Key Points
Siltuximab leads to rapid normalization of symptomatology and most abnormal laboratory parameters and prolongs PFS in patients with iMCD.
The findings in this study reinforce the use of siltuximab as the first iMCD treatment choice, as recommended by international guidelines.
Visual Abstract
Abstract
Idiopathic multicentric Castleman disease (iMCD) is a rare heterogeneous disorder involving multicentric lymphadenopathy, systemic inflammation, and cytokine-driven organ dysfunction. Despite the approval of siltuximab, a monoclonal antibody against interleukin-6, for the treatment of iMCD, it is not known how long patients should receive siltuximab before determining whether the treatment is beneficial and should be continued. We performed post hoc analyses of the phase 2 randomized double-blind placebo-controlled trial of siltuximab for the treatment of patients with iMCD to determine the sequence of normalization of laboratory, clinical, and lymph node responses in patients who responded to siltuximab. Seventy-nine patients were enrolled in the trial (siltuximab, n = 53; placebo plus best supportive care, n = 26). Progression-free survival (PFS) was significantly improved in siltuximab-treated patients compared with those receiving placebo (P = .0001). The median PFS was 14.5 months (95% confidence interval, 13.6 months to upper bound not reached) for patients receiving placebo but was not reached for patients receiving siltuximab. In siltuximab-treated patients who achieved durable tumor (radiologic) and symptomatic responses (18 [34%] of 53), the median time to normalization of abnormal laboratory tests and clinical end points occurred in the following sequence: thrombocytosis, symptomatic response, elevated C-reactive protein, hypoalbuminemia, anemia, lymph node response, hyperfibrinogenemia, and elevated immunoglobulin G. Siltuximab treatment prolongs PFS, rapidly improves symptomatology, and provides meaningful clinical benefit despite some laboratory tests and enlarged lymph nodes taking months to normalize in treatment responders. These data support the continued frontline use of siltuximab for iMCD, as recommended by international guidelines. This trial was registered at www.clinicaltrials.gov as #NCT01024036.
Introduction
Human herpesvirus-8–negative idiopathic multicentric Castleman disease (iMCD) is a rare heterogeneous disorder involving multicentric lymphadenopathy, systemic inflammation, and cytokine-driven organ dysfunction.1 Although iMCD etiology remains to be elucidated, dysregulated interleukin-6 (IL-6) signaling is an established pathogenic mechanism.2 Patients display heterogeneous clinical and laboratory abnormalities; some present with mild symptoms, whereas others present with acute episodes of multiorgan dysfunction requiring intensive care.1 Because iMCD is a rare disease with features resembling malignancies or autoimmune/infectious disorders, there are often delays in diagnosis and treatment initiation.1 iMCD carries a high mortality and overall disease burden. Recent claims database research found that patients with iMCD may have an increased risk of cancer,3 possibly because of ongoing and uncontrolled immune dysregulation.4 iMCD has a worse prognosis than many cancers: 35% of patients with iMCD die within 5 years of diagnosis, and 60% die within 10 years.5 Early targeted treatment is essential to control immune dysregulation and improve outcomes in patients with iMCD.
Siltuximab, a monoclonal antibody that targets IL-6, is the only therapy for iMCD approved by the US Food and Drug Administration and European Medicines Agency. In a phase 1 dose-finding study involving 37 adults with symptomatic CD (registered at www.clinicaltrials.gov as #NCT00412321),6 32 (86%) of the 37 patients treated with siltuximab demonstrated a clinical benefit response and sustained suppression of C-reactive protein (CRP), which is used as a surrogate measure of IL-6 activity. In a randomized phase 2 trial,7 siltuximab administered at a dose of 11 mg/kg every 3 weeks provided durable tumor (radiologic) and symptomatic responses in 18 (34%) of the 53 patients, compared with 0% in the placebo group. Siltuximab showed an acceptable safety profile across the clinical trials, with few serious adverse events or discontinuations and no evidence of new or cumulative toxicity.6-8
Despite the encouraging results reported with siltuximab and its recommendation as a first-line treatment for iMCD, a recent epidemiologic study found that only 8.7% of patients with iMCD received siltuximab. Moreover, corticosteroid monotherapy was the most commonly used treatment, despite it not being recommended in iMCD and a lack of data supporting its effectiveness.4 Because the pivotal trial indicated that 34% of patients respond to siltuximab,7 it is critical that responders are identified and maintained on siltuximab therapy, at least until the treatment fails, which occurs rarely in responders (response defined by the modified Cheson criteria and maintaining improvement or stabilization of disease-related symptoms for at least 18 weeks during blinded treatment). More importantly, nonresponders must be identified quickly to enable their treatment to be adjusted to control immune dysregulation as effectively as possible.
We conducted a detailed post hoc analysis of the phase 2 trial of siltuximab in iMCD7 to help patients and physicians understand the kinetics of clinical response to siltuximab and know how long to keep patients on therapy before deciding whether it is beneficial. We also analyzed disease progression and survival data to enable physicians to have informed discussions with their patients around treatment benefits and long-term outcomes.
Methods
Study design and participants
We performed post hoc analyses of data obtained from the phase 2 randomized double-blind placebo-controlled trial of siltuximab. The details of the trial have been described previously.7
Eligible adults age ≥18 years with symptomatic iMCD were diagnosed based on detailed patient history, physical examination, assessment of laboratory abnormalities, pathologic diagnosis, radiologic imaging, and histologically confirmed diagnosis from an excisional lymph node biopsy acquired before enrollment. Patients included in the study were confirmed to be HIV seronegative and human herpesvirus-8 negative, as determined by a polymerase chain reaction test. Written informed consent was obtained from participants. The institutional review board or independent ethics committee at each site approved the protocol. The study was carried out according to the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice.
Patients were randomly assigned to receive siltuximab or placebo every 3 weeks along with best supportive care. Best supportive care could include up to 1 mg/kg per day of prednisone, as well as other supportive measures such as antipruritic, antihistamine, antipyretic, and analgesic drugs and clinical management of effusions, transfusions, infections, and infusion reactions. Not permitted was use of other biologic treatments, antitumor agents, or erythropoietin-stimulating agents or an increase in steroid dose from baseline or initiation of new steroid treatment. Treatment continued until treatment failure, discontinuation of treatment, withdrawal from the study, or 48 weeks after the last patient started treatment. Patients assigned to siltuximab discontinued study treatment at treatment failure. Patients who were randomly assigned to receive placebo and had treatment failure were able to cross over to siltuximab.
Clinical outcomes
We compared progression-free survival (PFS) in patients treated with siltuximab and placebo. PFS was defined as the time from randomization until death resulting from any cause or clinical and/or radiologic progression assessed by a computerized tomography (CT) scan, as measured by the modified Cheson criteria.9
Overall survival, lymph node response, durable symptomatic response, durable tumor (radiologic) and symptomatic responses, and treatment failure were also analyzed as part of these exploratory analyses and are defined in the data supplement.
Laboratory measures
Normalization of 16 laboratory values was evaluated (hemoglobin, CRP, immunoglobulin G [IgG], fibrinogen, albumin, elevated platelets, alanine transaminase, alkaline phosphatase, aspartate transaminase, creatinine, hepcidin, ferritin, erythrocyte sedimentation rate, activated partial thromboplastic time, prothrombin time, and IgA).
For hemoglobin and albumin, normalization was defined as an abnormally low value at baseline rising to meet or exceed the lower limit of normal measured during ≥1 on-treatment evaluation. The reference ranges for normal hemoglobin and albumin varied by patient; based on factors such as the age and sex of the patient, the ranges of lower limits of normal used were 11 to 14 g/dL for hemoglobin and 32 to 40 g/L for albumin. For CRP (surrogate marker for IL-6), IgG, platelets, and fibrinogen, normalization was defined as an abnormally high value at baseline falling to meet or exceed the upper limit of normal (ULN) measured during ≥1 on-treatment evaluation. Reference values used to define ULN or lower were as follows: CRP, ≤4 mg/L (same ULN for all patients); IgG, ≤17.65 g/L (same for all patients); platelets, range of ≤ ≤300 to 500 × 109/L (variable by patient demographics); and fibrinogen, ≤4 μmol/L (same ULN for all patients). Information about additional laboratory biomarkers is included in the data supplement. Hemoglobin, albumin, CRP, and platelets were generally sampled on the first day of each cycle (every 3 weeks). IgG and fibrinogen were sampled at baseline and then generally at the start of every fourth cycle, starting with the first day of cycle 4 (between days 60 and 78 of the study, most commonly corresponding to day 64 of the study).
Comparably, the 34-point symptomatic score was generally assessed on the first day of each cycle, whereas lymph node size was generally assessed on a schedule that included assessments at the following study time points: weeks 9, 18, and 27 and then approximately every 13 weeks thereafter.
Statistical analyses
The Kaplan-Meier and cumulative incidence with competing risks methods with corresponding log-rank tests were used to estimate and compare the PFS, overall survival, time to treatment failure, time to lymph node response, time to durable symptomatic response, and time to normalization of laboratory values. A P value of ≤.05 was considered significant.
Results
Baseline characteristics
A total of 79 patients were enrolled in the phase 2 trial: 53 were assigned to siltuximab, and 26 were assigned to placebo. The median duration of follow-up was 422 (range, 55-1051) days. Patients were categorized into groups with so-called normal or abnormal parameters at baseline, including laboratory values, 34-point MCD symptom score, and sum of the product of the diameters (SPD) of the index lymph node (Table 1). Aside from the 34-point disease score, patients in the placebo arm tended to have higher rates of normal-level features, although only the rate of low SPD was significantly different between study arms (SPD <1400 mm2 in 20 [41%] of 49 vs 17 [68%] of 25 patients, respectively; P = .026; Table 1).
Table 1.
Baseline characteristics
| Factor | All patients (N = 79) |
Placebo + BSC (n = 26) |
Siltuximab + BSC (n = 53) |
P * |
|---|---|---|---|---|
| Hemoglobin normal at baseline (defined as ≤LLN, range 11-14 g/dL) | 37/79 (47) | 15/26 (58) | 22/53 (42) | .175 |
| CRP normal at baseline (defined as ≤ULN, ≤4 mg/L) | 37/79 (47) | 16/26 (62) | 21/53 (40) | .066 |
| IgG normal at baseline (defined as ≤ULN, ≤17.65 g/L) | 43/79 (54) | 15/26 (58) | 28/53 (53) | .683 |
| Fibrinogen normal at baseline (defined as ≤ULN, ≤4 μmol/L) | 30/79 (38) | 12/26 (46) | 18/53 (34) | .297 |
| Albumin normal at baseline (defined as ≥LLN, range 32-40 g/L) | 44/79 (56) | 16/26 (62) | 28/53 (53) | .463 |
| Platelets normal at baseline (defined as ≤ULN, range 300-500 × 109/L) | 55/79 (70) | 19/26 (73) | 36/53 (68) | .638 |
| 34-point disease score ≥7 | 40/79 (51) | 17/26 (65) | 23/53 (43) | .065 |
| SPD at baseline <1400 mm2 | 37/74 (50) | 17/25 (68) | 20/49 (41) | .026 |
| Best response (DTSyR) of ≥PR (postbaseline) | 18/79 (23) | 0/26 (0) | 18/53 (34) | NA |
Data given as n/N (%), where n indicates number with factor and N indicates number with valid data for factor. Bold font indicates significance.
BSC, best supportive care; DTsyR, durable tumor and systematic response; LLN, lower limit of normal; NA, not applicable; PR, partial response.
P values represent comparison between groups, not against the overall population.
PFS
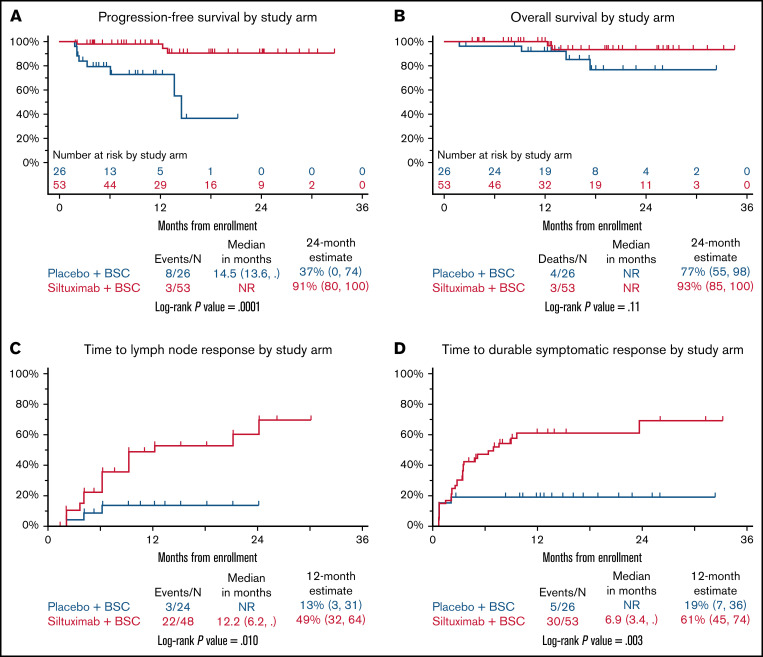
PFS was significantly improved in patients treated with siltuximab compared with those treated with placebo (P = .0001; Figure 1A). The 2-year estimates for PFS were 91% (95% confidence interval [CI], 80% to 100%) for patients treated with siltuximab and 37% (95% CI, 0% to 74%) for patients receiving placebo. The numbers of deaths and disease-progression events, which were used to calculate PFS, were higher in the placebo arm (8 in 26 patients) than in the siltuximab arm (3 in 53 patients). Of the 53 patients in the siltuximab arm, there were 2 deaths reported without disease progression. No deaths were reported after disease progression. One patient who was alive at last contact experienced disease progression while on study. Of the 26 patients in the placebo arm, there were 2 deaths reported without disease progression and 2 deaths reported after disease progression. Four patients in the placebo arm, who were alive at last contact, experienced disease progression while on study. The median PFS was 14.5 (95% CI, 13.6 to upper bound NR) months for patients in the placebo arm. The median PFS was NR for patients in the siltuximab arm.
Figure 1.
Clinical and lymph node responses. Kaplan-Meier curves for PFS (A) and overall survival in patients treated with siltuximab or placebo (B). Cumulative incidence curves for time to normalization of lymph node response (C) and time to durable symptomatic response (D) in patients treated with siltuximab or placebo. BSC, best supportive care; NR, not reached.
Overall survival
Because there are limited survival data published on patients with iMCD, we analyzed overall survival as an exploratory analysis despite the limited power to detect a difference. The 2-year estimates for overall survival were 93% (95% CI, 85% to 100%) for the siltuximab arm and 77% (95% CI, 55% to 98%) for the placebo arm. The difference between the 2 groups was not statistically significant (P = .11), but there was a trend toward improved survival for patients treated with siltuximab compared with placebo (Figure 1B). Given that this is a rare disease and that few deaths were observed during this study (2 in the siltuximab arm and 4 in the placebo arm), additional follow-up or a larger sample size may be necessary to discern a difference. Of note, no deaths occurred in the siltuximab-treated patients who achieved the primary end point of durable tumor (radiologic) and symptomatic responses. All 6 deaths occurred among placebo-treated patients and siltuximab patients who did not achieve the primary end point.
Time to treatment failure
The time to treatment failure (defined as disease progression or a lack of symptom improvement; data supplement6) was significantly improved with siltuximab vs placebo (P = .005; supplemental Figure 1). In total, 20 (37.7%) of 53 patients in the siltuximab arm experienced treatment failure, compared with 16 (61.5%) of 26 patients in the placebo arm. Seven patients experienced treatment failure as a result of tumor (radiologic) progression (1 in the siltuximab arm vs 6 in the placebo arm; supplemental Table 1). The median time to treatment failure was 4.8 months (95% CI, 3.3 to NR) with placebo and was NR with siltuximab. The 6-month estimates for treatment failure were 20% (95% CI, 11% to 32%) in the siltuximab arm and 52% (95% CI, 32% to 68%) in the placebo arm.
Time to lymph node response
The time to lymph node response (≥50% decrease in SPD from baseline) was significantly improved in the siltuximab arm compared with placebo (P = .01; Figure 1C). The percentage of patients treated with siltuximab demonstrating a lymph node response was greater than that of those receiving placebo (22 [45.8%] of 48 vs 3 [12.5%] of 24, respectively). The median time to SPD normalization was 12.2 months (95% CI, 6.2 to NR) with siltuximab treatment and was NR in the placebo arm. The 12-month estimates for SPD normalization were 49% (95% CI, 32% to 64%) with siltuximab and 13% (95% CI, 3% to 31%) with placebo.
Time to durable symptomatic response
The time to achieve a durable symptomatic response (≥50% decrease in the 34-point symptom score from baseline that was maintained for a minimum of 18 weeks) was significantly improved in the siltuximab arm compared with the placebo arm (P = .003; Figure 1D). More patients treated with siltuximab than with placebo demonstrated a durable symptomatic response (30 [56.6%] of 53 vs 5 [19.2%] of 26). The median time to achieve a durable symptomatic response was 6.9 months (95% CI, 3.4 to NR) with siltuximab and was NR with placebo. The 12-month estimate of durable symptomatic response was 61% (95% CI, 45% to 74%) in the siltuximab arm compared with 19% (95% CI, 7% to 36%) in the placebo arm.
Taken together, these analyses of PFS, overall survival, time to treatment failure, time to lymph node response, and time to durable symptomatic response indicate that siltuximab provides clinically meaningful benefits for patients with iMCD.
Time to normalization of abnormal laboratory parameters
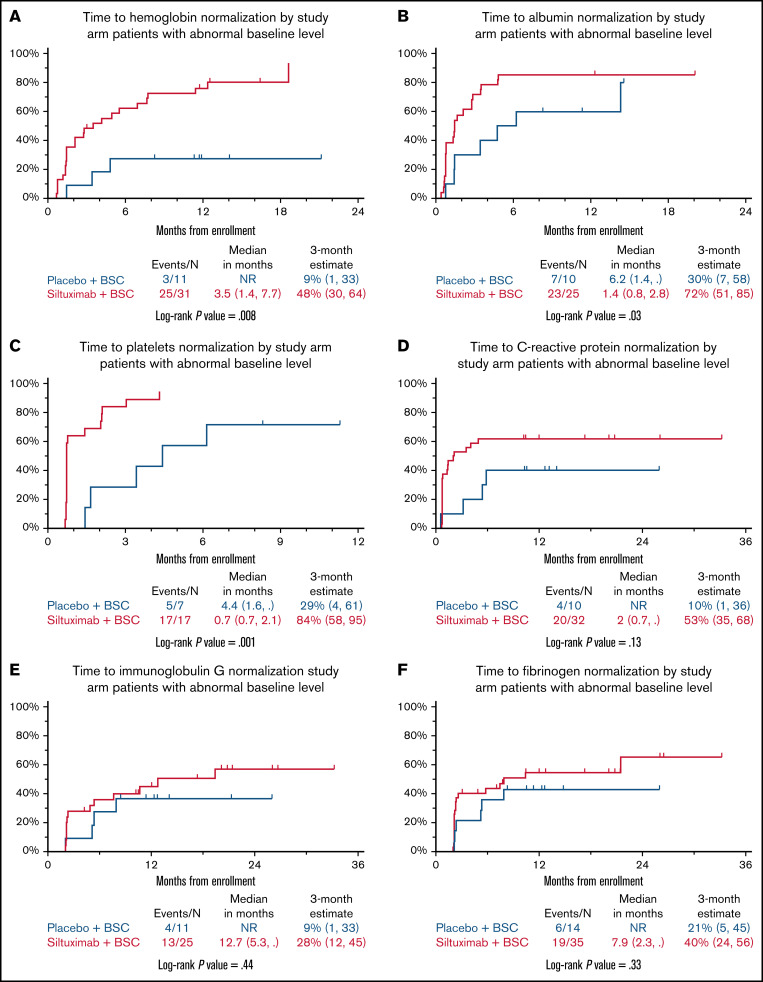
Next, we sought to understand the time to normalization for 6 laboratory parameters. We focused on patients with abnormal values at baseline in the siltuximab- and placebo-treated groups; both siltuximab responders and nonresponders were included in the analysis. The time to normalization of anemia (Figure 2A), hypoalbuminemia (Figure 2B), and thrombocytosis (Figure 2C) was significantly improved in patients treated with siltuximab compared with placebo (P = .008 for anemia, P = .03 forhypoalbuminemia, and P = .001 for thrombocytosis). The time to CRP normalization also seemed to be improved in patients treated with siltuximab vs placebo, but this did not achieve statistical significance (P = .13; Figure 2D). The times to normalization of elevated IgG (P = .44; Figure 2E) and hyperfibrinogenemia (P = .33; Figure 2F) were not significantly different between the 2 arms.
Figure 2.
Laboratory responses. Cumulative incidence curves for time to normalization for anemia (A), hypoalbuminemia (B), thrombocytosis (C), CRP (D), IgG (E), and hyperfibrinogenemia (F) in patients treated with siltuximab or placebo. BSC, best supportive care.
Looking in more detail at anemia normalization, 25 (80.6%) of 31 patients in the siltuximab arm with abnormal baseline levels achieved normalization, compared with 3 (27.3%) of 11 patients in the placebo arm (Figure 2A). The median time to anemia normalization was 3.5 (95% CI, 1.4-7.7) months in patients treated with siltuximab and was NR in those receiving placebo. The 3-month estimates for anemia normalization were 48% (95% CI, 30% to 64%) and 9% (95% CI, 1% to 33%), respectively.
For hypoalbuminemia, 23 (92.0%) of 25 patients in the siltuximab arm with abnormal baseline levels achieved normalization, compared with 7 (70.0%) of 10 patients in the placebo arm (Figure 2B). The median time to normalization was 1.4 (95% CI, 0.8-2.8) months with siltuximab and 6.2 (95% CI, 1.4 to NR) months with placebo. The 3-month estimates for hypoalbuminemia normalization were 72% (95% CI, 51% to 85%) and 30% (95% CI, 7% to 58%), respectively.
With regard to thrombocytosis, 17 (100%) of 17 patients in the siltuximab arm with abnormal baseline levels achieved normalization, compared with 5 (71.4%) of 7 in the placebo arm (Figure 2C). The median time to normalization was 0.7 (95% CI, 0.7-2.1) months with siltuximab vs 4.4 (95% CI, 1.6 to NR) months in the placebo arm. The 3-month estimates for thrombocytosis normalization were 84% (95% CI, 58% to 95%) and 29% (95% CI, 4% to 61%), respectively.
Finally, for CRP, 20 (62.5%) of 32 patients in the siltuximab arm with abnormally high baseline levels achieved normalization vs 4 (40.0%) of 10 in the placebo arm (Figure 2D). The median time to CRP normalization was 2.0 (95% CI, 0.7 to NR) months in patients treated with siltuximab and was NR for those in the placebo arm. The 3-month estimates for CRP normalization were 53% (95% CI, 35% to 68%) and 10% (95% CI, 1% to 36%), respectively.
Sequence of normalization for laboratory values and clinical features in siltuximab responders
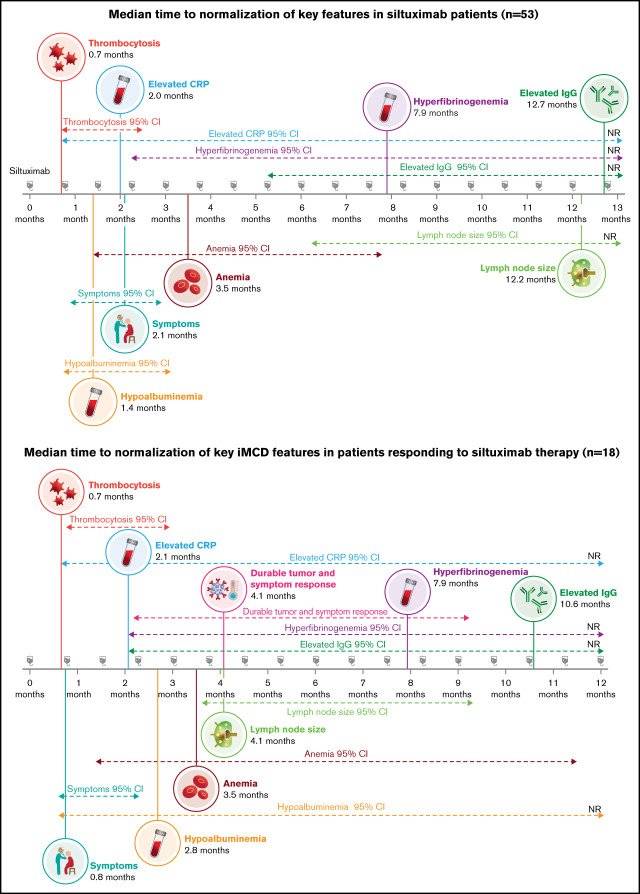
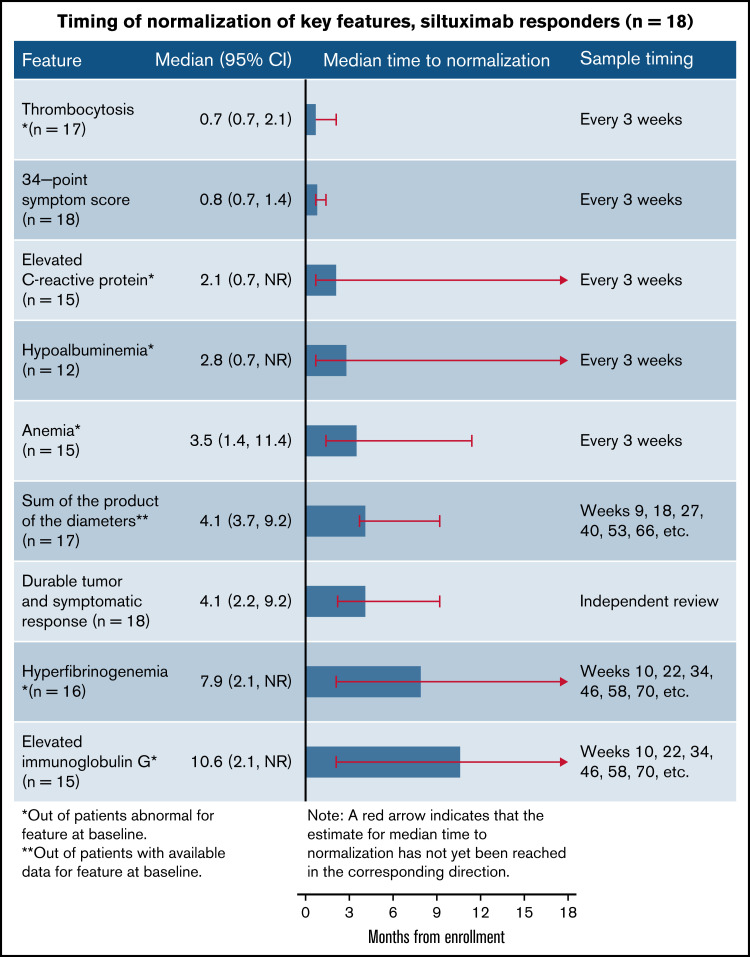
In order to understand the sequence of normalization in laboratory parameters and clinical end points in siltuximab responders, we focused our next analysis on the 18 patients with iMCD who achieved durable tumor (radiologic) and symptomatic responses while treated with siltuximab (the primary end point of the phase 2 study). The median time to normalization of 6 abnormal laboratory tests and 2 clinical end points occurred in the following sequence: thrombocytosis (0.7 [95% CI, 0.7-2.1] months), symptomatic response (0.8 [95% CI, 0.7-1.4] months), elevated CRP (2.1 [95% CI, 0.7 to NR] months), hypoalbuminemia (2.8 [95% CI, 0.7 to NR] months), anemia (3.5 [95% CI, 1.4-11.4] months), lymph node response (4.1 [95% CI, 3.7-9.2] months), hyperfibrinogenemia (7.9 [95% CI, 2.1 to NR] months), and elevated IgG (10.6 [95% CI, 2.1 to NR] months; Figure 3). It should be noted, however, that the timing and frequency of evaluation differed among these 8 parameters, as described in the Methods section.
Figure 3.
Sequence of normalization of laboratory, clinical, and lymph node responses in siltuximab responders. Responders were those achieving durable tumor (radiologic) and symptomatic responses (n = 18).
Among those treated with siltuximab who achieved durable tumor and symptomatic responses, the parameter that was met in the most patients after 3 months was symptomatic response (estimated 94% of patients; supplemental Figure 2). This was followed by normalization of thrombocytosis (estimated 84% of patients), normalization of hypoalbuminemia (67%), CRP normalization (53%), and anemia normalization (47%). At 3 months, the estimated rate of lymph node response was 24%, but this was >50% by 6 months (53%). However, estimated rates of hyperfibrinogenemia and elevated IgG normalization required 12 months to exceed 50% (58% and 56%, respectively; supplemental Figure 2). Together, these results indicate that some laboratory parameters and clinical end points normalize rapidly, whereas others take more time.
Discussion
We report for the first time that treatment with siltuximab significantly improves PFS compared with placebo in the setting of a randomized clinical trial. Patients treated with siltuximab benefited from a 2-year PFS of 91% vs only 37% in those receiving placebo (P = .001). There was also a trend toward superior overall survival in the siltuximab arm (93% vs 77%; P = .11), although this study was not powered or designed to address this question. Time to treatment failure in the placebo arm was only 4.8 months and NR with siltuximab (P = .005). The significantly improved PFS observed with siltuximab is a meaningful finding for patients who face lifelong treatment to control iMCD. This notion is further underscored by our previously reported observation that among patients who initially benefit from siltuximab, >70% have a sustained response during long-term follow-up, with an acceptable toxicity profile.8 There has been a paucity of data regarding PFS in iMCD for siltuximab and other treatments used off label for iMCD. In a single-center real-world study, siltuximab was found to prolong PFS compared with rituximab.10 A systematic review of patients treated with siltuximab in clinical trials estimated a 5-year overall survival rate of nearly 96.4%, whereas historical case series using traditional immunosuppressive and cytotoxic treatment methods reported 5-year overall survival ranging from 55% to 77%.11 Recently, 2 single-arm studies were published in which combination chemotherapy was used to treat iMCD. One study used thalidomide, cyclophosphamide, and prednisone and observed a 1-year PFS rate of 60%. A second study found a 1-year PFS rate of 79% using the 3-drug regimen of bortezomib, cyclophosphamide, and dexamethasone. These studies seemed to indicate inferior PFS, although obviously caution is required when cross-comparing studies.12
We previously reported that patients exhibiting a significant IL-6–induced inflammatory state who have a higher symptom burden or more pronounced laboratory features are most likely to derive benefit from siltuximab therapy.1 More recently, we found that a model incorporating the baseline laboratory values of hemoglobin, CRP, fibrinogen, and IgG had an 86% predictive ability to separate responders from nonresponders.8 In this study, we examined the temporal response to siltuximab therapy to provide clinicians with more insight regarding both the timing and nature of cardinal features to be monitored. Such information is of particular importance to the treating clinician in deciding whether to persist with siltuximab or switch to an alternative therapy and at which time point response can be deemed to be insufficient.
In comparison with those receiving placebo, siltuximab-treated patients rapidly normalized thrombocytosis, hypoalbuminemia, and anemia at a median of 0.7, 1.4, and 3.5 months, respectively. These improvements were rapid, bearing in mind that siltuximab was administered every 3 weeks. Similar effects were observed in the interim analysis of patients with CD who participated in the phase 1 study, where suppression of erythrocyte sedimentation rate, fibrinogen, CRP, and IgG was observed as early as week 3.13 The median time to a durable symptomatic response was longer, at 6.9 months, whereas lymph node regression occurred in a more delayed fashion (median time, 12.2 months). Only 49% of siltuximab-treated patients had lymph node regression at 12 months. When confining this analysis to siltuximab responders, rapid normalizations occurred in thrombocytosis, elevated CRP, hypoalbuminemia, and anemia (median times, 0.7, 2.1, 2.8, and 3.5 months, respectively), whereas other parameters such as hyperfibrinogenemia and elevated IgG returned to normal more slowly (median times, 7.9 and 10.6 months, respectively). It is not certain whether this delayed response in IgG and fibrinogen reflects a biologic difference or whether it can be explained by less frequent measurements during the study (every 9 weeks rather than every 3 weeks). However, the median time to first normalization of symptoms in siltuximab responders was swift, at just 0.8 months.
These data suggest that when monitoring patients during siltuximab therapy, one can expect significant improvements or normalizations of biochemical parameters such as CRP, hypoalbuminemia, thrombocytosis, and anemia to occur any time between the first and fourth doses in patients who will go on to respond. It should be noted that the analyses of laboratory values performed in this study were all one directional, and although a majority of patients (n = 24) in the siltuximab group had thrombocytosis, a small number did have thrombocytopenia (n = 6). Analysis of the patients with thrombocytopenia was not performed because of the very low sample size; however, there is evidence that patients with iMCD with low platelet levels are at increased risk of fatal outcomes.5,14
Similarly, we observed that iMCD-related symptoms abated relatively quickly in a large portion of patients. This is consistent with a real-world Polish study involving 11 patients treated with siltuximab, in which best symptomatic response was observed in most cases after the fourth treatment cycle (range, 3-9 cycles). Furthermore, 72.7% of patients had an objective response, and time to best response was 6.5 months (range, 2-34 months).15 However, siltuximab is not a cytoreductive therapy; it neutralizes IL-6. As a result, the time of lymph node involution is much slower and may take ≥1 year. A caveat in the interpretation of these results is that the clinical trial excluded patients with significant organ dysfunction, and these findings may not readily translate to the most severely afflicted patients. It is also possible that administration of concomitant steroids may have had some effect on the early responses observed. At baseline, concomitant corticosteroid use was 25% in the siltuximab arm and 35% in the placebo arm.7 Although some improvement initially observed may have been attributed to steroid use, sustained improvement with steroids is unusual in patients with iMCD, and overall steroid use was lower in the siltuximab group than in the placebo group. Also, in clinical practice, the recommended regimen in first-line treatment for patients with iMCD, as per internationally recognized Castleman Disease Collaborative Network treatment guidelines, is siltuximab (in combination with steroids in the most severe cases), so one would expect timings of response reported in these analyses to be applicable to the recommended clinical approach.16
We found that siltuximab treatment prolongs PFS and significantly reduces treatment failures while quickly improving symptomatology, resulting in often rapid and meaningful clinical benefit for patients. Moreover, prior studies have shown that the response to treatment with siltuximab is durable, with few patients losing response over time. The present study also highlights the importance of monitoring disease activity in patients with iMCD receiving siltuximab therapy using parameters indicative of clinical, laboratory, and lymph node responses. The description of suitable indicators to measure response to siltuximab can be used to assist clinician decision making in order to ensure patients are given optimal therapy for iMCD. Key laboratory markers that clinicians should follow are platelet count, albumin, and hemoglobin; these should be measured every cycle to help identify responders early. CRP is an excellent marker of iMCD disease activity, likely because of its role as a biomarker of IL-6 signaling, but it should be noted that the time to CRP normalization in both study arms was not significantly different in this study, and therefore, it may not be a good biomarker of response to therapy. The lymph node response lags significantly behind others, and imaging studies such as CT or CT/positron emission tomography should be conducted every 3 to 6 months. A lack of reduction in lymph node size soon after treatment initiation should therefore not drive clinical decisions on therapy. Our study had sample-size limitations because of the rarity of the disease. However, the phase 2 study remains the largest randomized clinical trial to date, involving 79 patients with iMCD. Overall, these data support the continued first-line use of siltuximab for iMCD, as recommended by international guidelines.12
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who volunteered to participate in this study and the staff members of the study sites who cared for them. The authors also thank Sheila Pierson for her valuable feedback and Azhaar Ashraf and Lisa Grant of TVF Communications (London, United Kingdom) for providing medical writing assistance.
EUSA Pharma provided funding for the development of the manuscript.
Authorship
Contribution: All authors meet the criteria for authorship defined in the International Committee of Medical Journal Editors recommendations, have had full access to all the data in the study, and accept responsibility to submit for publication. F.v.R. and D.C.F. defined the concept of the study; data analysis was provided by A.R., K.N., and A.H; and all authors critically discussed and commented on the paper.
Conflict-of-interest disclosure: F.v.R. reports a consultancy relationship with Takeda, Sanofi Genzyme, EUSA Pharma, Adicet Bio, Kite Pharma, and Karyopharm Therapeutics. K.K. and R.M. are employees of EUSA Pharma. D.C.F. received research funding from EUSA Pharma for the ACCELERATE registry, consulting fees from EUSA Pharma, and study drug from Pfizer for a clinical trial of sirolimus and holds pending provisional patents for “Methods of treating idiopathic multicentric Castleman disease with JAK1/2 inhibition” and “Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease.” The remaining authors declare no competing financial interests.
Correspondence: Frits van Rhee, Myeloma Center, University of Arkansas for Medical Sciences, West Markham St, Little Rock, AR 72205; e-mail: vanrheefrits@uams.edu.
References
- 1.Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129(12):1646-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshizaki K, Murayama S, Ito H, Koga T. The role of interleukin-6 in Castleman disease. Hematol Oncol Clin North Am. 2018;32(1):23-36. [DOI] [PubMed] [Google Scholar]
- 3.Liu AY, Nabel CS, Finkelman BS, et al. Idiopathic multicentric Castleman’s disease: a systematic literature review. Lancet Haematol. 2016;3(4):e163-e175. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee S, Martin R, Sande B, Paige J, Fajgenbaum DC. Epidemiology and treatment patterns of idiopathic multicentric Castleman disease in the era of IL-6 directed therapy. Blood Adv. 2022;6(2):359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dispenzieri A, Armitage JO, Loe MJ, et al. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87(11):997-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurzrock R, Voorhees PM, Casper C, et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res. 2013;19(13):3659-3670. [DOI] [PubMed] [Google Scholar]
- 7.van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15(9):966-974. [DOI] [PubMed] [Google Scholar]
- 8.van Rhee F, Casper C, Voorhees PM, et al. Long-term safety of siltuximab in patients with idiopathic multicentric Castleman disease: a prespecified, open-label, extension analysis of two trials. Lancet Haematol. 2020;7(3):e209-e217. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Tu M, Cortes J, et al. Clinical and pathological characteristics of HIV- and HHV-8-negative Castleman disease. Blood. 2017;129(12): 1658-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitenga J, Aird G, Ahmed A, Silberstein PT. Impact of siltuximab on patient-related outcomes in multicentric Castleman’s disease. Patient Relat Outcome Meas. 2018;9:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Zhang M-Y, Cao X-X, et al. A prospective, multicenter study of bortezomib, cyclophosphamide, and dexamethasone in relapsed/refractory iMCD. Leuk Lymphoma. 2022;63(3):618-626. [DOI] [PubMed] [Google Scholar]
- 13.van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010; 28(23):3701-3708. [DOI] [PubMed] [Google Scholar]
- 14.Fajgenbaum DC, Pierson SK, Kanhai K, et al. The disease course of Castleman disease patients with fatal outcomes in the ACCELERATE registry [published online ahead of print 4 May 2022]. Br J Haematol. doi:10.1111/bjh.18214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrowska B, Szymczyk A, Olszewska-Szopa M, et al. Efficacy of siltuximab in the treatment of idiopathic multicentric castleman disease, the first Polish, real-world experience with long-term observation. Leuk Lymphoma. 2021;62(12):3031-3034. [DOI] [PubMed] [Google Scholar]
- 16.van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132(20):2115-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.