Abstract

Introducing new materials with low cost and superior solar harvesting efficiency requires urgent attention to solve energy and environmental challenges. Titanium carbide (Ti3C2Tx) MXene, a 2D layered material, is a promising solution to solve the issues of existing materials due to their promising conductivity with low cost to function as a cocatalyst/support. On the other hand, metal–organic frameworks (MOFs) are emerging materials due to their high surface area and semiconducting characteristics. Therefore, coupling them would be promising to form composites with higher solar harvesting efficiency. Thus, the main objective of this work to disclose recent development in Ti3C2Tx-based MOF nanocomposites for energy conversion applications to produce renewable fuels. MOFs can generate photoinduced electron/hole pairs, followed by transfer of electrons to MXenes through Schottky junctions for photoredox reactions. Currently, the principles, fundamentals, and mechanism of photocatalytic systems with construction of Schottky junctions are critically discussed. Then the basics of MOFs are discussed thoroughly in terms of their physical properties, morphologies, optical properties, and derivatives. The synthesis of Ti3C2Tx MXenes and their composites with the formation of surface functionals is systematically illustrated. Next, critical discussions are conducted on design considerations and strategies to engineer the morphology of Ti3C2Tx MXenes and MOFs. The interfacial/heterojunction modification strategies of Ti3C2Tx MXenes and MOFs are then deeply discussed to understand the roles of both materials. Following that, the applications of MXene-mediated MOF nanotextures in view of CO2 reduction and water splitting for solar fuel production are critically analyzed. Finally, the challenges and a perspective toward the future research of MXene-based MOF composites are disclosed.
1. Introduction
In the modern age, the major issues faced by mankind is the energy crisis and the rapid depletion of fossil fuels. There is a growing urgency to look for alternative fuels and energy sources to partially fulfill the increasing energy demand. Thus, researchers are devoted to discovering sustainable technologies to overcome the energy crisis.1,2 For instance, renewable energy production such as steam reforming for H2 energy production3 and CO2 hydrogenation reactions4 to produce fuels such as hydrocarbons or alcohols are mature technologies that have already reached commercial scale operations. However, these approaches are highly energy-intensive as they require high thermal energy input to initiate the reactions. In turn, photocatalytic H2 production and CO2 reduction are emerging as promising advances in generating fuels and energy as they rely solely on naturally available solar energy to drive the reactions.5−7 Not only that, abundantly available CO2 and H2O function as low-cost feed stocks for the photocatalytic reactions. Hence, solar-driven CO2 reduction and water splitting is an up-and-coming approach to generate renewable fuels due to their inexpensiveness and environmentally benign process.8−10
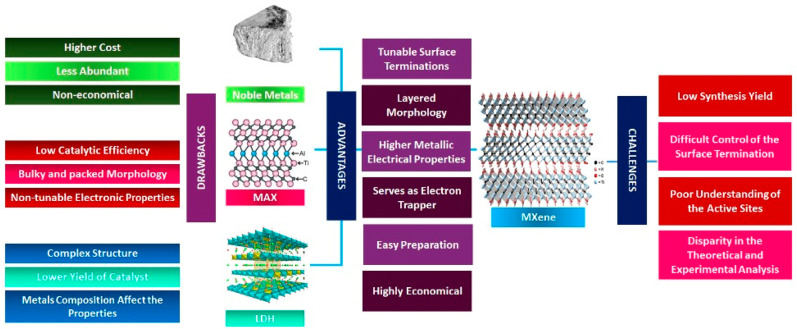
In 2011, MXenes were a class of novel materials first discovered by Gogotsi and co-workers.11 MXenes are promising photocatalytic materials that are derived from MAX via acid etching to remove the Al layers, leaving behind the M (transition metals) and X (C or N) with a general formula of Mn+1Xn. MXenes exist in either 0D quantum dots (QDs) or as 2D layered structures, which provides a higher specific surface area, reaction sites, ameliorated light harvesting, and electron acceptance capability as well as the prolonged lifetime of charge carriers.12,13 Additionally, they have good mechanical and chemical stability as well as easily tunable valence electrons via preparation using lighter and heavier transition metals.14 Due to its metallic properties, MXenes are able to act as a cocatalyst for photocatalytic reactions due to their ability to form a Schottky junction, which acts as an electron trap and mediator.15 By pairing the MXenes with other photoresponsive materials, a built-in internal electric field is induced, thus electron separation and migration are promoted.16 Also, several studies reported that some MXenes exhibit semiconducting properties with band gaps from 0.05 to 2.87 eV.17 Thus, MXenes are promising cocatalysts and can be coupled with semiconductors to boost reactivity.
Among the semiconductors, metal–organic frameworks (MOF) are a promising class of materials that have different morphologies and dimensionalities, ranging from 1D rods to 2D layered sheets and 3D network structures.18 Yaghi’s group pioneered the discovery and successful preparation of MOFs in 1999.19 The existence of highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) separated by an energy band gap (Ebg) makes them suitable candidates as photocatalysts due to their ability to harvest solar energy. Not only that, MOFs also have various beneficial properties such as extremely large specific surface area,20 high porosity,21 and a tunable structure. The light harvesting capability of MOFs is augmented due to the large surface area, which increases the exposed surface area of the material to light irradiation. Moreover, the desirable porosity makes it an excellent CO2 adsorbent that eases the subsequent conversion to solar fuels.22
Researchers all around the world are devoted to discovering highly active catalysts for solar-energy-driven renewable energy evolution. Pristine Ti3C2Tx MXene alone cannot be utilized as a photocatalyst for either solar-driven H2 production or CO2 reduction due to its inability to generate electron/hole pairs for photoredox reactions. Conversely, they are effective as cocatalysts due to their blackbody, which facilitates light absorption and harvesting.23 Not only that, Ti3C2Tx MXenes are able to function as electron reservoirs, in which they can efficiently trap photogenerated electrons.24 Hence, Ti3C2Tx MXenes must be coupled with other photoresponsive materials to unleash their full potential as a cocatalyst. In the past, Ti3C2Tx-based composite photocatalysts have been prepared, in which Ti3C2-based semiconductor composites were widely employed. For instance, Li et al. paired Ti3C2 QDs with a g-C3N4 semiconductor as a photocatalyst for H2 production, where the MXene effectively trapped the photogenerated electrons from the g-C3N4 nanosheets.25 Similarly, a ternary semiconductor-based TiO2/C3N4/Ti3C2 composite was utilized for solar-driven CO2 reduction. An S-scheme heterojunction was first obtained between the TiO2/C3N4 interface due to formation of an internal electric field, band bending, and Coulomb force, where the electrons in TiO2 combined with the holes in the valence band (VB) of C3N4. Subsequently, the electrons produced in C3N4 were trapped by Ti3C2Tx MXenes.26 However, the composite pairing with semiconductors presents a glaring disadvantage, which is the fast recombination of charge carriers due to large energy band gaps. In efforts to overcome this shortcoming, Ti3C2Tx-based MOF nanotextures are gaining attention as photocatalysts for solar fuel production. To date, 90,000 different MOF structures have been discovered, each with their own unique properties and energy band gap. This means that the potential combinations of Ti3C2Tx/MOF composites are limitless, in turn presenting a huge potential and research interest in maximizing solar fuel generation.
Herein, we reviewed the various applications of Ti3C2Tx MXene-based MOF composites on the photocatalytic production of solar fuels. First, an in-depth discussion on the principles and mechanism of photocatalysis is conducted. Then the fundamentals of photocatalytic CO2 reduction and water splitting are discussed by understanding the various possible oxidation and reduction reactions involved. The various preparation method of Ti3C2Tx MXenes are thoroughly reviewed, which includes HF etching, acid-containing fluoride ions, water-free etching, alkali etching, electrochemical etching, and molten salt substitution. Then a comprehensive discussion on the preparation of Ti3C2Tx MXene-based MOF composites is conducted. Numerous applications of Ti3C2Tx MXene-based MOF composites are reviewed and discussed for the sustainable production of solar fuels. Finally, the challenges and comparative analysis of Ti3C2Tx MXenes are conducted over various materials. Despite limited studies conducted on the Ti3C2Tx–MOF composites, they are definitely promising photocatalysts for solar fuel production and should be further explored for various applications.
2. Fundamentals and Principles
2.1. Principles of Photocatalysis
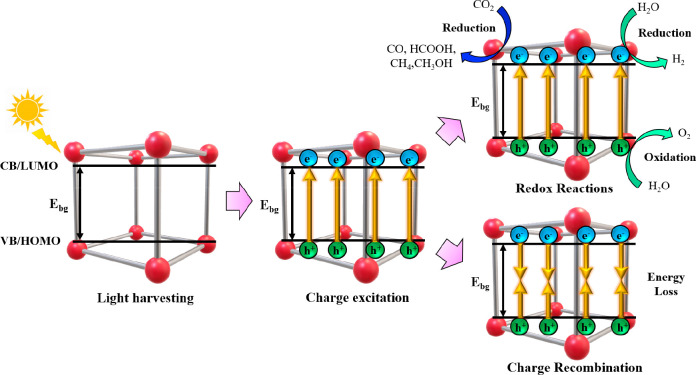
Photocatalysis is an up-and-coming sustainable approach to induce catalysis as it only utilizes naturally available solar energy as energy input to the reaction system. The photocatalysis pathway consists of four crucial steps, as shown in Figure 1. They are light harvesting, charge excitation, occurrence of redox reaction, and the recombination of charge carriers.27 Initially, the photoresponsive materials are exposed to light irradiation. There are three main radiations from solar energy, each with varying energy levels, with UV radiations having the highest energy, followed by visible light and infrared radiation. Hence, UV radiations show the highest efficiency in generating electron/hole pairs, whereas the infrared radiations only function to provide a heating effect.28 The light harvesting step can be further enhanced through multiple reflections and a light scattering effect over materials with rough surfaces as a result of meso- and macropores.29 Upon successful light harvesting, electrons are excited with sufficient energy to transverse the Ebg from the VB or HOMO to the conduction band (CB) or LUMO. On the other hand, the photogenerated holes are then left behind in the VB or HOMO.30 Following the charge excitation step where electron/hole pairs are formed, there are two possibilities that may occur, which is the favorable utilization of electron/hole pairs for redox reactions or the unfavorable recombination of charge carriers leading to loss of energy. The CO2 reduction reaction occurs on the CB/LUMO of the material, where the electrons are utilized to convert CO2 into solar fuels such as CO, HCOOH, CH4, and CH3OH, whereas the oxidation half-reaction occurs over the VB/HOMO.31 Similarly, photocatalytic water splitting reactions consist of two half-reactions, which is the reduction and oxidation of water to produce H2 and O2 gas, respectively. There are two types of recombinations that occur, namely, volume recombination where the charge carriers undergo recombination at the bulk of the material or surface recombination where the electron/hole pairs recombine on the surface of the material.32 The recombination of charge carriers will then lead to the loss of energy in the form of heat. Hence, the recombination should be prevented at all costs via different approaches, such as the formation of heterojunctions and surface sensitization, which can trap electrons and provide spatial separation, which prolongs the lifetime of the photogenerated electron/hole pairs.
Figure 1.
Schematic illustrating the overall mechanism for photocatalysis of CO2 reduction and hydrogen production. Adapted with permission from ref (30). Copyright 2022 Elsevier.
2.2. Fundamentals of Photocatalytic Solar Fuel Production
Recently, photocatalytic solar fuel production has been gaining traction due to its simplicity, cost-effectiveness, and environmentally benign process. There are two widely known and sustainable technologies to produce renewable fuels, namely, photocatalytic CO2 reduction and water splitting to produce H2. For photocatalytic reactions to occur, there are two main processes occurring, which is the reduction and oxidation half-reactions. For a particular reduction half-reaction to occur, the CB/LUMO of the material must be more negative compared to the redox potential. Conversely, the VB/HOMO of the photoresponsive material should be greater than that of the redox potential of the oxidation half-reaction for it to be feasible.33Equation 1 shows one electron reduction of CO2 to the CO2– radical.34 However, the reaction is deemed unfeasible due to it possessing a high redox potential. Not only that, it is impossible for a photocatalyst to supply enough potential to transfer a single electron to a CO2 molecule.35 Hence, reactions with low redox potentials are proton-assisted multielectron reactions, making them achievable over a wide range of materials.36 As shown in eqs 2–5, CO2 can be reduced to a wide variety of products, namely, CO, HCOOH, CH3OH, and CH4, respectively. The product selectivity depends on the amount of the number of electrons supplied to the CO2 molecule. For instance, photocatalytic CO2 reduction usually favors the production of CO and HCOOH due to it only requiring two electrons to initiate the reaction. On the other hand, the formation of CH3OH and CH4 requires more electrons and protons (6 and 8, respectively) to facilitate the reaction. For the photocatalytic water splitting to produce H2, it involves two crucial steps. The first step is shown in eq 6, where two holes are utilized to oxidize the water molecules to produce oxygen and two protons. Subsequently, the protons and electrons proceed via eq 7 to produce H2.37,38
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
2.3. Overview, Fundamentals, and Properties of MXenes
MXenes are a type of two-dimensional (2D) nanomaterial obtained through selective etching of MAX phases. The general formula of MXene is Mn+1XnTX, in which the M stands for the early transitional metal elements such as Sc, Ti, Hf, Mo, Ta, Nb, Cr, and so on. The acronym A represents the Al or Si layer, while X constitutes either C or N elements. The chemical exfoliation of the A element of MAX results in the termination of the M surface with abundant of functional groups, Tx such as −F, −OH, and −O. The first MXene material ever synthesized was in 2011 which is titanium carbide MXene (Ti3C2Tx) by Naguib and co-workers via chemical etching with hydrofluoric acid (HF).11 It was known that the mechanical method was not able to exfoliate the Mn+1Xn layers due to the strong metallic M–A bonds in MAX phase. Therefore, the selective etching is considered a preferable method to break the M–A bonds owing to the different chemical activities of M–A and M–X bonds in MAX.39 The n in the formula of MXene represents the atomic layer in a unit cell. A different compositional formula of MXene with different atomic layers can be obtained such as M2XTx, M3X2Tx, and M4X3Tx, as presented in Figure 2a. The typical structure of MXenes could be categorized as mono-M MXenes and double-M MXenes based on their compositional configurations.40 In mono-M MXenes, the compositional arrangement is distinguished by the position of an early transitional metal covering (M) the C or N (X) elements. On the other hand, double-M MXenes are subcategorized into solid solution and ordered type. In solid solution of double-M MXenes, two early transitional metals, M1 and M2, are randomly distributed into the MXenes, while for ordered type, one of the M metals is positioned between the second transition M metal.
Figure 2.
(a) Different compositional formula of Ti3C2Tx MXene with different atomic layers. Reprinted with permission from ref (40). Copyright 2019 Wiley. (b) Different electronic properties of Ti3C2Tx MXene. Reprinted from ref (15). Copyright 2021 American Chemical Society.
Typically, Ti3C2Tx MXenes are metallic materials that exhibit excellent electrical conductivity of 6000–8000 S cm–1, comparable to noble metal and graphene materials. This makes available as significant cocatalysts to promote the photocatalysis process. Additionally, the electrical properties of Ti3C2Tx MXenes can also be tailored through different M metal compositions, morphological modulation, and tuning the termination properties.15 For instance, delaminating the multilayer Ti3C2Tx MXene layers into their individual layer is reported to exhibit higher electrical conductivity up to 15000 S cm–1.41 The findings also suggested that the thickness of the Ti3C2Tx MXenes flakes is significantly linked to the electrical conductivity by which the monolayer Ti3C2Tx MXenes exhibited higher electrical conductivity compared to that of the bilayers and trilayers.42
In the perspective of electronic conductivity, Ti3C2Tx MXenes are metallic in nature and thus unable to generate electrons and holes. Therefore, they are typically used as a cocatalyst to assist photocatalytic activity due to the Schottky barrier effects. A higher work function is one of the distinct properties of Ti3C2Tx MXenes that give them a significant role as a photocatalytic enhancer and favorable as a noble metal substitution. A work function as high as 6.25 eV could be achieved, which is as high as other renowned metal cocatalysts such as Ag (∼4.5 eV), Au (∼5.38 eV), and Pt (∼6.10 eV).43−45 In the photocatalysis process, incorporating metal with a higher work function could improve the carrier dynamics and induce a stronger redox reaction.
Nevertheless, the work function of Ti3C2Tx MXenes can be varied depending on the tailored surface termination groups. This is because their work function is mainly controlled by the induced dipole moments arising from the transfer of charges between the termination group of Ti3C2Tx MXenes and the changes in the total surface dipole moments due to surface relaxation. In this context, the findings suggested that Ti3C2Tx MXenes with an −OH terminal group were observed to exhibit a work function of 1.6–2.8 eV. On the other hand, −O-terminated Ti3C2Tx MXenes displayed a higher work function between 5.75 and 6.25 eV.46 Furthermore, the termination of −OH groups caused the decrease of the work function compared to that with −O and −F termination, where the work function was shown to either increase or decrease depending on the M metal constituted.47 However, theoretical analysis through first-principles calculations indicated that some MXenes can exhibit semiconducting properties depending on the type of M metal constitution, surface terminating group, and the number of the MXene layers, such as that presented in Figure 2b. It was suggested that Ti3C2Tx manifests metallic properties regardless of any surface functionalization. The same findings were observed on the MXenes with thicker sheets and a large number of transition metals. Therefore, the probability of MXenes to undergo the shifting of the electronic properties into semiconducting is high in thinner MXenes, such as those with n = 2. Therefore, not all MXenes are metallic in nature, and some can exhibit semiconducting properties by configuring their termination groups, morphological structure, and types of metal composition. The feasibility of tailoring the electronic and electrical properties is one of the distinct properties of MXenes that favors them in a wide range of scientific applications.
2.4. Fundamental and Principles of Metal–Organic Frameworks
MOFs are a class of crystalline materials with metal nodes held together by organic linkers, forming highly sophisticated porous structures.48 Initially, MOFs were mainly used in the field of gas storage, but slowly their role expanded into various applications such as gas/liquid adsorption and separation,49 electrode development for supercapacitors,50,51 and batteries52 as well as heterogeneous catalysis.53−55 MOFs are able to be used in broad applications mainly due to their unique properties and characteristics, such as an unprecedented specific surface area, high porosity, and easily tunable chemistry.56 MOFs exists in a variety of morphology and dimensionality, from 1D rod structures to 2D layers and finally 3D network structures. Additionally, MOFs can also act as sacrificial templates to produce a different material such as porous metal oxides and layered double hydroxides. Similar to semiconductor materials, MOFs are also bestowed with impressive optical properties, making them able to induce electron/hole pairs upon irradiation with a light source. Table 1 summarizes the various MOFs discovered over the years with their respective metal nodes, linkers, and physical and optical properties.
Table 1. Various Classification of MOFs and Their Properties.
| MOF | metal node | linkers | dimension | surface area (m2/g) | HOMO (eV) | LUMO (eV) | Ebg (eV) | refs |
|---|---|---|---|---|---|---|---|---|
| ZIF-67 | Co | 2-methylimidazole | 3D | 1139.00 | 1.18 | –0.74 | 1.92 | (95,96) |
| Co-MOF-74 | Co | 2,5-dihydroxyterephthalic acid (H4DOBDC) | 1D | 1025.00 | (97) | |||
| MOF-71 | Co | terephthalate acid (H2BDC) | 2D | 56.50 | (98) | |||
| MOF-5 | Zn | terephthalic acid (H2BDC) | 3D | 1101.00 | 3.82 | (87) | ||
| ZIF-7 | Zn | benzimidazole | 3D | 167.00 | (99) | |||
| ZIF-7-NH2 | Zn | 2-aminobenzimidazole | 3D | 417.00 | (99) | |||
| ZIF-8 | Zn | dimethylimidazole | 3D | 1123.00 | 2.10 | –1.20 | 3.30 | (20,100) |
| HKUST-1 | Cu | 1,3,5-tricarboxylic acid (H3BTC) | 3D | 1143.06 | 2.01 | –0.58 | 2.59 | (101) |
| Cu-MOF | Cu | 1,3,5-trimesic acid | 3D | 19.00 | 1.64 | –0.80 | 2.44 | (102) |
| Cu3(L)2(4,4′-bipy) | Cu | 4,4′-bipyridine (4,4′-bipy) | 3D | 83.86 | 2.82 | (103) | ||
| UiO-66 | Zr | terephthalic acid (H2BDC) | 3D | 835.00 | 3.19 | –0.72 | 3.91 | (86) |
| UiO-66-NH2 | Zr | 2-aminoterephthalic acid (H2BDC-NH2) | 3D | 917.00 | 1.82 | –1.01 | 2.83 | (86) |
| UiO-67 | Zr | biphenyl-4,4′-dicarboxylate (H2BPDC) | 3D | 2500.00 | 2.43 | –1.20 | 3.63 | (63) |
| MIL-101 | Fe | 1,4-benzenedicarboxylic acid (H2BDC) | 3D | 1413.00 | 2.59 | (54,104) | ||
| NH2-MIL-101 | Fe | 2-aminoterephthalic acid (NH2-BDC) | 3D | 1237.40 | 0.97 | –1.75 | 2.72 | (105) |
| PCN-250 | Fe | 3,3′5,5′-azobenzene tetracarboxylic acid (H4abtc) | 3D | 1240.00 | 1.19 | –0.89 | 2.08 | (88) |
| MOF-901 | Ti | 4-aminobenzoid acid | 2D | 550.0 | 2.65 | (106) | ||
| NH2-MIL-125 | Ti | 2-aminoterephthalic acid (H2BDC-NH2) | 3D | 405.40 | 2.10 | –0.40 | 2.50 | (53) |
| MIL-125 | Ti | terephthalic acid | 3D | 1510.00 | 3.43 | 0.16 | 3.27 | (66,107) |
| NTU-9 | Ti | 2,5-dihydroxyterephthalic acid (H4DOBDC) | 2D | 1205.00 | 0.32 | –1.58 | 1.90 | (108,109) |
| PCN-415 | Ti | terephthalic acid | 3D | 1050.00 | 3.31 | (110) |
2.4.1. Chemistry of Metal–Organic Frameworks
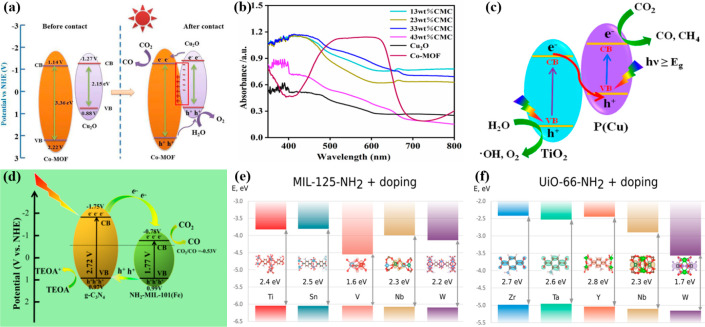
MOFs are composed of two main building blocks, including metal nodes and the bridging organic linkers. More than 60,000 types of MOFs can be discovered due to the endless possibilities of combination between different metal precursors with organic linkers. Figure 3 summarizes the general chemistry of MOFs. One of the more straightforward ways to classify and identify MOFs is through their metal nodes. For instance, Co, Zn, Cu, Zr, Fe, and Ti are some of the typical metal nodes present in monometallic MOFs. Co-based MOFs are highly utilized due to their relatively low cost and simple preparation methods.57 In the field of catalysis, Co metals were reported to show impressive activity in renewable fuel production.58 Zn-based MOFs such as ZIF-7 and ZIF-8 are referred to as noble-metal-free systems due to Zn being a cheaper and abundant alternative to noble metals, as well as showing promising performance under light irradiation.59 Cu is another transition metal that has shown beneficial properties in promoting photocatalytic performance. A study reported that the doping of Cu can enhance the reception of visible light by 35% as well as reduce the energy band gap significantly.60 Zr-based MOFs possess great structural stability with a high specific surface area due to Zr-carboxylate coordination bonds.61 UiO-6662 and UiO-6763 are two examples of well-known Zr-based MOFs that have been utilized in various photocatalytic reactions. In the case of Fe-MOFs, oxo-iron clusters are present which makes them potential candidates as photocatalysts as they exhibit good visible light harvesting ability.64 Finally, Ti-based MOFs consist of tetravalent Ti4+ cations which can form tough frameworks due to strong metal–ligand bonding. The presence of Ti-oxo clusters contributes to the photocatalytic activity by enhancing the redox reactions.65
Figure 3.
Illustration of the chemistry of MOFs in terms of metal node classification and organic linkers.
The second building blocks that are used to prepare MOFs are organic linkers. The linkers are the bridges between metal nodes that originate from organic acids such as terephthalic acid or trimesic acid. Usually, functionality can be introduced to MOFs via the use of functionalized linkers or via postsynthetic modifications. Most notably, the Ti-based MIL-125 and NH2-MIL-125 have a relatively similar synthesis method with similar metal precursors and solvents used, in which only the organic acids deployed are different. In the case of preparing MIL-125, terephthalic acid is utilized, whereas amine-functionalized terephthalic acid (2-amino terephthalic acid) is used to prepare NH2-MIL-125.66 Hence, NH2-MIL-125 will have amine functional groups on the linkers of the MOF. Another way to induce functionalization is through the postsynthetic modification (PSM) of the linkers.67 Su’s group reported an improvement in water resistance of Mg-MOF-74 via PSM by functionalizing it with tetraethylenepentamine (TEPA). Hence, amine functional groups were introduced to Mg-MOF-74, which inhibits the damaging effects of water entering into the framework.68
2.4.2. Physical Property of Metal–Organic Frameworks
MOFs are materials that have been widely researched in various fields and applications due to their desirable and unique physical properties. Here, the physical properties of MOFs such as their surface area, porosity, and various synthesis methods are critically discussed, which is then summarized, as shown in Figure 4. First, MOFs tend to boast incredibly high specific surface area, usually in the range of hundreds if not thousands. Titanium-based MIL-125 has a surface area of 1510 m2/g,66 whereas its metal oxide counterpart TiO2 only has a mere surface area of 35 m2/g.69 Moreover, MOFs are also bestowed with numerous pores with large openings. These large pores are beneficial for enabling them to function as effective materials for gas adsorption and capture. A chromium-based MIL-101 features a high pore volume of 1.63 cm3/g, in turn promoting a huge N2 uptake capacity of 1003 cm3/g.70 Interestingly, the porosity of MOFs not only is limited to gas adsorption but also enables them to capture large heavy metal molecules, as well. Nimbalkar et al. noticed that UiO-66 with a free carboxylic group is riddled with micropores of 0.4–0.7 nm, in turn providing a high lead and methylene blue adsorption capacity of 100 and 169 mg/g, respectively.71 Hence, a large surface area and pore volume is a highly sought-after property as it opens doors to various applications such as catalysis and adsorption.
Figure 4.
Illustration of the physical properties and preparation method of MOFs.
Even though most of the MOFs exist as three-dimensional (3D) polyhedrons, there are also some MOFs exhibiting 1D rods and 2D sheet structures. Recently, a novel 1D chiral imine Zr-MOF (DUT-136) was synthesized via the use of Schiff base of (R,R)-1,2-diphenylethylenediamine and 4-formylbenzoic acid linkers.72 Similarly, a number of 2D MOFs have been discovered by researchers, such as NUS-173 and MIL-169.74 In the past, various preparation methods for MOFs have been discovered. The most common approach to prepare MOFs is through the solvothermal method. Here, the metal precursors and linkers were first dissolved in solvent, such as methanol and N,N-dimethylformamide (DMF). Then the mixture was placed in a Teflon-lined stainless-steel autoclave where heat was introduced to the system to initiate the chemical reaction. Finally, the obtained slurry was washed and dried to obtain the MOF crystals.75 The solvothermal synthesis step usually utilizes a reaction temperature of not more than 200 °C, with a reaction time ranging from 12 to 72 h. A titanium-based NH2-MIL-125 was prepared via a conventional solvothermal method with reaction conditions of 150 °C and 72 h.76 Correspondingly, an Fe-MOF was prepared by first dissolving FeCl3·6H2O and H2BDC precursors in DMF solvent, followed by reaction for 24 h at 110 °C.77 Despite the easy process of the solvothermal synthesis method, it is limited by various factors such as a long synthesis time and inhomogeneous heating of reactants.78
MOFs can also be prepared via a straightforward co-precipitation method where the reactants are mixed until precipitates are formed without involving external energy sources.79 Co-precipitation provides a uniform distribution of components. ZIF-67 is a prime example of a MOF prepared via the co-precipitation method. The synthesis step is relatively straightforward: Co precursors were mixed with linkers (2-methylimidazole) in methanol and stirred for 6 h. Then the resulting solution was washed and dried to obtain 3D purple dodecahedral crystals.80 However, to date, there is only a limited number of MOFs prepared using this method. MOFs are also prepared using an energy-free approach, which is the slow evaporation method. In this method, the solvent is gradually evaporated at room conditions, leaving behind the dried MOF powders. Even though this process does not consume any energy, the synthesis requires a long period of time. Ghosh and co-workers reported a preparation period of 1 week for the synthesis of an Fe-MOF ([C18H22FeN2O6]NO3) via the slow evaporation method.81 In another study, 2 weeks were consumed to prepare purple Co-MOF crystals via the slow evaporation approach.82 Finally, microwave-assisted synthesis was also employed to achieve rapid synthesis of MOFs. This technique utilizes microwaves to provide a heating effect to initiate the chemical reaction, in turn producing nanosized crystals.83 The microwave-assisted approach is almost similar to the conventional solvothermal method but provides additional benefits such as fast crystallization time and facile morphology control and produces MOFs with narrow particle size distribution.64 Most notably, Reza et al. discovered that using a microwave-assisted method to prepare UiO-67 required a much shorter preparation time (2.5 h) compared to that with the conventional solvothermal method (24 h). This is because the microwave heating couples directly with the solvent molecules, causing a rapid rise in temperature at the reaction media, which induces localized superheating. This provides a much more efficient heat transfer compared to conventional heating, resulting in a much shorter reaction time.84
2.4.3. Optical Property of Metal–Organic Frameworks
Solar energy is a renewable source of energy that can be easily accessed. To fully utilize this abundant source of energy, researchers have been devoted to discovering materials that can effectively harvest and adsorb solar energy. Solar energy mainly comprises three main irradiations, namely, infrared, visible light, and ultraviolet (UV) radiations. Here, UV radiation has the highest energy content (shortest wavelength), followed by visible light and infrared radiation. Only high energy UV and visible light can generate electron/hole pairs over photoresponsive materials. However, despite the highest energy content of UV radiation, it only accounts for less than 4% of the solar spectrum, whereas visible light and infrared radiation constitutes 43 and 53% of solar energy, respectively.85 Hence, to effectively utilize solar energy for photocatalysis, suitable materials must be explored to efficiently harvest visible light radiations.
In this context, MOFs are highly sought-after materials due to their desirable optical properties, which makes them photoresponsive materials. To address the energy crisis, MOFs are employed as photocatalysts to harvest solar energy to generate renewable fuels. First, MOFs have a narrow energy band gap (Ebg) which allows electron to migrate over from the VB/HOMO to the CB/LUMO. The migration of charge carriers is only possible when photons with energy equal to or greater than Ebg strikes the surface of the MOF.64 Second, there has been a lot of MOFs discovered over the years, each with their own unique Ebg, as shown in Figure 5a. Some MOFs tend to have a wider band gap, such as UiO-6686 and MOF-587 with Ebg of 3.91 and 3.82 eV, respectively. However, there are also MOFs with narrow band gaps, such as PCN-25088 (2.08 eV) and NTU-9102 (1.90 eV). A narrow Ebg means that photons with lower energy are required to induce charge carrier formation. This means that visible light irradiation is sufficient to facilitate the photocatalytic reaction. Inversely, a wide Ebg requires more energy to generate electron/hole pairs, implying that UV irradiation will be more effective compared to visible light to drive photocatalysis. The relationship between the Ebg, the solar spectrum, and light wavelengths is illustrated in Figure 5b.
Figure 5.
(a) Band structures of various metal–organic frameworks. (b) Relationship between Ebg of photoresponsive materials (eV), solar spectrum, and light wavelength. Reprinted with permission from ref (64). Copyright 2021 Elsevier.
2.4.4. Metal–Organic Frameworks as Sacrificial Templates
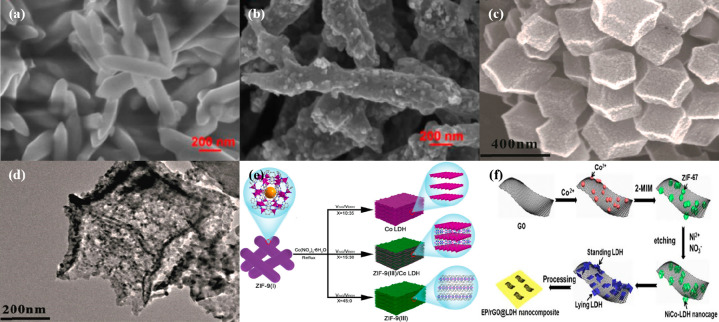
MOFs in their original form are proper candidates as photocatalysts but can also serve as sacrificial templates to produce other materials. One of the more common MOF derivatives is metal oxides, which are obtainable by simple thermal annealing of MOFs. By calcinating the MOF at elevated temperature, the organic linkers will be removed, leaving behind the metal nodes which are then oxidized, producing metal oxides which inherit the morphology, porosity, and surface area of the parent MOF.89 An Fe-MOF was synthesized via a solvothermal method with FeCl3·6H2O as the precursor and 1,4-benzenedicarboxylic acid linkers. The as-prepared MOF showed a well-defined nanospindle structure with a smooth surface, as illustrated in Figure 6a. Upon annealing the Fe-MOF in a N2 atmosphere, Fe2O3 was obtained while retaining the spindle structure of the parent MOF. From Figure 6b, it was noticed that the surface of the Fe-MOF-derived Fe2O3 was rougher with nanoparticles on the surface.90 Similarly, a Co-based MOF ZIF-67 was successfully converted into Co3O4 hollow polyhedrons via calcination treatment at 300 °C for 3 h. From the SEM image in Figure 6c, ZIF-67 particles have well-defined polyhedral shapes with a width of around 200 nm. After thermal treatment, the Co3O4 retained a similar polyhedral morphology with a specific surface area of 44.28 m2/g but had a hollow structure which is clearly observed in the TEM image in Figure 6d.91 Recently, there have been numerous reports on MOFs as templates to produce layered double hydroxides (LDHs). One study conducted by Chen et al. successfully converted 3D ZIF-9 into 2D Co LDH by varying the water/ethanol solvent volume ratio. According to the scheme in Figure 6e, when a 45:0 water/ethanol ratio was used, pure ZIF-9 with a 3D structure was obtained. However, as the ethanol amount increased from 15:30 to 10:35, ZIF-9 changed to ZIF-9/Co LDH and finally to pure 2D Co LDH.92 A bimetallic Ni–Co LDH-grown reduced graphene oxide (rGO) was also prepared via the etching of ZIF-67 by H+ upon the hydrolysis of nickel nitrate. As depicted in Figure 6f, ZIF-67 was first grown on GO. After successfully growing the ZIF-67 particles, the addition of nickel nitrate caused the framework of ZIF-67 to collapse into LDH platelets, resulting in the platelets inheriting a 3D structure of the parent MOF, forming standing and lying LDH on the GO.93 Another study reported an almost similar procedure to prepare Ni–Co LDH nanosheets, in which Co-MOF nanosheets were gradually decomposed and transformed into LDH via a hydrolyzed etching step.94 Thus, MOF templating is a useful way to produce other materials such as metal oxides and LDHs as the derivatives tend to retain the beneficial properties of the parent MOF, in turn promoting an enhanced photocatalytic activity.
Figure 6.
SEM images of (a) Fe-MOF and (b) Fe2O3 derived from Fe-MOF. Reprinted from ref (90). Copyright 2020 American Chemical Society. (c) SEM image of ZIF-67 and (d) TEM image of Co3O4 hollow polyhedrons. Reprinted with permission from ref (91). Copyright 2018 Elsevier. (e) Scheme of solvent ratio influence on formation of ZIF-9, ZIF-9/Co LDH, and Co LDH. Reprinted with permission from ref (92). Copyright 2021 Elsevier. (f) Preparation scheme of Ni–Co LDH on reduced graphene oxide. Reprinted with permission from ref (93). Copyright 2017 Elsevier.
2.5. Fabrication of a MXene-Based Schottky Junction
The performance efficiency of single semiconductor materials such as MOFs in photocatalytic solar fuel conversion can be enhanced through engineering modifications such as hybridization with other semiconductor materials, forming heterojunction composites. Other modifications included engineering the morphology of the semiconductor into different dimensions, defect engineering, and creation of vacancies.27 However, one of the techniques that has been proven effective in creating higher-performance semiconductor materials is by the addition of metallic material. In this regard, the performance efficiency can be enhanced through an improvement in the carriers’ dynamics. This indicates that Ti3C2Tx MXene possesses a higher metallic electrical conductivity, which provides them with the ability to form a potential barrier at the metal–semiconductor interfaces, known as the Schottky junction.111 Ti3C2Tx MXenes are, in general, unable to generate electrons and holes as they are naturally a metallic material. Therefore, they are mostly employed as a cocatalyst and paired with other semiconductor materials such as MOFs to promote the photoactivity through the synergy of a metal–semiconductor junction. The role of adding Ti3C2Tx MXene material is to improve the separation efficiency and promote the transfer of electrons in single semiconductor materials.
Fundamentally, the charge transfer mechanism in Ti3C2Tx MXene-based composites was achieved through the difference in the work function between Ti3C2Tx MXene and the semiconductor materials.112 The built-in internal electric field at the metal–semiconductor junction called Schottky barrier was formed through the band alignment when both the semiconductor and Ti3C2Tx MXene are in contact with each other. The Ti3C2Tx MXenes normally serve as electron acceptors due to their large metal work function. Typically, the electrons will migrate from higher Fermi level to lower Fermi level materials, which is the case for MOF-Ti3C2Tx MXene composites, where the electrons will migrate from a higher Fermi level of the MOF to a lower Fermi level for Ti3C2Tx MXene.113 Note that the metal work function value (ϕ) is defined as the energy required to transfer an electron from the Fermi level to the vacuum level, where the vacuum level is the reference level at which the electrons possess an energy level outside of the metal with zero kinetic energy. It is worth mentioning that the larger the difference of the work function between the two hybrid materials, the stronger the Schottky barrier that offers excellent separation of the photocarriers. Therefore, metal with a higher work function value such as noble metals and Ti3C2Tx MXene are highly sought after to optimize the solar to fuel conversion.114Figure 7 illustrates the electron transfer mechanism in Schottky junction composite materials. It can be observed that the electrons migrated to the surfaces of the Ti3C2Tx MXene are trapped and unable to return back to the semiconductor MOF; therefore, they reserve more electrons to undergo the redox reaction. The creation of a Schottky junction between MOF-Ti3C2Tx MXene interfaces prevents the reverse injection of electrons, hastens the electrons transfer to the Ti3C2Tx MXene surfaces, and inhibits the recombination of photogenerated charges.115
Figure 7.
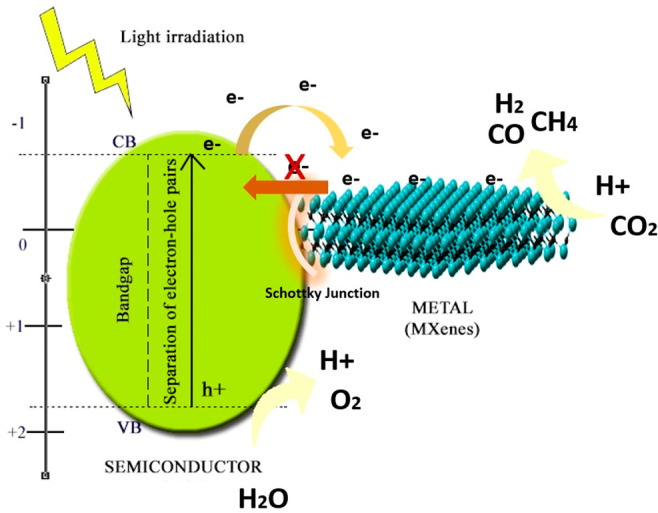
Construction of a Schottky junction and electron transfer mechanism.
3. Different Synthesis Techniques of MXene
In general, several different techniques are available to synthesize Ti3C2Tx MXene. One of the common and most applied techniques for synthesizing Ti3C2Tx MXene is HF etching, which utilizes hydrofluoric acid as a primary etchant. The utilization of an etchant is significant, especially in breaking the Ti–Al bond in Ti3AlC2 MAX, as the mechanical method cannot exfoliate the Al layer. Since the pioneering study of Ti3C2Tx MXene synthesis through HF etching, several other techniques have been developed to combat the utilization of concentrated HF acid, which is non-ecological, especially in wide-scale industrial production. Therefore, safer routes such as acid-containing fluoride etchant, alkali etching, electrochemical etching, halogen etching, water-free etching, and molten salt substitution techniques are among the alternatives to synthesize Ti3C2Tx MXene. Among them, the etching process through the utilization of acid-containing fluoride etchant, known as the in situ HF technique, has been broadly employed after the HF etching technique, unlike other newly developed techniques that have not been deeply researched.116Figure 8 summarizes the various preparation methods together with their reaction conditions for the synthesis of Ti3C2Tx MXenes, and Table 2 provides the reaction parameters for the different Ti3C2Tx synthesis methods.
Figure 8.
Different synthesis techniques of Ti3C2Tx MXenes. Reprinted from ref (117). Copyright 2022 American Chemical Society.
Table 2. Summary of Preparation Methods and Parameters of Ti3C2 MXenes.
| synthesis method | catalyst | etching agents | termination group | synthesis parameters | comments | refs |
|---|---|---|---|---|---|---|
| HF etching | TiO2/Ti3C2 | HF | O, OH, F | 20 mL of 48% HF, 15 h etching time | multilayer MXene produced with lower photocatalytic efficiency compared to monolayer counterpart | (138) |
| 2D/2D HCN/MXene | HF | 39% HF, 24 h etching time | high purity of MXene was produced with well-defined multilayer structure | (139) | ||
| 39% HF, 48 h etching time | the growth of anatase TiO2 on exfoliated multilayers MXene | |||||
| 39% HF, 96 h etching time | the growth of anatase and rutile TiO2 was observed on MXene layer; more exfoliated layer and increase in the growth of TiO2 NP with increasing etching time | |||||
| TiO2/Ti3C2 | HF | O, OH, F | 49% HF, 24 h etching time (35 °C); ethyl alcohol as a washing agent; hydrothermally treated at 450 °C | the formation of TiO2/Ti3C2 safflower-like morphology with ∼1 μm safflower size and shorter nanorods formation | (140) | |
| 49% HF, 24 h etching time (35 °C); ethyl alcohol as a washing agent; hydrothermally treated at 550 °C | average safflower size ∼2 μm upon heat increase heat treatment; well-formed safflower morphology | |||||
| 49% HF, 24 h etching time (35 °C); ethyl alcohol as a washing agent; hydrothermally treated at 650 °C | average safflower size ∼2 μm with lower spatial density of TiO2/Ti3C2 nanorods compared to at 550 °C heat treatment; the safflower morphology slightly deformed at higher temperature | |||||
| BQ/TiC/UCN | HF | 40% HF, 72 h etching time, 20 mL DMSO, 24 h intercalation time | ultrathin MXene sheet is produced, effectively function as the electron mediator | (141) | ||
| Ti3C2-QD/Ni-MOF | HF | 20 mL HF, 24 h etching time, DMF intercalating agent | MXene QD constructed with enhance charge transfer and higher separation capabilities of the photocarriers | (142) | ||
| 2D/2D g-C3N4/Ti3C2TA/R (CN/TCT) MXene | HF | 39% HF, 24 h etching time | less distribution of TiO2 NPs on the surface of MXene | (121) | ||
| 39% HF, 96 h etching time | higher amount of TiO2 growth/conversion with more of rutile phase compared to anatase | |||||
| 49% HF, 24 h etching time | higher distribution of TiO2 on the MXene surface when employing more concentrated etchants | |||||
| 49% HF, 96 h etching time | both TiO2 (rutile) and TiO2 (anatase) having higher concentration due to more oxidation | |||||
| acid-containing fluoride ions | TiO2/Ti3C2 | LiF + HCl | O, OH, F | LiF with 6 M HCl, 24 h etching time | MXene flakes exhibit excellent hydrophilicity and dispersity with flakes size range of 10–100 nm | (138) |
| ZnCdS/TiO2/Na-MXene | LiF + HCl | LiF with 9 M of HCl, 24 h etching time (35 °C) | the preintercalation of Na+ and attachment of ZnCdS nanoparticles on the MXene flakes improve the oxidation stability and slowing the oxidation to TiO2 | (143) | ||
| 2D-Bi2MoO6@2D MXene | LiF + HCl | O, OH | LiF with 9 M of 20 mL HCl, 24 h etching time (35 °C) | 2D MXene serves as effective platform for impeding the agglomeration and support the growth of Bi2MoO6 constructing a hierarchical composite structure | (144) | |
| Ti3AlC2/Ti3C2Tx | LiF + HCl | 3.08 M LiF/HCl, 6 h etching time; 1, 3, 6, 24, and 36 h | delaminated MXene with more conversion to Ti3C2Tx than 1 h etched | (145) | ||
| 3.08 M LiF/HCl, 24 h etching time | delaminated MXene are formed with residual of Ti3AlC2 was observed | |||||
| 3.08 M LiF/HCl, 36 h etching time | delaminated MXene with residual of Ti3AlC2 was observed; partial delamination occurs even at 36 h etched time | |||||
| Ti3C2Tx | LiF + HCl | O, OH, F | LiF with 9 M HCl, 24 h etching time (30 °C) | c-lattice parameter of 25.75 Å | (146) | |
| LiF with 9 M HCl, 24 h etching time (35 °C), ethanol as washing agent | the highest c-lattice parameter of 30.99 Å obtained with ethanol as washing agent with improved delamination ratio | |||||
| alkaline etching | Ti3C2Tx | KOH | O, OH | 5 M KOH, 120 °C hydrothermal treatment | less developed of accordion-like structure of MXene; more exposed of Ti-OH sites with with alkali etching | (147) |
| Ti3C2Tx | NaOH | O, OH | 27.5 M NaOH treatment at 270 °C | higher purity (92%) of multilayer MXene produced with removal of −F termination group | (129) | |
| 27.5 M NaOH treatment at 250 °C | Ti3C2Tx yield decrease with reaction temperature | |||||
| 5–10 M NaOH treatment at 270 °C | formation of Na/K–Ti–O compounds (NTOs) due to lower concentration of NaOH, increasing the water content and facilitate oxidation | |||||
| NaOH treatment at 100–220 °C | no formation of MXene as reation cannot happen at lower temperature regardless of any concentration | |||||
| electrochemical etching | Ti3C2Tx | NH4Cl, TMAOH | O, OH | 1 M NH4Cl, 0.2 M TMAOH | higher yield of single or bilayer (>90%) with larger average dimension | (126) |
| Ti2C | HCl | –Cl, −O, −OH | 1 M HCl, 0.6 V, 1 day etching | less conversion of MXene sheets. | (128) | |
| 2 M HCl, 0.6 V, 5 days etching | the formation of carbon-derived-carbide (CDC) as the results of overetching | |||||
| molten salt substitution | Ti3C2Tx | CuCl2 | –Cl, −O | Ti3SiC2 in CuCl2 molten salt (750 °C) | delamination of the layer proceed with the aid of SiCl4 gas molecules produced through etching process | (136) |
| Ti3C2Tx | ZnCl2 | –Cl, −O | Ti3ZnC2 + ZnCl2 (annealed time: 0.5 h, 1 h, 1.5 h, 3.0 h) | the increasing of the ratio of molten salt, ZnCl2 in the starting precursor, a gradual conversion to Ti3C2Cl2 can be observed | (134) | |
| Ti3C2Tx | NaCl2, KCl, CuCl2 | –I, −Br, −Cl | NaCl2, KCl, CuCl2 (mixed ratio of 1:2:2:3), 10 h annealed time in Ar (700 °C) | well-configured Lewis acidic etching route could tailored the surface chemistry of MXene | (148) |
3.1. HF Etching
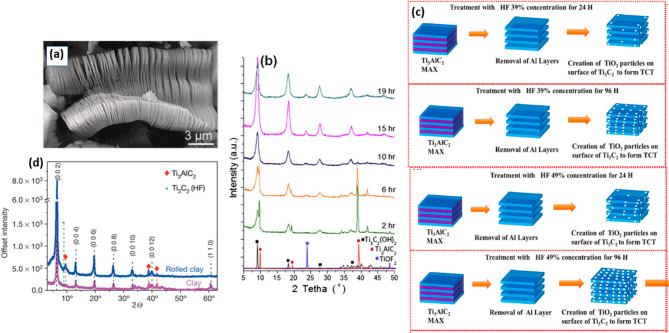
The HF etching technique is considered to be a pioneering technique and is an extensively used method to synthesize Ti3C2Tx MXene. HF was initially chosen as the primary etching agent due to its ability to break the large particle of MAX into smaller grains and its distinct properties to be one of the few selected etchants that can etch the titanium oxide layer typically present as a protective layer of Ti3C2Tx MAX.118 It was suggested that Ti3C2Tx MXene could be formed by treating the Ti3AlC2 MAX with 50% of HF acid under continuous stirring at room temperature.11,119 From the morphological perspectives, as presented in Figure 9a, the SEM image reveals an accordion-like structure, suggesting that the 2D structures of Ti3C2Tx MXene are formed after 2 h etching with HF. Studies also suggested that different morphologies of Ti3C2Tx MXene such as rolls, nanotubes, and multilayers were obtained after sonication. Moreover, the X-ray diffraction (XRD) pattern in Figure 9b shows that other parameters such as etching time could affect the removal of the Al layer of MAX. In this regard, the (104) peak at 2θ = 39, subjected to the MAX phase, was observed to slowly diminish with increasing etching time and was shown to completely disappear after 15 h of etching. This further revealed that increasing the etching time could assist the removal of the Al layer and the formation of Ti3C2Tx MXene. Further details on the mechanism of the Ti–Al bond breaking and the formation of Ti3C2Tx MXene is presented in eqs 8–10.120
| 8 |
| 9 |
| 10 |
Figure 9.
(a) SEM image of the 2D structure of Ti3C2Tx MXene after 2 h etching with HF. Reprinted from ref (119). Copyright 2012 American Chemical Society. (b) XRD analysis on Ti3C2Tx MXene at different etching times. Reprinted with permission from ref (125). Copyright 2013 Elsevier. (c) Formation of TiO2 on the Ti3C2Tx MXene sheets at different etching times. Reprinted with permission from ref (121). Copyright 2021 Elsevier. (d) XRD analysis on the formation of Ti3C2Tx MXene through reaction with LiF with HCl solution. Reprinted with permission from ref (122). Copyright 2014 Springer.
Generally, Ti3C2Tx MXene is formed by removing the Al layer sandwiched between the individual layer of Ti3C2Tx. As presented in eq 8, Ti3AlC2 MAX will react with three molecules of HF to form white small solid particles of AlF3 with the generation of H2 gas. The loss of the metallic bonding leads to the formation of layered Ti3C2Tx MXene, where the surface of Ti is terminated with functional groups such as −F and −OH such as that shown in eqs 9 and 10. It has been noted that the termination groups present are dependent on the type of etchants utilized. Therefore, the utilization of HF as a primary etching agent successfully etched away the Al layer of MAX. This technique has been favored due to the cost-effective and straightforward procedures. However, our research group found that the etching time could affect the creation of TiO2 on the Ti3C2Tx MXene surfaces.121 In this context, the etching of Ti3C2Tx MXene was carried out by utilizing 39 and 49% HF concentration, and both were etched at 24 and 96 h. It was suggested that increasing etching time with HF assisted the formation of TiO2 nanoparticles (NPs) on the surfaces of Ti3C2Tx MXene, as represented in Figure 9c. In this regard, the increasing number of active TiO2 semiconductor formation suggests a robust photocatalytic activity due to metal–semiconductor synergy. Even though the HF etching technique is highly utilized, the synthesis parameters, such as the concentration of HF and the etching time, are significant to ensure the purity of the produced Ti3C2Tx MXene. Moreover, controlling the synthesis parameters could essentially affect the photocatalytic activity of the Ti3C2Tx MXene-based materials.
3.2. Acid-Containing Fluoride Ions
Another synthesis technique that garnered scientific attention for the synthesis of Ti3C2Tx MXene is the acid-containing fluoride ion method. In order to minimize the use of concentrated HF acid, researchers have sought a new environmentally safe technique to synthesize Ti3C2Tx MXene. In this context, acid-containing fluoride ion etchants have been utilized to etch Al layers of Ti3AlC2 MAX, which at the same time delaminates their multilayer structure into the individual compartment.122 In this regard, the synthesis and delamination of Ti3C2Tx MXene can be achieved in a single step, unlike those by HF etching which requires intercalating agents to delaminate the multilayered structure of Ti3C2Tx MXene. The pioneering study reported that the single-step preparation method could be achieved by reacting Ti3C2Tx MXene with 6 M HCl with LiF at 45 °C for 45 h. Further analysis by powder XRD observed the removal of the Al layer from the Ti3AlC2 MAX, where the disappearance of Ti3AlC2 can be seen from Figure 9d. In this method, different types of acid and fluoride ions can be utilized, such as using sulfuric acid (H2SO4) containing NaF, KF, NH3F, CsF, and tetrabutylammonium fluoride.123 However, each combination might affect the properties of the synthesized Ti3C2Tx MXene. For instance, a study found that utilizing HCl might improve the electrochemical capacity of Ti3C2Tx MXene compared to those by HF.124 Additionally, each fluoride salt utilized might require different synthesis parameters such as the reaction temperatures and etching time. It has been suggested that utilizing fluoride salts such as NaF and KF might require 48 h etching time at 40 °C to ensure a complete etching process. However, acid-containing NH4F shows complete delamination after 24 h at 30 °C. A similar morphology can be observed in all synthesized Ti3C2Tx MXenes regardless of any types of fluoride ions. In this regard, the accordion-like structure is present, suggesting that different acid-containing fluoride ions successfully form the Ti3C2Tx MXene.
3.3. Electrochemical Etching
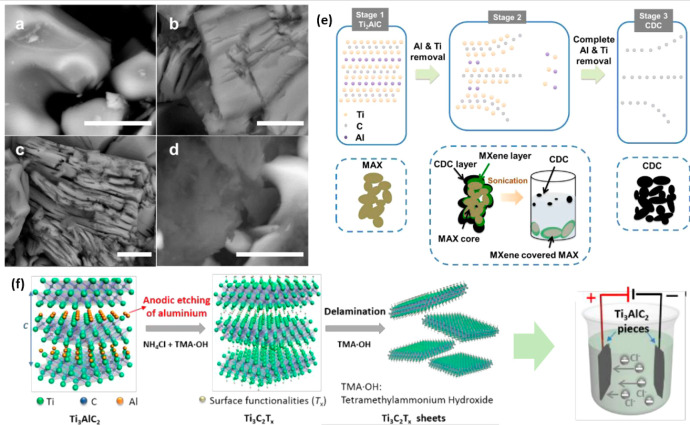
One of the newly developed techniques in synthesizing Ti3C2Tx MXene is the electrochemical etching by which the fluoride-based etchant can be eliminated. In this synthesized process, the electrode and electrolytes are required for the reaction to occur. The Al layer will be selectively etched with the assistance of electrolytes through anodic etching.126 In general, the Ti3AlC2 MAX will serve as a working electrode where the etching reaction will take place. Particularly, chemical and electrochemical etching is part of the surface reaction involving electron transfer, suggesting the possibility of forming Ti3C2Tx MXene by electrochemically etching the MAX phase. A study found that the Ti2AlC MAX can be electrochemically etched to form Ti2CTx MXene by utilizing an aqueous HCl (2 M) electrolyte. The absence of fluoride ions significantly yields fluoride-free terminated Ti2CTx MXene with the presence of −Cl, −O, and −OH terminal groups. In this approach, a three-layered structure of Ti2CTx MXene is formed, consisting of carbon-derived carbide (CDC), Ti2CTx MXene, and unetched MAX. Parameter study such as voltage, etching time, and electrolyte concentration was observed to affect the morphology and properties of Ti2CTx MXene, such as that shown in Figure 10a–d. In particular, increasing the concentration of the HCl and etching time shows pronounced delamination surfaces. However, overetching of Ti2CTx MXene might lead to the formation of CDC. Even though the three-layered structures consisting of CDC, MXene, and MAX are formed, bath sonication could separate the layered structure to obtain Ti2CTx MXene. The synthesis process of Ti2CTx MXene through this electrochemical etching is demonstrated in Figure 10e.
Figure 10.
(a) SEM images MAX, (b) Ti2CTx MXene etched with 1 M of HCl at 0.6 V for 1 day, (c) 2 M of HCl at 0.6 V for 5 days, (d) 2 M of HCl at 0.6 V for 14 days. (e) Synthesis illustration of the Al removal from MAX phase and formation of three-layered Ti2CTx MXene structure. Reprinted with permission from ref (128). Copyright 2017 Royal Society of Chemistry. (f) Synthesis process of electrochemical etching of Ti2CTx MXene in two-electrode system. Reprinted with permission from ref (126). Copyright 2018 Wiley.
Another study revealed that Ti3C2Tx MXene was successfully formed by utilizing binary aqueous electrolytes in a two-electrode system, such as that present in Figure 6f.126 In this method, the electrolyte consisting of 1 M NH4Cl and 0.2 M tetramethylammonium hydroxide (TMAOH) at pH >9 was employed for a 5 h reaction time to etch the Al layer and undergo intercalation to extract the carbide flakes. The presence of a Cl ion in the electrolyte expedites the anodic Al etching and breaks the Ti–Al bond. Intercalation by the ammonium hydroxide (NH4OH) promotes the etching underlying the surfaces. This approach successfully yields 90% of single and double Ti3C2Tx MXene layers with flakes larger than those in the conventional HF technique. On the other hand, the fluoride-free Ti3C2Tx MXene offers better capacitance (220 mF cm–2) than those conventionally prepared. Even though this synthesis method is shown to offer Ti3C2Tx MXene with Cl termination and successfully eliminate the −F functional, the formation of CDC required much attention. As mentioned, overetching the MAX phase in dilute HF, HCl, and NaCl electrolytes might lead to the removal of both Al and Ti layers.127 Moreover, the newly developed technique requires in-depth scientific study to ensure a successful formation of Ti3C2Tx MXene with excellent catalytic properties.
3.4. Alkali Etching
Aside from acid utilization as the etching agent, the study has found that alkali can also be employed to eliminate the Al layer of Ti3AlC2 MAX.129 A study on alkali etching revealed that the hydrothermal-assisted technique utilizing NaOH as the etching agent successfully etched the Al layer of Ti3AlC2 MAX to form Ti3C2Tx MXene. Alkali etching is one of the techniques to synthesize a fluorine-free Ti3C2Tx MXene as it does not utilize any fluoride-containing chemicals. Additionally, this newly developed preparation method was also revealed to yield high-purity (∼92%) Ti3C2Tx MXene with a multilayered structure. The strong binding ability of the NaOH with the Al element assists the etching process, thus making it possible. It has been noted that the synthesis parameters such as the reaction temperature and etching concentration are significant to ensure successful elimination of the Al layer. This is because, as demonstrated in Figure 11a, the low temperature might prevent the Al extraction process by the Al (oxide) hydroxides. Moreover, the inability to form Al4(OH)− due to lattice confinement from the Ti layer, known as the “jamming effect”, blocks formation of Ti3C2Tx MXene. On the other hand, utilizing a higher concentration of NaOH at a higher temperature leads to the oxidation of Ti3C2Tx MXene and the formation of NTOs such as Na2Ti3O7 and Na2Ti5O11. Higher water content promotes the oxidation of Ti to form NTOs. Therefore, a suitable reaction temperature and alkali concentration are required to ensure a complete formation of Ti3C2Tx MXene. The hydrothermal reaction at 270 °C with 27.5 M NaOH concentration successfully yielded Ti3C2Tx MXene with high purity compared to HF-etched MXene. In this hydrothermal-assisted NaOH etching method, controlling the temperature is essential to ensure a complete formation of Ti3C2Tx MXene, while the optimum concentration of Ti3C2Tx MXene controlled the purity of the produced Ti3C2Tx MXene. Even though the alkali-assisted, hydrothermal synthesis method has been shown to successfully generate Ti3C2Tx MXene, preventing the Ti3C2Tx MXene from overetching to form NTOs is one of the challenges and requires extra attention. In addition, this method needs in-depth research to confirm the successful formation of fluoride-free Ti3C2Tx MXene. Through this technique, −O and −OH terminal groups can be formed, and their role in stimulating photocatalytic activity and fuel conversion can be further extended.
Figure 11.
(a) Synthesis process of Ti3C2Tx MXene through alkali etching. Reproduced with permission from ref (129). Copyright 2018 Wiley. (b) Schematic representation of water-free etching of MXene by utilizing propylene carbonate. Reproduced with permission from ref (137). Copyright 2020 Elsevier. (c) SEM image of Ti3C2Tx MXene prepared through molten salt substitution. Reproduced from ref (134). Copyright 2019 American Chemical Society. (d–f) SEM image of Ti3C2Tx MXene by utilizing different types of MAX with chloride molten salts. Reproduced with permission from ref (136). Copyright 2020 Springer.
3.5. Water-Free Etching
Water-free etching is another alternative in producing Ti3C2Tx MXene with outstanding oxidation stability.130 In this synthesis process, the employment of water as a primary solvent is replaced by the polar organic solvent to ensure no presence of water. The exceptional potential of Ti3C2Tx MXenes in a wide array of applications indicates that they are one of the most favored materials. However, their hydrophilicity and tendency to oxidize limit their potential in applications requiring a longer storage period. Therefore, water-free etching was found to improve oxidation stability and broadened their role in any water-sensitive applications. In this preparation method, ammonium dihydrogen fluoride was utilized as the etching agent due to its capability to dissociate into NH4F and HF when dissolved in the polar solvents. Three reaction steps are required: etching, washing, and delamination. Specifically, as presented in Figure 11b, the etching process occurred at which Ti3AlC2 MAX underwent a reaction with NH4HF2, where the Al layer was etched out in the form of AlF3 and (NH4)3Al6. Reaction with propylene carbonate (PC) promotes delamination into single-layer Ti3C2Tx MXene. It has also been suggested that higher interlayer spacing of Ti3C2Tx MXene is obtained compared to water-etched Ti3C2Tx MXene.131 Controlling the termination groups are favorable as it has been revealed that, through this technique, approximately 70% of −F and −30% of −O/–OH containing MXene was obtained.132 In short, this method utilizes polar organic solvent, PC to replace water which was found to reduce the presence of the −OH terminating group. The control of the terminating group significantly enhances the oxidation stability, which raises Ti3C2Tx MXene potential in the water-sensitive applications as the finding suggested that more exposure of the Ti–OH species of Ti3C2Tx MXene expedites the oxidation process.133
3.6. Molten Salt Substitution
Another alternative in producing −Cl-terminated Ti3C2Tx MXene is through molten salt substitution.134 This method utilizes molten salts such as ZnCl2 and CuCl2 to undergo a substitution reaction with Ti3AlC2 MAX for the removal of the Al layer. This reaction process significantly produces nanolaminated Ti3C2Tx MXene with −Cl termination, which was found to be more stable than −F-terminated Ti3C2Tx MXene.135 The reaction mechanism is presented in eqs 11–13, which generally involves the replacement of the A element of MAX with the metal salts.
| 11 |
| 12 |
| 13 |
In eqs 11–13, the Zn2+ from the molten salts undergoes elemental replacement with the Al3+ from the Ti3AlC2 MAX. Stronger Lewis acidity by ZnCl2 promotes the substitution of Zn2+ with Al3+, forming Ti3ZnC2 MAX. Additionally, Zn2+ acts as a Lewis acid in the molten salt as it is a stronger Cl– and electron acceptor. Specifically, molten salt of ZnCl2 was ionized into Zn2+ and ZnCl42– in its molten state, while Al from Ti3AlC2 MAX undergoes redox conversion into Al3+. The formation of AlCl3 occurred through the bonding of ionic Al3+ with Cl–, where it will be evaporated. Rapid evaporation of AlCl3 facilitates the outward diffusion of the Al atom to undergo a substitution reaction. The elemental replacement further takes place when the reduced Zn atoms intercalate into the Al layer in the MAX phase, forming Ti3ZnC2, which is further exfoliated into Ti3C2–Cl2 MXene. The SEM image in Figure 11c reveals the morphology of the Cl-terminated MXene, where it shows a more compact structure with a less distinct accordion sheet compared to other conventional preparation techniques. Another study by Li et al. revealed that the synthesis of Ti3C2Tx MXene from different types of MAX phases could be achieved by employing different types of chloride molten salts such as CuCl2, FeCl2, NiCl2, and AgCl.136 Different morphology of MXenes etched by different chloride molten salts can be observed in Figure 11d–f, by which most of them show a prominent accordion-like structure of MXene.
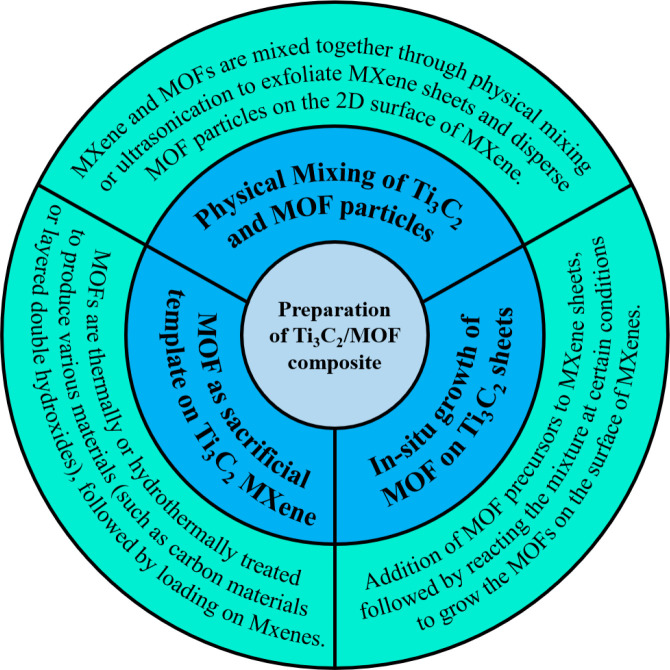
4. Design Principles and Considerations for Ti3C2Tx MXene-Based MOF Composite
4.1. Morphological Design and Engineering of Ti3C2Tx MXenes and MOFs
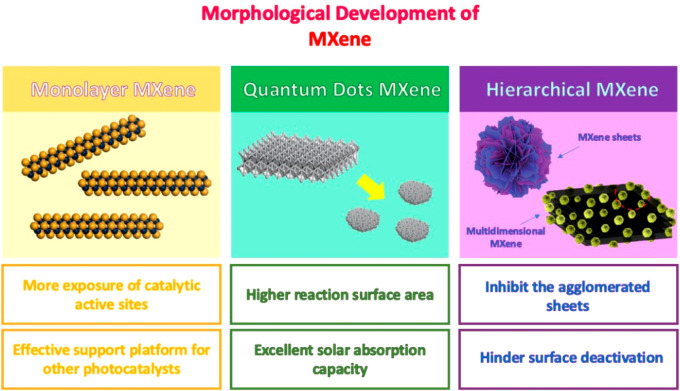
In the context of morphological development, Ti3C2Tx MXenes intrinsically manifest a unique accordion-like multilayer structure. However, their morphology can be modified into different dimensional structures, such as 0D quantum dots, 2D delaminated flakes, and 3D hierarchical/multidimensional structures. Delaminating Ti3C2Tx MXenes into single flakes was shown to supply more exposed catalytic active sites and was observed to be highly efficient in driving the redox reaction compared to their multilayer morphology.149 Typically, delaminating of Ti3C2Tx MXene can be mainly performed through direct delamination or a two-step delaminating process. It has to depend on the preparation method of the Ti3C2Tx MXene itself. Direct delamination can be achieved via an acid-containing fluoride ion etching technique.150 In this regard, no intercalating agents are required to separate the layer as the fluoride ions can serve as intercalants to aid in the delamination process. On the other hand, the HF etching technique is regarded as a two-step process, requiring intercalating agents to assist the layer separation. Commonly used intercalating agents include dimethyl sulfoxide (DMSO), dimethylformamide (DMF), tetrabutylammonium hydroxide (TBAOH), and tetramethylammonium hydroxide (TMAOH).151−153 Interestingly, a comparison study by Su et al. shows that monolayer Ti3C2Tx MXene is more reactive toward the photoredox reaction due to its thinner sheet structure.138 This is because the size and structure of Ti3C2Tx MXenes distinctly affect their photocatalytic property and are correlated with their Fermi level energy. Hydrogen yield with 2.5 times enhancement was disclosed by employing monolayer Ti3C2Tx MXene compared to their multilayer counterpart. Even though the precise location of active sites in Ti3C2Tx MXenes is yet to be confirmed, through structural analysis, it was inferred to be positioned at the edge of the monolayer flakes. This corroborates with the experimental study where Ti3C2Tx MXenes with smaller flake sizes were observed to have an exceptional catalytic activity compared to that of a denser multilayer structure. More exposure of the catalytic active sites due to size effects and exposed flake edges supports the conjecture. Correspondingly, delaminated Ti3C2Tx MXenes through intercalation with TMAOH were revealed to have an apparent effect on the photocatalytic activity with 1.49 times activity improvement over that of the standard HF-etched multilayered Ti3C2Tx MXene.154 Moreover, the 2D geometry of monolayer Ti3C2Tx MXene offers excellent support for other semiconductor photocatalysts, especially for nanoparticle materials from agglomeration, providing uniform dispersion. Therefore, the solar absorption performance can be augmented owing to a larger light receiving area.155
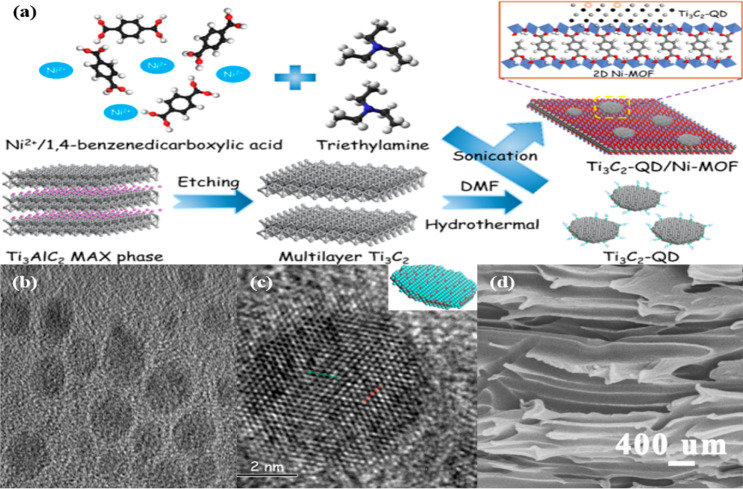
Additionally, the tunability of their morphology indicates that they are a highly selective cocatalyst for photocatalytic enhancement. For instance, quantum dot Ti3C2Tx MXene can be constructed through layer cutting by DMSO intercalant and thermal treatment with PEI.25,156 Another study reported the preparation of Ti3C2Tx MXene QD through an ammonia-assisted hydrothermal reaction.157 Moreover, hydrothermal intercalation with DMF was also found to convert the nanosheets into QD structures.142 A benefit in converting into smaller nanosize QDs is to offer better solvent solubility and to expose more active edge sites. As mentioned, dimensional size has a great impact on the photoreaction activity, which highlights the significance of well-controlled morphology. The smaller dimensional size of the Ti3C2Tx MXene QD is shown to provide an effective coating for stabilizing the oxidation of other semiconductor materials. For instance, the Ti3C2Tx MXene QD was found to cover the porous surface of Cu2O NWs by coating them and hindering the easy oxidation of the NWs.25 In this regard, the photoreaction stability was observed to maintain up to six long cycles and retain as high as 86% methanol yield. The relevance of morphologically tuning the Ti3C2Tx MXene into a QD structure can prevail with their outstanding photoreaction activity for hydrogen generation, successfully obtaining their 2D nanosheet structure. A study found that the Ti3C2Tx MXene QD successfully produced solar hydrogen generation with as high as 10 times enhancement than that of Ti3C2Tx MXene nanosheets and 3 times higher than that incorporated with Pt, disclosing the novelty of the Ti3C2Tx MXene QD as a noble metal replacement.25
Aside from morphologically constructing Ti3C2Tx MXene into single flakes and quantum dots, hierarchical and multidimensional Ti3C2Tx MXene constructed through the combination of different dimensional domains was observed to benefit them through a supportive platform. Typically, multidimensional Ti3C2Tx MXene can be constructed by hybridizing them with other dimensional photocatalysts. For instance, the Ti3C2Tx MXene safflower shape was successfully generated by hydrothermal oxidation followed by ion exchange to form TiO2/Ti3C2Tx MXene with a 3D porous framework.140 Thermally oxidized Ti3C2Tx MXene could support the formation of TiO2 nanoparticles and, through the ionic exchange process, form the safflower-like-shaped TiO2–Ti3C2Tx. The merit of having a 3D structure framework could hinder the agglomeration of the nanoparticles and sheet aggregation, which could reduce the absorption efficiency. Thinner and smaller sizes of Ti3C2Tx MXene are highly beneficial for the carrier’s transmission where shorter distances are required to carry out the redox reaction, but the implication of agglomerated sheets is one of the serious drawbacks.158 The creation of a multidimensional structure of a Ti3C2Tx MXene-based composite with a 1D/2D structure highly benefits the carrier dynamics as different dimensional domains have an individual contribution for photocatalytic enhancement. For instance, 1D/2D CdS/Ti3C2Tx MXene constructed through in situ assembly and solvothermal treatment was found to give an eminent improvement in the photocatalytic activity compared to that of their intrinsic counterpart.149 The effective platform of 2D Ti3C2Tx MXene sheets for CdS nanorod growth provides intimate contact and aids in the separation of charges. For instance, the longitudinal oriented charge transmission with high length-to-diameter ratio semiconductors could improve the separation efficiency as a longer time is needed for the charges to travel back and recombine. On the other hand, hierarchical nanoflower-like core–shell morphology is constructed by in situ hydrothermal LDH on Ti3C2Tx MXene.159 The growth of smaller LDH nanoflakes creates a Ti3C2Tx MXene with abundant active sites for photocatalytic CO2 reduction. The well-dispersed LDH nanoflakes covering the Ti3C2Tx MXene surfaces in the core–shell-like shape improve the light absorption and hinder the serious agglomeration typically observed in LDH nanoflakes. Therefore, designing Ti3C2Tx MXene into different morphological structures is one of the promising engineering considerations for producing a very efficient photocatalyst for solar fuel conversion. Figure 12 summarizes the development of Ti3C2Tx MXene into different morphological structures.
Figure 12.
Morphological development of Ti3C2Tx MXene into monolayer, quantum dots, and hierarchical/multidimensional structure.
As mentioned previously, MOFs tend to naturally occur in 1D rods, 2D sheets, or 3D polyhedrons, in which the latter tend to be the more common morphology. 1D MOFs are usually identified by their nanometric diameter together with an extended length, adopting chains or rod structures. On the other hand, 2D MOFs exist as sheets or layers that have a nanoscale thickness. 2D MOFs have a few beneficial properties as an efficient photocatalyst, such as high exposed surface area to light irradiation and reactants, as well as excellent electron transferability.160,161 Finally, 3D MOFs are held together by strong chemical bonds, which come together to form a highly complicated network structure. These polyhedrons boast an extremely high surface area, with uniform pore size distributed all over the surface of the MOF.30 Nevertheless, the morphology and dimensions of MOFs can be further regulated via two main approaches which is (1) modification of synthesis parameters and (2) introduction of additives. First, various synthesis parameters were reported to modify the morphology of MOFs, such as temperature, reaction time, and solvent ratio. Yuan et al. noticed that the structure of Ni-MOF alters according to the solvothermal synthesis temperature. At room temperature and 60 °C, the Ni-MOF exhibits an urchin-like structure with a diameter of approximately 200 nm. As the temperature increased to 100 °C, one-dimensional nanowires with 10 nm diameter were obtained. The change in morphology was mainly due to the dynamic viscosity of 2-methylimidazole linkers, which limits the mass transfer through the temperature change.162 In another study, the effects of reaction temperature and time were studied with a Co-MOF. The original 2D nanoplates of Co-MOF were transformed into 1D layered microrods as both the reaction temperature (120 to 160 °C) and time (5 to 30 min) increased.163 The solvent ratio can also effectively alter the structure of the MOF due to the role of solvent as a directing agent as well as ligands, where they can be incorporated onto the lattice of the MOF. It was previously reported that by manipulating the DMF/ethanol solvent ratio from 3 to 1, the 3D structure of the MOF was converted into 2D nanosheets.164
Second, the use of additives such as modulators and surfactants is effective at tuning the morphology of MOFs. For example, a 3D hollow porous concave octahedral bimetallic Fe–Zn MOF was prepared via a conventional solvothermal method using FeCl3·6H2O, Zn(NO3)2·6H2O, and terephthalic acid as linkers, with DMF and C2H5OH alcohol as solvent. Upon introduction of polyvinylpyrrolidone (PVP), the morphology of the MOF showed a drastic change, from 3D octahedrons to 1D hollow porous nanorods.165 Additionally, Sarawade et al. investigated the effect of various surfactants on the morphology on a Co-MOF with linkers of 2,6-naphthalenedicarboxylic acid (H2ndc) and trans-1,2-bis(4-pyridyl)ethylene (bipyen). Without the use of surfactant, the Co-MOF exhibited a spindle-like microrod structure. However, upon the use of cetyltrimethylammonium bromide, sodium dodecyl sulfate (SDS), and Pluronic triblock copolymer (P123) surfactants, the original morphology was converted into 2D nanoplates, nanosheets, and 1D nanorods, respectively.163 The morphology engineering of MOFs was then summarized, as shown in Figure 13. MOFs tend to exist mainly in 3D structures, with some MOFs naturally existing in 1D rods or 2D sheets. However, the morphology and dimensions of MOFs can be further engineered either by varying the synthesis parameters or by using additives.
Figure 13.
Scheme illustrating the general morphology and morphological engineering of MOFs.
4.2. Active Sites over Ti3C2Tx MXene-Based MOF Composite
Active sites are the locations or specific sites where a certain reaction has the highest activity. It is of utmost importance to identify the active sites over photocatalysts to gain a deeper understanding of how to quantify and optimize the active sites with respect to a specific chemical reaction. This is because the types of active sites present on the surface of the catalyst can influence the overall selectivity of the products. The role of active sites is especially evident in thermal and photothermal catalysis. For example, the utilization of Ni166 and Co80 metals tends to yield a higher production of CH4 from the CO2 hydrogenation reaction. However, the use of Fe promotes the Fischer–Tropsch reaction, in turn exhibiting a higher C2+ hydrocarbon evolution.167,168 From these examples, we can get a general idea on the active sites of MOFs. MOFs are made up of a variety of metal nodes, such as Co, Zr, and Ti and can be regarded as isolated quantum dots that can be excited upon light irradiation.169 Second, the organic linkers can also function as active sites or be functionalized to anchor active sites. Most notably, UiO-66 linkers modified with Cu(II) porphyrins not only enhanced the overall photocatalytic activity but also boosted the light adsorption of the photocatalyst.170 Similarly, MOF-525, which consists of porphyrin linkers anchored Co metal, successfully incorporated the Co active sites onto the framework of the MOF. The introduction of CO active sites to the framework prolonged the lifetime of charge carriers and improved the CH4 production from photocatalytic CO2 reduction.171 Lastly, the huge cavity of MOFs is a suitable host for encapsulating photoactive metal nanoparticles. Zhao’s group successfully encapsulated Ni nanoparticles within the pores of UiO-66, which limited the growth of Ni via agglomeration.172 Interestingly, the encapsulation is not limited to only metals. Li et al. reportedly boosted the photocatalytic CO2 reduction by embedding carbon dots inside NH2–UiO-66 particles. These carbon dots function as electron receptors and photosensitizers, which promote charge separation and transfer.173 The discussion on the active sites of MOFs are summarized in Figure 14, where they exist either at the metal nodes, organic linkers, or within the cavity of MOFs.
Figure 14.
Overview of the active sites present over metal–organic frameworks.
Up to the current development, the exact active sites of Ti3C2Tx MXene are still under exploration. Some studies inferring the position of the active sites might be located at the edges of the Ti3C2Tx MXene sheet. This has been experimentally hypothesized by which delaminating MXene into thinner flakes exposed more of the active sites and induced higher reaction activity compared to that with the multilayer structure.138 Therefore, engineering the morphology Ti3C2Tx MXene was observed to affect the photoreaction activity which is highly linked to the degree of exposure of the catalytic active sites. Nevertheless, despite the vagueness in specifying the active site of MXene, an analysis study revealed that the termination group has a prominent role in tailoring the stability and catalytic properties of Ti3C2Tx MXene. The type of the termination group can be qualitatively controlled through parameter regulation. Designing Ti3C2Tx MXene with suitable termination groups is significant to fit the application requirement and control its environmental stability. For instance, the formation of −F termination has been linked with poor ambient stability in a colloidal dispersion, which led to the formation of −F free terminated Ti3C2 MXene by Shi et al.174 On the other hand, Ti3C2Tx MXene can easily be oxidized under ambient environments, and the exposure of the Ti atom with oxygen could lead to the formation of TiO2. Worth mentioning is that the functional group is the active oxidative site which could affect the oxidation stability of Ti3C2Tx MXene. For instance, computational analysis on each type of termination group revealed that the termination group is linked with the defect formation energy. In this regard, the −OH termination group relating to lower oxidation stability is due to lower energy formation of the vacancy. However, =O termination is oxidatively stable due to higher energy formation of the vacancy. Moreover, it was confirmed by the study conducted indicating that the Ti–OH group is highly reactive owing to the lower work function.175 The abundance of reactive sites in Ti–OH further facilitates the oxidation of Ti3C2Tx MXene. The removal of −OH through hydrogen annealing was found to improve the oxidation stability of Ti3C2Tx MXene.133 In additional, preparing Ti3C2Tx MXene with −Cl termination was observed to possess higher oxidation stability compared to that of −F- and =O-terminated Ti3C2Tx MXene.176 Therefore, perfect control of the surface termination is significant, especially for ensuring a highly stable Ti3C2Tx MXene in an ambient environment. This is because it was noticed that Ti3C2Tx MXene could easily be oxidized during the thermal treatment even with no support of oxidizing agents due to the presence of surface terminating groups.177
4.3. Modification Strategies through Interfacial/Heterojunction Engineering
4.3.1. Ti3C2Tx MXene
Another design consideration to increase the potential of Ti3C2Tx MXene is through interfacial/heterojunction engineering. The metallic nature and excellent electrical conductivity in Ti3C2Tx MXene make it the most influential cocatalyst to assist the solar fuel conversion.178−181 Ti3C2Tx MXene could serve as an electron trapper through the creation of an electrostatic potential barrier at the Ti3C2Tx MXene/semiconductor junction. This phenomenon is termed the Schottky barrier, where electrons that transfer from the semiconductor to Ti3C2Tx MXene are trapped and unable to transfer back to the semiconductor counterpart and recombine with holes. The Schottky barrier can be constructed through the contact formation between Ti3C2Tx MXene with other semiconductor photocatalysts. Ti3C2Tx MXene with a large work function could highly benefit from the carrier separation. This is because the higher the difference between the metal work function and the semiconductor, the stronger the potential barrier created at the metal–semiconductor junction, making the electrons from the Ti3C2Tx MXene unable to travel back. The Schottky barrier induced by Ti3C2Tx MXene is a distinction that aids photocatalytic enhancement.
In addition to the creation of the Schottky barrier, Ti3C2Tx MXene could also serve as a mediator to assist electron transmission and is prominent in the construction of the Z-scheme heterojunction system.182 For the first time, Ti3C2Tx MXene was employed as a mediator in the three-phase Z-scheme system catalyst of CdS@Ti3C2@TiO2 by Liu et al.183 The electron sinking effects by Ti3C2Tx MXene with a well-matched band configuration give a substantial improvement in the photocatalytic activity through the creation of a Z-scheme electron transfer system. In this system process, Ti3C2Tx MXene serves as an electron-trapping center and as the mediator. The role of Ti3C2Tx MXene as the electron mediator facilitates the electrons from the CB of TiO2 to transfer to the VB of CdS, hastening the electron migration. Another study disclosed the significance of Ti3C2Tx MXene in mediating the electron transfer between g-C3N4 and MoSe2, constructing a Z-scheme heterojunction system.184 Separation of photocarriers was enhanced, and the electrons effectively transferred from one semiconductor to another semiconductor to undergo a redox reaction. The vital role of Ti3C2Tx MXene as a trapping center and a mediator provides greater reductive/oxidative ability as more electrons are available and less recombination occurs. Nevertheless, the employment of Ti3C2Tx MXene as an electron mediator in the Z-scheme system is still not widely explored compared to other cocatalysts such as reduced graphene oxide (rGO), which might be due to their thicker sheet reducing the contact formation with multiple semiconductor photocatalysts.183
In general, thermally oxidized Ti3C2Tx MXene could generate a Ti3C2Tx MXene–TiO2 hybrid.185 Moreover, our group also investigated the construction of anatase/rutile TiO2 formation by regulating the etching time with HF.121 Therefore, employing a Ti3C2Tx MXene–TiO2 hybrid with other semiconductor photocatalysts such as g-C3N4, CdS, and MoS2 could induce a hybrid Schottky–Z-scheme/Schottky–type II heterojunction system. In this context, the electrons will migrate following the Z-scheme/type II configuration to the TiO2-induced MXene and will then migrate to the reductive Ti3C2Tx MXene to undergo a reduction reaction.121,139,186,187 The Schottky barrier will be formed at the TiO2–Ti3C2Tx junction and thus suppress the backward reversal of electrons back to TiO2. Therefore, the combination of Schottky–Z-scheme/Schottky–type II heterojunction is highly propitious for boosting the photocatalytic performance of Ti3C2Tx MXene in the solar fuel conversion. Figure 15 presents the electron transfer mechanism of MXene as an electron mediator, in a Schottky barrier and a hybrid Schottky heterojunction system.
Figure 15.
Overall representation of the electron transfer mechanism in MXene as a cocatalyst.
4.3.2. MOFs
Since MOFs are photoresponsive materials that can generate electron/hole pairs when irradiated with solar energy, their photocatalytic activity can be enhanced via two main approaches, which is the prolongation of the lifetime of charge carriers and narrowing of band gap energy (Ebg). The lifetime of charge carriers can be prolonged by adopting various strategies to provide spatial separation of the electron/hole pairs. Additionally, the light harvesting ability of MOFs in the visible light region can be enhanced by modulating the Ebg of MOFs. Here, several approaches to promote the photocatalytic activity of MOFs will be discussed, such as the formation of heterojunctions, surface sensitization, MOF functionalization, doping of MOFs, and utilization of photosensitizers.
Heterojunctions are formed upon contact of two photoresponsive materials, in which different heterojunction systems have been discovered such as type I, type II, type III, Z-scheme, and step-scheme heterojunctions. The formation of heterojunctions not only promotes the adsorption of visible light but also provides spatial separation of charge carriers.188Figure 16a shows a type II heterojunction between Co-MOF and Cu2O (x-CMC). Here, electrons migrate from the CB of Cu2O to the LUMO of Co-MOF, whereas the holes from HOMO of Co-MOF transfers to the VB of Cu2O, achieving good spatial separation of charge carriers. Additionally, the formation of heterojunctions drastically improved the visible light adsorption of pristine Cu2O, as shown in the UV–vis diffuse reflectance spectra in Figure 16b.189 In another study, a Z-scheme heterojunction system (Figure 16c) was achieved over PCN-224(Cu)/TiO2 photocatalysts which improved the overall photocatalytic reaction.190 Surface sensitization is achieved when the Schottky junction is formed upon introduction of metals. Xiao’s group successfully encapsulated Cu nanoparticles within the pores of UiO-66 through an advanced double-solvent approach. Due to the intimate contact between the metals and MOF, a Schottky junction was generated, in which solar energy utilization and separation of charge carriers were ameliorated.191 By combining MOFs with MXenes, Schottky junction together with heterojunctions can be generated as reported by Wu et al. A Ti3C2-modulated MIL-125-NH2 nanohybrid exhibited both type II heterojunctions and Schottky junctions. This is because Ti3C2Tx MXenes tend to oxidize into the TiO2 semiconductor, allowing the heterojunction between TiO2 and MIL-125-NH2, while the metallic Ti3C2Tx MXenes induce a Schottky junction. Hence, the photogenerated electron is efficiently separated via migration to Ti3C2Tx, while the holes are left behind in the HOMO of the MOF.192 Functionalization is another promising strategy to enhance visible light adsorption as it can reduce the energy band gap of MOF. For instance, pristine UiO-66 has an Ebg of 3.91 eV, which decreased to 2.83 eV upon functionalization with an amine group.86 Dao’s group reported an efficient type II heterojunction system over NH2-MIL-125/g-C3N4 for photocatalytic CO2 reduction, as shown in Figure 16d. The −NH2 functional group not only enhanced CO2 adsorption but also promoted light adsorption in the visible light range.105
Figure 16.
(a) Type II heterojunction system between Co-MOF and Cu2O. (b) UV–vis diffuse reflectance spectra of Co-MOF and Cu2O composites (x-CMC). Reprinted with permission from ref (189). Copyright 2022 Elsevier. (c) Z-scheme heterojunction system on PCN-224(Cu)/TiO2 photocatalysts. Reprinted from ref (190). Copyright 2019 American Chemical Society. (d) Type II heterojunction scheme over NH2-MIL-125/g-C3N4. Reprinted from ref (105). Copyright 2020 American Chemical Society. Influence of different metal dopant on the Ebg of (e) MIL-125-NH2 and (f) UiO-66-NH2. Reprinted from ref (194). Copyright 2019 American Chemical Society.
Doping is a type of point defect where impurities or foreign atoms are introduced, which disrupts the original lattice structure of the material.193 A study conducted by Syzgantseva et al. reported that the doping of metals into the lattice of MOFs is able to modulate the energy band gap of MOFs. Here, two MOFs, namely, MIL-125-NH2 and UiO-66-NH2, were doped with different types of metals, and their Ebg were observed. From Figure 16e, the doping of MIL-125-NH2 with Sn increased the Ebg, but doping with Nb, W, and V narrowed the Ebg to 2.3, 2.2, and 1.6 eV, respectively. Similarly, UiO-66-NH2 (Figure 16f) doped with Y element caused a slight increase in Ebg, whereas Ta, Nb, and W dopants further reduced the Ebg to 2.6, 2.3, and 1.7 eV, respectively.194 A narrower band gap is able to promote a broader light adsorption spectrum. Finally, utilizing photosensitizers can promote light harvesting especially in the visible region due to them possessing a strong optical absorption over a wide range of wavelengths.195 When photosensitizers are used together with MOFs, MOFs benefit by obtaining energy from the incident light that is absorbed by the photosensitizer.196 Mu’s group coated CdS nanorods with Co(BDC) MOF and applied a [Co(bpy)3]2+ photosensitizer. The MOF layer successfully bridges the heterogeneous photosensitizer and molecular cocatalyst, which enhanced the CO2 reduction reaction and prevented photocorrosion of the photosensitizer.197 In another study, the Ru(bpy)2]Cl2 photosensitizer and Re(CO)3Cl molecular catalyst were grafted into the framework of MOF-808. It was noted that the covalent grafting of the photosensitizer not only enhanced the light harvesting ability of the photocatalyst but also improved the lifetime of electron/hole pairs and shortened the transport distance of charge carriers.198 Therefore, it is clear that the photocatalytic activity of MOFs in terms of spatial separation of charge carriers and light adsorption ability can be further regulated with various modification strategies.
5. Synthesis of MXene-Based MOF Composites and Their Characterization
In the past, there have been various ways to prepare Ti3C2 MXene-based MOF composites for various applications. The composite can be prepared by two main ways, which is either through physical mixing of both MOF and MXene together or by growing the MOF particles on the surface of MXenes. Also, MOFs can be used as sacrificial templates to prepare various materials such as carbon materials and layered double hydroxides, which is then dispersed on the surface of the MXenes. Herein, a thorough discussion on the various synthesis approaches and their characterization are explored, where the overview of the approaches is summarized as shown in Figure 17.
Figure 17.
Overview of approaches to prepare Ti3C2 MXene-based MOF composites.
One of the more common approaches to prepare the Ti3C2 MXene-based MOF composite is by physically mixing both Ti3C2 and MOF together. By mixing them, the MOFs and Ti3C2 MXenes will be randomly bound and be in contact with each other. Ultrasonication is a promising way to successfully synthesize MXene/MOF composites. This is because, under ultrasonication, the Ti3C2 sheets can be exfoliated into multilayers.199 For instance, Qin et al. prepared a Ti3C2 MXene-based MOF composite (Ti3C2-QD/Ni-MOF) through a facile ultrasonic method, as illustrated in Figure 18a. Ti3C2 quantum dots were prepared in two steps, which is the etching of the MAX phase to Ti3C2 multilayers, followed by hydrothermal treatment to obtain the Ti3C2 QDs. Then the prepared Ni-MOF was mixed with Ti3C2 QDs under continuous ultrasonication for 4 h. High-resolution transmission electron microscopy (HRTEM) images in Figure 18b,c show the successful preparation of the composite, in which a uniform dispersion of quantum dots on the 2D Ni-MOF was observed. Also, point defects are observed over the surface of Ti3C2, supplying coordination-unsaturated sites for catalytic reactions.142 Similarly, Liu and co-workers adopted the sonication method to prepare the MXene/MOF composite. First, NiCo-MOF nanosheets were prepared via a precipitation method. Upon obtaining the MOF powders, they were dispersed into a Ti3C2 aqueous solution under sonication. Due to the two-dimensional structure of both MOF and MXene, the ultrasonication promotes the interlacing of both materials, as studied over the field emission scanning electron microscopy (FE-SEM) image in Figure 18d. The interconnected porous networks which are developed between the MXene/MOF composite restricts the self-restacking of the NiCo MOF and Ti3C2 sheets.200
Figure 18.
(a) Illustration of Ti3C2-QD/Ni-MOF synthesis via ultrasonication approach. (b,c) HRTEM images of Ti3C2-QD/Ni-MOF. Reprinted from ref (142). Copyright 2020 American Chemical Society. (d) FE-SEM image of layered Ti3C2/NiCo-MOF. Reprinted with permission from ref (200). Copyright 2020 MDPI.
Another way to prepare the composite is through in situ growth of the MOF on the surface of MXenes. By growing the MOF particles on the MXenes, a more intimate contact between the two materials can be achieved. Zheng et al. anchored a 3D pillared Ni-MOF on the surface of 2D layered Ti3C2 MXenes, as illustrated in Figure 19a. The 4,4′-bipyridine (Bpy) and thiophene-2,5-dicarboxylate (Tdc) linkers were first introduced to the Ti3C2 sheets, followed by subsequent addition of NiCl2·6H2O into the solution. The addition of the linkers to the MXene bound the organic ligands to the interlayer functional groups of MXene. Then the addition of NiII coordinated the organic ligands over the Ti3C2 surface to produce a Ti3C2@Ni-MOF composite. The HRTEM images in Figure 19b display the successful growth of Ni-MOF on the surface of the 2D Ti3C2 sheets. The growth of Ni-MOF on MXene can prevent the oxidation of MXene due to the ample interlayer functional groups which diminish the exposure of surface atoms on MXenes.201 In another study, dual heterojunction NH2-MIL-125 modulated with a Ti3C2 hybrid was prepared using an in situ construction approach. Here, Ti3C2 nanosheets were added dropwise into the precursor aqueous solution for NH2-MIL-125, followed by subsequent hydrothermal synthesis at 150 °C for 1 day. Interestingly, as shown in the TEM images in Figure 19c, TiO2 is formed in the process, which is due to the hydrolysis of unreacted tetrabutyltitanate. Thus, Figure 19d shows that a type II heterojunction was formed between NH2-MIL-125 and TiO2, whereas a Schottky junction is formed where Ti3C2 acts as cocatalyst to trap photogenerated electrons.192 Long’s group also grew NH2-MIL-88B particles on the surface of Ti3C2 MXenes. Figure 19e,f depicts the accordion layered structure of Ti3C2 MXene and hexagonal prism structure of NH2-MIL-88B, respectively. Upon successful in situ growth of MOF on MXene, it can be observed that an optimal dispersion of NH2-MIL-88B is achieved over the Ti3C2 surface, where the MXene provides growth platforms for the MOF particles, as shown in Figure 19g.202
Figure 19.
(a) Scheme of in situ growth for Ti3C2 MXene@Ni-MOF composite. (b) HRTEM image of MXene@Ni-MOF. Reprinted with permission from ref (201). Copyright 2022 Elsevier. (c) HRTEM image and (d) energy level diagram for NH2-MIL-125/TiO2/Ti3C2 composite. Reprinted with permission from ref (192). Copyright 2020 Elsevier. SEM images of (e) Ti3C2 MXene, (f) NH2-MIL-88B, and (g) MXene/NH2-MIL-88B composites. Adapted with permission from ref (202). Copyright 2021 Elsevier.
Finally, MXene-based MOF composites can also be prepared by using MOFs as sacrificial templates to prepare various materials. For example, MOFs can be used to derive various carbon materials that are usually used for electrochemical applications such as batteries, supercapacitors, and electrochemical detection.203 Nitrogen-doped porous carbon (N-PC) was prepared by carbonizing a Zn-based MOF-5-NH2 in an Ar atmosphere at 900 °C over 2 h. Then N-PC (Figure 20a) was introduced to the Ti3C2 MXene (Figure 20b) via mixing and ultrasound treatment, obtaining the final MOF-derived composite denoted as Ti3C2/N-PC. From Figure 20c, it is noticed that the interlamellar spacing of the Ti3C2 MXene was enlarged, due to the successful insertion of N-PC between the gaps of the sheets.204 Likewise, Jiang and co-workers reported the in situ decoration of MOF-derived carbon on N-doped Ti3C2 nanosheets for applications in Li–S batteries. Ti3C2 solution and PVP solution were first mixed together for 10 min, followed by the addition of Co(NO3)2·6H2O and 2-methylimidazole for the growth of ZIF-67 on the surface of the MXene. Figure 20d illustrates the SEM image of Ti3C2/ZIF-67, indicating the effective growth of MOF on the MXene surface. Upon carbonizing the composite at 800 °C for 2 h, the 2D nanosheet morphology of N–Ti3C2/C inherited from the parent Ti3C2/ZIF-67 composite can be seen in Figure 20e. Also, in situ growth of ZIF-67-derived porous carbon mitigated the restacking of Ti3C2 sheets, as observed in the weaker XRD peak intensity of N–Ti3C2/C compared to that of Ti3C2 (Figure 20f).205 Not only that, MOFs can also act as precursors to derived LDHs.206 Hu’s work reported the growth of ZIF-67 on Ti3C2/nickel foam (Ti3C2/NF), followed by an etching–doping process, where Fe(SO4)2·7H2O and urea were added. The etching–doping process then yielded a MOF-derived LDH/MXene composite denoted as CoFe MLDH/NF. The LDH was obtained due to the etching of the Co ions due to the addition of urea, in which Fe salt was gradually co-precipitated with Co ions and some anions, producing CoFe LDH.207
Figure 20.
SEM images of (a) N-PC, (b) Ti3C2 MXene, and (c) Ti3C2/N-PC. Reprinted with permission from ref (204). Copyright 2020 Elsevier. (d) Ti3C2/ZIF-67 and (e) N–Ti3C2/C, and (f) XRD plot of N–Ti3C2/C and Ti3C2. Reprinted with permission from ref (205). Copyright 2019 Elsevier.
6. Ti3C2Tx MXene-Based MOF Composites for Solar Fuel Production
In the past, there have been numerous studies conducted on Ti3C2Tx MXene-based composites and MOF-based composites for the photocatalytic production of solar fuels.208 For instance, MOFs are materials that have HOMO and LUMO separated by an energy band gap, indicating their capability to generate charge carriers upon light irradiation. Even though pristine MOFs are reported to show photocatalytic activity for solar fuel production,209,210 their efficiency is very low due to the fast recombination of charge carriers. On the other hand, due to the metallic properties of Ti3C2Tx MXene, they can act as a cocatalyst to form a Schottky junction with other photoactive materials to effectively trap photogenerated electrons. It was observed that the Schottky junction between the CdZnS/Ti3C2 nanocomposite (Figure 21a) successfully ameliorated the photocatalytic reduction of CO2 to form CH4 and CO due to a highly efficient charge carrier separation.211 Ti3C2Tx MXene-based MOF composites present a number of advantageous synergies. First, MOFs which possess high surface area and porosity are able to host MXenes and prevent the restacking and agglomeration of MXene layers. The composites are also able to show improved stability due to the synergistic effects between the surface terminal groups of MXenes and functionalities of MOFs.212 Not only that, a built-in electric field between the MXene and MOF known as the Schottky junction is induced, where efficient separation, rapid mobility, and transportation of charge carriers are promoted.213 Ti3C2Tx MXene-based MOF composites can function as attractive photocatalysts for CO2 reduction and H2 production via water splitting. However, there are only a handful of studies conducted on the composite for solar fuel production.
Figure 21.
(a) Scheme of Schottky junction between CdZnS@Ti3C2 nanocomposite for CO2 reduction. Reprinted with permission from ref (211). Copyright 2021 Elsevier. (b) Schematic diagram of the preparation of MOF-derived Co–Co LDH/Ti3C2Tx nanosheets. (c) Photocatalytic stability over Co–Co LDH/Ti3C2Tx for CO2 reduction reaction. (d) Mechanism of the CO2 reduction over the nanocomposite with [Ru(bpy)3]Cl2 photosensitizer and TEOA sacrificial agent. Reprinted with permission from ref (206). Copyright 2020 Elsevier.
Chen and co-workers prepared a Ti3C2Tx composite with MOF-derived Co–Co LDH nanosheets, as shown in Figure 21b, for visible light CO2 reduction in the presence of a [Ru(bpy)3]Cl2 photosensitizer. The bulk Ti3AlC2 MAX was subjected to etching to remove the Al layers, followed by ultrasonic exfoliation to obtain Ti3C2Tx nanosheets (TNS). Then in situ growth of ZIF-67 was conducted on the Ti3C2Tx nanosheets. Upon successful loading of ZIF-67, the nanocomposite was subjected to solvothermal treatment, in which Co–Co LDH/TNS nanosheets were obtained. Pristine Ti3C2Tx nanosheets did not show any photocatalytic CO2 reduction activity. However, upon optimal loading of 15 mg of TNS on the MOF-derived Co–Co LDH, maximum CO generation rate of 1.25 × 104 μmol h–1 g–1 was achieved. The nanocomposite also exhibited an excellent stability, maintaining good photocatalytic performance for up to five cycles, as illustrated in Figure 21c. Figure 21d depicts the photocatalytic mechanism of the Co–Co LDH/TNS nanocomposite in the presence of a photosensitizer. Upon visible light irradiation, the photosensitizer is excited but is subsequently quenched by the TEOA electron donor to form a [Ru(bpy)3]Cl2– reduced state. The electrons are then transmitted to the nanocomposite, where the electrons are rapidly migrated to the Co active sites for the reduction of CO2 to CO.206
Ti3C2Tx MXene-based composites are also widely used as catalysts for photocatalytic water splitting to produce H2. Ti3C2Tx MXenes are two-dimensional sheets that boast a large surface area/volume ratio as well as strong hydrophilicity, which improves the interaction between the photocatalyst and water molecules.214 Tian et al. modified Ti3C2Tx nanosheets by introducing a water-stable and porous Zr-based UiO-66-NH2 for efficient photocatalytic hydrogen evolution reaction. It was observed that the pristine UiO-66-NH2 MOF displayed limited H2 production at only 25.6 μmol h–1 g–1. Upon introduction of Ti3C2 nanosheets, the photocatalytic activity of the composite (denoted as TU10) increased 8-fold, achieving H2 formation of 204 μmol h–1 g–1. This is obviously due to the formation of a Schottky junction between Ti3C2Tx and UiO-66-NH2. As shown in the photoluminescence plot in Figure 22a, the Ti3C2Tx-modified UiO-66-NH2 sample showed an intensity lower than that of unmodified UiO-66-NH2, elucidating a lower recombination rate over the composite. Figure 22b illustrates the energy level diagram of TU10, in which the electrons are first excited from the VB to CB of the UiO-66-NH2, followed by migration of electrons from CB of the MOF to Ti3C2. The efficient migration and spatial separation of electrons was mainly attributed to the O-terminated Ti3C2 having a low Gibbs free energy and highly positive Fermi level, which makes it trap electrons from the MOF for H2 production.215
Figure 22.
(a) Photoluminescence plot of pristine UiO-66-NH2 and TU10. (b) Mechanism of the Schottky junction between UiO-66-NH2 and Ti3C2Tx MXenes. Reprinted with permission from ref (215). Copyright 2019 Elsevier. (c) Photocatalytic H2 production over different catalysts, (d) Proposed mechanism of the hydrogen evolution reaction over Ti3C2/TiO2/UiO-66-NH2. Reprinted with permission from ref (216). Copyright 2019 Elsevier. (e) SEM image of Ti3C2@MIL-NH2. (f) Stability test of photocatalytic H2 formation over Ti3C2@MIL-NH2. Reprinted with permission from ref (217). Copyright 2021 Elsevier.
Similarly, Ti3C2 MXenes were first annealed in a N2 atmosphere to obtain TiO2 layers, followed by coating UiO-66-NH2 on the surface of Ti3C2Tx layers, obtaining a Ti3C2/TiO2/UiO-66-NH2 composite. From the activity test shown in Figure 22c, the annealing of Ti3C2Tx to produce TiO2 increased the photocatalytic production by 1.5 times compared to that of its Ti3C2/UiO-66-NH2 counterpart. This is because the formation of TiO2 induced a negative shift in the Mott–Schottky plots, elucidating an enhanced electron/hole pair separation as well as stronger reducibility. Not only that, the intimate contact as well as the favorable Fermi level and band gaps of Ti3C2, UiO-66-NH2, and TiO2 constructed various pathways for accelerating the transfer of electron/hole pairs. As illustrated in Figure 22d, the first and second pathway is via the Schottky junction between Ti3C2 nanosheets with UiO-66-NH2 and TiO2, respectively. The third pathway is the type II heterojunction system between UiO-66-NH2 and TiO2, followed by the transfer of electrons from the CB of TiO2 to Ti3C2 MXene.216 Fascinatingly, Li and co-workers successfully coordinated the Ti atom in Ti3C2 MXenes with MIL-NH2 via in situ growth, producing a Ti3C2@MIL-NH2 composite. Compared to conventional physical mixing of Ti3C2 with MIL-NH2, the in situ grown Ti3C2@MIL-NH2 boasted a 5-fold H2 production rate compared to that of the former. This is due to the intimate contact between the Ti3C2 and MIL-NH2, as shown in the SEM image in Figure 22e. Also, the self-aggregation of the MIL-NH2 is inhibited due to the even immobilization of MIL-NH2 on the surface of the MXene. Figure 22f displays that Ti3C2@MIL-NH2 also exhibited excellent stability, where the H2 production rate showed insignificant decrease even after four cycles. The excellent photoactivity and stability are attributed to the rapid photoinduced electrons from the MOF to Ti3C2 via the Ti–N path. This in turn enriches the Ti3C2 with electrons, where H+ is effectively adsorbed and reduced to H2.217 Nevertheless, despite limited studies conducted, 2D Ti3C2 sheets coupled with MOF composites show great promise as a photocatalyst for efficient solar fuel production and should be studied intensively in future works. Table 3 presents the summary of Ti3C2 MXene-based MOF photocatalysts for the production of solar fuels.
Table 3. Summary of Ti3C2 MXene-Based MOF Photocatalyst for Production of Solar Fuels.
| catalysts | operating conditions | results | ref |
|---|---|---|---|
| MOF-derived Co–Co LDH/Ti3C2Tx | •0.5 mg of catalyst | •CO = 6.248 μmol h–1 | (206) |
| •[Ru(bpy)3]Cl2·6H2O photosensitizer | •CO = 1.25 × 104 μmol h–1 g–1 | ||
| •MeCN/H2O/TEOA = 3/2/1 mL | •AQE = 0.92% | ||
| •5 W LED lamp | |||
| •light intensity = 32.3 mW cm–2 | |||
| Ti3C2/UiO-66-NH2 | •20 mg of catalyst | •formation of Schottky junction | (215) |
| •0.1 M Na2S and 0.1 M Na2SO3 (50 mL) | •H2 = 204 μmol h–1 g–1 | ||
| •350 W Xe lamp | |||
| •atmospheric temperature and pressure | |||
| •light intensity = 10 mW cm–2 | |||
| Ti3C2/TiO2/UiO-66-NH2 | •20 mg of catalyst | •formation of Schottky junction and heterojunctions | (216) |
| •0.1 M Na2S and 0.1 M Na2SO3 (50 mL) | |||
| •300 W Xe lamp | •H2 = 1980 μmol h–1 g–1 | ||
| •T = 5 °C | |||
| •light intensity = 10 mW cm–2 | |||
| in situ grown Ti3C2@MIL-NH2 | •70 mg of catalyst | •H2 = 4383.1 μmol h–1 g–1 | (217) |
| •20 mL of CH3OH and 0.3 mL of TEOA | |||
| •300 W Xe arc lamp | |||
| •light intensity = 552 mW cm–2 | |||
| TiO2–Ti3C2–CoSx | •20 mg of catalyst | •CoSx derived from ZIF-67 | (218) |
| •40 mL of distilled water and 10 mL of methanol | |||
| •300 W Xe arc lamp | •H2 = 0.95 mmol h–1 g–1 | ||
| •UV–visible irradiation | |||
| UiO-66-NH2(Zr/Ti)/ carboxyl-functionalized MXene (UZR/CFMX) | •10 mg of catalyst | •decorated carboxyl group on Ti3C2 MXene | (219) |
| •10 vol % triethanolamine and 400 μL of H2PtCl6 | |||
| •300 W Xe lamp | •H2 = 2187 μmol g–1 h–1 |
7. Challenges and Comparative Analysis of Ti3C2Tx MXene
Here, the challenges and comparative analysis of Ti3C2 MXenes with other materials are discussed and summarized, as shown in Figure 23. Higher work function in Ti3C2Tx MXene and their role as an electron trapper gave them potential to maximize the solar fuel production. However, some challenges that require attention for employing Ti3C2Tx MXene as photoactivity enhancer include the control of their termination functional groups. The tunable termination groups, Tx, have a significant influence in the electronic and optical properties. Studies suggested that MXene materials could either exhibit semiconducting or metallic properties depending on the type of termination groups present.47 Different termination properties might affect their photocatalytic efficiency due to differences in the work function value. The difference in the work function based on terminating groups was theoretically explicated due to changes in the total surface dipole moments and the transfer of charge between active sites of Ti3C2Tx MXene with the termination groups.46 Theoretical studies divulged that the −O-terminated Ti3C2Tx MXene exhibits a work function of 5.75–6.25 eV, whereas −OH-terminated Ti3C2Tx MXene was found to possess a work function of 1.6–2.8 eV.220 In photocatalysis processes, such as that in hydrogen production, studies suggested that a large difference in the metal work function between the main catalyst and the metal cocatalyst could optimally stimulate the solar to hydrogen conversion.114 However, experimental evaluation revealed that a perfect single termination group is not possible to achieve, causing a great disparity with the theoretical analysis. Moreover, it is difficult to control the distribution of the termination groups in the MXene materials.11 Therefore, the synthesis of mixed terminating Ti3C2Tx MXene might offer different results and catalytic efficiency. Other challenges can be observed in the thicker layer of Ti3C2Tx MXene, which is found to be difficult to employ as an electron mediator, resulting in weak contact formation between two photocatalysts.183 In the context for commercializing Ti3C2Tx MXene in high-scale production, it was noticed that the yield is still low, and up to the current development, many preparation methods are still unable to develop high-yield Ti3C2Tx MXene which can par up to high scalability industrial production.
Figure 23.
Drawbacks of other cocatalysts and advantages of MXenes.
Nevertheless, in the regards of their beneficial attribution, most of the studies suggested that employing Ti3C2Tx MXene as a cocatalyst offers an indisputable and positive result in ameliorating the performances of the semiconductors.121,139,199,221 Higher metal electrical conductivity and unique properties prevailed them as one of the potentially influence materials to substitute non-economic and low efficiency cocatalysts. Moreover, a tailorable work function to fit specific application requirements is one of the distinct properties of Ti3C2Tx MXene compared to other metallic materials such as noble metals. Not to mention, their economical synthesis cost and easily accessible precursor is one of the positive attributes which leads the way for commercialization. On the other hand, Ti3C2Tx MXene material is new and is still under exploration where the their physical, catalytic, and chemical properties is still undergoing thorough research for more improvement which may facilitate more promising discoveries. Their unique morphology and possible conversion to different morphological dimension is one of the compelling characteristics of Ti3C2Tx MXene. This is due to the ability of their multilayer structure to delaminate into monolayer flakes, convert to quantum dots, and form a hierarchical structure, extending their performance in the field of photocatalysis.
Other materials that have similar properties of Ti3C2Tx MXene include noble metals such as Ag, Au, and Pt. The compelling characteristics of noble metal in the energy conversion field such as surface plasmon resonance effects and their potential to create the Schottky barrier enable them to be one of the leading metal cocatalysts. However, the main drawback in employing noble metals despite their positive influence on the photocatalytic activity is due to the exorbitant prices.222 Comparatively, Ti3C2Tx MXene is regarded as the best substitution for noble metals with similar functionality and affordable material cost, which is commercially viable. Therefore, a high-scale production of semiconductor materials is more economic by employing Ti3C2Tx MXene compared to noble metals. Moreover, the work function of Ti3C2Tx MXene is highly comparable to those of noble metals ranging from 3.9 to 6.25 eV.223 In parallel, functional properties similar to those of noble metal including the creation of a potential energy barrier between the metal–semiconductor enhance the carrier dynamics and efficiently mediate the electrons transfer.
In terms of the morphological structure, Ti3C2Tx MXenes are known to be morphologically layered with a 2D structure, which is often referred to as an accordion-like shape. The accordion-like structure of Ti3C2Tx MXene offers a large surface area compared to that of the Ti3AlC2 MAX precursor, which is bulky, compact, and has dense layers.224 Loose layers with a bigger space interval of Ti3C2Tx MXene provide efficient site attachment for other semiconductors compared to MAX material, which exhibits more packed layers. Stronger interfacial contact between the semiconductor and Ti3C2Tx MXene supports the charge transmission and expedites the redox reaction. Moreover, Ti3C2Tx MXene offers a stable platform for a uniform distribution of the particulate semiconductors and inhibits the agglomeration. In addition, the well-defined layers of Ti3C2Tx MXene with a space interval support the redox reaction taking place at the inner and outer layers.139 Even though numerous studies suggested that Ti3AlC2 MAX could promote the solar to fuel conversion, their catalytic efficiency is still not comparable to that of Ti3C2Tx MXene.225 As previously mentioned, the removal of an Al layer through chemical etching could terminate the surfaces of Ti with functional groups such as −OH, −F, and −O. These functional groups known as surface terminations provide excellent contact with water molecules and exhibit stronger hydrophilicity. Compared to Ti3AlC2 MAX, the presence of functional group such as −OH stimulate the hydrogen generation through the reduction into hydrogen through water capture.
In different material studies, similar layered structures often utilized as a cocatalyst known as layered double hydroxide exhibit the flexibility and diversity in their compositional matrix. Similar to MXene materials, LDH existed in a multilayered structure form, and their electronic properties are easily tuned according to the types of metal cations and anions present in their matrix structures.27 LDH consisted of layers that stacked onto each other, where they exhibit negatively and positively charged layer. Generally, the positively charged layer of LDH consisted of metal cations while the negatively charged layers, known as interlamellar spaces, are composed of the intercalated anions. Therefore, the unique structure of LDHs attracts research attention to further explore their potential in photocatalytic fuel conversion field. However, the LDH structure, the metal coordination, and their nature need to be meticulously studied. In addition, their complicated structure and design criterion are some of the great challenges in employing LDH as a cocatalyst. Some criteria that require extra attention in designing LDH material include the nature of metal cations incorporated into the host layer of LDH, the compositional control of each metal cations, and their valence states and the anion intercalation. The mentioned design parameters could affect the fabrication of the LDH materials and their photocatalytic efficiency.226 For instance, mismatched metal cation pairing will lower the photocatalytic performances, and some might be inactive. Even though their energy gap can be tuned to meet specific application requirements, their complicated synthesis process and design criteria limit their role as compelling semiconductors. Therefore, the best alternative is to employ Ti3C2Tx MXene, which is economically affordable, is easily fabricated, and exhibits tunable electronic properties, unique structural properties, and excellent photocatalytic efficiency.
8. Conclusion
In conclusion, Ti3C2Tx MXenes are a class of materials that has seen widespread use in the field of photocatalysis. MXenes tend to exist in 0D quantum dots but usually adopt 2D layered morphologies that are able to provide a high surface area/volume ratio. However, pristine MXenes are unable to initiate any photocatalytic activity for solar fuel production as they do not have energy band gaps. The absence of a band gap means that there will not be any formation of electron/hole pairs despite light irradiation. Nevertheless, Ti3C2Tx is an excellent cocatalyst for photocatalytic fuel production due to its metallic properties, which allows it to function as an efficient electron sink and trap. Ti3C2Tx MXenes are usually hybridized with other materials containing energy band gaps such as semiconductors. This is due to the photogenerated electrons from the CB of the semiconductor to the Ti3C2Tx MXene, which has a more positive Fermi level. The efficient transfer of electrons will then ameliorate and prolong the lifetime of charge carriers, enhancing the photocatalytic formation of solar fuels.
The employment of Ti3C2Tx MXene in the solar fuel conversion field offers a sustainable approach for producing a clean and renewable energy source. Excellent metallic electrical conductivity of Ti3C2Tx MXene endows them with the ability to construct an electrostatic potential barrier which aids in the improvement of carrier dynamics and elevates their potential as highly sought-after cocatalysts. Mainly, HF etching is the pioneering technique for synthesizing Ti3C2Tx MXene with a −F termination group. However, their prominent role as a photocatalytic driver led to the development of various synthesis techniques, which facilitate tailoring their surface terminating groups. It is worth mentioning the blossoming of the synthesis techniques favoring the morphological development of Ti3C2Tx MXene into different structural growth. Tailorable functional groups with modifiable structure boosted their photoredox activity and improved the overall photocatalytic efficiency.
Metal–organic frameworks are another class of materials that possess a wide range of unique properties that make them promising candidates as photocatalysts. First, they have an extremely high surface area, usually in the range of a few thousand square meters per gram. Pores with large volume and uniform size are riddled all over the surface of the MOF, allowing them to effectively adsorb small gas molecules as well as large dye molecules. Interestingly, despite the usual 3D polyhedrons of MOFs, they can also naturally exist in 1D rods or 2D sheets, as well. These multidimensional MOFs can be obtained via different synthesis steps, namely, the solvothermal synthesis, the co-precipitation method, the slow evaporation method, and the microwave-assisted approach. Under solar energy irradiation, MOFs are able to generate photoinduced charge carriers. This is because they have a narrow energy band gap, where electrons can be excited from the HOMO to LUMO when photons with sufficient energy strike the surface of the MOF. Finally, MOFs can function as a sacrificial template to form other derivatives. Metal oxides are derived when MOFs are subjected to thermal treatment, whereas LDHs can be obtained when the MOFs undergo the hydrolyzed etching step.
In an effort to further improve the photocatalytic activity of both Ti3C2Tx MXenes and MOFs, various design principles and considerations were taken into account. In this context, the design considerations were discussed in three main criteria, namely, the morphological engineering of Ti3C2Tx and MOFs, the active sites over Ti3C2Tx-based MOF nanocomposites, and the interfacial/heterojunction engineering. In recent years, huge strides have been made in modifying the conventional 2D accordion-like multilayer of Ti3C2Tx, such as the formation of 0D quantum dots, 2D delaminated flakes, and 3D multidimensional structures. These can be achieved via various approaches such as delamination with or without intercalating agents and combining with various domains of different dimensions. Similarly, the morphology and dimensions of MOFs can be tuned between 1D rods, 2D sheets, or 3D polyhedrons by adjusting the synthesis parameters such as reaction time, temperature, and solvent ratio. Also, the use of surfactants such as polyvinylpyrrolidone and cetyltrimethylammonium bromide can effectively modulate the structure of MOFs. The active sites of MOFs were also discussed, where active sites can be found naturally on the MOF or by further introduction of the MOF by anchoring them on the organic ligands or cavity of the MOF.
The metallic nature of Ti3C2Tx MXenes make them highly promising cocatalysts due to their ability to trap electrons via the formation of a Schottky barrier with other photoresponsive materials. Interestingly, a Ti3C2Tx MXene–TiO2 hybrid could be generated as a result of oxidation, opening the door to hybrid Schottky–Z-scheme/Schottky–type II heterojunction systems. Also, there are reports that Ti3C2Tx can also serve as an electron mediator for Z-scheme heterojunction systems. Similar to semiconductors, MOFs are able to generate electron/hole pairs upon light irradiation, making them serviceable photocatalysts. However, their efficiency can be further improved via a series of strategies, such as formation of heterojunctions with other photoresponsive materials, surface sensitization with materials with metallic properties, functionalization of MOFs, doping, and use of photosensitizers. By deploying these strategies, the photocatalytic activity of MOFs can be enhanced as the light harvesting especially in the visible spectrum is ameliorated, and a narrower Ebg is achieved, and spatial charge carrier separation is realized. By preparing Ti3C2Tx MXene-based MOF photocatalysts, the photocatalytic activity can be effectively enhanced due to various synergistic effects such as boosted stability and formation of a built-in electric field known as the Schottky junction. Despite promising findings from Ti3C2-based MOF composites for solar fuel production, there is still very limited studies conducted. Hence, it is recommended to further explore Ti3C2 with various MOFs for photocatalytic CO2 reduction and water splitting reactions.
9. Future Perspective
In short, Ti3C2Tx and MOFs have made huge progress over the years. For instance, various new synthesis approaches were discovered, in turn producing a wide range of structures for both Ti3C2Tx MXene and MOFs. Similarly, both materials have seen an increasing role as photocatalysts in the production of renewable solar fuels. Hence, Ti3C2Tx MXene-based MOF nanotextures are highly promising photocatalysts for the production of green and renewable solar fuels. This is due to the synergistic effect between the two materials, in which MOFs are able to generate electron/hole pairs, whereas MXenes can effectively trap the electrons. This in turn will prolong the lifetime of charge carriers and ameliorate the production of solar-driven fuels. However, up to now, there is only a handful of research done on Ti3C2 MXene-based MOF composite on the applications of CO2 reduction and solar-driven H2 production. Thus, the future prospects are as follows:
-
1.
Different type of MXenes as well as morphologies should be utilized for the photocatalytic production of solar fuels. For example, different MXenes such as Nb2C, V2C, and Mo2C should be explored as cocatalysts for renewable fuel production. Other than 2D accordion layered structures of MXenes, facile synthesis approaches for 0D MXene quantum dots and exfoliated monolayer MXenes should also be researched intensively.
-
2.
The exact active sites of Ti3C2Tx MXene are yet to be confirmed. It is suggested for more studies to be conducted to identify, quantify, and gain a deeper understanding on the active sites over MXenes. Also, the study on the active sites can provide more insights into ways to further boost the role of Ti3C2Tx MXene as a cocatalyst for solar fuel production.
-
3.
Over the years, MXenes are mainly prepared using HF etching, which is detrimental to the environment due to the use of harsh chemicals. Also, formation of QDs of monolayered MXenes utilizes various intercalating agents like DMSO TMAOH, which may pose environmental threats. Therefore, more research must be conducted on the green synthesis of MXenes to reduce the negative impact on the environment.
-
4.
A solvothermal synthesis method is most commonly used to prepare a wide range of MOFs. However, they are severely limited by their long reaction time, inhomogeneous heating, and huge heat loss. Thus, it is recommended that more studies be conducted on other synthesis methods such as the microwave-assisted method to not only speed up the preparation period but also improve the efficiency and homogeneity of heating.
-
5.
More combinations of MOFs must be explored by either experimenting with different metal nodes, metal clusters, or linkers to obtain MOFs with enhanced photocatalytic activity for solar fuel production. Also, energy band gap engineering should also be conducted to further improve the light harvesting and utilization of MOFs especially in the visible light spectrum.
-
6.
Lastly, more research is needed on Ti3C2Tx MXene-based MOF nanotextures to produce renewable solar fuels. Despite its promising properties and characteristics, there are still limited studies on MXene/MOF for CO2 reduction and production of H2 solar fuels. Different types of MOFs can be paired together with Ti3C2Tx MXene to further unveil and gain a better understanding on the interaction and mechanism of the overall photocatalytic process.
Acknowledgments
The financial supports for this work were provided by Ministry of Higher Education (MOHE) under Fundamental Research Grant Scheme (FRGS, R.J130000.7351.4L952).
The authors declare no competing financial interest.
References
- Lingampalli S. R.; Ayyub M. M.; Rao C. N. R. Recent Progress in the Photocatalytic Reduction of Carbon Dioxide. ACS Omega 2017, 2 (6), 2740–2748. 10.1021/acsomega.7b00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W. K.; Tahir M. Structured clay minerals-based nanomaterials for sustainable photo/thermal carbon dioxide conversion to cleaner fuels: A critical review. Sci. Total Environ. 2022, 845, 157206. 10.1016/j.scitotenv.2022.157206. [DOI] [PubMed] [Google Scholar]
- Ranjekar A. M.; Yadav G. D. Steam Reforming of Methanol for Hydrogen Production: A Critical Analysis of Catalysis, Processes, and Scope. Ind. Eng. Chem. Res. 2021, 60 (1), 89–113. 10.1021/acs.iecr.0c05041. [DOI] [Google Scholar]
- Fan W. K.; Tahir M. Investigating the product distribution behaviour of CO2 methanation through thermodynamic optimized experimental approach using micro/nano structured titania catalyst. Energy Convers. Manage. 2022, 254, 115240. 10.1016/j.enconman.2022.115240. [DOI] [Google Scholar]
- Hurtado L.; Mohan A.; Ulmer U.; Natividad R.; Tountas A. A.; Sun W.; Wang L.; Kim B.; Sain M. M.; Ozin G. A. Solar CO2 hydrogenation by photocatalytic foams. Chem. Eng. J. 2022, 435, 134864. 10.1016/j.cej.2022.134864. [DOI] [Google Scholar]
- Qureshi W. A.; Hong X.; He X.; Liu Q.; Xu D.; Maouche C.; Sun Z.; Yang J. Dual plasmonic Au and TiN cocatalysts to boost photocatalytic hydrogen evolution. Chemosphere 2022, 291, 132987. 10.1016/j.chemosphere.2021.132987. [DOI] [PubMed] [Google Scholar]
- Tahir M.; Fan W. K.; Hasan M. Investigating influential effect of methanol-phenol-steam mixture on hydrogen production through thermodynamic analysis with experimental evaluation. Int. J. Energy Res. 2022, 46, 964–979. 10.1002/er.7216. [DOI] [Google Scholar]
- Li G.; Yang C.; He Q.; Liu J. Ag-based photocatalytic heterostructures: Construction and photocatalytic energy conversion application. J. Environ. Chem. Eng. 2022, 10, 107374. 10.1016/j.jece.2022.107374. [DOI] [Google Scholar]
- Tasleem S.; Tahir M. Investigating the performance of liquid and gas phase photoreactors for dynamic H2 production over bimetallic TiO2 and Ni2P dispersed MAX Ti3AlC2 monolithic nanocomposite under UV and visible light. J. Environ. Chem. Eng. 2021, 9 (4), 105351. 10.1016/j.jece.2021.105351. [DOI] [Google Scholar]
- Zhang C.; Zhang W.; Qian J.; Cheng H.; Ren S.; Zhang C.; Ma J.; Guo Z. Simple Preparation of Hierarchically Porous Ce/TiO2/Graphitic Carbon Microspheres for the Reduction of CO2 with H2O under Simulated Solar Irradiation. ACS Omega 2019, 4 (16), 16833–16839. 10.1021/acsomega.9b01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib M.; Kurtoglu M.; Presser V.; Lu J.; Niu J.; Heon M.; Hultman L.; Gogotsi Y.; Barsoum M. W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23 (37), 4248–53. 10.1002/adma.201102306. [DOI] [PubMed] [Google Scholar]
- Yang W.; Ma G.; Fu Y.; Peng K.; Yang H.; Zhan X.; Yang W.; Wang L.; Hou H. Rationally designed Ti3C2 MXene@TiO2/CuInS2 Schottky/S-scheme integrated heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 429, 132381. 10.1016/j.cej.2021.132381. [DOI] [Google Scholar]
- Biswal L.; Mohanty R.; Nayak S.; Parida K. Review on MXene/TiO2 nanohybrids for photocatalytic hydrogen production and pollutant degradations. J. Environ. Chem. Eng. 2022, 10 (2), 107211. 10.1016/j.jece.2022.107211. [DOI] [Google Scholar]
- Tahir M.; Ali Khan A.; Tasleem S.; Mansoor R.; Fan W. K. Titanium Carbide (Ti3C2) MXene as a Promising Co-catalyst for Photocatalytic CO2 Conversion to Energy-Efficient Fuels: A Review. Energy Fuels 2021, 35 (13), 10374–10404. 10.1021/acs.energyfuels.1c00958. [DOI] [Google Scholar]
- Sherryna A.; Tahir M. Role of Ti3C2 MXene as Prominent Schottky Barriers in Driving Hydrogen Production through Photoinduced Water Splitting: A Comprehensive Review. ACS Appl. Energy Mater. 2021, 4 (11), 11982–12006. 10.1021/acsaem.1c02241. [DOI] [Google Scholar]
- Tariq A.; Ali S. I.; Akinwande D.; Rizwan S. Efficient Visible-Light Photocatalysis of 2D-MXene Nanohybrids with Gd(3+)- and Sn(4+)-Codoped Bismuth Ferrite. ACS Omega 2018, 3 (10), 13828–13836. 10.1021/acsomega.8b01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. P.; Tuan Nguyen D. M.; Tran D. L.; Le H. K.; Vo D.-V. N.; Lam S. S.; Varma R. S.; Shokouhimehr M.; Nguyen C. C.; Le Q. V. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. 10.1016/j.mcat.2020.110850. [DOI] [Google Scholar]
- Xia B.; Yang Y.; Zhang Y.; Xia Y.; Jaroniec M.; Yu J.; Ran J.; Qiao S.-Z. Metal–organic framework with atomically dispersed Ni–N4 sites for greatly-raised visible-light photocatalytic H2 production. Chem. Eng. J. 2022, 431, 133944. 10.1016/j.cej.2021.133944. [DOI] [Google Scholar]
- Li H.; Eddaoudi M.; O’Keeffe M.; Yaghi O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402 (6759), 276–279. 10.1038/46248. [DOI] [Google Scholar]
- Yang X.; Wen Z.; Wu Z.; Luo X. Synthesis of ZnO/ZIF-8 hybrid photocatalysts derived from ZIF-8 with enhanced photocatalytic activity. Inorg. Chem. Front. 2018, 5 (3), 687–693. 10.1039/C7QI00752C. [DOI] [Google Scholar]
- Chen D.; Xing H.; Wang C.; Su Z. Highly efficient visible-light-driven CO2 reduction to formate by a new anthracene-based zirconium MOF via dual catalytic routes. J. Mater. Chem. A 2016, 4 (7), 2657–2662. 10.1039/C6TA00429F. [DOI] [Google Scholar]
- Fan W. K.; Tahir M. Current Trends and Approaches to Boost the Performance of Metal Organic Frameworks for Carbon Dioxide Methanation through Photo/Thermal Hydrogenation: A Review. Ind. Eng. Chem. Res. 2021, 60 (36), 13149–13179. 10.1021/acs.iecr.1c02058. [DOI] [Google Scholar]
- Huang K.; Li C.; Li H.; Ren G.; Wang L.; Wang W.; Meng X. Photocatalytic Applications of Two-Dimensional Ti3C2 MXenes: A Review. ACS Appl. Nano Mater. 2020, 3 (10), 9581–9603. 10.1021/acsanm.0c02481. [DOI] [Google Scholar]
- Tang R.; Xiong S.; Gong D.; Deng Y.; Wang Y.; Su L.; Ding C.; Yang L.; Liao C. Ti3C2 2D MXene: Recent Progress and Perspectives in Photocatalysis. ACS Appl. Mater. Interfaces 2020, 12 (51), 56663–56680. 10.1021/acsami.0c12905. [DOI] [PubMed] [Google Scholar]
- Li Y.; Ding L.; Guo Y.; Liang Z.; Cui H.; Tian J. Boosting the Photocatalytic Ability of g-C3N4 for Hydrogen Production by Ti3C2 MXene Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11 (44), 41440–41447. 10.1021/acsami.9b14985. [DOI] [PubMed] [Google Scholar]
- He F.; Zhu B.; Cheng B.; Yu J.; Ho W.; Macyk W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal., B 2020, 272, 119006. 10.1016/j.apcatb.2020.119006. [DOI] [Google Scholar]
- Sherryna A.; Tahir M.; Nabgan W. Recent advancements of layered double hydroxide heterojunction composites with engineering approach towards photocatalytic hydrogen production: A review. Int. J. Hydrogen Energy 2022, 47 (2), 862–901. 10.1016/j.ijhydene.2021.10.099. [DOI] [Google Scholar]
- Fan W. K.; Tahir M. Recent developments in photothermal reactors with understanding on the role of light/heat for CO2 hydrogenation to fuels: A review. Chem. Eng. J. 2022, 427, 131617. 10.1016/j.cej.2021.131617. [DOI] [Google Scholar]
- Li X.; Yu J.; Jaroniec M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45 (9), 2603–2636. 10.1039/C5CS00838G. [DOI] [PubMed] [Google Scholar]
- Fan W. K.; Tahir M. Recent advances on cobalt metal organic frameworks (MOFs) for photocatalytic CO2 reduction to renewable energy and fuels: A review on current progress and future directions. Energy Convers. Manage. 2022, 253, 115180. 10.1016/j.enconman.2021.115180. [DOI] [Google Scholar]
- Tahir M.; Fan W. K.; Tahir B.. MOF-Based Catalysts for Production of Value-Added Fine Chemicals from Carbon Dioxide. Metal–Organic Frameworks for Carbon Capture and Energy; American Chemical Society, 2021; pp 155–171. [Google Scholar]
- Khan A. A.; Tahir M. Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. 10.1016/j.jcou.2018.12.008. [DOI] [Google Scholar]
- Zhang W.; Mohamed A. R.; Ong W. J. Z-Scheme Photocatalytic Systems for Carbon Dioxide Reduction: Where Are We Now?. Angew. Chem., Int. Ed. 2020, 59 (51), 22894–22915. 10.1002/anie.201914925. [DOI] [PubMed] [Google Scholar]
- Costentin C.; Robert M.; Savéant J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42 (6), 2423–2436. 10.1039/C2CS35360A. [DOI] [PubMed] [Google Scholar]
- Habisreutinger S. N.; Schmidt-Mende L.; Stolarczyk J. K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem., Int. Ed. 2013, 52 (29), 7372–7408. 10.1002/anie.201207199. [DOI] [PubMed] [Google Scholar]
- Barton Cole E.; Lakkaraju P. S.; Rampulla D. M.; Morris A. J.; Abelev E.; Bocarsly A. B. Using a one-electron shuttle for the multielectron reduction of CO2 to methanol: kinetic, mechanistic, and structural insights. J. Am. Chem. Soc. 2010, 132 (33), 11539–11551. 10.1021/ja1023496. [DOI] [PubMed] [Google Scholar]
- Sherryna A.; Tahir M. Role of surface morphology and terminating groups in titanium carbide MXenes (Ti3C2Tx) cocatalysts with engineering aspects for modulating solar hydrogen production: A critical review. Chem. Eng. J. 2022, 433, 134573. 10.1016/j.cej.2022.134573. [DOI] [Google Scholar]
- Huang S.; Long Y.; Ruan S.; Zeng Y. J. Enhanced Photocatalytic CO2 Reduction in Defect-Engineered Z-Scheme WO3-x/g-C3N4 Heterostructures. ACS Omega 2019, 4 (13), 15593–15599. 10.1021/acsomega.9b01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoko A. D.; Steinmann S. N.; Seh Z. W. Theory-guided materials design: two-dimensional MXenes in electro- and photocatalysis. Nanoscale Horizons 2019, 4 (4), 809–827. 10.1039/C9NH00100J. [DOI] [Google Scholar]
- Li Z.; Wu Y. 2D Early Transition Metal Carbides (MXenes) for Catalysis. Small 2019, 15 (29), 1804736. 10.1002/smll.201804736. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Kong N.; Uzun S.; Levitt A.; Seyedin S.; Lynch P. A.; Qin S.; Han M.; Yang W.; Liu J.; Wang X.; Gogotsi Y.; Razal J. M. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32 (23), 2001093. 10.1002/adma.202001093. [DOI] [PubMed] [Google Scholar]
- Lipatov A.; Goad A.; Loes M. J.; Vorobeva N. S.; Abourahma J.; Gogotsi Y.; Sinitskii A. High electrical conductivity and breakdown current density of individual monolayer Ti3C2T MXene flakes. Matter 2021, 4 (4), 1413–1427. 10.1016/j.matt.2021.01.021. [DOI] [Google Scholar]
- Uda M.; Nakamura A.; Yamamoto T.; Fujimoto Y. Work function of polycrystalline Ag, Au and Al. J. Electron Spectrosc. Relat. Phenom. 1998, 88–91, 643–648. 10.1016/S0368-2048(97)00236-3. [DOI] [Google Scholar]
- Agresti A.; Pazniak A.; Pescetelli S.; Di Vito A.; Rossi D.; Pecchia A.; Auf der Maur M.; Liedl A.; Larciprete R.; Kuznetsov D. V.; Saranin D.; Di Carlo A. Titanium-carbide MXenes for work function and interface engineering in perovskite solar cells. Nat. Mater. 2019, 18 (11), 1228–1234. 10.1038/s41563-019-0478-1. [DOI] [PubMed] [Google Scholar]
- Derry G. N.; Ji-Zhong Z. Work function of Pt(111). Phys. Rev. B Condens Matter 1989, 39 (3), 1940–1941. 10.1103/PhysRevB.39.1940. [DOI] [PubMed] [Google Scholar]
- Chertopalov S.; Mochalin V. N. Environment-Sensitive Photoresponse of Spontaneously Partially Oxidized Ti3C2 MXene Thin Films. ACS Nano 2018, 12 (6), 6109–6116. 10.1021/acsnano.8b02379. [DOI] [PubMed] [Google Scholar]
- Khazaei M.; Ranjbar A.; Arai M.; Sasaki T.; Yunoki S. Electronic properties and applications of MXenes: a theoretical review. Journal of Materials Chemistry C 2017, 5 (10), 2488–2503. 10.1039/C7TC00140A. [DOI] [Google Scholar]
- Dutta A.; Pan Y.; Liu J.-Q.; Kumar A. Multicomponent isoreticular metal-organic frameworks: Principles, current status and challenges. Coord. Chem. Rev. 2021, 445, 214074. 10.1016/j.ccr.2021.214074. [DOI] [Google Scholar]
- Yoo D. K.; Bhadra B. N.; Jhung S. H. Adsorptive removal of hazardous organics from water and fuel with functionalized metal-organic frameworks: Contribution of functional groups. J. Hazard. Mater. 2021, 403, 123655. 10.1016/j.jhazmat.2020.123655. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Dai H.; Huang X.; Ren Y.; Wang Q.; Wang W.; Huang W.; Dong X. Porous trimetallic fluoride Ni–Co–M (M = Mn, Fe, Cu, Zn) nanoprisms as electrodes for asymmetric supercapacitors. Materials Today Energy 2020, 17, 100429. 10.1016/j.mtener.2020.100429. [DOI] [Google Scholar]
- Kang C.; Ma L.; Chen Y.; Fu L.; Hu Q.; Zhou C.; Liu Q. Metal-organic framework derived hollow rod-like NiCoMn ternary metal sulfide for high-performance asymmetric supercapacitors. Chem. Eng. J. 2022, 427, 131003. 10.1016/j.cej.2021.131003. [DOI] [Google Scholar]
- Hao R.; Chen J.; Wang Z.; Huang Y.; Liu P.; Yan J.; Liu K.; Liu C.; Lu Z. Trimetallic Zeolitic imidazolite framework-derived Co nanoparticles@CoFe-nitrogen-doped porous carbon as bifunctional electrocatalysts for Zn-air battery. J. Colloid Interface Sci. 2021, 586, 621–629. 10.1016/j.jcis.2020.10.130. [DOI] [PubMed] [Google Scholar]
- Ikreedeegh R. R.; Tahir M. Indirect Z-scheme heterojunction of NH2-MIL-125(Ti) MOF/g-C3N4 nanocomposite with RGO solid electron mediator for efficient photocatalytic CO2 reduction to CO and CH4. J. Environ. Chem. Eng. 2021, 9 (4), 105600. 10.1016/j.jece.2021.105600. [DOI] [Google Scholar]
- Guo Y.; Feng C.; Qiao S.; Wang S.; Chen T.; Zhang L.; Zhao Y.; Wang J. Magnetic Fe3O4-encapsulated VAN@ MIL-101 (Fe) with mixed-valence sites and mesoporous structures as efficient bifunctional water splitting photocatalysts. Nanoscale 2020, 12 (23), 12551–12560. 10.1039/D0NR02230F. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Wang Y.; Li Z.; Song Y.; Zhang J.; Wang J.; He X.; Wang C.; Lin W. Highly Dispersed Ni Catalyst on Metal-Organic Framework-Derived Porous Hydrous Zirconia for CO2 Methanation. ACS Appl. Mater. Interfaces 2020, 12 (15), 17436–17442. 10.1021/acsami.9b23277. [DOI] [PubMed] [Google Scholar]
- Li X.; Yang X.; Xue H.; Pang H.; Xu Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem. 2020, 2 (2), 100027. 10.1016/j.enchem.2020.100027. [DOI] [Google Scholar]
- Sreedhar I.; Varun Y.; Singh S. A.; Venugopal A.; Reddy B. M. Developmental trends in CO2 methanation using various catalysts. Catal. Sci. Technol. 2019, 9 (17), 4478–4504. 10.1039/C9CY01234F. [DOI] [Google Scholar]
- Yan S.; Ouyang S.; Xu H.; Zhao M.; Zhang X.; Ye J. Co-ZIF-9/TiO2 nanostructure for superior CO2 photoreduction activity. J. Mater. Chem. A 2016, 4 (39), 15126–15133. 10.1039/C6TA04620G. [DOI] [Google Scholar]
- Dou Y.; Zhou J.; Zhou A.; Li J.-R.; Nie Z. Visible-light responsive MOF encapsulation of noble-metal-sensitized semiconductors for high-performance photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5 (36), 19491–19498. 10.1039/C7TA06443H. [DOI] [Google Scholar]
- Mahmoud M. S.; Ahmed E.; Farghali A. A.; Zaki A. H.; Abdelghani E. A. M.; Barakat N. A. M. Influence of Mn, Cu, and Cd–doping for titanium oxide nanotubes on the photocatalytic activity toward water splitting under visible light irradiation. Colloids Surf., A 2018, 554, 100–109. 10.1016/j.colsurfa.2018.06.039. [DOI] [Google Scholar]
- Deria P.; Yu J.; Balaraman R. P.; Mashni J.; White S. N. Topology-dependent emissive properties of zirconium-based porphyrin MOFs. Chem. Commun. (Camb) 2016, 52 (88), 13031–13034. 10.1039/C6CC07343C. [DOI] [PubMed] [Google Scholar]
- Shi L.; Wang T.; Zhang H.; Chang K.; Ye J. Electrostatic Self-Assembly of Nanosized Carbon Nitride Nanosheet onto a Zirconium Metal-Organic Framework for Enhanced Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2015, 25 (33), 5360–5367. 10.1002/adfm.201502253. [DOI] [Google Scholar]
- Katz M. J.; Brown Z. J.; Colon Y. J.; Siu P. W.; Scheidt K. A.; Snurr R. Q.; Hupp J. T.; Farha O. K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49 (82), 9449–9451. 10.1039/c3cc46105j. [DOI] [PubMed] [Google Scholar]
- Ikreedeegh R. R.; Tahir M. A critical review in recent developments of metal-organic-frameworks (MOFs) with band engineering alteration for photocatalytic CO2 reduction to solar fuels. J. CO2 Util. 2021, 43, 101381. 10.1016/j.jcou.2020.101381. [DOI] [Google Scholar]
- Chen X.; Peng X.; Jiang L.; Yuan X.; Yu H.; Wang H.; Zhang J.; Xia Q. Recent advances in titanium metal–organic frameworks and their derived materials: Features, fabrication, and photocatalytic applications. Chem. Eng. J. 2020, 395, 125080. 10.1016/j.cej.2020.125080. [DOI] [Google Scholar]
- Kim S.-N.; Kim J.; Kim H.-Y.; Cho H.-Y.; Ahn W.-S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. 10.1016/j.cattod.2012.08.014. [DOI] [Google Scholar]
- Bavykina A.; Kolobov N.; Khan I. S.; Bau J. A.; Ramirez A.; Gascon J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120 (16), 8468–8535. 10.1021/acs.chemrev.9b00685. [DOI] [PubMed] [Google Scholar]
- Su X.; Bromberg L.; Martis V.; Simeon F.; Huq A.; Hatton T. A. Postsynthetic Functionalization of Mg-MOF-74 with Tetraethylenepentamine: Structural Characterization and Enhanced CO2 Adsorption. ACS Appl. Mater. Interfaces 2017, 9 (12), 11299–11306. 10.1021/acsami.7b02471. [DOI] [PubMed] [Google Scholar]
- Baamran K. S.; Tahir M.; Mohamed M.; Hussain Khoja A. Effect of support size for stimulating hydrogen production in phenol steam reforming using Ni-embedded TiO2 nanocatalyst. J. Environ. Chem. Eng. 2020, 8 (1), 103604. 10.1016/j.jece.2019.103604. [DOI] [Google Scholar]
- Xie Y.; Fang Z.; Li L.; Yang H.; Liu T. F. Creating Chemisorption Sites for Enhanced CO2 Photoreduction Activity through Alkylamine Modification of MIL-101-Cr. ACS Appl. Mater. Interfaces 2019, 11 (30), 27017–27023. 10.1021/acsami.9b09436. [DOI] [PubMed] [Google Scholar]
- Nimbalkar M. N.; Bhat B. R. Simultaneous adsorption of methylene blue and heavy metals from water using Zr-MOF having free carboxylic group. J. Environ. Chem. Eng. 2021, 9 (5), 106216. 10.1016/j.jece.2021.106216. [DOI] [Google Scholar]
- Nguyen K. D.; Ehrling S.; Senkovska I.; Bon V.; Kaskel S. New 1D chiral Zr-MOFs based on in situ imine linker formation as catalysts for asymmetric C C coupling reactions. J. Catal. 2020, 386, 106–116. 10.1016/j.jcat.2020.03.034. [DOI] [Google Scholar]
- Zhang M.; Feng G.; Song Z.; Zhou Y. P.; Chao H. Y.; Yuan D.; Tan T. T.; Guo Z.; Hu Z.; Tang B. Z.; Liu B.; Zhao D. Two-dimensional metal-organic framework with wide channels and responsive turn-on fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc. 2014, 136 (20), 7241–4. 10.1021/ja502643p. [DOI] [PubMed] [Google Scholar]
- Assi H.; Pardo Pérez L. C.; Mouchaham G.; Ragon F.; Nasalevich M.; Guillou N.; Martineau C.; Chevreau H.; Kapteijn F.; Gascon J.; et al. Investigating the case of titanium (IV) carboxyphenolate photoactive coordination polymers. Inorg. Chem. 2016, 55 (15), 7192–7199. 10.1021/acs.inorgchem.6b01060. [DOI] [PubMed] [Google Scholar]
- Kashyap A.; Singh N. K.; Soni M.; Soni A.. Deposition of thin films by chemical solution-assisted techniques. Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Elsevier, 2021; pp 79–117. [Google Scholar]
- Sun D.; Ye L.; Li Z. Visible-light-assisted aerobic photocatalytic oxidation of amines to imines over NH2-MIL-125(Ti). Appl. Catal., B 2015, 164, 428–432. 10.1016/j.apcatb.2014.09.054. [DOI] [Google Scholar]
- Xu W.; Xue W.; Huang H.; Wang J.; Zhong C.; Mei D. Morphology controlled synthesis of α-Fe2O3-x with benzimidazole-modified Fe-MOFs for enhanced photo-Fenton-like catalysis. Appl. Catal., B 2021, 291, 120129. 10.1016/j.apcatb.2021.120129. [DOI] [Google Scholar]
- González C. M. O.; Morales E. M. C.; Tellez A. d. M. N.; Quezada T. E. S.; Kharissova O. V.; Méndez-Rojas M. A.. CO2 Capture by MOFs. Handbook of Greener Synthesis of Nanomaterials and Compounds; Elsevier, 2021; pp 407–448. [Google Scholar]
- Kumar V.; Mohapatra T.; Dharmadhikari S.; Ghosh P. A Review Paper on Heterogeneous Fenton Catalyst: Types of Preparation, Modification Techniques, Factors Affecting the Synthesis, Characterization, and Application in the Wastewater Treatment. Bull. Chem. React. Eng. Catal. 2020, 15 (1), 1–34. 10.9767/bcrec.15.1.4374.1-34. [DOI] [Google Scholar]
- Chen X.; Li Q.; Zhang M.; Li J.; Cai S.; Chen J.; Jia H. MOF-Templated Preparation of Highly Dispersed Co/Al2O3 Composite as the Photothermal Catalyst with High Solar-to-Fuel Efficiency for CO2 Methanation. ACS Appl. Mater. Interfaces 2020, 12 (35), 39304–39317. 10.1021/acsami.0c11576. [DOI] [PubMed] [Google Scholar]
- Ghosh M. K.; Pathak S.; Ghorai T. K. Synthesis of Two Mononuclear Schiff Base Metal (M = Fe, Cu) Complexes: MOF Structure, Dye Degradation, H2O2 Sensing, and DNA Binding Property. ACS Omega 2019, 4 (14), 16068–16079. 10.1021/acsomega.9b02268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murinzi T. W.; Hosten E.; Watkins G. M. Synthesis and characterization of a cobalt-2,6-pyridinedicarboxylate MOF with potential application in electrochemical sensing. Polyhedron 2017, 137, 188–196. 10.1016/j.poly.2017.08.030. [DOI] [Google Scholar]
- Hindelang K.; Vagin S. I.; Anger C.; Rieger B. Tandem post-synthetic modification for functionalized metal-organic frameworks via epoxidation and subsequent epoxide ring-opening. Chem. Commun. (Camb) 2012, 48 (23), 2888–90. 10.1039/c2cc16949e. [DOI] [PubMed] [Google Scholar]
- Vakili R.; Xu S.; Al-Janabi N.; Gorgojo P.; Holmes S. M.; Fan X. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): Optimization and gas adsorption. Microporous Mesoporous Mater. 2018, 260, 45–53. 10.1016/j.micromeso.2017.10.028. [DOI] [Google Scholar]
- Chen X.; Zhu H. Catalysis by supported gold nanoparticles. Comprehensive nanoscience and technology 2011, 1–10. 10.1016/B978-0-12-374396-1.00095-7. [DOI] [Google Scholar]
- Mu X.; Jiang J.; Chao F.; Lou Y.; Chen J. Ligand modification of UiO-66 with an unusual visible light photocatalytic behavior for RhB degradation. Dalton Trans 2018, 47 (6), 1895–1902. 10.1039/C7DT04477A. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhou J.; Chen X.; Feng Q.; Cai W. MOF-derived C-doped ZnO composites for enhanced photocatalytic performance under visible light. J. Alloys Compd. 2019, 777, 109–118. 10.1016/j.jallcom.2018.10.383. [DOI] [Google Scholar]
- Dong H.; Zhang X.; Lu Y.; Yang Y.; Zhang Y.-P.; Tang H.-L.; Zhang F.-M.; Yang Z.-D.; Sun X.; Feng Y. Regulation of metal ions in smart metal-cluster nodes of metal-organic frameworks with open metal sites for improved photocatalytic CO2 reduction reaction. Appl. Catal., B 2020, 276, 119173. 10.1016/j.apcatb.2020.119173. [DOI] [Google Scholar]
- Zhao X.; Wu W.; Jing G.; Zhou Z. Activation of sulfite autoxidation with CuFe2O4 prepared by MOF-templated method for abatement of organic contaminants. Environ. Pollut. 2020, 260, 114038. 10.1016/j.envpol.2020.114038. [DOI] [PubMed] [Google Scholar]
- Li Y.; Xia Y.; Liu K.; Ye K.; Wang Q.; Zhang S.; Huang Y.; Liu H. Constructing Fe-MOF-Derived Z-Scheme Photocatalysts with Enhanced Charge Transport: Nanointerface and Carbon Sheath Synergistic Effect. ACS Appl. Mater. Interfaces 2020, 12 (22), 25494–25502. 10.1021/acsami.0c06601. [DOI] [PubMed] [Google Scholar]
- Xiong C.; Zhang T.; Kong W.; Zhang Z.; Qu H.; Chen W.; Wang Y.; Luo L.; Zheng L. ZIF-67 derived porous Co3O4 hollow nanopolyhedron functionalized solution-gated graphene transistors for simultaneous detection of glucose and uric acid in tears. Biosens. Bioelectron. 2018, 101, 21–28. 10.1016/j.bios.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Chen W.; Wang C.; Su S.; Wang H.; Cai D. Synthesis of ZIF-9(III)/Co LDH layered composite from ZIF-9(I) based on controllable phase transition for enhanced electrocatalytic oxygen evolution reaction. Chem. Eng. J. 2021, 414, 128784. 10.1016/j.cej.2021.128784. [DOI] [Google Scholar]
- Pan Y.-T.; Wan J.; Zhao X.; Li C.; Wang D.-Y. Interfacial growth of MOF-derived layered double hydroxide nanosheets on graphene slab towards fabrication of multifunctional epoxy nanocomposites. Chem. Eng. J. 2017, 330, 1222–1231. 10.1016/j.cej.2017.08.059. [DOI] [Google Scholar]
- Zhou J.-J.; Li K.; Wang W.; Lei Y.; Wu J.; Zhou C.; Zhang P.; Liu J.; Hua Z.; Chen L.; Han L. Boosting Specific Capacity for Supercapattery by In Situ Formation of Amorphous Ni–Co–Borate on MOF-Derived Ni–Co–LDH Nanosheet Array. ACS Appl. Energy Mater. 2020, 3 (12), 12046–12053. 10.1021/acsaem.0c02181. [DOI] [Google Scholar]
- Gong H.; Zhang X.; Wang G.; Liu Y.; Li Y.; Jin Z. Dodecahedron ZIF-67 anchoring ZnCdS particles for photocatalytic hydrogen evolution. Mol. Catal. 2020, 485, 110832. 10.1016/j.mcat.2020.110832. [DOI] [Google Scholar]
- Shao W.; Chen Y. R.; Xie F.; Zhang H.; Wang H. T.; Chang N. Facile construction of a ZIF-67/AgCl/Ag heterojunction via chemical etching and surface ion exchange strategy for enhanced visible light driven photocatalysis. RSC Adv. 2020, 10 (63), 38174–38183. 10.1039/D0RA06842J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu B.; Yang C.; Guo W.; Xu Y.; Liang Z.; Ma D.; Zou R. Highly dispersed Co-based Fischer–Tropsch synthesis catalysts from metal–organic frameworks. J. Mater. Chem. A 2017, 5 (17), 8081–8086. 10.1039/C7TA02128C. [DOI] [Google Scholar]
- Abrori S. A.; Septiani N. L. W.; Hakim F. N.; Maulana A.; Suyatman S.; Nugraha S.; Anshori I.; Yuliarto B. Non-Enzymatic Electrochemical Detection for Uric Acid Based on a Glassy Carbon Electrode Modified With MOF-71. IEEE Sensors Journal 2020, 21 (1), 170–177. 10.1109/JSEN.2020.3014298. [DOI] [Google Scholar]
- Xiang L.; Sheng L.; Wang C.; Zhang L.; Pan Y.; Li Y. Amino-functionalized ZIF-7 nanocrystals: improved intrinsic separation ability and interfacial compatibility in mixed-matrix membranes for CO2/CH4 separation. Adv. Mater. 2017, 29 (32), 1606999. 10.1002/adma.201606999. [DOI] [PubMed] [Google Scholar]
- Li P.; Li J.; Feng X.; Li J.; Hao Y.; Zhang J.; Wang H.; Yin A.; Zhou J.; Ma X.; Wang B. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10 (1), 2177. 10.1038/s41467-019-10218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Li X.; Zhao H.; Yao F.; Cao J.; Chen Z.; Wang D.; Yang Q. Core-shell structured Cu2O@HKUST-1 heterojunction photocatalyst with robust stability for highly efficient tetracycline hydrochloride degradation under visible light. Chem. Eng. J. 2021, 426, 131255. 10.1016/j.cej.2021.131255. [DOI] [Google Scholar]
- Cao Y.; Wang G.; Liu H.; Li Y.; Jin Z.; Ma Q. Regular octahedron Cu-MOFs modifies Mn0.05Cd0.95S nanoparticles to form a S-scheme heterojunction for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46 (10), 7230–7240. 10.1016/j.ijhydene.2020.11.214. [DOI] [Google Scholar]
- Shi C.; Zhou X.; Liu D.; Li L.; Xu M.; Sakiyama H.; Muddassir M.; Wang J. A new 3D high connection Cu-based MOF introducing a flexible tetracarboxylic acid linker: Photocatalytic dye degradation. Polyhedron 2021, 208, 115441. 10.1016/j.poly.2021.115441. [DOI] [Google Scholar]
- Li G.; Li F.; Liu J.; Fan C. Fe-based MOFs for photocatalytic N2 reduction: Key role of transition metal iron in nitrogen activation. J. Solid State Chem. 2020, 285, 121245. 10.1016/j.jssc.2020.121245. [DOI] [Google Scholar]
- Dao X.-Y.; Xie X.-F.; Guo J.-H.; Zhang X.-Y.; Kang Y.-S.; Sun W.-Y. Boosting Photocatalytic CO2 Reduction Efficiency by Heterostructures of NH2-MIL-101(Fe)/g-C3N4. ACS Appl. Energy Mater. 2020, 3 (4), 3946–3954. 10.1021/acsaem.0c00352. [DOI] [Google Scholar]
- Nguyen H. L.; Gandara F.; Furukawa H.; Doan T. L.; Cordova K. E.; Yaghi O. M. A Titanium-Organic Framework as an Exemplar of Combining the Chemistry of Metal- and Covalent-Organic Frameworks. J. Am. Chem. Soc. 2016, 138 (13), 4330–3. 10.1021/jacs.6b01233. [DOI] [PubMed] [Google Scholar]
- Jiang W.; Li Z.; Liu C.; Wang D.; Yan G.; Liu B.; Che G. Enhanced visible-light-induced photocatalytic degradation of tetracycline using BiOI/MIL-125(Ti) composite photocatalyst. J. Alloys Compd. 2021, 854, 157166. 10.1016/j.jallcom.2020.157166. [DOI] [Google Scholar]
- Kaur R.; Rana A.; Singh R. K.; Chhabra V. A.; Kim K.-H.; Deep A. Efficient photocatalytic and photovoltaic applications with nanocomposites between CdTe QDs and an NTU-9 MOF. RSC Adv. 2017, 7 (46), 29015–29024. 10.1039/C7RA04125J. [DOI] [Google Scholar]
- Yu Y.; Li S.; Huang L.; Yu J.; Zhang H.; Song S.; Zeng T. Solar-driven CO2 conversion promoted by MOF – on – MOF homophase junction. Catal. Commun. 2021, 150, 106270. 10.1016/j.catcom.2020.106270. [DOI] [Google Scholar]
- Yuan S.; Qin J. S.; Xu H. Q.; Su J.; Rossi D.; Chen Y.; Zhang L.; Lollar C.; Wang Q.; Jiang H. L.; Son D. H.; Xu H.; Huang Z.; Zou X.; Zhou H. C. [Ti8Zr2O12(COO)16] Cluster: An Ideal Inorganic Building Unit for Photoactive Metal-Organic Frameworks. ACS. Cent. Sci. 2018, 4 (1), 105–111. 10.1021/acscentsci.7b00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Xiao R.; Huang J.; Jiang Y.; Zhao C.; Yang X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42 (1), 107–114. 10.1016/S1872-2067(20)63559-8. [DOI] [Google Scholar]
- Zhang L.; Yang J.; Xie T.; Feng S.; Xu L. Boosting visible-light-driven photocatalytic activity of BiPO4 via constructing Schottky junction with Ti3C2 MXene. Materials & Design 2020, 192, 108772. 10.1016/j.matdes.2020.108772. [DOI] [Google Scholar]
- Li J.; Li J.; Wu C.; Li Z.; Cai L.; Tang H.; Zhou Z.; Wang G.; Wang J.; Zhao L.; Wang S. Crystalline carbon nitride anchored on MXene as an ordered Schottky heterojunction photocatalyst for enhanced visible-light hydrogen evolution. Carbon 2021, 179, 387–399. 10.1016/j.carbon.2021.04.046. [DOI] [Google Scholar]
- Fajrina N.; Tahir M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44 (2), 540–577. 10.1016/j.ijhydene.2018.10.200. [DOI] [Google Scholar]
- Ding M.; Xiao R.; Zhao C.; Bukhvalov D.; Chen Z.; Xu H.; Tang H.; Xu J.; Yang X. Evidencing Interfacial Charge Transfer in 2D CdS/2D MXene Schottky Heterojunctions toward High-Efficiency Photocatalytic Hydrogen Production. Solar RRL 2021, 5 (2), 2000414. 10.1002/solr.202000414. [DOI] [Google Scholar]
- Alhabeb M.; Maleski K.; Anasori B.; Lelyukh P.; Clark L.; Sin S.; Gogotsi Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29 (18), 7633–7644. 10.1021/acs.chemmater.7b02847. [DOI] [Google Scholar]
- Tahir M.; Sherryna A.; Mansoor R.; Khan A. A.; Tasleem S.; Tahir B. Titanium Carbide MXene Nanostructures as Catalysts and Cocatalysts for Photocatalytic Fuel Production: A Review. ACS Appl. Nano Mater. 2022, 5 (1), 18–54. 10.1021/acsanm.1c03112. [DOI] [Google Scholar]
- Anasori B.; Gogotsi Y.. Metal Carbides and Nitrides (MXenes) Structure, Properties and Applications; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- Naguib M.; Mashtalir O.; Carle J.; Presser V.; Lu J.; Hultman L.; Gogotsi Y.; Barsoum M. W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. 10.1021/nn204153h. [DOI] [PubMed] [Google Scholar]
- Feng A.; Yu Y.; Wang Y.; Jiang F.; Yu Y.; Mi L.; Song L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Materials & Design 2017, 114, 161–166. 10.1016/j.matdes.2016.10.053. [DOI] [Google Scholar]
- Khan A. A.; Tahir M. Well-designed 2D/2D Ti3C2TA/R MXene coupled g-C3N4 heterojunction with in-situ growth of anatase/rutile TiO2 nucleates to boost photocatalytic dry-reforming of methane (DRM) for syngas production under visible light. Appl. Catal., B 2021, 285, 119777. 10.1016/j.apcatb.2020.119777. [DOI] [Google Scholar]
- Ghidiu M.; Lukatskaya M. R.; Zhao M. Q.; Gogotsi Y.; Barsoum M. W. Conductive two-dimensional titanium carbide ’clay’ with high volumetric capacitance. Nature 2014, 516 (7529), 78–81. 10.1038/nature13970. [DOI] [PubMed] [Google Scholar]
- Liu F.; Zhou A.; Chen J.; Jia J.; Zhou W.; Wang L.; Hu Q. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. 10.1016/j.apsusc.2017.04.239. [DOI] [Google Scholar]
- Kvashina T. S.; Uvarov N. F.; Korchagin M. A.; Krutskiy Y. L.; Ukhina A. V. Synthesis of MXene Ti3C2 by selective etching of MAX-phase Ti3AlC2. Materials Today: Proceedings 2020, 31, 592–594. 10.1016/j.matpr.2020.07.107. [DOI] [Google Scholar]
- Mashtalir O.; Naguib M.; Dyatkin B.; Gogotsi Y.; Barsoum M. W. Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid. Mater. Chem. Phys. 2013, 139 (1), 147–152. 10.1016/j.matchemphys.2013.01.008. [DOI] [Google Scholar]
- Yang S.; Zhang P.; Wang F.; Ricciardulli A. G.; Lohe M. R.; Blom P. W. M.; Feng X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem., Int. Ed. 2018, 57 (47), 15491–15495. 10.1002/anie.201809662. [DOI] [PubMed] [Google Scholar]
- Lukatskaya M. R.; Halim J.; Dyatkin B.; Naguib M.; Buranova Y. S.; Barsoum M. W.; Gogotsi Y. Room-temperature carbide-derived carbon synthesis by electrochemical etching of MAX phases. Angew. Chem., Int. Ed. 2014, 53 (19), 4877–80. 10.1002/anie.201402513. [DOI] [PubMed] [Google Scholar]
- Sun W.; Shah S. A.; Chen Y.; Tan Z.; Gao H.; Habib T.; Radovic M.; Green M. J. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. A 2017, 5 (41), 21663–21668. 10.1039/C7TA05574A. [DOI] [Google Scholar]
- Li T.; Yao L.; Liu Q.; Gu J.; Luo R.; Li J.; Yan X.; Wang W.; Liu P.; Chen B.; Zhang W.; Abbas W.; Naz R.; Zhang D. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T = OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57 (21), 6115–6119. 10.1002/anie.201800887. [DOI] [PubMed] [Google Scholar]
- Carey M.; Hinton Z.; Natu V.; Pai R.; Sokol M.; Alvarez N. J.; Kalra V.; Barsoum M. W. Dispersion and Stabilization of Alkylated 2D MXene in Nonpolar Solvents and Their Pseudocapacitive Behavior. Cell Reports Physical Science 2020, 1 (4), 100042. 10.1016/j.xcrp.2020.100042. [DOI] [Google Scholar]
- Halim J.; Lukatskaya M. R.; Cook K. M.; Lu J.; Smith C. R.; Naslund L. A.; May S. J.; Hultman L.; Gogotsi Y.; Eklund P.; Barsoum M. W. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26 (7), 2374–2381. 10.1021/cm500641a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Radovic M.; Green M. J. Synthesizing MXene Nanosheets by Water-free Etching. Chem. 2020, 6 (3), 544–546. 10.1016/j.chempr.2020.02.013. [DOI] [Google Scholar]
- Lee Y.; Kim S. J.; Kim Y.-J.; Lim Y.; Chae Y.; Lee B.-J.; Kim Y.-T.; Han H.; Gogotsi Y.; Ahn C. W. Oxidation-resistant titanium carbide MXene films. J. Mater. Chem. A 2020, 8 (2), 573–581. 10.1039/C9TA07036B. [DOI] [Google Scholar]
- Li M.; Lu J.; Luo K.; Li Y.; Chang K.; Chen K.; Zhou J.; Rosen J.; Hultman L.; Eklund P.; Persson P. O. A.; Du S.; Chai Z.; Huang Z.; Huang Q. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141 (11), 4730–4737. 10.1021/jacs.9b00574. [DOI] [PubMed] [Google Scholar]
- Anasori B.; Lukatskaya M. R.; Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. 10.1038/natrevmats.2016.98. [DOI] [Google Scholar]
- Li Y.; Shao H.; Lin Z.; Lu J.; Liu L.; Duployer B.; Persson P. O. A.; Eklund P.; Hultman L.; Li M.; Chen K.; Zha X. H.; Du S.; Rozier P.; Chai Z.; Raymundo-Pinero E.; Taberna P. L.; Simon P.; Huang Q. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19 (8), 894–899. 10.1038/s41563-020-0657-0. [DOI] [PubMed] [Google Scholar]
- Natu V.; Pai R.; Sokol M.; Carey M.; Kalra V.; Barsoum M. W. 2D Ti3C2Tz MXene Synthesized by Water-free Etching of Ti3AlC2 in Polar Organic Solvents. Chem. 2020, 6 (3), 616–630. 10.1016/j.chempr.2020.01.019. [DOI] [Google Scholar]
- Su T.; Hood Z. D.; Naguib M.; Bai L.; Luo S.; Rouleau C. M.; Ivanov I. N.; Ji H.; Qin Z.; Wu Z. Monolayer Ti3C2Tx as an Effective Co-catalyst for Enhanced Photocatalytic Hydrogen Production over TiO2. ACS Appl. Energy Mater. 2019, 2 (7), 4640–4651. 10.1021/acsaem.8b02268. [DOI] [Google Scholar]
- Tahir M. Investigating the Influential Effect of Etchant Time in Constructing 2D/2D HCN/MXene Heterojunction with Controlled Growth of TiO2 NPs for Stimulating Photocatalytic H2 Production. Energy Fuels 2021, 35 (8), 6807–6822. 10.1021/acs.energyfuels.1c00204. [DOI] [Google Scholar]
- My Tran N.; Thanh Hoai Ta Q.; Noh J.-S. Unusual synthesis of safflower-shaped TiO2/Ti3C2 heterostructures initiated from two-dimensional Ti3C2 MXene. Appl. Surf. Sci. 2021, 538, 148023. 10.1016/j.apsusc.2020.148023. [DOI] [Google Scholar]
- Song T.; Hou L.; Long B.; Ali A.; Deng G. J. Ultrathin MXene ″bridge″ to accelerate charge transfer in ultrathin metal-free 0D/2D black phosphorus/g-C3N4 heterojunction toward photocatalytic hydrogen production. J. Colloid Interface Sci. 2021, 584, 474–483. 10.1016/j.jcis.2020.09.103. [DOI] [PubMed] [Google Scholar]
- Qin J.; Liu B.; Lam K.-H.; Song S.; Li X.; Hu X. 0D/2D MXene Quantum Dot/Ni-MOF Ultrathin Nanosheets for Enhanced N2 Photoreduction. ACS Sustainable Chem. Eng. 2020, 8 (48), 17791–17799. 10.1021/acssuschemeng.0c06388. [DOI] [Google Scholar]
- Qin X.; Cao R.; Gong W.; Luo L.; Shi G.; Ji L.; Zhu A. Hydrothermal growth of ZnCdS/TiO2 nanoparticles on the surface of the Ti3C2 MXene sheet to enhance photocatalytic performance under visible light. J. Solid State Chem. 2022, 306, 122750. 10.1016/j.jssc.2021.122750. [DOI] [Google Scholar]
- Zuo G.; Wang Y.; Teo W. L.; Xie A.; Guo Y.; Dai Y.; Zhou W.; Jana D.; Xian Q.; Dong W.; Zhao Y. Enhanced photocatalytic water oxidation by hierarchical 2D-Bi2MoO6@2D-MXene Schottky junction nanohybrid. Chem. Eng. J. 2021, 403, 126328. 10.1016/j.cej.2020.126328. [DOI] [Google Scholar]
- Kim Y.-J.; Kim S. J.; Seo D.; Chae Y.; Anayee M.; Lee Y.; Gogotsi Y.; Ahn C. W.; Jung H.-T. Etching Mechanism of Monoatomic Aluminum Layers during MXene Synthesis. Chem. Mater. 2021, 33 (16), 6346–6355. 10.1021/acs.chemmater.1c01263. [DOI] [Google Scholar]
- Zhang T.; Pan L.; Tang H.; Du F.; Guo Y.; Qiu T.; Yang J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl+LiF etchant: Enhanced exfoliation and delamination. J. Alloys Compd. 2017, 695, 818–826. 10.1016/j.jallcom.2016.10.127. [DOI] [Google Scholar]
- Zhang Q.; He J.; Fu X.; Xie S.; Fan R.; Lai H.; Cheng W.; Ji P.; Sheng J.; Liao Q.; Zhu W.; Li H. Fluorine-free strategy for hydroxylated Ti3C2/Ti3AlC2 catalysts with enhanced aerobic oxidative desulfurization and mechanism. Chem. Eng. J. 2022, 430, 132950. 10.1016/j.cej.2021.132950. [DOI] [Google Scholar]
- Khan U.; Luo Y.; Kong L. B.; Que W. Synthesis of fluorine free MXene through lewis acidic etching for application as electrode of proton supercapacitors. J. Alloys Compd. 2022, 926, 166903. 10.1016/j.jallcom.2022.166903. [DOI] [Google Scholar]
- Xiao R.; Zhao C.; Zou Z.; Chen Z.; Tian L.; Xu H.; Tang H.; Liu Q.; Lin Z.; Yang X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal., B 2020, 268, 118382. 10.1016/j.apcatb.2019.118382. [DOI] [Google Scholar]
- Su T.; Hood Z. D.; Naguib M.; Bai L.; Luo S.; Rouleau C. M.; Ivanov I. N.; Ji H.; Qin Z.; Wu Z. 2D/2D heterojunction of Ti3C2/g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Nanoscale 2019, 11 (17), 8138–8149. 10.1039/C9NR00168A. [DOI] [PubMed] [Google Scholar]
- Zhuang Y.; Liu Y.; Meng X. Fabrication of TiO2 nanofibers/MXene Ti3C2 nanocomposites for photocatalytic H2 evolution by electrostatic self-assembly. Appl. Surf. Sci. 2019, 496, 143647. 10.1016/j.apsusc.2019.143647. [DOI] [Google Scholar]
- Wang W.-T.; Batool N.; Zhang T.-H.; Liu J.; Han X.-F.; Tian J.-H.; Yang R. When MOFs meet MXenes: superior ORR performance in both alkaline and acidic solutions. J. Mater. Chem. A 2021, 9 (7), 3952–3960. 10.1039/D0TA10811A. [DOI] [Google Scholar]
- Munir S.; Rasheed A.; Rasheed T.; Ayman I.; Ajmal S.; Rehman A.; Shakir I.; Agboola P. O.; Warsi M. F. Exploring the Influence of Critical Parameters for the Effective Synthesis of High-Quality 2D MXene. ACS Omega 2020, 5 (41), 26845–26854. 10.1021/acsomega.0c03970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.; Rahman M. M.; Kareem S.; Dong H.; Qiao F.; Xiong W.; Liu X.; Li N.; Zhao X. Facile synthesis of CuS/MXene nanocomposites for efficient photocatalytic hydrogen generation. CrystEngComm 2020, 22 (11), 2060–2066. 10.1039/D0CE00104J. [DOI] [Google Scholar]
- Yang Y.; Zhang D.; Fan J.; Liao Y.; Xiang Q. Construction of an Ultrathin S-Scheme Heterojunction Based on Few-Layer g-C3N4 and Monolayer Ti3C2Tx MXene for Photocatalytic CO2 Reduction. Solar RRL 2021, 5 (2), 2000351. 10.1002/solr.202000351. [DOI] [Google Scholar]
- Zeng Z.; Yan Y.; Chen J.; Zan P.; Tian Q.; Chen P. Boosting the Photocatalytic Ability of Cu2O Nanowires for CO2 Conversion by MXene Quantum Dots. Adv. Funct. Mater. 2019, 29 (2), 1806500. 10.1002/adfm.201806500. [DOI] [Google Scholar]
- Du X.; Zhao T.; Xiu Z.; Xing Z.; Li Z.; Pan K.; Yang S.; Zhou W. BiVO4@ZnIn2S4/Ti3C2 MXene quantum dots assembly all-solid-state direct Z-Scheme photocatalysts for efficient visible-light-driven overall water splitting. Applied Materials Today 2020, 20, 100719. 10.1016/j.apmt.2020.100719. [DOI] [Google Scholar]
- Liu L.; Ying G.; Wen D.; Li Y.; Zhang K.; Min H.; Hu C.; Sun C.; Wang C. High-performance copper-matrix materials reinforced by nail board-like structure 2D Ti3C2T MXene with in-situ TiO2 particles. Materials Science and Engineering: A 2022, 832, 142392. 10.1016/j.msea.2021.142392. [DOI] [Google Scholar]
- Zhao S.; Pan D.; Liang Q.; Zhou M.; Yao C.; Xu S.; Li Z. Ultrathin NiAl-Layered Double Hydroxides Grown on 2D Ti3C2Tx MXene to Construct Core–Shell Heterostructures for Enhanced Photocatalytic CO2 Reduction. J. Phys. Chem. C 2021, 125 (19), 10207–10218. 10.1021/acs.jpcc.1c00017. [DOI] [Google Scholar]
- Zhao M.; Huang Y.; Peng Y.; Huang Z.; Ma Q.; Zhang H. Two-dimensional metal-organic framework nanosheets: synthesis and applications. Chem. Soc. Rev. 2018, 47 (16), 6267–6295. 10.1039/C8CS00268A. [DOI] [PubMed] [Google Scholar]
- Zhuang L.; Ge L.; Liu H.; Jiang Z.; Jia Y.; Li Z.; Yang D.; Hocking R. K.; Li M.; Zhang L.; et al. and Scalable General Strategy for Synthesizing Ultrathin Two-Dimensional Metal-Organic Framework Nanosheets for the Oxygen Evolution Reaction. Angew. Chem., Int. Ed. Engl. 2019, 58 (38), 13565–13572. 10.1002/anie.201907600. [DOI] [PubMed] [Google Scholar]
- Yuan M.; Wang R.; Sun Z.; Lin L.; Yang H.; Li H.; Nan C.; Sun G.; Ma S. Morphology-Controlled Synthesis of Ni-MOFs with Highly Enhanced Electrocatalytic Performance for Urea Oxidation. Inorg. Chem. 2019, 58 (17), 11449–11457. 10.1021/acs.inorgchem.9b01124. [DOI] [PubMed] [Google Scholar]
- Sarawade P.; Tan H.; Polshettiwar V. Shape- and Morphology-Controlled Sustainable Synthesis of Cu, Co, and In Metal Organic Frameworks with High CO2 Capture Capacity. ACS Sustainable Chem. Eng. 2013, 1 (1), 66–74. 10.1021/sc300036p. [DOI] [Google Scholar]
- Wan H.; Wang Y.; Chen J.; Meng H. M.; Li Z. 2D Co-MOF nanosheet-based nanozyme with ultrahigh peroxidase catalytic activity for detection of biomolecules in human serum samples. Mikrochim. Acta 2021, 188 (4), 130. 10.1007/s00604-021-04785-2. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Yuan Y.; Zhang Q.; Chang F.; Yang L.; Chen Z.; Bai Z. Morphology-controlled synthesis of metal-organic frameworks derived lattice plane-altered iron oxide for efficient trifunctional electrocatalysts. Nano Energy 2021, 82, 105699. 10.1016/j.nanoen.2020.105699. [DOI] [Google Scholar]
- Mateo D.; Morlanes N.; Maity P.; Shterk G.; Mohammed O. F.; Gascon J. Efficient Visible-Light Driven Photothermal Conversion of CO2 to Methane by Nickel Nanoparticles Supported on Barium Titanate. Adv. Funct. Mater. 2021, 31, 2008244. 10.1002/adfm.202008244. [DOI] [Google Scholar]
- Song C.; Liu X.; Xu M.; Masi D.; Wang Y.; Deng Y.; Zhang M.; Qin X.; Feng K.; Yan J.; Leng J.; Wang Z.; Xu Y.; Yan B.; Jin S.; Xu D.; Yin Z.; Xiao D.; Ma D. Photothermal Conversion of CO2 with Tunable Selectivity Using Fe-Based Catalysts: From Oxide to Carbide. ACS Catal. 2020, 10 (18), 10364–10374. 10.1021/acscatal.0c02244. [DOI] [Google Scholar]
- Chen G.; Gao R.; Zhao Y.; Li Z.; Waterhouse G. I. N.; Shi R.; Zhao J.; Zhang M.; Shang L.; Sheng G.; Zhang X.; Wen X.; Wu L. Z.; Tung C. H.; Zhang T. Alumina-Supported CoFe Alloy Catalysts Derived from Layered-Double-Hydroxide Nanosheets for Efficient Photothermal CO2 Hydrogenation to Hydrocarbons. Adv. Mater. 2018, 30 (3), 1704663. 10.1002/adma.201704663. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy A.; Li Z.; Garcia H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47 (22), 8134–8172. 10.1039/C8CS00256H. [DOI] [PubMed] [Google Scholar]
- Wang L.; Jin P.; Duan S.; She H.; Huang J.; Wang Q. In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci. Bull. 2019, 64 (13), 926–933. 10.1016/j.scib.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wei J.; Dong J.; Liu G.; Shi L.; An P.; Zhao G.; Kong J.; Wang X.; Meng X.; Zhang J.; Ye J. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal-Organic Framework. Angew. Chem., Int. Ed. Engl. 2016, 55 (46), 14310–14314. 10.1002/anie.201608597. [DOI] [PubMed] [Google Scholar]
- Zhao Z.-W.; Zhou X.; Liu Y.-N.; Shen C.-C.; Yuan C.-Z.; Jiang Y.-F.; Zhao S.-J.; Ma L.-B.; Cheang T.-Y.; Xu A.-W. Ultrasmall Ni nanoparticles embedded in Zr-based MOFs provide high selectivity for CO2 hydrogenation to methane at low temperatures. Catal. Sci. Technol. 2018, 8 (12), 3160–3165. 10.1039/C8CY00468D. [DOI] [Google Scholar]
- Li S.; Ji K.; Zhang M.; He C.; Wang J.; Li Z. Boosting the photocatalytic CO2 reduction of metal-organic frameworks by encapsulating carbon dots. Nanoscale 2020, 12 (17), 9533–9540. 10.1039/D0NR01696A. [DOI] [PubMed] [Google Scholar]
- Shi H.; Zhang P.; Liu Z.; Park S.; Lohe M. R.; Wu Y.; Shaygan Nia A.; Yang S.; Feng X. Ambient-Stable Two-Dimensional Titanium Carbide (MXene) Enabled by Iodine Etching. Angew. Chem., Int. Ed. 2021, 60 (16), 8689–8693. 10.1002/anie.202015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.-J.; Rajavel K.; Li X.-M.; Wang X.-Y.; Liao S.-Y.; Lin Z.-Q.; Zhu P.-L.; Sun R.; Wong C.-P. Electromagnetic interference shielding of Ti3C2T MXene modified by ionic liquid for high chemical stability and excellent mechanical strength. Chem. Eng. J. 2021, 408, 127303. 10.1016/j.cej.2020.127303. [DOI] [Google Scholar]
- Cao F.; Zhang Y.; Wang H.; Khan K.; Tareen A. K.; Qian W.; Zhang H.; Agren H. Recent Advances in Oxidation Stable Chemistry of 2D MXenes. Adv. Mater. 2022, 34 (13), 2107554. 10.1002/adma.202107554. [DOI] [PubMed] [Google Scholar]
- Seredych M.; Shuck C. E.; Pinto D.; Alhabeb M.; Precetti E.; Deysher G.; Anasori B.; Kurra N.; Gogotsi Y. High-Temperature Behavior and Surface Chemistry of Carbide MXenes Studied by Thermal Analysis. Chem. Mater. 2019, 31 (9), 3324–3332. 10.1021/acs.chemmater.9b00397. [DOI] [Google Scholar]
- Zhong C.; Shang Z.; Zhao C.; Luo H. a.; Cao Y.; Yan D.; You K. Co-Catalyst Ti3C2TX MXene-Modified ZnO Nanorods Photoanode for Enhanced Photoelectrochemical Water Splitting. Top. Catal. 2022, 10.1007/s11244-022-01619-0. [DOI] [Google Scholar]
- Almusattar W.; Tahir M.; Madi M.; Tahir B. Fabricating Ti3C2 MXene cocatalyst supported NiAl-LDH/g-C3N4 ternary nanocomposite for stimulating solar photocatalytic H2 production. J. Environ. Chem. Eng. 2022, 10 (4), 108010. 10.1016/j.jece.2022.108010. [DOI] [Google Scholar]
- Ma Y.; Xu D.; Chen W.; Tang Y.; Wang X.; Li L.; Wang J. Oxygen-vacancy-embedded 2D/2D NiFe-LDH/MXene Schottky heterojunction for boosted photodegradation of norfloxacin. Appl. Surf. Sci. 2022, 572, 151432. 10.1016/j.apsusc.2021.151432. [DOI] [Google Scholar]
- Zhong Q.; Li Y.; Zhang G. Two-dimensional MXene-based and MXene-derived photocatalysts: Recent developments and perspectives. Chem. Eng. J. 2021, 409, 128099. 10.1016/j.cej.2020.128099. [DOI] [Google Scholar]
- Bao Y.; Liu Y.; Pan J.; Chen P.; Liu X.; Li Y.; Tang X.; Zhang W.; Liu B.; Liu J. Constructing 2D layered PCN/Ti3C2/Bi2MoO6 heterojunction with MXene as charge mediator for enhanced photocatalytic performance. Appl. Surf. Sci. 2022, 589, 152883. 10.1016/j.apsusc.2022.152883. [DOI] [Google Scholar]
- Liu Q.; Tan X.; Wang S.; Ma F.; Znad H.; Shen Z.; Liu L.; Liu S. MXene as a non-metal charge mediator in 2D layered CdS@Ti3C2@TiO2 composites with superior Z-scheme visible light-driven photocatalytic activity. Environmental Science: Nano 2019, 6 (10), 3158–3169. 10.1039/C9EN00567F. [DOI] [Google Scholar]
- Zhou Y.; Yu M.; Zhan R.; Wang X.; Peng G.; Niu J. Ti3C2 MXene-induced interface electron separation in g-C3N4/Ti3C2 MXene/MoSe2 Z-scheme heterojunction for enhancing visible light-irradiated enoxacin degradation. Sep. Purif. Technol. 2021, 275, 119194. 10.1016/j.seppur.2021.119194. [DOI] [Google Scholar]
- Yang J.-X.; Yu W.-B.; Li C.-F.; Dong W.-D.; Jiang L.-Q.; Zhou N.; Zhuang Z.-P.; Liu J.; Hu Z.-Y.; Zhao H.; Li Y.; Chen L.; Hu J.; Su B.-L. PtO nanodots promoting Ti3C2 MXene in-situ converted Ti3C2/TiO2 composites for photocatalytic hydrogen production. Chem. Eng. J. 2021, 420, 129695. 10.1016/j.cej.2021.129695. [DOI] [Google Scholar]
- Khan A. A.; Tahir M.; Bafaqeer A. Constructing a Stable 2D Layered Ti3C2 MXene Cocatalyst-Assisted TiO2/g-C3N4/Ti3C2 Heterojunction for Tailoring Photocatalytic Bireforming of Methane under Visible Light. Energy Fuels 2020, 34 (8), 9810–9828. 10.1021/acs.energyfuels.0c01354. [DOI] [Google Scholar]
- Huang K.; Li C.; Meng X. In-situ construction of ternary Ti3C2 MXene@TiO2/ZnIn2S4 composites for highly efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 580, 669–680. 10.1016/j.jcis.2020.07.044. [DOI] [PubMed] [Google Scholar]
- Fang J.; Xie K.; Kang Q.; Gou Y. Facile fabrication of g-C3N4/CdS heterojunctions with enhanced visible-light photocatalytic degradation performances. Journal of Science: Advanced Materials and Devices 2022, 7 (1), 100409. 10.1016/j.jsamd.2021.100409. [DOI] [Google Scholar]
- Dong W.-W.; Jia J.; Wang Y.; An J.-R.; Yang O.-Y.; Gao X.-J.; Liu Y.-L.; Zhao J.; Li D.-S. Visible-light-driven solvent-free photocatalytic CO2 reduction to CO by Co-MOF/Cu2O heterojunction with superior selectivity. Chem. Eng. J. 2022, 438, 135622. 10.1016/j.cej.2022.135622. [DOI] [Google Scholar]
- Wang L.; Jin P.; Huang J.; She H.; Wang Q. Integration of Copper(II)-Porphyrin Zirconium Metal–Organic Framework and Titanium Dioxide to Construct Z-Scheme System for Highly Improved Photocatalytic CO2 Reduction. ACS Sustainable Chem. Eng. 2019, 7 (18), 15660–15670. 10.1021/acssuschemeng.9b03773. [DOI] [Google Scholar]
- Xiao L.; Zhang Q.; Chen P.; Chen L.; Ding F.; Tang J.; Li Y.-J.; Au C.-T.; Yin S.-F. Copper-mediated metal-organic framework as efficient photocatalyst for the partial oxidation of aromatic alcohols under visible-light irradiation: Synergism of plasmonic effect and schottky junction. Appl. Catal., B 2019, 248, 380–387. 10.1016/j.apcatb.2019.02.012. [DOI] [Google Scholar]
- Wu Y.; Li X.; Yang Q.; Wang D.; Yao F.; Cao J.; Chen Z.; Huang X.; Yang Y.; Li X. Mxene-modulated dual-heterojunction generation on a metal-organic framework (MOF) via surface constitution reconstruction for enhanced photocatalytic activity. Chem. Eng. J. 2020, 390, 124519. 10.1016/j.cej.2020.124519. [DOI] [Google Scholar]
- Tahir M.; Sherryna A.; Khan A. A.; Madi M.; Zerga A. Y.; Tahir B. Defect Engineering in Graphitic Carbon Nitride Nanotextures for Energy Efficient Solar Fuels Production: A Review. Energy Fuels 2022, 36 (16), 8948–8977. 10.1021/acs.energyfuels.2c01256. [DOI] [Google Scholar]
- Syzgantseva M. A.; Ireland C. P.; Ebrahim F. M.; Smit B.; Syzgantseva O. A. Metal Substitution as the Method of Modifying Electronic Structure of Metal-Organic Frameworks. J. Am. Chem. Soc. 2019, 141 (15), 6271–6278. 10.1021/jacs.8b13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MS R.; Shanmuga Priya S.; Freudenberg N. C.; Sudhakar K; Tahir M. Metal-organic framework-based photocatalysts for carbon dioxide reduction to methanol: A review on progress and application. J. CO2 Util. 2021, 43, 101374. 10.1016/j.jcou.2020.101374. [DOI] [Google Scholar]
- Gomez Alvarez E.; Wortham H.; Strekowski R.; Zetzsch C.; Gligorovski S. Atmospheric photosensitized heterogeneous and multiphase reactions: from outdoors to indoors. Environ. Sci. Tehcnol. 2012, 46 (4), 1955–1963. 10.1021/es2019675. [DOI] [PubMed] [Google Scholar]
- Mu Q.; Su Y.; Wei Z.; Sun H.; Lian Y.; Dong Y.; Qi P.; Deng Z.; Peng Y. Dissecting the interfaces of MOF-coated CdS on synergized charge transfer for enhanced photocatalytic CO2 reduction. J. Catal. 2021, 397, 128–136. 10.1016/j.jcat.2021.03.018. [DOI] [Google Scholar]
- Karmakar S.; Barman S.; Rahimi F. A.; Maji T. K. Covalent grafting of molecular photosensitizer and catalyst on MOF-808: effect of pore confinement toward visible light-driven CO2 reduction in water. Energy Environ. Sci. 2021, 14 (4), 2429–2440. 10.1039/D0EE03643A. [DOI] [Google Scholar]
- Tahir M.; Tahir B. 2D/2D/2D O-C3N4/Bt/Ti3C2Tx heterojunction with novel MXene/clay multi-electron mediator for stimulating photo-induced CO2 reforming to CO and CH4. Chem. Eng. J. 2020, 400, 125868. 10.1016/j.cej.2020.125868. [DOI] [Google Scholar]
- Liu Y.; He Y.; Vargun E.; Plachy T.; Saha P.; Cheng Q. 3D Porous Ti3C2 MXene/NiCo-MOF Composites for Enhanced Lithium Storage. Nanomaterials (Basel) 2020, 10 (4), 695. 10.3390/nano10040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.; Zhou H.; Xue H.; Braunstein P.; Pang H. Pillared-layer Ni-MOF nanosheets anchored on Ti3C2 MXene for enhanced electrochemical energy storage. J. Colloid Interface Sci. 2022, 614, 130–137. 10.1016/j.jcis.2022.01.094. [DOI] [PubMed] [Google Scholar]
- Long R.; Yu Z.; Tan Q.; Feng X.; Zhu X.; Li X.; Wang P. Ti3C2 MXene/NH2-MIL-88B(Fe): Research on the adsorption kinetics and photocatalytic performance of an efficient integrated photocatalytic adsorbent. Appl. Surf. Sci. 2021, 570, 151244. 10.1016/j.apsusc.2021.151244. [DOI] [Google Scholar]
- Luo X.; Abazari R.; Tahir M.; Fan W. K.; Kumar A.; Kalhorizadeh T.; Kirillov A. M.; Amani-Ghadim A. R.; Chen J.; Zhou Y. Trimetallic metal–organic frameworks and derived materials for environmental remediation and electrochemical energy storage and conversion. Coord. Chem. Rev. 2022, 461, 214505. 10.1016/j.ccr.2022.214505. [DOI] [Google Scholar]
- Huang R.; Liao D.; Chen S.; Yu J.; Jiang X. A strategy for effective electrochemical detection of hydroquinone and catechol: Decoration of alkalization-intercalated Ti3C2 with MOF-derived N-doped porous carbon. Sens. Actuators, B 2020, 320, 128386. 10.1016/j.snb.2020.128386. [DOI] [Google Scholar]
- Jiang G.; Zheng N.; Chen X.; Ding G.; Li Y.; Sun F.; Li Y. In-situ decoration of MOF-derived carbon on nitrogen-doped ultrathin MXene nanosheets to multifunctionalize separators for stable Li-S batteries. Chem. Eng. J. 2019, 373, 1309–1318. 10.1016/j.cej.2019.05.119. [DOI] [Google Scholar]
- Chen W.; Han B.; Xie Y.; Liang S.; Deng H.; Lin Z. Ultrathin Co-Co LDHs nanosheets assembled vertically on MXene: 3D nanoarrays for boosted visible-light-driven CO2 reduction. Chem. Eng. J. 2020, 391, 123519. 10.1016/j.cej.2019.123519. [DOI] [Google Scholar]
- Hu L.; Xiao R.; Wang X.; Wang X.; Wang C.; Wen J.; Gu W.; Zhu C. MXene-induced electronic optimization of metal-organic framework-derived CoFe LDH nanosheet arrays for efficient oxygen evolution. Appl. Catal., B 2021, 298, 120599. 10.1016/j.apcatb.2021.120599. [DOI] [Google Scholar]
- Li S.; Wu F.; Lin R.; Wang J.; Li C.; Li Z.; Jiang J.; Xiong Y. Enabling photocatalytic hydrogen production over Fe-based MOFs by refining band structure with dye sensitization. Chem. Eng. J. 2022, 429, 132217. 10.1016/j.cej.2021.132217. [DOI] [Google Scholar]
- Li N.; Liu J.; Liu J. J.; Dong L. Z.; Xin Z. F.; Teng Y. L.; Lan Y. Q. Adenine Components in Biomimetic Metal-Organic Frameworks for Efficient CO2 Photoconversion. Angew. Chem., Int. Ed. Engl. 2019, 58 (16), 5226–5231. 10.1002/anie.201814729. [DOI] [PubMed] [Google Scholar]
- Liao W. M.; Zhang J. H.; Wang Z.; Lu Y. L.; Yin S. Y.; Wang H. P.; Fan Y. N.; Pan M.; Su C. Y. Semiconductive Amine-Functionalized Co(II)-MOF for Visible-Light-Driven Hydrogen Evolution and CO2 Reduction. Inorg. Chem. 2018, 57 (18), 11436–11442. 10.1021/acs.inorgchem.8b01265. [DOI] [PubMed] [Google Scholar]
- Saeed A.; Chen W.; Shah A. H.; Zhang Y.; Mehmood I.; Liu Y. Enhancement of photocatalytic CO2 reduction for novel Cd0.2Zn0.8S@Ti3C2 (MXenes) nanocomposites. J. CO2 Util. 2021, 47, 101501. 10.1016/j.jcou.2021.101501. [DOI] [Google Scholar]
- Najam T.; Shah S. S. A.; Peng L.; Javed M. S.; Imran M.; Zhao M.-Q.; Tsiakaras P. Synthesis and nano-engineering of MXenes for energy conversion and storage applications: Recent advances and perspectives. Coord. Chem. Rev. 2022, 454, 214339. 10.1016/j.ccr.2021.214339. [DOI] [Google Scholar]
- Yu L.; Liu B.; Wang Y.; Yu F.; Ma J. Recent progress on MXene-Derived material and its’ application in energy and environment. J. Power Sources 2021, 490, 229250. 10.1016/j.jpowsour.2020.229250. [DOI] [Google Scholar]
- Sharma K.; Hasija V.; Patial S.; Singh P.; Nguyen V.-H.; Nadda A. K.; Thakur S.; Nguyen-Tri P.; Nguyen C. C.; Kim S. Y. Recent progress on MXenes and MOFs hybrids: Structure, synthetic strategies and catalytic water splitting. Int. J. Hydrogen Energy 2022, 10.1016/j.ijhydene.2022.01.004. [DOI] [Google Scholar]
- Tian P.; He X.; Zhao L.; Li W.; Fang W.; Chen H.; Zhang F.; Huang Z.; Wang H. Ti3C2 nanosheets modified Zr-MOFs with Schottky junction for boosting photocatalytic HER performance. Sol. Energy 2019, 188, 750–759. 10.1016/j.solener.2019.06.060. [DOI] [Google Scholar]
- Tian P.; He X.; Zhao L.; Li W.; Fang W.; Chen H.; Zhang F.; Huang Z.; Wang H. Enhanced charge transfer for efficient photocatalytic H2 evolution over UiO-66-NH2 with annealed Ti3C2Tx MXenes. Int. J. Hydrogen Energy 2019, 44 (2), 788–800. 10.1016/j.ijhydene.2018.11.016. [DOI] [Google Scholar]
- Li Y.; Liu Y.; Wang Z.; Wang P.; Zheng Z.; Cheng H.; Dai Y.; Huang B. In-situ growth of Ti3C2@MIL-NH2 composite for highly enhanced photocatalytic H2 evolution. Chem. Eng. J. 2021, 411, 128446. 10.1016/j.cej.2021.128446. [DOI] [Google Scholar]
- Zhao J.-H.; Liu L.-W.; Li K.; Li T.; Liu F.-T. Conductive Ti3C2 and MOF-derived CoSx boosting the photocatalytic hydrogen production activity of TiO2. CrystEngComm 2019, 21 (14), 2416–2421. 10.1039/C8CE02050G. [DOI] [Google Scholar]
- Shi L.; Wu C.; Wang Y.; Dou Y.; Yuan D.; Li H.; Huang H.; Zhang Y.; Gates I. D.; Sun X.; Ma T. Rational Design of Coordination Bond Connected Metal Organic Frameworks/MXene Hybrids for Efficient Solar Water Splitting. Adv. Funct. Mater. 2022, 32 (30), 2202571. 10.1002/adfm.202202571. [DOI] [Google Scholar]
- Sherryna A.; Tahir M. Role of Surface Morphology and Terminating Groups in Titanium Carbide MXenes (Ti3C2Tx) Cocatalysts with Engineering Aspects for Modulating Solar Hydrogen Production: A Critical Review. Chem. Eng. J. 2022, 433, 134573. 10.1016/j.cej.2022.134573. [DOI] [Google Scholar]
- Tahir M. Binary Ni2P/Ti3C2 Multilayer Cocatalyst Anchored TiO2 Nanocomposite with Etchant/Oxidation Grown TiO2 NPs for Enhancing Photocatalytic H2 Production. Energy Fuels 2021, 35 (17), 14197–14211. 10.1021/acs.energyfuels.1c01340. [DOI] [Google Scholar]
- Afroz K.; Moniruddin M.; Bakranov N.; Kudaibergenov S.; Nuraje N. A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6 (44), 21696–21718. 10.1039/C8TA04165B. [DOI] [Google Scholar]
- Schultz T.; Frey N. C.; Hantanasirisakul K.; Park S.; May S. J.; Shenoy V. B.; Gogotsi Y.; Koch N. Surface Termination Dependent Work Function and Electronic Properties of Ti3C2Tx MXene. Chem. Mater. 2019, 31 (17), 6590–6597. 10.1021/acs.chemmater.9b00414. [DOI] [Google Scholar]
- Tahir M.; Sherryna A.; Zakaria Z. Y. Facile Synthesis of MAX Modified Graphitic Carbon Nitride Nanocomposite for Stimulating Hydrogen Production Through Photocatalytic Water Splitting. Chem. Eng. Trans. 2021, 89, 571–576. [Google Scholar]
- Tasleem S.; Tahir M.; Zakaria Z. Y. Fabricating structured 2D Ti3AlC2 MAX dispersed TiO2 heterostructure with Ni2P as a cocatalyst for efficient photocatalytic H2 production. J. Alloys Compd. 2020, 842, 155752. 10.1016/j.jallcom.2020.155752. [DOI] [Google Scholar]
- Sherryna A.; Tahir M. Recent developments in layered double hydroxide structures with their role in promoting photocatalytic hydrogen production: A comprehensive review. Int. J. Energy Res. 2022, 46, 2093–2140. 10.1002/er.7335. [DOI] [Google Scholar]