Abstract

The utilization of Mg–O–F prepared from Mg(OH)2 mixed with different wt % of F in the form of (NH4F·HF), calcined at 400 and 500 °C, for efficient capture of CO2 is studied herein in a dynamic mode. Two different temperatures were applied using a slow rate of 20 mL·min–1 (100%) of CO2 passing through each sample for only 1 h. Using the thermogravimetry (TG)-temperature-programed desorption (TPD) technique, the captured amounts of CO2 at 5 °C were determined to be in the range of (39.6–103.9) and (28.9–82.1) mgCO2·g–1 for samples of Mg(OH)2 mixed with 20–50% F and calcined at 400 and 500 °C, respectively, whereas, at 30 °C, the capacity of CO2 captured is slightly decreased to be in the range of (32.2–89.4) and (20.9–55.5) mgCO2·g–1, respectively. The thermal decomposition of all prepared mixtures herein was examined by TG analysis. The obtained samples calcined at 400 and 500 °C were characterized by X-ray diffraction and surface area and porosity measurements. The total number of surface basic sites and their distribution over all samples was demonstrated using TG- and differential scanning calorimetry-TPD techniques using pyrrole as a probe molecule. Values of (ΔH) enthalpy changes corresponding to the desorption steps of CO2 were calculated for the most active adsorbent in this study, that is, Mg(OH)2 + 20% F, at 400 and 500 °C. This study’s findings will inspire the simple preparation and economical design of nanocomposite CO2 sorbents for climate change mitigation under ambient conditions.
1. Introduction
The world is on the verge of an environmental catastrophe due to global warming as a result of the continuous increase in atmospheric CO2. According to the data released by the Scripps Institute of Oceanography at the University of California in May 2022, the daily average concentration of atmospheric CO2 reached a record high of 421.37 parts per million (ppm).1 Numerous climate scientists and researchers are interested in experimenting with various CO2-capturing materials. Currently, the CO2 adsorption technique is used on some highly active materials, such as basic metal oxides, to combat global warming. In the pure form or promoted with other materials, MgO is regarded as a cornerstone component in all prepared materials for CO2 capture purposes, among these basic metal oxides.2−9 This characteristic relates to the abundance and strength of basic sites over the MgO surface.10−12 Other types of adsorbents, including the reduced graphene oxide–MnO2 nanocomposite,13 nickel–lithium silicate for CO2 capture and subsequent production of CH4,14 Li4SiO4-based sorbents for high-temperature CO2 capture,15 NiO-functionalized ultra-stable Y zeolite,16 Mg–Al mixed metal oxides for high-temperature CO2 capture,17 alumina-supported layered double hydroxides,18 CuAl2O4 nanoplates,19 Ni–CaO dual function materials,20 and CaO–Fe2O3–SiO2 composite, have been extensively examined.21 Few articles have focused on the study of capturing CO2 in a dynamic mode at different temperatures by flowing CO2 gas at different rates through the adsorbents.22,23 Many review articles with a forward-looking perspective discussed various CO2 capture techniques24−27 utilizing different adsorbent materials.
Rarely, published articles on using MgO–MgF2 mixtures for CO2 capture are cited in the literature. In this regard, this study can be viewed as proactive research into using these mixtures. Furthermore, pure MgF2 is considered to be a suitable and effective sintering additive for the preparation of certain types of ceramics,28,29 in manufacturing transparent IR windows,30 in solar thermal technology,31 and as a catalyst in the form of MgF2–x(OH)x during aldol condensation of furfural and acetone.32
On the occasion of the 27th UN Climate Change Conference of the Parties (COP27), which will be held in Sharm el-Sheikh, Egypt, in November 2022, two research teams from South Valley University (Egypt) and Queen’s University Belfast (UK) collaborated in a vital effort to combat global warming. Herein, our samples were prepared using a direct and simple method and exhibited a relatively high surface area and a superior reactivity toward CO2 capture at 5 and 30 °C, despite a limited CO2 flow time (1 h) throughout the samples. The applied method is based on two environmentally friendly materials, that is, Mg(OH)2 and NH4F·HF, which were used to prepare the required mixtures without releasing harmful greenhouse gases.
Early on, it was determined that the generation of MgCO3 from MgO without a promoter has poor kinetics and is limited to a few surface carbonate layers, resulting in only a small fraction (<2%) of the theoretical CO2 sorption capacity of MgO. The slowdown kinetics is caused by the high lattice enthalpy of MgO and the formation of a layer of magnesium carbonate, which acts as a barrier for CO2 molecules. Alkali metal salts are the most effective promoters for MgO-based sorbents.2 This work focuses on the preparation of Mg–O–F nanocomposite catalysts with a high surface area, a large population of surface basic sites, and a high CO2 capture efficiency using environmentally friendly materials without emitting harmful greenhouse gases. Compared to recently published articles utilizing other adsorbents, these samples appear to be more effective CO2 adsorbents under ambient conditions.
2. Experimental
2.1. Preparation of Mg–O–F Nanocomposite Mixtures
Mixed samples of Mg(OH)2 with 20–50% F (by weight) as ammonium hydrogen difluoride (AHDF) NH4F·HF were prepared as follows: calculated amounts of ammonium hydrogen difluoride (NH4F·HF, Hopkin & Williams) were dissolved in deionized water in Teflon beakers and mixed well with the corresponding weights of Mg(OH)2 (BDH, chemicals). These mixtures were then evaporated until dry in a water bath. The resulting mixtures were dried at 100 °C overnight. All these mixtures were also calcined at two temperatures of 400 and 500 °C in static air for 3 h.
2.2. Characterization
A 50H thermogravimetric analyzer and differential scanning calorimetry (DSC) instruments (Shimadzu—Japan) were used in this work. A slow heating rate of 3 °C min–1 was used in the He atmosphere (40 mL·min–1) during the study of the thermal stability of all prepared mixtures, as well as the pure compounds of Mg(OH)2 and NH4F·HF. Thermogravimetry (TG) and DSC experiments of the temperature-programed desorption (TPD) of CO2 over all samples under study, at different temperatures of CO2 adsorption, were accomplished using a heating rate of 10 °C min–1 in a dynamic N2 flow (40 mL·min–1). The instrument is equipped with a data acquisition and handling system (TA-50WSI), and highly sintered α-Al2O3 was applied as reference material in DSC experiments.
X-ray diffraction (XRD) patterns of the calcined samples at 400 and 500 °C were measured by powder XRD using a Brucker AXS-D8 Advance diffractometer (Germany), equipped with a copper anode generating Ni-filtered CuKa radiation (k = 1.5406 Å) from a generator operating at 40 kV and 40 mA in the 2θ range between 10 and 80°. The instrument is supported with interfaces of DIFFRACplus SEARCH and DIFFRACplus EVA to facilitate an automatic search and match of the crystalline phases for identification purposes with the ICDD database.
The Brunauer–Emmett–Teller (BET) surface area and porosity of all samples calcined at 400 and 500 °C were measured by N2 adsorption/desorption isotherms at the liquid nitrogen temperature (−196 °C) using a Micromeritics ASAP 2020 system, equipped with an online data acquisition and handling system operating BET and Barrett–Joyner–Halenda analytical software. All samples were degassed at 200 °C and 10–5 Torr for 2 h before measurements (1 Torr = 133.3 Pa).
The total number of basic sites (sites·g–1) over each sample was measured by the TPD of pyrrole (99%-ACROS Organics, New Jersey, USA) as a probe molecule using TG and DSC techniques. The experimental details can be explained as follows:33,34 60 mg of each sample is preheated at 390 °C for 1 h in the air before the probe molecule is exposed. 20 ± 2 mg covered samples with pyrrole were subjected to thermogravimetric analysis (TGA) and another sample was subjected to DSC analyses at a heating rate of (10 °C·min–1) in a dry N2 flow (40 mL·min–1), using a 50H Shimadzu thermal analyzer (Japan). The thermal analyzer is equipped with a data acquisition and handling system (TA-50WSI). α-Al2O3 was used as the reference material in DSC measurements. The mass loss due to the desorption of pyrrole during TG experiments from the basic sites was determined to measure the total surface basicity as sites per gram. Equation 1 is used to estimate the total number of surface basic sites (Nbasic):35
| 1 |
where npyrrole is the number of moles of desorbed pyrrole, NA is Avogadro’s number (sites/mol), and wTG is the weight of the TG sample (g).
2.3. CO2 Capture Experiments in a Dynamic Mode under Ambient Pressure
Before the CO2 capture experiment, each sample was activated in a homemade fixed-bed U-tube Pyrex reactor by heating 100 mg of the sample at 390–400 °C for 2 h in an oxygen flow (150 mL·min–1). The sample is cooled down to room temperature (RT), and the reactor is then transferred to a thermostatic water bath set to the desired temperature, that is, at 5, 10, or 30 °C, as shown in Figure 1. The CO2 gas stream (99.9%) is allowed to pass over the catalyst bed for 1 h, with a flow rate = 20 mL·min–1, permeating between catalyst particles inside the reactor to exit from the opposite side of the reactor. The residual CO2 flow is trapped by passing through an aqueous 5% solution of KOH. The quantity of CO2 molecules captured by each sample was measured immediately, employing the TPD technique, using the above-mentioned TG and DSC units as follows: 20–25 mg of each sample was subjected to TG and DSC analyses on heating to 425 °C, in the case of samples calcined at 400 °C, or to 525 °C in the case of the other samples treated at 500 °C (at a 10 °C·min–1 heating rate) in dry N2 (flow rate = 40 mL·min–1). In TG curves, the mass loss due to CO2 desorption was measured as the capturing efficiency of each sample at the specified temperature. Values of the molar enthalpy change (ΔHdes in J mol–1) corresponding to the desorption of CO2 in each step, at different temperatures, of the most active samples were calculated from the DSC curves.36
Figure 1.
Schematic diagram of CO2 captured in a dynamic mode at different temperatures with a flow rate of 20 mL·min–1.
3. Results and Discussion
3.1. Thermogravimetric Analysis
A slow heating rate was chosen (3 °C·min–1) to examine the thermal decomposition of both Mg(OH)2 and ammonium hydrogen difluoride (AHDF) NH4F·HF as pure precursors, as well as all their mixtures in the He atmosphere, as presented in Figure 2. Due to the loss of physically adsorbed water molecules, Mg(OH)2 began to lose weight upon heating immediately and continuously up to 200 °C. This step is followed by another weight loss at approximately 270 °C, which is ascribed to the dehydroxylation of Mg(OH)2.37,38 At 400 °C, the calculated mass loss was 34.5%, whereas at 500 °C, it was 37.7%. Due to the liberation of physically adsorbed water molecules associated with Mg(OH)2, these values exceeded the theoretical value38−40 for the conversion of anhydrous Mg(OH)2 to MgO (ca. 30.86%), as presented in eq 2:
| 2 |
on the other hand, NH4F·HF undergoes melting at 125 °C, followed by a subsequent fast step due to its decomposition around 150 °C,41 as shown in eq 3:
| 3 |
the TG profiles of the mixed samples can be divided into four temperature zones, as shown in Figure 2. The first zone in the temperature range RT–150 °C, includes both melting and decomposition of AHDF (see eq 3). Zone-II in the temperature range 150–250 °C exhibits the reaction of the HF liberated by eq 3 with Mg(OH)2 in these mixtures to produce magnesium fluoride and magnesium fluoride hydroxide42 as follows:
| 4 |
and
| 5 |
Notably, the % mass loss associated with this step in Zone-II increased from 5.1 to up to 15.1% as the % (x) of AHDF added to Mg(OH)2 increased from 20 to 50%, respectively. This may be attributable to the increased amounts of the liberated HF that participate in reactions 4 and 5 in these mixtures. Furthermore, melting NH4F·HF as a fluorinating agent in these mixtures facilitates the formation of both MgF2 and magnesium fluoride hydroxide Mg(OH)F at a low-temperature range in all prepared samples.42,43 It is a benefit of this method that MgF2 can be prepared more easily. This phenomenon has a negative impact on these samples’ surface area, as will be discussed later. The third zone (250–390 °C) could be related to both dehydroxylation and dehydration processes,42 followed by a slow weight loss step at a temperature above 390 °C (Zone-IV) due to the removal of any residual H2O molecules still bonded on the surface of the solid samples, as follows:
| 6 |
according to the TG curves of the last two samples, the final product at 400 °C, Mg(OH)2-40% F and Mg(OH)2-50% F, suggests the formation of the Mg–O–F nanocomposite as a mixture of MgF2 and MgO. XRD analysis will provide further explanation to confirm this hypothesis.
Figure 2.
TG curves of Mg(OH)2, NH4F·HF (AHDF), and their mixtures were performed in 40 mL·min–1 He, with a 3 °C·min–1 heating rate.
3.2. XRD Analysis
XRD analysis of the prepared nanocomposite mixtures at 400 and 500 °C was performed in the range of 10–80° (2θ) to confirm the nanocomposite structure of these samples. Figure 3a,b demonstrates the XRD patterns of all samples. The diffraction patterns of all samples calcined at 400 °C (Figure 3a) showed two main diffraction peaks belonging to the polycrystalline cubic structure of MgO (ICCD file: 74-1225)12,44,45 at 42.785 and 62.233°, with Miller indices values of (200) and (220), respectively. These diffraction peaks were sharp and predominant in the case of Mg(OH)2-20% F, then their intensity decreased gradually with increasing (x) % F added to Mg(OH)2, see Figure 3a. On the other hand, a group of diffraction peaks associated with the formation of tetragonal MgF2 (ICCD file: 6-0290) in these mixtures43,46−49 was recorded along with the crystallographic planes (110), (101), (111), (201), (211), and (220), which correspond to the Bragg reflections at 2θ values of 27.265, 35.174, 40.405, 43.613, 53.468, and 56.149°, respectively. The sharpness and intensity of these diffractions increased steadily from Mg(OH)2-20% F up to Mg(OH)2-50% F due to the formation of further MgF2 in these nanocomposite mixed samples. The same diffraction peaks were recorded in all patterns of samples calcined at 500 °C, as shown in Figure 3b, as previously explained in the samples calcined at 400 °C. These diffractions were characterized by a strong intensity and sharpness. Furthermore, the predominance of MgF2 was clearly observed in these samples at 500 °C, particularly in those containing more than 20% F, see Figure 3b. The XRD analysis of our samples reveals that the Mg(OH)2-20% F sample, whether calcined at 400 or 500 °C, contains more MgO than MgF2. This phenomenon will significantly impact the sample’s surface area and CO2 capturing efficiency, as will be discussed later. The mean crystallite size of MgO in all samples was calculated using the (200) reflection to be within the range of 7.9–9.4 nm and between 8.6 and 12.0 nm for samples calcined at 400 and 500 °C, respectively. Furthermore, the crystallite size of MgF2 in all samples was estimated using the main reflection (111) and was in the range of 3.2–19.1 and 21.6–26.7 nm for samples calcined at 400 and 500 °C, respectively. The calculated values of the crystallite size using the Scherrer equation of all samples are cited in Table 1. Notably, increasing the calcination temperature from 400 to 500 °C resulted in the formation of larger MgO and MgF2 crystallite phases in all samples.
Figure 3.
XRD patterns of Mg(OH)2 mixed with 20–50% F (wt by wt) calcined in static air for 3 h at (a) 400 and (b) 500 °C.
Table 1. Textural Properties of Mg(OH)2 Modified with x % F (by wt) That Was Calcined at 400 and 500 °C for 3 h in Air and the Crystallite Size of the Obtained Phases, as Calculated from XRD Patterns.
| XRD
crystallite size (nm)a |
||||||||
|---|---|---|---|---|---|---|---|---|
| adsorbent composition | MgO* cubic | MF2** tetragonal | SBET(m2/g) | external surface area (m2/g) | micropore area (m2/g) | total pore volume (cm3/g) | mesopore volume (cm3/g) | average pore diameter (nm) |
| Mg(OH)2 + 20% F-400 | 9.4 | 3.2 | 153.22 | 145.79 | 7.43 | 0.23 | 0.23 | 3.7 |
| Mg(OH)2 + 30% F-400 | 7.9 | 8.9 | 117.18 | 111.49 | 5.68 | 0.26 | 0.26 | 3.7, 13.0 |
| Mg(OH)2 + 40% F-400 | 8.6 | 14.6 | 88.27 | 79.39 | 8.88 | 0.29 | 0.29 | 3.6, 16.0 |
| Mg(OH)2 + 50% F-400 | 19.1 | 72.22 | 61.59 | 10.62 | 0.31 | 0.30 | 3.6, 18.9 | |
| Mg(OH)2 + 20% F-500 | 12.0 | 22.7 | 103.06 | 96.25 | 6.81 | 0.24 | 0.24 | 3.5, 6.1 |
| Mg(OH)2 + 30% F-500 | 8.6 | 22.8 | 81.93 | 80.74 | 1.18 | 0.16 | 0.16 | 3.6 |
| Mg(OH)2 + 40% F-500 | 9.9 | 21.6 | 58.32 | 54.31 | 4.00 | 0.13 | 0.13 | 3.7, 33.0 |
| Mg(OH)2 + 50% F-500 | 26.7 | 39.70 | 33.83 | 5.87 | 0.12 | 0.11 | 3.5, 33.8 | |
The position of the most intense peak is at (*) 2θ = 42.84° (200) in the case of MgO and (**) = 40.38° (111) in the case of MgF2.
3.3. Surface Area and Porosity Measurements
The textural properties of the samples calcined at 400 and 500 °C were investigated using the BET method. Figure 4 exhibits the N2 adsorption/desorption isotherms that were recorded at −196 °C and the pore volume profiles of all samples. It is concluded from Figure 4a,b that all samples, whether calcined at 400 or 500 °C, display type IV isotherms, as recommended by the IUPAC,50,51 with type H3 hysteresis loops.52 The calculated values of the surface area (SBET) and the corresponding values of the external surface area of samples calcined at 400 °C, as shown in Table 1, were approximately 1.4–1.5 times higher than those calcined at 500 °C, except for samples containing 50% F, which experienced a clear reduction in the surface area. The gradual decrease of the surface area of all samples with the increase in the added amounts of (x)% F as NH4F·HF is probably due to the melting of this fluorinating agent41 at an early stage in the calcination process of these mixtures. This phenomenon has caused the surface area of all samples calcined for 3 h at 400 and 500 °C to decrease. The adsorption hysteresis of all samples was located in the P/P° region of 0.40–0.45, except for one sample, that is, Mg(OH)2 mixed with 50% F and calcined at 500 °C, see Figure 4b. This is interpreted as evidence that all samples are mesoporous.51 The pore volume profiles of these samples strongly supported these findings; see Figure 4a(1),b(1). All samples showed pores with a 3.5–3.7 nm diameter, see Table 1. Increasing the mixing ratios of (F), that is, > 20% F, has resulted in the generation of new wide pores with diameters of 13, 16, and 18.9 nm in the case of samples calcined at 400 °C. Two samples, namely, Mg(OH)2-40% F and Mg(OH)2-50% F, calcined at 500 °C, recorded wider mesopores with diameters equal to 33 and 33.8 nm, respectively, see Figure 4a(1),b(1). Finally, the calculated values of both the total pore volume and mesopore volume in cubic centimeter per gram, as shown in Table 1, varied steadily with the addition of (x) % F in these nanocomposite mixtures. This is due to the release of more NH3 during the decomposition of molten NH4F·HF, as shown in eq 3, resulting in larger pore volumes in samples calcined at 400 °C. In contrast, samples calcined at 500 °C exhibited a gradual decrease in the total pore volume and mesopore volume. This may be a result of the formation of larger crystallites at 500 °C at the expense of the pore volumes in these mixtures, see Table 1.
Figure 4.
N2 adsorption–desorption isotherms and pore diameter profiles of samples of Mg(OH)2-(x) wt % F calcined at 400 °C [a,a(1)] and at 500 °C [b,b(1)].
3.4. Assessment of the Surface Basicity Using TG and DSC-TPD Techniques of Pyrrole
The surface basicity of any adsorbent is critical for achieving a high catalytic activity in the dehydrogenation reaction of alcohols53 and enhancing its capacity to capture CO2.54 The catalytic surface with the highest concentrations of basic sites is the most effective in capturing CO2. Using TG- and DSC-TPD techniques, pyrrole was used as a probe molecule to determine the number and strength of surface basic sites over all samples in this work. Numerous articles have studied the TPD technique of pyrrole55−57 to categorize the different types of basic sites over solid catalysts.
Others investigated the use of pyrrole as an IR spectroscopic molecular probe in a surface basicity determination of MgF2 and metal oxides,58,59 as well as employing pyrrole as an NMR probe molecule to determine the basic sites’ strength over solid catalysts.60 Very recently, Chen and his co-workers33 proposed some possible intermediates of pyrrole due to its reactions with the O/Cu (100) surface, based on the TPD curves of pyrrole.
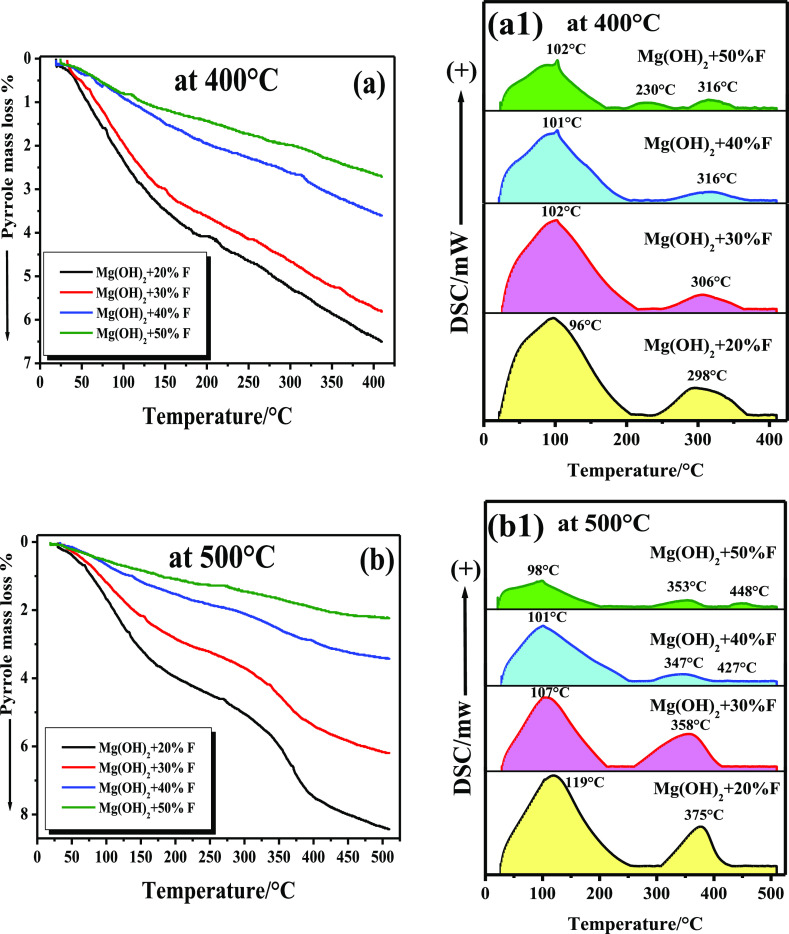
TG and DSC-TPD profiles of pyrrole recorded for all samples, calcined at 400 and 500 °C, are shown in Figure 5. The total number of basic sites over each sample is calculated from the TG curve, while the DSC profile exhibits the distribution and strength of the different types of these basic sites over its surface. The weight loss due to the desorption of pyrrole from the surface of each sample, in milligrams or pyrrole per gram and the total number of molecules of pyrrole desorbed per gram corresponding to the indicated temperature range were calculated and are shown in Table 2. Increasing the (x) % F from 20 to 50% added to Mg(OH)2 in these mixtures was accompanied by the formation of more MgF2 in these samples, as shown in XRD patterns, see Figure 3. It is known that MgF2 particles possess a strong Lewis acidity during their formation in magnesium hydroxyl fluoride.58,61 Consequently, a steady reduction of the total basic sites can be observed through these samples, whether calcined at 400 or 500 °C, as shown in the TG curves (Figure 5a,b) and the calculated values in Table 2. The calculated values of all samples’ total number of basic sites are fully compatible with the surface area measurement, see Table 2. Observing the DSC-TPD profiles of samples calcined at 500 °C, shown in Figure 5b(1), reveals that the basic sites over these samples are more pronounced than those over samples calcined at 400 °C. The recorded Tmax values of the strong basic sites, over samples calcined at 500 °C, were shifted to higher temperatures in the range of 347–375 °C. Furthermore, a new type of strong basic sites clearly appeared in the case of Mg(OH)2-50% F that was calcined at 400 and 500 °C at higher temperatures, see Figure 5a(1),b(1). From the results mentioned above, it is notable that all samples contain a higher ratio of weak basic sites, in addition to strong basic sites with a low ratio at higher temperatures, see the DSC-TPD profiles in Figure 5a(1),b(1). As is discussed in the following section, this distribution of basic sites will further support samples’ efficiency in capturing CO2 at low temperatures.
Figure 5.
Pyrrole TG and DSC-TPD curves recorded for Mg(OH)2 mixed with 20–50% (wt/wt) F, as NH4·HF, calcined at 400 °C [a,a(1)] and calcined at 500 °C [b,b(1)].
Table 2. Basicity Measurements Calculated Based on TG-TPD Curves of Pyrrole over Mg(OH)2 + x % F Adsorbents Calcined, for 3 h in Air, at 400 and 500 °C.
| calcined
at 400 °C |
calcined
at 500 °C |
|||||
|---|---|---|---|---|---|---|
| adsorbent | SBET(m2·g–1) | apyrrole RT–400 °C(mgpyrr·g–1) | btotal no. of basic sites(site·g–1) | SBET(m2·g–1) | apyrrole RT–500 °C(mgpyrr·g–1) | btotal no. of basic sites (site·g–1) |
| Mg(OH)2 + 20% F | 153.22 | 62.5 | 5.61 × 1020 | 103.06 | 82.4 | 7.39 × 1020 |
| Mg(OH)2 + 30% F | 117.16 | 56.7 | 5.09 × 1020 | 81.93 | 60.2 | 5.40 × 1020 |
| Mg(OH)2 + 40% F | 88.27 | 35.1 | 3.15 × 1020 | 58.32 | 33.4 | 3.00 × 1020 |
| Mg(OH)2 + 50% F | 72.22 | 26.3 | 2.36 × 1020 | 39.7 | 21.6 | 1.93 × 1020 |
Mass loss calculated from TG-TPD curves.
Total number of basic sites (sites per gsolid).
3.5. CO2 Capture Study
In order to adjust the temperature at which CO2 capture experiments yield logical and good results. A preliminary test was carried out at three different temperatures, that is, 5, 10, and 30 °C. TG- and DSC-TPD profiles (Figure 6) represent the results where CO2 permeation of the sample under test, that is, Mg(OH)2-20% F at 400 °C, with the highest surface area using a slow flow rate (20 mL·min–1) of CO2 in a vertical U-tube reactor is conducted. Two different temperatures, 5 and 30 °C, were selected for all CO2-capturing tests due to the overlap of results recorded at 10 °C with those at 5 °C, especially at temperatures <350 °C. At 5 °C, the sample captured 103.9 mgCO2·g–1, which is equivalent to 2.36 mmolCO2·g–1, while at 10 °C, it captured 111.9 mgCO2·g–1 and 2.54 mmolCO2·g–1, respectively. Furthermore, at 30 °C, the results were clearly different, as shown in Table 3. This test shed light on the efficiency of our samples in capturing CO2 at low and relatively high temperatures, that is, 30 °C, as will be discussed later. Figures 7 and 8 exhibit the collected TG and DSC-TPD profiles of CO2 captured by Mg(OH)2-x % F samples calcined at 400 and 500 °C at 5 and 30 °C, respectively. Table 3 lists the total amounts of CO2 captured by each sample as % mass loss (due to the desorption of CO2) in milligrams of CO2 per gram and millimoles of CO2 per gram, respectively, in the temperature ranges of RT–420 °C and RT–520 °C, in the case of samples calcined at 500 °C.
Figure 6.

CO2 capture at different temperatures by Mg(OH)2-20% F, calcined at 400 °C, in a dynamic mode using a CO2 flow rate of 20 mL·min–1 (a,b).
Table 3. CO2 Capture Capacity at 5 and 30 °C, Calculated Based on TG-TPD Curves, of Mg(OH) + x % F Adsorbents Calcined, for 3 h in Air, at 400 and 500 °Ca.
| calcined
at 400 °C |
calcined
at 500 °C |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| adsorbent | total % mass loss (RT–420 °C) | *total mgCO2·g–1(RT–420 °C) | **total (mmolCO2·g–1)(RT–420 °C) | total % mass loss (RT–420 °C) | *total mgCO2·g–1(RT–420 °C) | **total (mmolCO2·g–1)(RT–420 °C) | total % mass loss (RT–520 °C) | total mgCO2·g–1(RT–520 °C) | total (mmolCO2·g–1)(RT–520 °C) |
| CO2 Capture at 5 °C | |||||||||
| Mg(OH)2 + 20% F | 10.39 | 103.9 | 2.36 | 7.73 | 77.3 | 1.76 | 8.21 | 82.1 | 1.86 |
| Mg(OH)2 + 30% F | 7.76 | 77.6 | 1.76 | 7.28 | 72.8 | 1.65 | 7.78 | 77.8 | 1.77 |
| Mg(OH)2 + 40% F | 4.77 | 49.5 | 1.13 | 4.47 | 44.7 | 1.02 | 4.73 | 47.3 | 1.07 |
| Mg(OH)2 + 50% F | 3.94 | 39.6 | 0.90 | 2.78 | 27.8 | 0.63 | 2.89 | 28.9 | 0.66 |
| CO2 Capture at 30 °C | |||||||||
| Mg(OH)2 + 20% F | 8.94 | 89.4 | 2.03 | 5.24 | 52.4 | 1.19 | 5.56 | 55.5 | 1.26 |
| Mg(OH)2 + 30% F | 6.20 | 61.9 | 1.41 | 4.64 | 46.4 | 1.05 | 4.99 | 49.9 | 1.13 |
| Mg(OH)2 + 40% F | 4.00 | 39.9 | 0.91 | 2.97 | 29.7 | 0.68 | 3.14 | 31.4 | 0.71 |
| Mg(OH)2 + 50% F | 3.22 | 32.2 | 0.73 | 1.99 | 19.9 | 0.45 | 2.09 | 20.9 | 0.47 |
*,** for comparison at the same temperature range (RT–420 °C).
Figure 7.

Capture of CO2 in a dynamic mode, using a flow rate of 20 mL·min–1, by Mg(OH)2 + x% F (wt/wt) calcined at 400 °C for 3 h in air, at 5 °C (a,b), and at 30 °C (c,d).
Figure 8.

Capture of CO2 in a dynamic mode, using a flow rate of 20 mL·min–1, by Mg(OH)2 + x% F (wt/wt) calcined at 500 °C for 3 h in air, at 5 °C (a,b), and at 30 °C (c,d).
Due to the continuous desorption of CO2 from weak and strong basic sites, all TG-TPD curves exhibited two continuous mass loss steps. This behavior is evident whether CO2 was captured at 5 or 30 °C. The total % mass loss due to desorption of CO2, in the range of RT–420 °C, dramatically decreased as the (x) % F added to Mg(OH)2 steadily increased. This phenomenon is correlated with the decrease in the surface area of these samples due to the formation of MgF2 in these nanocomposite mixtures, as shown in Table 1, and consequently a reduction of some of the total number of basic sites over the sample’s surface (see Table 2). Comparing the amount of CO2 desorbed within the same temperature range reveals an additional effect of calcination temperature on the CO2-capturing efficiency of the samples. Heating samples up to 420 °C, the total amount of desorbed CO2 (as mgCO2·g–1 and mmolCO2·g–1) for samples calcined at 400 °C were always higher than those calculated for samples at 500 °C in the same temperature range; see Table 3. Calcinating samples at 500 °C liberates OH groups from the surface of these mixtures62 and eliminates surface defects of MgO. This clearly reduces both weak and strong basic sites over all samples. From the data of CO2 capture at 5 and 30 °C shown in Table 3 over samples calcined at 400 and 500 °C and the curves (a,c) in Figures 7 and 8, it is evident that the amount of CO2 captured by any sample is inversely proportional to the temperature at which the capturing process is carried out. This is ascribed to the exothermic nature of the adsorption of CO2 over the MgO surface, as confirmed recently.23
DSC-TPD profiles, as presented in Figures 7 and 8 (profiles b,d), revealed that our samples mainly include two different basic sites. Weak basic sites are observed in the temperature range of 29–200 °C, where CO2 is associated with these weak sites in the form of bicarbonate species.54 Furthermore, strong basic sites are recorded at a higher temperature range,62,63 that is, 290–450 °C, where CO2 interacts with the strong basic sites to form monodentate and bridged carbonate carbonates.54,62 This is clearly exhibited in the case of Mg(OH)2-20% F calcined at 400 °C and to somewhat in the case of the other samples, see Figure 7.
From the above-discussed results, one can conclude that the most active sample in capturing CO2 at 5 and 30 °C was Mg(OH)2-20% F, whether calcined at 400 or 500 °C. Its reactivity could be associated with the presence of surface interfaces between both MgF2 with low concentrations distributed in the bulk of MgO throughout its high surface area.
The calculated values of the molar enthalpy change (ΔH, J mol–1) corresponding to each desorption step, based on TG- and DSC-TPD profiles in Figures 7 and 8, for the most active adsorbent Mg(OH)2-20% F are listed in Table 4. It is obvious from Table 4 that values of ΔH calculated for step-1, in the case of Mg(OH)2-20% F at 400 °C, are always higher than the corresponding values calculated for step-2 during CO2 capture at 5 and 30 °C. This agrees with the desorbed amounts of CO2 in each step, see Table 4. This behavior does not match with that of the sample calcined at 500 °C. Our values of ΔH for both steps, as presented in Table 4, for desorption of CO2 after (1 h) capturing the pure gas, are in close agreement with those recently published.12
Table 4. Enthalpy Changes Corresponding to the Desorption Steps of CO2 Captured at 5 and 30 °C, Calculated Based on TG- and DSC-TPD Profiles, of the Most Active Adsorbent Mg(OH)2 + 20% F Calcined, for 3 h in Air, at 400 and 500 °C.
| Mg(OH)2 + 20% F at 400 °C |
Mg(OH)2 + 20% F at 500 °C |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| step-1(29–225 °C) |
step-2(290–450 °C) |
step-1(29–225 °C) |
step-2(290–450 °C) |
|||||||||
| experimental condition | Desor. CO2mgCO2·g–1 | ΔH1(J·mol–1) | Tmax1(°C) | Desor. CO2mgCO2·g–1 | ΔH2(J·mol–1) | Tmax2(°C) | Desor. CO2mgCO2·g–1 | ΔH1(J·mol–1) | Tmax1(°C) | Desor. CO2mgCO2·g–1 | ΔH2(J·mol–1) | Tmax2(°C) |
| CO2 capture at 5 °C | 55.2 | 599.5 | 105 | 41.8 | 561.1 | 374, 410 | 49.9 | 528.5 | 101 | 27.0 | 572.6 | 379 |
| CO2 capture at 30 °C | 43.1 | 825.1 | 111 | 41.0 | 503.8 | 357, 400 | 32.6 | 526.3 | 108 | 15.3 | 584.1 | 359 |
Finally, Table 5 compares the CO2-capturing capacities of some adsorbents, under the same conditions, from previously published articles to the obtained results using the most active sample Mg(OH)2-20% F calcined at 400 and 500 °C.
Table 5. Comparison of CO2 Adsorption Capacity by Different MgO Adsorbents at Different Conditions as Cited in the References, and the Obtained Values of Our Adsorbent Mg(OH)2-20% F Calcined at 400 and 500 °C as the Most Active Onea.
| experimental
conditions |
|||||
|---|---|---|---|---|---|
| adsorbent | SBETm2·g–1 | temp. (°C) | CO2(vol %) | mgCO2·g–1 and mmolCO2·g–1 | reference |
| Mg(OH)2-20%F-400 °C | 153.22 | at 5 | 100% | 103.9 mgCO2 | this work |
| 2.36 mmolCO2 | |||||
| at 30 | 100% | 89.4 mgCO2 | this work | ||
| 2.03 mmolCO2 | |||||
| 103.06 | at 5 | 100% | 82.1 mgCO2 | this work | |
| Mg(OH)2-20%F-500 °C | 1.86 mmolCO2 | ||||
| at 30 | 100% | 55.5 mgCO2 | this work | ||
| 1.26 mmolCO2 | |||||
| metal–organic framework (MOF) | N.C. | at 25 | 100% | 114.96 mgCO2 | (64) |
| glass industry waste | |||||
| waste SiO2 | 16.82 | at 20 | 50% | 0.71 mmolCO2 | (65) |
| waste Al2O3 | 7.28 | at 20 | 50% | 0.68 mmolCO2 | (65) |
| *MgO | 350.0 | at 30 | 100% | 30.0 mgCO2 | (66) |
| *MgO-APTES | 134.0 | at 30 | 100% | 65.7 mgCO2 | (66) |
| *MgO-DETA | 91.0 | at 30 | 100% | 47.7 mgCO2 | (66) |
| *MgO-PEI | 72.0 | at 30 | 100% | 23.9 mgCO2 | (66) |
| **MgO-SR adsorbent | 100.0 | at 200 | 10% | 2.39 mmolCO2 | (67) |
| CeO2 | 333.0 | at 30 | 100% | 10.4 wt % | (68) |
N.C.-not calculated, *MgO modified with 3-aminopropyl-triethoxysilane (APTES), diethylenetriamine (DETA), and polyethylenimine (PEI), **Prepared by a solid-state reaction.
The performance of our CO2 capture adsorbents appears to be superior to that of many adsorbents previously evaluated for the same application (see Table 5). This adsorbent includes MgO modified with 3-aminopropyl-triethoxysilane (APTES), diethylenetriamine (DETA), and polyethylenimine (PEI), MgO prepared by a solid–state reaction, and CeO2. This adsorbent also includes adsorbents derived from waste materials, such as “glass industry waste: waste SiO2 and waste Al2O3,” for the circular economy and waste management approach.65−68 Therefore, it is not surprising that our samples, which were prepared by mixing Mg(OH)2 with different wt % of NH4F·HF, particularly those containing 20–30% F, whether calcined at 400 or 500 °C, demonstrated a high capacity in capturing a large number of CO2 molecules in a short period of time (1 h), see Table 3, at different temperatures of 5 and 30 °C.
Conclusions
-
1
Using environmentally friendly materials, such as Mg(OH)2 and NH4F·HF, a direct and simple method has been developed for the preparation of nanocomposite mixtures of Mg(OH)2-(x) wt % F.
-
2
The obtained samples were mixtures of MgO and MgF2 with a high surface area and a mesoporous structure. Using the DSC-TPD technique with pyrrole, all samples, whether calcined at 400 or 500 °C, exhibited two surface basic sites as weak and strong basic sites, as measured by pyrrole as a probe molecule.
-
3
We correlated the high efficiency of these samples toward CO2 capture at 5 and 30 °C to their high surface area, the high population of surface basic sites with weak and strong basic sites, and the presence of a low concentration of MgF2 composing active interfaces in MgO as the main component in these samples, as seen from XRD patterns. On the other hand, the efficiency was inversely proportional to the temperature at which the samples were calcined.
-
4
The Mg(OH)2-20% F sample was the most active in our investigation because it had a larger surface area and a greater total number of basic sites than the other samples (30–50% F). Consequently, the Mg(OH)2-20% F sample is the most effective in capturing CO2.
Future work will utilize the prepared Mg–O–F samples from this study and design MgO-based looping new systems for CO2 capture in the flue gas of diluted CO2 concentration and containing steam (H2O) and gas impurities (e.g., SO2, NO, CO, and HCl), which are commonly present in coal plants and other industrial applications, to investigate the effects of these impurities on the sorption performance. In addition, the future work will include increasing the operating pressure from 1 to 20 or 30 atm and the temperature to various values to examine the effect of increasing pressure and temperature on the sorption performance.
Acknowledgments
The authors dedicate this work to the spirit of the distinguished Egyptian professor Samih A. Halawy, who passed away on September 2, 2022. The authors acknowledge the support of The Bryden Centre (Project ID VA5048). The Bryden Centre is supported by the European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB).
The authors declare no competing financial interest.
Author Status
§ Died on September 2, 2022.
References
- https://www.downtoearth.org.in/news/climate-change/global-co2-concentration-in-atmosphere-hit-new-high-in-may-2nd-week-report-82907. (Accessed 18 May 2022)
- Donat F.; Müller C. R. Prospects of MgO-based sorbents for CO2 capture applications at high temperatures. Curr. Opin. Green Sustainable Chem. 2022, 36, 100645. 10.1016/j.cogsc.2022.100645. [DOI] [Google Scholar]
- Liu W.; Cai Y.; Luo M.; Yang Y.; Li P. Potential Application of Alkaline Metal Nitrate-Promoted Magnesium-Based Materials in the Integrated CO2 Capture and Methanation Process. Ind. Eng. Chem. Res. 2022, 61, 2882–2893. 10.1021/acs.iecr.1c04615. [DOI] [Google Scholar]
- Hu P.; Wang S.; Zhuo Y. Strengthened CO2 adsorption over Ce/Al-promoted MgO for fast capture. Sep. Purif. Technol. 2022, 287, 120518. 10.1016/j.seppur.2022.120518. [DOI] [Google Scholar]
- Sun S.; Sun H.; Guan S.; Xu S.; Wu C. Integrated CO2 capture and methanation on Ru/CeO2-MgO combined materials: Morphology effect from CeO2 support. Fuel 2022, 317, 123420. 10.1016/j.fuel.2022.123420. [DOI] [Google Scholar]
- Guo X.; Ding J.; Wu Y.; Zhang J.; Guo G. Feasible fabrication of highly dispersed La2O3 promoted MgO composites for CO2 capture at mid-temperature. Mater. Chem. Phys. 2022, 279, 125734. 10.1016/j.matchemphys.2022.125734. [DOI] [Google Scholar]
- Bang G.; Kim K.-M.; Jin S.; Lee C.-H. Dynamic CO2 sorption on MgO-based sorbent in the presence of CO and H2O at elevated pressures. Chem. Eng. J. 2022, 433, 134607. 10.1016/j.cej.2022.134607. [DOI] [Google Scholar]
- Pang H.; Xu H.; Sun A.; Xiao G. Characteristics of MgO-based sorbents for CO2 capture at elevated temperature and pressure. Appl. Surf. Sci. 2022, 598, 153852. 10.1016/j.apsusc.2022.153852. [DOI] [Google Scholar]
- Nagarajan L.; Saravanan P.; Kumaraguru K.; Joo S.-W.; Vasseghian Y.; Rajeshkannan R.; Rajasimman M. Synthesis of magnesium nanocomposites decked with multilayer graphene (MG) and its application for the adsorptive removal of pollutant. Chemosphere 2022, 298, 134121. 10.1016/j.chemosphere.2022.134121. [DOI] [PubMed] [Google Scholar]
- Fan A. Acid-base bifunctional magnesium oxide catalyst prepared from a simple hydrogen peroxide treatment for highly selective synthesis of jasminaldehyde. Energy Sources, Part A 2020, 42, 2501–2515. 10.1080/15567036.2019.1607949. [DOI] [Google Scholar]
- Fan A.; Gao H. Synthesis of MgO nanostructures through simple hydrogen peroxide treatment for carbon capture. Process Saf. Environ. Prot. 2021, 156, 361–372. 10.1016/j.psep.2021.10.024. [DOI] [Google Scholar]
- Halawy S. A.; Mohamed M. A.; El-Nahas S. A prolonged evaluation of air contamination level with CO2 in a college student’s laboratory using nanosized MgO. Int. J. Environ. Sci. Technol. 2020, 17, 1551–1566. 10.1007/s13762-019-02584-0. [DOI] [Google Scholar]
- Aquatar Md. O.; Bhatia U.; Rayalu S. S.; Krupadam R. J. Reduced graphene oxide-MnO2 nanocomposite for CO2 capture from flue gases at elevated temperatures. Sci. Total Environ. 2022, 816, 151522. 10.1016/j.scitotenv.2021.151522. [DOI] [PubMed] [Google Scholar]
- Jo S.; Lee J. H.; Kim T. Y.; Woo J. H.; Ryu H.-J.; Hwang B.; Lee S. C.; Kim J. C.; Gilliard-AbdulAziz K. L. A fundamental study of CO2 capture and CH4 production in a rapid cyclic system using nickel-lithium-silicate as a catal-sorbent. Fuel 2022, 311, 122602. 10.1016/j.fuel.2021.122602. [DOI] [Google Scholar]
- Chen S.; Dai J.; Qin C.; Yuan W.; Manovic V. Adsorption and desorption equilibrium of Li4SiO4-based sorbents for high-temperature CO2 capture. Chem. Eng. J. 2022, 429, 132236. 10.1016/j.cej.2021.132236. [DOI] [Google Scholar]
- Dong J.; Wang F.; Chen G.; Wang S.; Ji C.; Gao F. Fabrication of nickel oxide functionalized zeolite USY composite as a promising adsorbent for CO2 capture. Chin. J. Chem. Eng. 2022, 46, 207–213. 10.1016/j.cjche.2021.10.011. [DOI] [Google Scholar]
- Lund A.; Manohara G. V.; Song A.-Y.; Jablonka K. M.; Ireland C. P.; Cheah L. A.; Smit B.; Garcia S.; Reimer J. A. Characterization of Chemisorbed Species and Active Adsorption Sites in Mg-Al Mixed Metal Oxides for High-Temperature CO2 Capture. Chem. Mater. 2022, 34, 3893–3901. 10.1021/acs.chemmater.1c03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Ye Q.; Wang L.; Meng F.; Dai H. Mesoporous alumina-supported layered double hydroxides for efficient CO2 capture. J. CO2 Util. 2022, 60, 101982. 10.1016/j.jcou.2022.101982. [DOI] [Google Scholar]
- Xu J.; Liu C.; Zhiani R. CuAl2O4 nanoplates as a nanocatalyst for carbon dioxide adsorption at high temperatures. Inorg. Chem. Commun. 2022, 139, 109421. 10.1016/j.inoche.2022.109421. [DOI] [Google Scholar]
- Wang G.; Guo Y.; Yu J.; Liu F.; Sun J.; Wang X.; Wang T.; Zhao C. Ni-CaO dual function materials prepared by different synthetic modes for integrated CO2 capture and conversion. Chem. Eng. J. 2022, 428, 132110. 10.1016/j.cej.2021.132110. [DOI] [Google Scholar]
- Hashim Z. H.; Kuwahara Y.; Hanaki A.; Mohamed A. R.; Yamashita H.. Synthesis of a CaO-Fe2O3-SiO2 composite from a dephosphorization slag for adsorption of CO2. Catal. Today 2022, in press. 10.1016/j.cattod.2022.03.030 [DOI]
- Tan C.; Guo Y.; Sun J.; Li W.; Zhang J.; Zhao C.; Lu P. Structurally improved MgO adsorbents derived from magnesium oxalate precursor for enhanced CO2 capture. Fuel 2022, 278, 118379. [Google Scholar]
- Alkadhem A. M.; Elgzoly M. A. A.; Alshami A.; Onaizi S. A. Kinetics of CO2 capture by novel amine-functionalized magnesium oxide adsorbents. Colloids Surf., A 2021, 616, 126258. 10.1016/j.colsurfa.2021.126258. [DOI] [Google Scholar]
- Chang R.; Wu X.; Cheung O.; Liu W. Synthetic solid oxide sorbents for CO2 capture: state-of-the art and future perspectives. J. Mater. Chem. A. 2022, 10, 1682–1705. 10.1039/d1ta07697c. [DOI] [Google Scholar]
- Sun S.; Sun H.; Williams P. T.; Wu C. Recent advances in integrated CO2 capture and utilization: a review. Sustain. Energy Fuels 2021, 5, 4546–4559. 10.1039/d1se00797a. [DOI] [Google Scholar]
- Dunstan M. T.; Donat F.; Bork A. H.; Grey C. P.; Müller C. R. CO2 capture at medium to high temperature using solid oxide-based sorbents: Fundamental aspects, mechanistic insights, and recent advances. Chem. Rev. 2021, 121, 12681–12745. 10.1021/acs.chemrev.1c00100. [DOI] [PubMed] [Google Scholar]
- Wang X.; He T.; Hu J.; Liu M. The progress of nanomaterials for carbon dioxide capture via the adsorption process. Environ. Sci.: Nano 2021, 8, 890–912. 10.1039/d0en01140a. [DOI] [Google Scholar]
- Nečina V.; Hostaša J.; Pabst W.; Veselý M. Magnesium fluoride (MgF2) – A novel sintering additive for the preparation of transparent YAG ceramics via SPS. J. Eur. Ceram. Soc. 2022, 42, 3290–3296. 10.1016/j.jeurceramsoc.2022.02.003. [DOI] [Google Scholar]
- Chen H.; Sun Q.; Zhang J.; Sheng J. Effect of MgF2 addition on sinterability and mechanical properties of fluorapatite ceramic composites fabricated by wollastonite and phosphate glass. Ceram. Int. 2022, 48, 20400–20408. 10.1016/j.ceramint.2022.03.325. [DOI] [Google Scholar]
- Tavakoli M.; Movahedi B.; Alhaji A. Fluorination synthesis of MgF2 nanoparticles synthesized for manufacturing IR windows by hot-pressing. Ceram. Int. 2021, 47, 21285–21292. 10.1016/j.ceramint.2021.04.135. [DOI] [Google Scholar]
- Motamedi M.; Crisostomo F.; Yao Y.; Mofarah S. S.; Chen W.-F.; Koshy P.; Taylor R. A. Single-layer, anti-reflective thin films of porous MgF2 for solar thermal applications. J. Phys. D: Appl. Phys. 2019, 52, 315501. 10.1088/1361-6463/ab1f5e. [DOI] [Google Scholar]
- Xu M.; Célérier S.; Comparot J. D.; Rousseau J.; Corbet M.; Richard F.; Clacens J. M. Upgrading of furfural to biofuel precursors via aldol condensation with acetone over magnesium hydroxide fluorides MgF2–x(OH)x. Catal. Sci. Technol. 2019, 9, 5793–5802. 10.1039/c9cy01259a. [DOI] [Google Scholar]
- Chen Y.-J.; You Z.-J.; Lee S.-S.; Chang L.-C.; Lin H.-S.; Liu Y.-F.; Liu Y.-X.; Lin J.-L. Comparison of adsorption and reactions of pyrrole on Cu(100) and O/Cu(100). Surf. Sci. 2021, 706, 121787. 10.1016/j.susc.2020.121787. [DOI] [Google Scholar]
- Osman A. I.; Abu-Dahrieh J. K.; Rooney D. W.; Halawy S. A.; Mohamed M. A.; Abdelkader A. Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl. Catal., B 2012, 127, 307–315. 10.1016/j.apcatb.2012.08.033. [DOI] [Google Scholar]
- Halawy S. A.; Osman A. I.; Abdelkader A.; Nasr M.; Rooney D. W. Assessment of Lewis-Acidic Surface Sites Using Tetrahydrofuran as a Suitable and Smart Probe Molecule. ChemistryOpen 2022, 11, e202200021 10.1002/open.202200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail H. M.; Mansour S. A. A.; Zaki M. I. Acid properties of silica and alumina surfaces as probed by thermogravimetry and differential scanning calorimetry of temperature-programmed desorption of pyridine. Thermochim. Acta 1992, 202, 269–280. 10.1016/0040-6031(92)85171-q. [DOI] [Google Scholar]
- Perera-Solis D. D.; Zholobenko V. L.; Whiting A.; Greenwell H. C. Heterogeneous ketonic decarboxylation of dodecanoic acid: studying reaction parameters. RSC Adv. 2021, 11, 35575–35584. 10.1039/d1ra06871g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q.; Li J.; Xudong L.; Xie Z.; An D. Development and Characterization on the Isothermal Kinetics of Mg(OH)2-sol Synthesized by Chemical Method. J. Australas. Ceram. Soc. 2022, 10, 130–137. 10.1080/21870764.2021.2019376. [DOI] [Google Scholar]
- Zhang R.; Arrigoni A.; Panesar D. K. Could reactive MgO cement be a green solution? The effect of CO2 mineralization and manufacturing route on the potential global warming impact. Cement Concr. Compos. 2021, 124, 104263. 10.1016/j.cemconcomp.2021.104263. [DOI] [Google Scholar]
- Iwasaki S.; Kodani S.; Koga N. Physico-Geometrical kinetic modeling of the thermal decomposition of magnesium hydroxide. J. Phys. Chem. C 2020, 124, 2458–2471. 10.1021/acs.jpcc.9b09656. [DOI] [Google Scholar]
- Resentera A. C.; Rosales G. D.; Esquivel M. R.; Rodriguez M. H. Thermal and structural analysis of the reaction pathways of α-spodumene with NH4HF2. Thermochim. Acta 2020, 689, 178609. 10.1016/j.tca.2020.178609. [DOI] [Google Scholar]
- Booster J. L.; Voncken J. H. L.; van Sandwijk A.; Reuter M. A. Characterization of hydroxyl-bearing magnesium fluoride containing physically bound water. Powder Diffr. 2002, 17, 112–118. 10.1154/1.1458940. [DOI] [Google Scholar]
- Kokane R. S.; Acham V. R.; Kulal A. B.; Kemnitz E.; Dongare M. K.; Umbarkar S. B. Palladium Supported on Fluorinated Magnesium Hydroxide: An Efficient Catalyst for Hydrogenation under Ambient Conditions. ChemistrySelect 2017, 2, 10618–10627. 10.1002/slct.201702217. [DOI] [Google Scholar]
- Thomele D.; Baumann S. O.; Schneider J.; Sternig A. K.; Shulda S.; Richards R. M.; Schwab T.; Zickler G. A.; Bourret G. R.; Diwald O. Cubes to Cubes: Organization of MgO Particles into One-Dimensional and Two-Dimensional Nanostructures. Cryst. Growth Des. 2021, 21, 4674–4682. 10.1021/acs.cgd.1c00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan G.; Velavan R.; Mujasam Batoo K. M.; Raslan E. H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. 10.1016/j.rinp.2020.103013. [DOI] [Google Scholar]
- Bindhu M. R.; Umadevi M.; Kavin Micheal M. K.; Arasu M. V.; Abdullah Al-Dhabi N. A. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater. Lett. 2016, 166, 19–22. 10.1016/j.matlet.2015.12.020. [DOI] [Google Scholar]
- Kokane R.; Corre Y.; Kemnitz E.; Dongare M. K.; Agbossou-Niedercorn F.; Michon C.; Umbarkar S. B. Palladium supported on magnesium hydroxyl fluoride: an effective acid catalyst for the hydrogenation of imines and N-heterocycles. New J. Chem. 2021, 45, 19572–19583. 10.1039/d1nj03760a. [DOI] [Google Scholar]
- Chen M.; Jin J.-M.; Lin S.-D.; Li Y.; Liu W.-C.; Guo L.; Li L.; Li X. N. SiO2-template synthesis of mesoporous MgF2 highly effective for Cl/F exchange reaction. J. Fluorine Chem. 2013, 150, 46–52. 10.1016/j.jfluchem.2013.03.003. [DOI] [Google Scholar]
- Jacobsohn L. G.; Roy A. L.; McPherson C. L.; Kucera C. J.; Oliveira L. C.; Yukihara E. G.; Ballato J. Rare earth-doped nanocrystalline MgF2: synthesis, luminescence and thermoluminescence. Opt. Mater. 2013, 35, 2461–2464. 10.1016/j.optmat.2013.06.045. [DOI] [Google Scholar]
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- Yang F.; Guo Z. Engineering NiO sensitive materials and its ultra-selective detection of benzaldehyde. J. Colloid Interface Sci. 2016, 467, 192–202. 10.1016/j.jcis.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Sing K. S.; Williams R. T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. 10.1260/0263617053499032. [DOI] [Google Scholar]
- Halawy S. A.; Osman A. I.; Rooney D. W. Highly basic and active ZnO–x% K2O nanocomposite catalysts for the production of methyl ethyl ketone biofuel. Energy Sci. Eng. 2022, 10, 2827–2841. [Google Scholar]
- Ruhaimi A. H.; Ab Aziz M. A. High-performance flake-like mesoporous magnesium oxide prepared by eggshell membrane template for carbon dioxide capture. J. Solid State Chem. 2021, 300, 122242. 10.1016/j.jssc.2021.122242. [DOI] [Google Scholar]
- Alsawalha M.; Ratemi E. Properties of raw Saudi Arabian grey kaolin studied by pyrrole adsorption and catalytic conversion of methylbutynol. J. Chem. 2018, 2018, 8656207. 10.1155/2018/8656207. [DOI] [Google Scholar]
- Nuttinee S.; Jatuporn W.; Sanchai P.; Wojciech S.; Frank R. Basic properties of potassium oxide supported on zeolite Y studied by pyrrole-TPD and catalytic conversion of methylbutynol. Quim. Nova 2012, 35, 1719–1723. 10.1590/s0100-40422012000900003. [DOI] [Google Scholar]
- Förster H.; Fuess H.; Geidel E.; Hunger B.; Jobic H.; Kirschhock C.; Klepel O.; Krause K. Adsorption of pyrrole derivatives in alkali metal cation-exchanged faujasites: comparative studies by surface vibrational techniques, X-ray diffraction and temperature-programmed desorption augmented with theoretical studies Part I. Pyrrole as probe molecule. Phys. Chem. Chem. Phys. 1999, 1, 593–603. 10.1039/a807296e. [DOI] [Google Scholar]
- Wuttke S.; Vimont A.; Lavalley J.-C.; Daturi M.; Kemnitz E. Infrared Investigation of the Acid and Basic Properties of a Sol–Gel Prepared MgF2. J. Phys. Chem. C 2010, 114, 5113–5120. 10.1021/jp911584h. [DOI] [Google Scholar]
- Binet C.; Jadi A.; Lamotte J.; Lavalley J. C. Use of pyrrole as an IR spectroscopic molecular probe in a surface basicity study of metal oxides. J. Chem. Soc., Faraday Trans. 1996, 92, 123–129. 10.1039/ft9969200123. [DOI] [Google Scholar]
- Yi X.; Li G.; Huang L.; Chu Y.; Liu Z.; Xia H.; Zheng A.; Deng F. An NMR scale for measuring the base strength of solid catalysts with pyrrole probe: a combined solid-state NMR experiment and theoretical calculation study. J. Phys. Chem. C 2017, 121, 3887–3895. 10.1021/acs.jpcc.6b11518. [DOI] [Google Scholar]
- Wuttke S.; Coman S. M.; Scholz G.; Kirmse H.; Vimont A.; Daturi M.; Schroeder S. L.; Kemnitz E. Novel Sol-Gel Synthesis of Acidic MgF2–x(OH)x Materials. Chem.—Eur. J. 2008, 14, 11488–11499. 10.1002/chem.200801702. [DOI] [PubMed] [Google Scholar]
- Di Cosimo J. I.; Díez V. K.; Ferretti C.; Apesteguía C. R. Basic catalysis on MgO: generation, characterization and catalytic properties of active sites. Catalysis 2014, 26, 1–28. 10.1039/9781782620037-00001. [DOI] [Google Scholar]
- Jin S.; Bang G.; Lee C. H. Unusual morphology transformation and basicity of magnesium oxide controlled by ageing conditions and its carbon dioxide adsorption. J. CO2 Util. 2020, 41, 101273. 10.1016/j.jcou.2020.101273. [DOI] [Google Scholar]
- Mabokela T. E.; Somo T. R.; Maponya T. C.; Hato M. J.; Makhado E.; Makgopa K.; Modibane K. D. Dynamic carbon dioxide uptake capacity of metal organic framework using thermogravimetrical evaluation at different CO2 pressure. Mater. Lett. 2022, 317, 132086. 10.1016/j.matlet.2022.132086. [DOI] [Google Scholar]
- Ramos P. B.; Ponce M. F.; Jerez F.; Barreto G. P.; Bavio M. A. Assessment of industrial waste for adsorption and capture of CO2: Dynamic and static capture system. J. Environ. Chem. Eng. 2022, 10, 107521. 10.1016/j.jece.2022.107521. [DOI] [Google Scholar]
- Alkadhem A. M.; Elgzoly M. A.; Onaizi S. A. Novel amine-functionalized magnesium oxide adsorbents for CO2 capture at ambient conditions. J. Environ. Chem. Eng. 2020, 8, 103968. 10.1016/j.jece.2020.103968. [DOI] [Google Scholar]
- Guo Y.; Tan C.; Wang P.; Sun J.; Li W.; Zhao C.; Lu P. Structure-performance relationships of magnesium-based CO2 adsorbents prepared with different methods. Chem. Eng. J. 2020, 379, 122277. 10.1016/j.cej.2019.122277. [DOI] [Google Scholar]
- Jin S.; Bang G.; Liu L.; Lee C. H. Synthesis of mesoporous MgO-CeO2 composites with enhanced CO2 capture rate via controlled combustion. Microporous Mesoporous Mater. 2019, 288, 109587. 10.1016/j.micromeso.2019.109587. [DOI] [Google Scholar]