Key Points
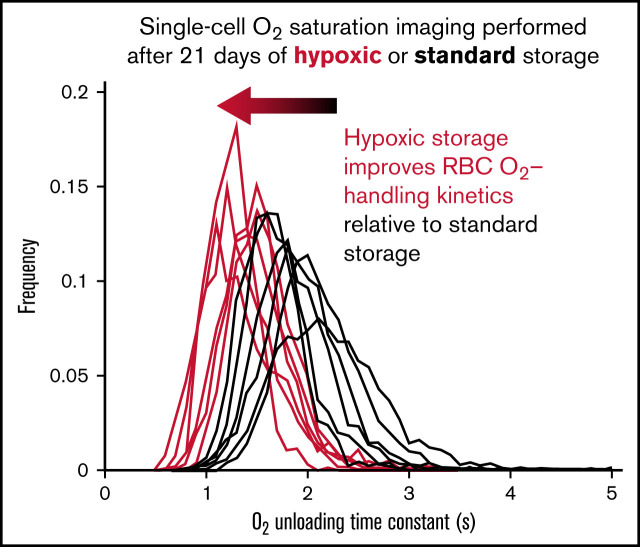
Relative to standard blood-bank protocols, hypoxic storage preserves faster O2 unloading from red cells through metabolic remodelling.
Functional appraisal of O2 handling demonstrates a beneficial effect of hypoxic storage on the quality and shelf life of blood products.
Visual Abstract
Abstract
Stored red blood cells (RBCs) incur biochemical and morphological changes, collectively termed the storage lesion. Functionally, the storage lesion manifests as slower oxygen unloading from RBCs, which may compromise the efficacy of transfusions where the clinical imperative is to rapidly boost oxygen delivery to tissues. Recent analysis of large real-world data linked longer storage with increased recipient mortality. Biochemical rejuvenation with a formulation of adenosine, inosine, and pyruvate can restore gas-handling properties, but its implementation is impractical for most clinical scenarios. We tested whether storage under hypoxia, previously shown to slow biochemical degradation, also preserves gas-handling properties of RBCs. A microfluidic chamber, designed to rapidly switch between oxygenated and anoxic superfusates, was used for single-cell oxygen saturation imaging on samples stored for up to 49 days. Aliquots were also analyzed flow cytometrically for side-scatter (a proposed proxy of O2 unloading kinetics), metabolomics, lipidomics, and redox proteomics. For benchmarking, units were biochemically rejuvenated at 4 weeks of standard storage. Hypoxic storage hastened O2 unloading in units stored to 35 days, an effect that correlated with side-scatter but was not linked to posttranslational modifications of hemoglobin. Although hypoxic storage and rejuvenation produced distinct biochemical changes, a subset of metabolites including pyruvate, sedoheptulose 1-phosphate, and 2/3 phospho-d-glycerate, was a common signature that correlated with changes in O2 unloading. Correlations between gas handling and lipidomic changes were modest. Thus, hypoxic storage of RBCs preserves key metabolic pathways and O2 exchange properties, thereby improving the functional quality of blood products and potentially influencing transfusion outcomes.
Introduction
Each year in Europe and North America, ∼35 units of blood are transfused per 1000 inhabitants. To support this demand, red blood cells (RBCs) are stored extracorporeally under conditions designed to preserve quality.1 However, long-term storage results in donor-dependent2,3 biochemical and morphological changes4-7 that are sensitive to the processing method.1,8,9 Although multiple clinical trials found no effect of storage duration on clinical outcomes,10-13 a recent analysis of large real-world data showed that transfusion of RBC units stored for more than 1 to 2 weeks is associated with higher recipient mortality.14 This seminal finding suggests a negative impact of storage lesions on transfusion efficacy and highlights the need for optimized storage conditions.
Any efforts to improve storage regimes must be guided by appropriate functional readouts of RBC quality. Because the primary physiological role of RBCs is to transport and exchange oxygen, the most appropriate means of gauging quality should explicitly relate to this process. Our current understanding of the effect of storage on RBCs has been based on biochemical studies as gas-handling assays have not been routinely performed. An emerging paradigm of storage lesion formation is that of oxidative damage,15 manifesting as posttranslational changes in proteins (eg, hemoglobin,16 peroxiredoxin 2,16-18 anion exchanger 1 [SLC4A1],19 catalase, pyruvate kinase, glucose 6-phosphate dehydrogenase20) and depletion of redox metabolites (eg, glutathione).21 Specifically, the oxidation of glyceraldehyde 3-phosphate dehydrogenase has been linked to the depletion of 2,3-diphosphoglycerate (2,3-DPG) during the first 2 weeks of storage.5,8,9,22,23 Based on these observations, hypoxic storage has been proposed as a way of evading oxidative damage.24,25 Indeed, storage under hypoxia has been shown to preserve 2,3-DPG,26 protect proteins against oxidative damage (eg, hemoglobin26), and improve end-of-storage cell morphology.25 Part of these benefits is due to the alkalinizing effect of hypoxic/hypocapnic storage, which boosts the activity of bisphosphoglycerate mutase.27 Previously, alkaline additives have been shown to improve the biochemical and biophysical qualities of stored RBCs,28,29 although a recent study found no effect on posttransfusion recovery.29 In contrast, transfusion of RBCs stored under hypoxia produces better outcomes in a rodent model of trauma and hemorrhage compared with standard storage,30 indicating a more complex effect of hypoxic storage, beyond alkalinization.
Although the biochemical consequences of hypoxic storage are well described, their relationship with the primary physiological function of RBCs remains elusive. Certain metabolites, such as 2,3-DPG have known effects on Hb-O2 stability; however, another factor influencing gas exchange relates to the cytoplasmic diffusion distance,31 which expands under storage because of spherical remodeling6,32; its metabolic dependence, however is less understood Thus, it remains to be tested whether the sum of all metabolic responses to hypoxic storage truly improves RBC gas-handling function. Moreover, it is unclear which metabolic changes should be prioritized for normalization by improved storage regimes. To address these issues, we developed single-cell oxygen saturation imaging that measures the O2 storage capacity and unloading time constant from individual RBCs.31 Previously, we used this approach to describe how standard storage implemented by the English National Health Service Blood and Transplant (NHSBT), among others, impairs O2 unloading.33 Slower O2 release to respiring tissues may reduce the efficacy of transfusions when an immediate restoration of gas transport is required, for instance, after a profound hemorrhage or in critical care. The kinetic derangement inflicted by standard storage could be rescued by biochemical rejuvenation using a formulation based on adenine, inosine, and pyruvate, marketed as Rejuvesol. This striking effect indicated that impaired gas handling relates to a deterioration in RBC biochemistry, but the dominant influence remains unclear as numerous metabolites respond to rejuvenation.34
This study tested whether hypoxic storage is superior to standard conditions in preserving physiological levels of O2 handling by RBCs. To address this question, we performed kinetic measurements at various timepoints during storage and benchmarked the effect of hypoxic storage against biochemical rejuvenation. We used a purpose-built microfluidics device capable of rapid superfusate exchange in an air-impermeable system. Impermeability of the chamber is necessary to maintain anoxia in 1 of the streams for driving O2 unloading from cells. Rapid solution exchange between anoxic and normoxic streams was achieved by a system of coordinated valves. This ensured that switching is rapid and not rate-limiting for the evoked cellular response. Rapid fluid exchange between microstreams was previously achieved using a double-barreled micropipette positioned near the cells on a coverslip,31 which was technically challenging. The innovative engineering solution offers a more robust and higher throughput alternative that outperforms the previously used technique. In parallel, samples were processed for flow cytometric, proteomic, metabolomic, and lipidomic analyses to seek correlations and identify common patterns with the effects of biochemical rejuvenation. We show profound improvement in RBC O2 handling during the first 35 days of storage under hypoxic conditions, reaching an efficacy comparable to rejuvenation, and implicating a set of common biochemical responses. We also corroborate that side-scatter (SSC) is a good proxy of RBC gas handling.
Methods
Blood storage
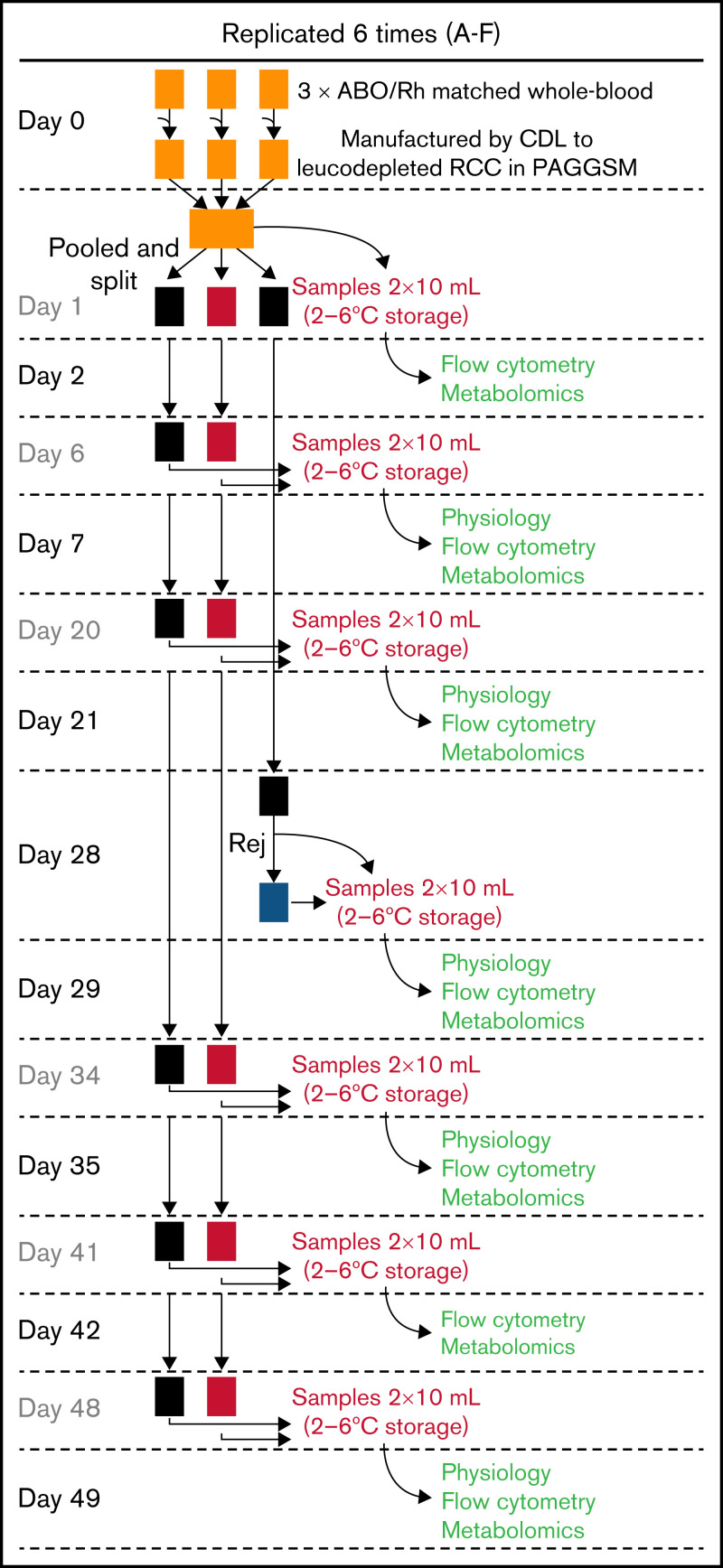
Donors gaving fresh blood samples gave consent for use of their blood in research. Data were anonymized and not traceable. Whole-blood units were collected in citrate phosphate dextrose, leukocyte depleted and manufactured as “leukocyte-reduced red cell concentrates” in isotonic phosphate-adenine-glucose-guanosine-saline-mannitol (Fresenius Kabi AG, Homburg, Germany) within 27 hours of venipuncture, according to standard NHSBT protocol. A total of 6 pools were prepared, each by pooling 3 immunocompatible leukocyte-reduced red cell concentrates units. Pools were split 3 ways: 2 stored in standard bags and the third prepared for storage under hypoxic conditions (Hemanext Inc, Lexington, MA).35 The hypoxic bag and 1 of the standard storage bags were sampled at various time points, whereas the second standard storage bag was used for biochemical rejuvenation at 28 days. See Figure 2 for sample processing schematic. Sampling was via needle-free spike (Origen Biomedical, Austin, TX) into syringes (confirmed as gas-tight) that were stored overnight at 4 ± 2°C and analyzed the following day. Blood bank quality measures were made as previously described.33,36 Freshly obtained venous blood from 6 volunteers was used for reference values.
Figure 2.
Protocol for storing RBC units. A total of 6 pools of blood were produced, each from 3 donors to reduce variation. Pools were split so that 1 set was stored under standard conditions, 1 under hypoxic conditions, and a third under standard conditions for end-point rejuvenation. Color coding of units is consistent throughout the data figures: standard storage in black, hypoxic storage in red, biochemically rejuvenated units in blue. At the time points indicated, RBC units were sampled for measurements the day after.
Design and fabrication of microfluidic devices
The microfluidic device for superfusing RBCs consisted of 2 input lines, each branching into a network of 8 capillary channels laid on a plane of 2 circles. Channels delivered solution to a central chamber (2.4 mm diameter, 0.2 mm height) with an open bottom, onto which a borosilicate coverslip is attached. See supplemental Methods for further details and supplemental Figure 1 for a schematic diagram.
Single-cell oxygen saturation imaging
O2 handling was measured using a modified version of a technique developed in our laboratory31 (see supplemental Methods).
Flow cytometry
Blood samples were analyzed on Sysmex XN1000, involving treatment with Cellpack DFL (Sysmex Europe GmbH, Norderstedt, Germany). RET-RBC-Y and RET-RBC-Z channels, corresponding to RBC forward-scatter (FSC) and SSC, were measured.
Metabolomics, lipidomics, and proteomics
Metabolomics were performed as described previously.37 Lipidomics were performed via ultra-high-performance liquid chromatography-tandem mass spectrometry.37 Proteomics were performed by FASP digestion and nano ultra-high-performance liquid chromatography-tandem mass spectrometry identification.38 See supplemental Methods for further details.
Statistical analysis
Summary data are presented as mean ± standard error of the mean unless stated otherwise. Blood bank measures were tested by repeated measures 2-way analysis of variance (ANOVA) with a multiple comparisons posttest. Large datasets were tested for significant effects of treatment and storage duration by 2-way ANOVA (Metaboanalyst). Correlations were performed against the change in time constant or SSC using Pearson test. Multiple regression analysis was performed using MATLAB.
Results
Measuring RBC oxygen handling using a microfluidic device and fluorescence imaging
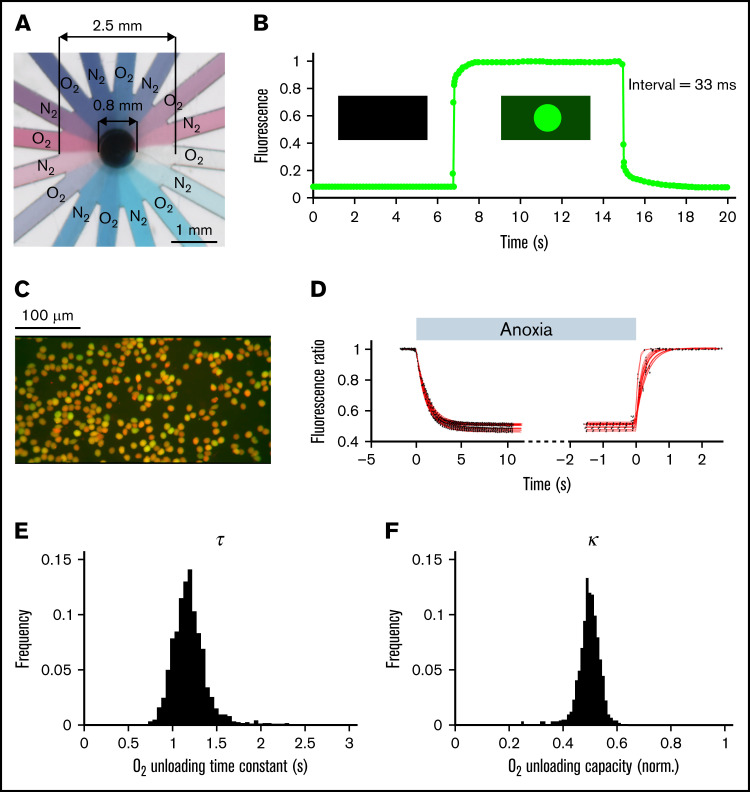
To interrogate oxygen-handling kinetics, RBCs were exposed to alternate flows of normoxic and anoxic solutions controlled by a system of electronically operated valves. Anoxic solutions were produced and maintained by continuous bubbling with N2 and the addition of 2 mM dithionite, with fresh solution was prepared every 2 hours. The 2 solutions were gravity fed to the inputs of the microfluidic device, where each line branched into 8 radially positioned channels feeding into a central chamber wherein cells are plated on a glass coverslip (Figure 1A; supplemental Figure 1). This microfluidic system produced rapid solution exchange, with a time constant faster than 33 ms as determined by labeling 1 solution with fluorescein (ie, not rate-limiting relative to the rate of O2 unloading from RBCs) (Figure 1B). To image O2 handling, RBCs were dually loaded with CellTracker DeepRed and Calcein and imaged using a fast camera system during superfusion with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered media (Figure 1C). At their respective optimal emission peaks, red fluorescence is strongly quenched by oxyhemoglobin; thus, the red/green ratio provides a readout of the RBCs’ oxygenation state.31 The rate and degree of O2 unloading was determined by analyzing the fluorescence response to a rapid and transient decrease in extracellular O2 tension, from atmospheric to anoxic and back. Best-fitting to a monoexponential curve informed the time constant and extent of O2 unloading (Figure 1D). Because the method has single-cell resolution, it can describe a population of RBCs in terms of frequency histograms of O2 unloading time constant (τ; Figure 1E) and capacity (κ; Figure 1F), illustrated here using venous blood drawn from a nonanemic volunteer.
Figure 1.
Measuring oxygen handling by RBCs. (A) Microfluidic chamber showing 16 channels supplied from 2 different solutions, and feeding into a central chamber where cells are superfused. (B). Rate of solution exchange was gauged by including 15 μM fluorescein in 1 of the solutions. The exchange time constant is better than 33 ms. (C) Imaging of RBCs loaded dually with CellTracker DeepRed and Calcein. (D) Fluorescence ratio (DeepRed/Calcein) during a rapid solution exchange, from normoxia to anoxia and back. The datapoints (black) are fitted to a monoexponential curve (red). (E) Histogram of time constant (τ) and (F) oxygen carrying capacity (κ) obtained for RBCs drawn from a nonanemic donor. A total of 1263 cells imaged from 18 fields of view and 2 loadings.
Oxygen-handling properties of RBCs are improved under hypoxic storage
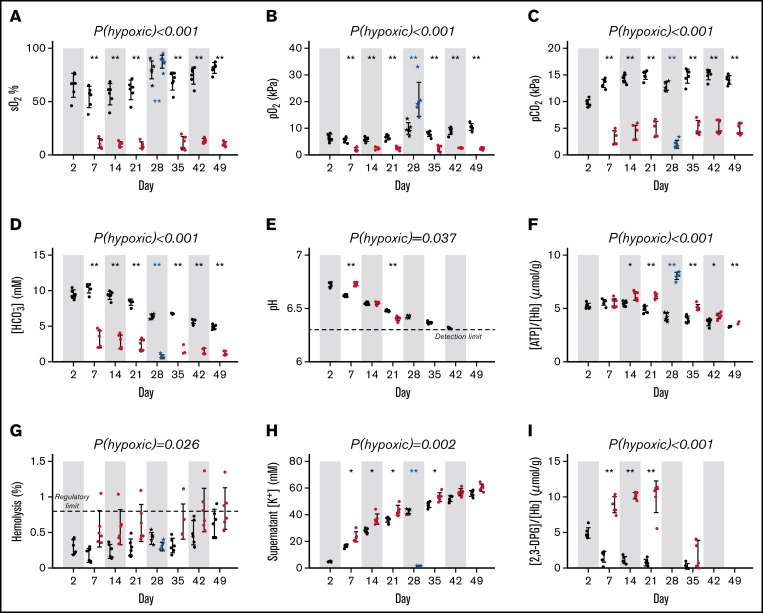
Single-cell oxygen saturation imaging was performed on samples of blood taken at various points during hypoxic and standard storage (Figure 2). The effect of hypoxic storage was benchmarked against biochemical rejuvenation of blood stored for 28 days. Oxygen saturation in standard bags was 54.4 ± 9.9% (mean ± standard deviation) on day 7, rising to 81.6 ± 4.8% on day 49; in contrast, hypoxic storage system maintained significantly lower levels throughout storage (eg, 10.4 ± 2.1% at day 49; Figure 3A). A similar pattern was reported for O2 partial pressure (Figure 3B). Hypoxic units had lower CO2 partial pressure (Figure 3C), lower bicarbonate (Figure 3D), and a more rapid decline in pH (Figure 3E). Starting from day 14, cellular ATP levels were significantly higher in hypoxic vs standard pools (Figure 3F). Hypoxic storage trended with ∼0.2% more hemolysis than standard storage for 5 of the pools (A-E), whereas the sixth hypoxic pool (F) appeared to be an outlier (Figure 3G). RBCs leaked potassium at a slightly increased rate under hypoxia (Figure 3H). Units were confirmed to be free of bacterial contamination at the end of storage. Hypoxic storage was associated with a profound increase in 2,3-DPG levels, peaking at day 21, followed by depletion by day ∼35; in contrast, 2,3-DPG levels became depleted within only a week of standard storage (Figure 3I).
Figure 3.
Standard quality measurements of units. Red cell concentrates (RCC) upon pooling or when subsequently split and stored under the hypoxic storage system (red dots), or standard normoxic storage (black dots) and rejuvenated (blue dots). (A) Oxygen saturation (sO2; %). (B) Partial pressure of O2 or (C) carbon dioxide (pCO2; kPa). (D) bicarbonate concentration ([HCO3−] mmol/L). (E) pH; note the lower detection limit is 6.3. (F) Cellular (ATP) (μmol/gHb) measured by assay. (G) Degree of hemolysis. (H) Supernatant potassium concentration (mmol/L). (I) [2,3-DPG] (μmol/gHb) measured by assay (measured for storage days 2, 7, 14, 21, and 35 only). Error bars represent mean ± standard deviation (n = 6). Significance values for statistical tests were denoted *P < .05, **P < .01 for hypoxic vs standard storage (repeated measures 2-way ANOVA, followed by multiple comparisons test) and for pre- and postrejuvenation (paired 2-sample t-test).
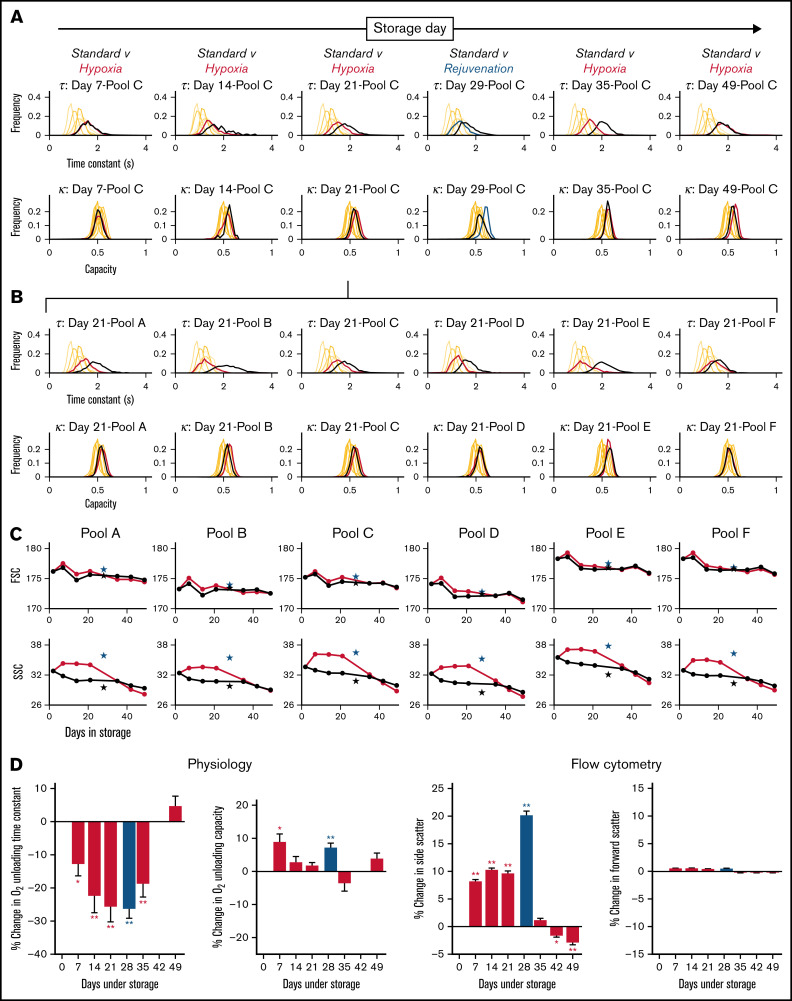
Exemplar histograms for the evolution of τ and κ at various time points along 49-day storage under hypoxic and standard conditions are shown in Figure 4A for 1 of the 6 blood pools. Measurements from freshly drawn venous blood from 6 nonanemic volunteers are included for comparison (supplemental Table 1 shows additional donor information). Compared with standard conditions, hypoxic storage was able to preserve faster O2 unloading τ for up to 35 days, indicated by a left shift in the histograms. The effect of hypoxic storage did not alter the width of the distribution, implying a uniform effect on all RBCs rather than a specific subpopulation. The peak effect of hypoxic storage was comparable to that of biochemical rejuvenation performed at 4 weeks. The beneficial effect of hypoxic storage was detected in all 6 blood pools studied, as illustrated for day 21 of storage in Figure 4B. In terms of capacity κ, the effect of hypoxic storage was less profound; with modest increases in capacity observed in some pools.
Figure 4.
Hypoxic storage improved RBC physiological function. (A) Histograms of O2 unloading time constant (top) and O2 carrying capacity (bottom) for bloods in pool C, sampled at various time points indicated during 49-day storage under standard condition (black lines) or hypoxia (red lines). Rejuvenation at 28 day was included as a benchmark (blue lines). Each histogram is constructed from at least 500 cells collected from at least 2 loadings and measurements from 6 fields of view. (B) Histograms of O2 unloading time constant (top row) and O2 carrying capacity (bottom row) for all 6 pools at day 21 of storage. (C) Effect of hypoxic storage and rejuvenation on flow cytometric parameters: FSC (top) and SSC (bottom). Mean of 2 flow cytometric experiments per time point. Star symbols show effect of rejuvenation relative to its control. (D) Average data from 6 pools for physiological and flow cytometric indices. *P < .05; **P < .01 (t-test).
Aliquots of blood were also tested flow cytometrically for changes in FSC and SSC, on the basis that the latter was previously correlated with changes in oxygen unloading kinetics.33 Whereas FSC remained constant over the duration of storage and largely unaffected by hypoxia, SSC was significantly higher under hypoxic storage, compared with standard conditions, to at least 21 days of storage (Figure 4C). Although the red cell factors determining this SSC response are not fully elucidated, its significance as a quality marker is supported by the fact that biochemical rejuvenation also increased SSC.
The effect of hypoxia and rejuvenation on physiological (τ, κ) and flow cytometric (SSC, FSC) parameters is presented in Figure 4D as the percentage change relative to time-matched controls under standard storage. A beneficial effect of hypoxia was measurable within a week of storage, and the maximum effect was observed around day 21. By day 49, however, there was no difference between standard and hypoxic storage. It is noteworthy that hypoxic storage maintained a favorable effect on RBC gas-handling kinetics for 35 days, whereas 2,3-DPG levels were protected for a shorter period. This observation indicates that the beneficial effect of hypoxic storage on RBC function is not solely related to 2,3-DPG, but that other processes and metabolites are of significance. In contrast to kinetics, the O2 carrying capacity (κ) was less responsive to hypoxic storage and did not change in a consistent manner. In agreement with earlier observations, the responses of τ and SSC correlated negatively. FSC, in contrast, was relatively unaffected by storage regime.
The effect of hypoxic storage on physiological function is not related to posttranslational modifications of relevant proteins
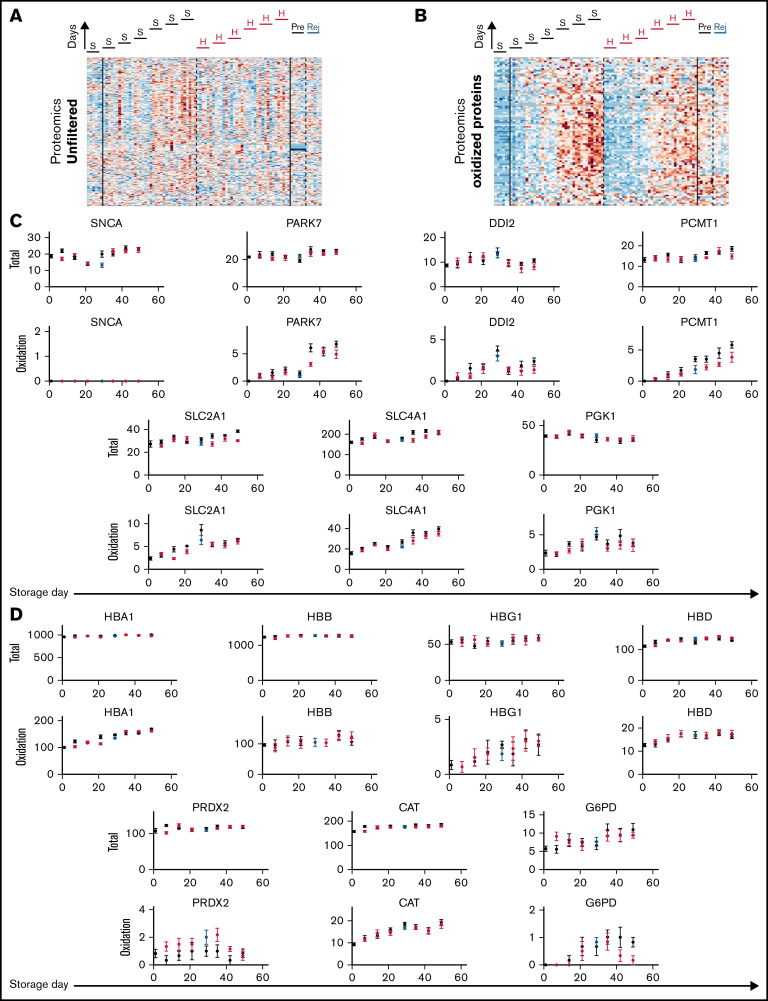
Standard storage protocols are associated with a depletion of antioxidants, which may affect the posttranslational state of proteins implicated in gas handling. To test whether differences in the levels of proteins, or their posttranslational modifications, underpin the effect of hypoxic storage on RBC physiological function, proteomic analyses were performed on samples normalized to input total protein (Figure 5A-B). There was no consistent effect of storage regime on the level of any protein detected, except for a modest effect on synuclein α (SNCA; Figure 5C). In terms of posttranslational modifications, the only statistically significant effect of hypoxic storage was on the oxidation of SLC4A1, parkinsonism-associated deglycase (PARK7), DNA-damage inducible homolog 2 (DDI2), protein-l-isoaspartate O-methyltransferase (PCMT1), glucose transporter 1 (SLC2A1), and phosphoglycerate kinase 1 (PGK1) (Figure 5C). Although the effect size was small, these catalytically active proteins are among the most abundant components in the proteome of mature RBCs. Of note, some of these components, specifically SLC4A1 and PCMT1, play a role in RBC metabolic reprogramming in response to oxidant stress and had been previously identified as targets of the storage lesion under normoxic, but not hypoxic storage.19,38 Hemoglobin isoforms (HBA1, HBB, HBG1, and HBD), catalase (CAT), and glucose 6-phosphate dehydrogenase (G6PD) showed a progressive increase in oxidation, consistent with prior observations39; however, hypoxic storage or biochemical rejuvenation did not significantly affect this specific posttranslational modification (Figure 5D). The implemented hypoxic storage protocol did not change the degree of methylation nor cysteine conversion to dehydroalanine (via β-elimination of thiols), which had been noted in prior studies.40 Thus, the observed beneficial effect of hypoxic storage on RBC gas handling does not relate to the levels of relevant proteins or their posttranslational modifications.
Figure 5.
Posttranslational modifications to proteins do not correlate with changes in RBC oxygen-handling function. (A) Proteomic analysis of RBC lysates (under S = standard or H = hypoxia storage, or in Rej = rejuvenated and Pre = its control). Heatmap shows signal filtered for proteins in the oxidized state. (B) Proteins for which oxidation was significantly affected by hypoxic storage. Top row shows total protein signal (unfiltered), and bottom row shows peptides gated for oxidized state. Note that these do not relate directly to oxygen transport. (C) Analysis of proteins directly involved in oxygen transport or shown previously to undergo oxidative damage during standard storage. Hypoxic storage had no effect on this posttranslational modification.
Biochemical analyses define the metabolic signature of restored gas handling
To seek biochemical mechanisms for the preservation of RBC physiological function under hypoxic storage, metabolomic and lipidomic analyses were performed on samples normalized to total protein. Because both hypoxic storage and biochemical rejuvenation increased O2 unloading rate and SSC, concordant changes in metabolites and lipids were sought as potential candidates for the underlying functional rescue.
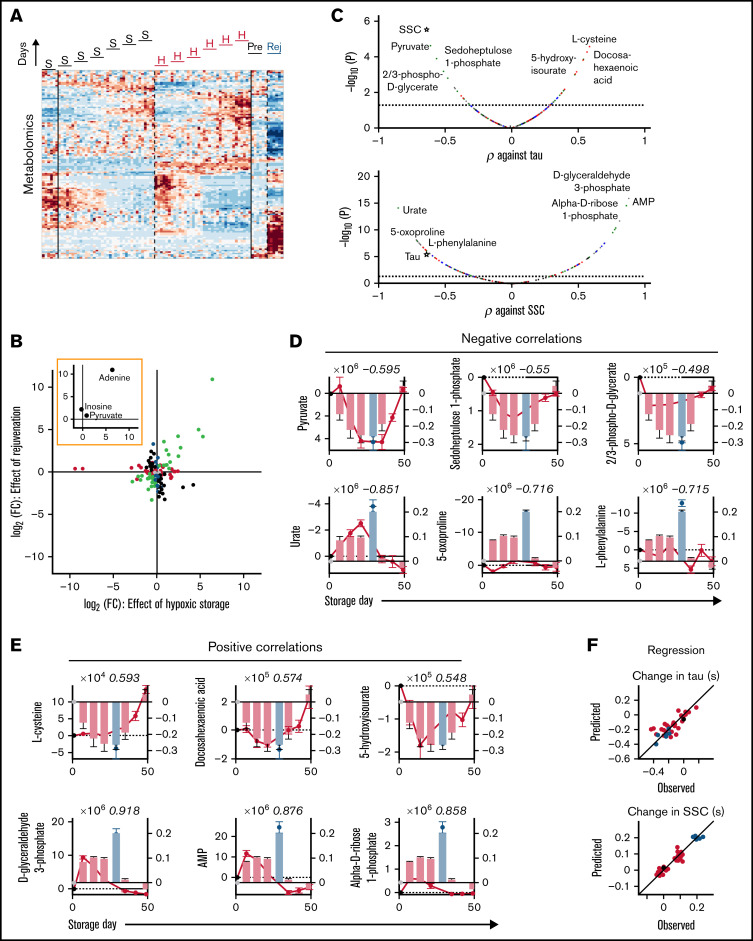
Relative to standard storage, hypoxic storage or biochemical rejuvenation affected a total of 126 metabolites (Figure 6A; supplemental Table 2). Of these, 35 were significantly affected by hypoxic storage but not rejuvenation; notable examples in this set included 3-phosphonopyruvate and phosphoenolpyruvate. Eighteen metabolites were affected by rejuvenation but not hypoxia, and 34 responded in an opposite manner to hypoxic and rejuvenation treatments. Thirty-nine metabolites responded concordantly under hypoxic storage and with rejuvenation. Among the 3 key ingredients of rejuvenation solution, adenine and pyruvate were substantially increased under hypoxia and following rejuvenation, whereas inosine was not significantly affected. Enrichment analysis of the concordantly responding set identified the Warburg effect as the most significant pathway affected (false discovery rate = 3 × 10-5). Substances with the greatest fold-change included adenine, sedoheptulose 1-phosphate, 1,3-bisphosphoglycerate, 2/3-phosphoglycerate, d-fructose 1,6-bisphosphate, and 2-oxoglutarate (Figure 6B). Many of these substances correlated significantly with effects of storage on gas handling (Figure 6C). The most significantly correlating metabolites were pyruvate, sedoheptulose 1-phosphate, 2/3-phosphoglycerate (negatively; Figure 6D), and l-cysteine, docosahexaenoic acid, and 5-hydroxyisourate (positively; Figure 6E). The best metabolite correlates with the effect of storage on SSC were noted for urate, 5-oxoproline, l-phenylalanine (negative; Figure 6D), d-glyceraldehyde 3-phosphate, AMP, and α-d-ribose 1-phosphate (positive; Figure 6E). Multivariate regression using the top 3 negative and top 3 positive metabolic correlates produced good predictions for the O2-unloading time constant and SSC (Figure 6F). Overall, the single best correlate to kinetic properties was pyruvate, yet this was not as strong as the correlation between τ and SSC. In turn, SSC could be very accurately described ratiometrically from the ratio of d-glyceraldehyde 3-phosphate to urate, which was superior to τ alone.
Figure 6.
Biochemical correlates of changes in RBC oxygen-handling function. (A) Metabolomics of RBC lysates. Heatmap shows 130 metabolites significantly affected by hypoxic storage or rejuvenation (2-way ANOVA: time and treatment with P < .05). (B) Scatter plot shows effect of hypoxic storage or rejuvenation for the differentially abundant metabolites. Green symbols show concordantly responding metabolites. Black symbols show metabolites that respond oppositely to hypoxia and rejuvenation. Red symbols show metabolites affected by hypoxic storage only. Blue symbols show metabolites affected by rejuvenation only. Paired t-test, P < .05. Inset highlights the 3 chemical components of rejuvenation solution. (C) Pearson’s correlation coefficient between specific metabolite and time constant (τ; top) or SSC (bottom). Color coding refers to scheme described in panel E. (D) Top 3 negative correlations to time constant (top) or SSC (bottom). Bars show time constant or SSC data from Figure 2; circles show metabolite levels. Number in italics refers to correlation coefficient. (E) Analysis for the top 3 positive correlations. (F) Multivariate regression analysis using the top 3 negative and top 3 positive correlates. For time constant τ: R2 = 0.6482, F = 10.75, P < .0001. For SSC: R2 = 0.9128, F = 71.53, P < .0001.
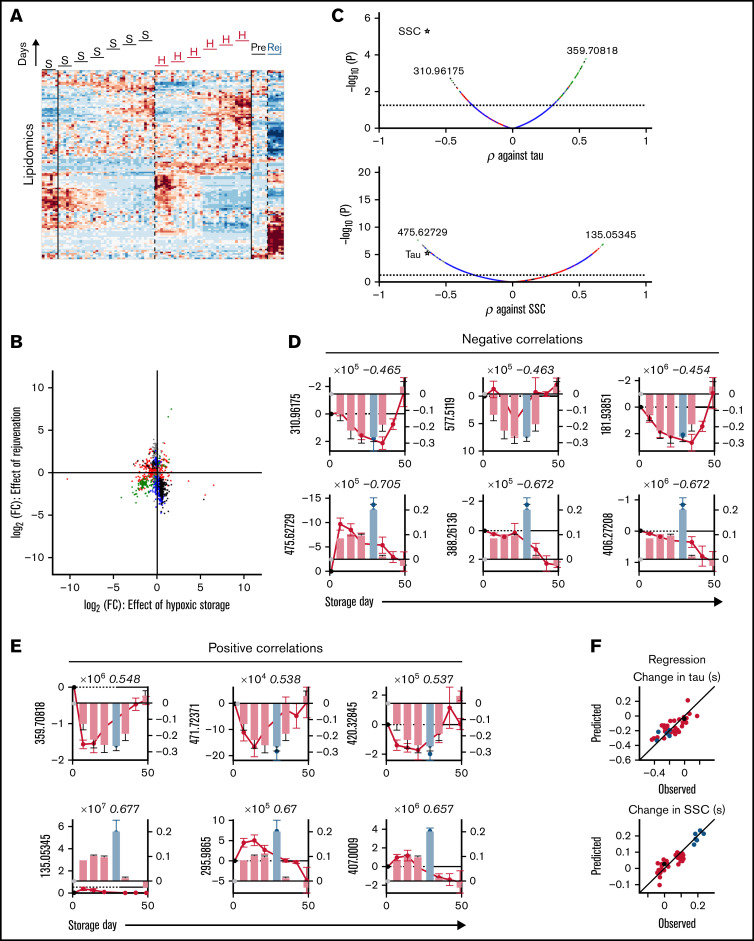
Hypoxic storage or biochemical rejuvenation affected more than 1200 lipids, relative to standard storage (Figure 7A). Many of the lipidomic responses could be divided into those that decreased during hypoxic storage only (n = 236; for example, m/z = 757.55597 [decrease], 258.1833 and 406.27198 [increase]) or changed selectively after rejuvenation (n = 276), indicating that these treatments produced distinct lipid signatures (Figure 7B; supplemental Table 3). In this group, multiple lipid peroxidation products (oxylipins) are noted (supplemental Table 2). As many as 237 lipids responded in opposite directions to hypoxic storage and rejuvenation and are thus unlikely correlates to physiological function. However, 110 lipids showed a coordinated response to hypoxic storage and rejuvenation (eg, α-amino-β,γ-dihydroxybutyrate [increase]). Most of these concordantly responding lipids were among substances that correlate positively with the change in τ (ie, decreased as O2 unloading accelerated under hypoxic storage) (Figure 7C). The top lipid correlates to τ and SSC are shown in Figure 7D (negative) and Figure 7E (positive). Using regression analysis, the top 3 negative and positive lipids produced good predictions for the effects of storage on SSC or τ (Figure 7F). Overall, however, metabolites yielded better correlations with SSC/τ compared with lipids.
Figure 7.
Lipidomic correlates of changes in RBC oxygen-handling function. (A) Lipidomics of RBC lysates. Heatmap shows ∼1200 lipids identified as responding to hypoxic storage or rejuvenation (2-way ANOVA: time and treatment with P < .05). (B) Scatter plot shows effect of hypoxic storage or rejuvenation for the differentially abundant lipids. Same color coding as in Figure 6B. (C) Pearson correlation coefficient between specific metabolite and time constant (top) or SSC (bottom). (D) Top 3 negative correlations to time constant (top) or SSC (bottom). Bars show time constant or SSC data from Figure 2; circles show lipid levels. Number in italics refers to correlation coefficient. (E) Analysis for the top 3 positive correlations. (F) Multivariate regression analysis using the top 3 negative and top 3 positive correlates. For time constant τ: R2 = 0.6415, F = 10.44, P < .0001. For SSC: R2 = 0.8133, F = 29.77, P < .0001.
Discussion
The primary physiological rationale for RBC transfusions is to improve the delivery of oxygen to respiring tissues. Consequently, the superiority of alternative storage regimes should be assessed based on functional measures of RBC quality, rather than on biochemical changes, which are merely indirect proxies of cellular physiology. Our previous work31 established that O2 release from intact RBCs is a diffusion-reaction phenomenon, in which both Hb-O2 binding kinetics and cytoplasmic diffusion distance influence cellular gas handling kinetics. Under standard storage, the functional attrition of RBCs continued even after 2,3-DPG depletion,33 which indicates that O2 unloading is not solely a function of HbO2 affinity, but also determined by other factors, such as diffusion distance that expands in spherically remodeled cells. Considering this complexity, it has not been possible to predict whether the sum of metabolic changes observed with an alternative storage regime has a net beneficial effect on O2 unloading, nor to identify which specific metabolic normalization has the greatest impact on gas handling. Such information would allow a more targeted approach to selecting the best storage regimes. Previous studies have correlated metabolomic data to posttransfusion recovery20,41,42 and hemolysis,3,43 but no study has undertaken a functional characterization of O2 release from RBCs stored under alternative storage regimes. Here, we applied single-cell oxygen saturation imaging to compare the physiological outcomes of storage under standard vs hypoxia, a newly available storage regime. With this approach, we established a beneficial effect of hypoxic storage on oxygen unloading from RBCs and identified the metabolites that correlate best with this functional improvement.
RBC units stored under hypoxic conditions preserved faster O2 unloading kinetics compared with the standard storage protocols implemented by a large national blood provider. The most profound effect of hypoxic storage was noted for the first 35 days of storage; that is, the longest permissible shelf-life of blood in the United Kingdom and many European jurisdictions. In clinical practice, the median storage duration of transfused units is 21 days, and at this point, the beneficial effect of hypoxic storage was consistent in all 6 blood pools studied. Storage under hypoxia produced similar effects on O2 unloading to that of biochemical rejuvenation following standard storage. Both interventions share many common metabolic responses, but for logistic and regulatory reasons, the former is more readily implementable for clinical use, as it can be conducted on units in bulk and before dispatch to hospitals.
Overall, 41 metabolites and 343 lipids correlated with the O2 unloading time constant, of which 18 metabolites and 30 lipids showed concordant responses to hypoxic storage and rejuvenation. Because gas handling is an ensemble diffusion-reaction process that involves multiple biochemicals and proteins, correlations with individual metabolites or lipids will be, at best, modest, but such analysis highlights the most critical biochemical processes for normalization during storage. The most prominent biochemical correlate against physiological function was for pyruvate, which highlights its importance as an ingredient of the rejuvenation cocktail. An emerging pattern from these observations is that pathways related to high-energy purine (eg, ATP) breakdown and deamination (eg, urate) correlated with the degradation in physiological function. This is relevant, considering the association of these pathways with poorer posttransfusion recoveries.44 Additionally, hypoxic storage prevented the accumulation of storage-induced lipid peroxidation products, a marker of poor posttransfusion recovery in rodent models of storage quality.42
Preservation of physiological function correlated strongly with SSC, a flow cytometric composite measure relating to the shape and contents of RBCs (ie, a gauge of their diffusive milieu). This observation strengthens the emerging case for using SSC as a proxy of gas handling in clinical laboratories. Interestingly, several metabolites correlated very strongly with SSC, which sheds new insights into the biochemical basis of changes underpinning this flow cytometric measure. Overall, 110 metabolites and 1843 lipids correlated with SSC, of which 30 and 93, respectively, showed a concordant response under hypoxia and rejuvenation. Changes in SSC could be very accurately described in terms of the ratio of urate to d-glyceraldehyde 3-phosphate. The effect of hypoxic storage on increasing SSC may relate to the preservation of more discoid cellular shape, which favors smaller diffusion distances31 and a reduced degree of vesiculation and subsequent altered deformability.6 This may benefit transfusion efficacy because prolonged storage under normoxic conditions is linked to a higher rate of intravascular hemolysis.45
Among routinely measured blood-banking parameters, pO2, pCO2 (and consequently [HCO3−]) were maintained at low levels by the hypoxic storage protocol. We observed higher mean levels of hemolysis in the hypoxic storage arm, compared with standard storage following the oxygen-reduction step that involves agitation. We saw more hemolysis than previously reported under hypoxic storage35 possibly from operator inexperience and a particularly high value in 1 unit. This outlier had a higher degree of hemolysis than the regulatory limit of 0.8% hemolysis at day 35, although our study was not designed to assess production compliance to that specification. Supernatant potassium levels were slightly raised in the hypoxic arm compared with standard, although within a range comparable to historical reference data for our laboratory.35,46
Interventions that accelerate O2 unloading are predicted to benefit respiring tissues because RBCs have only a few seconds to complete gas exchange during their capillary sojourn.47,48 Because of the diffusion-reaction nature of O2 unloading, tissue acidosis (which destabilizes HbO2) may not be sufficient to fully restore O2 release because low pH is not expected to improve gas diffusion across cytoplasm. Mathematical models31 based on O2 unloading rates measured in fresh blood predict that tissue oxygenation can become rate-limiting at higher perfusion rates or when the O2 unloading process is slowed (eg, in spherical RBCs). Given that standard storage can slow the O2 unloading time constant to 2 to 3 seconds, we speculate that inferior gas-handling kinetics may impact the efficacy of transfusions for patients requiring an immediate improvement to tissue oxygenation, such as those in critical care. In these instances, the ∼30% acceleration of O2 release attained under hypoxic storage may bring tangible benefits to tissue oxygenation. Indeed, recent studies have shown superiority of hypoxic storage in 2-arm cross-sectional studies on posttransfusion recovery in healthy autologous volunteers.41 It must be noted that numerous clinical trials have failed to detect an effect of storage duration on clinical outcomes,10-13 but these studies have typically recruited people with stable anemia for whom a prompt restoration of oxygen release is not critical,49 rather than patients requiring massive transfusion to restore tissue oxygenation (ie, cohorts for whom impaired gas exchange kinetics is most relevant). Moreover, these trials have typically correlated clinical outcomes with storage duration, which is not the most appropriate choice of independent variable because of its complex relationship with RBC quality. Indeed, the molecular age of a unit is affected by donor biology44 and genetics,50 processing strategies,51 and even exposures (eg, alcohol, caffeine, smoking, and drugs52). The progression of storage lesion is not a strict function of duration, thus relying on storage time for treatment allocation introduces noise and compromises the statistical power to resolve effects in randomized clinical trials.49
Based on our findings, we argue that the preservation of O2 unloading may enhance the physiological quality of stored blood units, particularly those intended for restoring gas exchange immediately on transfusion such as with major trauma or intensive care, or in patients with sickle cell disease who would benefit from transfusates with better O2 release to compensate for HbS RBCs. The impact of O2 release kinetics on transfusion outcomes is yet to be tested. Our results provide a framework for assessing the functional quality of stored RBCs for future clinical trials testing the transfusion efficacy of alternative storage regimes.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Supported by the John Fell Fund (to P.S.) and European Research Council Proof of Concept grant “BLOODMARK” funded through UKRI as grant EP/X021548/1 (to P.S.), First Team grant (POIR.04.04.00-00-3FEF/17-00) of the Foundation for Polish Science cofinanced by the EU under the Smart Growth Operational Programme (to P.M.K.), and from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL146442, R01HL149714, R01HL148151, R01HL161004, and R21HL150032 to A.D.). These studies were in part supported by funds from Hemanext Inc., including National Institutes of Health, National Heart, Lung, and Blood Institute R44HL149579 to Hemanext Inc and Omix Technologies Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Authorship
Contribution: J.R., S.B., A.M., D.Z., D.S., M.D., and D.N. performed the research and analyses; P.M.K. and S.B. designed devices; S.S.-C. provided resources and performed 2,3-DPG analyses for Figure 3; A.D. and P.S. analyzed the data; and R.C., S.S.-C., P.M.K., P.A.S., A.D., and P.S. supervised the study.
Conflict-of-interest disclosure: Resources for this study were provided by Hemanext Inc, and S.S.-C. is an employee of Hemanext Inc. Hemanext supplied the equipment, materials, and instructions for hypoxic storage to NHSBT and performed 2,3-DPG analysis on samples supplied to them, but the company and its staff had no role or influence on other data collection, nor its analysis and interpretation. A.D. is a founder of Omix Technologies Inc and Altis Biosciences LLC, a science advisory board member for Hemanext Inc. and FORMA Therapeutics Inc, and consultant for Rubius Therapeutics. R.C. is a science advisory board member for Terumo BCT. The remaining authors declare no competing financial interests.
Correspondence: Pawel Swietach, Department of Physiology, Anatomy & Genetics, University of Oxford, Parks Rd, Oxford OX1 3PT, United Kingdom; e-mail: pawel.swietach@dpag.ox.ac.uk.
References
- 1.Shih AW, Apelseth TO, Cardigan R, et al. ; QMiP Investigators on behalf of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative . Not all red cell concentrate units are equivalent: international survey of processing and in vitro quality data. Vox Sang. 2019;114(8):783-794. [DOI] [PubMed] [Google Scholar]
- 2.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56(6):1274-1286. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro A, Fu X, Kanias T, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2021;106(5):1290-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104(43): 17063-17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107(1):1-9. [DOI] [PubMed] [Google Scholar]
- 6.Roussel C, Dussiot M, Marin M, et al. Spherocytic shift of red blood cells during storage provides a quantitative whole cell-based marker of the storage lesion. Transfusion. 2017;57(4):1007-1018. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17(1): 27-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Meer PF, Cancelas JA, Cardigan R, et al. ; BEST Collaborative . Evaluation of overnight hold of whole blood at room temperature before component processing: effect of red blood cell (RBC) additive solutions on in vitro RBC measures. Transfusion. 2011;51(suppl 1):15S-24S. [DOI] [PubMed] [Google Scholar]
- 9.Wilsher C, Garwood M, Sutherland J, Turner C, Cardigan R. The effect of storing whole blood at 22 degrees C for up to 24 hours with and without rapid cooling on the quality of red cell concentrates and fresh-frozen plasma. Transfusion. 2008;48(11):2338-2347. [DOI] [PubMed] [Google Scholar]
- 10.Heddle NM, Cook RJ, Arnold DM, et al. Effect of Short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016; 375(20):1937-1945. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DJ, McQuilten ZK, Nichol A, et al. ; TRANSFUSE Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Age of red cells for transfusion and outcomes in critically ill adults. N Engl J Med. 2017;377(19):1858-1867. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix J, Hébert PC, Fergusson DA, et al. ; Canadian Critical Care Trials Group . Age of transfused blood in critically ill adults. N Engl J Med. 2015;372(15):1410-1418. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Stanworth SJ, Alexander JH, et al. Clinical trials evaluating red blood cell transfusion thresholds: an updated systematic review and with additional focus on patients with cardiovascular disease. Am Heart J. 2018;200:96-101. [DOI] [PubMed] [Google Scholar]
- 14.Bruun-Rasmussen P, Andersen PK, Banasik K, Brunak S, Johansson PI. Intervening on the storage time of RBC units and its effects on adverse recipient outcomes using real-world data. Blood. 2022;139(25):blood.2022015892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rael LT, Bar-Or R, Ambruso DR, et al. The effect of storage on the accumulation of oxidative biomarkers in donated packed red blood cells. J Trauma. 2009;66(1):76-81. [DOI] [PubMed] [Google Scholar]
- 16.Wither M, Dzieciatkowska M, Nemkov T, Strop P, D’Alessandro A, Hansen KC. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56(2):421-426. [DOI] [PubMed] [Google Scholar]
- 17.Bayer SB, Hampton MB, Winterbourn CC. Accumulation of oxidized peroxiredoxin 2 in red blood cells and its prevention. Transfusion. 2015;55(8):1909-1918. [DOI] [PubMed] [Google Scholar]
- 18.Rinalducci S, D’Amici GM, Blasi B, Vaglio S, Grazzini G, Zolla L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51(7):1439-1449. [DOI] [PubMed] [Google Scholar]
- 19.Issaian A, Hay A, Dzieciatkowska M, et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica. 2021;106(11):2971-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis RO, D’Alessandro A, Eisenberger A, et al. Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J Clin Invest. 2020;130(5):2270-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325-336. [DOI] [PubMed] [Google Scholar]
- 22.Dern RJ, Brewer GJ, Wiorkowski JJ. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J Lab Clin Med. 1967;69(6):968-978. [PubMed] [Google Scholar]
- 23.Valeri CR, Hirsch NM. Restoration in vivo of erythrocyte adenosine triphosphate, 2,3-diphosphoglycerate, potassium ion, and sodium ion concentrations following the transfusion of acid-citrate-dextrose-stored human red blood cells. J Lab Clin Med. 1969;73(5):722-733. [PubMed] [Google Scholar]
- 24.Högman CF, de Verdier CH, Ericson A, Hedlund K, Sandhagen B. Effects of oxygen on red cells during liquid storage at +4 degrees C. Vox Sang. 1986;51(1):27-34. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, AuBuchon JP, Tryzelaar L, Foster KY, Bitensky MW. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92(1):22-31. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8(4):220-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, Yoshida T. CO2-dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion. 2016;56(2):392-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancelas JA, Dumont LJ, Maes LA, et al. Additive solution-7 reduces the red blood cell cold storage lesion. Transfusion. 2015;55(3):491-498. [DOI] [PubMed] [Google Scholar]
- 29.de Bruin S, Peters AL, Wijnberge M, et al. Storage of red blood cells in alkaline PAGGGM improves metabolism but has no effect on posttransfusion recovery. Blood Adv. 2022;bloodadvances.2022006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AT, Jani VP, Nemkov T, et al. Transfusion of anaerobically or conventionally stored blood after hemorrhagic shock. Shock. 2020;53(3):352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson SL, Hulikova A, Proven M, et al. Single-cell O2 exchange imaging shows that cytoplasmic diffusion is a dominant barrier to efficient gas transport in red blood cells. Proc Natl Acad Sci USA. 2020;117(18):10067-10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22(2):90-96. [DOI] [PubMed] [Google Scholar]
- 33.Donovan K, Meli A, Cendali F, et al. Stored blood has compromised oxygen unloading kinetics that can be normalized with rejuvenation and predicted from corpuscular side-scatter. Haematologica. 2022;107(1):298-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Alessandro A, Gray AD, Szczepiorkowski ZM, Hansen K, Herschel LH, Dumont LJ. Red blood cell metabolic responses to refrigerated storage, rejuvenation, and frozen storage. Transfusion. 2017;57(4):1019-1030. [DOI] [PubMed] [Google Scholar]
- 35.Sowemimo-Coker SO, Fast LD. Effects of hypoxic storage on the efficacy of gamma irradiation in abrogating lymphocyte proliferation and on the quality of gamma-irradiated red blood cells in additive solution 3. Transfusion. 2021;61(12):3443-3454. [DOI] [PubMed] [Google Scholar]
- 36.Keitt AS. Reduced nicotinamide adenine dinucleotide-linked analysis of 2,3-diphosphoglyceric acid: spectrophotometric and fluorometric procedures. J Lab Clin Med. 1971;77(3):470-475. [PubMed] [Google Scholar]
- 37.Stefanoni D, Shin HKH, Baek JH, et al. Red blood cell metabolism in Rhesus macaques and humans: comparative biology of blood storage. Haematologica. 2020;105(8):2174-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion. 2018;58(12):2978-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128(12):e32-e42. [DOI] [PubMed] [Google Scholar]
- 41.D'Alessandro A, Yoshida T, Nestheide S, et al. Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion. 2020;60(4):786-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howie HL, Hay AM, de Wolski K, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019;3(15):2272-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van ’t Erve TJ, Wagner BA, Martin SM, et al. The heritability of hemolysis in stored human red blood cells. Transfusion. 2015;55(6):1178-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103(2):361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. 2017;127(1):375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meli A, McAndrew M, Frary A, et al. Familial pseudohyperkalemia induces significantly higher levels of extracellular potassium in early storage of red cell concentrates without affecting other standard measures of quality: a case control and allele frequency study. Transfusion. 2021;61(8):2439-2449. [DOI] [PubMed] [Google Scholar]
- 47.Wagner PD. Diffusion and chemical reaction in pulmonary gas exchange. Physiol Rev. 1977;57(2):257-312. [DOI] [PubMed] [Google Scholar]
- 48.Wexler J, Whittengerger JL, Himmelfarb S. An objective method for determining circulation time from pulmonary to systemic capillaries by the use of the oximeter. J Clin Invest. 1946;25(3):447-450. [PubMed] [Google Scholar]
- 49.Trivella M, Stanworth SJ, Brunskill S, Dutton P, Altman DG. Can we be certain that storage duration of transfused red blood cells does not affect patient outcomes? BMJ. 2019;365:l2320. [DOI] [PubMed] [Google Scholar]
- 50.Page GP, Kanias T, Guo YJ, et al. ; National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology Donor Evaluation Study–III (REDS-III) program . Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage. J Clin Invest. 2021;131(13):146077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Alessandro A, Culp-Hill R, Reisz JA, et al. ; Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) . Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2019;59(1):89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemkov T, Stefanoni D, Bordbar A, et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight. 2021; 6(3):e146715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.