Abstract
The biochemical composition of the apical membranes of epithelial M cells overlying the gut-associated lymphoid tissues (GALT) is still largely unknown. We have prepared monoclonal antibodies (MAbs) directed against carbonate-washed plasma membranes from epithelial cells detached with EDTA from rabbit appendix, a tissue particularly rich in GALT. As determined by immunofluorescence microscopy, several MAbs specifically recognized either M cells or enterocyte-like cells of the domes from rabbit appendix, sacculus rotundus, and Peyer’s patches. M cells were identified by their large ventral pocket containing lymphoid cells and by specific labeling with antivimentin. Among various characterized MAbs, MAb 104 recognized rabbit immunoglobulins and was used as an apical marker for M cells in the rabbit appendix, MAb 58 selectively stained an integral membrane glycoprotein of greater than 205 kDa located at the apex of M cells, and MAb 214 stained a smaller soluble glycoprotein associated with the apical surfaces from neighboring enterocytes. In addition, both MAbs 58 and 214 also labeled luminal mucus and secretory granules in goblet cells. The selective association of mucin-related molecules at the surfaces of either M cells or enterocyte-like cells of the follicle-associated epithelium suggests that specific carbohydrate antigens are differentially expressed by epithelial cells and could account for the differential binding properties of pathogens.
The gastrointestinal tract is a major site of entry for pathogens such as bacteria, viruses, and parasites. Penetration of these pathogens into internal tissues and fluids is normally prevented by the epithelial barrier (29). Intestinal enterocytes are protected by the filamentous brush border glycocalyx (13, 20, 32, 33), and the entire mucosal surface is protected by secreted mucus components (35). Certain pathogens can disrupt the continuity of the epithelial barrier and transit through the epithelium (28). It is important, therefore, for the immune system to be alerted to the presence of pathogens in the intestinal tract. Sampling of luminal antigens and pathogens is achieved by specialized epithelial cells, the M cells, which bind and rapidly transport macromolecules and microorganisms across the follicle-associated epithelium (FAE) toward underlying gut-associated lymphoid tissues that contain all of the cells necessary for activation of the mucosal immune system (10). As a result, specific B lymphoblasts differentiate, migrate systemically, and home to local and distant mucosal and glandular tissues where they secrete dimeric immunoglobulin A (IgA) molecules. Dimeric IgA is actively transported to the intestinal lumen via the dimeric IgA receptor (for a review, see reference 6).
The M-cell transport mechanism, however, is sometimes used by pathogens to circumvent the intact intestinal barrier and invade the underlying tissues (31). Therefore, although efficient mucosal protection depends upon M-cell sampling, transport of pathogens must be restricted and controlled to prevent massive infection. This is probably why M cells are a very small minority in the epithelium of the gastrointestinal tract and are located only in the FAE over immune inductive sites through the gut.
M cells are well defined by morphological features (37) and by their propensity to transport certain pathogens (for a review, see reference 36), but their molecular features are still poorly understood. The cytoskeleton of M cells is unusual for epithelial cells in that it contains vimentin (15) and specific cytokeratins (15, 16). Villin, a cytoskeletal protein concentrated in the microvilli of enterocytes (4), is diffusely distributed in the cytosol of mouse M cells (22). Certain integral membrane proteins normally present on the apical surfaces of intestinal epithelial cells are not expressed on M cells. This is the case for alkaline phosphatase (38) and aminopeptidase (43a). Membrane proteins specific to M-cell apical surfaces have not been identified, although β1 integrin has recently been proposed to be such a protein (7). Igs of the A type bind to the apical surfaces of M cells and are transported through the epithelium (45, 48), but the corresponding receptor remains unknown. It has recently been shown that some of the structural and functional features of M cells could result from interactions between epithelial cells and lymphocytes (23).
Monoclonal antibodies (MAbs) that labeled apical surfaces of M cells have been described (39) and were thought to increase microsphere uptake by rabbit Peyer’s patches (40), but the antigens involved were not characterized. It is possible that they recognized carbohydrate structures, since the apical membranes of FAE cells display specific carbohydrate patterns as demonstrated by the binding of different lectins and antibodies. For instance, the lectins of Ulex europaeus (UEA-I) and Psophocarpus tetragonolobus (WBA II) are specific for M cells in BALB/c mice (8). However, the expression of these lectin-detected glycoconjugates in FAE varies among species, from one region of the intestine to the other (14), and among M cells within the same dome (17). The UEA-I epitopes are also found within vesicles of M cells and along the basolateral membranes (17). These observations are of particular interest, since glycoconjugates play key roles in pathogen-target cell interactions on one hand (for a review, see reference 12) and in the protection of the epithelial cells through the highly glycosylated glycocalyx (20) and the mucus blanket (35) on the other hand.
In this paper, we describe MAbs which recognize mucin-related epitopes differently expressed on apical surfaces of either M cells or enterocyte-like cells of the rabbit FAE. The M-cell-associated antigen behaves as an integral membrane glycoprotein and is also associated with endocytic vesicles and the Golgi complex.
(This work was presented in part at the 6th International Congress on Cell Biology, San Francisco, Calif., 1996 [34a].)
MATERIALS AND METHODS
Animals.
New Zealand albino rabbits weighing 2 to 3 kg were obtained from the Institut National de la Recherche Agronomique, Montpellier, France. Female BALB/c mice (2 to 8 weeks old) were obtained from the Centre d’Elevage Janvier, Le Genest-Saint-Isle, France. Animals were housed and cared for according to French regulations 87-848 and EEC-L358.
Reagents.
Cell culture reagents, including fetal calf serum, were purchased from GIBCO BRL (Paisley, Scotland); glutaraldehyde was from Ladd Research Industries (Burlington, Vt.); protein A-Sepharose was from Pharmacia (Saint Quentin, France); Immobilon-P was from Millipore (Bedford, Mass.); the ECL kit was from Amersham (Buckinghamshire, United Kingdom); Lowicryl K4M was from Electron Microscopy Sciences (Fort Washington, Pa.); Epon was from TAAB Laboratories Equipment Ltd. (Berks, United Kingdom); Mowiol was from Calbiochem Corp. (La Jolla, Calif.); and rhodamine isothiocyanate (RITC)–UEA-I, fluorescein isothiocyanate (FITC)-VVA (Vicia villosa agglutinin), and 4,6-dichlorotriazinylaminofluorescein (DTAF) were from Sigma Chemical Co. (St. Louis, Mo.). All other chemicals were reagent grade.
Antibodies.
Goat anti-mouse IgGs coupled to horseradish peroxidase, FITC, or tetramethyl rhodamine isothiocyanate were from Biosys (Compiègne, France), gold-coupled protein A (PAG10) was purchased from the Utrecht University School of Medicine (Utrecht, The Netherlands), rabbit anti-mouse IgG and mouse antivimentin (clone V9) were obtained from Dako (Glostrup, Denmark), FITC–goat anti-rabbit IgA was from Nordic Immunology (Tilburg, The Netherlands), and purified normal rabbit IgG was from Miles (Elkhart, Ind.).
Cell culture.
Gerbil fibroblasts cells (CCL146) and nonsecreting myeloma-1 cells (NS-1) were obtained from the American Type Culture Collection and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g of glucose per liter, 10% fetal calf serum, 20 mM l-glutamine, and 25 U of penicillin-streptomycin, per ml. Hybridomas were grown on the same Dulbecco’s modified Eagle’s medium containing in addition 100 μM hypoxanthine, 0.4 μM aminopterine, and 16 μM thymidine. All cells were grown at 37°C with 5% CO2–95% air.
Cell dissociation.
The appendix epithelium was detached with EDTA by a modification of previously described methods (1, 2, 41, 44). The animals were fasted overnight and were anesthetized by injection of 25% (wt/vol) urethane (10 ml/kg) in phosphate-buffered saline (PBS). The appendix was removed, quickly washed with PBS, inverted, and tied on a 5-ml plastic pipette. It was incubated with 40 ml of dissociation buffer (200 mM sucrose, 76 mM Na2HPO4, 19 mM KH2PO4, 60 mM NaOH, 40 mM EDTA [pH 7.4]) containing a cocktail of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg of antipain per ml, 1 μg of pepstatin per ml, 0.5 μg of leupeptin per ml, and 15 μg of benzamidine per ml) for 4 h at room temperature, with the buffer changed every 30 min. The dissociated epithelium preparation, which contained many free lymphocytes, was filtered through a 100-mesh nylon cloth (Nitex Tobler Co., New York, N.Y.). Lymphocytes passed through and were discarded. Epithelial sheets were retained on the filter and were used immediately for immunolabeling or stored at −20°C for further biochemical analysis.
Membrane preparation.
Epithelial sheets were homogenized at 4°C in 9 volumes of 20 mM Tris-HCl buffer (pH 7.4) containing 150 mM sucrose, 5 mM MgCl2, 20 mM Na2HPO4, and the cocktail of protease inhibitors described above, using 75 strokes of a Dounce type B homogenizer in ice (27, 46). The homogenate was centrifuged at 1,000 × g (2,700 rpm in an HS4 rotor) for 10 min at 4°C in a Sorvall (Newtown, Conn.) RS5C centrifuge. The pellet was rehomogenized in 2.5 volumes with 50 strokes of a Dounce type B homogenizer and centrifuged as previously. The two supernatants were pooled, and a crude membrane fraction was prepared by centrifugation at 100,000 × gav (31,000 rpm in a 60 Ti rotor) in an ultracentrifuge (Beckman Instruments, Palo Alto, Calif.). In order to extract peripheral proteins and mucus, the pellet was resuspended in 10 volumes of 100 mM Na2CO3 (pH 11) (19) containing antiproteases as described above, using a Vibracell Sonifier (Sonics and Materials Inc., Danbury, Conn.) set at 600 W for five 10-s periods on ice, and then centrifuged at 100,000 × g for 1 h (50 Ti rotor, 38,500 rpm). The procedure was repeated twice. The membranes were stored as a pellet at −20°C. They were resuspended by sonication in 1 volume of PBS.
Immunization procedure.
Mice were injected intraperitoneally with 100 μg of carbonate-washed membrane proteins emulsified in complete Freund’s adjuvant. They were boosted 3 and 5 weeks later with the same amount of membrane proteins in incomplete adjuvant. After 7 weeks, the sera were tested in an immunofluorescence assay on cryostat sections from rabbit appendix, and mice were boosted again at 9 weeks with the same amount of membrane proteins in PBS. They were sacrificed 4 days later.
MAbs.
Mice were aseptically splenectomized, and spleen cells were fused with mouse NS-1 myeloma cells by the method of Kohler and Milstein (24) except that the fusion was mediated by polyethylene glycol (21). Hybridomas were screened by immunofluorescence on rabbit appendix cryostat sections. Positive hybridomas were cloned twice by limiting dilution on confluent CCL146 cells as feeders. Ascite tumors were produced in Pristane-treated BALB/c mice by injection of 3 × 106 monoclonal cells. The ascitic fluid was purified on protein A-Sepharose and eluted with 100 mM glycine-HCl buffer, pH 3.0. Purified MAbs were coupled to fluorescein by using DTAF as described previously (18).
Immunofluorescence microscopy.
Tissues were fixed for 2 h with 2% formaldehyde in 100 mM phosphate buffer (pH 7.4), rinsed and infused for several hours in 1 M sucrose in the same buffer, mounted on a cork tissue holder, and frozen in OCT compound (Miles). Sections 6 μm thick were cut in a Reichert 2700 cryostat, collected on polylysine-coated slides, and stored at 4°C. After permeabilization with 0.2% Triton X-100 for 3 min, they were stained with primary antibodies followed by FITC- or RITC-coupled secondary antibodies. In inhibition assays with rabbit Ig, MAb 104 was preincubated for 1 h with 100 μg of purified rabbit IgG per ml before the labeling was performed with the mixture and analyzed by immunofluorescence. In competition assays, sections were preincubated with an irrelevant sheep anti-rabbit Ig serum (dilution, 1:100) for 1 h prior to labeling with MAb 104. For inhibition studies with monosaccharides, the MAb 58 or lectins were preincubated for 2 h with 30 to 300 mM sugar and then used for labeling. Slides were mounted with Mowiol containing 1.4-diazabicyclo[2.2.2]octane (DABCO) and observed on a Reichert-Jung Polyvar fluorescence microscope equipped with a cooled charge-coupled digital camera (Princeton, Trenton, N.J.) or on a Leica (Wetzlar, Germany) TCS 4D confocal microscope equipped with an argon-krypton laser. Digitized micrographs were processed with the Adobe Photoshop 4.0 software and printed on an Epson Stylus Photo Ex color printer.
Electron microscopy.
Tissues were fixed for 1 h at room temperature with 2% formaldehyde–1% glutaraldehyde in 100 mM phosphate buffer (pH 7.2), dehydrated in graded alcohol, and embedded in Epon or in Lowicryl K4M by the methods described by the manufacturer. Sections were obtained with an RMC (Tucson, Ariz.) MT7000 ultramicrotome and immunolabeled for 90 min with MAb 58 followed by rabbit anti-mouse IgG and by protein A-gold 10. They were stained with uranyl acetate and lead citrate and observed on a Hitachi H7100 electron microscope. Semithin sections were stained with 0.1% toluidine blue in 0.1% sodium borate.
For scanning electron microscopy, tissues were freed from mucus with a flow of PBS, fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer–0.1 M sucrose (pH 7.2) for 24 h at room temperature, rinsed in the same buffer, and postfixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.2) for 2 h. Samples where gradually dehydrated in ethanol, critical point dried with CO2, and sputtered with gold-palladium (1.45 nm thick). The samples were observed in a Hitachi S4000 scanning electron microscope at 15 kV.
Gel electrophoresis and immunoblotting.
Fifty micrograms of membranes was dissolved in sample buffer and subjected to sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis as described by Laemmli (26). For electrophoresis under reducing conditions, the sample buffer contained 100 mM dithiothreitol and the samples were boiled for 3 min before loading. Molecular weight markers were myosin, β-galactosidase, phosphorylase b, and bovine serum albumin. Separated proteins were electrotransferred from the gel to Immobilon-P sheets (5). The entire sheets were stained with Coomassie blue, and the antigens were detected by using hybridoma culture supernatants or diluted (1/1,000) ascites fluid followed by horseradish peroxidase-conjugated goat anti-mouse IgG and revealed by enhanced chemiluminescence (ECL) as described by the manufacturer (Amersham).
Protein assays.
Membrane proteins solubilized in PBS were quantified by the method of Bradford (3), using bovine serum albumin as a standard.
Chemical hydrolysis.
Periodate oxidation was performed either with electroblotted proteins as described by Woodward et al. (49) or with appendix sections as described by Falk et al. (11) by using 20 mM sodium metaperiodate–50 mM sodium acetate (pH 4.5) for 1 h at room temperature. Saponification was performed by incubation of tissue sections in 0.5% KOH in 70% ethanol for 5 to 15 min at room temperature. This mild alkaline treatment was eventually followed by periodate oxidation (9).
RESULTS
Isolation of FAE sheets from rabbit appendix as an enriched source of M cells for generation of MAbs.
Rabbit appendix epithelium could be detached with EDTA to produce monolayer sheets which retained the morphological features of the epithelium in situ. In these sheets, M cells were recognizable by their pocket filled with lymphocytes (Fig. 1). After epithelial dissociation, the remaining lymphoid follicle and the lamina propria were mainly free from epithelium (Fig. 1a). By electron microscopy, the basal lamina was intact but displayed some gaps, which presumably were sites of lymphocyte penetration (not shown). From these epithelial sheets, a total membrane fraction was prepared, washed extensively with sodium carbonate (pH 11) to reduce contaminating peripheral proteins and mucus, and used to immunize mice and generate MAbs. Three clones were selected on the basis of their immunofluorescence staining of rabbit appendix cryostat sections; all three stained apical surfaces of subpopulations of epithelial cells of the FAE.
FIG. 1.
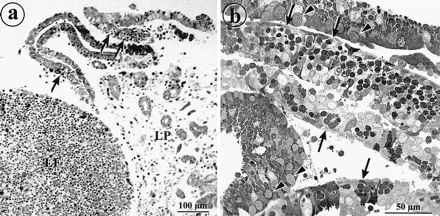
Epithelial sheets detach from rabbit appendix after treatment with EDTA. (a) Light micrograph of rabbit appendix tissue after treatment with EDTA for 2 h. Epithelial sheets are being released from the lamina propria (LP). M cells (arrows) are recognizable by their pockets filled with lymphocytes. The lymphocytes in the follicle are retained in the mucosal tissue (LF). (b) Light micrograph of a sectioned pellet of isolated epithelial sheets. The cells are still attached to form monolayer sheets containing M cells (arrows). Some layers from the villus epithelium display goblet cells (arrowheads).
The apical membranes of rabbit appendix M cells display IgA.
MAb 104 labeled the apical surfaces of M cells (Fig. 2a), identified by the presence of a basolateral pocket filled with lymphoid cells (Fig. 2c) and staining with antivimentin antibodies (Fig. 2b). Lymphocytes in the follicles, in the basolateral pockets of M cells, and in the lamina propria were also labeled. The specificity of MAb 104 was determined to be anti-rabbit Ig antibody, since unrelated anti-rabbit Ig competed with MAb 104 in immunofluorescence staining and since prior incubation of MAb 104 with normal rabbit Ig extinguished the staining, as described in Materials and Methods section (not shown). Moreover, the staining pattern of anti-rabbit IgA antibodies on sections of rabbit appendix mimicked that of MAb 104 (not shown). Anti-IgA labeling on M-cell apical surfaces was consistently strong in rabbit appendix, whereas it was less uniform on the domes of sacculus rotundus and ileum Peyer’s patches. MAb 104 or anti-IgA labeling was used throughout this study as a reliable marker for M cells in rabbit appendix.
FIG. 2.
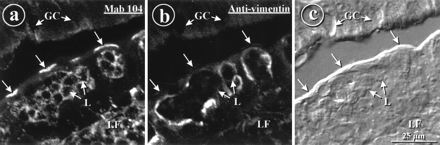
M cells of rabbit appendix are labeled with MAb 104 and antivimentin antibodies. (a and b) Double indirect immunofluorescence labeling of a cryostat section of rabbit appendix with MAb 104 (a) and antivimentin (b), viewed by confocal microscopy. The labeling with MAb 104 was concentrated at the apices of M cells (unlabeled arrows), on lymphocytes in the follicle (LF), and in the pockets (L). Antivimentin antibodies labeled intermediate filaments concentrated at the peripheries of the pockets of the same cells. GC, unlabeled goblet cells. (c) Differential interference contrast view of the same field shown in panels a and b.
Mucin-related epitopes are differentially expressed at the apices of M cells and enterocytes of the FAE.
Despite the fact that the membranes used for immunization were carbonate washed, many of the MAbs obtained recognized mucus in the lumen and in the secretory granules of goblet cells, as seen by immunofluorescence on cryosections of rabbit appendix. However, some of these MAbs also specifically stained the apical surface of the dome epithelium. This was not surprising, since oligosaccharides found on membrane glycoproteins such as the filamentous brush border glycocalyx may be shared by mucus glycoproteins. Among these MAbs, two MAbs showed a differential expression pattern on FAE. MAb 58 stained the apical surfaces of M cells (Fig. 3 and 4), whereas MAb 214 labeled dome enterocyte surfaces (Fig. 4). In contrast, MAb 1-5 labeled goblet cells and luminal mucus but not the apical surfaces of either M cells or enterocytes in the FAE (Fig. 4). MAb 1-5 was useful, however, to distinguish the labeling of MAbs 58 and 214 from that of luminal mucus occasionally adherent to the domes. The antigens recognized by MAbs 58, 214, and 1-5 were called antigens 58, 214, and 1-5, respectively. The specificity of MAb 58 for M cells (Fig. 3a and c) was confirmed by the identification of M cells from the morphological criteria reported above, by anti-IgA (Fig. 3b), and by U. europaeus UEA-I lectin (Fig. 3d). The apices of M cells of Peyer’s patches (Fig. 3e and f) and of the sacculus rotundus (not shown) were also stained with MAb 58 but more sparsely. A few rabbits were negative for antigen 58 in M cells, but this was mainly correlated with diarrhea and/or inflammation of the intestine. Such an effect could be due to a change of the glycosylation pattern of the epitope or to its destruction after massive endocytosis upon infection.
FIG. 3.
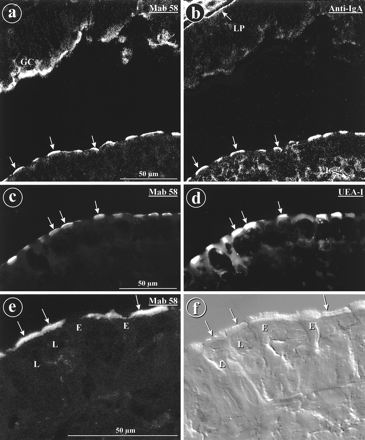
MAb 58, anti-IgA antibodies, and lectin UEA-I label the apices of M cells. Indirect immunofluorescence labeling on cryostat section from rabbit appendix, viewed by confocal microscopy is shown. (a to d) The labeling of the dome epithelium with MAb 58 (a) (arrows) was identical to that obtained with anti-IgA (b) (unlabeled arrows). However, MAb 58 also labeled goblet cells (GC), while anti-IgA also labeled lymphocytes (L) and the matrix of the lamina propria (LP). UEA-I lectin (d) labeled the same cells (arrows) as MAb 58 (c), but the labeling with UEA-I was significantly more extended within the cells. (e) On a cryostat section from a rabbit ileal Peyer’s patch, MAb 58 labeled the apices of M cell (arrows). (f) Differential interference contrast view of the same field shown in panel e. E, dome enterocytes; L, lymphocytes.
FIG. 4.
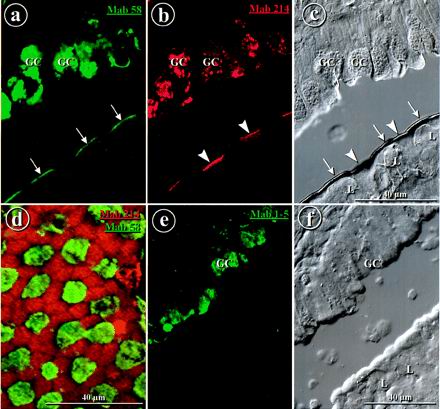
Antimucus MAbs 58 and 214 label the apices of different cell types in the FAE, whereas MAb 1-5 does not label the FAE. Double immunofluorescence labeling of a cryostat section from rabbit appendix is shown. (a) Direct labeling with DTAF-coupled MAb 58 was concentrated on the apices of M cells (arrows) and in goblet cells (GC). (b) Indirect labeling with MAb 214 was concentrated on the apices of enterocytes (arrowheads) and in goblet cells. (c) Differential interference contrast view of the same field as in panels a and b. L, lymphocytes. (d) Double immunofluorescence labeling with DTAF-coupled MAb 58 (green) and indirect labeling with MAb 214 (red), in dorsal view, showing complementary labeling. (e) MAb 1-5 labels goblet cells but not the surface of the FAE. (f) Differential interference contrast view of the same field as in panel e. Bars, 40 μm.
Along the lateral sides of the domes, staining by MAb 58 (or anti-IgA [not shown]) displayed a mosaic-like pattern absolutely complementary to that of MAb 214 (Fig. 4a to d). Occasionally, cells at the necks of the crypts at the bases of the domes were also decorated by MAb 58 or by MAb 214 (not shown), but this was sometimes difficult to assess due to mucus adhesion at these sites that could be monitored with MAb 1-5.
Electron microscopic localization of antigen 58.
In rabbit appendix and Peyer’s patches, MAb 58 heavily labeled microvilli and apical vesicles of M cells (Fig. 5 and 6). Some labeling was occasionally observed in the basolateral extracellular space but not on lymphocytes (Fig. 5a). The trans-side cisternae of the Golgi complexes of M cells as well as some vesicles and multivesicular bodies in the vicinity of the Golgi complex were also faintly labeled (Fig. 5c). Occasionally, secondary lysosomes in phagocytic cells inside the follicle were also labeled (not shown). A few cells with the morphological features of M cells were not labeled. Neighboring enterocytes were not labeled (Fig. 5b), except at the very tips of the microvilli when some mucus was adherent (Fig. 7). MAb 58 labeled the matrix of the secretory granules from goblet cells (Fig. 7) and, more faintly, differentiating goblet cells at the necks of the crypts (not shown).
FIG. 5.
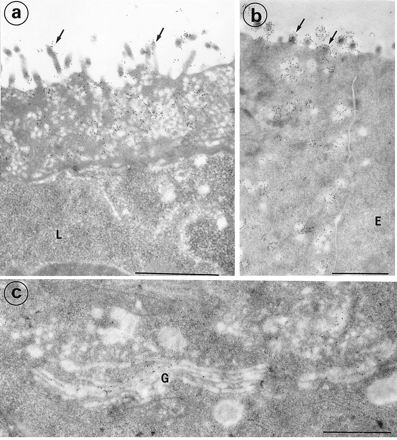
Electron micrographs of M cells from rabbit appendix labeled with MAb 58. (a and b) The labeling (gold 10) was concentrated on the microvilli (arrows) and on the membranes of apical vesicles from M cells. The lymphocytes (L) in the basolateral pocket are devoid of significant labeling. The dome enterocytes (E) were not labeled (b). (c) The trans-side saccules of the Golgi complex (G) and some cytoplasmic vesicles in the vicinity of the Golgi complex were labeled. Bars, 1 μm.
FIG. 6.
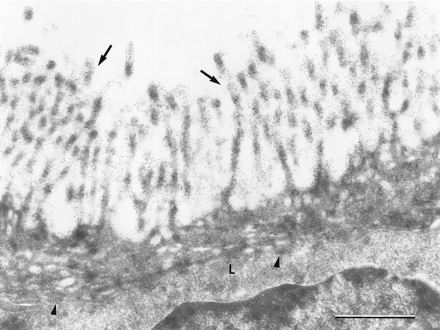
Electron micrograph of an M cell from a rabbit Peyer’s patch, labeled with MAb 58. The labeling was concentrated on the microvilli (arrows) and on endocytic vesicles of M cells. The basolateral lymphocytes (L) were not significantly labeled, whereas the pocket membrane was occasionally labeled (arrowheads). Magnification, ×32,000. Bar, 1 μm.
FIG. 7.
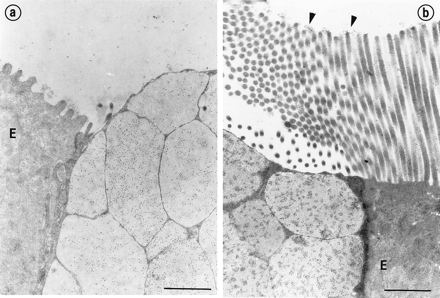
Electron micrograph of rabbit appendix and Peyer’s patch goblet cells labeled with MAb-58. The labeling was concentrated in the matrix of the secretory granules in appendix (a) and Peyer’s patches (b). The enterocytes (E) were not labeled, but adherent mucus was labeled (arrowheads). Note the longer microvilli on enterocytes in Peyer’s patches compared to those in appendix. Magnification, ×18,500. Bars, 1 μm.
Biochemical characterization of antigens 1-5, 58, and 214.
In Western blot analysis of reduced proteins of crude membranes, MAb 58 recognized a large broad band above 205 kDa and, occasionally, another band of 140 kDa (Fig. 8). When the membranes were washed twice with 100 mM sodium carbonate (pH 11), antigen 58 was still detected in the membrane fraction (Fig. 8). These results indicated that antigen 58 may be an integral membrane glycoprotein. Although antigen 58 partitioned in the aqueous phase after extraction with Triton X-114 (not shown), this does not rule out an integral membrane antigen, because such behavior has been described for glycosylated membranes proteins with large hydrophilic domains (42).
FIG. 8.
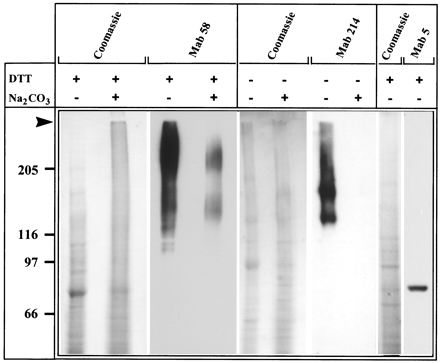
Immunoblot characterization of the antigens recognized in rabbit appendix epithelial membranes by MAbs 58, 214, and 1-5. Fifty micrograms of total membranes untreated or treated with sodium carbonate (100 mM, pH 11) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon sheets before staining with Coomassie blue and Western blotting with MAb 58, MAb 214, and MAb 1-5, followed by ECL detection. Proteins were run under reducing conditions, except for the lanes stained by MAb 214. The arrow points to the stacking gel limit. Molecular weights (in thousands) are indicated. DTT, dithiothreitol.
In Western blotting in the absence of a reducing agent, MAb 214 recognized a large smear extending from the stacking gel to two main bands at 180 and 140 kDa (Fig. 8). The labeling was abolished after reduction with DTT but not after boiling, indicating that the MAb was directed against a conformational epitope. Antigen 214 was no longer detected in membranes that had been extracted with sodium carbonate, indicating that it is a soluble glycoprotein. In Western blot analysis of reduced membrane proteins, MAb 1-5 recognized a single band at 80 kDa (Fig. 8).
Immunofluorescence staining of FAE sections by MAb 58 was conserved after periodate oxidation of sugar vicinal diols. However, it was abolished after mild alkaline removal of sugar ester substituents (not shown). This indicated that the epitope recognized by MAb 58 is a carbohydrate epitope. Under the same conditions, MAb 214 and 1-5 labeling was maintained.
The labeling of sections by MAb 58 was not abolished by competition with the following monosaccharides commonly found in membrane glycoproteins, either alone or mixed: l-fucose, d-(+)-galactose, N-acetyl-d-glucosamine, and N-acetyl-d-galactosamine. In contrast, as a control, the binding of fluorescent UEA-I lectin and VVA lectin was abolished after preincubation with l-fucose and N-acetyl-d-galactosamine, respectively (not shown). This ruled out the possibility that the MAb 58 epitope is similar to the l-fucose-containing UEA-1 or the N-acetyl-d-galactosamine-containing VVA binding sites previously described for rabbit and mouse M cells (14).
Antigen 58 is still present at the apices of M cells from EDTA-detached epithelial sheets.
MAb 104 and MAb 58 labeled apical surfaces of M cells in isolated epithelial sheets from rabbit appendix (Fig. 9). This indicated that M cells in the isolated epithelium still retained some apical surface properties and MAb binding capacity. Such binding was also observed on some isolated cells (not shown).
FIG. 9.
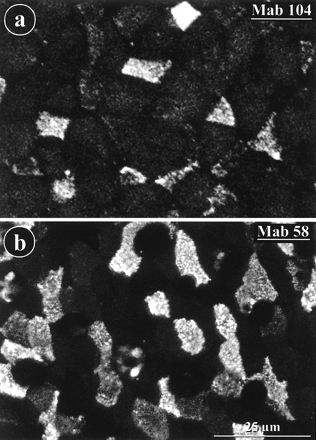
MAb 104 and MAb 58 label the surfaces of epithelial sheets detached from rabbit appendix with EDTA. The detached and fixed epithelium was labeled by indirect immunofluorescence with MAb 104 (a) or MAb 58 (b) and viewed from above with a confocal microscope.
DISCUSSION
In this study, we have modified a method for dissociation of FAE from rabbit appendix by using EDTA (1, 2, 41, 44), with the aim of using purified, washed plasma membranes to generate MAbs. This produced monolayered epithelial sheets that retained some apical membrane properties of M cells, such as the binding of two of the MAbs produced. Determination of whether the sheets are still functional requires further experiments, but the binding of the antibodies on isolated cells indicates that they probably can be used in cell sorting experiments to purify M cells.
Using this strategy, we generated MAbs that discriminate between the two types of epithelial cells comprising the FAE in the rabbit intestine. Of the four MAbs described (Table 1), three (MAbs 58, 214, and 1-5) recognized epitopes present in mucus glycoproteins; two of these also showed selective specificity for the two types of dome epithelial cells, and one (MAb 104) was directed against rabbit Igs. Two (MAbs 58 and 104) labeled the apices of M cells, another (MAb 214) recognized the apices of the dome enterocytes, and the fourth (MAb 1-5) recognized mucus but did not stain any cell in the FAE.
TABLE 1.
Summary of immunofluorescence labeling of rabbit appendix mucosa by MAbs raised against FAE plasma membranes
| MAb | Labeling of:
|
|||
|---|---|---|---|---|
| M cells | Enterocytes | Goblet cells | Lymphocytes | |
| 104 | + | − | − | + |
| 58 | + | − | + | − |
| 214 | − | + | + | − |
| 1-5 | − | − | + | − |
MAb 104 labeling confirmed that IgA molecules bind to the apices of M cells and are transcytosed to the basolateral pocket (45, 48), a process that may contribute to the mucosal immune response (25). The fact that apical labeling of M cells with anti-IgA antibody was most consistent in the rabbit appendix and was irregular in other intestinal FAE might reflect a differential efficiency of IgA secretion and recapture in different segments of the intestine (34). MAb 104 was used as a selective marker for M cells in rabbit appendix.
MAb 58 recognized apical membranes of M cells, where it was found concentrated on microvilli and on intracellular, presumably endocytic, vesicles. MAb 58 is directed against a membrane-associated protein with a mucin-like epitope, presumably an oligosaccharide. This antigen behaved as an integral membrane protein, since it was retained after carbonate treatment at alkaline pH. The glycosylated nature of antigen 58 was confirmed by its sensitivity to potassium hydroxyde treatment and by a high-Mr smear on Western blots. The characterization of antigen 58 as an integral membrane protein is consistent with its localization by electron microscopy on intracellular vesicles of M cells. Its localization within Golgi stacks supports the idea that antigen 58 is synthesized in M cells as a membrane glycoprotein, although we cannot exclude the possibility that another cross-reacting protein may be localized in the Golgi. The fact that two other types of mucus-related antigens (214 and 1-5) were never detected within intracellular vesicles of M cells indicates that it is unlikely that the intracellular presence of antigen 58 resulted from nonspecific endocytosis of mucins or other antigens by M cells. Whether antigen 58 is taken up from M-cell surfaces together with endocytosed pathogens remains to be established. Such uptake and transport would explain the labeling occasionally observed on a few phagocytic cells within the follicle.
MAb 214 recognized a soluble protein associated with the apices of FAE enterocytes. Labeling with MAb 214 was complementary to that with MAb 58. Biochemical characterization of antigen 214 suggests a mucin-cross-reactive antigen. It is not known whether it is secreted by goblet cells and subsequently specifically bound to dome enterocytes or whether it is synthesized by the dome enterocytes and then specifically associated with its surfaces. The latter possibility seems most likely, because MAb 1-5, which also clearly labeled secreted mucus, was not consistently associated with FAE or dome enterocytes. Therefore, our results show that two distinct mucus-related epitopes are expressed by either M cells or dome enterocytes but not by both.
It has been shown by lectin and antibody binding that M cells differ from enterocytes in their glycosylation pattern (8). It has also been established that the well-characterized glycoproteins of the enterocyte brush border (38) and the highly glycosylated glycocalyx proteins involved in the protection of enterocytes (20) may be absent from M cells (13). This may be of broad significance for understanding the function of M cells, since it has been proposed that glycosylation plays an important role in pathogen recognition by epithelial cells (for a review, see reference 12). It has been shown that several pathogens, such as Campylobacter upsaliensis, Shigella, and Yersinia enterocolitica, bind purified rabbit and human mucins through the mucin carbohydrate moiety and that respiratory pathogens also interact with purified mucins (30, 43, 47). Interactions with mucin-cross-reactive carbohydrates could explain how most of the pathogen may be trapped in the mucus gel while a small amount could be sampled by M cells and transcytosed to the mucosal immune system.
ACKNOWLEDGMENTS
We thank Jean Pierre Kraehenbuhl and Christian Roy for helpful discussions, Alain Sahuquet for digital art, Brigitte Nguyen for excellent technical help, and Paul Paulet for photographic work. Electron microscopy was performed at the Centre Régional d’Imagerie Cellulaire, Montpellier, France.
This work was supported by the Centre National de la Recherche Scientifique (UMR 5539 and Cell Biology project 96033), the Association pour la Recherche sur le Cancer (contract 6844), and the Ligue Nationale contre le Cancer. H.L. was a fellow from the Délégation à la Recherche et au Développement.
REFERENCES
- 1.Amerongen H M, Mack J A, Wilson J M, Neutra M R. Membrane domains of intestinal epithelial cells: distribution of Na+K+-APTase and the membrane skeleton in adult rat intestine during fetal development and after epithelial isolation. J Cell Biol. 1989;109:2129–2138. doi: 10.1083/jcb.109.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjerknes M, Cheng H. Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec. 1981;199:565–574. doi: 10.1002/ar.1091990412. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher A, Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980;20:839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- 5.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecylsulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 6.Cebra J J, Gearheart P J, Kamat R, Robertson S M, Tseng J. Origin and differentiation of lymphocytes involved in the secretory IgA response. Cold Spring Harbor Symp Quant Biol. 1976;41:201–215. doi: 10.1101/sqb.1977.041.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark M A, Hirst B H, Jepson M A. M-cell surface B1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark M A, Jepson M A, Simmons N L, Booth T A, Hirst B H. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993;41:1679–1687. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- 9.Culling C F, Reid P E, Clay M G, Dunn W L. The histochemical demonstration of O-acylated sialic acid in gastrointestinal mucins. Their association with the potassium hydroxide-periodic acid-Schiff effect. J Histochem Cytochem. 1974;22:826–831. doi: 10.1177/22.8.826. [DOI] [PubMed] [Google Scholar]
- 10.Ermak T H, Owen R L. Differential distribution of lymphocytes and accessory cells in mouse Peyer’s patches. Anat Rec. 1986;215:144–152. doi: 10.1002/ar.1092150208. [DOI] [PubMed] [Google Scholar]
- 11.Falk P, Roth K A, Boren T, Westblom T U, Gordon J I, Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc Natl Acad Sci USA. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 13.Frey A, Giannasca K T, Weltzin R, Giannasca P J, Reggio H, Lencer W I, Neutra M R. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebert A, Hach G. Differential binding of lectins to M cells and enterocytes in the rabbit cecum. Gastroenterology. 1993;105:1350–1361. doi: 10.1016/0016-5085(93)90139-4. [DOI] [PubMed] [Google Scholar]
- 15.Gebert A, Hach G, Bartels H. Co-localization of vimentin and cytokeratins in M cells of rabbit gut-associated lymphoid tissue (GALT) Cell Tissue Res. 1992;269:331–340. doi: 10.1007/BF00319625. [DOI] [PubMed] [Google Scholar]
- 16.Gebert A, Rothkötter H J, Pabst R. Cytokeratin 18 is an M cell marker in porcine Peyer’s patches. Cell Tissue Res. 1994;276:213–221. doi: 10.1007/BF00306106. [DOI] [PubMed] [Google Scholar]
- 17.Giannasca P J, Giannasca K T, Falk P, Gordon J I, Neutra M R. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol. 1994;267:G1108–G1121. doi: 10.1152/ajpgi.1994.267.6.G1108. [DOI] [PubMed] [Google Scholar]
- 18.Goding J W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13:215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard A L, Ma A. Isolation of rat hepatocyte plasma membranes. II. Identification of membrane-associated cytoskeletal proteins. J Cell Biol. 1983;96:230–239. doi: 10.1083/jcb.96.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S. Form and function of the glycocalyx on free cell surfaces. Philos Trans R Soc London B. 1974;268:55–66. doi: 10.1098/rstb.1974.0015. [DOI] [PubMed] [Google Scholar]
- 21.Kennett R. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- 22.Kerneis S, A, Bogdanova A, Colucci-Guyon E, Kraehenbuhl J-P, Pringault E. Cytosolic distribution of villin in M cells from mouse Peyer’s patches correlates with the absence of a brush border. Gastroenterology. 1996;110:515–521. doi: 10.1053/gast.1996.v110.pm8566599. [DOI] [PubMed] [Google Scholar]
- 23.Kerneis S, Bogdanova A, Kraehenbuhl J-P, Pringault E. Conversion by Peyer’s patches lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 24.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature (London) 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 25.Kraehenbuhl J-P, Neutra M R. Transepithelial transport and mucosal defence. II. Secretion of IgA. Trends Cell Biol. 1992;2:170–174. doi: 10.1016/0962-8924(92)90036-m. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Le Bivic A, Hirn M, Reggio H. Apical membrane marker is expressed early in colonic epithelial cells. Biol Cell. 1987;60:209–216. doi: 10.1111/j.1768-322x.1987.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 28.Madara J L. The chameleon within: improving antigen delivery. Science. 1997;277:910–911. doi: 10.1126/science.277.5328.910. [DOI] [PubMed] [Google Scholar]
- 29.Madara J L. Tight junctions dynamics: is paracellular transport regulated? Cell. 1988;53:497–498. doi: 10.1016/0092-8674(88)90562-4. [DOI] [PubMed] [Google Scholar]
- 30.Mantle M, Husar S D. Binding of Yersinia enterocolitica to purified, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect Immun. 1994;62:1219–1227. doi: 10.1128/iai.62.4.1219-1227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcial M, Madara J L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986;90:583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- 32.Maury J, Bernadac A, Rigal A, Maroux S. Expression and glycosylation of the filamentous brush border glycocalyx (FBBG) during rabbit enterocyte differentiation along the crypt-villus axis. J Cell Sci. 1995;108:2705–2713. doi: 10.1242/jcs.108.7.2705. [DOI] [PubMed] [Google Scholar]
- 33.Maury J, Nicoletti C, Guzzo-Chambraud L, Maroux S. The filamentous brush border glycocalyx, a mucin-like marker of enterocyte hyper-polarization. Eur J Biochem. 1995;228:323–331. [PubMed] [Google Scholar]
- 34.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 34a.Montcourrier P, Lelouard H, Mangeat P, Reggio H. Apical membranes from M cells and enterocytes have different binding capacity for monoclonal antibodies in rabbit appendix and Peyer’s patches. Mol Biol Cell. 1996;7:89a. [Google Scholar]
- 35.Neutra M R, Forstner J-P. Gastrointestinal mucus: synthesis, secretion and function. In: Johnson L R, editor. Physiology of the gastrointestinal tract. New York, N.Y: Raven Press; 1987. pp. 975–1009. [Google Scholar]
- 36.Neutra M R, Pringault E, Kraehenbuhl J-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 37.Owen R L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer’s patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977;72:440–451. [PubMed] [Google Scholar]
- 38.Owen R L, Bhalla D K. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer’s patch M cells. Am J Anat. 1983;168:199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- 39.Pappo J. Generation and characterization of monoclonal antibodies recognizing follicle epithelial M cells in rabbit gut-associated lymphoid tissues. Cell Immunol. 1989;120:31–41. doi: 10.1016/0008-8749(89)90172-x. [DOI] [PubMed] [Google Scholar]
- 40.Pappo J, Ermak T H. Uptake and translocation of fluorescent latex particles by rabbit Peyer’s patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol. 1989;76:144–148. [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips T E, Phillips T H, Neutra M R. Regulation of intestinal goblet cell secretion. III. Isolated intestinal epithelium. Am J Physiol. 1984;247:G674–G681. doi: 10.1152/ajpgi.1984.247.6.G674. [DOI] [PubMed] [Google Scholar]
- 42.Pryde J G. Triton X-114: a detergent that has come in from the cold. Trends Biochem Sci. 1986;11:160–163. [Google Scholar]
- 43.Rajkumar R, Devaraj H, Niranjali S. Binding of Shigella to rat and human intestinal mucin. Mol Cell Biochem. 1998;178:261–268. doi: 10.1023/a:1006844125976. [DOI] [PubMed] [Google Scholar]
- 43a.Reggio, H., and M. R. Neutra. Unpublished data.
- 44.Roy M J, Ruiz A. Dome epithelial cells dissociated from rabbit gut-associated lymphoid tissues. Am J Vet Res. 1986;47:2577–2583. [PubMed] [Google Scholar]
- 45.Roy M J, Varvayanis M. Development of dome epithelium in gut-associated lymphoid tissues: association of IgA with M cells. Cell Tissue Res. 1987;248:645–651. doi: 10.1007/BF00216495. [DOI] [PubMed] [Google Scholar]
- 46.Stieger B, Marxer A, Hauri H P. Isolation of brush-border membranes from rat and rabbit colonocytes: is alkaline phosphatase a marker enzyme? J Membr Biol. 1986;91:19–31. doi: 10.1007/BF01870211. [DOI] [PubMed] [Google Scholar]
- 47.Sylvester F A, Philpott D, Gold B, Lastovica A, Forstner J F. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, Campylobacter upsaliensis. Infect Immun. 1996;64:4060–4066. doi: 10.1128/iai.64.10.4060-4066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weltzin R, Lucia-Jandris P, Michetti P, Fields B N, Kraehenbuhl J-P, Neutra M R. Binding and transepithelial transport of immunoglobulin by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989;108:1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodward M P, Young W W, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]


