Abstract
Purpose:
Novel effective therapies are urgently needed in recurrent osteosarcoma. GD2 is expressed in human osteosarcoma tumors and cell lines. This study evaluated the disease control rate (DCR) in patients with recurrent osteosarcoma treated with the anti-GD2 antibody dinutuximab plus cytokine therapy as compared to historical outcomes.
Methods:
AOST1421 was a single-arm phase 2 study for patients with recurrent pulmonary osteosarcoma in complete surgical remission. Patients received up to five cycles of dinutuximab (70mg/m2/cycle) with GM-CSF. Two different dinutuximab infusion schedules were studied: 35mg/m2/day over 20 hours (2-day) and 17.5mg/m2/day over 10 hours (4-day). Primary end point was DCR, defined as proportion of patients event-free at 12 months from enrollment. The historical benchmark was 12-month DCR of 20% (95% CI 10–34%). Dinutuximab would be considered effective if ≥16/39 patients remained event-free. Secondary objectives included toxicity evaluation and pharmacokinetics (PK).
Results:
Thirty-nine eligible patients were included in the outcome analysis. Dinutuximab did not demonstrate evidence of efficacy as 11/39 patients remained event-free for a DCR of 28.2% (95% CI 15–44.9%). One of 136 administered therapy cycles met criteria for unacceptable toxicity when a patient experienced sudden death of unknown cause. Other ≥ Grade 3 toxicities included pain, diarrhea, hypoxia and hypotension. PK parameters were similar in the two schedules.
Conclusions:
The combination of dinutuximab with GM-CSF did not significantly improve DCR in recurrent osteosarcoma. Dinutuximab toxicity and PK in adolescent and young adult osteosarcoma patients were similar to younger patients. Other strategies for targeting GD2 in osteosarcoma are being developed.
Keywords: Dinutuximab, recurrent osteosarcoma, GD2
INTRODUCTION
Treatment of patients with recurrent osteosarcoma remains challenging with very few effective therapeutic options available. A subset of patients with relapsed disease achieves subsequent surgical remission, but the majority of these patients will nevertheless relapse with a poor overall survival (OS). The main predictors of survival after osteosarcoma recurrence include the time to first recurrence, number of prior recurrences, disease burden and ability to achieve complete surgical remission (CR) after recurrence. Five-year OS for patients who are able to achieve second CR is reported at 39% versus 0% for patients who do not. Further, both event-free survival (EFS) and OS continue to decline rapidly after each subsequent recurrence (1), (2). Therefore, identification of novel effective therapies in this disease is an urgent need.
The disialoganglioside GD2 is a rational target in osteosarcoma. GD2 is expressed in >95% osteosarcoma tumors and cell lines (3,4). Prior experience from neuroblastoma clinical trials showed that the anti-GD2 antibody dinutuximab combined with cytokine therapy significantly improves outcomes in the setting of minimal residual disease (5). In neuroblastoma trials, dinutuximab has typically been administered at 17.5mg/m2/day for four days over a minimum of 10 hours each day with a cumulative dose of 70mg/m2/cycle (4-day schedule). Neuropathic pain is a frequent and major adverse effect associated with dinutuximab infusion necessitating hospitalization for continuous opiate administration. Modeling data using simulations in fourteen neuroblastoma patients with different administration schedules of dinutuximab with the same total dose per cycle showed no difference in the overall area under the curve (AUC) with a 48-hour infusion cycle, suggesting that this schedule of administration should not impact the efficacy of dinutuximab (6). Since minimizing hospital time is desirable in an older adolescent osteosarcoma patient population, an alternative shorter 2-day infusion schedule was used in this study with the same overall total dose per cycle.
To evaluate the efficacy of novel agents expeditiously in this rare disease, the Bone Tumor Committee of Children’s Oncology Group (COG) undertook a retrospective analysis of all osteosarcoma patients enrolled in prior Phase 2 trials conducted within the COG. The goal of this analysis was to characterize the median progression-free survival (PFS) of recurrent osteosarcoma patients to identify criteria for efficacy that were supported by prospectively collected data. This analysis estimated that 12-month PFS was 20% (CI 10–34%) for patients who were able to achieve a surgical CR (7). These data have since been used as the historic efficacy standard for a series of COG Phase 2 trials in recurrent osteosarcoma including AOST1321 and the current study AOST1421. This benchmark has allowed for trials testing novel agents with rapid accrual of small numbers of patients to detect an efficacy signal that would then provide justification for a larger Phase 3 study.
In this context, we conducted the single arm phase 2 trial (NCT02484443) to evaluate disease control rate (DCR) in patients with recurrent osteosarcoma, who achieved a pre-enrollment complete resection, when treated with dinutuximab plus cytokine therapy as compared to our historical benchmark experience. We evaluated a novel infusion schedule and focused on patients in a minimal residual disease state.
PATIENTS AND METHODS
Patient Population and Eligibility
Patients with recurrent osteosarcoma between the ages of 0–30 years who had isolated pulmonary recurrence and were able to achieve a CR within 4 weeks prior to enrollment were eligible for the study. Other key eligibility criteria included an ECOG performance score of 0, 1 or 2 and adequate organ function requirements as defined in the protocol. Key exclusion criteria included a history of bone metastases or any concurrent extra-pulmonary metastases, primary refractory disease which progressed on upfront therapy, any prior GD2-based therapy or known allergy to protocol drugs and pregnancy or breastfeeding.
The study was open to enrollment groupwide in the COG. The National Cancer Institute’s Central Institutional Review Board approved this study. All patients or their legal representatives signed informed consent and assent (as per local institutional policy) prior to enrollment.
Study Design
This was a single arm Phase 2 study of dinutuximab in combination with GM-CSF in patients with pulmonary recurrence of osteosarcoma. Patients were enrolled after complete surgical resection of disease and received five 28-day cycles of protocol therapy unless they progressed on therapy or experienced unacceptable toxicity.
The study schema is shown in Figure 1a. All patients received GM- CSF on days 1–14 subcutaneously at 250mcg/m2/day (max dose 500mcg/day). At study inception, a shorter experimental schedule of dinutuximab infusion was administered. While the total dose per cycle (70mg/m2) was identical to that used in neuroblastoma, dinutuximab was infused over 2 days as a 20-hour infusion each day on days 4 and 5 (35mg/m2/day in this 2-day schedule) in contrast to the 4-day schedule. The administration schedule was changed to a 4-day schedule (17.5mg/m2/day) after the first 32 patients were enrolled due to a grade 5 adverse event of sudden death of a patient on protocol therapy. The last 9 patients on study received dinutuximab on this 4-day schedule.
Figure 1a:
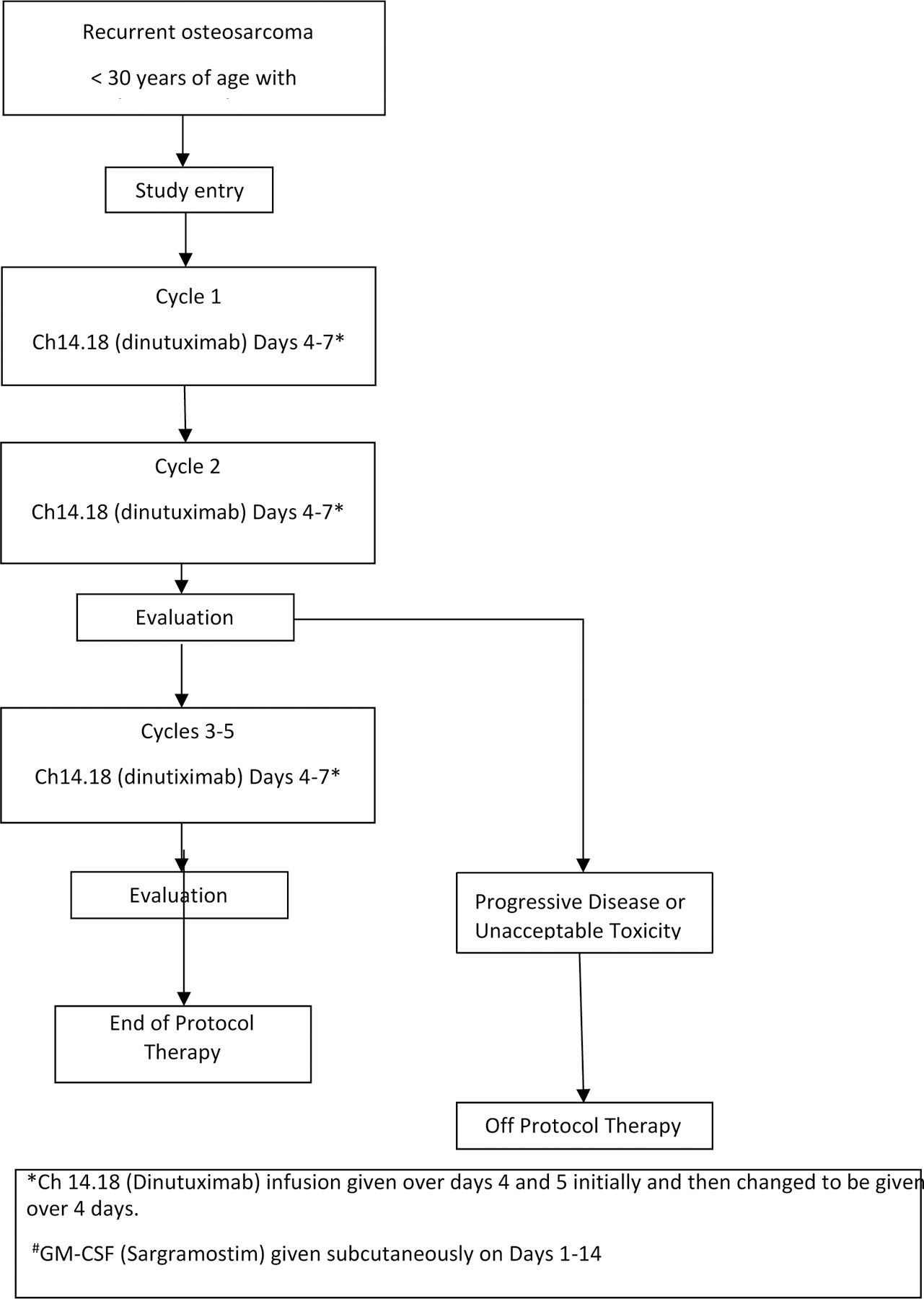
Study design schema for AOST1421
Endpoints
Primary Endpoint:
Patients had disease evaluations at baseline, following cycle 2 and 5 and then at 8 and 12 months from start of therapy. The primary endpoint was occurrence of an event which was defined as (1) disease progression - appearance of new lesions as per RECISTv1.1 (8); (2) diagnosis of a second malignant neoplasm; or (3) death regardless of cause. A patient who did not experience any of these three events in the 12-month interval after enrollment was considered to have experienced a disease control success (DCS). All other eligible patients were considered not to have experienced DCS. Patients who were not eligible could be replaced to fulfill the design criteria.
Secondary Endpoints:
Toxicities were graded according to CTCAEv4.0. Protocol-defined unacceptable toxicities (UT) included: anaphylaxis; unexpected death on protocol therapy or within 30 days of last dose of protocol therapy not related to disease progression; grade ≥ 4 allergic reaction, capillary leak syndrome, pain requiring narcotics/lidocaine AND persisting ≥ 4 days after the end of ch14.18 (dinutuximab) infusion; or grade ≥ 3 peripheral motor neuropathy for ≥ 2 weeks duration.
Dinutuximab concentrations were measured in plasma on days 4 and 7 of cycle 1 dinutuximab infusion and prior to start of cycle 2 infusion. Samples were analyzed using a validated electrochemiluminescence method at BioAgilytix Labs, Durham, NC, as previously published (9).
Exploratory Endpoints:
Several biological correlates that might potentially impact the response to immunocytokine therapy were explored in our study. These include development of human anti-chimeric antibodies (HACA) and presence of specific FcγR gene polymorphisms and natural killer cell isoforms. Given the small number of patients enrolled in this study, these analyses were exploratory.
Since dinutuximab is a human-mouse chimeric antibody, patients can develop potentially neutralizing human anti-chimeric antibodies (HACA). In neuroblastoma patients, dinutuximab is typically administered in an adjuvant setting after patients have received high dose chemotherapy followed by autologous stem cell transplant. These patients are frequently immunosuppressed and therefore the rate of developing HACA is low. The risk of developing HACA in our more immunocompetent study population was unknown. Therefore, we collected blood samples prior to start of each cycle to assess HACA titers in all patients. This analysis was performed using a validated electro chemiluminescent method (Bioagilytix Labs, Durham, NC). A 3-step process of screening assay, confirmatory assay and titration assay was utilized, as previously published (9).
FcγR gene polymorphisms can affect the immune cells’ ability to mediate antibody-dependent cytotoxicity by affecting the affinity of IgG for the FcγR (10). In neuroblastoma patients receiving immunocytokine therapy (hu14.18-IL2), a trend towards higher response rate was seen in patients with FcγR2A 131-H/H genotype (p=0.06). Therefore, we explored the different FcγR genotypes to evaluate any potential correlation with outcome. FcγR genotypes were determined at polymorphic codon 158 of FcγRIIIA (FCGR3A, rs396991) and codon 131 of FcγRIIA (FCGR2A, rs1801274) via selective gene amplification, purification and direct sequence analysis, as described previously (11).
Natural killer cells play an important role in response to immunocytokine therapy. In patients with GIST and neuroblastoma, an immunosuppressive natural killer cell isoform, NKp30C is associated with worse prognosis (12,13). We performed natural cytotoxicity receptor 3 (NCR3, rs986475) genotyping and isoform prediction using both DNA and RNA. Isoform quantifications were performed by real-time PCR using NKp30 and B2M primers as previously described (12). The relative quantity of each isoform was measured as a percentage of the total of the A, B, and C isoforms. Patients were characterized as harboring an immunosuppressive profile when the immunosuppressive isoform C (which is usually <10% of total isoform population) was ≥25%.
Statistical Analysis
Primary Objective:
The primary objective of the study was to compare DCR at 12 months in patients with completely resected pulmonary metastatic osteosarcoma, with a benchmark developed by Lagmay et al (7). Since that analysis demonstrated that the upper 95% confidence bound for the 12-month EFS probability to be approximately 30%, the null hypothesis for this study was a disease control response (DCR) probability ≤ 30%. We were to enroll 39 eligible patients. If 15 or fewer patients experienced DCS, we would conclude that dinutuxumab did not offer sufficient disease control to warrant further study; otherwise, we would consider dinutuxumab for further study. If the true DCR probability is 30%, the therapy would not be considered for further development with probability 0.91. If the true DCR is 50%, the therapy would be considered for further development with probability 0.90.
Secondary Objectives:
PK evaluation:
PK parameters such as volume of distribution, clearance, half-life (t1/2 α and t1/2 β), area under the curve (AUC) and maximum plasma concentration (Cmax) were calculated for each schedule of administration. Equality of the median of those six PK parameters across the two schedules was tested using the Kruskal-Wallis test (14).
Toxicity evaluation:
The analytic unit for UT monitoring was each patient-cycle. Any cycle in which the patient received at least 85% of planned protocol therapy, or in which a UT is observed was considered as a UT-evaluable cycle. If no UT was observed in an evaluable cycle, the cycle was considered free of a toxicity event; otherwise, the cycle had a toxicity event. We used a Bayesian rule to monitor for excessive toxicity. We assumed a beta prior distribution with α=0.4 and β=1.6 for the UT probability. The posterior probability of the chance of UT exceeded 30% was calculated monthly. If this posterior probability exceeded 80%, the COG Data and Safety Monitoring Committee was to be notified and a plan for possible dose modification developed.
The maximum grade of each adverse event in the NCI CTCAE version 4 that was grade 3, 4 or 5 that was experienced by each individual while on protocol therapy was determined. These maximum grades were tabulated according to regimen received. The p-value from the exact conditional test of equality of proportion of patients with the particular toxicity when comparing the two dosing regimens was calculated. In order to control the false discovery rate at 5%, we used the Bonferroni correction. We identified a difference in the rate of any particular toxicity as significant only if the exact conditional p-value was 0.00625 (=0.05/8) or less.
Exploratory Objectives:
The determination of HACA positivity in this study corresponds to that used for a recent COG neuroblastoma trial using dinutuximab (9). Briefly, patients were considered HACA evaluable if they had at least one sample available for analysis after cycle 1, day 4 (C1D4). Any patient with a confirmed HACA titer of >100 ng/ mL (prior studies determined this cutoff to be clinically meaningful) any time after C1D4 was deemed as HACA positive; the remaining were considered HACA negative. Time to development of HACA was assessed for evaluable patients with serial samples available.
FcγR gene polymorphisms and the presence of NKp30 isoform were categorized only for eligible patients with evaluable specimens. The association between DCS category and the biological measures noted above was assessed using the exact conditional methods.
For secondary and exploratory outcomes, a P-value of 0.05 or less were considered indicative of a significant association.
RESULTS
Patient Characteristics
AOST1421 enrolled 41 patients between November 2015 and August 2018. Data current to 31 December 2020 were used for this analysis. Accrual rate was greater than expected (2.1 patients vs. 1.6 patients per month). Two patients were ineligible, leaving 39 eligible and evaluable patients for this analysis. Figure 1b summarizes the allocation of treatment and disposition status of the patients enrolled on this study. Table 1 lists the patient characteristics of these 39 patients and compared by two infusion schedules. Median age was 15 years (range: 7 to 26 years). The majority of patients (74%) were non-metastatic at their initial diagnosis and 72% were in their first recurrence.
Figure 1b:
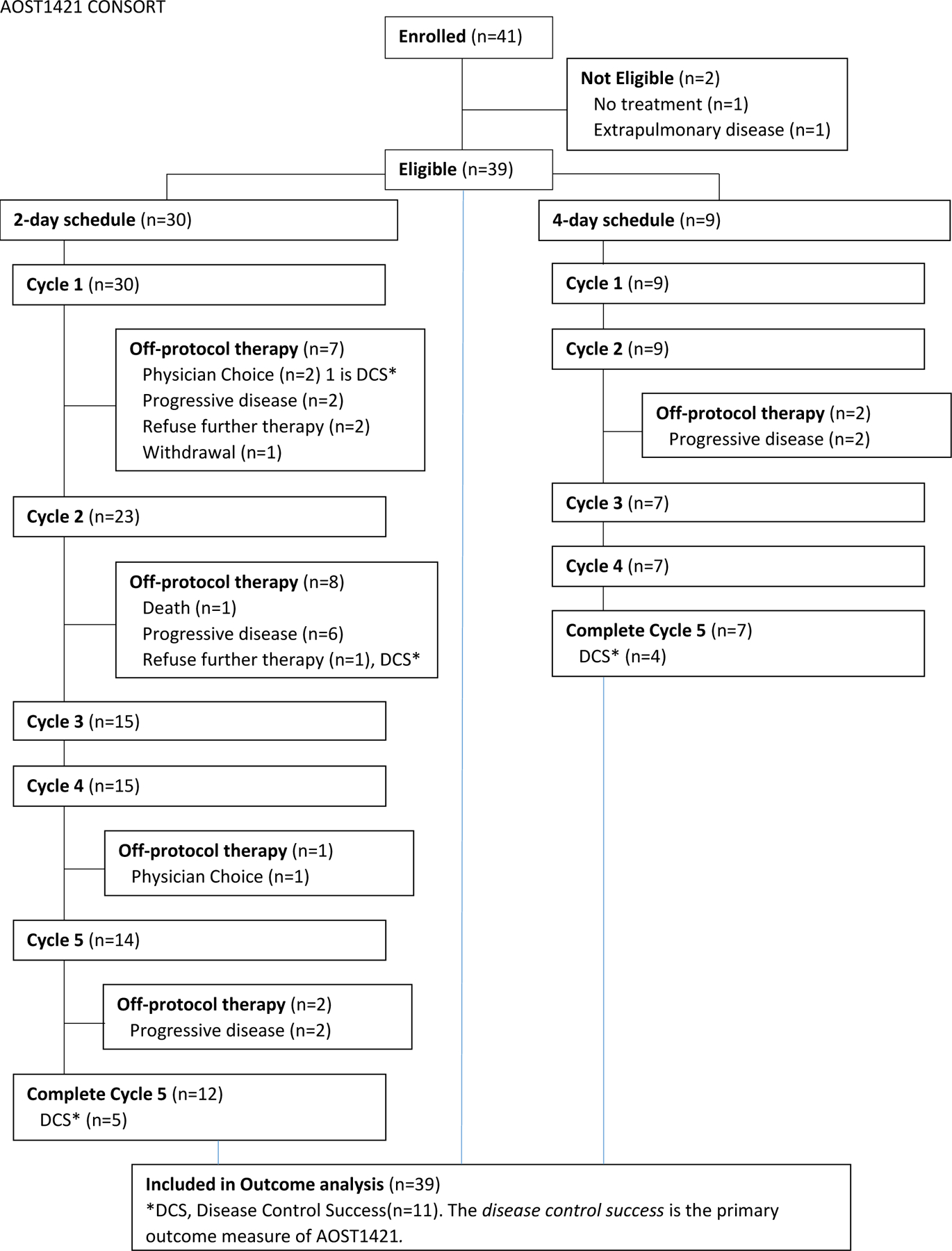
CONSORT diagram showing patient allocation and disposition
Table 1:
Characteristics of 39 eligible and evaluable patients (by dinutuximab infusion schedule and total)
| 2-day schedule (N=30) | 4 –day schedule (N=9) | All (N=39) | |||||
|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | ||
| Age at enrollment | |||||||
| <21 years | 25 | 83% | 8 | 89% | 33 | 85% | |
| 21+ | 5 | 17% | 1 | 11% | 6 | 15% | |
| Sex | |||||||
| Male | 18 | 60% | 4 | 44% | 22 | 56% | |
| Female | 12 | 40% | 5 | 56% | 17 | 44% | |
| Race | |||||||
| White | 21 | 70% | 6 | 67% | 27 | 69% | |
| Black | 3 | 10% | 1 | 11% | 4 | 10% | |
| Other | 1 | 3% | 2 | 22% | 3 | 8% | |
| Unknown/not reported | 5 | 17% | 0 | 0% | 5 | 13% | |
| Metastatic disease at initial diagnosis | |||||||
| Yes | 9 | 30% | 1 | 11% | 10 | 26% | |
| No | 21 | 70% | 8 | 89% | 29 | 74% | |
| Number of recurrences prior to enrollment | |||||||
| 1 | 21 | 70% | 7 | 78% | 28 | 72% | |
| 2 | 9 | 30% | 2 | 22% | 11 | 28% | |
Disease Control Rate
For the primary outcome, DCR, 28 of 39 patients experienced an event within 12 months for a DCR of 28.2% (95% CI 15–44.9%). One event was sudden death of unknown cause and the remaining events were incidents of disease progression. The EFS at 12 months was 28.2% (95% CI: 15.3–42.7%) and OS was 88% (95% CI: 72.9–95%) (Figure 2). All 11 patients with DCS were patients in their first recurrence and the 12-month EFS for the 28 patients with first recurrence was 39.3% (95% CI 21.7–56.5%; Table 2). The DCR was not significantly related to dinutuximab infusion schedule (Table 2).
Figure 2:
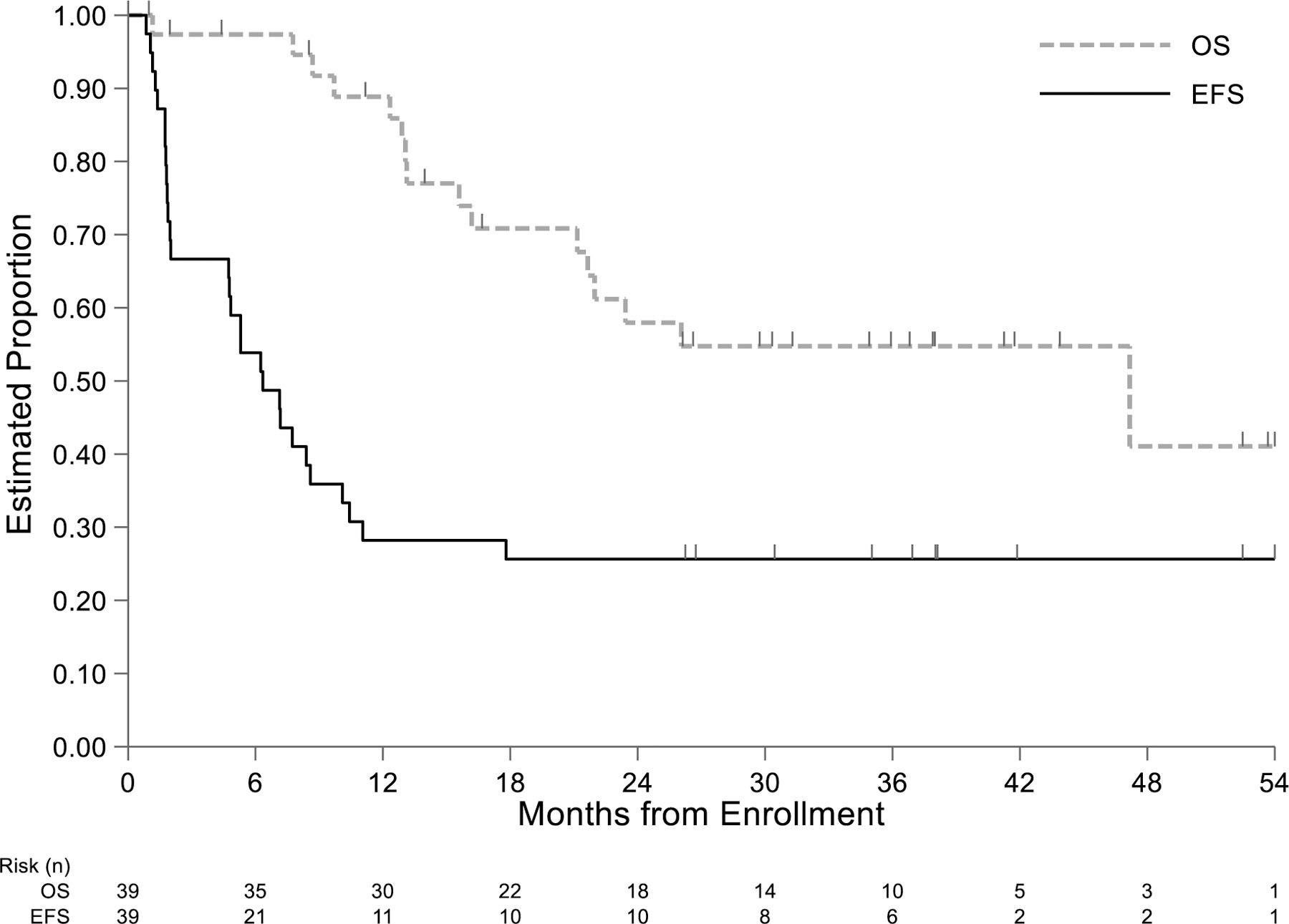
Kaplan-Meier estimates of event-free and overall survival in 39 patients with relapsed osteosarcoma treated with dinutuximab after complete surgical resection of recurrent disease.
Table 2:
Disease control success (DCS) rates by clinical, treatment, and biomarker parameters
| Disease Control Response (DCR) | Total | Fisher’s exact p-value1 | ||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| n | (%) | n | (%) | n | ||
| Dinutuximab Infusion schedule | 7 | (23) | 23 | (77) | 30 | 0.238 |
| 2-day schedule | ||||||
| 4-day schedule | 4 | (44) | 5 | (56) | 9 | |
| Age less than or 21 or greater at enrollment | 9 | (27) | 24 | (73) | 33 | 1.000 |
| No | ||||||
| Yes | 2 | (33) | 4 | (67) | 6 | |
| Sex | 4 | (18) | 18 | (82) | 22 | 0.158 |
| Male | ||||||
| Female | 7 | (41) | 10 | (59) | 17 | |
| Race | 7 | (26) | 20 | (74) | 27 | 0.323 |
| White | ||||||
| Black | 1 | (25) | 3 | (75) | 4 | |
| Other | 2 | (67) | 1 | (33) | 3 | |
| Unknown/ Not Reported | 1 | 4 | 5 | |||
| Ethnicity | 4 | (44) | 5 | (56) | 9 | 0.393 |
| Hispanic or Latino | ||||||
| Not Hispanic or Latino | 6 | (23) | 20 | (77) | 26 | |
| Unknown/ Not Reported | 1 | 3 | 4 | |||
| Metastatic disease at initial diagnosis. | 9 | (31) | 20 | (69) | 29 | 0.693 |
| No | ||||||
| Yes | 2 | (20) | 8 | (80) | 10 | |
| Number of recurrences prior to enrollment. | 11 | (39) | 17 | (61) | 28 | 0.017 |
| 1 | ||||||
| 2 | 0 | 11 | (100) | 11 | ||
| NKp30 isoform Final | 6 | (25) | 18 | (75) | 24 | 0.232 |
| AB | ||||||
| C | 5 | (50) | 5 | (50) | 10 | |
| no data | 0 | 5 | 5 | |||
| FCGR2A | 5 | (24) | 16 | (76) | 21 | 0.681 |
| high | ||||||
| low | 4 | (36) | 7 | (64) | 11 | |
| no data | 2 | 5 | 7 | |||
| FCGR3A | 6 | (40) | 9 | (60) | 15 | 0.448 |
| high | ||||||
| low | 4 | (22) | 14 | (78) | 18 | |
| no data | 1 | 5 | 6 | |||
| Siebert, Fc affinity profile (20) | 3 | (33) | 6 | (67) | 9 | 0.685 |
| high | ||||||
| low | 6 | (26) | 17 | (74) | 23 | |
| no data | 2 | 5 | 7 | |||
Excluding categories Unknown / Not reported or no data.
Adverse Effects
One of 136 administered therapy cycles met criteria for UT when a patient receiving the 2-day schedule died after cycle 2 of infusion due to an unknown cause, attributed as probably related to protocol therapy. The patient completed 2-day infusion of dinutuximab without serious adverse events and was discharged home. He died in his sleep that night at home. Despite extensive review of the medical records, no cause of death could be determined, and the family declined autopsy. Since the patient received the 2-day experimental infusion schedule, the study was paused and the protocol was amended to allow only the standard 4-day schedule. Three patients who were on protocol therapy at this time were taken off therapy prematurely. No further grade 5 events occurred in the subsequent nine patients enrolled on study. Other ≥ Grade 3 toxicities occurring in > 10 % participants were known dinutuximab toxicities such as pain, diarrhea, hypoxia and hypotension. Table 3 summarizes these common adverse events. Of note, 23/30 patients in the 2-day schedule did not experience any Grade 3–5 toxicities as compared to 100% of patients in the 4-day schedule experiencing at least one Grade 3–4 toxicity. Abdominal pain (56% vs. 10%) and hypoxia (44% vs. 23%) were more common in the 4-day schedule vs. the 2-day schedule. None of the differences in rates were considered significant after controlling the false discovery rate at 5%.
Table 3:
Grade 3–5 Adverse Events Observed by Infusion Schedule: one grade 5 event of sudden death seen in 2-day schedule; 23% of patients in 2-day schedule had no Grade 3–5 events
| Organ systems, Toxicity Type | Dosing Schedule1 | ||||
|---|---|---|---|---|---|
| 2-day schedule (N=30) | 4-day schedule (N=9) | ||||
| n | Percent | n | Percent | ||
| None | 7 | (23) | |||
| Gastrointestinal disorders | Abdominal pain | 3 | (10) | 5 | (56) |
| Diarrhea | 3 | (10) | 1 | (11) | |
| Nausea | 1 | (3.3) | |||
| Vomiting | 2 | (6.7) | |||
| Vascular disorders | Hypotension | 8 | (27) | 2 | (22) |
| Respiratory, thoracic and mediastinal disorders | Hypoxia | 7 | (23) | 4 | (44) |
| General disorders and administration site conditions | Pain | 5 | (17) | 2 | (22) |
| Sudden death NOS | 1 | (3.3) | |||
None of the comparisons between the two schedules of the rates of specific toxicity were significant at the 0.05 level after the Bonferroni correction was applied.
Pharmacokinetics Parameters
PK data were available on 20 patients with the 2-day schedule and 9 patients with the 4-day schedule. Table 4 summarizes the PK parameters for patients in each infusion schedule and for the overall cohort. Volume of distribution (V1), clearance (CL), t 1/2 alpha and t1/2 beta were not statistically different according to the criteria described above. The 4-day schedule demonstrated significantly greater AUC0−∞ and significantly smaller Cmax when compared with the 2-day schedule.
Table 4:
PK Parameters by Infusion Schedule
| 4-day schedule (N=9) |
2-day schedule (N=20) |
Overall (N=29) |
Test p-value Median* | |
|---|---|---|---|---|
| BSA (m 2 ) | ||||
| Mean (SD) | 1.59 (0.331) | 1.82 (0.317) | 1.75 (0.333) | |
| Median [Min, Max] | 1.65 [0.860, 2.04] | 1.79 [1.31, 2.39] | 1.70 [0.860, 2.39] | |
| Delivered Dose (mg) | ||||
| Mean (SD) | 111 (23.2) | 120 (29.4) | 118 (27.5) | |
| Median [Min, Max] | 116 [60.0, 143] | 119 [55.0, 169] | 116 [55.0, 169] | |
| CL (L/Day/m 2 ) | ||||
| Mean (SD) | 0.746 (0.0537) | 0.852 (0.153) | 0.819 (0.139) | |
| Median [Min, Max] | 0.729 [0.693, 0.829] | 0.792 [0.666, 1.27] | 0.788 [0.666, 1.27] | 0.053 |
| V1 (L/m 2 ) | ||||
| Mean (SD) | 1.58 (0.320) | 2.43 (1.41) | 2.17 (1.24) | |
| Median [Min, Max] | 1.46 [1.27, 2.09] | 1.84 [1.13, 6.84] | 1.81 [1.13, 6.84] | 0.053 |
| T1/2 Alpha (days) | ||||
| Mean (SD) | 0.720 (0.106) | 0.946 (0.339) | 0.876 (0.304) | |
| Median [Min, Max] | 0.684 [0.615, 0.885] | 0.810 [0.566, 1.89] | 0.800 [0.566, 1.89] | 0.053 |
| T1/2 Beta (days) | ||||
| Mean (SD) | 7.62 (0.138) | 7.48 (0.162) | 7.53 (0.165) | |
| Median [Min, Max] | 7.65 [7.42, 7.76] | 7.49 [7.25, 7.86] | 7.50 [7.25, 7.86] | 0.059 |
| AUC (mg * hr/L) | ||||
| Mean (SD) | 2261 (158) | 1911 (419) | 2019 (392) | |
| Median [Min, Max] | 2304 [2027, 2440] | 2038 [904, 2514] | 2136 [904, 2514] | 0.018 |
| Cmax (mg/L) | ||||
| Mean (SD) | 14.5 (2.54) | 19.7 (5.74) | 18.1 (5.51) | |
| Median [Min, Max] | 13.6 [11.7, 19.3] | 21.3 [7.58, 26.3] | 18.4 [7.58, 26.3] | 0.010 |
Kruskal-Wallis test with a null hypothesis that medians by dose schedule are equal.
Development of HACA
Assessment of HACA was mandatory on this study and results were available for 36/39 patients (no samples were received on the remaining 3 patients for analysis). Of these 36 patients, 30 were evaluable as previously defined. The majority (17/30; 57%) of patients developed HACA after exposure to dinutuximab while 13/30 (43%) remained HACA negative. Of the 17 HACA + patients, 15 were evaluable for the course when HACA first developed; 11 (73%) developed HACA before the end of course 2 and 4 (24%) developed HACA from course 3 onwards.
NKp30 isoform and FcγR Analysis
No significant associations between DCR and any quantification of NKp30 isoform or FcγR were identified (Table 2).
DISCUSSION
While dinutuximab in combination with GM-CSF did not meet the design efficacy criteria for 12-month DCR in patients with completely resected recurrent osteosarcoma, there are several key takeaways from our study. First, there remains a tremendous need for novel therapies in patients with recurrent osteosarcoma as demonstrated by faster than expected accrual in our study even with concurrent accrual to another trial within COG (AOST1321) for the same patient population. Second, our current phase 2 trial design using a benchmark approach remains a highly effective and efficient mechanism to discern signal of activity of novel agents in patients. Using this approach, we confirmed that our study showed a definite lack of activity. An updated analysis of EFS outcomes in three recent COG trials for patients with measurable disease at enrollment (AOST1322, AOST1321 cohort 1 and AOST1521) as well as two trials for patients with CR at enrollment (AOST1321 cohort 2, AOST1421) suggests that the previously established historical benchmarks for outcomes have not changed with time and can continue to be used in these screening studies in the future (15,16). However, some refinement may be needed in future trials for patients in first recurrence based on our data in those patients. Third, pharmacokinetic parameters of dinutuximab in adolescent and young adult patients with osteosarcoma are similar to those seen in younger patients with neuroblastoma (17). Our data show that AUC and Cmax were affected by duration of infusion in contrast to the simulated data in neuroblastoma patients. Consistent with previous reports in neuroblastoma trials (18,19), most patients who were to develop HACA did so early on in therapy by the end of cycle 2. This suggests that duration of therapy is likely not a significant factor in HACA development but rather an inherent propensity of the host. The overall rate of HACA positivity in this patient population was higher than that reported in neuroblastoma patients where it is rarely seen, likely due to a more immunocompromised state when dinutuximab is administered after autologous stem cell transplant (11).
In this study we also attempted to determine if some of the known predictors of response to immunotherapy in neuroblastoma such as NKp30 isoforms and FcγR genotype affinities would predict response in osteosarcoma. NKp30 isoform C is associated with an immunoinhibitory effect and hypothesized to be associated with a poor response to immunotherapy whereas AB isoform is immunostimulatory. Similarly, high affinity of FcγR genotypes 2A and 3A is predicted to be associated with good response to immunotherapy while low affinity is associated with poor response (20). In our cohort, no difference in DCS was seen based on these biological markers.
In addition, apart from the one sudden unexpected death of unknown cause in a patient receiving the 2-day infusion schedule, this shorter infusion schedule was well tolerated in the majority of patients and some adverse effects such as abdominal pain and hypoxia tended to be less severe in this group. These data may provide the basis for designing future trials with dinutuximab in adolescent and young adult cancer patients.
Previous studies have suggested that GD2 is expressed in majority of osteosarcoma patient tumors in diagnostic, metastatic as well as recurrent disease but degree of expression can be variable (21). Based on these data, presence of GD2 expression was not a required eligibility criterion in our study. Instead, one of the proposed objectives of the study was to evaluate GD2 expression in tumor material retrospectively and correlate with clinical outcome. However, this aim could not be completed as proposed as it was not possible to develop a validated test to determine GD2 expression in formalin-fixed tissues with the current available antibodies. One of the specific challenges in osteosarcoma specimens is the need to decalcify tissues which can hamper the immunohistochemical staining of proteins. In addition, significant variability exists in institutional protocols for decalcification. Our inability to complete this objective in this study was one of the limiting factors to determine the role of degree of GD2 expression in dinutuximab efficacy. Future studies should take this into consideration and collect fresh frozen tissue or touch preparation of the tumor tissue for GD2 evaluation. The other limitation of our study was that while all of our DCS patients were those with first recurrence, due to small numbers we could not definitively ascertain whether dinutuximab is more efficacious in patients with first recurrence or whether this was due to the natural history of the disease. It may be prudent to consider enrolling a larger cohort of patients in first recurrence in future trials with dinutuximab.
Despite the fact that GD2 is highly expressed on the osteosarcoma cell surface, dinutuximab as a monotherapy was not adequate to improve outcome in relapsed patients. This is likely not a reflection of the target but instead the mechanism of anti-tumor activity of dinuximab (antibody-dependent complement mediated cytotoxicity) being not sufficient. Therefore, novel approaches are being developed to enhance efficacy of anti-GD2 antibodies. A combination of anti-CD47 antibody with dinutuximab in OS xenografts showed significant tumor growth delay in osteosarcoma xenografts as well as decrease in metastatic burden in a pulmonary metastatic model whereas single agent anti-GD2 or anti-CD47 had no anti-tumor effects. The investigators hypothesized that GD2 is a macrophage checkpoint capable of inhibiting tumor cell phagocytosis (22). This combination is now being evaluated in a clinical trial for recurrent osteosarcoma. Similarly, GD2-based CAR T cell therapy is also being studied in patients with recurrent osteosarcoma (NCT04539366; NCT03373097). Another strategy being investigated is an anti-CD3 x anti-GD2 bispecific antibody (NCT02173093) with the rationale that the simultaneous recruitment of T-cells using anti-CD3 would significantly enhance the tumor kill effect of anti-GD2 antibody. In addition, chemoimmunotherapy combining irinotecan and temozolomide with dinutuximab has shown pronounced activity in neuroblastoma and combination of dinutuximab with chemotherapy may similarly warrant evaluation in osteosarcoma (23,24). In summary, data from our initial dedicated study of dinutuximab in osteosarcoma will inform future trials with dinutuximab in patients with osteosarcoma.
HIGHLIGHTS.
GD2 is expressed in most osteosarcoma tumors and cell lines.
Dinutuximab with GM-CSF in patients with recurrent osteosarcoma was evaluated.
Dinutuximab failed to improve 12-month disease control rate in these patients.
Study provides safety and PK of dinutuximab in adolescent & young adults patients.
Novel therapeutics to target GD2 are being evaluated.
Acknowledgements
AOST1421 study was supported by United Therapeutics Corporation.
Funding
This study was funded by NCTN Operations Center Grant U10CA180886, NCTN Statistics & Data Center Grant U10CA180899, St. Baldrick’s Foundation, and the Quad W Foundation. Dinutuximab was supplied by United Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CRediT Author Statement
Pooja Hingorani: Conceptualization, Investigation, Writing- Original Draft, Review and Editing, Visualization, Project Administration
Mark Krailo: Methodology, Software, Formal Analysis, Writing-review and editing, data curation
Allen Buxton: Formal analysis, writing-review and editing, data curation
Paul Hutson: Investigation, writing- review and editing
Paul Sondel: Investigation, writing- review and editing
Mitchell Diccianni: Investigation, writing-review and editing
Alice Yu: Investigation, writing- review and editing
Carol Morris: writing- review and editing
Richard Womer: writing- review and editing
Brian Crompton: investigation, writing- review and editing
R Lor Randall: writing- review and editing
Lisa Teot: writing- review and editing
Steven G. Dubois: Investigation, Writing- review and editing, supervision
Katherine Janeway: Investigation, Writing- review and editing, supervision
Richard Gorlick: Conceptualization, Methodology, Writing- review and editing, supervision
Michael Isakoff: Investigation, Writing- review and editing, supervision
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
SGD has received consulting fees from Amgen, Bayer, and Jazz as well as travel expenses from Loxo, Roche, and Salarius
MDK has received consulting fees from Merck, Sharpe and Dhome
KAJ has received consulting fees from Bayer and Ipsen and honoraria from Takeda and Foundation Medicine
The remaining authors have no conflicts of interest
References
- 1.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer 2003;98(11):2447–56 doi 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 2.Bielack SS, Kempf-Bielack B, Branscheid D, Carrle D, Friedel G, Helmke K, et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol 2009;27(4):557–65 doi 10.1200/JCO.2008.16.2305. [DOI] [PubMed] [Google Scholar]
- 3.Roth M, Linkowski M, Tarim J, Piperdi S, Sowers R, Geller D, et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 2014;120(4):548–54 doi 10.1002/cncr.28461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiner JP, Miraldi F, Kallick S, Makley J, Neely J, Smith-Mensah WH, et al. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res 1987;47(20):5377–81. [PubMed] [Google Scholar]
- 5.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363(14):1324–34 doi 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai AV, Fox E, Smith LM, Lim AP, Maris JM, Balis FM. Pharmacokinetics of the chimeric anti-GD2 antibody, ch14.18, in children with high-risk neuroblastoma. Cancer Chemother Pharmacol 2014;74(5):1047–55 doi 10.1007/s00280-014-2575-9. [DOI] [PubMed] [Google Scholar]
- 7.Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O, 3rd, et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol 2016;34(25):3031–8 doi 10.1200/JCO.2015.65.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Desai AV, Gilman AL, Ozkaynak MF, Naranjo A, London WB, Tenney SC, et al. Outcomes following anti-GD2 antibody-based post-consolidation therapy after cessation of randomization on ANBL0032: a report from the Children’s Oncology Group. JCO; submitted 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado DC, Hank JA, Kolesar J, Lorentzen D, Gan J, Seo S, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res 2010;70(23):9554–61 doi 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu AL, Gilman AL, Ozkaynak MF, Naranjo A, Diccianni MB, Gan J, et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin Cancer Res 2021;27(8):2179–89 doi 10.1158/1078-0432.CCR-20-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011;17(6):700–7 doi 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 13.Semeraro M, Rusakiewicz S, Minard-Colin V, Delahaye NF, Enot D, Vely F, et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci Transl Med 2015;7(283):283ra55 doi 10.1126/scitranslmed.aaa2327. [DOI] [PubMed] [Google Scholar]
- 14.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Amer Statist Assoc 1952;47:583–621. [Google Scholar]
- 15.Chou AJ, Krailo M, Han R, Buxton A, Reed DR, Gorlick R, et al. Updated outcomes for patients with completely resected pulmonary recurrent osteosarcoma: A report from the Children’s Oncology Group. JCO 2021;39:15_suppl.10023. [Google Scholar]
- 16.Rao S, Han R, Krailo M, Buxton A, Hingorani P, Chou AJ, et al. Outcome of patients with recurrent/ refractory osteosarcoma enrolled in three recent phase II trials: A report from the Children’s Oncology Group. JCO 2021;39:15_suppl.11530. [Google Scholar]
- 17.Uttenreuther-Fischer MM, Huang CS, Yu AL. Pharmacokinetics of human-mouse chimeric anti-GD2 mAb ch14.18 in a phase I trial in neuroblastoma patients. Cancer Immunol Immunother 1995;41(6):331–8 doi 10.1007/BF01526552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol 2014;32(14):1445–52 doi 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hank JA, Gan J, Ryu H, Ostendorf A, Stauder MC, Sternberg A, et al. Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin Cancer Res 2009;15(18):5923–30 doi 10.1158/1078-0432.CCR-08-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siebert N, Jensen C, Troschke-Meurer S, Zumpe M, Juttner M, Ehlert K, et al. Neuroblastoma patients with high-affinity FCGR2A, −3A and stimulatory KIR 2DS2 treated by long-term infusion of anti-GD2 antibody ch14.18/CHO show higher ADCC levels and improved event-free survival. Oncoimmunology 2016;5(11):e1235108 doi 10.1080/2162402X.2016.1235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon VI, Roth M, Piperdi S, Geller D, Gill J, Rudzinski ER, et al. Ganglioside GD2 expression is maintained upon recurrence in patients with osteosarcoma. Clin Sarcoma Res 2015;5(1):4 doi 10.1186/s13569-014-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theruvath JL, Smith B, Linde MH, Sotillo E, Heitzeneder S, Marjon K, et al. GD2 is a macrophage checkpoint molecule and combined GD2/CD47 blockade results in synergistic effects and tumor clearance in xenograft models of neuroblastoma and osteosarcoma. AACR Special Conference on the Advances in Pediatric Cancer Research 2020. doi 10.1158/1538-7445. [DOI] [Google Scholar]
- 23.Mody R, Yu AL, Naranjo A, Zhang FF, London WB, Shulkin BL, et al. Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children’s Oncology Group. J Clin Oncol 2020;38(19):2160–9 doi 10.1200/JCO.20.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol 2017;18(7):946–57 doi 10.1016/S1470-2045(17)30355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


