Abstract
Background
Blood transfusions are common medical procedures and every age group requires detailed insights and treatment bundles. The aim of this study was to examine the association of anaemia, co-morbidities, complications, in-hospital mortality, and transfusion according to age groups to identify patient groups who are particularly at risk when undergoing surgery.
Methods
Data from 21 Hospitals of the Patient Blood Management Network Registry were analysed. Patients were divided into age subgroups. The incidence of preoperative anaemia, co-morbidities, surgical disciplines, hospital length of stay, complications, in-hospital mortality rate, and transfusions were analysed by descriptive and multivariate regression analysis.
Results
A total of 1 117 919 patients aged 18–108 years were included. With increasing age, the number of co-morbidities and incidence of preoperative anaemia increased. Complications, hospital length of stay, and in-hospital mortality increased with age and were higher in patients with preoperative anaemia. The mean number of transfused red blood cells (RBCs) peaked, whereas the transfusion rate increased continuously. Multivariate regression analysis showed that increasing age, co-morbidities, and preoperative anaemia were independent risk factors for complications, longer hospital length of stay, in-hospital mortality, and the need for RBC transfusion.
Conclusion
Increasing age, co-morbidities, and preoperative anaemia are independent risk factors for complications, longer hospital length of stay, in-hospital mortality, and the need for RBC transfusion. Anaemia diagnosis and treatment should be established in all patients.
The association between age, preoperative anaemia, and co-morbidities on perioperative outcome in surgical patients, was performed to augment our knowledge on each age decade. This big data set, including 1 117 919 patients aged 18–108 years, gives detailed information and can be used to plan prospective studies in the setting of surgical intervention, age, complications, and transfusion management. To conclude, increasing age, preoperative anaemia and the sum of co-morbidities have been proven to be independent risk factors for complications, mortality, length of in-hospital stay, and the need for blood products.
Introduction
Despite recent advances in surgical techniques and medical procedures, major surgery is still commonly associated with subsequent anaemia, substantial blood loss, and the need for blood transfusion. As age is considered a risk factor for severe adverse events1 and a predictor for poor outcome with significantly higher mortality rate and postoperative complications2–5, patients at older age need higher care resources during and after the in-hospital stay. In addition, co-morbidities often lead to complications and prolonged cure.
Anaemia is one of the most common diseases worldwide. The major cause in more than 50 per cent is altered erythropoiesis due to iron deficiency, leading to iron deficiency anaemia6. The second most common is anaemia of chronic disease representing up to 45 per cent, also called anaemia of inflammation and caused by cancer, acute or chronic diseases, which is predominant in hospitalized patients. Less than 10 per cent of anaemia is caused by genetic inheritance, vitamin B12 or folate deficiency, or diseases of the bone marrow6. Among elderly patients, renal disease and chronic inflammation account for approximately one-third of all anaemia incidences7. Anaemia leads, independent of age, to increased use of allogenic blood products and is associated with an increased complication rate, hospitalization, and mortality rate8–11.
The aim of this study was to examine the association of anaemia, co-morbidities, complications, in-hospital mortality, and transfusion according to age groups to identify patient groups who are particularly at risk when undergoing surgery in a large data set of more than 1 million patients.
Methods
A sub-analysis of an ongoing prospective multicentre observational trial12 focusing on the implementation and establishment of patient blood management (PBM) in surgical patients (registration number: NCT02147795; http://www.clinicaltrials.gov) was performed. The study was approved by the ethics committee of the University Hospital Frankfurt (reference number 318/17) and the Hessian data protection office (reference number 43.60; 60.01.21-ga). The requirement for written informed consent by patients was waived. The data were extracted from the respective electronic hospital information systems by the local information technology staff and anonymized for further analysis.
Patients and procedures
All adult (18 years or more) in-house patients with defined sex undergoing surgery (German OPS code 5-01 to 5-99) were included in the analysis. The surgeries covered all surgical disciplines: visceral and endocrine surgery, cardiac surgery, thoracic surgery, otorhinolaryngology, gynaecology, neurosurgery, obstetrics, dermatology, and ophthalmology, oral and maxillofacial surgery, surgery of the haematopoietic and lymphatic system, trauma and orthopaedic surgery, urology, vascular surgery, and mixed surgery (OPS codes from at least two different surgical disciplines) (Table S1). All patients received standard German perioperative care. Patients were divided into subgroups according to their age: 18–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, 90–99, and 100–109 years.
Anaemia was defined according to the WHO as haemoglobin (Hb) concentration of less than 12.0 g/dl in women and less than 13.0 g/dl in men. Anaemia was categorized into mild (Hb 11.0–11.9 g/dl for women and Hb 11.0–12.9 g/dl for men); moderate (Hb 8.0–10.9 g/dl); and severe (Hb less than 8.0 g/dl).
All allogeneic blood products (red blood cell (RBC) concentrates, platelet concentrates, fresh frozen plasma, fibrinogen, and prothrombin complex concentrate) were administered in accordance with the previous German transfusion guideline13,14.
Existing co-morbidities (diabetes, hypertension, chronic kidney disease, chronic pulmonary disease, neurological disorder, heart insufficiency, and malignancy) were defined by their respective ICD codes (Table S2). Hospital-acquired complications indicated by new diagnoses during the in-hospital stay (pneumonia, sepsis, acute renal failure, myocardial infarction, and ischaemic stroke) were also defined by their respective ICD codes (Table S2). In-hospital mortality was analysed based on existing discharge codes and thereby defined as mortality during the in-hospital stay.
Statistical analysis
The aim of the analysis was to describe patient characteristics (for example co-morbidities, sex, surgery groups, and preoperative anaemia) and clinical outcomes (for example length of hospital stay (LOS), in-hospital mortality, complications, postoperative and hospital-acquired anaemia, and blood product consumption) for adult surgical patients divided into age groups of 10 years. In addition, multivariate regression analysis was performed to identify dependence of clinical outcomes on the chosen patients’ characteristics and hence to give indications for risk factors in clinical routine that could be targeted for effective patient care.
Values for the descriptive analysis are reported as rates with 95 per cent confidence interval (c.i.) for categorical variables, and as mean(s.e.m.) or median and interquartile range for continuous variables. Patient characteristics (age, sex, co-morbidities, preoperative anaemia, and surgery type) and outcomes (postoperative and hospital-acquired anaemia rates and severity, postoperative complication rates, in-hospital mortality rates, LOS, blood product transfusion rates, and mean consumptions) within the different age groups are shown. A descriptive sub-analysis (regarding patient groups with and without preoperative anaemia) for chosen outcomes (composite endpoint of complications, LOS, and in-hospital mortality) was performed. Another descriptive sub-analysis was performed for patients with and without RBC transfusions for the outcome of in-hospital mortality.
The P values from the multivariate analysis were derived from mixed logistic regression models for categorical binary variables and from mixed linear regression models for continuous variables, including the hospitals as random variables and age group, sex, surgery discipline, preoperative anaemia, and sum of co-morbidities as fixed effects. The strength and direction of the included influence factors was estimated by the OR with 95 per cent c.i. for categorical binary variables and by the difference of the mean(s.e.m.) for continuous variables.
Analysis was performed using R version 3.6 for windows (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
Results
Age, sex, surgery disciplines, co-morbidities, and prevalence of preoperative anaemia
A total of 1 117 919 patients from 21 German hospitals underwent surgery between 1 January 2010 and 20 September 2019 and were included in the analysis. The minimum age was 18 years and the maximum age was 108 years. Overall, 51.3 per cent (573 816) of the patients were female. The proportion of female patients varied between the different age groups and was dominant at the ages of 20–49 years and 80–109 years (Table S3). Types of surgery performed varied between age groups: the majority of patients aged between 18–19 years underwent trauma and orthopaedic surgery (21.3 per cent); those aged between 20–29 and 30–39 years underwent obstetric surgery (34.0 per cent and 43.3 per cent); those aged between 40–49, 50–59, 60–69 years underwent visceral and endocrine surgery (18.7 per cent, 19.8 per cent and 18.4 per cent); those aged between 70–79 and 80–89 years underwent mostly dermatological and ophthalmological surgery (18.3 per cent and 22.5 per cent); and those aged between 90–109 years underwent trauma and orthopaedic surgery (28.1 per cent and 39.4 per cent) (Table S3 and Fig. S1).
The total number of co-morbidities increased with age (Table S4 and Fig. S2). The mean total number of co-morbidities increased from 0.1(0.0) at the age of 18–39 years up to 1.8(0.0) at the age of 90–99 years. The rate of patients with at least one co-morbidity rose from 9.1 per cent (8.6 to 9.6 per cent) for patients aged 18–19 years up to 83.7 per cent (83.1 to 84.2 per cent) for patients aged 90–99 years. The rate of each co-morbidity, except neurological diseases and chronical kidney diseases, increased only until a certain age and then stabilized or even decreased again (Table S4 and Fig. S3).
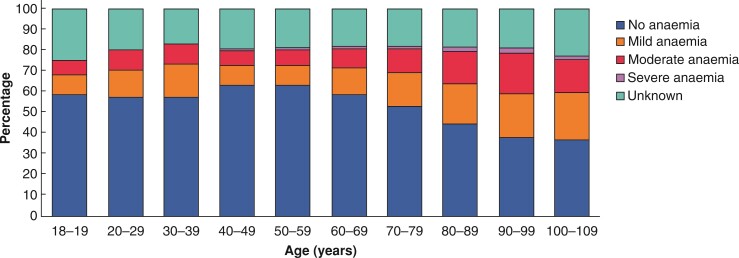
Overall prevalence of preoperative anaemia increased from the age group of 18–19 years (22.3 per cent; 21.5 to 23.2 per cent) until a maximum at 90–99 years (53.3 per cent; 52.5 to 54.1 per cent) (Table S3). The prevalence of mild preoperative anaemia increased from 9.5 per cent (aged 40–49 years) up to 22.5 per cent (aged 100–109 years), of moderate preoperative anaemia from 7.3 per cent (aged 40–49 years) up to 19.5 per cent (aged 90–99 years), and of severe anaemia from 1.0 per cent (aged 40–49 years) up to 2.9 per cent (aged 90–99 years) (Table S3 and Fig. 1).
Fig. 1.
Distribution of preoperative anaemia severities according to age groups
Complications, in-hospital mortality, length of stay, and incidence of hospital-acquired anaemia
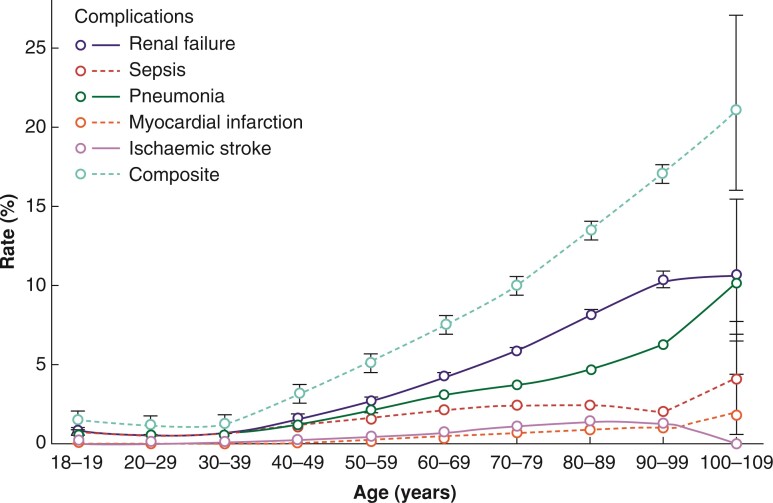
The mean total number of complications increased slightly from 0.1(0.0) at the age of 18–49 years up to 0.3(0.0) at the age of 100–109 years (Table S5). The rate of patients with at least one hospital-acquired complication rose from 1.0 per cent (1.0 to 1.1 per cent) for patients aged 20–29 years up to 21.1 per cent (15.9 to 27.1 per cent) for patients aged 100–109 years (Table S5 and Fig. 2).
Fig. 2.
Perioperative complication rates according to age groups: single and composite rate of complications with 95 per cent confidence interval per age group
The composite complication rate was higher in patients with preoperative anaemia than in patients without preoperative anaemia (Table 1).
Table 1.
Complications, length of in-hospital stay, and mortality rate according to presence of preoperative anaemia by age groups. Postoperative and hospital-acquired anaemia rates according to age groups
| Age (years) | 18–19 n = 12 485 |
20–29 n = 100 762 |
30–39 n = 136 847 |
40–49 n = 112 220 |
50–59 n = 171 687 |
60–69 n = 198 869 |
70–79 n = 238 172 |
80–89 n = 128 631 |
90–99 n = 18 028 |
100–109 n = 218 |
|---|---|---|---|---|---|---|---|---|---|---|
| Composite complication rate*‡ | 1.6 | 1.0 | 1.2 | 3.3 | 5.1 | 7.8 | 10.0 | 13.5 | 17.0 | 21.1 |
| (1.4–1.8) | (1.0–1.1) | (1.2–1.3) | (3.2–3.4) | (5.0–5.2) | (7.6–7.9) | (9.9–10.2) | (13.3–13.6) | (16.4–17.5) | (15.9–27.1) | |
| n = 201 | n = 1028 | n = 1664 | n = 3728 | n = 8738 | n =15 422 | n =23 896 | n =17 302 | n = 3060 | n = 46 | |
| With preoperative anaemia | 5.8 | 2.4 | 2.6 | 10.1 | 14.7 | 17.6 | 18.5 | 19.9 | 22.4 | 29.5 |
| (4.9–6.9) | (2.2–2.6) | (2.4–2.8) | (9.7–10.5) | (14.3–15.1) | (17.2–17.9) | (18.2–18.8) | (19.5–20.2) | (21.5–23.4) | (20.3–40.2) | |
| n = 123 of 2114 | n = 567 of 23 605 | n = 917 of 35 501 | n = 2023 of 20 043 | n = 4561 of 31 055 | n = 8225 of 46 818 | n =12 978 of 70 298 | n = 9569 of 48 195 | n = 1760 of 7846 | n = 26 of 88 | |
| Without preoperative anaemia | 0.9 | 0.6 | 0.8 | 2.1 | 3.3 | 5.4 | 7.6 | 11.6 | 15.8 | 18.5 |
| (0.7–1.1) | (0.6–0.7) | (0.7–0.9) | (2.0–2.2) | (3.2–3.4) | (5.3–5.5) | (7.4–7.7) | (11.4–11.9) | (15.0–16.7) | (10.8–28.7) | |
| n = 66 of 7349 | n = 370 of 57 801 | n = 642 of 79 253 | n = 1457 of 70 814 | n = 3608 of 108 682 | n = 6303 of 117 035 | n = 9560 of 126 279 | n = 6631 of 56 972 | n = 1086 of 6870 | n = 15 of 81 | |
| Length of hospital stay (days)† | 5.5 (±0.1) |
5.0 (±0.0) | 5.2 (±0.0) | 6.6 (±0.0) | 7.9 (±0.0) | 9.2 (±0.0) | 9.7 (±0.0) | 10.1 (±0.0) | 10.1 (±0.1) | 8.8 (±0.5) |
| With preoperative anaemia | 9.9 (±0.4) | 6.8 (±0.1) | 6.9 (±0.1) | 11.7 (±0.1) | 14.2 (±0.1) | 14.9 (±0.1) | 14.0 (±0.1) | 12.9 (±0.1) | 11.8 (±0.1) | 9.6 (±0.9 ) |
| Without preoperative anaemia | 5.1 (±0.1) | 4.8 (±0.0) | 4.9 (±0.0) | 6.1 (±0.0) | 7.2 (±0.0) | 8.2 (±0.0) | 9.0 (±0.0) | 9.9 (±0.0) | 10.5 (±0.1) | 9.7 (±0.8) |
| Mortality rate* | 0.3 | 0.2 | 0.3 | 0.8 | 1.4 | 2.1 | 2.7 | 3.9 | 6.3 | 8.7 |
| (0.2–0.5) | (0.2–0.3) | (0.2–0.3) | (0.8–0.9) | (1.3–1.5) | (2.0–2.1) | (2.6–2.8) | (3.7–4.0) | (6.0–6.7) | (5.3–13.3) | |
| n = 42 | n = 234 | n = 374 | n = 932 | n = 2408 | n = 4140 | n = 6402 | n = 4955 | n = 1140 | n = 19 | |
| With preoperative anaemia | 1.2 | 0.6 | 0.6 | 2.9 | 4.8 | 5.5 | 5.6 | 6.1 | 8.1 | 11.4 |
| (0.8–1.7) | (0.5–0.7) | (0.5–0.7) | (2.7–3.2) | (4.5–5.0) | (5.3–5.7) | (5.4–5.8) | (5.9–6.3) | (7.5–8.7) | (5.6–19.9) | |
| n = 25 of 2114 | n = 139 of 23 605 | n = 222 of 35 501 | n = 586 of 20 043 | n = 1476 of 31 055 | n = 2564 of 46 818 | n = 3945 of 70 298 | n = 2922 of 48 195 | n = 633 of 7846 | n = 10 of 88 | |
| Without preoperative anaemia | 0.1 | 0.1 | 0.1 | 0.4 | 0.7 | 1.1 | 1.6 | 2.9 | 6.0 | 7.4 |
| (0.1–0.3) | (0.1–0.1) | (0.1–0.2) | (0.3–0.4) | (0.6–0.7) | (1.0–1.2) | (1.5–1.7) | (2.7–3.0) | (5.4–6.6) | (2.8–15.4) | |
| n = 11 of 7349 | n = 65 of 57 801 | n = 110 of 79 253 | n = 268 of 70 814 | n = 750 of 108 682 | n = 1287 of 117 035 | n = 2037 of 126 279 | n = 1628 of 56 972 | n = 412 of 6870 | n = 6 of 81 | |
| With RBC transfusion | 9.0 | 8.0 | 8.3 | 12.4 | 13.3 | 13.3 | 13.6 | 14.8 | 14.2 | 24.4 |
| (6.5–12.1) | (6.9–9.2) | (7.4–9.3) | (11.5–13.2) | (12.7–13.9) | (12.8–13.7) | (13.2–14.0) | (14.3–15.3) | (13.0–15.4) | (12.9–39.5) | |
| n = 40 | n = 184 | n = 284 | n = 712 | n = 1734 | n = 2917 | n = 4267 | n = 2754 | n = 477 | n = 11 | |
| Without RBC transfusion | 0.0 | 0.1 | 0.1 | 0.2 | 0.4 | 0.7 | 1.0 | 2.0 | 4.5 | 4.6 |
| (0.0–0.1) | (0.0–0.1) | (0.1–0.1) | (0.2–0.2) | (0.4–0.5) | (0.7–0.7) | (1.0–1.1) | (1.9–2.1) | (4.2–4.9) | (2.0–8.9) | |
| n = 2 | n = 50 | n = 90 | n = 220 | n = 674 | n = 1223 | n = 2135 | n = 2201 | n = 663 | n = 8 | |
| Postoperative anaemia rate | 43.6 | 56.5 | 61.2 | 44.3 | 47.8 | 57.2 | 64.3 | 71.4 | 77.9 | 86.9 |
| (42.5–44.6) | (56.1–56.9) | (60.9–61.5) | (44.0–44.7) | (47.5–48.1) | (56.9–57.4) | (64.1–64.5) | (71.1–71.7) | (77.2–78.6) | (80.9–91.5) | |
| n = 3519 of 8080 | n = 41 144 of 72 827 | n = 64 768 of 105 810 | n = 35 415 of 79 857 | n = 58 899 of 123 179 | n = 84 918 of 148 561 | n = 116 547 of 181 252 | n = 69 910 of 97 926 | n = 11 166 of 14 335 | n = 152 of 175 | |
| Hospital-acquired anaemia rate | 26.6 | 39.1 | 45.1 | 29.2 | 33.6 | 41.1 | 46.1 | 49.9 | 56.3 | 75.0 |
| (25.4–27.8) | (38.7–39.6) | (44.7–45.5) | (28.8–29.5) | (33.3–33.9) | (40.8–41.4) | (45.8–46.4) | (49.4–50.3) | (55.1–57.6) | (63.4–84.5) | |
| n = 1392 of 5241 | n = 17 152 of 43 848 | n = 28 683 of 63 630 | n = 16 102 of 55 219 | n = 28 708 of 85 443 | n = 39 347 of 95 739 | n = 48 994 of 106 278 | n = 23 987 of 48 087 | n = 3397 of 6030 | n = 54 of 72 |
The denominator is only shown for the subgroup analysis, otherwise it is equal to n of each age subgroup displayed in the first line of the table. *Values are % (95% c.i.). †Values are mean(±s.e.m.). ‡At least one complication present. RBC, red blood cell.
The mean LOS raised from 5.0(±0.0) days in patients aged 20–29 years up to 10.1(0.0) in patients aged 80–89 years. With the additional diagnosis of preoperative anaemia, mean LOS was prolonged (Table 1 and Fig. S4).
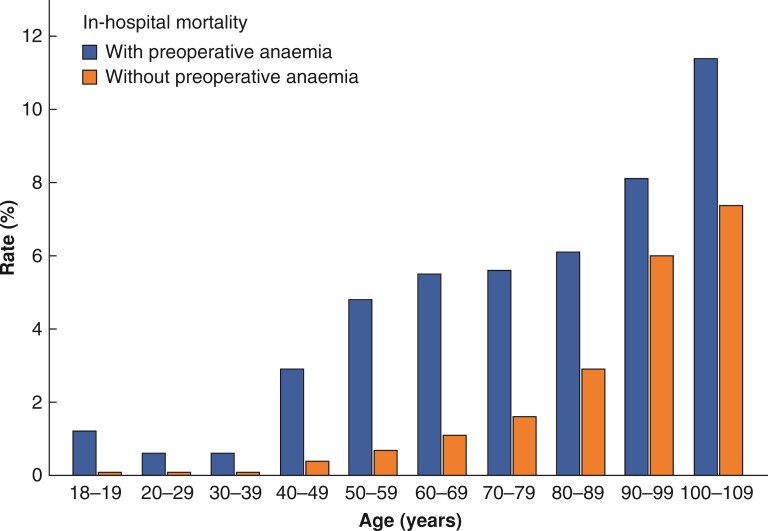
In-hospital mortality rate increased from 0.2 per cent (0.2 to 0.3 per cent) in patients aged 20–29 years to 8.7 per cent (5.3 to 13.3 per cent) in patients aged more than 100 years (Table 1). In-hospital mortality rate was higher in patients with preoperative anaemia (Table 1 and Fig. 3).
Fig. 3.
In-hospital mortality rate with and without preoperative anaemia according to age groups
Postoperative anaemia rate ranged from 43.6 per cent (42.5 to 44.6 per cent) in patients aged 18–19 years up to 86.9 per cent (80.9 to 91.5 per cent) in patients over 100 years (Table 1 and Table S5). The rate of hospital-acquired anaemia ranged from 26.6 per cent (25.4 to 27.8 per cent) in patients aged 18–19 years up to 75.0 per cent (63.4–84.5 per cent) in patients more than 100 years (Table 1). The severity of hospital-acquired anaemia was categorized mostly as mild (dominant for patients aged 18–69 years) or moderate (dominant for patients aged 70–109 years) (Table S5 and Fig. S5).
Blood product consumption with special focus on in-hospital mortality
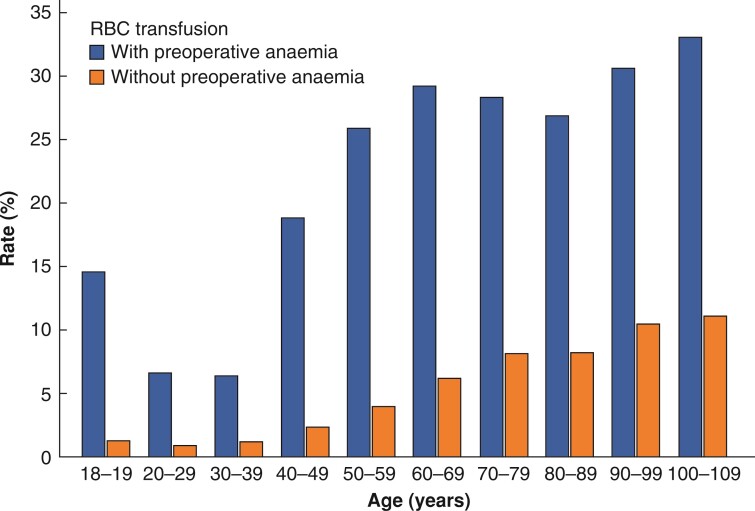
The data indicate that receipt of RBC transfusion is more probable at an older age, whereas the number of RBCs per patient decreases at older age. The RBC transfusion rate increased continuously from 2.3 per cent (2.2 to 2.4 per cent) for patients aged 20–29 years until 20.6 per cent (15.5 to 26.6 per cent) (100–109 years) (Table 2). The mean number of RBCs per 1000 patients increased from 156 ± 6 units (20–29 years) until 678 ± 6 units (70–79 years). At all ages, the RBC transfusion rate and mean number of RBCs per 1000 patients were higher in patients with preoperative anaemia than in patients without preoperative anaemia (approximately 3.4- to 10.1-fold) (Table 2, Fig. 4). RBC transfusion is associated with higher in-hospital mortality in every age group, but causality cannot be proven (Table 2 and Fig. 4). While in non-transfused patients, in-hospital mortality increased from 0.0 per cent (0.0 to 0.1 per cent) in patients aged 18–19 years continuously to 4.6 per cent (2.0 to 8.9 per cent) in patients aged 100–109 years; in-hospital mortality of transfused patients ranged from 8.0 per cent (6.9 to 9.2 per cent) at 20–29 years of age up to 24.4 per cent (12.9 to 39.5 per cent) at 100–109 years of age (Table 1).
Table 2.
Mean consumption and transfusion rates of allogeneic red blood cell (RBC) units with dependence on preoperative anaemia according to age groups
| Age (years) | 18–19 n = 12 485 | 20–29 n = 100 762 | 30–39 n = 136 847 | 40–49 n = 112 220 | 50–59 n = 171 687 | 60–69 n = 198 869 | 70–79 n = 238 172 | 80–89 n = 128 631 | 90–99 n = 18 028 | 100–109 n = 218 |
|---|---|---|---|---|---|---|---|---|---|---|
| RBC units mean per 1000 patients† | 268(±22) | 156(±6) | 163(±6) | 384(±10) | 506(±8) | 663(±8) | 678(±6) | 567(±6) | 502(±11) | 477(±92) |
| With preoperative anaemia | 1126(±107) | 448(±22) | 436(±18) | 1481(±43) | 1857(±36) | 1868(±26) | 1518(±17) | 1074(±14) | 852(±21) | 875(±208) |
| Without preoperative anaemia | 111(±21) | 68(±6) | 70(±5) | 152(±8) | 227(±7) | 328(±7) | 380(±6) | 305(±7) | 253(±11) | 198(±69) |
| RBC transfusion rate* | 3.5 | 2.3 | 2.5 | 5.1 | 7.6 | 11.0 | 13.2 | 14.5 | 18.6 | 20.6 |
| (3.2–3.9) | (2.2–2.4) | (2.4–2.6) | (5.0–5.3) | (7.5–7.7) | (10.9–11.2) | (13.1–13.3) | (14.3–14.7) | (18.1–19.2) | (15.5–26.6) | |
| n = 442 | n = 2302 | n = 3403 | n = 5758 | n = 13 063 | n = 21 966 | n = 31 413 | n = 18 657 | n = 3359 | n = 45 | |
| With preoperative anaemia | 14.6 | 6.6 | 6.4 | 18.8 | 25.9 | 29.2 | 28.3 | 26.9 | 30.6 | 33.0 |
| (13.1–16.1) | (6.3–6.9) | (6.2–6.7) | (18.3–19.4) | (25.4–26.4) | (28.7–29.6) | (27.9–28.6) | (26.5–27.3) | (29.5–31.6) | (23.3–43.8) | |
| n = 308 | n = 1560 | n = 2278 | n = 3778 | n = 8052 | n = 13 649 | n = 19 880 | n = 12 945 | n = 2397 | n = 29 | |
| Without preoperative anaemia | 1.3 | 0.9 | 1.2 | 2.4 | 4.0 | 6.2 | 8.1 | 8.2 | 10.5 | 11.1 |
| (1.1–1.6) | (0.9–1.0) | (1.1–1.2) | (2.3–2.5) | (3.9–4.1) | (6.1–6.4) | (7.9–8.2) | (8.0–8.4) | (9.8–11.2) | (5.2–20.0) | |
| n = 98 | n = 547 | n = 917 | n = 1678 | n = 4334 | n = 7303 | n = 10 201 | n = 4670 | n = 719 | n = 9 |
*Values are % (with 95% c.i.). †Values are mean(±s.e.m.). RBC, red blood cell.
Fig. 4.
RBC transfusion rate with and without preoperative anaemia according to age groups
RBC, red blood cell
Multivariate analysis of potential independent risk factors (age, preoperative anaemia, and co-morbidities) on transfusion outcomes, LOS, postoperative complications, and in-hospital mortality
To identify risk factors independently associated with postoperative outcome, linear and logistic multivariate regression models, including the hospitals as random variables and all other variables such as age group, sex, surgery discipline, preoperative anaemia, and sum of co-morbidities as fixed effects, were performed. Age, preoperative anaemia, co-morbidities, surgery group, and sex were independent risk factors for the following outcomes: composite complications, single complications (renal failure, sepsis, pneumonia, ischaemic stroke, and myocardial infarction), postoperative anaemia, in-hospital mortality, LOS, RBC transfusion rate, RBC mean consumption, and hospital-acquired anaemia. In patients with preoperative anaemia, the mean difference in LOS and the OR of in-hospital mortality rate, composite complication rate, single complication rates, and RBC transfusion rate were significantly higher (P < 0.001). A higher sum of co-morbidities contributed significantly to a higher OR of all outcomes (P < 0.001). In women, mean LOS, in-hospital mortality, and event rates of complications were significantly lower, but RBC transfusion rate, RBC mean consumption, and hospital-acquired anaemia rate were significantly higher compared with men (P <0.001) (Table 3, Table S6 and Table S7).
Table 3.
Multivariate analysis of risk factors age, preoperative anaemia, sum of co-morbidities on outcomes LOS, mortality, composite complications, and red blood cell transfusion rate
| Length of hospital stay (days)* | Mortality rate† | Composite complication rate† | RBC transfusion rate† | ||
|---|---|---|---|---|---|
| Age (years) | 20–29 | −0.38(±0.12)* | NS | 0.82 (0.72–0.92)* | 0.74 (0.68–0.80) |
| 30–39 | −0.41(±0.12) | NS | NS | 0.73 (0.67–0.79) | |
| 40–49 | NS | 1.71 (1.42–2.04) | 1.41 (1.26–1.57) | NS | |
| 50–59 | 0.66(±0.12) | 2.29 (1.92–2.72) | 1.52 (1.36–1.69) | 1.27 (1.18–1.37) | |
| 60–69 | 0.93(±0.12) | 2.70 (2.78–3.21) | 1.64 (1.47–1.82) | 1.50 (1.40–1.62) | |
| 70–79 | 0.85(±0.12) | 3.22 (2.72–3.82) | 1.71 (1.54–1.91) | 1.57 (1.46–1.68) | |
| 80–89 | 0.77(±0.12) | 4.62 (3.90–5.49) | 2.13 (1.91–2.37) | 1.51 (1.50–1.73) | |
| 90–99 | 0.48(±0.15) | 9.14 (7.64–10.94) | 3.10 (2.76–3.48) | 2.40 (2.21–2.61) | |
| >99 | NS | 15.90 (10.11–25.01) | 5.71 (4.09–7.99) | 3.34 (2.72–4.09) | |
| Preoperative anaemia | 3.46(±0.03) | 2.92 (2.82–3.01) | 2.10 (2.07–2.14) | 5.21 (5.12–5.29) | |
| Sum of co-morbidities | Per increase of 1 co-morbidity | 2.19(±0.01) | 1.39 (1.38–1.41) | 1.77 (1.76–1.78) | 1.41 (1.40–1.42) |
*Values are mean(±s.e.m.). †Values are OR (95% c.i.). For the age groups, all values are with respect to age group 18–19 years, for co-morbidities all values represent the values after augmentation by 1 unit (that means for 1 co-morbidity more) with respect to before the augmentation, and for preoperative anaemia with respect to without anaemia. All significant P values were P < 0.001, except for * (P = 0.001). RBC, red blood cell.
Discussion
In the present multicentre analysis of more than 1 million surgical patients, anaemia, co-morbidities, and the need for allogeneic blood products was more common with rising age. Rates of perioperative complications, LOS, and in-hospital mortality also increased with age. Age, preoperative anaemia, and co-morbidities could be identified as independent risk factors for a longer LOS and higher in-hospital mortality, postoperative anaemia, and RBC transfusion rate as well as a higher mean consumption of RBCs. RBC transfusion was associated with a higher in-hospital mortality in every age group, but causality could not be proven. The data indicate that anaemia is associated with increased in-hospital mortality in all age groups, preoperative anaemia is an independent risk factor for postoperative complications, and patients with increasing age are more likely to receive an RBC unit.
In 2013, Loor et al. found that both anaemia and RBC transfusions may have an impact on patients’ health15. The first conclusion could be that anaemia should be treated, even before a surgical intervention. But in Germany, anaemia screening and treatment is still not standard care in most hospitals and should therefore be supported. A second conclusion could lead to the aim to avoid unnecessary RBC transfusions during the entire stay in hospital. Therefore, rational transfusion regimens should be followed according to current guidelines, including in older patient cohorts13.
The high percentage of visceral surgery in patients aged 40–60 years is explained by the high incidence of acute appendicitis and symptomatic gallstones or acute cholecystitis at the age of 40–60 years16. The number of co-morbidities increased continuously until the age of 80 years. In contrast, malignancies peaked at 60–69 years, and patients with malignancy have increased mortality. In addition, RBC consumption has been shown to be associated with increased mortality17. For colon cancer18, gastric cancer19, and bladder cancer20 increased cancer recurrence rates have been reported in transfused compared with non-transfused patients. In addition, malignancy can lead to anaemia. The anaemia of chronic disease is highly prevalent in malignancy, but also in other chronic diseases such as heart failure and renal insufficiency21. As chronic diseases are more prevalent at higher age, anaemia is also more prevalent and the diagnosis of anaemia requires additional investigations to determine the underlying cause of anaemia22.
Overall, the present data shows a higher incidence of the complications renal failure, sepsis, myocardial infarction, ischaemic stroke, and pneumonia in patients at increasing age. Age itself has been described as an independent risk factor in the development of acute kidney injury. Further explanations for the increase might be the decreasing glomerular filtration rate with increasing age, inflammation, and co-morbidities such as chronic kidney disease, congestive heart failure, and diabetes mellitus23,24. Likewise, these complications had an increasing incidence in the study population with increasing age.
In the present analysis, in-hospital mortality also increased significantly with increasing patient age, which is expected and in line with the results of recent studies. Caterino et al. explored about 75 600 patients with traumatic injuries and found a significantly higher mortality rate in patients older than 70 years25. Milzman et al. analysed 7789 patients for pre-existing disease and showed that pre-existing diseases were an independent predictor of mortality. The median age of patients with pre-existing diseases was higher. The mortality rate for patients with two or more co-morbidities was higher26. Baron et al. analysed about 40 000 patients with moderate or severe anaemia and demonstrated higher in-hospital mortality and prolonged hospital LOS as well as a higher number of postoperative admissions to intensive care units in 27 European countries8.
It should be mentioned that anaemia in women is still defined as Hb levels less than 12 g/dl, even though the classification of anaemia in non-pregnant women by WHO has been the subject of many discussions in recent years27. By accepting lower Hb levels in women, transfusions with all their possible side effects and complications are more likely in women28,29, as shown in the present results, where RBC transfusion rate, RBC mean consumption, and hospital-acquired anaemia rate in women were significantly higher compared with men. In addition, women may be excluded from effective therapy of preoperative anaemia because of the ‘accepted’ Hb value30.
Although this study presents a large patient cohort, including more than 1 million patients, some limitations need to be considered. Routine data were used, thus data amount and quality varied between the hospitals. The utilized ICD codes do not allow a clear distinction between an underlying disease as an indication for surgery and a new diagnosis made during the inpatient stay. For this reason, the complications of ischaemic stroke and acute myocardial infarction were not investigated in the group of neurosurgical and cardiac surgery respectively.
The group of 18–19-year-olds is special, as patients in this age group are often severely injured trauma patients or are suffering from malignant diseases31 and are therefore only suitable for comparison to a limited extent. The group of patients aged 20–39 years includes a large number of healthy patients undergoing obstetric procedures. To some extent, LOS is certainly dependent on the days of the week, as more patients are usually discharged on weekdays rather than on weekends. Furthermore, the higher mortality rate in transfused patients compared with non-transfused patients is probably multifactorial. Patients who need transfusions may have a complicating surgical or medical condition, therefore, higher mortality cannot be directly attributed to RBC transfusion.
Collaborators
German PBM Network Collaborators
Klinikum Westmuensterland Bocholt: O. Baumhove, S. de Leeuw van Weenen (Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy). DONAUISAR Klinikum Deggendorf/Dingolfing/Landau: D. Narita (Institute of Laboratory Diagnostics und Transfusion Medicine), J. M. Huber (Institute of Laboratory Diagnostics, Immunhematology and Microbiology). University Hospital Frankfurt: E. Adam, S. Choorapoikayil, L. Hof, S. Isik, M. Krämer, H. Neb, V.Neef, F.Piekarski, E.Schmitt, K. Zacharowski (Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy), T. Walther, T. Holubec, (Department of Thoracic and Cardiovascular Surgery), A. Schnitzbauer, W. O. Bechstein (Department of General and Visceral Surgery), W. Derwich, T.Schmitz-Rixen (Department of Vascular and Endovascular Surgery), B. Steffen, H. Serve (Department of Haematooncology), J. Bojunga, S. Zeuzem (Department of Gastroenterology and Hepatology), J. Konczalla, V. Seifert (Department of Neurosurgery), F. Roos, F. Chun (Department of Urology), C. Nau, I. Marzi (Department of Trauma, Hand and Reconstructive Surgery), M. Leinung, T. Stöver (Department of Otorhinolaryngology), S. Ghanaati, R. Sader (Department of Oral Maxillofacial and Plastic Facial Surgery), F. Louwen, S. Becker (Department of Gynecology and Obstetrics). Protestant Deaconess Hospital Freiburg: J. Ernst, Joachim Sauter, C. Wiesenack (Department of Anaesthesiology, Protestant Deaconess Hospital Freiburg, Freiburg, Germany), M. Gerber (Department of Gynecology and Obstetrics, Protestant Deaconess Hospital Freiburg, Freiburg, Germany). Agatharied Hospital Hausham: A. Bayer (Department of Anaesthesiology and Intensive Care Medicine). SLK-Kliniken Heilbronn: H. Weigt (Department of Anaesthesiology). University Hospital Jena: A. Raadts (Department of Anaesthesiology and Intensive Care Medicine). University Hospital Schleswig-Holstein Kiel: J. Duemmler, M. Gruenewald, Lars Hummitzsch, U. Lorenzen, J. Renner, M. Sokirjanski, M. Steinfath (Department of Anaesthesiology and Intensive Care), M. Pagel (Information-Technology UKSH), A. Haneya, T. Puehler (Department of Cardiovascular Surgery), R. Berndt, R. Rusch (Department of Vascular Surgery), T. Becker, J. Pochhammer (Department of General and Visceral Surgery), T. Klueter, A. Seekamp (Department of Trauma, Hand and Reconstructive Surgery), H. Ahmeti, A. Helmers (Department of Neurosurgery), Daniar Osmonow (Department of Urology), Dirk Bauerschlag (Department of Gynecology and Obstetrics), Henning Wieker (Department of Oral Maxillofacial and Plastic Facial Surgery), Markus Hoffmann (Department of Otorhinolaryngology). Klinikum Leverkusen: J. Friedrich, Gerd Molter (Department of Anaesthesiology and Intensive Care Medicine). Marienhaus Klinikum St. Wendel/Ottweiler: M. Bier, M. Gutjahr (Department of Anaesthesiology and Intensive Care Medicine). Muenchen Klinik Bogenhausen: R. Bauer (Department of Clinical Chemistry and Transfusion Medicine), B. Bräutigam (Business Unit Hospital Controlling), P. Friederich (Department of Anaesthesiology, Critical Care Medicine, Pain Therapy). University Hospital Muenster: H. K Van Aken, A. Boanta, J. Becker, M. Bomert, A. Bueckmann, K. Boerner, A. Ceanga, M. Nunez Cortés, I. Duran, T. Fraune, R. Gallaby, D. Goncalves, M.l Heßler, M. Hawari, D. J. Jenke, K. Kieserling, J. Korhonen, J. Krycki, F. Lehmann, T. Maeßen, V. Mocanu, P.k Naber, S.n Opas, F.Peters, M. Rauer, H. Rieder, R. Van Ohlen, B. Schilling, D. Scholle, J. Schulz, D.Schwellenbach, A.U. Steinbicker, I. Thoene, A. Ueding, G. Varelas, L. Warnken, C. Wempe, I. Wisudanto, A. Zarbock, N. Zurheiden (Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy), W. Stummer, B. Brokinkel (Department of Neurosurgery), S. Martens (Department of Thoracic and Cardiovascular Surgery), N. Senniger, H. Wolters, A. Pascher (Department of General-, Visceral and Transplant Surgery), G. Gosheger, B. Moellenbeck, N. Deventer (Department of General Orthopaedics and Cancer Orthopaedics), A. Oberhuber, B. Kasprzak (Clinic for Vascular and Endovascular Surgery), E. Herrmann, A. Schrader (Clinic for Urology and Pediatric Urology), L. Kiesel (Department of Gynecology and Obstetrics), J. Kleinheinz (Department for Maxillo-, Oral and Facial Surgery), C. Koesters, M. J. Raschke (Department of Traumatology, Hand and Plastic Surgery), T. Latal, D. Niehoff (Department of Medical Controlling), R. G. Geissler, H. Hillmann (Institute of Transfusion Medicine), M. Stelljes, A. Kerkhoff, W. Berdel, G. Lenz (Medical Clinic A, Haematology, Pneumology, Haemostaseology and Oncology). Diakonie Hospital Nuremberg: K. Schwendner (Department of Anaesthesiology and Operative Intensive Care Medicine). Ortenau Klinikum Offenburg-Kehl: J. Thoma (Department of Anaesthesiology and Operative Intensive Care). University Hospital Wuerzburg: P. Helmer, S. Hottenrott, P. Kranke, P. Meybohm, D. Roeder, T. Schlesinger, M. Sitter, J. Stumpner (Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine). Klinikum Mittelmosel Zell: P. Stark (Department of Visceral and Vascular Surgery). German PBM Network Collaborators are listed in the Supplement.
Supplementary Material
Contributor Information
Lea Valeska Blum, Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany.
Elke Schmitt, Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany; Department of Medicine, Institute of Biostatistics and Mathematical Modelling, Goethe University, Frankfurt, Germany.
Suma Choorapoikayil, Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany.
Olaf Baumhove, Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, Klinikum Westmuensterland, Bocholt, Germany.
Alexandra Bayer, Department of Anaesthesiology and Intensive Care Medicine, Agatharied Hospital, Hausham, Germany.
Patrick Friederich, Department of Anaesthesiology, Operative Intensive Care Medicine and Pain Therapy, Munich, Germany.
Jens Friedrich, Department of Anaesthesiology and Intensive Care Medicine, Klinikum Leverkusen, Leverkusen, Germany.
Christof Geisen, German Red Cross, Institute for Transfusion Medicine and Immunohaematology, German Red Cross Baden-Wuertemberg–Hessen, Goethe University Frankfurt, Frankfurt am Main, Germany.
Matthias Gruenewald, Department of Anaesthesiology and Operative Intensive Care Medicine, University Hospital Schleswig-Holstein, Kiel, Germany.
Martin Gutjahr, Department of Anaesthesiology, Marienhaus, Ottweiler, Germany.
Eva Herrmann, Department of Medicine, Institute of Biostatistics and Mathematical Modelling, Goethe University, Frankfurt, Germany.
Markus Müller, German Red Cross, Institute for Transfusion Medicine and Immunohaematology, German Red Cross Baden-Wuertemberg–Hessen, Goethe University Frankfurt, Frankfurt am Main, Germany.
Diana Narita, Institute for Laboratory Diagnostics and Transfusion Medicine, Donauisarklinikum, Deggendorf, Germany.
Ansgar Raadts, Department of Anaesthesiology and Intensive Care Medicine, University Hospital Jena, Jena, Germany.
Klaus Schwendner, Department of Anaesthesiology and Operative Intensive Care Medicine, Diakonie Hospital Martha-Maria, Nuremberg, Germany.
Erhard Seifried, German Red Cross, Institute for Transfusion Medicine and Immunohaematology, German Red Cross Baden-Wuertemberg–Hessen, Goethe University Frankfurt, Frankfurt am Main, Germany.
Patrick Stark, Department of Visceral and Vascular Surgery, Klinikum Mittelmosel, Zell, Germany.
Josef Thoma, Department of Anaesthesiology and Operative Intensive Care Medicine, Ortenauklinikum, Gengenbach, Germany.
Henry Weigt, Department of Anaesthesiology, SLK-Kliniken, Heilbronn, Germany.
Christoph Wiesenack, Department of Anaesthesiology, Evangelisches Diakoniekrankenhaus Freiburg, Freiburg, Germany.
Andrea Ulrike Steinbicker, Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany; Department of Anaesthesiology, Intensive Care and Pain Medicine, University Hospital Muenster, Muenster, Germany.
Kai Zacharowski, Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany.
Patrick Meybohm, Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany; Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg, Wuerzburg, Germany.
German PBM Network Collaborators:
O Baumhove, S de Leeuw van Weenen, D Narita, J M Huber, E Adam, S Choorapoikayil, L Hof, S Isik, M Krämer, H Neb, V Neef, F Piekarski, E Schmitt, K Zacharowski, T Walther, T Holubec, A Schnitzbauer, W O Bechstein, W Derwich, T Schmitz-Rixen, B Steffen, H Serve, J Bojunga, S Zeuzem, J Konczalla, V Seifert, F Roos, F Chun, C Nau, I Marzi, M Leinung, T Stöver, S Ghanaati, R Sader, F Louwen, S Becker, J Ernst, Joachim Sauter, C Wiesenack, M Gerber, A Bayer, H Weigt, A Raadts, J Duemmler, M Gruenewald, Lars Hummitzsch, U Lorenzen, J Renner, M Sokirjanski, M Steinfath, M Pagel, A Haneya, T Puehler, R Berndt, R Rusch, T Becker, J Pochhammer, T Klueter, A Seekamp, H Ahmeti, A Helmers, Daniar Osmonow, Dirk Bauerschlag, Henning Wieker, Markus Hoffmann, J Friedrich, Gerd Molter, M Bier, M Gutjahr, R Bauer, B Bräutigam, P Friederich, H K Van Aken, A Boanta, J Becker, M Bomert, A Bueckmann, K Boerner, A Ceanga, M Nunez Cortés, I Duran, T Fraune, R Gallaby, D Goncalves, Ml Heßler, M Hawari, D J Jenke, K Kieserling, J Korhonen, J Krycki, F Lehmann, T Maeßen, V Mocanu, Pk Naber, Sn Opas, F Peters, M Rauer, H Rieder, R Van Ohlen, B Schilling, D Scholle, J Schulz, D Schwellenbach, A U Steinbicker, I Thoene, A Ueding, G Varelas, L Warnken, C Wempe, I Wisudanto, A Zarbock, N Zurheiden, W Stummer, B Brokinkel, S Martens, N Senniger, H Wolters, A Pascher, G Gosheger, B Moellenbeck, N Deventer, A Oberhuber, B Kasprzak, E Herrmann, A Schrader, L Kiesel, J Kleinheinz, C Koesters, M J Raschke, T Latal, D Niehoff, R G Geissler, H Hillmann, M Stelljes, A Kerkhoff, W Berdel, G Lenz, K Schwendner, J Thoma, P Helmer, S Hottenrott, P Kranke, P Meybohm, D Roeder, T Schlesinger, M Sitter, J Stumpner, and P Stark
Funding
The authors have no funding to declare.
Acknowledgements
The authors thank C. Füllenbach for his support to the study. We would like to thank the IT staff of every participating hospital for their support in this project by providing the necessary data. Conception was carried out by P.M., L.B., E.S., and K.Z. Writing of the manuscript was conducted by L.B., E.S., A.U.S., P.M., and K.Z. Data acquisition was the responsibility of E.S., K.Z., P.M., O.B., A.B., P.F., J.F., M. Gruenewald, M Gutjahr, D.N., A.R., K.S., P.S., A.U.S., J.T., H.W., and C.W. Data analysis and statistical interpretation was carried out by E.S. Medical interpretation was the responsibility of L.B., A.U.S., P.M., and K.Z. Revision of the manuscript was carried out by all authors. L.V.B. and E.S. contributed equally.
Disclosure
K.Z.’s department received grants from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of the German PBM programme. P.M. and K.Z. received honoraria for scientific lectures from B. Braun Melsungen, Vifor Pharma, Ferring, CSL Behring, and Pharmacosmos. M. Gruenewald received honoraria for scientific lectures from Vifor Pharma, Ferring, and CSL Behring. All other authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data set generated during and/or analysed during the present study are available from the corresponding author on reasonable request.
References
- 1. Earl-Royal E, Kaufman EJ, Hsu JY, Wiebe DJ, Reilly PM, Holena DN. Age and preexisting conditions as risk factors for severe adverse events and failure to rescue after injury. J Surg Res 2016;205:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nemunaitis G, Roach MJ, Claridge J, Mejia M. Early predictors of functional outcome after trauma. PM&R 2016;8:314–320 [DOI] [PubMed] [Google Scholar]
- 3. Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26:1091–1101 [DOI] [PubMed] [Google Scholar]
- 4. Sepehri A, Beggs T, Hassan A, et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg 2014;148:3110–3117 [DOI] [PubMed] [Google Scholar]
- 5. Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg 2003;96:583–589 [DOI] [PubMed] [Google Scholar]
- 6. Steinbicker AU, Muckenthaler MU. Out of balance—systemic iron homeostasis in iron-related disorders. Nutrients 2013;5:3034–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood 2018;131:505–514 [DOI] [PubMed] [Google Scholar]
- 8. Baron DM, Hochrieser H, Posch M, et al. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 2014;113:416–423 [DOI] [PubMed] [Google Scholar]
- 9. Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion 2007;47:1468–1480 [DOI] [PubMed] [Google Scholar]
- 10. Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011;378:1396–1407 [DOI] [PubMed] [Google Scholar]
- 11. Whitlock EL, Kim H, Auerbach AD. Harms associated with single unit perioperative transfusion: retrospective population-based analysis. BMJ 2015;350:h3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meybohm P, Fischer DP, Geisen C, et al. Safety and effectiveness of a patient blood management (PBM) program in surgical patients–the study design for a multi-centre prospective epidemiologic non-inferiority trial. BMC Health Serv Res 2014;14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bundesärztekammer (BÄK) . Guidelines for Therapy with Blood Components and Plasma Derivatives in Germany 2020. Available from: https://www.bundesaerztekammer.de/fileadmin/user_upload/_old-files/downloads/pdf-Ordner/MuE/Querschnitts-Leitlinien_BAEK_zur_Therapie_mit_Blutkomponenten_und_Plasmaderivaten-Gesamtnovelle_2020.pdf (accessed 05 March 2021)
- 14. BÄK . Guidelines for Therapy with Blood Components and Plasma Derivatives in Germany 2014. Available from: https://www.bundesaerztekammer.de/fileadmin/user_upload/_old-files/downloads/QLL_Haemotherapie_2014.pdf (accessed 05 March 2021)
- 15. Loor G, Rajeswaran J, Li L, et al. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg 2013;146:1480–1487 [DOI] [PubMed] [Google Scholar]
- 16. Gurusamy KS, Davidson C, Gluud C, et al. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev. 2013;(6):CD005440. 10.1002/14651858.CD005440.pub3 [DOI] [PubMed] [Google Scholar]
- 17. Hanna DN, Gamboa AC, Balch GC, et al. Perioperative blood transfusions are associated with worse overall survival but not disease-free survival after curative rectal cancer resection: a propensity score-matched analysis. Dis Colon Rectum 2021;64:946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 2012;256:235–244 [DOI] [PubMed] [Google Scholar]
- 19. Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg 2015;13:102–110 [DOI] [PubMed] [Google Scholar]
- 20. Volz Y, Eismann L, Pfitzinger PL, et al. Prognostic impact of perioperative blood transfusions on oncological outcomes of patients with bladder cancer undergoing radical cystectomy: a systematic review. Arab J Urol 2021;19:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011–1023 [DOI] [PubMed] [Google Scholar]
- 22. Alvarez-Payares JC, Rivera-Arismendy S, Ruiz-Bravo P, et al. Unexplained anemia in the elderly. Cureus 2021;13:e19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thongprayoon C, Hansrivijit P, Kovvuru K, et al. Diagnostics, risk factors, treatment and outcomes of acute kidney injury in a new paradigm. J Clin Med. 2020;9(4):1104. 10.3390/jcm9041104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int 2015;87:46–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caterino JM, Valasek T, Werman HA. Identification of an age cutoff for increased mortality in patients with elderly trauma. Am J Emerg Med 2010;28:151–158 [DOI] [PubMed] [Google Scholar]
- 26. Milzman DP, Boulanger BR, Rodriguez A, Soderstrom CA, Mitchell KA, Magnant CM. Pre-existing disease in trauma patients: a predictor of fate independent of age and injury severity score. J Trauma 1992;32:236–243 [PubMed] [Google Scholar]
- 27. Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017;72:233–247 [DOI] [PubMed] [Google Scholar]
- 28. Blaudszun G, Munting KE, Butchart A, Gerrard C, Klein AA. The association between borderline pre-operative anaemia in women and outcomes after cardiac surgery: a cohort study. Anaesthesia 2018;73:572–578 [DOI] [PubMed] [Google Scholar]
- 29. Miles LF, Larsen T, Bailey MJ, Burbury KL, Story DA, Bellomo R. Borderline anaemia and postoperative outcome in women undergoing major abdominal surgery: a retrospective cohort study. Anaesthesia 2020;75:210–217 [DOI] [PubMed] [Google Scholar]
- 30. Dugan C, MacLean B, Cabolis Ket al. The misogyny of iron deficiency. Anaesthesia 2021;76:56–62 [DOI] [PubMed] [Google Scholar]
- 31. Statistisches Bundesamt (Destatis) S . Todesursachen nach Altersgruppen und Geschlecht, 2019; https://www-genesis.destatis.de/genesis/online?sequenz=tabelleErgebnis&selectionname=23211-0004#abreadcrumb (accessed 01 February 2021)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set generated during and/or analysed during the present study are available from the corresponding author on reasonable request.