuracy. 6 New and emerging “smart pens,” with either integrated USB or Bluetooth connectivity or pens with removable connection-enabled caps, have features that help to facilitate dose calculations and track insulin use. 7 Insulin pumps have also evolved in the last decade with the introduction of “patch pumps,” which are small, lightweight, and free of infusion sets, instead attaching directly to the skin via an adhesive. 6 In addition, hybrid closed loop insulin systems now allow for the automation of basal insulin. 8
Glucose monitoring has also evolved, with the clinical application of the modern era of continuous glucose monitoring (CGM) beginning in 2000. 9 Real-time CGM (rtCGM) measures glucose subcutaneously in the interstitial fluid and generates an average glucose value every 5 minutes. 10 The use of rtCGM has made it easier for people with diabetes (PWD) and health care providers to visualize glucose patterns over time. 9 Recent advances in the use of rtCGM mean that patients are no longer required to calibrate with self-monitoring of blood glucose (SMBG) fingerstick values. 11 More recently, intermittently scanned CGM (isCGM) has also been developed. 11 In contrast to rtCGM, isCGM is less expensive but only provides glucose readings when the device is scanned. 12
Given all these advancements in insulin and technology, we undertook this study to update and expand upon our understanding of trends in insulin use, insulin delivery, and glucose monitoring from 2009 to 2018 for individuals with T1D and T2D.
Methods
This retrospective study utilizes data from the IBM® MarketScan® databases over the time period from January 1, 2009 to December 31, 2018. Specifically, the analyses utilize the Commercial Claims and Encounters (CCAE) and the Medicare Supplemental (MDCR) databases. The CCAE data include working individuals younger than 65 and their dependents while the MDCR data capture data from retirees covered by previous employers. 13 Both databases capture information from fully paid and adjudicated claims and include information on demographics, eligibility, benefits plan, financial information, inpatient and outpatient diagnoses and procedures and outpatient prescription drug use. 13 Inclusion of both databases allows for the study to capture a wide age-range of individuals with diabetes. All data are fully de-identified and Health Insurance Portability and Accountability Act (HIPAA) compliant. Given the use of retrospective and de-identified data, ethics committee approval was not required.
For each year from 2009 to 2018, prevalent insulin use was examined, where prevalence was defined as the filling of a prescription for any insulin during the specified year. Table 1 shows all insulins examined by regimen, strength, and delivery method. The analyses excluded inhaled insulin given its relatively limited use. For individuals who received multiple prescriptions for insulin, the method of delivery and the strength were based upon the last prescription filled in the year. 14
Table 1.
Insulin Examined: By Regimen, Strength and Delivery.
| Insulin—generic name | Strength | Delivery | |||
|---|---|---|---|---|---|
| Concentrated A | 100 u/mL | Vial | Disposable pen | Reusable pen | |
| Basal insulin | |||||
| Long-acting | |||||
| Insulin degludec | + | + | + | ||
| Insulin Degludec/liraglutide | + | + | |||
| Insulin detemir | + | + | + | ||
| Insulin glargine | + | + | + | + | + |
| Insulin glargine/lixisenatide | + | + | |||
| Intermediate-acting | |||||
| Insulin human isophane (NPH) | + | + | + | + | |
| Short/rapid insulin | |||||
| Short-acting | |||||
| Insulin human regular | + | + | + | + | + |
| Rapid-acting | |||||
| Insulin aspart | + | + | + | + | |
| Insulin glulisine | + | + | + | + | |
| Insulin lispro | + | + | + | + | + |
| Pre-mix insulin | |||||
| Insulin aspart protamine/insulin aspart | + | + | + | + | |
| Insulin human isophane (NPH)/Insulin human regular | + | + | + | + | |
| Insulin lispro protamine/insulin lispro | + | + | + | ||
This analysis excludes any use of Insulin Human Zinc (Lente), Insulin Human Zinc, Extended (Ultralente) and Insulin, Human Inhaled.
Concentrated insulin includes 200, 300 and 500 u/mL.
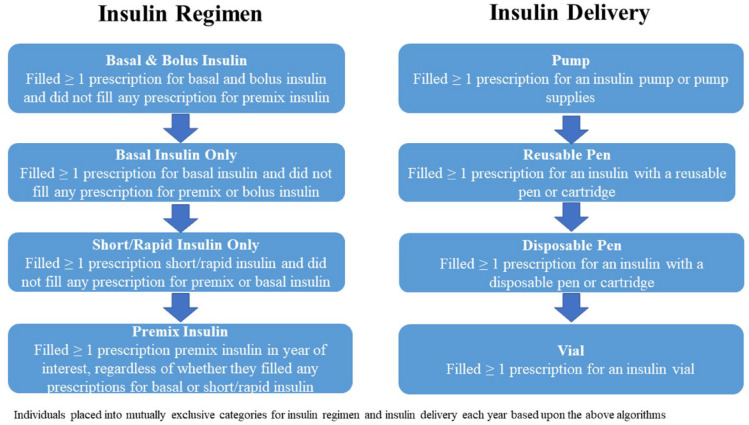
Individuals who received multiple types of insulin and/or had multiple methods of insulin delivery during the year were clustered into mutually exclusive groups. Specifically, insulin regimen was categorized as basal and bolus, basal only, short/rapid only, or premix. Patients were also categorized based upon insulin delivery as users of an insulin pump, reusable pen, disposable pen, or insulin vial. Note that while the use of pens or vials/syringes could be determined based upon the last insulin prescription(s) in a calendar year, use of an insulin pump was based upon examining all outpatient drug records during the year of interest. Figure 1 illustrates the hierarchical algorithms used to identify insulin regimen and delivery system. Use of glucose monitoring was also grouped into mutually exclusive categories, with PWD who filled a prescription for CGM, including isCGM, identified as CGM users independent of their filling a prescription for SMBG (meter or strips). Individuals who did not fill a prescription for CGM (device or supplies) but did fill a prescription for SMBG (meter or supplies) were identified as SMBG users.
Figure 1.
Algorithm for classifying individuals based upon insulin regimen and insulin delivery.
Given potential differences in insulin use among individuals with T1D or T2D, all analyses examined these cohorts separately. Consistent with previous research, 13 individuals were classified as having T2D if they received at least one diagnosis of T2D (ICD-9-CM of 250.×0 or 250.×2; ICD-10-CM of E11xx) and no receipt of any diagnosis of T1D (ICD-9-CM of 25.×1 or 250.×3; ICD-10-CM of E11xx) and as T1D if they received 1 or more diagnoses of T1D and no diagnoses of T2D. Individuals who received diagnoses of both T1D and T2D were identified as having T2D if they also filled a prescription for at least one oral glucose lowering agent (GLA). Individuals who received diagnoses of both T1D and T2D and did not fill any prescription for an oral GLA were classified as T1D only if they received more diagnoses of T1D than T2D; otherwise, they were classified as having T2D. Individuals were excluded if they were diagnosed with secondary diabetes (ICD-9-CM of 249.xx or ICD-10-CM of E08xx or E09xx), gestational diabetes (ICD-9-CM of 648.8× of ICD-10-CM of 0244×), or diabetes mellitus complicating pregnancy, childbirth, or the puerperium (ICD-9-CM of 648.0× or ICD-10-CM of 0249×).
The analyses were primarily descriptive in nature. Means and standard deviations are reported for all continuous variables, while frequencies and percentages are reported for categorical variables. Differences in continuous variables were examined using t-tests, and differences in categorical variables were reported using chi-square statistics. Trends over time were examined using generalized estimating equations (GEE) tests. All analyses were conducted using SAS, version 9.4, and a P value < .05 was considered, a priori, to be statistically significant.
Results
Total annual sample size ranged from 383,979 to 652,208. Given the large number of subjects in the analyses, demographic characteristics are reported for the years 2009, 2014, and 2018 only. Table 2 presents characteristics for prevalent insulin users with T1D or T2D. Consistent with the declining population of the IBM® MarketScan® data over the study period, there were fewer individuals included in both cohorts over time. For all years examined, there were more people with T2D than T1D (76.7%-81.4% of individuals classified as having T2D). Compared to individuals with T2D, those with T1D were found to be significantly younger and less likely to be captured in the Medicare Supplemental Data. For both cohorts, there were more males than females, and there was no statistically significant difference in the ratio of males to females when comparing the T1D and T1D and T2D cohorts.
Table 2.
Demographic Characteristics – Years 2009, 2014, and 2018.
| Characteristic | Type 1 diabetes | Type 2 diabetes | ||||
|---|---|---|---|---|---|---|
| 2009 | 2014 | 2018 | 2009 | 2014 | 2018 | |
| % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | |
| Sample size | 115,274 | 106,286 | 77,504 | 418,458 | 457,591 | 274,872 |
| Demographic characteristics | ||||||
| AgeA,B,C | 38.3 ± 19.0 | 36.8 ± 18.3 | 35.9 ± 17.2 | 57.9 ± 13.4 | 58.6 ± 13.0 | 56.8 ± 12.2 |
| Sex | ||||||
| MaleA,B | 52.3 | 53.3 | 53.8 | 52.7 | 53.8 | 54.4 |
| FemaleA,B | 47.8 | 46.7 | 46.2 | 47.3 | 46.2 | 45.6 |
| Region | ||||||
| NortheastA,B | 17.8 | 20.7 | 17.5 | 14.3 | 18.9 | 16.9 |
| North CentralA,B | 29.9 | 23.7 | 24.6 | 31.5 | 25.5 | 24.6 |
| SouthB,C | 34.5 | 35.7 | 41.1 | 38.1 | 40.8 | 45.6 |
| WestA,C | 15.9 | 16.9 | 16.6 | 14.3 | 12.4 | 12.7 |
| UnknownA,B,C | 1.9 | 3.0 | 0.2 | 1.8 | 2.4 | 0.2 |
| Insurance type | ||||||
| CommercialA,B,C | 92.3 | 94.3 | 97.0 | 72.3 | 71.7 | 82.0 |
| Medicare SupplementalA,B,C | 7.7 | 5.7 | 3.0 | 27.7 | 28.3 | 18.0 |
SD, standard deviation.
Statistically significant trend (P < .05) for individuals with type 1 diabetes.
Statistically significant trend (P < .05) for individuals with type 2 diabetes.
Statistically significant difference (P < .05) in trend between individuals with type 1 diabetes and type 2 diabetes.
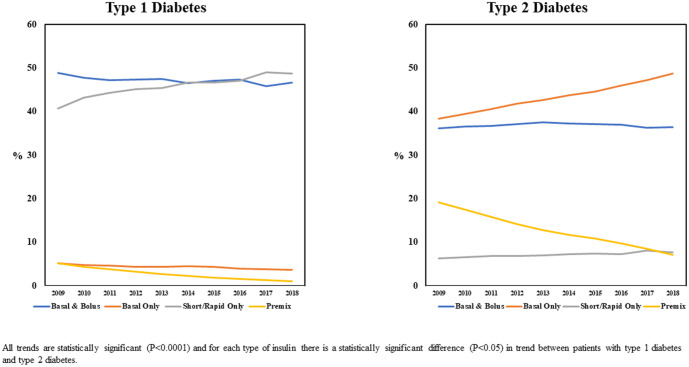
Figure 2 illustrates the type of insulin prescribed for individuals with T1D and T2D over time. As the figure shows, individuals with T1D were most commonly prescribed a basal and bolus insulin regimen or short/rapid insulin only, while those with T2D were most commonly prescribed a basal and bolus insulin regimen or basal insulin only. Over time, the use of basal-only increased in individuals with T2D, and for both cohorts there was a statistically significant increase in the use of short/rapid insulin only as well as a significant decline in the use of premix. While not presented in Figure 2, the analyses also revealed that for both groups, the use of U-200, U-300, or U-500 (concentrated) insulins increased over the study period as these products were introduced to the market. 5 For example, 0.2% of individuals with T1D and 0.7% of individuals with T2D were identified as prevalent users of concentrated insulin in 2009 when only U-500 insulin was available, and these percentages increased to 6.1% and 18.4% of people with T1D or T2D, respectively, by 2018.
Figure 2.
Trends in insulin regimen from 2009 to 2018 for individuals with type 1 or type 2 diabetes.
Figure 3 examines changes in insulin delivery over time. Results indicate that for those with T1D or T2D there was a statistically significant increase in the use of disposable pens. For example, from 2009 to 2018, disposable pen use increased from 14.4% to 29.2% for individuals with T1D and from 35.6% to 79.2% for people with T2D. Insulin pump use increased significantly (from 43.2% to 54.4%) over the study period for individuals with T1D, and more modestly (from 3.2% to 4.4%) among individuals with T2D. For both cohorts, there has been a statistically significant decline in the use of insulin vials without any pump use and a decrease in the use of reusable pens over the time period from 2009 to 2018.
Figure 3.
Trends in insulin delivery from 2009 to 2018 for individuals with type 1 or type 2 diabetes.
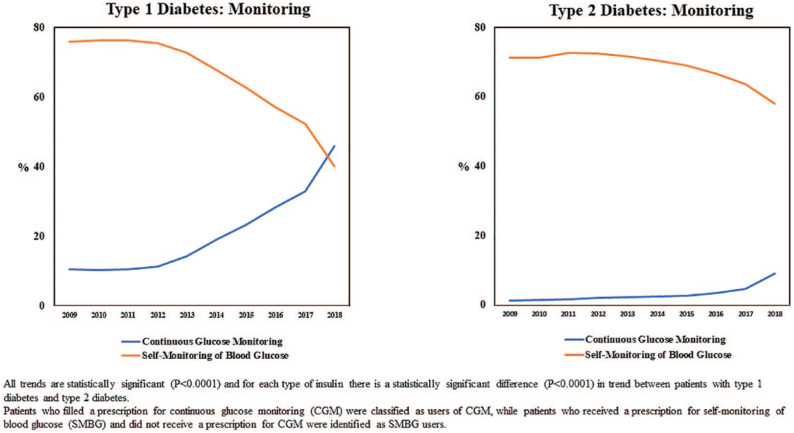
Figure 4 illustrates trends in glucose monitoring for individuals with T1D and T2D. Results reveal that a statistically significant increase in the use of CGM among individuals with T1D over the study period (from 10.5% to 45.9%), especially since 2012. CGM use increased significantly among individuals with T2D, as well (from 1.3% to 9.1%), although this uptake occurred later than for the T1D cohort, and overall use was considerably less for individuals with T2D relative to people with T1D. In both cohorts, the increase in CGM was accompanied by a corresponding statistically significant decrease in those classified as SMBG-only users.
Figure 4.
Trends in insulin monitoring from 2009 to 2018 for individuals with type 1 or type 2 diabetes.
Finally, as a test of the robustness of results, all analyses were conducted again for patients based upon the following age groups: (a) less than 18 years; (b) 18-44 years; (c) 45-64 years; (d) 65-74 years; and (e) 75 years or older. The general patterns reported for the cohorts overall with regard to insulin regimen, delivery, and monitoring for individuals with T1D or T2D were consistently found across all age groups. In addition, results revealed that for individuals with T1D, the use of basal-only insulin, premix insulin, and insulin delivery via a vial without a pump increased as age category increased, while the use of insulin pumps decreased as age category increased. For people with T2D, age category was found to be positively associated with the use of premix insulin and insulin via a vial without a pump and negatively associated with the use of insulin pumps and CGM.
Discussion
These longitudinal analyses revealed notable changes in the patterns of insulin use from 2009 to 2018. The increase in the use of basal-only insulin among people with T2D is consistent with American Diabetes Association (ADA) guidelines. which suggest basal insulin as the first type of insulin to add if an individual is not well-controlled on non-insulin GLAs. 15 In addition, the increased use of basal insulin coincides with the availability of newer basal insulin products that more closely mimic physiological basal insulin release and have a lower propensity for causing hypoglycemia. 4
During the study period, new insulin products which are only available via pens, such as insulin glargine (Basaglar®, Eli Lilly and Co, Indianapolis, IN; Toujeo®, Sanofi, Paris, France), insulin degludec (Tresiba®, Novo Nordisk, Bagsvaerd, Denmark), insulin degludec/liraglutide (Xultophy®, Novo Nordisk, Bagsvaerd, Denmark), and glargine/lixenatide (Soliqua®, Sanofi, Paris, France) received FDA approval.16,17 A wide range of research has found that adherence and persistence are improved when using an easier method of insulin delivery, such as insulin pens,18–20 and that improvements in adherence and persistence are associated with lower HbA1c, fewer hypoglycemic events, and lower annual treatment costs.18,19,21,22 Other research has shown economic and clinical benefits associated with the use of pens compared to vials.18,23 These studies may have influenced prescribing practices, resulting in the increased use of disposable pens observed in the present study.
Previous investigations have found that exogenous insulin replacement required for T1D does not on its own provide the metabolic regulation necessary to avoid diabetes-related complications, such as retinopathy, neuropathy, cardiovascular disease, and hypoglycemia. 24 As a result, the management of T1D often includes the use of technologies such as insulin pumps and continuous glucose monitors for improving metabolic control. 24 In our study, both insulin pumps and CGMs were more likely to be used by individuals with T1D, consistent with the increased metabolic regulation necessary for T1D. The use of both insulin pumps and CGM also increased over the study period for individuals with T2D, albeit the increase was less prominent in T2D relative to T1D. It remains to be seen whether technology use will rise to the same level in T2D as in T1D.
There are some limitations to our study. First, the use of a large insurance claims database limits the applicability of this data and introduces some potential biases. For example, the data are based upon a large convenience sample which is not random and hence, may not generalize to other populations. In addition, most data are obtained from large employers therefore small and medium firms may be underrepresented. 13 In addition, potential biases are introduced by the individual’s propensity to access health care services as well as the provider’s propensity to accurately detect, treat, and record patient health care status. 25
In addition to potential biases introduced by the use of administrative claims data, the number of evaluable subjects in the database declined over the duration of the study due to decreased market capture. However, there is no evidence that the decline in sample size resulted in systemic differences in patient cohorts. Third, the analyses were not able to examine isCGM separately from rtCGM due to the more recent introduction of isCGM to the market. Fourth, the data relies on diagnostic codes entered by the healthcare provider. A number of subjects had codes for both T1D and T2D at different times during the sampling period, and our algorithm for classifying PWD did not preclude the possibility of misclassification of diabetes type. Finally, the analyses focused exclusively on prescriptions filled for insulin products in a calendar year and did not include insulin which was prescribed at the end of the prior calendar year and used in the year of interest.
Conclusions
In conclusion, the results from this study illustrate a shift in the types of insulin used, insulin delivery systems, and glucose monitoring between 2009 and 2018. Specifically, the use of basal-only insulin increased among people with T2D, the use of short/rapid insulin only increased among people with T1D, and the use of premix insulin decreased in both cohorts. In addition, the use of disposable pens and insulin pumps increased over time, as did the utilization of CGM. These changes are consistent with both expert guidelines and medical research. Overall, the results suggest that diabetes management is changing in response to advances in technology and research.
Acknowledgments
The authors thank Patricia Platt for her assistance in the writing of this manuscript.
Footnotes
Abbreviations: ADA, American Diabetes Association; CCAE, commercial claims and encounters; CGM, continuous glucose monitoring; GEE, generalized estimating equation; GLA, glucose- lowering agent; HIPAA, Health Insurance Portability and Accountability Act; isCGM, intermittently scanned continuous glucose monitoring; MDCR, Medicare supplemental; PWD, people with diabetes; rtCGM, real-time continuous glucose monitoring; SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors Eby, Fan, Juneja, and Perez-Nieves conducted this research as employees and shareholders of Eli Lilly and Co. Meadows conducted this research as an employee/former employee and shareholder of Eli Lilly and Co. HealthMetrics Outcomes Research was compensated by Eli Lilly and Co. for their work on this research.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research was provided by Eli Lilly and Company.
ORCID iD: Maureen J Lage  https://orcid.org/0000-0003-4961-2027
https://orcid.org/0000-0003-4961-2027
References
- 1. Centers for Disease Control and Prevention. National diabetes statistics report, 2020 [Internet]. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 19, 2021. https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html. [Google Scholar]
- 2. Howard-Thompson A, Khan M, Jones M, George CM. Type 2 diabetes mellitus: outpatient insulin management. Am Fam Physician. 2018;97:29-37. [PubMed] [Google Scholar]
- 3. Subramanian S, Baidal D, Skyler JS, Hirsch IB. The management of type 1 diabetes. In: Feingold KR, Anawalt B, Boyce A, et al. eds. Endotext. MDText.com, Inc.; 2016. [Google Scholar]
- 4. Hirsch IB, Juneja R, Beals JM, Antalis CJ, Wright EE. The evolution of insulin and how it informs therapy and treatment choices. Endocr Rev. 2020;41:733-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson JL, Downes JM, Obi CK, Asante NB. Novel concentrated insulin delivery devices: developments for safe and simple dose conversions. J Diabetes Sci Technol. 2016;11:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kesavadev J, Saboo B, Krishna MB, Krishnan G. Evolution of insulin delivery devices: from syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. 2020;11:1251-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sangave NA, Aungst TD, Patel DK. Smart connected insulin pens, caps, and attachments: a review of the future of diabetes technology. Diabetes Spectrum. 2019;32:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knebel T, Neumiller JJ. Medtronic MiniMed 670G hybrid closed-loop system. Clin Diabetes. 2019;37:94-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patton SR, Clements MA. Continuous glucose monitoring versus self-monitoring of blood glucose in children with type 1 diabetes- are there pros and cons for both? US Endocrinol. 2012;8:27-29. [PMC free article] [PubMed] [Google Scholar]
- 11. Forlenza GP, Kushner T, Messer LH, Wadwa RP, Sankaranarayanan S. Factory-calibrated continuous glucose monitoring: how and why it works, and the dangers of reuse beyond approved duration of wear. Diabetes Technol Ther. 2019;21:222-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinemann L, Freckmann G. CGM versus FGM; or, continuous glucose monitoring is not flash glucose monitoring. J Diabetes Sci Technol. 2015;9:947-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. IBM Watson Health. IBM MarketScan Research Databases for life sciences researchers [Internet]. IBM Watson Health; 2020. Accessed April 27, 2021. https://www.ibm.com/downloads/cas/OWZWJ0QO. [Google Scholar]
- 14. Perez-Nieves M, Jiang D, Eby E. Incidence, prevalence, and trend analysis of the use of insulin delivery systems in the United States (2005 to 2011). Curr Med Res Opin. 2015;31:891-899. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S111-S124. [DOI] [PubMed] [Google Scholar]
- 16. Chung JW, Hartzler ML, Smith A, Hatton J, Kelley K. Pharmacological agents utilized in patients with type-2 diabetes: beyond lowering A1c. P T. 2018;43:214-227. [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Food and Drug Administration [Internet]. Drug approvals and databases. 2020. Accessed February 19, 2021. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases
- 18. Lasalvia P, Barahona-Correa JE, Romero-Alvernia DM, et al. Pen devices for insulin self-administration compared with needle and vial. J Diabetes Sci Technol. 2016;10:959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miao R, Wei W, Lin J, Xie L, Baser O. Does device make any difference? A real-world retrospective study of insulin treatment among elderly patients with type 2 diabetes. J Diabetes Sci Technol. 2014;8:150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheen HHM, Lim SH, Huang MC, Bee YM, Wee HL. Adherence to premixed insulin in a prefilled pen compared with a vial/syringe in people with diabetes in Singapore. Clin Ther. 2014;36:1043-1053. [DOI] [PubMed] [Google Scholar]
- 21. Cobden D, Lee WC, Balu S, Joshi AV, Pashos CL. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27:948-962. [DOI] [PubMed] [Google Scholar]
- 22. Grabner M, Chu J, Raparla S, Quimbo R, Zhou S, Conoshenti J. Clinical and economic outcomes among patients with diabetes mellitus initiating insulin glargine pen versus vial. Postgrad Med. 2013;125:204-213. [DOI] [PubMed] [Google Scholar]
- 23. Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: a review of the evidence. Diabetes Technol Ther. 2010;12:S101-S108. [DOI] [PubMed] [Google Scholar]
- 24. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terris DD, Litaker DG, Koroukian SM. Health state information derived from secondary databases was affected by multiple sources of bias. J Clin Epidemiol. 2007;60:734-741. [DOI] [PMC free article] [PubMed] [Google Scholar]