Abstract
Two genes encoding 97- to 99-kDa Chlamydia pneumoniae VR1310 outer membrane proteins (Omp4 and Omp5) with mutual similarity were cloned and sequenced. The proteins were shown to be constituents of the C. pneumoniae outer membrane complex, and the deduced amino acid sequences were similar to those of putative outer membrane proteins encoded by the Chlamydia psittaci and Chlamydia trachomatis gene families. By use of a monospecific polyclonal antibody against purified recombinant Omp4, it was shown that without heating, the protein migrated at 65 to 75 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoelectron microscopy showed that epitopes of Omp4 were exposed on the surface of C. pneumoniae elementary bodies, reticulate bodies, and outer membrane complex. Proteins encoded by the C. pneumoniae gene family seem to be dominant antigens in experimentally infected mice.
Chlamydia pneumoniae is a widespread pathogen of the upper respiratory tract. About 10% of cases of community-acquired pneumonia have been associated with C. pneumoniae, but asymptomatic or mildly symptomatic infections are also common results of current infection. Other illnesses associated with this bacterium are bronchitis, pharyngitis, sinusitis, otitis media, and fever of undetermined origin (15, 37).
Saikku et al. (36) were the first to report that chronic C. pneumoniae infection may be associated with cardiovascular diseases. By using the microimmunofluorescence test, they showed that sera from patients with acute myocardial infarction or chronic coronary heart disease contained higher levels of antibodies to C. pneumoniae TWAR than did sera from control patients (36). Other studies have shown that C. pneumoniae may be associated with disease of the coronary or carotid arteries (16, 23, 35).
The only well-characterized surface exposed component of C. pneumoniae is the genus-specific lipopolysaccharide epitope (9). Several studies have been performed to identify surface exposed and immunogenic proteins. Species-specific, immunogenic proteins of 98, 53, 46, and 43 kDa have been described (8, 20, 21), and immunoelectron microscopy studies have shown that the 53-kDa protein is located on the surface (31). However, none of these proteins are good markers for C. pneumoniae infection, because their recognition varies among patient serum samples (24). Species-specific monoclonal antibodies (MAbs) that react with the surface of C. pneumoniae elementary bodies (EBs) and with the outer membrane complex (OMC) in immunoelectron microscopy have been generated, but attempts to characterize the antigenic determinant by immunoblotting have been unsuccessful (9, 34).
Sarkosyl treatment of C. trachomatis EBs leaves a Sarkosyl-insoluble fraction named Chlamydia outer membrane complex (COMC) (6) in which the major outer membrane protein (MOMP) of 38 to 42 kDa is the dominant protein (18, 38). Moreover, COMC contains the cysteine-rich outer membrane protein 2 (Omp2) doublet of 60 to 62 kDa and the cysteine-rich outer membrane protein 3 (Omp3) of 12.5 to 15.5 kDa (1, 10). In addition to MOMP, Omp2, and Omp3, the protein profiles of C. pneumoniae and C. psittaci OMC contain proteins of approximately 98 kDa which are not seen in C. trachomatis OMC (8, 28, 30).
In the C. psittaci ovine abortion strain, a 98-kDa OMP migrated at 66 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) without heating (28). In later studies of the same strain, Longbottom et al. (27) identified five genes, named OMP90 gene family, encoding homologous OMPs of 90 to 98 kDa. Immunoblotting with postabortion sheep sera showed that these proteins, and especially the amino-terminal ends, were major immunogens (27). In addition, the protein family could be located to the surface of both EBs and reticulate bodies (RBs) by immunoelectron microscopy (26). This is in contrast to results obtained by Buendia et al. (5) which showed that a group of 80- to 90-kDa proteins from C. psittaci serotype 1 AB7 was located on the surface of RBs but not EBs.
The aim of this study was to identify the genes encoding the 97- to 99-kDa proteins present in the C. pneumoniae VR1310 OMC, to analyze the localization and antigenicity of the proteins, and to compare the gene sequences with those of the gene family from C. psittaci encoding the group of 90- to 98-kDa proteins.
MATERIALS AND METHODS
C. pneumoniae strain and cultivation conditions.
C. pneumoniae (CDC/CWL-029/VR-1310), purchased from the American Type Culture Collection (Rockville, Md.), was cultivated for 72 h in HeLa 229 cells (American Type Culture Collection) as described previously (11).
Enzymes and primers.
The restriction enzymes and enzymes used for PCR and cloning were purchased from Boehringer GmbH (Mannheim, Germany). DNase I (grade II; bovine pancreas), and RNase was obtained from Worthington Biochemical Corp. (Freehold, N.J.). DNA polymerase I was obtained from Gibco BRL Life Technologies (Gaithersburg, Md.), and Benzoase was obtained from Sigma (St. Louis, Mo.). Primers used for sequencing and PCR were purchased from DNA technology (Aarhus, Denmark).
Purification of C. pneumoniae.
EBs were purified from HeLa cells grown in 16 six-well plates (Nunc, Roskilde, Denmark). The infected HeLa cells were disrupted by sonication for 30 s. HeLa cell debris was removed by centrifugation (4 min at 200 × g). The supernatant was then ultracentrifuged for 1 h at 100,000 × g through a layer of 30% Urografin (Schering-Plough Corp., Madison, N.J.) and a layer of 50% sucrose. The pellet was dissolved in HEPES buffer (10 mM HEPES, 150 mM NaCl [pH 7.2]), sonicated briefly, and digested with 50 μg of RNase per ml and 40 μg of DNase per ml. The suspension was ultracentrifuged for 1 h at 200,000 × g through a discontinuous gradient consisting of 34, 40, 46, and 52% Urografin (Schering-Plough). Upon centrifugation, the three layers (an EB layer, an intermediate layer, and an RB layer) were transferred to separate vials, diluted in HEPES buffer, and ultracentrifuged for 30 min at 200,000 × g. Finally, supernatant was discharged, and each pellet was resuspended in HEPES buffer and stored at −70°C.
Purification of the C. pneumoniae OMC.
EBs were dissolved in phosphate-buffered saline (PBS) containing 2% sodium N-lauroylsarcosine (Sarkosyl) and 5 mM EDTA, sonicated briefly, and incubated for 30 min at 37°C. Insoluble material was pelleted by centrifugation. The pellet was dissolved in PBS containing 2% Sarkosyl and 5 mM MgCl2, sonicated briefly, and digested with Benzoase and RNase for 30 min at 37°C. Insoluble material was pelleted, washed twice in PBS, and resuspended in PBS.
SDS-PAGE, immunoblotting, and silver staining.
The concentrations of C. pneumoniae EB and OMC were estimated from a silver-stained SDS-PAGE gel, and about 1 μg was applied per lane. The concentration of recombinant Omp4 was measured with the bicinchoninic acid protein assay reagent kit (Pierce, Rockford, Ill.) as specified by the manufacturer. Approximately 2 μg was applied per lane.
C. pneumoniae EB and OMC solubilized in SDS sample buffer (62.5 mM Tris-HCl [pH 6.8], 2.3% [wt/vol] SDS, 10% [wt/vol] glycerol, 5% [wt/vol] β-mercaptoethanol, 0.05% [wt/vol] bromphenol blue) were either heated to 100°C for 5 min or incubated at room temperature for 15 min, separated by SDS-PAGE (25), and electrophoretically transferred to nitrocellulose membranes. Electrotransfer and immunodetection were carried out by a protein-blotting procedure (Bio-Rad, Richmond, Calif.).
MAb 24.1.44 was generated against purified C. pneumoniae EBs and shown by immunoelectron microscopy to react with the C. pneumoniae surface (9). For immunoblotting, MAb 24.1.44 was diluted 1:3 (tissue culture supernatant) and the polyclonal antisera were diluted 1:200 in antibody buffer (20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, 0.2% gelatin [pH 7.5]). Bound polyclonal antibodies were detected with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Bio-Rad). Bound MAb 24.1.44 was detected with alkaline phosphatase-conjugated rabbit anti-mouse IgG (Bio-Rad). The blots were stained with 5-bromo-4-chloro-3-indolyl phosphate–nitroblue tetrazolium (BCIP-NBT) color development solution (Bio-Rad).
Silver staining of the proteins separated by SDS-PAGE was performed by the method of Sambrook et al. (39). Negative Cu2+ staining of the polyacrylamide gel, excision of the band, and extraction of proteins were done as described previously (28).
Production of a polyclonal rabbit antiserum against C. pneumoniae OMC.
For each immunization, 10 μg of antigen was incubated for 15 min with 30 μl of SDS sample buffer, diluted in PBS, and emulsified in Freund’s incomplete adjuvant (Difco Laboratories, Detroit, Mich.). A New Zealand White rabbit was immunized intramuscularly on days 1 and 7 with SDS-treated antigen. On day 20 and 27, it was immunized intravenously with antigen in PBS. The rabbit was bled on day 69.
Cloning of C. pneumoniae DNA, production of libraries, and screening.
Genomic DNA was extracted from C. pneumoniae (29), centrifuged on a CsCl gradient, and dialyzed against TE buffer (39). For the expression library, the DNA was partially digested with Sau3AI and cloned in pEX1, pEX2, and pEX3 vectors (40), and the expressed peptides were screened by colony blotting with the polyclonal antiserum to denatured C. pneumoniae OMC as previously described (3).
For the production of a genomic library, 1 μg of genomic C. pneumoniae DNA was completely digested with 10 U of BamHI and cloned in the pBluescript vector (Stratagene, La Jolla, Calif.). Recombinant plasmids were electrotransformed into competent E. coli XL1-blue cells, and the colonies were blotted onto nitrocellulose (39). A 410-bp fragment (which covers bp 568 to 978 of omp5) was amplified by PCR on genomic C. pneumoniae DNA with two primers 5′-GGGAATAAATCGAGCGC-3′ and 5′-CAGCTGCCAGTATAGAAATGG-3′ and used for production of a radioactive probe ([α-32P]dATP was obtained from Amersham International, Little Chalfont, United Kingdom). The genomic library was screened as described previously (39).
The plasmid DNA was extracted from the recombinant E. coli by the alkali lysis method (39), except that an additional step of lysozyme digestion was included. When the plasmid DNA was used for automatic sequencing, the step involving phenol extraction was omitted.
DNA sequencing and sequence analysis.
Template DNA (0.5 μg) was mixed with primer and Thermo Sequenase (Amersham, Cleveland, Ohio) and thermocycled in a DNA thermal cycler as described by the manufacturer (Gene-Amp PCR system 9600; Perkin-Elmer, Warrington, United Kingdom). Sequencing was performed in a ABI PRISM Dye Terminator apparatus (Perkin Elmer). The sequences were analyzed with the programs in the Wisconsin Package, version 8.1 (Genetics Computer Group [GCG], Madison, Wis.).
High-fidelity PCR.
By use of the high-fidelity PCR kit (Boehringer GmbH), a fragment containing the 3′ end of the omp5 gene was amplified on genomic C. pneumoniae DNA with the primers 5′-GGCAGTCACTACGCCTTCTCTCCTATGTTTGAAGTGC-3′ (located at the 5′ end of the B8F3 fragment) and 5′-GCCTCCGAAGACAATATAAGGTACCGTCATAACAGCGG-3′ (located in an expression clone [pEX3-29] that encodes a peptide with similarity to Omp4 and Omp5). A fragment comprising the omp4 and omp5 genes was amplified on genomic DNA with the primers 5′-GCACTTCAAACATAGGAGAGAAGGCGTAGTGACTGC-3′ and 5′-CCCTCAGTAAAAATGCCTGTCTTGAAAGATTGCCACCG-3′.
Purification of inclusion bodies and production of mouse polyclonal antibodies.
Fusion proteins from the pEX clones were expressed in E. coli, and the recombinant C. pneumoniae proteins were extracted as described previously (17). For immunization of BALB/c mice, the proteins were dissolved in PBS and emulsified in Freund’s incomplete adjuvant (Difco). Immunization was performed intramuscularly on days 1, 15, 22, and 35 with 50 μg of protein each time. The mice were bled on day 85.
Production of a hyperimmune antiserum against recombinant Omp4.
The omp4 gene was amplified by PCR with the primers 5′-GACGACGACAAGATGAAGACTTCGATTCCTTGGGTTTTAGTTTCC-3′ and 5′-GAGGAGAAGCCCGGTTAGAATCGGAGTTTGGTACCAACATCTACATTG-3′, which contained LIC sites, and the PCR products were cloned into the pET-30 LIC vector (Novagen, Madison, Wis.) as described by the manufacturer. The histidine-tagged fusion protein was expressed and purified, and a New Zealand White rabbit was immunized six times intramuscularly (with the antigen dissolved in PBS and emulsified in Freund’s incomplete adjuvant) and three times intravenously (with the antigen dissolved in PBS) with 8 μg of protein each time. The rabbit was bled on day 60.
IMF.
HeLa cell monolayers were infected with 0.5 inclusion-forming unit of C. pneumoniae per HeLa cell and cultivated for 72 h. Indirect immunofluorescence microscopy (IMF) was performed as described previously (11), except that when the cells were incubated with the mouse polyclonal antibodies (PAbs), 20% fetal calf serum (Gibco) in PBS was used as the antibody buffer. Mouse PAbs were used at 1:100, and MAbs (tissue culture supernatant) were used at 1:10.
Immunoelectron microscopy.
A 10-μl drop of purified C. pneumoniae EB, RB, or OMC suspension was placed for 1 min on top of a 400-mesh nickel grid covered with freshly glow-discharged carbon film. After being washed in 3 drops of PBS, the grid was incubated for 5 min at room temperature with PBS containing 0.5% ovalbumin, transferred to a drop containing the MAb, and incubated for 30 min at 37°C. MAb 24.1.44 (tissue culture supernatant) was diluted 1:5 in PBS–0.5% ovalbumin. As the secondary antibody, 10-nm-colloidal-gold-labeled rabbit anti-mouse Ig (British Biocell, Cardiff, United Kingdom) diluted 1:20 in PBS with 1% cold fish gelatin was used. The grids were stained with 2 drops of 0.3% phosphotungstic acid (pH 7) and blotted dry. Electron microscopy was done with a JEOL 1010 transmission electron microscope at 40 kW.
Experimental infection of C57 black mice.
Four C57 black mice were inoculated intranasally with 107 inclusion-forming units of C. pneumoniae under a light ether anaesthesia (32). After 14 days of infection, the serum samples were obtained and used for immunoblotting (1:100).
Nucleotide sequence accession numbers.
The nucleotide and amino acid sequences of C. pneumoniae omp4 and omp5 have been submitted to the GenEMBL database and assigned accession no. AJ001311.
RESULTS
Protein profile and antigenicity of the C. pneumoniae OMC.
In addition to the proteins present in the C. trachomatis OMC, proteins of 98 kDa have been found in the C. pneumoniae OMC (8, 30). To obtain antibodies reacting with these proteins, purified C. pneumoniae OMC was denatured with SDS and used for immunization of a rabbit. Comparison of the protein profiles of C. pneumoniae EBs and OMC is shown in Fig. 1A. As expected, bands corresponding in size to MOMP (39.5 kDa) and Omp2 (60 to 62 kDa) were seen in C. pneumoniae OMC (Omp3 was not detected because of its low molecular mass). In addition, a band of 97 to 99 kDa was detected (lane 2). By immunoblotting of EBs, it was shown that the polyclonal antiserum generated against C. pneumoniae OMC recognized bands corresponding in size to MOMP, Omp2, and the 97- to 99-kDa proteins (Fig. 1B). A band of 75 kDa, which probably represented DnaK (3), was also recognized.
FIG. 1.
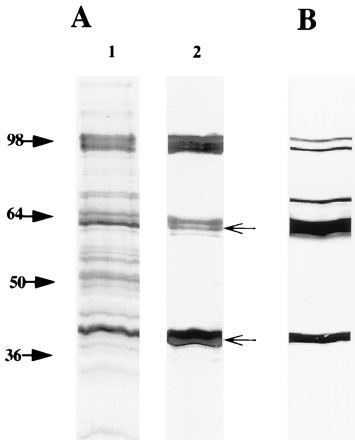
(A) Silver-stained SDS–10% polyacrylamide gel of C. pneumoniae EB (lane 1) and OMC (lane 2). The samples were boiled in SDS sample buffer. (B) Immunoblotting of C. pneumoniae EBs reacted with the polyclonal rabbit antiserum to C. pneumoniae OMC. The arrows indicate the migration of Omp2 (60 to 62 kDa) and MOMP (39.5 kDa).
Detection of recombinant clones.
Since the polyclonal antiserum to C. pneumoniae OMC recognized the 97- to 99-kDa proteins, it was used to screen an expression library. A total of 49 immunoreactive clones were obtained and sequenced from the 5′ end of the cloned insert. By using the FASTA program from the GCG program package, 10 clones were shown to contain inserts of the well-known C. pneumoniae omp genes (momp, omp2, and omp3) and 7 clones were shown to represent the dnaK gene. The generation of DnaK antibodies is probably due to the peptide binding ability of this protein (4), whereby it was copurified in the outer membrane preparation. The other 32 clones had an insert without homology to these genes. These inserts were further sequenced until a stop codon was found in frame or the entire sequence of the insert was obtained.
Identification of two novel C. pneumoniae genes.
To obtain the complete open reading frames encoding the proteins represented by the expression clones, a genomic C. pneumoniae BamHI library of 1,000 clones was produced and screened by colony hybridization with a radioactive probe representing a 410-bp fragment of the pEX1-1 clone (Fig. 2). A BamHI fragment of 4.5 kbp (B8F3) was obtained. Sequencing of this fragment showed that it overlapped with three additional pEX clones (pEX3-36, pEX2-10, and pEX2-18). The complete sequence of the pEX2-18 clone and the B8F3 clone comprised 6.5 kbp that contained two open reading frames (Fig. 2). These two genes were named omp4 and omp5, since they most probably encoded OMC-associated proteins of C. pneumoniae. A potential stem-loop structure was detected 7 bp downstream of omp4. Due to the presence of a BamHI site in the omp5 sequence, the 3′ end of this gene was not contained within the B8F3 fragment (Fig. 2). By performing PCR with primers obtained from the sequences of the expression clones, the 3′ end of the omp5 gene was determined. Computer analyses did not predict any stem-loop or termination structures downstream of omp5.
FIG. 2.
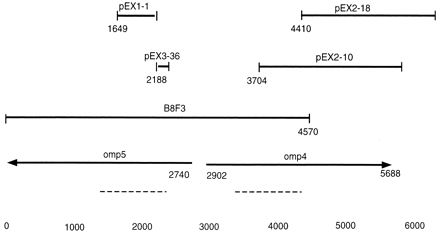
Diagram of the overlapping pEX clones and pBluescript clones. The two open reading frames that were named omp4 and omp5 are shown (the arrows indicate the direction of transcription). Dotted lines show the regions where the nucleotides encoding the repeated amino acid motifs (GGAI) were found. Base numbers are designated at the bottom.
Use of PCR on genomic C. pneumoniae DNA with primers that annealed to the 3′ ends of omp4 and omp5 confirmed that the two genes are adjacent on the C. pneumoniae genome (results not shown).
Sequence analysis of the two OMPs Omp4 and Omp5.
The two identified open reading frames, each comprising 2,784 bp, encoded two proteins, named Omp4 and Omp5, with calculated molecular masses of 98.9 and 97.2 kDa, respectively. As illustrated (Fig. 2), omp4 and omp5 were transcribed in opposite directions. Potential promoter elements were identified in the intergenic region between omp4 and omp5 (19). Potential −10 elements were identified for both genes, but a potential −35 element was identified only for omp5. In addition, putative ribosomal binding sites were present in both genes. The amino-terminal parts of Omp4 and Omp5 were relatively hydrophobic, and signal peptidase II cleavage sites were predicted in both proteins, which indicated lipid modification (Fig. 3).
FIG. 3.

Multiple alignment of C. pneumoniae Omp4 and Omp5 and C. psittaci Pomp98 (27). Amino acid sequence alignment was done with the PILEUP program (GCG 8.1 package). Dots represent gaps, the black areas indicate amino acid identity, and the grey areas indicate amino acid similarity. The repeated amino acid motifs are boxed. The arrow shows the putative signal peptidase II cleavage sites in C. pneumoniae Omp4 and Omp5. The beginnings and ends of peptides expressed from the pEX clones are indicated by boxes (pEX3-36), asterisks (pEX1-1), and triangles (pEX2-10), respectively.
The sequences of omp4 and omp5 and the derived amino acid sequences were compared. The percentage of identical nucleotides between the two genes was 53%, and the amino acid similarity was 61%. In addition, the amino acid motif glycine-glycine-alanine-isoleucine (GGAI) was repeated six times in Omp4. Omp5 contained these repeats as well, except that in one of the repeats the isoleucine was replaced by a leucine (Fig. 3). Both Omp4 and Omp5 contained cysteine (8 and 16 residues, respectively).
Further alignment showed that two additional pEX clones encoded peptides that were similar but not identical to the Omp4 and Omp5 sequences. PCR performed on genomic C. pneumoniae DNA with primers located in each of the two additional pEX clones revealed that they did not represent two different regions of the same gene (results not shown). Thus, a family of at least four genes encoding homologous OMPs exists in C. pneumoniae.
Database search in the GenEMBL database showed that Omp4 and Omp5 were similar to the OMP90 (formerly POMP) family of five 90- to 98-kDa OMPs from C. psittaci (27). A DNA alignment between omp4 and omp5 from C. pneumoniae and the pomp genes from C. psittaci showed that there were 49 to 54% identical nucleotides. Moreover, the similarity between one of the C. psittaci proteins (Pomp98) and C. pneumoniae Omp5 was 63%, which is even higher than the similarity between Omp4 and Omp5. The repeated amino acid motif GGAI was conserved in Pomp98, except that in one of the repeats an isoleucine was replaced by a valine (Fig. 3).
A similar gene family encoding nine putative membrane proteins (PmpA to PmpI) of 70 to 200 kDa has also been identified in C. trachomatis (41). Although the repeated amino acid motif (GGAI) was found in these proteins, the overall similarity between the C. pneumoniae and the C. trachomatis membrane proteins was low.
Surface localization of Omp4 and Omp5.
To determine the localization of Omp4 and Omp5 in C. pneumoniae, polyclonal mouse antisera to purified recombinant proteins from the pEX clones representing fragments of Omp4 and Omp5 were generated. The reaction of the mouse PAbs was investigated by IMF of infected HeLa cells fixed with formaldehyde 72 h after infection. Antibodies to the peptides expressed by pEX2-10 (which covers amino acids 271 to 914 of Omp4 [Fig. 3]) and pEX3-36 (which covers amino acids 149 to 184 of Omp5 [Fig. 3]) reacted strongly in IMF with the surface of C. pneumoniae, as shown by the presence of fluorescent chlamydial inclusions (Fig. 4A and B). It is generally assumed that fixation of the cells with formaldehyde makes the chlamydiae impermeable to antibodies due to cross-linking of the OMPs. To verify this, MAb 18.1, which reacts with C. trachomatis and C. pneumoniae DnaK in immunoblotting (4), was also used in IMF (Fig. 4C and D). The epitope of MAb 18.1 was shown to be formaldehyde insensitive (results not shown). It reacted strongly with C. pneumoniae when the cells were methanol fixed and thus permeabilized (Fig. 4C) but not when the cells were fixed with formaldehyde (Fig. 4D). No reaction was seen when the infected cells were incubated with normal mouse serum (Fig. 4E). Thus, IMF showed that epitopes of Omp4 and Omp5 were exposed on the surface of formaldehyde-fixed C. pneumoniae.
FIG. 4.
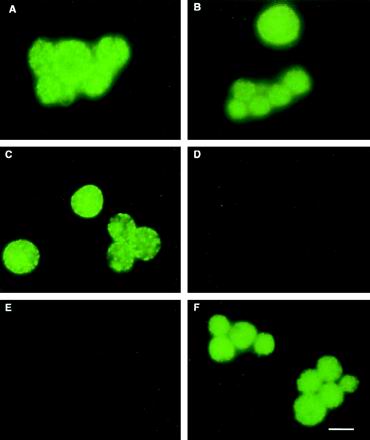
IMF of C. pneumoniae inclusions. C. pneumoniae-infected HeLa cells were grown for 72 h, fixed with 3.7% formaldehyde (A, B, and D through F) or methanol (C), and reacted with antibodies. (A) PAb-Omp4271–914; (B) PAb-Omp5149–184; (C and D) MAb 18.1 (against DnaK); (E) normal mouse serum; (F) MAb 24.1.44 (9). Bar, 10 μm.
Migration pattern of Omp4 in SDS-PAGE.
When the mouse PAbs that reacted in IMF (Fig. 4A and B) were used in immunoblotting of denatured C. pneumoniae EB proteins, no reaction was seen, indicating that these antibodies recognized nonlinear epitopes (results not shown). We therefore amplified, cloned, and expressed the entire omp4 gene. The recombinant Omp4 (rOmp4) was purified under denaturing conditions and used for immunization of a rabbit to obtain an antibody reacting with linear epitopes. When the hyperimmune serum against rOmp4 (PAbOmp4) was used in immunoblotting of C. pneumoniae OMC, a strong reaction was seen with a 99-kDa band and a weak reaction was seen with a 97-kDa band (Fig. 5, lane 1). In addition, when a panel of MAbs specific for the C. pneumoniae surface (9) were tested for reaction in immunoblotting with rOmp4, MAb 24.1.44 reacted (lane 2). In IMF of C. pneumoniae inclusions, MAb 24.1.44 recognized a surface-localized epitope (Fig. 4F) that was shown by transmission immunoelectron microscopy of native, unfixed samples to be present both on the surface of RBs, EBs, and purified OMC (Fig. 6). This indicated that Omp4 had surface-exposed epitopes.
FIG. 5.
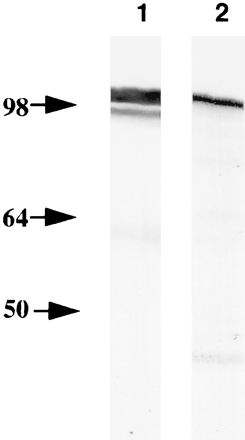
Immunological identification of Omp4 by immunoblotting. Purified C. pneumoniae OMC was reacted with PAbOmp4 (lane 1), and recombinant Omp4 was reacted with MAb 24.1.44 (lane 2). The proteins were boiled in SDS sample buffer and separated by SDS-PAGE.
FIG. 6.
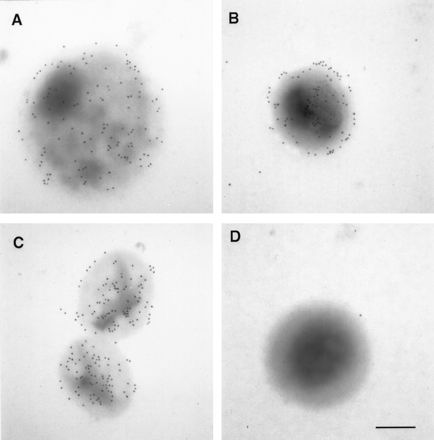
Immunoelectron microscopy of C. pneumoniae RB (A), EB (B), and OMC (C) incubated with MAb 24.1.44 and of C. pneumoniae EB reacted with a MAb against C. trachomatis MOMP (negative control) (D). Bar, 0.2 μm.
To determine the migration of Omp4 in boiled and unboiled samples, MAb 24.1.44 was reacted in immunoblots with C. pneumoniae OMC proteins. In the sample that was boiled prior to SDS-PAGE, a reaction with a band of 99 kDa corresponding in size to Omp4 was seen (Fig. 7A, lane 1). A reaction with a 40-kDa band was also seen. To determine whether this band was MOMP, we reacted recombinant MOMP in immunoblots with MAb 24.1.44 and a MAb (of the same Ig class) against Mycoplasma hominis PG21. Both MAbs gave a weak reaction with MOMP. This was also the case when we performed an immunoblot analysis in which the primary antibody was omitted (results not shown). A nonspecific reaction with MOMP has previously been noticed in immunoblot experiments (33). Thus, even though the MAb 24.1.44 recognized the 99-kDa band and MOMP with the same intensity, only the reaction with the 99-kDa band is specific.
FIG. 7.
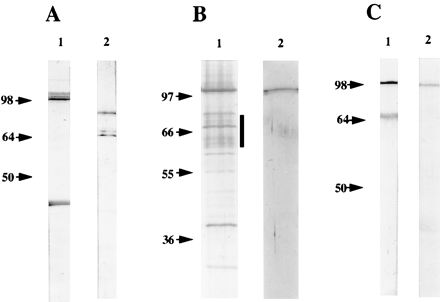
Analysis of the migration pattern of C. pneumoniae OMC proteins in SDS-PAGE. (A) C. pneumoniae OMC proteins were solubilized in SDS sample buffer. Then boiled (lane 1) and unboiled (lane 2) proteins were separated by SDS-PAGE and subjected to immunoblotting with MAb 24.1.44. (B) Silver staining of SDS-PAGE-separated C. pneumoniae OMC proteins solubilized in SDS sample buffer without boiling (lane 1). The bands marked with a vertical bar were excised from a Cu2+-stained gel, boiled in SDS sample buffer, rerun in SDS-PAGE, and silver stained (lane 2). (C) Alternatively, the bands marked with the bar in panel B were excised, boiled and subjected to immunoblotting with PAbOmp4 (lane 1) and MAb 24.1.44 (lane 2).
When the MAb was reacted with C. pneumoniae OMC proteins separated by SDS-PAGE without boiling, two bands of 65 and 75 kDa were detected (Fig. 7A, lane 2). The migration of Omp4 was thus similar to that seen in the ovine abortion strain of C. psittaci, where a 98-kDa OMP migrated as 66 kDa when the protein was not heated (28).
The migration pattern of unboiled C. pneumoniae OMC proteins was analyzed by silver staining of SDS-PAGE-separated proteins. Several protein bands of 65 to 75 kDa were seen (Fig. 7B, lane 1). To investigate whether these bands contained proteins which changed migration upon boiling, the bands were excised from a polyacrylamide gel of C. pneumoniae OMC proteins separated without prior boiling and visualized by negative staining (28). When the protein content of these bands was extracted, boiled, and reanalyzed, it produced only one band of 99 kDa (lane 2), similar to what was seen for the C. psittaci protein (28). Immunoblotting of the extracted and boiled protein sample showed that both PAbOmp4 (Fig. 7C, lane 1) and MAb 24.1.44 (lane 2) recognized the 99-kDa protein. Thus, Omp4 was contained within the excised bands of 65 to 75 kDa. Immunoblotting of the excised and reanalyzed protein was also performed with PAbs against C. pneumoniae Omp2 and MOMP, but no reaction with these antibodies was seen (results not shown). These results demonstrated that the excised protein bands contained Omp4 but not MOMP or Omp2. Thus, without complete denaturation, Omp4 migrated as bands corresponding to 65 to 75 kDa that, upon heating, changed to a band of 99 kDa.
Antibody response of mice experimentally infected with C. pneumoniae.
To analyze the humoral immune response to C. pneumoniae in experimentally infected mice and to determine whether Omp4 and Omp5 were immunogenic in such infections, four mice were experimentally infected with purified C. pneumoniae EBs. Sera were obtained from the mice 14 days after infection. The sera were reacted in immunoblot analyses of SDS-PAGE-separated proteins from C. pneumoniae EBs (Fig. 8A and B). Mouse serum 1 reacted with a 54-kDa band (Fig. 8A, lane 1), and mouse sera 2 and 3 reacted strongly with a band corresponding in size to Omp2 (lanes 2 and 3). This is in agreement with the size of proteins previously described to be immunogenic in human C. pneumoniae infections (8, 20, 21). The four mouse sera reacted weakly with several bands, of which 98- and 40-kDa bands were seen in all four lanes. Without boiling of the samples, all four mouse sera reacted strongly with a broad band that migrated as 65 kDa (Fig. 8B) and mouse serum 1 reacted with two additional bands (lane 1). Mouse serum 2 (Fig. 8A and B, lanes 2) was used in immunoblotting to analyze the reaction with C. pneumoniae OMC. Figure 8C (lane 1 [boiled] and 2 [unboiled]) showed the same reaction pattern as with the EB proteins. Thus, the immunogenic proteins are present in the C. pneumoniae OMC.
FIG. 8.
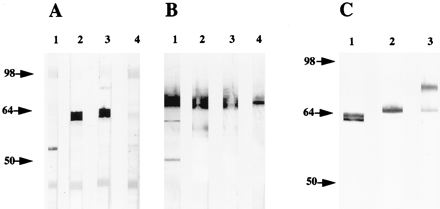
Immunoblot analysis of four mouse sera obtained 14 days after nasal inoculation with 107 inclusion-forming units of C. pneumoniae. (A) C. pneumoniae EB proteins were solubilized in SDS sample buffer, boiled, separated by SDS-PAGE, and subjected to immunoblot analyses with the four sera. (B) C. pneumoniae EB proteins were suspended in SDS sample buffer without boiling and subjected to immunoblotting with the same four sera as used in panel A. (C) C. pneumoniae OMC proteins were solubilized in SDS sample buffer. The boiled (lane 1) and unboiled (lanes 2 and 3) proteins were subjected to immunoblotting. Lanes 1 and 2 were incubated with serum from mouse 2 (as in Fig. 8A and B, lanes 2), whereas lane 3 was incubated with PAbOmp4.
When the PAbOmp4 was reacted with unboiled C. pneumoniae OMC, two bands of 65 and 75 kDa were recognized (Fig. 8C, lanes 3). This result is similar to the immunoblotting results with MAb 24.1.44 (Fig. 7A, lane 2). Thus, the immunoblot analyses (Fig. 7 and 8) demonstrated that without boiling the Omp4 migrated as bands corresponding to sizes between 65 and 75 kDa, demonstrating incomplete denaturation of the unboiled proteins that results in a different conformation of Omp4. In addition, the results indicate that in experimentally infected mice, linear epitopes of Omp2 and conformational epitopes of Omp4 are dominant.
DISCUSSION
Components of the envelope of Chlamydia are important for attachment of the EBs to the host cell membrane and their subsequent uptake. Moreover, the outer membrane protects Chlamydia EBs in the extracellular environment against inactivation by antibodies. Of the three OMPs known to be present in COMC, Omp2 and Omp3 are not believed to be surface exposed (13). In contrast to MOMP from C. trachomatis and C. psittaci, the C. pneumoniae MOMP is genetically stable (14), is not a major immunogen (7), and is probably not surface exposed. Additional components of approximately 98 kDa were known to be present in the C. pneumoniae envelope (7, 30), but not until this study have the genes encoding proteins of this size been identified. Since previous studies indicated that antigenic reactivity is directed primarily against conformational epitopes of proteins at the C. pneumoniae surface (9, 34), we generated a polyclonal antiserum against SDS-denatured C. pneumoniae OMC and identified the genes encoding the two OMPs Omp4 and Omp5, of 98.9 and 97.2 kDa, respectively. Due to the content of cysteine residues in Omp4 (8 cysteines) and Omp5 (16 cysteines), these proteins probably correspond to the [35S]cysteine-labeled OMPs of 98 kDa described by Melgosa et al. (30). We showed that Omp4 and Omp5 are located at the surface and that conformational epitopes of Omp4 seem to be dominant in experimentally infected mice. Therefore, this is the first study identifying genes encoding surface-exposed outer membrane proteins of C. pneumoniae.
Two additional expression clones encoding polypeptides with similarity to Omp4 and Omp5 were identified, and thus the genes constitute a gene family. Similar gene families exist in C. psittaci (27) and C. trachomatis (41), and a variable number of repeats of the amino acid motif Gly-Gly-Ala-Ile is a common feature of the deduced proteins. The repeated motif was not reflected at the DNA level, and therefore the origin of the gene family was probably not gene duplication. In general, the function of gene families in other pathogenic bacteria is to maintain antigenic variation in response to the host immune system. One example is the genes encoding the Opa proteins of Neisseria gonorrhoea, where phase variation generates switching among antigenically different proteins, allowing gonococci to avoid the host immune response (2). It is at present not known whether a similar mechanism by which EBs could switch the expression of the homologous OMPs and thus change their surface exists in C. pneumoniae.
Surface (S) layers have been detected on the outside of numerous gram-negative bacteria. In Campylobacter fetus, a family of S-layer proteins are encoded by multiple genes (12). The existence of silent genes in this gene family offers the possibility of varying the S layer. Thus, inversion of a chromosomal segment containing the promoter for a S-layer protein gene leads to the expression of another S-layer protein gene. By this mechanism the expression of the S-layer protein genes can be switched on and off, so that the C. fetus bacteria have the ability to vary both the antigenicity and structure of the S layer (12). The Omp4 and Omp5 gene family shows similarities to S-layer proteins, such as the amino acid composition and the requirement for boiling to permit complete denaturation. However, crystalline structure present at the surface of C. pneumoniae is required before the criteria for the proteins to be S-layer proteins can be fulfilled.
The C. trachomatis genes encoding PmpA to PmpI are located mainly in two clusters on the genome (41). Localization of related genes in clusters frequently indicates that they are important for pathogenicity, as described for the fim gene cluster from E. coli K-12 (22).
Despite the existence of similar gene families encoding surface-exposed proteins, our studies indicate that there are structural differences between the C. pneumoniae and C. psittaci envelopes. By immunoelectron microscopy, Longbottom et al. showed that proteins of the OMP90 family were exposed on the surface of both EBs and RBs (26), while the 80- to 90-kDa proteins from C. psittaci serotype 1 AB7 described by Buendia et al. (5) were exposed only on the surface of RBs. IMF with mouse PAbs indicated that C. pneumoniae Omp4 and Omp5 were located on the surface of the microorganism. However, these studies did not enable us to differentiate whether EBs, RBs, or both reacted. Use of immunoelectron microscopy, however, showed that epitopes of MAb 24.1.44, which reacted with recombinant Omp4, were located on the surface of EBs, RBs, and OMC.
In C. psittaci and C. trachomatis, MOMP is a major surface-exposed immunogen, but in C. psittaci the proteins of 90 to 98 kDa are also major antigens recognized by postabortion sera from ewes experimentally infected with C. psittaci (27). Even though a 98-kDa protein has been found to be immunogenic in C. pneumoniae infections (8, 21), a protein of this size was not a major immunogen when patient sera were reacted with purified C. pneumoniae EBs in immunoblot analyses (20). This is in agreement with our findings, since immunoblotting with sera from experimentally infected mice showed that the 97- to 99-kDa proteins were immunogenic only when the proteins were not fully denatured and thus migrated as 65 kDa. This is similar to the C. psittaci 98-kDa OMP, which migrates as 66 kDa in a SDS-PAGE when the protein is not heated; also, the 66-kDa band was shown to be immunogenic when reacted with pooled postabortion sheep sera (28).
Thus, conformational epitopes of Omp4 and possibly the other OMPs encoded by the C. pneumoniae gene family are likely to be the target for the humoral immune response in C. pneumoniae infections. The identification of this novel gene family may therefore facilitate the determination of the pathogenicity of this microorganism.
ACKNOWLEDGMENTS
We are grateful to Karin Skovgaard Sørensen and Inger Andersen for skillful technical assistance.
The work was financially supported by the Danish Health Research Council (grants 12-0850-1, 12-0150-1), the Danish Veterinary and Agricultural Research Council (grants 20-3503-1), Aarhus University Research foundation, “Nationalforeningen til Bekæmpelse af Lungesygdomme,” The Velux Foundation, The Danish Pasteur Society, and the “Direktør Jacob Madsen og hustru Olga Madsen” foundation.
REFERENCES
- 1.Allen J E, Cerrone M C, Beatty P R, Stephens R S. Cysteine-rich outer membrane proteins of Chlamydia trachomatis display compensatory sequence changes between biovariants. Mol Microbiol. 1990;4:1543–1550. doi: 10.1111/j.1365-2958.1990.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhat K S, Gibbs C P, Barrera O, Morrison S G, Jähnig F, Stern A, Kupsch E-M, Meyer T F, Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 3.Birkelund S, Lundemose A G, Christiansen G. Characterization of native and recombinant 75-kilodalton immunogens from Chlamydia trachomatis serovar L2. Infect Immun. 1989;57:2683–2690. doi: 10.1128/iai.57.9.2683-2690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkelund S, Mygind P, Holm A, Larsen B, Beck F, Christiansen G. Characterization of two conformational epitopes of the Chlamydia trachomatis serovar L2 DnaK immunogen. Infect Immun. 1996;64:810–817. doi: 10.1128/iai.64.3.810-817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buendia A J, Salinas J, Sanchez J, Gallego M C, Rodolakis A, Cuello F. Localization by immunoelectron microscopy of antigens of Chlamydia psittaci suitable for diagnosis or vaccine development. FEMS Microbiol Lett. 1997;150:113–119. doi: 10.1111/j.1574-6968.1997.tb10358.x. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell L A, Kuo C-C, Grayston T. Structural and antigenic analysis of Chlamydia pneumoniae. Infect Immun. 1990;58:93–97. doi: 10.1128/iai.58.1.93-97.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell L A, Kuo C-C, Wang S-P, Grayston T. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol. 1990;28:1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen G, Østergaard L, Birkelund S. Molecular biology of the Chlamydia pneumoniae surface. Scand J Infect Dis Suppl. 1997;104:5–10. [PubMed] [Google Scholar]
- 10.Clarke I N, Ward M E, Lambden P R. Molecular cloning and sequence analysis of a developmentally regulated cysteine-rich outer membrane protein from Chlamydia trachomatis. Gene. 1988;71:307–314. doi: 10.1016/0378-1119(88)90047-9. [DOI] [PubMed] [Google Scholar]
- 11.Clausen J D, Christiansen G, Holst U, Birkelund S. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol Microbiol. 1997;25:441–449. doi: 10.1046/j.1365-2958.1997.4591832.x. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin J, Blaser J. Generation of Campylobacter fetus S-layer protein diversity utilizes a single promoter on an invertible DNA segment. Mol Microbiol. 1996;19:1241–1253. doi: 10.1111/j.1365-2958.1996.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 13.Everett K D, Hatch T P. Architecture of the cell envelope of Chlamydia psittaci 6BC. J Bacteriol. 1995;177:877–882. doi: 10.1128/jb.177.4.877-882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaydos C A, Quinn T C, Bobo L D, Eiden J J. Similarity of Chlamydia pneumoniae strains in the variable domain IV region of the major outer membrane protein gene. Infect Immun. 1992;60:5319–5323. doi: 10.1128/iai.60.12.5319-5323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grayston J T. Infections caused by Chlamydia pneumoniae strain TWAR. Clin Infect Dis. 1992;15:757–763. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- 16.Grayston J T, Kuo C-C, Coulston A S, Campbell L A, Lawrence R D, Lee M J, Strandness E D, Wang S. Chlamydia pneumoniae (TWAR) in atherosclerosis of the carotid artery. Circulation. 1995;92:3397–3400. doi: 10.1161/01.cir.92.12.3397. [DOI] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 18.Hatch T P, Vance D W, Jr, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol. 1981;146:426–429. doi: 10.1128/jb.146.1.426-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iijima Y, Miyashita N, Kishimoto T, Kanamoto Y, Soejima R, Matsumoto A. Characterization of Chlamydia pneumoniae species-specific proteins immunodominant in humans. J Clin Microbiol. 1994;32:583–588. doi: 10.1128/jcm.32.3.583-588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanamoto Y, Iijima Y, Miyashita N, Matsumoto A, Sakano T. Antigenic characterisation of Chlamydia pneumoniae isolated in Hiroshima, Japan. Microbiol Immunol. 1993;37:495–498. doi: 10.1111/j.1348-0421.1993.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 22.Klemm P, Krogfelt K A, Hedegaard L, Christiansen G. The major subunit of Escherichia coli type 1 fimbriae is not required for d-mannose-specific adhesion. Mol Microbiol. 1990;4:553–559. doi: 10.1111/j.1365-2958.1990.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuo C-C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 24.Kutlin A, Roblin P M, Hammerschlag M R. Antibody response to Chlamydia pneumoniae infection in children with respiratory illness. J Infect Dis. 1998;177:720–724. doi: 10.1086/514223. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Longbottom D, Findlay J, Vretou E, Dunbar S M. Immunoelectron microscopic localisation of the OMP90 family on the outer membrane surface of Chlamydia psittaci. FEMS Microbiol Lett. 1998;164:111–117. doi: 10.1111/j.1574-6968.1998.tb13075.x. [DOI] [PubMed] [Google Scholar]
- 27.Longbottom D, Russell M, Dunbar S M, Jones G E, Herring A J. Molecular cloning and characterization of the genes coding for the highly immunogenic cluster of 90-kilodalton envelope proteins from the Chlamydia psittaci subtype that causes abortion in sheep. Infect Immun. 1998;66:1317–1324. doi: 10.1128/iai.66.4.1317-1324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCafferty M C, Herring A J, Andersen A A, Jones G E. Electrophoretic analysis of the major outer membrane protein of Chlamydia psittaci reveals multimers which are recognized by protective monoclonal antibodies. Infect Immun. 1995;63:2387–2389. doi: 10.1128/iai.63.6.2387-2389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClenaghan M, Herring A J, Aitken I D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984;45:384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melgosa M P, Kuo C-C, Campbell L A. Outer membrane complex proteins of Chlamydia pneumoniae. FEMS Microbiol Lett. 1993;112:199–204. doi: 10.1111/j.1574-6968.1993.tb06448.x. [DOI] [PubMed] [Google Scholar]
- 31.Miyashita N, Matsumoto A. Microbiology of chlamydiae with emphasis on physiochemistry, antigenicity and drug susceptibility of Chlamydia pneumoniae. Kawasaki Med J. 1994;20:1–17. [Google Scholar]
- 32.Moazed T, Kuo C-C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 33.Newhall W J, Batteiger B, Jones R B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982;38:1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puolakkainen M, Parker J, Kuo C-C, Grayston J T, Campbell L A. Further characterisation of Chlamydia pneumoniae specific monoclonal antibodies. Microbiol Immunol. 1995;39:551–554. doi: 10.1111/j.1348-0421.1995.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 35.Saikku P, Leinonen M, Tenkanen L, Linnanmaki E, Ekman M-R, Manninen V, Manttari M, Frick M H, Huttunen J K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki heart study. Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 36.Saikku P, Matilla K, Nieminen M S, Leinonen M, Ekman M-R, Mäkelä P H, Valtonen V. Serological evidence of an association of a novel Chlamydia TWAR with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 37.Saikku P, Wang S, Kleemola M, Brander E, Rusanen E, Grayston J T. An epidemic of mild pneumoniae due to an unusual strain of Chlamydia psittaci. J Infect Dis. 1985;151:832–839. doi: 10.1093/infdis/151.5.832. [DOI] [PubMed] [Google Scholar]
- 38.Salari S H, Ward M E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981;123:197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J E, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Stanley K K, Luzio J P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984;3:1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephens R S, Kalman S, Fenner C, Davis R. Chlamydia Genome Project. 1998. http://chlamydia-www.berkeley.edu:4231 ( http://chlamydia-www.berkeley.edu:4231). ). [Google Scholar]


