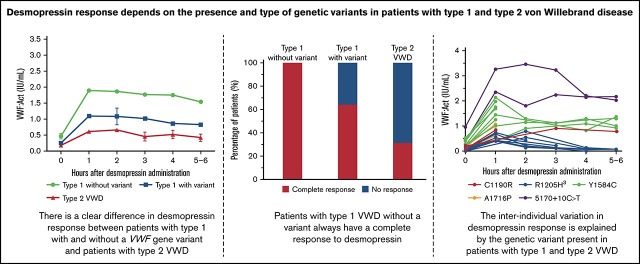
Key Points
All type 1 VWD patients without a VWF gene variant have a complete response to desmopressin.
In type 1 and type 2 VWD patients with a VWF gene variant, desmopressin response highly depends on the causative VWF gene variant.
Visual Abstract
Abstract
Patients with type 1 and type 2 von Willebrand disease (VWD) can be treated with desmopressin. Although a previous study has shown that the location of the causative VWF gene variant is associated with desmopressin response in type 1 VWD, the association between variants in the VWF gene and desmopressin response is not yet fully understood. Our primary aim was to compare desmopressin response in type 1 VWD patients with and without a VWF gene variant. Secondly, we investigated whether desmopressin response depends on specific VWF gene variants in type 1 and type 2 VWD. We included 250 patients from the Willebrand in the Netherlands study: 72 type 1 without a VWF gene variant, 108 type 1 with a variant, 45 type 2A, 16 type 2M, and 9 type 2N patients. VWF gene was analyzed with ion semiconductor sequencing and Multiplex Ligation-dependent Probe Amplification. Complete response to desmopressin was observed in all type 1 VWD patients without a variant, 64.3% of type 1 patients with a variant, and 31.3% of type 2 patients (P < .001). Despite a large interindividual variability in desmopressin response, patients with the same variant had comparable desmopressin responses. For instance, in 6 type 1 patients with exon 4 to 5 deletion, mean VWF activity at 1 hour after desmopressin was 0.81 IU/mL, with a coefficient of variation of 22.9%. In conclusion, all type 1 VWD patients without a VWF gene variant respond to desmopressin. In type 1 and type 2 VWD patients with a VWF variant, desmopressin response highly depends on the VWF gene variants.
Introduction
von Willebrand Disease (VWD) is characterized by a reduced or abnormal function of von Willebrand factor (VWF).1 VWF is responsible for platelet adhesion and aggregation.2 Therefore, patients with VWD have a reduced clot formation and an increased bleeding phenotype.1 VWF also serves as carrier protein for coagulation factor VIII (FVIII), explaining the reduced FVIII levels observed in VWD patients.2 Clinical manifestations of VWD include mucosa-associated bleeding, such as menorrhagia, gingival bleeding, and postsurgical bleeding.3 VWD can be classified in 3 types.1 Type 1 VWD is most prevalent and characterized by reduced levels of VWF. Type 2 VWD is characterized by an abnormal function of VWF, whereas type 3 VWD is characterized by a complete absence of VWF.1
Treatment of VWD consists of increasing VWF and FVIII levels by infusion of exogenous VWF-containing concentrates or by administration of desmopressin, which stimulates the release of endogenous VWF from vascular endothelial cells.4-6 Treatment with desmopressin is preferred above VWF-containing concentrates as it is more convenient because desmopressin can be administered intranasally and subcutaneously and is less expensive. However, not all VWD patients have a sufficient increase in VWF and FVIII after desmopressin administration.6 The majority of patients with type 1 VWD respond well to desmopressin, whereas only a small number of type 2 VWD patients respond.7 In type 2 VWD patients, VWF antigen usually increases after desmopressin, but VWF activity remains low.8 Therefore, a desmopressin test dose is required in patients diagnosed with VWD to assess the magnitude and duration of response to desmopressin.6 In patients with type 2B VWD, desmopressin is contraindicated because desmopressin administration may lead to thrombocytopenia.1,4
VWF levels in circulation are largely determined by the VWF gene, which is located on chromosome 12.9 A broad spectrum of variants in the VWF gene are found in patients with VWD.9-12 However, ∼30% of type 1 VWD patients do not have a variant in the VWF gene.10,13-15 The European MCMDM-1VWD study has previously shown in 77 patients that the location of the causative VWF gene variant is associated with desmopressin response in type 1 VWD patients.16 The MCMDM-1VWD study also found that all of the 16 included type 1 VWD patients without a VWF gene variant had a complete response to desmopressin.16 Larger studies are needed to compare the desmopressin response of type 1 VWD patients with and without a VWF gene variant. Moreover, it is unknown whether specific VWF gene variants explain the variability in desmopressin response in type 1 and type 2 VWD patients. It is also unclear whether family members with a comparable phenotype of VWD have a similar response to desmopressin. By clarifying the association between genotype and desmopressin response, one may hypothetically be able to predict the desmopressin response of patients, and thereby, patients may not need a test dose of desmopressin.
Therefore, we have investigated the association between genotype and desmopressin response in a large cohort of type 1, type 2A, 2M, and 2N VWD patients. Our primary aim was to compare the desmopressin response between type 1 VWD patients with and without a VWF gene variant. Secondly, we aimed to investigate whether the desmopressin response depends on specific VWF gene variants in type 1 and type 2 VWD patients. Lastly, we aimed to compare the desmopressin response of index cases in whom the VWF gene was analyzed and affected family members in whom the VWF gene was not analyzed.
Methods
Patients
We included all type 1, type 2A, 2M, and 2N VWD patients from the Willebrand in the Netherlands (WiN) study in whom a desmopressin test was performed and in whom all exons of the VWF gene was analyzed.3,17 Affected family members from the WiN study in whom the VWF gene was not analyzed but who had the same type of VWD as an index case, including comparable historical and centrally measured VWF and FVIII levels, were also included. Inclusion criteria of the WiN study were historically lowest VWF antigen (VWF:Ag), VWF activity or VWF collagen binding (VWF:CB) ≤0.30 IU/mL or FVIII activity (FVIII:C) ≤0.40 IU/mL (in case of type 2N VWD), and a positive family history of VWD or personal bleeding diathesis.3,17 The WiN study was approved by the Erasmus Medical Center Medical Ethical Committee. The study was conducted in accordance with the Declaration of Helsinki.
Data assessment
During inclusion in the WiN study, blood was obtained, and all patients filled in a questionnaire containing a self-administered Tosetto bleeding score. VWF and FVIII levels prior and immediately after a test dose of desmopressin were obtained from the electronic patient files. Desmopressin was, in most cases, administered intravenously at a dosage of 0.3 μg/kg in 50 mL of sodium chloride 0.9% infused over 30 minutes or intranasally at a total dosage of 300 µg. Venous blood samples were routinely obtained according to the institutional protocols. This consisted routinely of samples before and 1 to 4 or 6 hours after desmopressin administration.
Laboratory measurements
VWF and FVIII levels were centrally measured at inclusion in the WiN study at the Erasmus University Medical Center as described before.3,18 VWF propeptide (VWFpp) was centrally measured at the Leiden University Medical Center as described before.18 The assessment methods of the WiN study have been described in detail previously.3,17,18
VWF:Ag, VWF activity (VWF:Act), VWF:CB, and FVIII:C before and after desmopressin response were measured at the local treatment centers and were obtained from the electronic patient files. VWF:Act measurements varied between centers and varied in each center over time. In short, VWF:Act was measured with the antibiotic ristocetin and platelets assay (VWF:RCo), ristoceting and recombinant glycoprotein Ib (GPIb) fragments (VWF:GPIbR), recombinant GPIb fragments with 2 gain-of-function mutations (VWF:GPIbM), and monoclonal antibody assay (VWF:Ab). Although the laboratory assays that were used in each center may differ, all centers participated in external quality controls. As such, VWF and FVIII measurements were obtained from standardized assays, which were used in routine diagnostic settings.
Genetic analysis
Data on genetic analysis were obtained from the WiN study, in which the 52 exons of VWF gene and plus or minus 20 bp exon-intron boundaries were analyzed with Ion semiconductor sequencing (Ion-Torrent) at the hematology laboratory of the Radboud University Medical Center in Nijmegen. All detected variants were confirmed with Sanger sequencing. In patients without VWF gene variants, Multiplex Ligation-dependent Probe Amplification was performed to detect large deletions or duplications. All presented variants in this manuscript are in heterozygous form unless specified as homozygous. Benign variants were not regarded as pathogenic and were therefore omitted from this manuscript.
Definitions
Complete response to desmopressin was defined according to the 2021 American Society of Hematology, International Society on Thrombosis and Haemostasis, National Hemophilia Foundation, and World Federation of Hemophilia VWD guidelines: 2 times increase in VWF:Act (from baseline) at 1 hour after desmopressin and VWF:Act and FVIII ≥0.50 IU/mL until 4 hours after desmopressin.6,19 In patients with VWF:Act ≥0.50 IU/mL measured immediately before desmopressin administration, complete response was defined as VWF:Act >1.00 IU/mL until 4 hours after desmopressin.
Reduced synthesis/secretion of VWF was defined as FVIII:C/VWF:Ag ratio ≥1.9, whereas increased clearance of VWF was defined as VWFpp/VWF:Ag ratio ≥2.2 as described before.18,20 FVIII:C/VWF:Ag ratio <1.9 and VWFpp/VWF:Ag ratio <2.2 was defined as undetermined pathophysiology of reduced VWF levels.18
Affected family members
Affected family members were only included in the analyses comparing desmopressin response of type 1 VWD patients with and without a VWF gene variant and type 2 VWD patients and in the analyses comparing the desmopressin response of index cases and affected family members.
Statistical analysis
Continuous data are described as median and interquartile range and categorical data as number and percentage. The normality of data was visually assessed with histograms. In case of >30 patients per group, we compared groups with parametric tests, such as independent sample t-test or analysis of variance. Categorical data were compared between groups using a χ2 test.
Desmopressin response between type 1 with and without variants and type 2 VWD patients were compared with an analysis of variance test. Proportion of responders to desmopressin were compared between groups with a χ-square test. Desmopressin response per VWF gene variant are presented descriptively, without statistical tests, because of the low number of patients per VWF gene variant. The variability in desmopressin response is presented as the coefficient of variation (CV), which is expressed in percentages. Statistical analyses were performed with SPSS Statistics version 25 (IBM Corp., Armonk, NY). P < 0.05 was considered significant.
Results
We included a total of 250 type 1, type 2A, 2M, and 2N VWD patients. In 208 patients, genetic analysis was performed, and 42 patients were affected family members with the same type of VWD as the index case with a known genetic variant and similar historical and centrally measured VWF and FVIII levels. The patient characteristics are presented in Table 1. Seventy-two patients had type 1 VWD without a VWF gene variant, 108 patients had type 1 VWD with a VWF gene variant, 45 patients had type 2A, 16 had type 2M, and 9 had type 2N VWD. The median age at desmopressin administration was 36 years (24-46) and did not differ among type 1 patients with a variant, type 1 patients without a variant, and type 2 patients (Table 1). Older age was associated with a better desmopressin response (supplemental Figure 1). For instance, in type 1 VWD patients with a variant, a complete response to desmopressin was observed in 10/26 (38.5%) patients younger than 18, 25/37 (67.6%) patients 18 to 40, and 27/33 (81.8%) patients older than 40 years (P = .002). In type 1 VWD patients without a variant, VWF and FVIII levels 1 hour after desmopressin were similar, but levels at 2 to 5 or 6 hours were lower in patients with blood group O compared with patients with blood group non-O, although not statistically significant (supplemental Figure 2).
Table 1.
Patient characteristics
| Characteristics | Type 1 without variant (n = 72) | Type 1 with variant (n = 108) | Type 2 VWD (n = 70) |
|---|---|---|---|
| Age at desmopressin | 37 [26-46] | 35 [18-45] | 38 [27-48] |
| Female, n (%) | 48 (66.7%) | 66 (61.1%) | 39 (55.7%) |
| Blood group O, n (%) | 54 (75.0%)* | 60 (60.6%)* | 38 (55.1%)* |
| Genetic analysis, n (%) | 63 (87.5%) | 86 (79.4%) | 59 (84.3%) |
| Included AFMs, n (%) | 9 (12.5%) | 22 (20.4%) | 11 (15.7%) |
| Bleeding score1 | 9 [5-15]* | 7 [4-12]* | 11 [6-14]* |
| Historically lowest VWF levels2 | |||
| VWF:Ag | 0.39 [0.30-0.46]* | 0.26 [0.15-0.36]* | 0.31 [0.19-0.43]* |
| VWF:Act | 0.24 [0.20-0.27]* | 0.18 [0.10-0.25]* | 0.09 [0.04-0.22]* |
| VWF:CB | 0.25 [0.19-0.33]* | 0.19 [0.10-0.26]* | 0.15 [0.03-0.27]* |
| FVIII:C | 0.50 [0.40-0.61]* | 0.44 [0.27-0.60]* | 0.38 [0.26-0.57]* |
| FVIII:C/VWF:Ag ratio1 | 1.6 [1.4-1.8]* | 2.0 [1.6-2.5]* | 1.5 [1.2-2.0]* |
| VWFpp/VWF:Ag ratio1 | 2.0 [1.7-2.3]* | 2.8 [1.9-5.0]* | 4.1 [3.1-5.6]* |
Data are presented as median [interquartile range] unless otherwise specified. AFMs affected family members. 1FVIII:C/VWF:Ag ratio and VWFpp/VWF:Ag ratio were obtained at the inclusion in the WiN study. 2Historically lowest levels were measured at the local laboratories were also VWF and FVIII levels before and after desmopressin administration were measured.
P < .05. between groups.
Desmopressin response in patients with and without a VWF gene variant
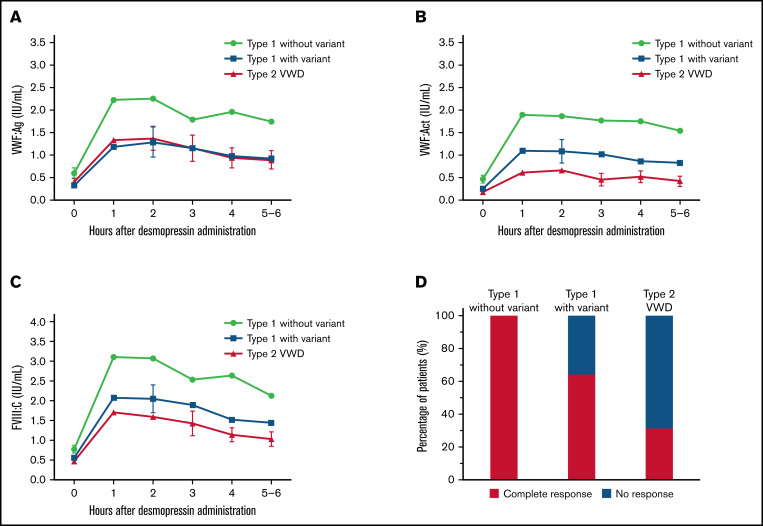
An overview of all VWF gene variants found in our cohort and their association with desmopressin response is provided in supplemental Table 1. Overall, we found a clear difference in VWF and FVIII levels after desmopressin between type 1 VWD patients with and without a variant and type 2 VWD (P < .001 for all variables at all measurements, Figure 1A-C). Desmopressin response was at all measurements after desmopressin significantly higher in type 1 VWD patients without a variant compared with type 1 patients with a variant (P < .001 at all measurements, Figure 1A-C). Even after adjustment for relevant confounders, these differences were present. For instance, in patients without a VWF gene variant, VWF:Act was at 1 hour after desmopressin β = 0.36 IU/mL higher (95% confidence interval, 0.16-0.56; P < .001) compared with type 1 VWD patients with a VWF gene variant (adjusted for historically lowest VWF:Act, VWF:Act immediately prior to desmopressin administration, FVIII:C/VWF:Ag ratio, and VWFpp/VWF:Ag ratio). As expected, VWF:Act was lower after desmopressin administration in type 2 VWD patients compared with type 1 patients with a variant (P < .01 at all measurements, Figure 1B), whereas VWF:Ag was not different between both groups (P > .3 at all measurements, Figure 1A).
Figure 1.
A large difference in desmopressin response was observed between type 1 VWD with and without a VWF gene variant and type 2 VWD. (A) VWF:Ag was higher after desmopressin in type 1 VWD patients without a variant compared with type 1 VWD patients with a variant and type 2 VWD patients. (B-C) VWF:Act and FVIII:C after desmopressin administration was highest in type 1 patients without a variant, followed by type 1 patients with a variant and type 2 VWD patients. (D) All type 1 VWD patients without a variant had a complete response to desmopressin, whereas 66.3% of type 1 patients with a variant and 31.1% of type 2 VWD patients had a complete response. In panels A-C, data are represented as mean and 95% CI. CI, confidence interval.
Based on the most recently defined desmopressin response criteria, 100% of type 1 patients without a VWF gene variant had a complete response, whereas 64.3% of type 1 VWD patients with a VWF gene variant and 31.3% of type 2 VWD patients had a complete response after desmopressin (P < .001, Figure 1D).
Desmopressin response depends on VWF gene variants in type 1 VWD
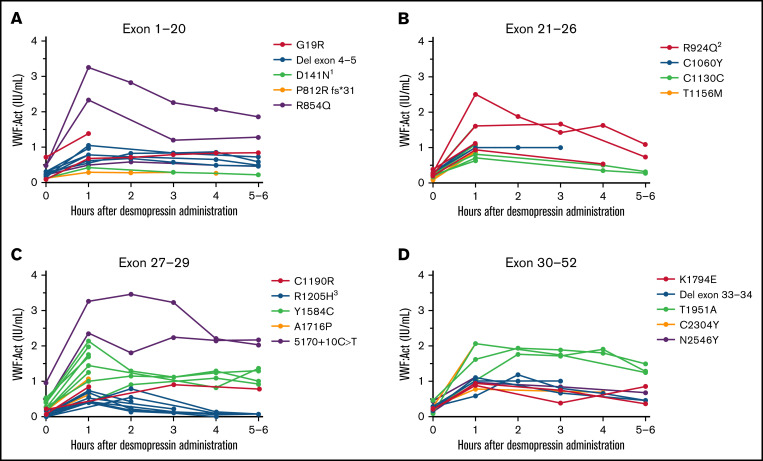
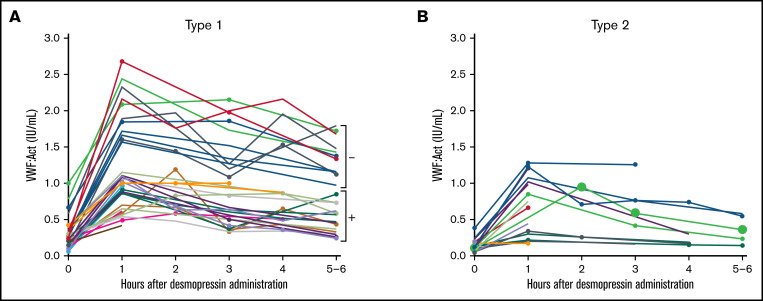
In all type 1 VWD patients in whom genetic analysis was performed, the interindividual variability in desmopressin response was large, with a CV of, respectively, 61.9% and 48.6% at 1 hour after desmopressin and 69.7% and 69.2% at 4 hours after desmopressin for VWF:Act and FVIII:C. However, patients with the same VWF gene variant had comparable desmopressin responses with small interindividual differences (Figure 2). For instance, in 6 type 1 VWD patients with an exon 4 to 5 deletion, mean VWF:Act at 1 hour after desmopressin was 0.81 IU/mL, with a CV of 22.9%, whereas at 4 hours mean VWF:Act was 0.73 IU/mL, with a CV of 27.0% (Figure 2A). Similar small interindividual CVs were observed for all other variants, except for R854Q and R924Q. One patient with R924Q had a lower desmopressin response compared with other patients with R924Q, which could be attributed to a second VWF gene variant (C1169W) present in this patient. One patient with R854Q had a lower desmopressin response compared with other patients with R854Q, for which no clear explanation could be found.
Figure 2.
Type 1 VWD patients with the same VWF gene variants have a comparable response to desmopressin. Each line represents a single patient. (A) Type 1 VWD patients with variants in exon 1 to 20. 1Both patients also had R2313C. (B) Type 1 VWD patients with variants in exon 21 to 26. 2The patient with lower desmopressin response also had C1169W. (C) Type 1 VWD patients with variants in exon 27 to 29. 3Two patients also had 5170+10C>T. (D) Type 1 VWD patients with variants in exon 30 to 52.
Desmopressin response depends on VWF gene variants in type 2 VWD
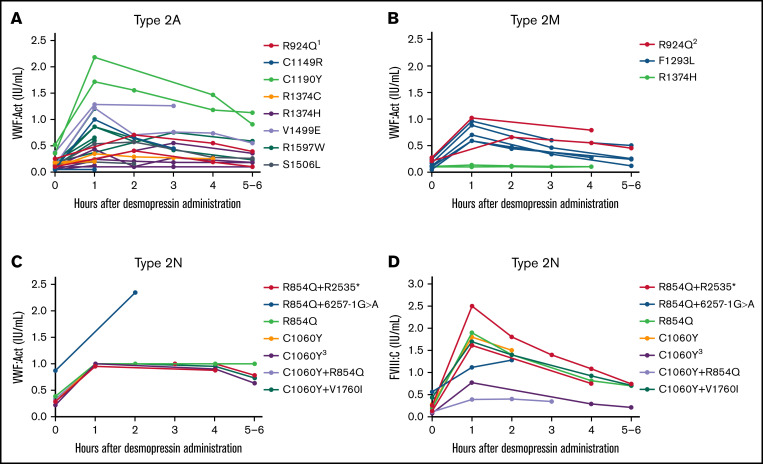
We included 36 type 2A VWD patients with 16 different VWF gene variants. Overall, a large interindividual variability was found, especially for VWF:Act with interindividual CVs of 80.7% and 38.1% at, respectively, 1 hour and 4 hours after desmopressin. However, the interindividual variability in type 2A patients with the same variant was low (Figure 3A). Patients with C1190Y had the highest VWF:Act after desmopressin, followed by V1499E. In all type 2A patients, desmopressin response was very comparable between patients with the same variant.
Figure 3.
Type 2 VWD patients with the same VWF gene variants have comparable responses to desmopressin. Each line represents a single patient. (A) Type 2A VWD. 1One patient also had G1609R. (B) Type 2M VWD. 2One patient also had R1374H. (C) VWF:Act in patients with type 2N VWD. VWF:Act was in most type 2N patients recorded as ≥1.00 IU/mL in the electronic patient files, in case of high levels, explaining the straight line from 1 to 4 hours in panel C. (D) FVIII:C in patients with type 2N VWD. 3Homozygous variant.
In type 2M VWD, 14 patients were included with 8 different VWF gene variants. Overall, also type 2M patients showed a variable response in VWF:Act at 1 hour and 4 hours after desmopressin, depending on the causative mutation (Figure 3B). However, in patients with the same variants, the interpatient variability in response was small because 2 patients with R1374H both had no increase in VWF:Act after desmopressin, whereas 2 patients with R924Q both had a complete response to desmopressin. In 5 patients with F1293L, desmopressin responses were very comparable, with low interindividual variance at measurements after desmopressin (Figure 3B).
In type 2N VWD, 6 patients had compound heterozygous variants, 2 patients had heterozygous variants, and 1 patient had a homozygous variant. Because there was a large variety of second variants in type 2N patients, we could not assess the variability in desmopressin response between patients with exactly the same genetic background, except for R854Q+R2535*, which was present in 2 patients with a comparable desmopressin response (Figure 3C-D). Although VWF:Act increased well after desmopressin in all type 2N patients, there was a large interindividual variability in FVIII:C response after desmopressin (Figure 3C-D).
The association between genotype and desmopressin response is mediated via the pathophysiological defects of VWF
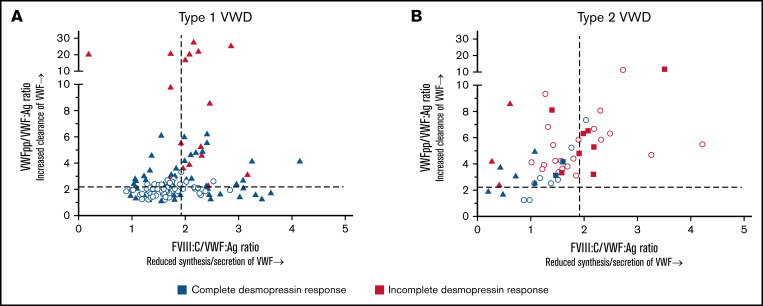
In type 1 VWD, all patients with reduced synthesis/secretion of VWF with normal clearance (FVIII:C/VWF:Ag ≥1.9 and VWFpp/VWF:Ag <2.2) had a complete response to desmopressin (Figure 4A). All patients with a rapid clearance of VWF with VWFpp/VWF:Ag ratio >7 had an incomplete response to desmopressin (Figure 4A). Lastly, all patients with undetermined pathophysiology of reduced VWF levels (FVIII:C/VWF:Ag <1.9 and VWFpp/VWF:Ag <2.2) had a complete response to desmopressin (Figure 4A).
Figure 4.
The association between genotype and desmopressin response is mediated via the pathophysiological defects of VWF. Each point represents 1 patient. Dashed lines represent the cut-off values for reduced synthesis/secretion and increase clearance of VWF. (A) Type 1 VWD. Triangles represent patients with a VWF gene variant, whereas dots represent patients without a VWF gene variant. (B) Type 2 VWD. Dots represent patients with type 2A, squares represent patients with type 2M and triangles represent patients with type 2N VWD.
In type 2 VWD, all patients with rapid clearance of VWF with VWFpp/VWF:Ag ratio ≥6.0 had an incomplete response to desmopressin (Figure 4B). Also, all patients with a combination of both reduced synthesis/secretion and increased clearance of VWF (FVIII:C/VWF:Ag ratio ≥1.9 and VWFpp/VWF:Ag ≥2.2) had an incomplete response to desmopressin, except for 1 patient with a borderline response (VWF:Act of 0.56 IU/mL 4 hours after desmopressin, Figure 4B). Lastly, all 4 patients with undetermined pathophysiology of reduced VWF levels (FVIII:C/VWF:Ag <1.9 and VWFpp/VWF:Ag <2.2) had a complete response to desmopressin (Figure 4B).
Desmopressin response in affected family members
VWF and FVIII levels after desmopressin were similar between index cases and affected family members at all measurements after desmopressin (all P values > .05). In 41 type 1 VWD patients, desmopressin response was very comparable between index cases in whom the VWF gene was analyzed and affected family members (9 families with a VWF gene variant and 5 families without a variant, Figure 5A). In 19 type 2 VWD patients from 7 families, desmopressin response also seemed comparable between index cases and affected family members (Figure 5B).
Figure 5.
Index cases and affected family members have a comparable desmopressin response. Each line represents a single patient. All family members are illustrated with the same color. Index patients are indicated with dots. (A) Desmopressine response in index cases and affected family members with type 1 VWD. –Type 1 patients without a VWF gene variant. +Type 1 patients with a VWF gene variant. (B) Desmopressine response in index cases and affected family members with type 2 VWD.
Discussion
In this large study in well-defined type 1 and type 2 VWD patients, we have investigated the association between genotype and desmopressin response. We found that all type 1 VWD patients without a VWF gene variant had a complete response to desmopressin, whereas in type 1 VWD patients with a VWF gene variant, only 64.3% had a complete response, and in type 2 VWD, only 31.3% had a complete response. Furthermore, despite a large interindividual variation in desmopressin response in type 1 and type 2 VWD, patients with the same VWF gene variants had very similar desmopressin responses, even in unrelated patients. Lastly, desmopressin response seemed comparable between index cases in whom the VWF gene was analyzed and affected family members.
The results of our study indicate that there is a clear difference in the desmopressin response of type 1 VWD patients with and without a VWF gene variant, confirming the results of the MCMDM-1VWD study.16 Because all type 1 VWD patients without a VWF gene variant had a complete response, desmopressin testing may not be needed in these patients. Similarly, it was previously found that 40/40 (100%) patients with historically lowest VWF levels of 0.30 to 0.50 IU/mL had a complete response to desmopressin, suggesting that desmopressin testing may not be needed in these patients.21 Together with our previous findings that there is a clear difference in the pathophysiology of type 1 VWD patients with and without a VWF gene variant and that type 1 VWD patients without a mutation have higher VWF and FVIII levels, the results of our current study indicates that type 1 VWD patients with and without a VWF gene variant are distinct groups.22 Although until now, genetic testing was not routinely performed in type 1 VWD patients, these new insights raise the question of whether we should perform genetic analyses in type 1 VWD patients. Especially, because genetic analysis may have therapeutic consequences as observed that all type 1 patients without a variant respond well to desmopressin and therefore do not need a desmopressin test.
Castaman et al have previously demonstrated in 77 patients that desmopressin response is influenced by the genotype in type 1 VWD patients.16 We have confirmed their results in type 1 VWD patients with various variants and additionally found the same association in type 2 VWD patients. Some of the variants that we describe in this manuscript have already been reported in relation to desmopressin response. As described in several previous studies, type 1 Vicenza (R1205H) is known for its poor response to desmopressin, due to fast clearance of VWF.23 We have also found that type 1 VWD patients with other variants associated with a rapid clearance of VWF based on a VWFpp/VWF:Ag ratio above 7.0, such as S2179R, also do not respond to desmopressin. Of note, despite that patients with an increased clearance of VWF may not have a complete desmopressin response, desmopressin treatment may still have a therapeutic role during minor interventions, as was previously shown for R1205H and C1130F.24 Also comparable to our study, patients with R1374H and R1374C were previously found not to respond to desmopressin.8,16 On the other hand, Y1584C and R854Q were previously shown to be associated with a good response to desmopressin, as confirmed in the current study.8,16 Importantly, in patients with R854Q who did not respond to desmopressin, a second variant was often identified. It was also previously shown that most patients with R1597W have a poor response to desmopressin.8,16 In summary, these findings illustrate that desmopressin response highly depends on the specific VWF gene variant in type 1 and type 2 VWD patients. This suggests that if the genetic variant in a certain patient is known in literature to be associated with no response or with a very good response to desmopressin, then a desmopressin test may not be required in that patient. In type 2N VWD, only a small number of patients had the same VWF gene variants. Therefore, we could not reliably assess the variability in desmopressin response of patients with the same variants. In line with our study, it was previously found that type 2N VWD patients have a good response to desmopressin.16,25 Moreover, it was also found that there is a large variability in FVIII:C increase after desmopressin in type 2N VWD patients.25
Older age was associated with a better desmopressin response. This could mean that desmopressin response is better in older patients, which may have to do with the age-related increase of VWF levels.26,27 It could also be associated with the fact that patients that are diagnosed at a younger age generally have a more severe bleeding phenotype, which is associated with a worse desmopressin response.28 Future studies are needed to investigate whether desmopressin response changes intraindividually with aging.
We have also observed that in index cases and affected family members with the same type of VWD and similar historical and centrally measured VWF and FVIII levels, desmopressin response was comparable between index cases and affected family members. This suggests that desmopressin tests may not be needed in affected family members with the same disease phenotype as an index case. Additional prospective studies are needed to confirm these findings. However, one may choose to perform a test dose of desmopressin to assess the side effects of desmopressin in a certain patient.
Furthermore, the pathophysiology of reduced VWF levels was strongly associated with desmopressin response, especially in type 1 VWD patients. We have previously shown that each VWF gene variant leads to specific synthesis/secretion and/or clearance defects of VWF and is associated with historically lowest and centrally measured VWF levels.22 Together, these findings lead to the working hypothesis that a specific VWF gene variant leads to specific defects in VWF synthesis/secretion and/or clearance, which determines VWF levels and subsequently determines whether a patient responds to desmopressin or not.
The main strength of this study is that extensive VWF and FVIII measurements were performed before and after desmopressin administration, and all exons of the VWF gene were analyzed, including Multiplex Ligation-dependent Probe Amplification, in a large cohort of well-defined type 1 and type 2 VWD patients. Therefore, we were able to investigate many VWF gene variants and to compare the desmopressin response between several patients with the same VWF gene variants. Also, we had data on the pathophysiology of reduced VWF levels and were therefore able to investigate the association between genetic variants, pathophysiological defects in VWF, and desmopressin response. A potential limitation is that VWF and FVIII levels after desmopressin were measured with different assays in different laboratories. However, we have demonstrated for the 4 most used VWF:Act assays that overall, the assays are very comparable.29 Moreover, we found a similar response to desmopressin in patients with the same variants in whom VWF and FVIII levels were measured in different laboratories. If VWF and FVIII levels would have been measured in the same laboratory, desmopressin responses would have probably been even more comparable. Another potential limitation is that some VWF gene variants may lead to a laboratory phenotype of type 1 VWD in some patients and type 2 VWD in other patients. Therefore, some variants are listed in this manuscript as causing both type 1 VWD and type 2 VWD. Lastly, it should be noted that a patient who is classified as a nonresponder according to the desmopressin response criteria may still benefit from desmopressin during, for instance, small bleeding such as epistaxis.
In conclusion, this study shows that type 1 VWD patients without a VWF gene variant always respond to desmopressin, and desmopressin response strongly depends on specific VWF gene variants in patients with type 1 and type 2 VWD. These results indicate that genetic analysis may have an important additional value for optimizing the therapeutic management of VWD patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by research funding from the Dutch Hemophilia Foundation (Stichting Haemophilia), Shire (Takeda), and CSL Behring (unrestricted grant).
Appendix: WiN study group members
The members of the WiN study group are: Karin J. Fijnvandraat, Michiel Coppens, Joke de Meris, Laurens Nieuwenhuizen, Karina Meijer, Rienk Y.J. Tamminga, Paula F. Ypma, Jeroen Eikenboom, Johanna G. van der Bom, Frans J.W. Smiers, Karin P.M. van Galen, Floor C.J.I. Heubel-Moenen, Paul Brons, Saskia E.M. Schols, Frank W.G. Leebeek (principal investigator), Marjon H. Cnossen, Ferdows Atiq, and Calvin B. van Kwawegen.
Authorship
Contribution: F.A. designed the study, performed statistical analysis, interpreted data, and wrote the manuscript; J.H., F.S., and E.K. collected data, interpreted data, and critically revised the manuscript; J.B., W.L.v.H., S.K., and S.C.S. designed the study, collected data, interpreted data, and critically revised the manuscript; J.d.M., D.M., S.E.M.S, K.G., J.B., M.C., K.M., K.F., and J.E. designed the study, interpreted data, and critically revised the manuscript; F.L. conceived of and designed the study, interpreted data, and critically revised the manuscript; and all authors gave their consent to the final version of the manuscript.
Conflict-of-interest disclosure: F.A. received the CSL Behring Professor Heimburger Award 2018 and a travel grant from Sobi. J.H. received the CSL Behring Professor Heimburger Award 2018. J.B. started working at Sobi after finishing this research project. W.L.v.H. reports speaker, consultant, and travel fees from Takeda, Bayer, CSL Behring, and Sobi. He is also cofounder and CSO of Enzyre. M.H.C. has received investigator-initiated research and travel grants over the years from the Netherlands Organisation for Scientific Research (NWO), the Netherlands Organization for Health Research and Development (ZonMw), the Dutch “Innovatiefonds Zorgverzekeraars,” Baxter/Baxalta/Shire, Pfizer, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, and Nordic Pharma and has served as a steering board member for Roche, Bayer, and Novartis. All grants, awards, and fees go to the Erasmus Medical Center as institution. The institution of K.F. has received unrestricted research grants from CSL Behring, Sobi, and NovoNordisk, and her institution received consultancy fees from Grifols, Takeda, Novo Nordisk, and Roche. K.M. received research support from Bayer, Sanquin, and Pfizer; speaker fees from Bayer, Sanquin, Boehringer Ingelheim, BMS, and Aspen; and consulting fees from uniQure, of which all fees go to the institution. S.E.M.S. has received travel grants from Bayer and Takeda and consultancy grants from Takeda and Novo Nordisk. J.E. received research support from CSL Behring, and he has been a teacher on educational activities of Roche. K.P.M.v.G. received unrestricted research support from CSL Behring and Bayer and speaker fees from Takeda. J.G.v.d.B. has been a teacher on educational activities of Bayer and received consultancy fees from Novo Nordisk, paid to the Leiden University Medical Center. F.W.G.L. received research support from CSL Behring and Shire/Takeda for performing the Willebrand in the Netherlands (WiN) study and Sobi and uniQure for studies not related to this manuscript; is a consultant for uniQure, Sobi, Biomarin, and Shire/Takeda, of which the fees go to the institution; and has received a travel grant from Sobi. He is also a DSMB member for a study by Roche. The remaining authors declare no competing financial interests.
A full list of members of the WiN study group appears in “Appendix.”
Correspondence: Frank W. G. Leebeek, Department of Hematology, Erasmus University Medical Center, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.
References
- 1.Leebeek FW, Eikenboom JC. Von Willebrand’s disease. N Engl J Med. 2016;375(21):2067-2080. [DOI] [PubMed] [Google Scholar]
- 2.Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10(12): 2428-2437. [DOI] [PubMed] [Google Scholar]
- 3.de Wee EM, Sanders YV, Mauser-Bunschoten EP, et al. ; WiN study group . Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108(4):683-692. [DOI] [PubMed] [Google Scholar]
- 4.Leebeek FWG, Atiq F. How I manage severe von Willebrand disease. Br J Haematol. 2019;187(4):418-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijdra JM, Cnossen MH, Leebeek FWG. Current and emerging options for the management of inherited von Willebrand Disease. Drugs. 2017;77(14):1531-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federici AB. The use of desmopressin in von Willebrand disease: the experience of the first 30 years (1977-2007). Haemophilia. 2008; 14(Suppl 1):5-14. [DOI] [PubMed] [Google Scholar]
- 8.Federici AB, Mazurier C, Berntorp E, et al. Biologic response to desmopressin in patients with severe type 1 and type 2 von Willebrand disease: results of a multicenter European study. Blood. 2004;103(6):2032-2038. [DOI] [PubMed] [Google Scholar]
- 9.de Jong A, Eikenboom J. Von Willebrand disease mutation spectrum and associated mutation mechanisms. Thromb Res. 2017;159:65-75. [DOI] [PubMed] [Google Scholar]
- 10.Veyradier A, Boisseau P, Fressinaud E, et al. ; French Reference Center for von Willebrand disease . A laboratory phenotype/genotype correlation of 1167 French patients from 670 families with von Willebrand disease: a new epidemiologic picture. Medicine (Baltimore). 2016;95(11):e3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borràs N, Batlle J, Pérez-Rodríguez A, et al. Molecular and clinical profile of von Willebrand disease in Spain (PCM-EVW-ES): comprehensive genetic analysis by next-generation sequencing of 480 patients. Haematologica. 2017;102(12):2005-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodeve A, Eikenboom J, Castaman G, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD). Blood. 2007;109(1):112-121. [DOI] [PubMed] [Google Scholar]
- 13.Cumming A, Grundy P, Keeney S, et al. ; UK Haemophilia Centre Doctors’ Organisation . An investigation of the von Willebrand factor genotype in UK patients diagnosed to have type 1 von Willebrand disease. Thromb Haemost. 2006;96(5):630-641. [PubMed] [Google Scholar]
- 14.James PD, Notley C, Hegadorn C, et al. The mutational spectrum of type 1 von Willebrand disease: results from a Canadian cohort study. Blood. 2007;109(1):145-154. [DOI] [PubMed] [Google Scholar]
- 15.Johansson AM, Halldén C, Säll T, Lethagen S. Variation in the VWF gene in Swedish patients with type 1 von Willebrand Disease. Ann Hum Genet. 2011;75(4):447-455. [DOI] [PubMed] [Google Scholar]
- 16.Castaman G, Lethagen S, Federici AB, et al. Response to desmopressin is influenced by the genotype and phenotype in type 1 von Willebrand disease (VWD): results from the European Study MCMDM-1VWD. Blood. 2008;111(7):3531-3539. [DOI] [PubMed] [Google Scholar]
- 17.de Wee EM, Mauser-Bunschoten EP, Van Der Bom JG, et al. ; Win Study Group . Health-related quality of life among adult patients with moderate and severe von Willebrand disease. J Thromb Haemost. 2010;8(7):1492-1499. [DOI] [PubMed] [Google Scholar]
- 18.Sanders YV, Groeneveld D, Meijer K, et al. ; WiN study group . von Willebrand factor propeptide and the phenotypic classification of von Willebrand disease. Blood. 2015;125(19):3006-3013. [DOI] [PubMed] [Google Scholar]
- 19.Connell NT, James PD, Brignardello-Petersen R, et al. von Willebrand disease: proposing definitions for future research. Blood Adv. 2021;5(2):565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eikenboom J, Federici AB, Dirven RJ, et al. ; MCMDM-1VWD Study Group . VWF propeptide and ratios between VWF, VWF propeptide, and FVIII in the characterization of type 1 von Willebrand disease. Blood. 2013;121(12):2336-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atiq F, van Heerde WL, Boender J, et al. Genetic analyses in 391 VWD patients: novel VWF gene variations, and important differences in the phenotype of type 1 VWD patients with- and without – a VWF gene mutation [abstract]. Res Pract Thromb Haemost. 2020;4(suppl 1). https://abstracts.isth.org/abstract/genetic-analyses-in-391-vwd-patients-novel-vwf-gene-variations-and-important-differences-in-the-phenotype-of-type-1-vwd-patients-with-and-without-a-vwf-gene-mutation/. Accessed 11 May 2022. [Google Scholar]
- 23.Haberichter SL, Castaman G, Budde U, et al. Identification of type 1 von Willebrand disease patients with reduced von Willebrand factor survival by assay of the VWF propeptide in the European study: molecular and clinical markers for the diagnosis and management of type 1 VWD (MCMDM-1VWD). Blood. 2008;111(10):4979-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castaman G, Tosetto A, Federici AB, Rodeghiero F. Bleeding tendency and efficacy of anti-haemorrhagic treatments in patients with type 1 von Willebrand disease and increased von Willebrand factor clearance. Thromb Haemost. 2011;105(4):647-654. [DOI] [PubMed] [Google Scholar]
- 25.Mazurier C, Gaucher C, Jorieux S, Goudemand M; Collaborative Group . Biological effect of desmopressin in eight patients with type 2N (‘Normandy’) von Willebrand disease. Br J Haematol. 1994;88(4):849-854. [DOI] [PubMed] [Google Scholar]
- 26.Rydz N, Grabell J, Lillicrap D, James PD. Changes in von Willebrand factor level and von Willebrand activity with age in type 1 von Willebrand disease. Haemophilia. 2015;21(5):636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders YV, Giezenaar MA, Laros-van Gorkom BA, et al. ; WiN study group . von Willebrand disease and aging: an evolving phenotype. J Thromb Haemost. 2014;12(7):1066-1075. [DOI] [PubMed] [Google Scholar]
- 28.Atiq F, Schütte LM, Looijen AEM, et al. von Willebrand factor and factor VIII levels after desmopressin are associated with bleeding phenotype in type 1 VWD. Blood Adv. 2019;3(24):4147-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boender J, Eikenboom J, van der Bom JG, et al. ; WiN Study Group . Clinically relevant differences between assays for von Willebrand factor activity. J Thromb Haemost. 2018;16(12):2413-2424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.