TO THE EDITOR:
Splenic marginal-zone lymphoma (SMZL) accounts for <2% of lymphoid neoplasms.1 Treatment is indicated for progressive and symptomatic splenomegaly and for cytopenias.2,3 The options include splenectomy, chemotherapy, rituximab with or without chemotherapy, and more recently, targeted therapies.4-6 Results from small cohorts suggest that the anti-CD20 monoclonal antibody (mAb) rituximab can achieve deep and durable responses in many patients (overall response rate [ORR], >80%, complete response [CR], >40%) as a single-agent therapy.7-10 Nevertheless, the management of relapsed and refractory SMZL remains an unmet clinical need. Novel strategies include BTK, PI3K, and immune check-point inhibitors; antibody-drug conjugates; and chimeric antigen receptor T cells.3
Ofatumumab is a fully humanized anti-CD20 mAb targeting a different CD20 epitope on B cells with slower release from the target, compared with rituximab. Less frequent and milder infusion-related reactions (IRRs) have been reported.11,12
We designed a phase 2 open-label, multicenter, nonrandomized trial to assess the efficacy and safety of ofatumumab monotherapy in patients with relapsed or refractory SMZL, including those previously treated with rituximab. Key eligibility criteria (detailed in supplemental Material) included age ≥18 years, diagnosis of SMZL, relapse after or refractoriness to 1 or 2 lines of therapy, and progressive disease requiring treatment. This trial is registered on Eudra-CT as #2013-004916-23. Patients with several exposures to anti-CD20 mAb were excluded because of the expected limited benefit of anti-CD20 rechallenge and the lack of data on the efficacy of ofatumumab in this setting. Splenectomy and/or treatment for hepatitis C virus infection were not considered a line of treatment. The study was approved by IRCCS Ospedale San Raffaele, Milano, Italy, and satellite sites, and it was conducted according to the Declaration of Helsinki and the Good Clinical Practice guidelines from the International Conference on Harmonization. All patients provided written informed consent. Ofatumumab was administered intravenously as follows: first dose of 300 mg, followed by 7 weekly doses of 1000 mg. The primary end point was the CR rate evaluated by investigators according to the 2007 International Working Group for non-Hodgkin Lymphoma Criteria.13 Based on an optimal Simon’s 2-stage design, ≥8 CR in the first 15 patients would be needed to confirm that ofatumumab was worth investigating. The interim analysis showed 7 CR (only 1 less than required) and, given that the patients were benefiting from treatment without relevant toxicity, we proceeded to enroll 22 patients. Drug development in Europe was then halted by the manufacturing authorization holder, and accrual was interrupted before reaching the total expected number of subjects (n = 43).
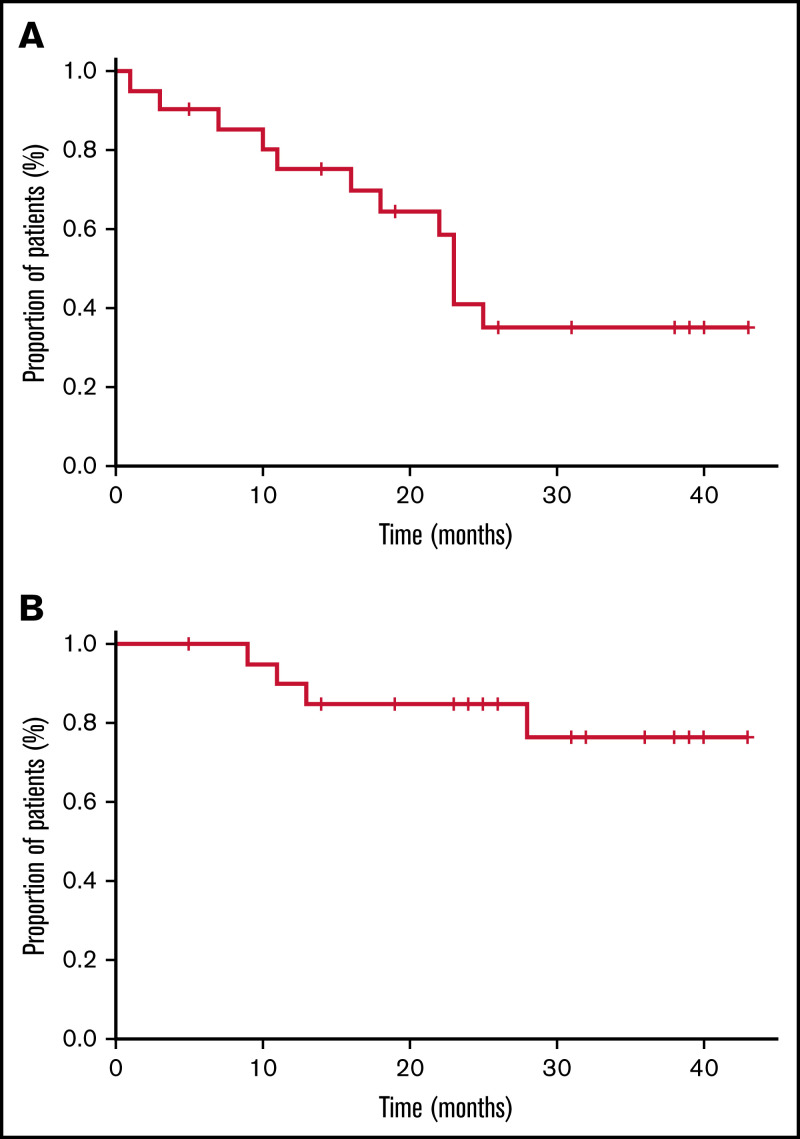
Twenty-two patients were enrolled from August 2014 through October 2018 at 5 sites in Italy. The database lock date was 31 May 2019. All patients received at least 1 dose of ofatumumab and were assessable for safety, whereas efficacy was assessed in patients who completed the full courses of ofatumumab or discontinued treatment earlier because of disease progression (n = 21 patients; 1 patient discontinued ofatumumab because of adverse events [AEs]). The median age of the 22 patients was 71 years (range, 48-91), and the median number of previous therapies was 1 (supplemental Table 1). Eighteen (82%) patients had been exposed to rituximab (best response to prior rituximab: CR in 8 cases, partial response [PR] in 8 cases); 3 of them refractory to rituximab, alone or combined with chemotherapy (1 patient with PR to rituximab in first-line therapy became rituximab refractory in second-line treatment). The median time to ofatumumab initiation after last prior therapy was 32 months (range, 3-57). The median time from last rituximab dose to first ofatumumab infusion (available for 16 rituximab-treated patients) was 34 months (range, 5-65). At study entry, 17 (77%) patients had at least 1 cytopenia, and 20 (91%) had stage IV disease. Two patients with stage II disease had treatment that was indicated for bulky abdominal lymph nodes (n = 1) and splenomegaly-related cytopenias (n = 1). All patients but 1 (95%) completed the planned course ofatumumab treatment. At a median follow-up of 26 months (range, 5-43), 11 of the 21 assessable patients achieved a CR (52%; 95% confidence interval [CI], 32-72), and 7 achieved a PR, with an ORR of 86% (95% CI, 65-95). Disease in 2 patients progressed during treatment, and 1 had stable disease for 5 months. Nine patients experienced relapse, with a median progression-free survival (PFS) of 23 months (95% CI, 21-24; Figure 1A). Median duration of response (first response assessment 30 days after ofatumumab completion) in 18 responding patients was 21 months (95% CI, 18-24). Median overall survival (OS) was not reached (Figure 1B), with a 2-year OS rate of 77% (95% CI, 55-97). At last follow-up, 18 patients were alive, 9 of them without evidence of disease. Four patients died, all from lymphoma of progression. Among the 18 patients who had been exposed to rituximab, ORR was 89% (16 of 18; 95% CI, 67-97), with 9 CR and 7 PR, including 3 rituximab-refractory patients who obtained 2 CR and 1 PR as best response to ofatumumab. Treatment with ofatumumab was very well tolerated, with a few mild AEs in the 22 patients. No dose delays and/or reductions were recorded; only 1 patient discontinued ofatumumab because of IRR. Twenty (91%) patients experienced at least 1 AE, for a total of 90 AEs (supplemental Table 2). No grade 5 AEs occurred; 19 grade 3 to 4 hematologic AEs occurred (8 neutropenia, 4 thrombocytopenia, and 2 anemia). Nonhematologic toxicity was limited to grade 3 events (1 each: chest pain, increased GGT, hypersensitivity reaction, and hypotension). Seven IRRs were recorded in 4 patients; only 1 was grade 3. No difference in white blood cell count at baseline was detected in the 4 patients experiencing IRR, compared with the others. Five serious AEs occurred in 3 patients (Table 1).
Figure 1.
Survival of patients who completed the planned course of ofatumumab treatment. The survival curves depict PFS (A) and OS (B) of the whole cohort.
Table 1.
Summary of adverse events (AEs) occurring in ≥2 patients and serious AEs in ≤2 patients
| Event | Grade 1-2 | Grade 3 | Grade 4 |
|---|---|---|---|
| AEs | |||
| Neutropenia | 0 | 6 | 2 |
| Infusion-related reaction | 6 | 1 | 0 |
| Thrombocytopenia | 1 | 2 | 2 |
| Asthenia | 4 | 0 | 0 |
| Fatigue | 4 | 0 | 0 |
| Nausea | 4 | 0 | 0 |
| Anemia | 1 | 2 | 0 |
| Dyspnea | 3 | 0 | 0 |
| Chills | 2 | 0 | 0 |
| Fever | 2 | 0 | 0 |
| Flushing | 2 | 0 | 0 |
| Hematoma | 2 | 0 | 0 |
| Itchy throat | 2 | 0 | 0 |
| Skin rash | 2 | 0 | 0 |
| Serious AEs | |||
| Hypersensitivity reaction | 1 | 1 | 0 |
| Chest pain | 0 | 1 | 0 |
| Dyspnea | 1 | 0 | 0 |
| Pleural effusion | 1 | 0 | 0 |
This is the first prospective study to address the efficacy and safety of ofatumumab monotherapy in patients with relapsed or refractory SMZL. Data on the best treatment strategies in patients with SMZL are scanty and are usually drawn from trials with different indolent lymphomas. Just considering MZLs, the 3 entities within this category (extra nodal MZL, nodal MZL, and SMZL) showed different disease biology and response to treatment, thus preventing firm conclusions on the best management of each of them. Our study has the advantage of enrolling a homogeneous population of patients with SMZL. Previous studies documented that treatment with the anti-CD20 mAb obtained long-term disease control with favorable tolerability and raised the question of the need for chemotherapy. Our study confirms the feasibility and benefit of anti-CD20 mAb alone, even in the relapsed/refractory setting after previous anti-CD20 mAb, achieving a notable 86% ORR with 52% CR. With the limitation of an indirect comparison, 1 series reported an ORR of 75% (9 of 12 patients) with 50% CR in a cohort of patients previously exposed to rituximab retreated with rituximab upon relapse.14 These findings suggest that anti-CD20 mAb with different target and eliciting different immune response mechanisms may control SMZL in a more effective way than rituximab. Of note, 3 of our patients who were refractory to rituximab, with or without chemotherapy, obtained a response to ofatumumab, thus supporting the advantage of rechallenging with different anti-CD20 mAbs in this subset. Our results also compare favorably with those of novel targeted agents, including BTK inhibitors. In 13 patients with relapsed or refractory SMZL treated with ibrutinib, ORR was 62%, with only 1 (8%) CR and a median PFS of 18.5 months.15,16 Similar results were obtained with the PI3Kδ inhibitor idelalisib (ORR, 47% in 15 patients with MZL)17 and the PI3Kαδ inhibitor copanlisib (ORR, 70% in 23 patients with MZL).18 In both cohorts, information on the number of patients with SMZL was not reported. Umbralisib (a dual PI3Kδ/CK1ε inhibitor) had an ORR of only 45% in the SMZL subgroup (n = 11) and a 2-year PFS of 50% in the whole cohort, with 15% of patients discontinuing treatment because of AEs.5 A better outcome was reported with zanubrutinib (MAGNOLIA trial), with a median 15-month PFS of 82.5%, although only 12 patients with SMZL were included, and the median follow-up was 15.7 months.6 Thus, the data should be reassessed with longer follow-up, taking into account the disadvantages of a continuous therapy (such as zanubrutinib, until progression or unacceptable toxicity) vs short treatment courses. In our study ofatumumab treatment was completed in 2 months. We acknowledge that the study has limitations, including the relatively short follow-up and the early stop in the accrual because of drug unavailability. Even if the use of ofatumumab in B-cell malignancies has been halted, these results further provide the rationale and reinforce the concept of future studies with newer anti-CD20 mAb (eg, obinutuzumab and ublituximab) pursuing an effective chemo-free approach in relapsed and refractory SMZL, even after rituximab.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Acknowledgments: The authors thank Clioss for its valuable support in conducting the study, collecting the data, and performing pharmacovigilance.
The MORE trial (COMB157DIT04T) received funding and drug, first from GSK and then from Novartis, and a research grant from Bando Reddito Operativo Lordo/Relazioni Internazionali (ROL/REL).
Contribution: P.G. and A.J.M.F. designed the study; P.G., L.S., S.F., A.M.F., M.T., A.B., E.S., M.C., P. Ranghetti, P.A., P. Ronchi, M.P., and A.J.M.F. provided study materials or patients; E.S., L.S., S.F., A.M.F., M.T., A.B., E.S., M.C., P. Ranghetti, P.A., and P. Ronchi collected and assembled the data; P.G., A.J.M.F., M.P., and L.S. analyzed and interpreted the data; L.S., P.G., A.J.M.F., and M.P. wrote the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: L.S. has received honoraria from AbbVie, AstraZeneca, and Janssen outside the submitted work. A.J.M.F. has received speaker fees from Adienne and Gilead. B.M.S. and research grants from Beigene, Pharmacyclics, Hutchison Medipharma, Amgen, Genmab, ADC Therapeutics, Roche, Gilead, Novartis, and Pfizer; has participated in Gilead, Novartis, Roche, Juno, and PletixaPharm advisory boards; and is an inventor on the following patents: NGR-hTNF/RCHOP for relapsed or refractory primary central nervous system lymphoma (PCNSL) and SNGR-hTNF for brain tumors. P.G. received honoraria from AbbVie, Arqule/MSD, AstraZeneca, Celgene/Juno/BMS, Janssen, Loxo/Lilly, and Roche; and research support from AbbVie, AstraZeneca, Janssen, Gilead, and Sunesis. The remaining authors declare no competing financial interests.
Correspondence: Paolo Ghia, Strategic Research Program on Chronic Lymphatic Leukemia (CLL), Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale San Raffaele and Università Vita Salute San Raffaele, Via Olgettina 58, 20132 Milan, Italy; e-mail: ghia.paolo@hsr.it.
References
- 1.Piris M, Isaacson P, Swerdlow SH, et al. Splenic marginal zone lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2017:223-225. [Google Scholar]
- 2.Zucca E, Arcaini L, Buske C, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(1):17-29. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni F, Rossi D, Raderer M, Zucca E. Marginal zone lymphomas. Cancer J. 2020;26(4):336-347. [DOI] [PubMed] [Google Scholar]
- 4.Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127(17):2072-2081. [DOI] [PubMed] [Google Scholar]
- 5.Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol. 2021;39(15):1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opat S, Tedeschi A, Linton K, et al. The MAGNOLIA trial: zanubrutinib, a next-generation bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res. 2021;27(23):6323-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalpadakis C, Pangalis GA, Dimopoulou MN, et al. Rituximab monotherapy is highly effective in splenic marginal zone lymphoma. Hematol Oncol. 2007;25(3):127-131. [DOI] [PubMed] [Google Scholar]
- 8.Bennett M, Sharma K, Yegena S, Gavish I, Dave HP, Schechter GP. Rituximab monotherapy for splenic marginal zone lymphoma. Haematologica. 2005;90(6):856-858. [PubMed] [Google Scholar]
- 9.Tsimberidou AM, Catovsky D, Schlette E, et al. Outcomes in patients with splenic marginal zone lymphoma and marginal zone lymphoma treated with rituximab with or without chemotherapy or chemotherapy alone. Cancer. 2006;107(1):125-135. [DOI] [PubMed] [Google Scholar]
- 10.Kalpadakis C, Pangalis GA, Sachanas S, et al. Rituximab monotherapy in splenic marginal zone lymphoma: prolonged responses and potential benefit from maintenance. Blood. 2018;132(6):666-670. [DOI] [PubMed] [Google Scholar]
- 11.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104(6):1793-1800. [DOI] [PubMed] [Google Scholar]
- 12.Hagenbeek A, Gadeberg O, Johnson P, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111(12):5486-5495. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 14.Kalpadakis C, Pangalis GA, Angelopoulou MK, Sachanas S, Vassilakopoulos TP. Should rituximab replace splenectomy in the management of splenic marginal zone lymphoma? Best Pract Res Clin Haematol. 2018;31(1):65-72. [DOI] [PubMed] [Google Scholar]
- 15.Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noy A, de Vos S, Coleman M, et al. Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: long-term follow-up and biomarker analysis. Blood Adv. 2020;4(22): 5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma [published correction appears in J Clin Oncol. 2018;36(5):521]. J Clin Oncol. 2017;35(35):3898-3905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.