Abstract
CD4+ T cells capture membrane and membrane-bound molecules from APCs directly from the immunological synapse in a process termed trogocytosis. The function and biological consequences of trogocytosis are largely unknown. In this study, we examine the biological significance of this phenomenon on the trogocytosis-positive T cell. We used murine fibroblasts expressing GFP-tagged I-Ek molecules loaded with a covalently attached antigenic peptide (moth cytochrome c 88–103) to present Ag to primary TCR transgenic T cells. Using a combination of high-resolution light microscopy and flow cytometry, we show that the trogocytosed molecules are retained on the surface of the T cell in association with the TCR and elevated phosphorylated ZAP-70, phosphorylated tyrosine, and phosphorylated ERK 1/2. Through the use of the Src inhibitor PP2, we demonstrate that trogocytosed molecules directly sustain TCR signaling. In addition, after removal of APC, trogocytosis-positive cells preferentially survive in culture over several days. These novel findings suggest that trogocytosed molecules continue to engage their receptors on the T cell surface and sustain intracellular signaling leading to selective survival of these cells.
Antigen recognition by CD4+ T lymphocytes leads to the acquisition of APC membrane lipids and membrane-bound proteins, including the cognate MHC:peptide complexes (1, 2). This phenomenon has been termed trogocytosis (3). The initial description of trogocytosis by T cells dates back to the early 1970s (4). Although there were several important publications in the 1980s (5–7), interest in this phenomenon and its potential immunomodulatory role was reignited in the late 1990s by the work of Mannie and colleagues (8–14), Sprent et al. (15–17), and Hudrisier and Joly (1, 3, 18–25). Although much has been learned about the molecules transferred and the mechanism of trogocytosis in the past decade, the biological consequences of this phenomenon remain largely unknown.
Initiation of trogocytosis coincides with the formation of an immunological synapse between an Ag-specific T lymphocyte and an APC (2, 13, 15, 16, 26). The formation of the immune synapse is the result of spatial and temporal rearrangement of molecules into distinct supramolecular activation complexes (SMACs) (27). The prototypical Th1 immune synapse is characterized as a bull’s-eye with TCR-MHC:peptide, TCR signaling associated molecules, and CD28/CD80 localized to the center (the cSMAC), whereas adhesion molecules, such as ICAM-1/LFA-1, are found in a surrounding, peripheral ring (the pSMAC) (27–29). The overall function of the immunological synapse is unclear; past results have shown that the immune synapse is the site of cytokine secretion (30, 31), cytolytic granule secretion (26), and TCR downmodulation and signaling (32). We have previously shown that the immune synapse is also the site of APC to CD4+ T cell trogocytosis (2). Trogocytosis is not unique to T cells, as both B cells (23, 33, 34) and NK cells (35–38) also form mature immune synapses and trogocytose molecules via these structures.
Trogocytosis leads to the capture of APC membrane and associated surface molecules by the T cell. It has been shown that T cells capture and integrate into their plasma membranes costimulatory molecules [including CD80 (2, 39, 40), CD86 (16), OX40 ligand (41), and programmed death ligand 1 (42)], adhesion molecules [including ICAM-1 (24)], and MHC:peptide complexes via trogocytosis (1, 2, 15, 16). Several studies have examined the possibility that the presence of these APC-derived immune synapse molecules on the T cell surface (1, 2, 15, 16) allow the trog+ T cells to present Ag and modulate the activation of responding T cells (43–49). Much less well studied is the possibility that these molecules could play a role in sustaining intracellular signaling in the trog+ cell by continual engagement of their receptors on the T cell surface.
The mechanism of trogocytosis from APC to T cells remains unclear. Transmission electron microscopy studies have shown that nanoscale membrane fusion events, so called “membrane bridges,” occur between APC and CTL during cell dissociation that may result in trogocytosis (26). More recently, Martinez-Martin et al. (50) found that TCR downmodulation at the immune synapse is due to phagocytosis by the T cell. They suggest that trogocytosis is the consequence of coincidental T cell phagocytosis of APC membrane during TCR downmodulation. These phagocytosed APC membrane fragments have been hypothesized to fuse with the endosomal membrane and recycle to the T cell plasma membrane, resulting in the presence of these APC-derived molecules on the T cell surface (51). The presence of captured MHC:peptide and costimulatory molecules on T cells suggest the possibility for autopresentation leading to continued signaling. Autopresentation by trogocytosed molecules may play a role in signal “summing” (52) [i.e., repeated short-duration encounters that have been observed to result in T cell activation (53, 54)]. Consistent with this, our earlier data show that several signaling-associated molecules, including phosphorylated tyrosine (pTyr) and Lck, colocalize with the trogocytosed molecules on the T cells (2). In this study, we have expanded these previous studies to examine the potential for sustained signaling in T cells after trogocytosis.
In this paper, we examined the biological effects of trogocytosis on CD4+ T cells. We hypothesized that the trogocytosed molecules on T cells would engage their receptors on the T cell surface and sustain intracellular signaling after removal of APC. Using a combination of wide-field deconvolution microscopy and flow cytometry, we found that the transfer of MHC:peptide complexes and other APC-derived membrane proteins to the T cell results in sustained TCR proximal and distal signaling events, as measured by ZAP-70 and ERK 1/2 phosphorylation, respectively. The addition of the Src family kinase inhibitor PP2 resulted in a cessation of intracellular signaling, and the signaling from the trogocytosed molecules resumed upon removal of the PP2. The trog+ cells maintained an activated phenotype and preferentially survived in culture after removal of APC, suggesting that trogocytosis may play an important role in the activation and survival of T cells during an immune response.
Materials and Methods
Animals
Heterozygous AD10 TCR transgenic mice (Vβ3+), specific for pigeon cytochrome c fragment 88–104 (55) and reactive against moth cytochrome c (MCC) fragment 88–103 on a B10.BR (H-2k) background, were provided by S. Hedrick (University of California, San Diego, CA) by way of D. Parker (Oregon Health and Science University, Portland, OR). Heterozygous Ag transgenic mice expressing GFP-tagged I-Ek β-chain with covalently attached MCC88–103 peptide under the control of the MHC class II promoter were provided by D. Parker. Both strains were maintained as heterozygotes by breeding to B10.BR mice, and transgenic mice were identified by PCR. Mice were housed in the University of Montana Laboratory Animal Resources facilities and were allowed food and water ad libitum. All procedures were supervised and in accordance with the University of Montana Institutional Animal Care and Use Committee.
Abs and staining reagents
The following conjugated or unconjugated Abs were purchased from BD Pharmingen: anti-TCR Vβ3 (clone KJ25), anti-ZAP70 PO4 (Y319; clone 17 A/P-ZAP-70), and anti-ERK PO4 (MAPK p44/42 T202/Y204; clone 20a). Chicken anti-rabbit IgG Alexa Fluor 647 was purchased from Life Technologies (Eugene, OR). Biotin conjugated anti-phosphotyrosine (4G10) was purchased from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal Abs specific for pLck (Y505; number 2751) and phosphroylated ZAP-70 (Y319 Syk PO4 Y352; number 2701) along with a mouse monoclonal anti-phosphroylated ERK 1/2 (MAPK p44/42 T202/Y204; number E10) were purchased from Cell Signaling Technology (Beverly, MA). Secondary staining reagents including aminomethylcoumarin acetate-conjugated streptavidin, Cy5-conjugated goat anti-Armenian hamster IgG, Texas Red-conjugated donkey anti-rabbit IgG, and Texas Red-conjugated anti-mouse IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-CD69 (H1.2F3), anti-CD4 (RM4–5), anti-C25 (PC61), and CD80 (16–10A1) were purchased from BioLegend (San Diego, CA). Anti–I-Ek (17–3–3) was purchased fro Southern Biotechnology Associates (Birmingham, AL).
APCs
MCC:GFP fibroblasts expressing enhanced GFP-tagged I-Ek β-chain with covalent antigenic MCC88–103 were described previously (56). A second transfected fibroblast line, MCC:FKBP, has the MCC:I-Ek β-chain fused to three repeats of the FK506-binding protein (Ariad Pharmaceuticals, Cambridge, MA) in place of the GFP tag (2). The MCC:GFP and MCC:FKBP express nearly identical levels of CD80, ICAM-1, and surface MCC:I-Ek. Cells were maintained in “complete DMEM” containing high-glucose DMEM (Life Technologies, Carlsbad, CA) supplemented with 10% FBS (Atlanta Biologicals, Atlanta, GA), 1 mM L-glutamine, 100 mg/ml sodium pyruvate, 50 μM 2-ME, essential and nonessential amino acids (Life Technologies), 100 U/ml penicillin G, 100 U/ml streptomycin, and 50 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO).
In vitro T cell priming
Single-cell suspensions of splenocytes from 6- to 12-wk-old AD10 transgenic mice were depleted of erythrocytes by hypotonic lysis and resuspended in “complete RPMI 1640” containing RPMI 1640 medium (Life Technologies) supplemented with 10% FBS (Atlanta Biologicals), 1 mM L-glutamine, 100 mg/ml sodium pyruvate, 50 μM 2-ME, essential and nonessential amino acids (Life Technologies), 100 U/ml penicillin G, 100 U/ml streptomycin, and 50 μg/ml gentamicin (Sigma-Aldrich). Cells were used directly ex vivo or were activated in vitro for 6 d by addition of 2.5 μM MCC88–103 peptide to splenic cell suspensions. Lymphocytes were isolated from priming cultures by density centrifugation using Lympholyte M (Cedarlane Laboratories, Burlington, NC). T cells were resuspended at 5 × 106/ml in phenol red-free complete RPMI 1640 medium and kept at 4°C until used.
Standard in vitro trogocytosis assay
To assess trogocytosis by the CD4+ AD10 T cells, we used our previously described standard in vitro trogocytosis assay (2). Briefly, 1 × 106 MCC: GFP or MCC:FKBP cells were plated into individual wells of a 6-well tissue culture plate (Greiner, Monroe, NC) and incubated overnight at 37°C. A total of 2.5 × 106 in vitro-primed T cells were added to each well, and the plates were centrifuged briefly (30 s at 200 × g) to promote T–APC interaction. The cells were then incubated for 90 min at 37°C. After the 90-min incubation, T cells were recovered from the cultures by rinsing with PBS. No additional dissociating reagents were added (e.g., EDTA or trypsin) to aid in T cell recovery. In this way, we collected only cells that had spontaneously dissociated from the APC. Greater than 70% of the input cells were routinely recovered from the culture at 90 min after the PBS wash. After two additional PBS washes, recovered T cells (containing both trog+ and trog−) were used immediately in microscopy or flow cytometry experiments or were resuspended in complete RPMI 1640 medium at very low density (104/ml) and cultured for additional time periods. At the end of the incubation period, cells were resuspended at 106/ml before fixation and staining for flow cytometry experiments or addition to poly-L-lysine–coated LabTek II chambered coverslips for imaging, as described below.
In vivo trogocytosis experiments
To examine in vivo trogocytosis, 106 naive AD10 T cells in 0.2 ml HBSS were injected i.v. into pairs of unstimulated, heterozygous MCC:GFP:I-Ek Ag transgenic or negative littermate controls. After 4 d, splenocytes were recovered from the mice. Injected AD10 cells were identified based on anti-CD4 and anti-Vβ3 staining. The donor AD10 cells were analyzed for trogocytosis (CD80) and activation (Vβ3 and CD62L downmodulation and upregulation of CD69, CD25, and CD44) using flow cytometry, as described below.
Flow cytometry
Cells were recovered from the cultures and resuspended at 106/ml in FACS buffer (pH 7.4 PBS containing 2% BSA Fraction V [Sigma-Aldrich] and 0.1% NaN3). The T cells were stained with the indicated reagents for 30 min at 4°C in FACS buffer. After three washes, cells were stained for 20 min with secondary reagents in FACS buffer, when necessary. Following a final set of three washes in FACS buffer, cell were resuspended in 500 μl FACS buffer and stored on ice until analyzed using a FACSAria II (BD Biosciences) in the UM Fluorescence Cytometry Core. Alternatively, after the final wash, cells were fixed by addition of ice-cold fixative (4% paraformaldehyde and 0.5% glutaraldehyde), followed by a 45-min incubation at room temperature. Following an additional set of washes, cells were resuspended in 500 μl FACS buffer and were stored at 4°C in the dark for up to 3 d until analysis.
To stain intracellular signaling molecules, we modified the protocol of Chow et al. (57). Briefly, after staining surface molecules on live cells as described above, cells were fixed for 10 min in ice-cold fixative (4% paraformaldehyde and 0.5% glutaraldehyde in PBS). After two washes in PBS plus 5% FBS, cells were permeabilized for 10–30 min with ice-cold 50% methanol in PBS and washed twice in PBS prior to staining. Cells were then stained with primary staining reagents for 30 min at 4°C in FACS buffer at a 1:100 dilution. After three washes, cells were stained for 20 min with secondary reagents in FACS buffer at a 1:200 dilution. After a final set of washes, cells were analyzed immediately or were stored at 4°C for up to 3 d before analysis on a FACSAria II. Data were analyzed with FlowJo 8.8.7 software (Tree Star, Ashland, OR).
Immune synapse microscopy
For examination of the immune synapse, one day prior to use 2.5 × 104 MCC:GFP cells were added per well in #1.5 LabTek II eight-chambered coverslips (Nunc, Rochester, NY) and incubated overnight at 37°C. The next day, 105 T cells were added to each well, and the dishes were spun briefly to initiate contact between T cells and APCs. After a 30-min incubation at 37°C, cells were fixed by addition of ice-cold fixative (4% paraformaldehyde and 0.5% glutaraldehyde in PBS) and incubated for 45 min at room temperature in the dark, followed by permeabilization with 0.2% Triton X-100 in PBS for 10 min. Cultures were stained with primary Abs at concentrations of 10 μg/ml in imaging buffer (PBS plus 2% BSA Fraction V) for 2 h at room temperature in a humidified chamber. After three 5-min PBS washes, cells were incubated with secondary Abs (1:500 dilution in imaging buffer) for 2 h at room temperature. After three additional PBS washes, SlowFade Gold antifade reagent (Molecular Probes) was added to the wells. Samples were stored at 4°C and protected from light until imaged.
T cell–APC conjugates to be imaged were chosen based upon their characteristic morphology in differential interference contrast of T cells in tight contact with and flattened against an APC. A stack of 50–90 fluorescent images spaced 0.2 μm apart in the z-axis was obtained with a × 60, 1.4 NA, oil immersion lens on the Applied Precision DeltaVisionRT image restoration microscopy system (Issaquah, WA).
Microscopic analysis of trogocytosis
To examine trogocytosis by microscopy, T cells recovered were from the standard in vitro trogocytosis assay after the 90 min incubation by vigorous rinsing with PBS. For imaging of cells directly from the trogocytosis assay, 106 recovered T cells were placed in poly-L-lysine–precoated #1.5 LabTek II eight-chambered coverslips for 10 min at 37°C. For time course experiments, recovered T cells (containing both trog+ and trog−) were incubated at very low density (104/ml) for the indicated times before being placed in the poly-L-lysine–coated chambered coverslips. Cells were fixed by addition of ice-cold fixative (4% paraformaldehyde and 0.5% glutaraldehyde in PBS) and incubated for 45 min at room temperature in the dark followed by permeabilization with 0.2% Triton X-100 in PBS for 10 min. Cells were Ab stained and imaged using an Applied Precision DeltaVisionRT microscopy system as described in the immune synapse section above. Cells were chosen for imaging in differential interference contrast by identifying isolated, individual T cells in the field (and without regard to potential fluorescence). T cells were imaged in the same manner as the T–APC conjugates, as described above.
Image analysis
Constrained, iterative deconvolution was performed using the API SoftWorx software package. Using SoftWorx, the integrated intensity of GFP, which is a measure of the amount of fluorescently labeled molecules accumulated, was obtained for areas more than or equal to two times above background fluorescence. For the analysis of phosphorylated signaling molecules, the integrated intensity and mean fluorescent intensity was obtained for areas ≥6-fold above background, unless otherwise noted. Three-dimensional reconstructions and image analysis were performed using the API SoftWorx software. For both recovered T cells and T–APC conjugates, 20–25 cells or conjugates were imaged for each treatment group in each experiment. Integrated intensity and mean fluorescence intensity (MFI) where measured using single slice images. Both MFI and integrated intensity were measured using a region of interest that encompassed the desired object. Colocalization was assessed by the Pearson’s correlation coefficient and overlap coefficients using the JACOP plug-in (58) in ImageJ (59).
TCR signaling inhibition
To determine whether sustained signaling was due to engagement of the TCR by trogocytosed material, TCR signaling inhibition experiments were performed similar to Faroudi et al. (60). Cells incubated at very low density (104/ml) were treated for 10 or 30 min with Src family tyrosine kinase inhibitor PP2 (Life Technologies) (61). Three treatment groups were set up for the TCR signaling inhibition experiments. In the first group, cells were incubated for 30 min in media only to serve as an untreated control. In the second group, cells were incubated with 20 μM PP2 for 10 min, followed by three washes to remove the PP2. Cells were incubated for 20 additional minutes in media alone for a total duration of 30 min. In the final group, cells were incubated for 30 min in the presence of 20 μM PP2 to confirm that the PP2 treatment inhibited both TCR proximal and distal signaling events. After incubation, cells were fixed and stained for pZAP-70 (TCR proximal signaling) and pERK 1/2 (TCR distal signaling) for flow cytometry and imaging, as described above.
Statistical analysis and graphing
Statistical analysis (Student t test) and graphing were performed using Prism 4 (GraphPad Software, La Jolla, CA). Significance was defined as p ≤ 0.05.
Results
In vitro trogocytosis system mimics in vivo trogocytosis
The presence of APC-derived molecules on the surface of T cells as a consequence of trogocytosis raises the possibility that these molecules play a role in immune modulation. It has previously been shown that trogocytosis may allow T cells to act as APCs (43–47), but the biological effect of trogocytosis on the individual T cell remains unclear. We have previously reported that trog+ T cells have an activated phenotype, with increased CD69 expression and TCR downmodulation (2). However, it is unclear whether there are biological consequences/advantages for the T cells that carry out trogocytosis. In support of a potential biologically significant role of trogocytosis on individual cells, we previously reported that the acquired MHC:peptide molecules colocalize with elevated pTyr and the TCR–proximal kinase Lck (2), suggesting that the trogocytosed molecules are associated with sustained intracellular signaling after dissociation from APC. In this paper, we examine the biological effects of trogocytosis on individual CD4+ T cells, specifically looking at sustained signaling and cell survival.
We began by extending our previous in vitro studies (2), examining in vivo trogocytosis using an adoptive transfer system. Naive I-Ek:MCC88–103-specific, Vβ3+ AD10 TCR transgenic T cells were injected i.v. into Ag transgenic mice. The recipients express the same GFP-tagged I-Ek:MCC88–103 construct used to generate the MCC:GFP fibroblasts (56) under the control of the endogenous MHC class II promoter. As a control, naive AD10 cells were injected into negative littermates. Spleen cells from the recipient animals were recovered on day 4 and examined for activation and trogocytosis by flow cytometry.
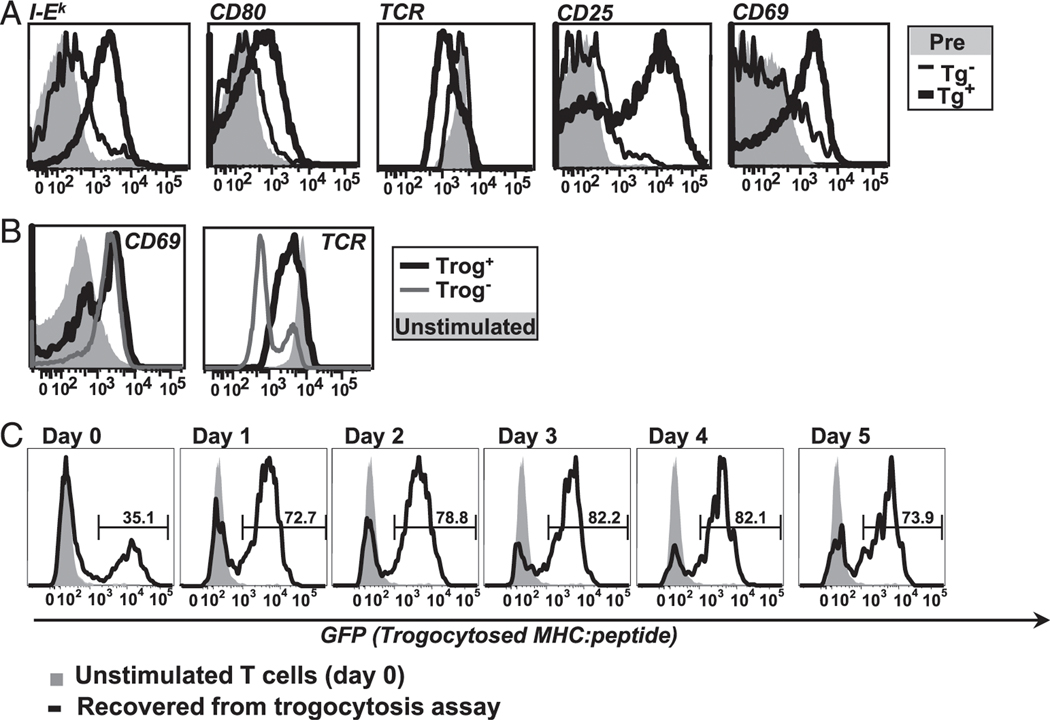
The results in Fig. 1A strongly suggest that the naive AD10 T cells recognized Ag in vivo and efficiently carried out trogocytosis. Nearly all of the AD10 T cells from the Ag transgenic recipients had trogocytosed I-Ek. By comparison, very few AD10 T cells from the nontransgenic recipient had trogocytosed I-Ek, and the I-Ek MFI was 6-fold lower than on the T cells recovered from the Ag transgenic recipients. Similarly, the T cells transferred into the Ag transgenic mice had 6-fold higher levels of trogocytosed CD80 compared with the Ag-negative littermate controls. Because murine T cells do not endogenously express CD80 (39), the appearance of CD80 on these T cells is also due to in vivo trogocytosis. The cells from the Ag transgenic recipients also expressed significantly higher levels of CD25 and CD69 along with significantly more TCR Vβ3 downmodulation than the cells from the nontransgenic recipients. The level of CD44 was significantly higher and the level of CD62L significantly lower on cells from the Ag-bearing recipients (data not shown). These data show that the T cells were recognizing Ag in vivo and performing trogocytosis in the Ag-bearing mice and not in the negative littermate controls. These results are similar to the in vivo data reported by Zhou et al. (48) and to our unpublished data using a similar covalent–MHC: peptide Ag transgenic mouse strain bearing MCC T102S (62). Thus, in the Ag transgenic mice, the naive CD4+ AD10 cells recognized Ag, were activated, and efficiently performed trogocytosis. In addition, the frequency of recovered Vβ3+CD4+ AD10 transgenic cells on day 4 was significantly higher in the Ag transgenic recipients (6.3 ± 1.3%) than in the nontransgenic recipients (0.53 ± 0.07%), suggesting that these cells may have expanded in vivo. Our data further suggest that the trogocytosis observed using our standard in vitro system using transfected fibroblast APC (2) is similar to in vivo trogocytosis from professional APC.
FIGURE 1.
In vitro trogocytosis mimics in vivo trogocytosis and is associated with the selective T cell survival after removal of APC. (A) In vivo trogocytosis. Histograms comparing level of trogocytosed I-Ek (far left) and CD80 (left) on recovered TCR transgenic T cells from MCC:GFP transgenic (black line) and negative littermates (thin black line) 4 d after adoptive transfer. The activation state of the recovered cells was determined by flow cytometric analysis of TCR downmodulation (center) and CD25 and CD69 (right, far right). Preinjection cells are shown for comparison (shaded histogram). Data are representative of three separate experiments (two mice per group for each experiment). (B) Both trog+ and trog− cells are activated during standard in vitro trogocytosis assay. CD69 (left) and TCR (right) expression on T cells recovered from fibroblast APC. Data are representative of six separate experiments. (C) Trog+ cells preferentially survive after removal of APC. T cells were recovered from standard trogocytosis assay and cultured at low density (104/ml). At indicated time points, the presence of trogocytosed GFP-tagged MHC on CD4+Vβ3+ gated cells was determined by flow cytometry. The level of trogocytosed MHC:peptide on day 0 unstimulated cells (gray shaded) and on recovered T cells at the indicated time point (black line) are shown. Horizontal region markers indicate the frequency of trog+ cells in the cultures. Data are representative of four separate experiments.
Trogocytosis negative cells recognize Ag and are activated similar to trog+ cells
After recovery from the in vitro trogocytosis assay, T cells are clearly trog+ or trog−. It is unclear whether this reflects differences in activation or the ability to perform trogocytosis. To examine the hypothesis that in vitro Ag recognition by trog− cells was not as efficient as Ag recognition by the trog+ cells, we used our standard in vitro trogocytosis assay and characterized the activation state of trog+ and trog− cells. In vitro-primed T cells were recovered from a 90-min incubation with the fibroblast APC, and the activation state was assessed by TCR (Vβ3) downmodulation and CD69 upregulation. As predicted, the trog+ T cells had elevated CD69 expression along with substantial TCR downmodulation (Fig. 1B), indicating that they recognized Ag and were activated. These results are consistent with our previously published data (2). Somewhat unexpectedly, the trog− T cells also expressed high levels of CD69 and TCR downmodulation, clearly showing that the trog− T cells also interacted with APCs and were activated. There were small experiment-to-experiment variations in the levels of CD69 and TCR downmodulation, but over the course of six separate experiments, these differences were not significant. Thus, the slight differences in TCR downmodulation and CD69 expression between the trog+ and trog− in Fig. 1B are not significant.
The results in Fig. 1B suggest that the difference between trog+ and trog− cells is not simply the lack of Ag recognition by the trog− cells. Because it appears that both trog+ and trog− T cells are similarly activated, in the remainder of this paper, we assessed whether there are potential biological advantages for the individual T cells that perform trogocytosis.
Selective survival of trog+CD4+ T cells in vitro after removal of APC
We initially determined that, although at early time points the naive trog+ cells preferentially divide, both trog+ and trog− cells are recognizing Ag and are being activated. We next examined whether there are any other selective advantages for trog+CD4+ T cells. First, we examined the fate of the trog+ and trog− cells after removal of APC over several days to assess the possibility that trogocytosis leads to sustained survival of trog+ cells. After the 90-min coculture of in vitro-primed AD10 T cells with MCC:GFP fibroblast APC, recovered T cells were placed in culture dishes at low density to limit potential T-T Ag presentation events. We monitored the frequency of trog+ (GFP+) and trog− (GFP−) cells daily over 5 d by flow cytometry. On day 0, the trog+ T cells made up ∼35.1% of the T cells in the cultures, whereas 64.8% of the CD4+ T cells were trog− (Fig. 1C). By day 1, a significant change in the proportion of trog+ and trog− T cells in the culture occurred, with the trog+ T cells more than doubling to 72.7% of the CD4+ cells. This trend continued on days 2 and 3, where the trog+ cells represented 78.8 and 82.2% of the viable CD4+ cells in culture. On day 3, the trog− T cell population represented only 17.5% of the viable CD4+ T cells. The population of trog+ cells peaked on days 3 and 4 at 82.2% of the viable CD4+ cells and decreased slightly on day 5 at 73.9%. Although there is an overall reduction of viable CD4+ cells over the 5-d culture period, the proportion of trog+ to trog− cells changes dramatically. The results in Fig. 1C strongly suggest that trog+ cells selectively survive in culture after removal of APC. There is a slight reduction in the GFP levels between days 0 and 1, but the level remains fairly constant after day 1. This is consistent with our previously published data (2). Thus, the trog+ cells do not appear to be proliferating significantly in this culture because the level of GFP on the trog+ cells is not significantly reduced on days 4 and 5, as would be expected if the GFP was equally distributed among daughter cells. Taken together, the results in Fig. 1 suggest that the trog+ cells are activated are the first to proliferate after Ag recognition and selectively survive after removal of APC.
Sustained TCR-proximal intracellular signaling in trog+ T cells
The results in Fig. 1 suggest that one potential advantage for trog+ cells is selective survival after removal of APC. It is unclear whether trogocytosed molecules drive the T cell survival or simply mark differentially activated cells that survive. One possibility, consistent with a direct role for the trogocytosed molecules in the selective survival of trog+ cells, is that these molecules are engaging the TCR and costimulatory receptors on the T cell surface and sustaining intracellular signaling. Consistent with this possibility, we previously showed that pTyr and Lck accumulate with trogocytosed molecules on T cells after removal of APC (2). To examine the hypothesis that the trogocytosed molecules sustain intracellular signaling leading to T cell activation and survival, we assessed TCR signaling in T cells recovered from APC.
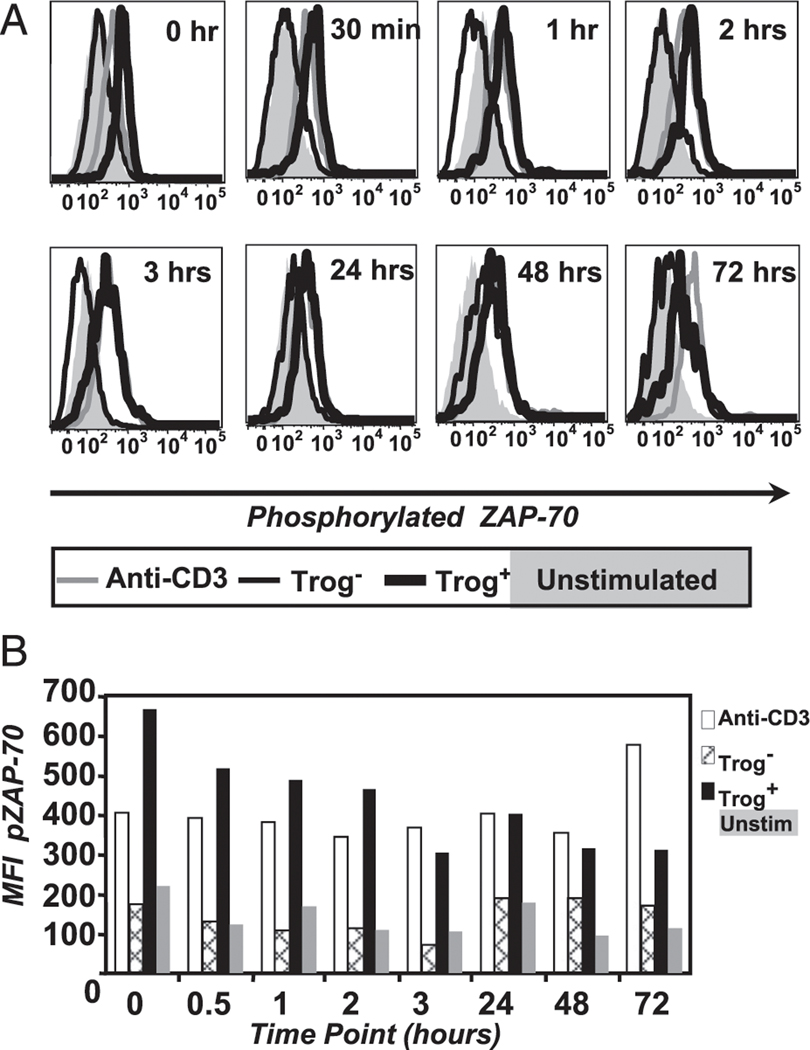
As a measure of TCR-proximal signaling events, we examined the phosphorylation and activation state of Syk family kinase, ZAP-70. To measure the phosphorylation of molecules in T cells recovered from the standard trogocytosis assay, we used phosphor-specific Abs and flow cytometry, so called phosflow (57, 63). This technique is as sensitive as traditional biochemical methods (63) and requires significantly fewer cells. As shown in Fig. 2, there are significant differences in the phosphorylation and activation state of ZAP-70 between trog+ and trog−CD4+ T cells. Immediately after removal from APC (time 0), the MFI of pZAP-70 is 3.03-fold higher in trog+ cells (thick black line) than in the trog− T cells (thin black line). The trog− cells are nearly identical to unstimulated T cell controls (shaded histogram). By 30 min, the pZAP-70 MFI of the trog+ T cells is 4.2-fold higher than the trog− T cells and is nearly identical to the anti–CD3-stimulated positive control (thick gray line). Over the next 24 h, there is a steady decrease in the pZAP-70 MFI for trog+ cells, but pZAP-70 remains 3- to 4-fold higher than trog− cells. The level of pZAP-70 in the trog+ cell remains very similar to the anti-CD3 positive control during this time frame. At 72 h, the trog+ T cells still have significantly higher pZAP-70 levels compared with the trog− T cells, which remain similar to the unstimulated control cells. Thus, there is sustained TCR-proximal signaling in trog+ T cells, which were shown to preferentially survive in culture after APC removal (Fig. 1C).
FIGURE 2.
TCR-proximal signaling is sustained in trog+CD4+ T cells. (A) T cells were recovered from the standard in vitro trogocytosis assay using MCC:FKBP APC and fixed immediately (0 h) or were cultured for the indicated times before fixation and Ab staining. The level of pZAP-70 in CD4+Vβ3+ gated trog+ (thick black line), trog− (thin black line), and unstimulated cells (shaded histogram) are shown for each time point. Cross-linked anti-CD3 (gray line) was used to stimulate TCR signaling as a positive control. (B) Mean fluorescence intensity values for the pZAP-70 flow cytometry data in (A). Data are representative of six separate experiments.
Trogocytosed MHC:peptide molecules colocalize with TCR-proximal signaling molecules in trog+ T cells
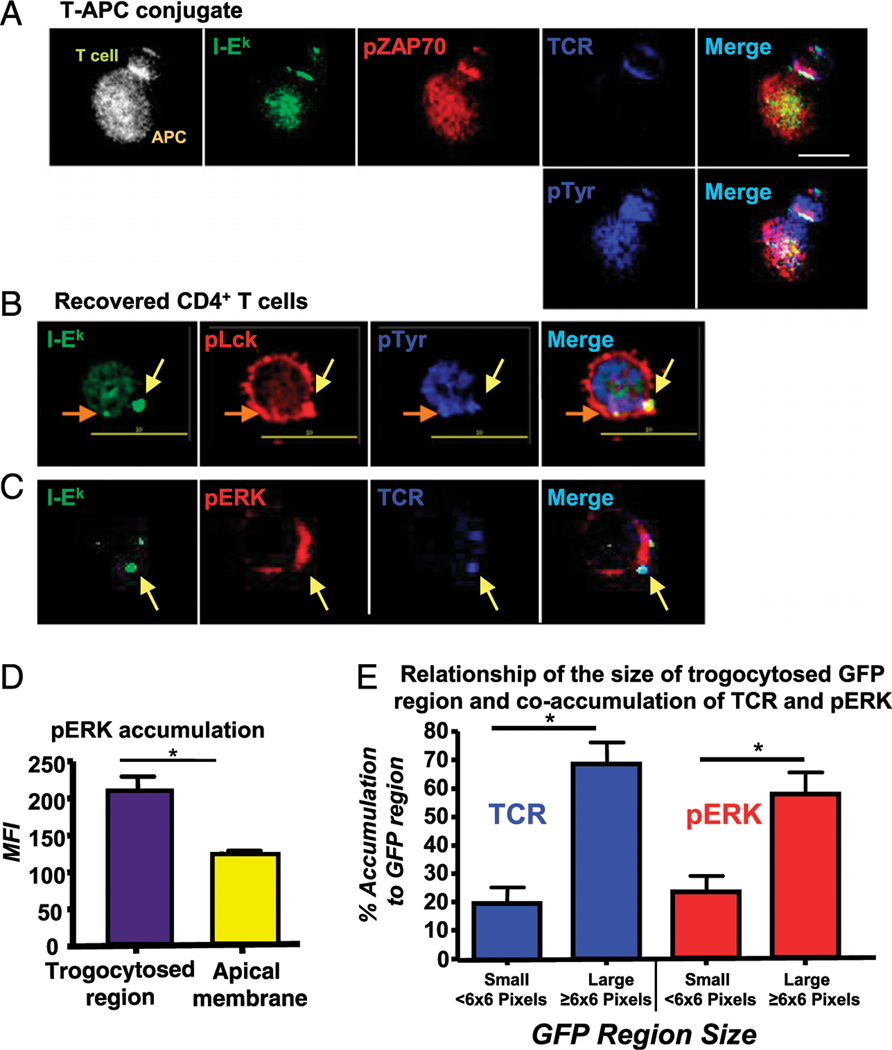
The flow cytometry data show that TCR-proximal signaling is maintained in the trog+ cells after removal of APC (Fig. 2). To demonstrate that the elevated pZAP-70 was associated with the trogocytosed molecules on the surface of the T cells, we used high-resolution microscopy to determine the spatial distribution and potential colocalization of TCR-proximal signaling molecules with trogocytosed, GFP-tagged MHC:peptide. To begin, we characterized the spatial distribution of the TCR, pZAP-70, total pTyr, and GFP-tagged MHC:peptide molecules at the immune synapse formed between AD10 T cells and MCC:GFP fibroblast APC. At the T–APC interface in Fig. 3A, cognate MHC:peptide (green) and the TCR (top row, blue) accumulate, indicating the presence of a mature immune synapse (56). At this T–APC interface, there is significant enrichment of pZAP-70 (red) and total pTyr (blue, bottom). These images are consistent with previous reports characterizing signaling at the IS (32, 56, 64–67). Interestingly, on the distal pole of the T cell (opposite the IS), there is a small spot of trogocytosed MHC:peptide. The presence of trogocytosed MHC: peptide complexes (≥2-fold above background) was observed in 24% of the T–APC conjugates imaged. These trogocytosed molecules accumulated at the distal pole on ˃82% of the trog+ T cells in T–APC conjugates. For T–APC conjugates imaged with the accumulation of trogocytosed MHC:peptide complexes at the distal pole of the T cell, these molecules localized with the TCR, elevated pZAP-70, and elevated total pTyr ˃ 99% of the time. The staining pattern is very similar to that of the immunological synapse at the T–APC interface and strongly suggests that these trogocytosed molecules sustain/initiate TCR signaling after transfer onto the T cell.
FIGURE 3.
TCR signaling-associated molecules are associated with trogocytosed molecules on the T cell surface. Cells were fixed and stained for the indicated molecules, as described in the Materials and Methods section. (A) Elevated pZAP-70 (red) and pTyr (blue, bottom row) levels colocalize with GFP-tagged MHC:peptide (green) and the TCR (blue, top row) at the immune synapse. These molecules also colocalize with trogocytosed MHC:peptide on the distal pole of the T cell. Fluorescence composite image shows position of T cell and APC. (B) pLck (red) and total pTyr (blue) colocalize with two trogocytosed MHC:peptide molecules (green) regions (indicated by yellow and orange arrows) on T cells recovered from the in vitro trogocytosis assay. Images are from a single focal plane. (C) pERK (red) coassociates with trogocytosed MHC:peptide (green) and TCR (blue) on the recovered trog+ cells. (D) MFI of pERK associated with the trogocytosed MHC:peptide compared with the apical membrane on the same cell. (E) The relationship of the size of the trogocytosed MHC:peptide region on the T cell with the coaccumulation of TCR (blue) and pERK (red). Small regions were defined as <6 × 6 pixels and large ≥6 × 6 pixels. For each condition, ˃100 individual T cell were imaged. Images were collected with a ×60 objective. Scale bars, 10 μm. Bars represent mean ± SEM. Horizontal lines indicate statistical comparison between indicated groups, *p ≤ 0.05.
The imaging data in Fig. 3A suggest that there is sustained TCR signaling from trogocytosed molecules in T–APC conjugates. To examine the possibility that the trogocytosed molecules sustain TCR signaling in T cells after dissociation from APC, we recovered T cells from the standard trogocytosis assay and fixed and stained them after an additional 30-min incubation. We examined the location of the TCR, along with the TCR-proximal signaling molecules, pLck and total pTyr. We imaged 111 T cells after recovery, and representative images are shown in Fig. 3B. Trogocytosis, defined as the presence of GFP-tagged MHC:peptide at least 2-fold above background fluorescence, was observed in 36% of the T cells imaged. The cell imaged in Fig. 3B has two regions of trogocytosed MHC:peptide (indicated by the orange and yellow arrows). These are similar in size and total MHC:peptide accumulation, but the bottom region appears smaller because this image is a single optical section, and the bottom spot is axially centered in a separate focal plane. For both regions, the trogocytosed MHC:peptide localizes with both pLck and pTyr. This staining pattern was observed in 45% of trog+ cells. These results are similar to our previously published data (2). The localization of TCR-proximal signaling molecules with transferred GFP in Fig. 3B suggests that after recovery from APCs the transferred molecules on the T cells sustain TCR-proximal signaling.
Sustained TCR-distal signaling in trog+ T cells
To examine whether the trogocytosis-associated TCR-proximal signaling observed in Figs. 2, 3A, and 3B leads to downstream signaling, the accumulation of pERK 1/2 (red) and its spatial distribution relative to the TCR (blue) and trogocytosed MCC:I-Ek:GFP (green) was analyzed. As in Fig. 3B, the cells in the Fig. 3C have two distinct trogocytosed spots (green) that are more than 2-fold above background fluorescence. These spots colocalize with accumulated TCR in 96.6% of the 111 trog+ T cells imaged. The TCR-distal signaling molecule pERK is also accumulated to the region adjacent to the trogocytosed molecules. Although there is little actual pERK colocalization with the TCR or MHC:peptide, this staining pattern is very similar to what we have previously reported for pERK accumulation at the mature immunological synapse (67) and is consistent with sustained TCR-distal signaling initiated by the trogocytosed molecules.
To further analyze the potential relationship between trogocytosed molecules and T cell intracellular signaling, we compared the MFI of elevated pERK adjacent to trogocytosed molecules to the amount at a region of the T cell apical membrane devoid of transferred material. The bar graph in Fig. 3D shows that the regions adjacent to the trogocytosed molecules have a 56% increase in the level of pERK staining compared with the apical membrane. Taken together, the flow cytometry and imaging data in Figs. 2 and 3 suggest that after the trogocytosis event, the transferred MHC: peptide continues to engage the TCR and sustains intracellular signaling. These results are also consistent with the selective survival of T cells seen in Fig. 1.
While examining the accumulation of the TCR and pERK with the trogocytosed MHC:peptide complexes, we observed a correlation between the area of the MHC:peptide and the frequency of TCR and pERK coaccumulation. Using three-dimensional image reconstruction, we analyzed 228 different GFP regions on 47 T cells to further analyze this potential relationship between the area of the GFP-tagged MHC:peptide (at least 6-fold above background) and the TCR and enhanced pERK (≥2 fold above background). We observed that ∼80% of larger GFP regions, defined as being ≥6 × 6 pixels in size, colocalized with elevated TCR, and ∼60% had adjacent elevated pERK staining. In comparison, small GFP regions (<6 × 6 pixels in size) colocalized with TCR only ∼20% of the time and only ∼22% were adjacent to elevated pERK (Fig. 3E). These data suggest a correlation between the size of the trogocytosed patches of APC molecules on the T cell and the engagement of the TCR leading to increased levels of TCR-associated signaling, specifically pERK.
Trogocytosed molecules induce sustained signaling in trog+ T cells
The results in Figs. 2 and 3 suggest that trogocytosed molecules sustain TCR signaling in T cells after the removal of APCs. However, these results could also be attributed to residual signaling initiated at the immune synapse while the T cells initially interacted with APCs. To determine whether the trogocytosed molecules were initiating TCR signaling, we adapted the PP2 interruption technique of Faroudi et al. (60). PP2 is a Src family kinase inhibitor that prevents the phosphorylation and activation of Lck during TCR engagement, terminating TCR signaling (60, 61). If the signaling detected by flow cytometry and imaging resulted from residual signaling from the immune synapse, it would not resume after the removal of the PP2. However, if the trogocytosed molecules were engaging their receptors on the T cell and sustaining/initiating intracellular signaling, this signaling would be expected to resume upon removal of PP2. To test this hypothesis, T cells were recovered from the standard 90-min trogocytosis assay and incubated for an additional 30 min, followed by flow cytometry and imaging analysis of potential TCR-proximal and TCR-distal signaling. Control cultures were treated with 20 μM PP2 or were untreated for the entire 30-min culture period. In parallel cultures, cells were incubated with 20 μM PP2 for 10 min at 37°C before the PP2 was washed out. These cells were incubated for an additional 20 min in the absence of PP2 before analysis. The results of these experiments are shown in Figs. 4 and 5.
FIGURE 4.
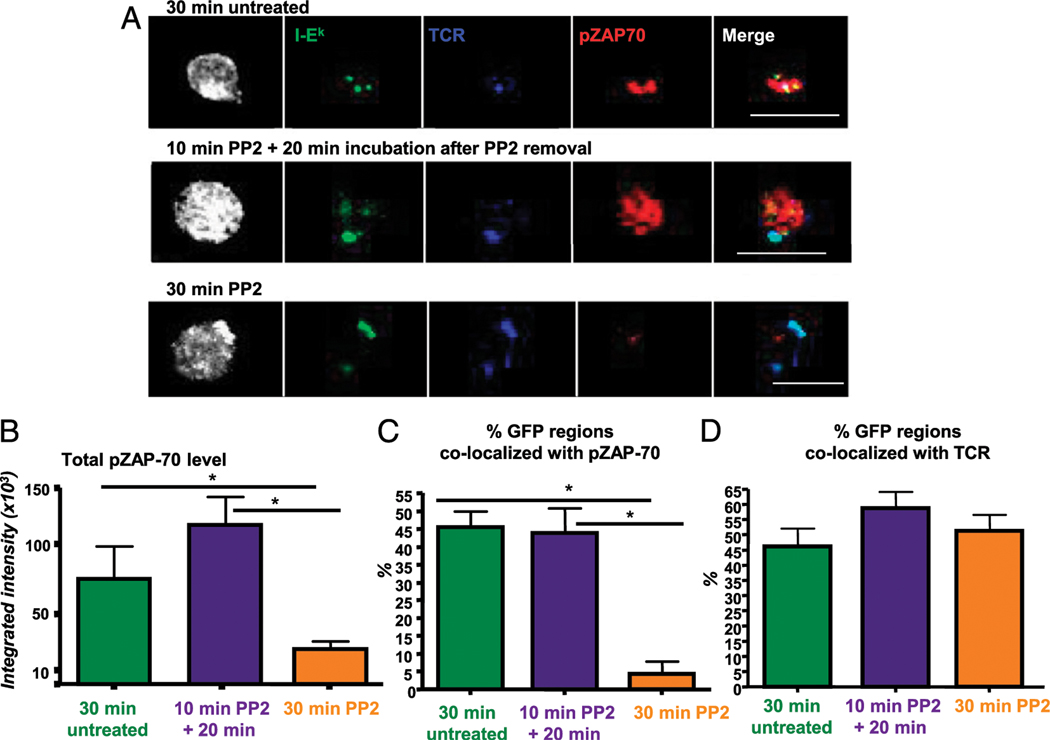
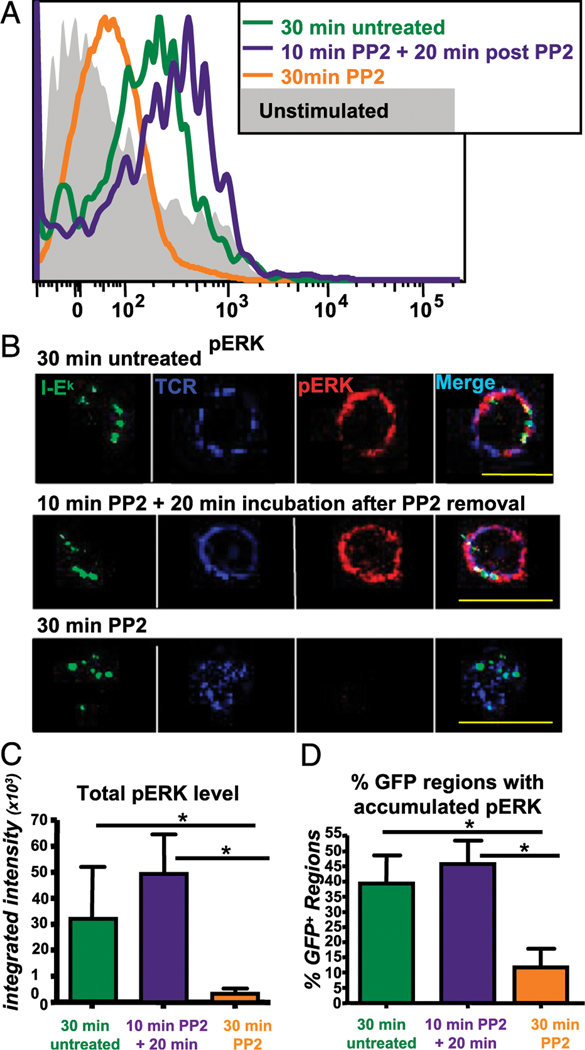
TCR-proximal signaling is sustained by trogocytosed molecules in trog+ cells. (A) Top panel, T cells were incubated for 30 min after recovery from standard trogocytosis assay before fixation and staining. In untreated cells, pZAP-70 (red) and the TCR (blue) colocalize with the trogocytosed MHC:peptide on the T cell surface. Bottom panel, The TCR and MHC:peptide colocalize on T cells treated with the Src inhibitor PP2 following the 30-min incubation period, but the there is minimal phospho-ZAP-70 detected. Middle panel, For cells with a 10-min PP2 treatment to extinguish signaling and before incubation for 20 min after PP2 removal, the pZAP-70 level rebounds and it colocalizes with the trogocytosed MHC:peptide and TCR. More than 100 individual T cell were imaged using a ×60 objective. Left image for each group is a fluorescence composite image to show the position of the T cell. Scale bars, 10 μm. (B) Integrated intensity of pZAP70 ≥6-fold above background in images as in (A) (top panels). Thirty-minute untreated T cells (green bar), T cells incubated with PP2 for 30 min (orange bar), or for treated for 10 min then incubated for 20 min after removal of PP2 (purple bar) are shown. (C) The frequency of pZAP-70 colocalizing with trogocytosed MHC:peptide for each treatment group is shown. (D) The frequency of TCR colocalizing with the TCR. Bars represent mean ± SEM. Horizontal lines indicate statistical comparison between indicated groups, *p ≤ 0.05.
FIGURE 5.
TCR-distal signaling is sustained by trogocytosed molecules in trog+ CD4+ T cells. (A) Phosflow data showing the level of pERK 1/2 in untreated cells (green line), cells treated with PP2 for 30 min (orange line) and cells treated with PP2 followed by a 20-min incubation after PP2 removal (purple line). Unstimulated cells (shaded histogram) are shown as a control. (B) Recovered T cells were incubated for 30 min as described for (A) before staining and imaging. The location of TCR (blue), MHC:peptide (green), and elevated pERK (red) are shown for each treatment group. Data are representative of three separate experiments with more than 100 cells imaged for each treatment group. Images were collected using a ×60 objective. Scale bars, 10 μm. (C) Total pERK integrated intensity for areas ≥ 6-fold above background for each treatment group. (D) The frequency of trogocytosed MHC:peptide molecules that coassociate with elevated pERK (≥2-fold above background) is shown. Bars represent mean ± SEM. Horizontal lines indicate statistical comparison between indicated groups, *p ≤ 0.05.
In the first set of experiments, TCR-proximal signaling was examined by assessing the level and localization of pZAP-70 in the various treated cells. In addition to representative images seen in Fig. 4A, quantitative data analysis of the imaging is shown in the bar graphs in Fig. 4B–D. In the top row of images in Fig. 4A, the untreated cells were incubated in media alone for 30 min after removal from APC. There are three distinct GFP-tagged MHC: peptide spots (green) on this particular T cell. These spots colocalized with the TCR Vβ3 (blue) and coassociated with pZAP-70 (red). In contrast, when cells were cultured in 20 μM PP2 for the 30-min incubation period (bottom row), the MHC:peptide and TCR colocalized normally, but there was minimal pZAP-70 staining and essentially none was associated with either the trogocytosed MHC:peptide or the accumulated TCR. Cells treated with PP2 for 10 min showed a reduction of total pZAP-70 and no pZAP-70 colocalization with either MHC:peptide or TCR, similar to the 30-min PP2 treatment group (data not shown). Finally, in the cells treated with PP2 for 10 min, followed by an additional 20-min incubation after PP2 removal, the single distinct GFP spot on the cell colocalized with both the TCR and elevated pZAP-70.
The bar graph in Fig. 4B shows the integrated intensity of pZAP-70 (a measure of fluorescence intensity) in areas more than or equal to six times above background on trog+ cells. After im- aging more than 110 trog+ cells, the 30-min PP2-treated cells had significantly reduced levels pZAP-70 staining compared with the 30-min untreated cells. For the 10-min PP2 plus 20-min incubation group, the level of pZAP-70 was restored. Interestingly, the levels were consistently higher for the 10-min PP2 plus 20-min incubation group than that found in the untreated cells. The trog− T cells showed an absence of pZAP-70 staining in all of the treatments (data not shown), consistent with the flow cytometry data in Fig. 2A. Thus, elevated pZAP-70 seen in the trog+ 10-min PP2 treatment plus 20-min incubation group is not simply due to PP2 treatment and removal, but also requires the presence of trogocytosed molecules.
We next examined the spatial relationship between elevated pZAP-70 and trogocytosed MHC:peptide molecules on the T cell surface for the different treatment groups (Fig. 4C). For ∼45% of both the 30-min untreated cells and the 10-min PP2 plus 20-min incubation cells, pZAP-70 colocalized with the trogocytosed MHC:peptide complexes. In contrast, <5% of the cells treated with PP2 for 30 min showed any MHC:peptide colocalization with pZAP-70. The significant reduction both in total pZAP-70 and colocalization with the TCR or MHC:peptide for the 30-min PP2 treatment group is likely due to the biochemical effects of PP2 on Lck activation and is not due to inhibition of TCR-MHC:peptide interactions, because these molecules colocalized equally in the presence or absence of PP2 (Fig. 4D). The results in Fig. 4 strongly support the hypothesis that the trogocytosed molecules continue to engage their receptors on the T cell surface and sustain TCR-proximal signaling.
After showing that the trogocytosed molecules induced reinitiation of TCR-proximal signaling after PP2 removal, we examined TCR-distal signaling, using the phosphorylation and activation of ERK 1/2 as a readout. T cells were recovered from the standard trogocytosis assay and treated with PP2 following the same regimen described for the ZAP-70 studies above. We initially examined pERK levels in the treatment groups by phosflow. Similar to the pZAP-70 data, 30-min PP2 treatment significantly reduced the amount of pERK in the cell (orange line) compared with the untreated cells (green line). The pERK 1/2 MFI for the 30-min PP2 treatment was 1.6-fold lower than the untreated control. In contrast, the cells treated with PP2 for 10 min, followed by an additional 20-min incubation in the absence of PP2 (purple line), showed a restoration of pERK 1/2 staining. Reminiscent of the pZAP-70 imaging data, the MFI for the 10-min PP2 plus 20-min incubation group was 30% higher than the untreated controls and was 1.9-fold higher than the 30-min PP2 treatment group. It was also 2.4-fold higher than the unstimulated T cells controls. These results are consistent with the TCR-proximal signaling seen in Fig. 4 and show that, after PP2 inhibition of TCR signaling is removed, TCR-distal signaling is reinitiated within the trog+ T cells.
To confirm that the recovery of TCR-distal signaling after PP2 removal seen in Fig. 5A is associated with the trogocytosed molecules, high-resolution imaging experiments were performed. In each row of images in Fig. 5B, the trogocytosed GFP-MHC: peptide (green), TCR (blue), and pERK 1/2 (red) are seen, along with a merged image of all three (right column). In the top row, for the untreated group, there are several distinct MHC:peptide spots on the recovered T cell. Each of these spots colocalize with the regions of significantly elevated TCR (blue). Adjacent to these TCR-MHC:peptide regions, there is accumulation of significantly elevated pERK. The pERK does not colocalize with either the MHC:peptide or TCR but rather is adjacent to these molecules on the T cell. The spatial distribution of the pERK in these trog+ cells is very similar to our previous report regarding pERK accumulation at the immunological synapse (67). When PP2 was present for the entire 30-min incubation period, the trogocytosed MHC: peptide colocalized with the TCR, but there were several regions on the cell with elevated TCR without apparent MHC:peptide. Similar to the pZAP-70 data, there was minimal pERK 1/2 staining in the 30-min PP2 group. In the cells incubated for 10 min with PP2 before its removal followed by an additional 20-min incubation, there were several regions of trogocytosed MHC:peptide complexes that colocalized with the TCR. There was also accumulation of pERK 1/2 adjacent to the trogocytosed molecules. This confirms the flow data (Fig. 5A), showing that removal of PP2 allows resumption of TCR-dependent distal signaling.
As with the pZAP-70 imaging data, we calculated the integrated intensity of pERK (Fig. 5C) and the frequency of pERK association with trogocytosed MHC:peptide (Fig. 5D) for ˃100 cells in each treatment group. As expected from the representative images in Fig. 5B, the cells treated with PP2 for 30 min showed an absence of pERK 1/2 staining in contrast to the untreated group. Cells treated for 10 min were identical to the 30-min PP2 treatment (data not shown). For the 10-min PP2 plus 20-min incubation group, there was a significant recovery in the integrated intensity for pERK 1/2, similar to pZAP-70. The pERK integrated intensity for cells treated for 10 min with PP2 treatment, followed by a 20-min post-PP2 incubation was 10-fold higher than the 30-min PP2 treatment. We also quantified the frequency of elevated pERK adjacent to the trogocytosed MHC:peptide for each of the treatment groups (Fig. 5D). In 39% of untreated samples and 47% of 10-min PP2 plus 20-min incubation samples, the trogocytosed GFP-tagged MHC:peptide was associated with elevated pERK staining. In contrast, only 8% of the 30-min PP2 cells show association of pERK with the trogocytosed molecules.
Taken together, the results in Figs. 4 and 5 show that a 30-min treatment with the Src inhibitor PP2 significantly reduces both pERK 1/2 and pZAP-70 levels in the trog+ cells. Upon PP2 removal, both TCR-proximal (pZAP-70) and TCR-distal (pERK 1/2) signaling is reinitiated by the trogocytosed molecules. These results strongly suggest that these trogocytosed molecules are engaging their receptors on the T cell surface and are sustaining intracellular signaling after dissociation from the APC.
Discussion
Ag presentation to CD4+ T cells results in the formation of an immunological synapse between the APC and the T cell. While T cells are conjugated with APC and upon cellular dissociation, MHC:peptide complexes, costimulatory molecules, plasma membrane lipids, and other membrane-bound molecules are acquired from the APC by the T cells via the immunological synapse in a process called trogocytosis (2, 3, 19). Over the past decade, there have been numerous studies that have examined the mechanism of trogocytosis and the complement of molecules that are transferred, but relatively little is currently known about the potential biological significance of capturing these molecules by the trog+ T cell. The presence of these APC-derived molecules on the T cell raises the potential that they could engage receptors on the T cell and sustain intracellular signaling. Sprent’s group has published several papers looking at the ability of APC-derived exosomes to stimulate T cells (68–71), but attachment of exosomes to the surface of the cell is fundamentally different from the integration of these molecules into the plasma membrane as a result of trogocytosis. In 2005, Zhou et al. (72) showed that CD80+ T cells had sustained NF-κB activation and production of selected cytokines. However, their results were most likely the result of T-T Ag presentation, not cell autonomous signaling (72). Other reports have looked at the transfer of constitutively activated receptor tyrosine kinases (73) and the Ig-like transcript 2 signaling after trogocytosis (74), but none have actually addressed sustained signaling from trogocytosed APC-derived molecules. In this study, we have examined the hypothesis that trogocytosed MHC:peptide complexes and other APC membrane molecules sustain intracellular signaling within CD4+ T cells after the cells dissociate from APC leading to sustained T cell activation and survival.
Because murine CD4+ T cells cannot express MHC class II endogenously (75), the presence of these molecules on T cells was due to a trogocytosis event. When we examined AD10 T cells isolated after a 90-min incubation with transfected MCC:GFP fibroblasts, we observed that the trog+ T cells had increased TCR downmodulation and high levels of CD69 (Fig. 1B), suggesting that these cells were activated. This is consistent with our previously published results (2). It is interesting to note that both trog+ and trog− T cells have a similar activation phenotype suggesting that both cell types recognize Ag and are activated. It is unclear why the trog− cells did not perform trogocytosis when even anergic T cells efficiently trogocytose molecules from APC (67). It is likely not due to differences in activation, because both populations are similarly activated upon Ag recognition. It is possible that it reflects differences in the avidity of the T cells, their adhesion molecule concentrations, and/or additional, uncharacterized attributes of the two populations of cells.
To confirm that our in vitro system reflects in vivo trogocytosis events, we adoptively transferred naive AD10 T cells into Ag transgenic mice expressing the same MCC88–103:I-Ek:GFP construct used to transfect our fibroblast APC. As seen in Fig. 1A, these AD10 cells were activated and efficiently trogocytosed I-Ek and CD80 in vivo. These results are similar to our in vitro studies and support the use of our well-defined in vitro system to examine the biological consequences of trogocytosis on the individual T cell. These results are also consistent with recent in vivo studies showing that T cells can capture HLA-G from tumor cells (76) and molecules from LCMV-infected cells (77). In addition, Zhou et al. (48) recently showed that in vivo trogocytosis occurred with Th cells and regulatory T cells in vaccinia virus infection model.
When cells were recovered from the standard 90-min in vitro trogocytosis assay and incubated at low density (104/ml), we observed a significant change in the frequency of trog+ and trog− cells over several days. Immediately after recovery of the T cells from the APC, the trog− cells represented almost two-thirds of the CD4+ cells. Over the course of several days, the frequency of trog+ and trog− cells had reversed so that by day 3 the trog+ cells made up ˃82% of the viable CD4+ T cells and the trog− represented <18% of the viable CD4+ cells. The level of GFP-tagged MHC remained fairly constant on the trog+ cells over 5 d, which suggests that the cells were not dividing, because proliferation would result in the dilution of trogocytosed molecules onto daughter cells. Rather, we interpret these results as showing that the trog+ cells were selectively surviving in the cultures while the trog− cells were dying.
One explanation for the preferential survival of the trog+ cells could be that the trogocytosed molecules were sustaining intra- cellular signaling. It would be expected that TCR signaling would cease in recovered cells unless there is TCR-MHC:peptide engagement. Using a combination of high-resolution light microscopy and flow cytometry, we observed that after the acquisition of cognate MHC:peptide complexes and other APC membrane molecules, these APC-derived molecules colocalize with the TCR. There was also elevated pTyr in the trog+ cells along with phosphorylation and activation of both TCR-proximal (pZAP-70) and TCR-distal signaling molecules (pERK 1/2) over several days. These TCR signaling molecules accumulated with the TCR and trogocytosed molecules, supporting the hypothesis that the trogocytosed molecules continue to engage their receptors on the T cell and that this “autopresentation” sustains intracellular signaling.
Although the detection of phosphorylated and activated signaling molecules in trog+ cells by flow cytometry and the colocalization of these molecules with both the TCR and trogocytosed MHC:peptide supports this model, it does not prove that the trogocytosed molecules are driving the observed signaling. It is possible that the sustained signaling is actually due to residual signaling carried over from the immune synapse between the APC and T cell. To show that this signaling was due to sustained signaling from the trogocytosed molecules, we adapted the approach of Faroudi et al. (60). Following the addition of the Src family of tyrosine kinase inhibitor, PP2, TCR-mediated signaling, both proximal (pZAP-70) and downstream (pERK 1/2), ceased. Upon removal of the PP2 and a subsequent 20-min incubation, both pZAP-70 and pERK 1/2 were detected and colocalized with the TCR and trogocytosed MHC:peptide molecules. This signaling was observed only in the trog+ cells, not in the trog− cells. Because the trog− cells were capable of recognizing Ag and responding (Fig. 1B), the lack of detectable signaling within these cells lead us to conclude that the signaling seen in the trog+ was cell autonomous and driven by the trogocytosed molecules. If this signaling had been the result of T-T presentation events, there would have been detectable signaling within the trog− cells as well, because the trog− cells make up the majority of the population and are more likely to encounter the trog+ cells in T-T conjugates. The role of specific trogocytosed molecules (i.e., MHC:peptide, costimulatory molecules, and so on) in the sustained signaling is unclear and current experiments are underway to characterize these critical molecular interactions.
Our results show that the trogocytosed molecules are responsible for initiating and sustaining signaling by engaging the TCR and suggest that this leads to the activation and selective survival of trog+ T cells. The result of trogocytosis could, therefore, play a role in immune modulation. This sustained signaling might lead to clonal exhaustion or activation-induced cell death, helping to turn off an ongoing immune response. Alternatively, this sustained signaling could result in alterations/increases in T cell effector functions. Further analysis of effector function and cytokine production by trog+ T cells will help clarify the potential role of trogocytosis in immune control.
The imaging experiments shown in Fig. 3A demonstrate a significant accumulation of the TCR and signaling-associated molecules at the T-APC interface as well as with trogocytosed molecules. Interestingly, the trogocytosed molecules move to a region of the T cell directly opposite the immune synapse ˃82% of the time. This area has been termed the distal pole complex (78, 79). The trogocytosed patches of APC membrane are usually arranged in a circular pattern at the distal pole forming a “trogocytosis crown.” The reason for this segregation of the trogocytosed molecules to the opposite pole of the T cell when conjugated to APC is unclear, but it raises the intriguing possibility that the trogocytosed molecules could play a role in asymmetric T cell division. Asymmetric T cell division involves the segregation of proteins and signaling molecules in a bipolar manner resulting differential cell fate (effector versus memory) in daughter cells (80). It is possible that the T cells integrate differential signaling from the immune synapse and from trogocytosed molecules, which may play a role in setting up the observed asymmetric T cell division and differential development of T cell fate.
Sustained signaling after trogocytosis could also resolve an apparent paradox between the duration of Ag stimulation necessary to fully activate CD4+ T cells. Iezzi et al. (81) found that T cell activation required sustained Ag stimulation for up to 6 h. However, intravital microscopy has shown that in an intact lymph node, the duration of initial T–DC interactions are on the order of minutes (82). A pair of previous in vitro studies has shown that T cells form multiple, short lived interactions with APC, and that signals are “summed” to fully activate the T cells (53, 54). These results are consistent with the signaling summation model proposed by Lanzavecchia and colleagues (52). However, Lanzavecchia’s model requires that the summed signaling events temporally overlap. The interval between successive T–APC encounters in both in vivo (82) and in vitro studies is on the order of minutes, during which time signaling would likely be terminated. Partially phosphorylated/activated signaling cascades are refractory to fur- ther stimulation leading to inactivation of the cells; a mechanism underlying the TCR antagonism phenomenon (83, 84). Thus, the short duration, repeated T–APC interactions observed by others are not strictly in line with the Lanzavecchia model. We propose that the sustained signaling observed here after trogocytosis (Figs. 2, 3), could function to sustain intracellular signaling between successive APC interactions allowing for full activation of the T cells via signal summing.
In conclusion, the data presented in this paper demonstrate that trogocytosed molecules continue to engage their receptors on the surface of trog+ T cells, which leads to sustained intracellular signaling. We observed selective survival of the trog+ T cells after recovery from the APC coculture, and we suggest this is likely due to sustained signaling associated with trogocytosed molecules. Thus, our results show that trogocytosis appears to provide a selective advantage for the trog+ cells.
Acknowledgments
We thank the University of Montana Fluorescence Cytometry core and Pam Shaw for expert technical assistance with flow cytometry. We also thank the University of Montana Molecular Histology and Fluorescence Imaging Core and Lou Herritt for technical assistance with imaging experiments. We also thank Joanna Kreitinger and Lindsay Thueson for additional technical assistance, and Dr. Mike Minnick, Dr. Jesse Hay, Andy Lester, and Dr. Stephanie Lathrop for critical review of the manuscript.
This work was supported by National Center for Research Resources Grant 2P20RR017670-06 Project 5 (to S.A.W.) and the University of Montana University Grant Program MRA 380 and MRA 404 (to S.A.W.). This work was also supported by National Center for Research Resources Grant 2P20RR017670, which supports the University of Montana Fluorescence Cytometry and Molecular Histology and Fluorescence Imaging core facilities. The Molecular Histology and Fluorescence Imaging core is also supported by National Center for Research Resources Grant P20RR015583.
Abbreviations used in this article:
- cSMAC
center supramolecular activation complex
- FKBP
FK506-binding protein
- Lck
p56Lck
- MCC
moth cytochrome c
- MFI
mean fluorescence intensity
- pSMAC
peripheral ring supramolecular activation complex
- pTyr
phosphorylated tyrosine
- SMAC
supramolecular activation complex.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hudrisier D, Riond J, Mazarguil H, Gairin JE, and Joly E. 2001. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J. Immunol 166: 3645–3649. [DOI] [PubMed] [Google Scholar]
- 2.Wetzel SA, McKeithan TW, and Parker DC. 2005. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J. Immunol 174: 80–89. [DOI] [PubMed] [Google Scholar]
- 3.Joly E, and Hudrisier D. 2003. What is trogocytosis and what is its purpose? Nat. Immunol 4: 815. [DOI] [PubMed] [Google Scholar]
- 4.Hudson L, Sprent J, Miller JF, and Playfair JH. 1974. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature 251: 60–62. [DOI] [PubMed] [Google Scholar]
- 5.Lorber MI, Loken MR, Stall AM, and Fitch FW. 1982. I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J. Immunol 128: 2798–2803. [PubMed] [Google Scholar]
- 6.Nepom JT, Benacerraf B, and Germain RN. 1981. Acquisition of syngeneic I-A determinants by T cells proliferating in response to poly (Glu60Ala30-Tyr10). J. Immunol 127: 888–892. [PubMed] [Google Scholar]
- 7.Sharrow SO, Mathieson BJ, and Singer A. 1981. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J. Immunol 126: 1327–1335. [PubMed] [Google Scholar]
- 8.Mannie MD, Rendall SK, Arnold PY, Nardella JP, and White GA. 1996. Anergy-associated T cell antigen presentation: a mechanism of infectious tolerance in experimental autoimmune encephalomyelitis. J. Immunol 157: 1062–1070. [PubMed] [Google Scholar]
- 9.Arnold PY, Davidian DK, and Mannie MD. 1997. Antigen presentation by T cells: T cell receptor ligation promotes antigen acquisition from professional antigen-presenting cells. Eur. J. Immunol 27: 3198–3205. [DOI] [PubMed] [Google Scholar]
- 10.Arnold PY, and Mannie MD. 1999. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur. J. Immunol 29: 1363–1373. [DOI] [PubMed] [Google Scholar]
- 11.Patel DM, Arnold PY, White GA, Nardella JP, and Mannie MD. 1999. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J. Immunol 163: 5201–5210. [PubMed] [Google Scholar]
- 12.Patel DM, Dudek RW, and Mannie MD. 2001. Intercellular exchange of class II MHC complexes: ultrastructural localization and functional presentation of adsorbed I-A/peptide complexes. Cell. Immunol 214: 21–34. [DOI] [PubMed] [Google Scholar]
- 13.Patel DM, and Mannie MD. 2001. Intercellular exchange of class II major histocompatibility complex/peptide complexes is a conserved process that requires activation of T cells but is constitutive in other types of antigen presenting cell. Cell. Immunol 214: 165–172. [DOI] [PubMed] [Google Scholar]
- 14.Walker MR, and Mannie MD. 2002. Acquisition of functional MHC class II/peptide complexes by T cells during thymic development and CNS-directed pathogenesis. Cell. Immunol 218: 13–25. [DOI] [PubMed] [Google Scholar]
- 15.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, and Cai Z. 1999. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 286: 952–954. [DOI] [PubMed] [Google Scholar]
- 16.Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, and Sprent J. 2000. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J. Exp. Med 191: 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang I, and Sprent J. 2001. Role of the actin cytoskeleton in T cell absorption and internalization of ligands from APC. J. Immunol 166: 5099–5107. [DOI] [PubMed] [Google Scholar]
- 18.Espinosa E, Tabiasco J, Hudrisier D, and Fournié JJ. 2002. Synaptic transfer by human γδ T cells stimulated with soluble or cellular antigens. J. Immunol 168: 6336–6343. [DOI] [PubMed] [Google Scholar]
- 19.Hudrisier D, and Bongrand P. 2002. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J. 16: 477–486. [DOI] [PubMed] [Google Scholar]
- 20.Hudrisier D, Riond J, Garidou L, Duthoit C, and Joly E. 2005. T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur. J. Immunol 35: 2284–2294. [DOI] [PubMed] [Google Scholar]
- 21.Hudrisier D, Aucher A, Puaux AL, Bordier C, and Joly E. 2007. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J. Immunol 178: 3637–3647. [DOI] [PubMed] [Google Scholar]
- 22.Riond J, Elhmouzi J, Hudrisier D, and Gairin JE. 2007. Capture of membrane components via trogocytosis occurs in vivo during both dendritic cells and target cells encounter by CD8+ T cells. Scand. J. Immunol 66: 441–450. [DOI] [PubMed] [Google Scholar]
- 23.Aucher A, Magdeleine E, Joly E, and Hudrisier D. 2008. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood 111: 5621–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daubeuf S, Aucher A, Bordier C, Salles A, Serre L, Gaibelet G, Faye JC, Favre G, Joly E, and Hudrisier D. 2010. Preferential transfer of certain plasma membrane proteins onto T and B cells by trogocytosis. PLoS ONE 5: e8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daubeuf S, Lindorfer MA, Taylor RP, Joly E, and Hudrisier D. 2010. The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. J. Immunol 184: 1897–1908. [DOI] [PubMed] [Google Scholar]
- 26.Stinchcombe JC, Bossi G, Booth S, and Griffiths GM. 2001. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15: 751–761. [DOI] [PubMed] [Google Scholar]
- 27.Monks CR, Freiberg BA, Kupfer H, Sciaky N, and Kupfer A. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395: 82–86. [DOI] [PubMed] [Google Scholar]
- 28.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, and Dustin ML. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285: 221–227. [DOI] [PubMed] [Google Scholar]
- 29.Thauland TJ, Koguchi Y, Wetzel SA, Dustin ML, and Parker DC. 2008. Th1 and Th2 cells form morphologically distinct immunological synapses. J. Immunol 181: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kupfer A, and Singer SJ. 1989. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu. Rev. Immunol 7: 309–337. [DOI] [PubMed] [Google Scholar]
- 31.Huse M, Lillemeier BF, Kuhns MS, Chen DS, and Davis MM. 2006. T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol 7: 247–255. [DOI] [PubMed] [Google Scholar]
- 32.Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, et al. 2003. The immunological synapse balances T cell receptor signaling and degradation. Science 302: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 33.Batista FD, and Neuberger MS. 2000. B cells extract and present immobilized antigen: implications for affinity discrimination. EMBO J. 19: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batista FD, Iber D, and Neuberger MS. 2001. B cells acquire antigen from target cells after synapse formation. Nature 411: 489–494. [DOI] [PubMed] [Google Scholar]
- 35.Carlin LM, Eleme K, McCann FE, and Davis DM. 2001. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J. Exp. Med 194: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjöström A, Eriksson M, Cerboni C, Johansson MH, Sentman CL, Kärre K, and Höglund P. 2001. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J. Exp. Med 194: 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabiasco J, Espinosa E, Hudrisier D, Joly E, Fourniç JJ, and Vercellone A. 2002. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur. J. Immunol 32: 1502–1508. [DOI] [PubMed] [Google Scholar]
- 38.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, and Strominger JL. 1999. The human natural killer cell immune synapse. Proc. Natl. Acad. Sci. USA 96: 15062–15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabzevari H, Kantor J, Jaigirdar A, Tagaya Y, Naramura M, Hodge J, Bernon J, and Schlom J. 2001. Acquisition of CD80 (B7–1) by T cells. J. Immunol 166: 2505–2513. [DOI] [PubMed] [Google Scholar]
- 40.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, and Sabzevari H. 2002. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J. Immunol 169: 6162–6169. [DOI] [PubMed] [Google Scholar]
- 41.Baba E, Takahashi Y, Lichtenfeld J, Tanaka R, Yoshida A, Sugamura K, Yamamoto N, and Tanaka Y. 2001. Functional CD4 T cells after intercellular molecular transfer of 0X40 ligand. J. Immunol 167: 875–883. [DOI] [PubMed] [Google Scholar]
- 42.Gary R, Voelkl S, Palmisano R, Ullrich E, Bosch JJ, and Mackensen A. 2012. Antigen-specific transfer of functional programmed death ligand 1 from human APCs onto CD8+ T cells via trogocytosis. J. Immunol 188: 744–752. [DOI] [PubMed] [Google Scholar]
- 43.Xiang J, Huang H, and Liu Y. 2005. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J. Immunol 174: 7497–7505. [DOI] [PubMed] [Google Scholar]
- 44.Shi M, Hao S, Chan T, and Xiang J. 2006. CD4+ T cells stimulate memory CD8+ T cell expansion via acquired pMHC I complexes and costimulatory molecules, and IL-2 secretion. J. Leukoc. Biol 80: 1354–1363. [DOI] [PubMed] [Google Scholar]
- 45.Umeshappa CS, and Xiang J. 2010. Tumor-derived HLA-G1 acquisition by monocytes through trogocytosis: possible functional consequences. Cell. Mol. Life Sci 67: 4107–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsang JYS, Chai JG, and Lechler R. 2003. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood 101: 2704–2710. [DOI] [PubMed] [Google Scholar]
- 47.Helft J, Jacquet A, Joncker NT, Grandjean I, Dorothçe G, Kissenpfennig A, Malissen B, Matzinger P, and Lantz O. 2008. Antigen-specific T-T interactions regulate CD4 T-cell expansion. Blood 112: 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou G, Ding ZC, Fu J, and Levitsky HI. 2011. Presentation of acquired peptide-MHC class II ligands by CD4+ regulatory T cells or helper cells differentially regulates antigen-specific CD4+ T cell response. J. Immunol 186: 2148–2155. [DOI] [PubMed] [Google Scholar]
- 49.Choi EY, Jung KC, Park HJ, Chung DH, Song JS, Yang SD, Simpson E, and Park SH. 2005. Thymocyte‑thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity 23: 387–396. [DOI] [PubMed] [Google Scholar]
- 50.Martínez-Martín N, Fernández-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, Irvine DJ, Huang B, Bustelo XR, Shaw A, and Alarcon B. 2011. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity 35: 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dopfer EP, Minguet S, and Schamel WW. 2011. A new vampire saga: the molecular mechanism of T cell trogocytosis. Immunity 35: 151–153. [DOI] [PubMed] [Google Scholar]
- 52.Rachmilewitz J, and Lanzavecchia A. 2002. A temporal and spatial summation model for T-cell activation: signal integration and antigen decoding. Trends Immunol. 23: 592–595. [DOI] [PubMed] [Google Scholar]
- 53.Gunzer M, Schäfer A, Borgmann S, Grabbe S, Zänker KS, Bröcker EB, Kämpgen E, and Friedl P. 2000. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity 13: 323–332. [DOI] [PubMed] [Google Scholar]
- 54.Underhill DM, Bassetti M, Rudensky A, and Aderem A. 1999. Dynamic interactions of macrophages with T cells during antigen presentation. J. Exp. Med 190: 1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaye J, Vasquez NJ, and Hedrick SM. 1992. Involvement of the same region of the T cell antigen receptor in thymic selection and foreign peptide recognition. J. Immunol 148: 3342–3353. [PubMed] [Google Scholar]
- 56.Wetzel SA, McKeithan TW, and Parker DC. 2002. Live-cell dynamics and the role of costimulation in immunological synapse formation. J. Immunol 169: 6092–6101. [DOI] [PubMed] [Google Scholar]
- 57.Chow S, Hedley D, Grom P, Magari R, Jacobberger JW, and Shankey TV. 2005. Whole blood fixation and permeabilization protocol with red blood cell lysis for flow cytometry of intracellular phosphorylated epitopes in leukocyte subpopulations. Cytometry A 67: 4–17. [DOI] [PubMed] [Google Scholar]
- 58.Bolte S, and Cordelieres FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc 224: 213–232. [DOI] [PubMed] [Google Scholar]
- 59.Rasband WS 1997-2009. ImageJ. National Institues of Health:http://rsb.info.nih.gov/ij/. [Google Scholar]
- 60.Faroudi M, Zaru R, Paulet P, Müller S, and Valitutti S. 2003. Cutting edge: T lymphocyte activation by repeated immunological synapse formation and intermittent signaling. J. Immunol 171: 1128–1132. [DOI] [PubMed] [Google Scholar]
- 61.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, and Connelly PA. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J. Biol. Chem 271: 695–701. [DOI] [PubMed] [Google Scholar]
- 62.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, and Parker DC. 2004. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J. Immunol 172: 6735–6743. [DOI] [PubMed] [Google Scholar]
- 63.Perez OD, and Nolan GP. 2006. Phospho-proteomic immune analysis by flow cytometry: from mechanism to translational medicine at the single-cell level. Immunol. Rev 210: 208–228. [DOI] [PubMed] [Google Scholar]
- 64.Huppa JB, and Davis MM. 2003. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol 3: 973–983. [DOI] [PubMed] [Google Scholar]
- 65.Huppa JB, Gleimer M, Sumen C, and Davis MM. 2003. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat. Immunol 4: 749–755. [DOI] [PubMed] [Google Scholar]
- 66.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, and Shaw AS. 2002. T cell receptor signaling precedes immunological synapse formation. Science 295: 1539–1542. [DOI] [PubMed] [Google Scholar]
- 67.Doherty M, Osborne DG, Browning DL, Parker DC, and Wetzel SA. 2010. Anergic CD4+ T cells form mature immunological synapses with enhanced accumulation of c-Cbl and Cbl-b. J. Immunol 184: 3598–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang I, Shen X, and Sprent J. 2003. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc. Natl. Acad. Sci. USA 100: 6670–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sprent J, and Surh CD. 2003. Cytokines and T cell homeostasis. Immunol. Lett 85: 145–149. [DOI] [PubMed] [Google Scholar]
- 70.Sprent J.2005. Direct stimulation of näıve T cells by antigen-presenting cell vesicles. Blood Cells Mol. Dis 35: 17–20. [DOI] [PubMed] [Google Scholar]
- 71.Kovar M, Boyman O, Shen X, Hwang I, Kohler R, and Sprent J. 2006. Direct stimulation of T cells by membrane vesicles from antigen-presenting cells. Proc. Natl. Acad. Sci. USA 103: 11671–11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou J, Tagaya Y, Tolouei-Semnani R, Schlom J, and Sabzevari H. 2005. Physiological relevance of antigen presentasome (APS), an acquired MHC/costimulatory complex, in the sustained activation of CD4+ T cells in the absence of APCs. Blood 105: 3238–3246. [DOI] [PubMed] [Google Scholar]
- 73.Rechavi O, Goldstein I, Vernitsky H, Rotblat B, and Kloog Y. 2007. Intercellular transfer of oncogenic H-Ras at the immunological synapse. PLoS ONE 2: e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.HoWangYin KY, Caumartin J, Favier B, Daouya M, Yaghi L, Carosella ED, and LeMaoult J. 2011. Proper regrafting of Ig-like transcript 2 after trogocytosis allows a functional cell-cell transfer of sensitivity. J. Immunol 186: 2210–2218. [DOI] [PubMed] [Google Scholar]
- 75.Benoist C, and Mathis D. 1990. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu. Rev. Immunol 8: 681–715. [DOI] [PubMed] [Google Scholar]
- 76.HoWangYin KY, Alegre E, Daouya M, Favier B, Carosella ED, and LeMaoult J. 2010. Different functional outcomes of intercellular membrane transfers to monocytes and T cells. Cell. Mol. Life Sci 67: 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenits K, Keppler SJ, Vucikuja S, and Aichele P. 2010. T cells acquire cell surface determinants of APC via in vivo trogocytosis during viral infections. Eur. J. Immunol 40: 3450–3457. [DOI] [PubMed] [Google Scholar]
- 78.Cullinan P, Sperling AI, and Burkhardt JK. 2002. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol. Rev 189: 111–122. [DOI] [PubMed] [Google Scholar]
- 79.Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, and Sperling AI. 2001. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15: 739–750. [DOI] [PubMed] [Google Scholar]
- 80.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. 2007. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315: 1687–1691. [DOI] [PubMed] [Google Scholar]
- 81.Iezzi G, Karjalainen K, and Lanzavecchia A. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8: 89–95. [DOI] [PubMed] [Google Scholar]
- 82.Mempel TR, Henrickson SE, and Von Andrian UH. 2004. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427: 154–159. [DOI] [PubMed] [Google Scholar]
- 83.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, and Germain RN. 1995. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science 267: 515–518. [DOI] [PubMed] [Google Scholar]
- 84.Kersh EN., Kersh GJ, and Allen PM. 1999. Partially phosphorylated T cell receptor zeta molecules can inhibit T cell activation. J. Exp. Med 190: 1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]