Abstract
Periodontitis is the inflammatory response in periodontal tissues elicited by bacterial colonization in periodontal pockets. In this response, pocket epithelial cells are the first cells to come into contact with bacteria. To elucidate this mechanism, we determined the adherence of the periodontopathic bacterium Eikenella corrodens 1073, which has a GalNAc-sensitive lectin-like adhesin (EcLS), to a human oral epithelial carcinoma cell line (KB) and the induction of proinflammatory cytokine production in the cells following exposure to this bacterium in vitro. In the adherence assay, EcLS played a role as the adhesin of this bacterium in adherence to KB cells. In a reverse transcriptase PCR, significant interleukin-8 (IL-8) and IL-6 mRNA levels were induced in response to exposure to this bacterium. In an enzyme-linked immunosorbent assay after an 8-h bacterial exposure, the IL-8 and IL-6 protein levels were 13.5- and 8.3-fold higher than those in the nonexposed controls, respectively. These protein responses were time dependent. Interestingly, when E. corrodens was separated from KB cells by cell culture inserts, a slight stimulation of the IL-6 and IL-8 mRNA and secreted protein levels was seen. These results imply that the direct contact of E. corrodens 1073 with oral epithelial cells is not necessarily required for the stimulation of IL-6 and IL-8 secretion. We suggest that E. corrodens induces the epithelial cells to secrete proinflammatory cytokines which serve as an early signaling system to host immune and inflammatory cells in underlying connective tissues.
Periodontitis is the inflammatory response in gingival and connective tissue elicited by bacterial colonization in periodontal pockets. In this response, pocket epithelial cells are the first cells to come into contact with bacteria. It is thus suspected that an interaction between bacteria and host epithelium cells is required for periodontal inflammation consisting of leukocyte inflammation and epithelium and connective tissue damage. However, the mechanism which initiates this response is not well understood.
The chemokines are a family of small polypeptides that have chemoattractant properties for inflammatory cells (35) and act as a signal for the emigration of blood cells. Several reports have suggested that cytokines play important roles in the pathogenesis of periodontitis (23, 52). Many cytokines found in periodontal tissue exhibit biological activities that are known to be involved in the progress of this disease, including the activation of osteoclasts and fibroblasts (23). For example, interleukin-8 (IL-8) is one of the C-X-C chemokines, which are considered the most important mediators of the accumulation of granulocytes (4, 35). IL-8 has been shown to be localized in gingival tissue sections of patients with periodontitis, and IL-8 mRNA was expressed at the same sites (15, 47). IL-8 mRNA levels were shown to correspond to the severity of periodontitis (47). IL-1β and IL-6 were detected in gingival crevicular fluid from patients with chronic periodontitis (17, 25, 37). Tumor necrosis factor alpha (TNF-α) has also been detected in gingival crevicular fluid (40). These cytokines are found at the levels of protein (20) and mRNA transcripts (26) in inflamed gingival tissue. The nature of the stimuli that induce cytokine production by pocket epithelium is as yet unknown, however.
Eikenella corrodens, a facultative gram-negative anaerobic rod, is found predominantly in subgingival plaque in patients with advanced periodontitis and may also cause extraoral infections (5, 6, 42, 43). For example, E. corrodens has been linked to a variety of disease states, including abscess, endocarditis, meningitis, osteomyelitis, keratitis, conjunctivitis, and cellutitis. The monoinfection of germfree or gnotobiotic rats with E. corrodens causes periodontal disease with severe alveolar bone loss. We found that E. corrodens 1073 has a cell-associated N-acetyl-d-galactosamine (GalNAc)-specific lectin-like substance (EcLS) that mediates its adherence to various host tissue cell surfaces (12, 31, 53, 54). We also found that EcLS mediates the GalNAc-inhibitable coaggregation of E. corrodens with some strains of Streptococcus sanguis and Actinomyces viscosus in vitro (11) and stimulates the proliferation response of murine B lymphocytes (34).
The ability of many pathogens to adhere to host tissues is correlated with their ability to agglutinate erythrocytes, suggesting that the hemagglutinins of these organisms function as adhesins during the infectious process. We reported that E. corrodens can agglutinate neuraminidase-treated erythrocytes through EcLS as a mediator (12, 54). We recently cloned and expressed in Escherichia coli the porin-like protein which is one of the components of EcLS (56). Several reports have suggested that bacterial porin induces cytokine release from host cells (16, 48, 49).
In the present study, we investigated whether EcLS mediates the adherence of E. corrodens to a human oral epidermoid carcinoma cell line, KB, and whether the adherence of E. corrodens including EcLS induces the secretion and expression of cytokines by KB cells, using an enzyme-linked immunosorbent assay (ELISA) and reverse transcriptase PCR (RT-PCR). It was recently reported that the direct contact of Helicobacter pylori with epithelial cells may be critical for IL-8 induction in vitro (39). Using the same model system, we investigated whether the direct contact of E. corrodens with epithelial cells is necessary for cytokine induction and whether E. corrodens, which diffuses in periodontal pockets and attaches to the surface of the tooth root, can induce epithelial cells to produce proinflammatory cytokines.
MATERIALS AND METHODS
Bacteria and growth conditions.
E. corrodens 1073 (provided by S. S. Socransky, Forsyth Dental Center, Boston, Mass.) and 612L were cultured at 37°C in tryptic soy broth containing 2 mg of KNO3 per ml and 5 mg of hemin per ml under anaerobic conditions (95% N2 and 5% CO2).
Cell culture.
The KB cell line (derived from a human oral epidermoid carcinoma) was provided by T. Okamoto, Hiroshima University School of Dentistry, Hiroshima, Japan. The KB cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, N.Y.) supplemented with 2 mM l-glutamine, 10% (vol/vol) fetal bovine serum (JRH Biosciences, Lenexa, Kans.), 50 IU of penicillin per ml, and 50 μg of streptomycin per ml at 37°C in a water-saturated atmosphere of 95% air and 5% CO2.
Quantitative adherence assay.
The adherence of the bacteria to the epithelial cells was quantitated by the counting of viable E. corrodens 1073 cells attached to KB monolayers, which was a modification of the procedures developed by Tokuda et al. (46). Approximately 105 KB cells in DMEM were seeded in wells of 24-well tissue culture plates and incubated until confluent monolayers developed. The bacteria were pelleted by centrifugation, washed twice in phosphate-buffered saline (PBS) (pH 7.2), and suspended in DMEM devoid of serum and antibiotics at a concentration of 1.0 × 108 to 2.0 × 108 CFU/ml. The bacterial concentrations were determined spectrophotometrically according to standard curves. KB cell layers were washed three times with Hanks’ balanced salt solution (BioWhittaker, Walkersville, Md.), inoculated with 500 μl of the microbial suspension, and incubated at 37°C for 1 h. Nonadherent bacteria were removed by being washed twice with PBS, and cell-attached bacteria were quantitated after the lysis of the cell layers in 500 μl of distilled water and subsequent dilution with distilled water. Appropriate dilutions were spread onto tryptic soy agar plates, and CFU were enumerated after incubation at 37°C in an anaerobic chamber for 2 days. To determine the effect of the EcLS on the adherence to this cell line, GalNAc (1 or 50 mM) was added to the microbial suspension.
Cytokine stimulation assay.
The cultivation of KB cells was carried out similarly to the above-described quantitative adherence assay. Briefly, KB cell monolayers were washed three times with Hanks’ balanced salt solution, inoculated with 500 μl of the microbial suspension, and incubated at 37°C. For the kinetics studies, cells were incubated for 0.5, 1, 2, 4, 8, and 26.5 h. At the end of these incubations, the culture medium was collected and centrifuged, and the supernatant was stored at −20°C until assayed. RNA was extracted immediately from the cells as described below. As a control, 10 μg of lipopolysaccharide (LPS) from E. coli O26:B6 (Sigma, St. Louis, Mo.) was added per ml.
The in vitro separation assay was performed as follows and was a modification of the procedures reported by Rieder et al. (39). First, 0.5 ml of E. corrodens suspension (stock [approximately 1.0 × 108 to 2.0 × 108 CFU/ml] or a 1:10 dilution in the same medium) was added either directly to the monolayer in 24-well tissue culture plates or to a Falcon cell culture insert (Becton Dickinson, Franklin Lakes, N.J.) placed in a 24-well tissue culture plate. With the use of the inserts, the bacteria were separated from the cells by a polyethylene terephthalate track-etched membrane (pore size, 0.4 μm). Then, 0.5 ml of DMEM devoid of serum and antibiotics was added to each well. As a control, 1.0 ml of DMEM devoid of serum and antibiotics was used instead of bacterial suspension. The cell cultures were incubated at 37°C for 4, 8, and 21 h. RNA was extracted immediately from the cells after the 4-h incubation, and the culture media were collected after the 8- and 21-h incubations. The RNA and culture media were used for the RT-PCR and ELISA, respectively.
RNA extraction and cDNA preparation.
Total RNA was extracted from KB cells, prepared as described above, with a Catrimox-14 (Iowa Biotechnology, Coralville, Iowa) according to the directions supplied by the manufacturer. cDNA was prepared in 20 μl of reverse transcription buffer (Toyobo, Osaka, Japan) supplemented with 50 pmol of random primer (nonadeoxyribonucleotide mixture; TaKaRa, Otsu, Japan) and 1.5 μl of total RNA. The reaction mixture was heated to 94°C for 2 min and cooled on ice. Following this cooling step, 24 U of RNase inhibitor (TaKaRa), 1.0 mM each deoxynucleoside triphosphate (TaKaRa), and 60 U of Moloney murine leukemia virus RT (Toyobo) were added, and the reaction mixture was incubated (first at 30°C for 30 min, next at 37°C for 60 min, and then at 94°C for 5 min to inactivate the RT), cooled on ice, and stored at −20°C.
PCR procedure.
Specific cytokine gene cDNA transcript solutions were amplified by PCR with a Program Temp Control System PC-800 (Astec, Fukuoka, Japan). The amplification reaction was carried out in a total volume of 50 μl of PCR buffer containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.4 μM (each) sense and antisense primers, and 1.25 U of Taq DNA polymerase (TaKaRa). The oligonucleotide primer sequences used in amplification reactions were provided by S. Murakami, Osaka University Faculty of Dentistry, Osaka, Japan, and are shown in Table 1. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene transcript was used as the control. After 2 min of predenaturation at 94°C, PCR was performed as follows: denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 1 min. The number of PCR cycles was 36 to ensure the detection of low-abundance mRNA. A sample (10 μl) of each amplified product was subjected to electrophoresis in a 0.9, 1.2, or 1.5% agarose gel (TaKaRa), stained with ethidium bromide, and visualized by UV illumination.
TABLE 1.
Oligonucleotide sequences of the 5′ and 3′ primers of the five target genes
| mRNA species | Primer | Sequence | Size of PCR fragment (bp) |
|---|---|---|---|
| GAPDH | 5′ | 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ | 985 |
| 3′ | 5′-CATGTGGGCCATGAGGTCCACCAC-3′ | ||
| IL-6 | 5′ | 5′-ATGAACTCCTTCTCCACAAGCGC-3′ | 628 |
| 3′ | 5′-GAAGAGCCCTCAGGCTGGACTG-3′ | ||
| IL-8 | 5′ | 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ | 294 |
| 3′ | 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ | ||
| TNF-α | 5′ | 5′-GAGTGACAAGCCTGTAGCCCATGTTGTAGCA-3′ | 446 |
| 3′ | 5′-GCAATGATCCCAAAGTAGACCTGCCCAGACT-3′ | ||
| IL-1β | 5′ | 5′-ATGGCAGAAGTACCTAAGCTCGC-3′ | 804 |
| 3′ | 5′-ACACAAATTGCATGGTGAAGTCAGTT-3′ |
For negative controls, the Moloney murine leukemia virus RT was omitted from the cDNA synthesis mixture to ensure amplification from genomic DNA.
Cytokine assays.
The concentrations of the cytokines in cell culture supernatants were determined by ELISA. Commercially available ELISA kits (Biosource, Camerillo, Calif.) for the quantitation of cytokines were used as described in the manufacturer’s instructions.
Electron and optical microscopy.
Examination by scanning electron microscopy (SEM) and optical microscopy of KB cell monolayers with E. corrodens attached was performed as follows. Briefly, approximately 105 KB cells in DMEM were seeded on cell desks (Cell Desk LF1; Sumitomo, Tokyo, Japan) placed in 24-well plates. The KB cell confluent monolayers were inoculated with microbial suspension (1.0 × 108 to 2.0 × 108 CFU/ml) and incubated at 37°C for 2 h. The medium was removed, the monolayers were washed with PBS, and the cell desks with attached KB cells were fixed by adding 2% glutaraldehyde (Wako, Osaka, Japan) in PBS dropwise to the monolayers. After the cell desks were left standing overnight at 4°C, the solution was removed and the monolayers were washed with 0.1 M PBS, pH 7.4. The cells were dehydrated through a graded series of ethanol solutions to 100% ethanol. After dehydration, the cells were treated with 50% t-butylalcohol for 10 min and twice with 100% t-butylalcohol for 10 min. The cell desks with fixed KB cells were lyophilized with a freeze-drying device (JFD 300; JEOL, Tokyo, Japan), and coated with Au ion at a thickness of 100 Å for the SEM analysis. The SEM was carried out with a JEOL 5300 electron microscope.
For optical microscopy after the removal and washing of the cell desks, the cell desks with attached KB cells were fixed by the addition of 100% methyl alcohol. After 10 min, the cells were stained with 20% Giemsa solution (Wako) and washed. Optical microscopy was carried out with an OPTIPHOTO-2 microscope (Nikon, Tokyo, Japan).
Statistical analysis.
All statistical analyses were performed by using an unpaired Student’s t test. Differences in the data were considered significant when the probability value was less than 5% (P < 0.05).
RESULTS
Attachment of E. corrodens to epithelial cells.
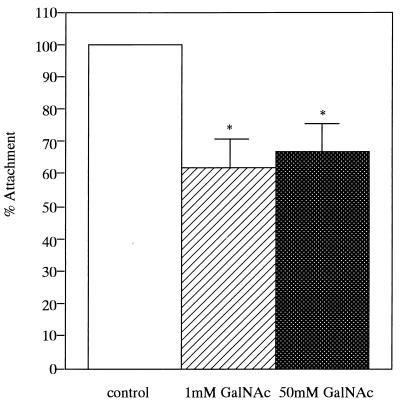
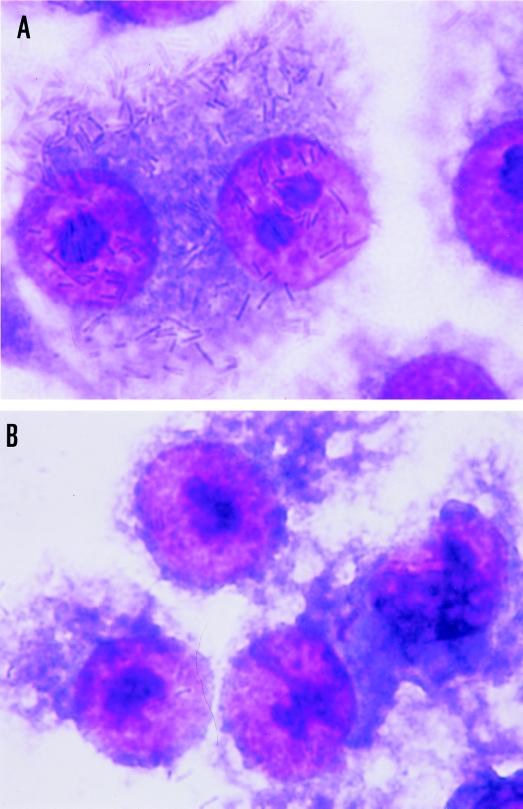
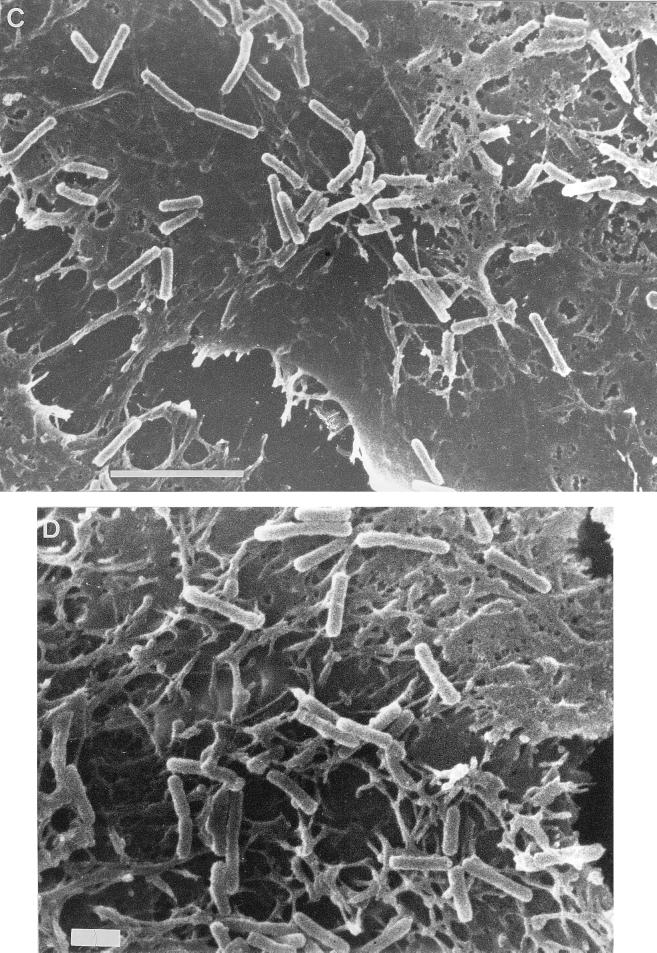
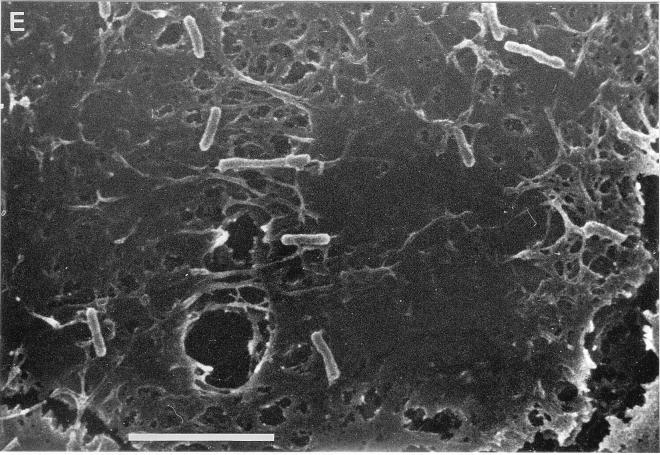
The binding of E. corrodens 1073 to epithelial cells was quantitated by the counting of viable E. corrodens cells attached to KB monolayers. For the determination of the effect of the EcLS on the adherence to this cell line, GalNAc was added to the microbial suspension. The results obtained in these assays are shown in Fig. 1. E. corrodens 1073 attachment to KB cells was inhibited by about 30% in the presence of 1 or 50 mM GalNAc. There was no significant difference between the effects of 1 and 50 mM GalNAc. This result corresponded to our observations by optical microscopy and SEM (Fig. 2). These findings suggest that EcLS plays a role in the adhesion of E. corrodens 1073 to KB cells and that other adhesins for adhesion to this cell line were present on the surface of E. corrodens 1073. We thus speculate that the adherence of E. corrodens 1073 is a multistep process mediated by different adhesins for different sites on the pocket epithelial cells. The SEM showed that E. corrodens 1073 adhered to the microvillus-like process on KB cells and that infected KB cells exhibited fibrillar protrusions (Fig. 2D).
FIG. 1.
Inhibition of the attachment of E. corrodens 1073 to KB cells. The results are expressed as the percent attachment of E. corrodens 1073 without and with 1 or 50 mM GalNAc (percent attachment = attachment with GalNAc/attachment without GalNAc [control]). The results are the means of triplicate determinations from three different experiments; error bars indicate SDs. Asterisks indicate significant differences (P < 0.01) versus the control.
FIG. 2.
Optical micrographs (A and B) and SEMs (C, D, and E) of KB cells with attached E. corrodens 1073 cells without (A, C, and D) and with (B and E) 1 mM GalNAc. Magnifications, ×625 (A and B), ×5,000 (C and E), and ×7,500 (D). Bars, 5 μm (C and E) and 1 μm (D). Samples were prepared as described in Materials and Methods.
Oral epithelial cells are stimulated by E. corrodens to secrete IL-6 and IL-8.
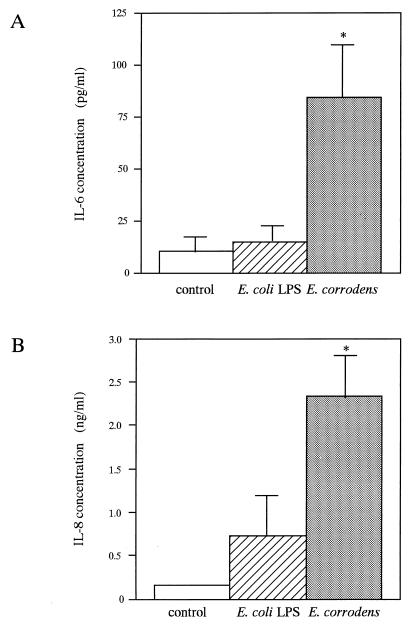
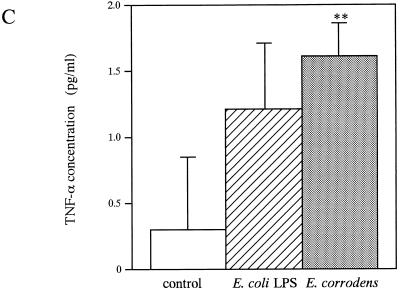
We investigated the production of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) secreted from KB cells in response to E. corrodens stimuli, since previous studies had shown that proinflammatory cytokines played a role in inflammatory responses induced by pathogenic bacteria. KB cells were incubated with E. corrodens for 8 h, and the levels of the cytokines in the culture supernatant were then measured by ELISA. In the absence of bacteria, KB cells released small amounts of TNF-α, IL-1β, IL-6, and IL-8, as reported by Eckmann et al. (14) and Madianos et al. (24). IL-1β was not generated above the constitutive levels from bacterium-stimulated KB cells (2.870 ± 1.217 pg/ml versus 1.429 ± 1.000 pg/ml for medium-alone controls and 1.524 ± 0.663 pg/ml for E. coli LPS), whereas the IL-8 and IL-6 protein levels in the stimulated cells were 13.5- and 8.3-fold higher than the respective levels in the nonexposed controls (Fig. 3). The TNF-α protein level was slightly increased compared with that for the nonexposed controls (Fig. 3). In contrast, LPS of E. coli O26:B6 did not stimulate significant secretion of IL-8 and IL-6 from KB cells in a similar assay (Fig. 3). In addition, when a microbial suspension diluted 1:10 was added to KB monolayers, the IL-8 protein level was decreased to 46% in comparison with that of the stock microbial suspension, and the IL-6 protein level was 1.4-fold higher than that of the stock microbial suspension (data not shown). Thus, the increase in IL-8 secretion showed a positive correlation with the amount of bacteria added, but the increase in IL-6 secretion showed an inverse correlation with the amount of bacteria added, ranging from 107 to 108 CFU/ml in the suspension.
FIG. 3.
IL-6 (A), IL-8 (B), and TNF-α (C) production by KB cells after stimulation with E. corrodens 1073. Supernatants were tested at 8 h of culture, and IL-6, IL-8, and TNF-α levels were determined by ELISA. The controls consisted of monolayers incubated in medium alone. Data are the means and SDs of triplicate determinations from three different experiments. LPS from E. coli O26:B6 (10 μg/ml) was also used. Asterisks indicate significant differences (∗, P < 0.01; ∗∗, P < 0.05) versus control values.
E. corrodens induces IL-6- and IL-8-specific mRNAs in KB cells.
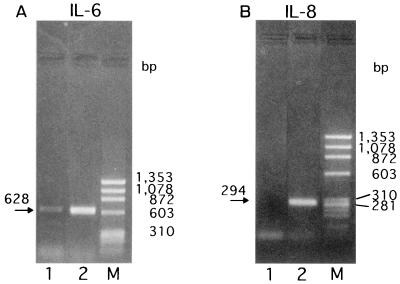
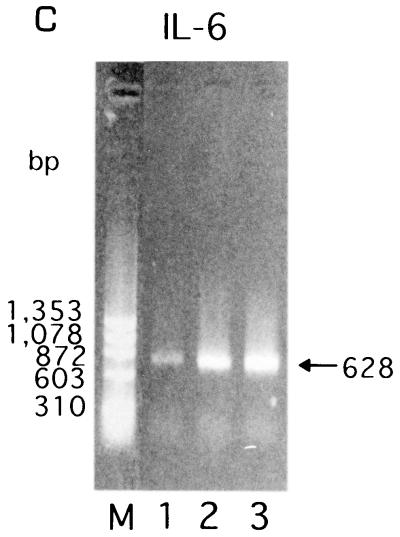
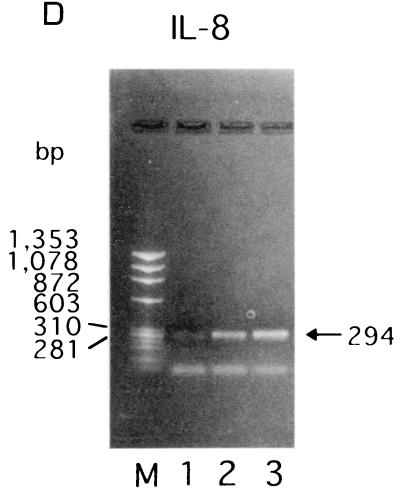
To assess the specific induction of these cytokines at the mRNA level, RT-PCR was performed with the RNA isolated from KB cells after bacterial stimulation for 3.5 h, and the amounts of cytokine mRNAs were semiquantitated by scanning densitometry of the gel with the NIH Image 1.62 image analysis software program, as reported by Darveau et al. (8). The levels of IL-8- and IL-6-specific mRNAs from E. corrodens-infected KB cells were significantly increased compared to those from uninfected control cells (Fig. 4). In contrast, TNF-α-specific mRNA from the E. corrodens-infected KB cells was faintly detected by RT-PCR, and IL-1β-specific mRNA was not increased in the infected KB cells (data not shown). These data are consistent with the ELISA data showing the protein secretion of these cytokines (Fig. 3).
FIG. 4.
IL-6 (A) and IL-8 (B) gene expression by KB cells after stimulation with E. corrodens 1073. RNA was isolated from KB cells after bacterial stimulation for 3.5 h. cDNA synthesis and RT-PCR were performed as described in Materials and Methods. The GAPDH housekeeping gene (C) was used as a control. Lanes: 1, control (medium alone); 2, E. corrodens 1073; M, markers. The amounts of IL-6 and IL-8 mRNAs, compared to that of GAPDH mRNA in the controls, were semiquantitated by scanning densitometry of the gel with NIH Image 1.62.
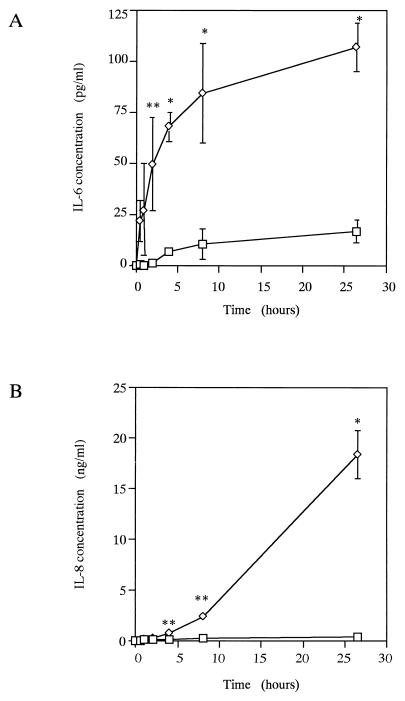
The secretion of IL-6 and IL-8 after E. corrodens stimulation is time dependent.
For the evaluation of the changes in cytokine secretion after E. corrodens stimulation, KB cells were incubated for up to 26.5 h with E. corrodens 1073. The results are shown in Fig. 5. Increases in the level of IL-6 protein were observed as early as 0.5 h after stimulation, and this concentration increased steadily over time. The IL-6 protein level was significantly increased within 4 h. At 26.5 h, the IL-6 protein level was 6.4-fold greater than the control level. At 8 h, the IL-6 protein level was maximal compared with that of the nonexposed control. In contrast, increases in the level of IL-8 protein were observed at 4 h after stimulation, and the level increased sharply from that point. At 26.5 h, the IL-8 protein level was 61.8-fold greater than the control level. Increases in the level of TNF-α protein were observed as early as 0.5 h after stimulation, and this concentration increased slightly over time. The concentrations of TNF-α protein were 1.534 pg/ml (standard deviation [SD], 0.555 pg/ml) and 2.267 pg/ml (SD, 0.287 pg/ml) with 4 and 26.5 h of bacterial stimulation, respectively. Without bacterial stimulation, the TNF-α protein concentrations were 0.254 pg/ml (SD, 0.179 pg/ml) and 0.673 pg/ml (SD, 0.400 pg/ml) at 4 and 26.5 h of culture, respectively. The IL-1β protein level was not significantly increased compared with that of the nonexposed control over time (data not shown).
FIG. 5.
Kinetics of IL-6 (A) and IL-8 (B) protein secretion induced from KB cells by E. corrodens 1073 stimulation. Supernatants were tested at 0, 0.5, 1, 2, 4, 8, and 26.5 h of culture, and the IL-6 and IL-8 levels were determined by ELISA. ◊, E. corrodens 1073; □, control. The controls consisted of monolayers incubated in medium alone. Data are the means and SDs of triplicate determinations from three different experiments. Asterisks indicate significant differences (∗, P < 0.01; ∗∗, P < 0.05) versus control values.
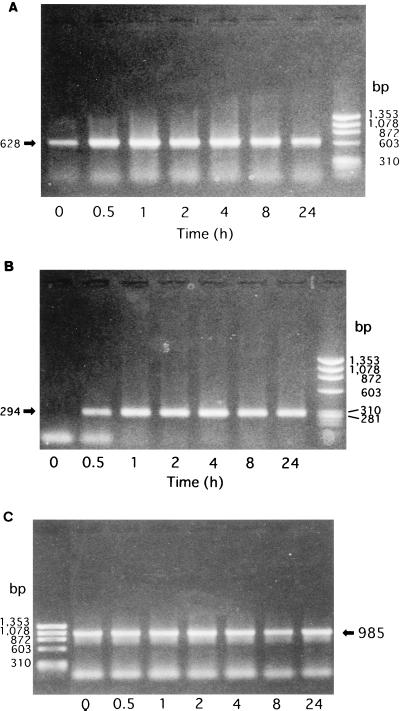
The patterns of IL-6 and IL-8 mRNA kinetics after E. corrodens stimulation are similar to those of the secreted protein levels.
The patterns of the IL-6, IL-8, and TNF-α mRNA changes after E. corrodens stimulation were similar to those of the secreted protein levels, as revealed by the semiquantitation method (see the legend to Fig. 4). However, different kinetic patterns for IL-6, IL-8, and TNF-α were observed after E. corrodens stimulation. Messages for IL-6 and IL-8 were detectable early (at 30 min) after the exposure to E. corrodens. The message for IL-6 peaked at 4 h and fell to near-baseline levels at 24 h after stimulation. In contrast, the mRNA levels for IL-8 peaked at 4 h and did not significantly decline until 24 h after stimulation (Fig. 6). The message for TNF-α peaked at 1 h and soon fell to near-baseline levels (data not shown).
FIG. 6.
Kinetics of IL-6 (A) and IL-8 (B) gene expression induced from KB cells by E. corrodens 1073 stimulation. RNA was isolated from KB cells after 0, 0.5, 1, 2, 4, 8, or 24 h of exposure to bacterial stimulation. cDNA synthesis and RT-PCR were performed as described in Materials and Methods. The GAPDH housekeeping gene (C) was used as a control. The amounts of IL-6 and IL-8 mRNAs, compared to that of GAPDH mRNA in the control, were semiquantitated by scanning densitometry of the gel with NIH Image 1.62.
Effects of EcLS on cytokine expression and secretion in KB cells exposed to E. corrodens.
For the investigation of the effects of EcLS on cytokine expression and secretion in KB cells exposed to E. corrodens 1073, GalNAc was added to the microbial suspension. We found that 50 mM GalNAc slightly downregulated the IL-8 and IL-6 mRNAs expressed in KB cells in response to E. corrodens 1073, as determined by RT-PCR and the semiquantitation method (data not shown). In the ELISA, 50 mM GalNAc inhibited the IL-8 and IL-6 protein levels by only about 20 to 30% (data not shown). These data corresponded to the effects of EcLS on the adherence to this cell line (Fig. 1).
We also tested E. corrodens 612L, an EcLS-negative strain, and this strain showed no GalNAc-sensitive hemagglutination (12). The IL-6 and IL-8 gene expression and secretion in KB cells exposed to E. corrodens 612L were similar to those induced by the 1073 strain (data not shown).
These data suggest that (i) EcLS plays a role as one of the inducers of the expression and secretion of cytokines from KB cells and (ii) other components from E. corrodens 1073 and 612L may induce these cytokines from the same cells.
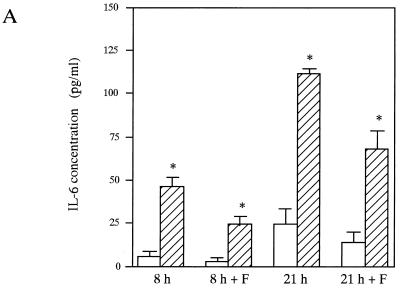
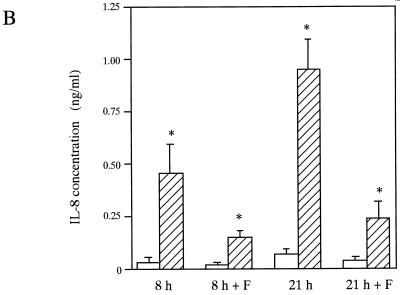
Effects of the attachment of E. corrodens on epithelial cytokine secretion.
To clarify the mechanism of cytokine secretion from the epithelial cells after bacterial stimulation and to test the possibility that components other than EcLS are inducers of cytokine production, we observed the effects of the attachment of E. corrodens on epithelial cytokine expression and secretion in vitro as described in Materials and Methods. Figure 7 shows the effects of E. corrodens on IL-6 and IL-8 secretion and the mRNA expression in KB cells at 8 and 21 h and at 4 h, respectively, with and without the cell culture insert. Significant increases in IL-6 and IL-8 production were observed at the mRNA and protein levels when E. corrodens attached directly to the epithelial cells. When bacteria in the stock solution were separated from the epithelial cells by a cell culture insert, slight stimulations of IL-6 and IL-8 were found at both levels compared to those of the controls, and the IL-8 protein levels were 25 to 33% of those of the nonseparated control (direct attachment condition) after 8- and 21-h exposures. The IL-6 protein levels were 52 to 61% of those of the nonseparated control (direct attachment condition) after 8- and 21-h exposures. These data suggest that the components secreted from E. corrodens 1073 may induce the expression and secretion of IL-6 and IL-8 by KB cells.
FIG. 7.
IL-6 (A and C) and IL-8 (B and D) production and gene expression by KB cells induced by E. corrodens 1073 stimulation without (8 and 21 h) and with (8 h + F and 21 h + F) a cell culture insert. (A and B) Supernatants were tested at 8 and 21 h of culture, and the IL-6 and IL-8 levels were determined by ELISA. ▨, E. corrodens 1073; □, control. The controls consisted of monolayers incubated in medium alone. Data are the means and SDs of triplicate determinations from three different experiments. Asterisks indicate significant differences (P < 0.01) versus control values. (C and D) RNA was isolated from KB cells after a 4-h bacterial stimulation. cDNA synthesis and RT-PCR were performed as described in Materials and Methods. The GAPDH housekeeping gene (E) was used as a control. Lanes: 1, control (medium alone); 2, E. corrodens 1073 stimulation with filter (cell culture insert); 3, E. corrodens 1073 stimulation without filter (cell culture insert); M, markers. The amounts of IL-6 and IL-8 mRNAs, compared to that of GAPDH mRNA in the control, were semiquantitated by scanning densitometry of the gel with NIH Image 1.62.
DISCUSSION
The bacterial colonization of gingival tissue and its penetration and destruction are important processes in the pathogenesis of periodontal disease. Periodontopathic bacteria possess a wide variety of virulence factors. One of these factors, adhesin, enables bacteria to attach to other bacteria, epithelial cells, and extracellular matrix proteins. In this study, we demonstrated that EcLS plays a role as the adhesin of E. corrodens 1073 to in adhesion KB cells (Fig. 1 and 2) and that its adherence was inhibited by about 30% in the presence of 1 or 50 mM GalNAc. These results suggested that other adhesins for adherence to this cell line are expressed on the surface of E. corrodens 1073. Renesto et al. reported that the adherence of Entamoeba histolytica, which has 260-kDa galactose- and GalNAc-sensitive lectins on the cell surface, to Caco-2 epithelial cells was inhibited by 42.9% in the presence of 100 mM GalNAc, and they found that E. histolytica has an 80-kDa membrane protein recognized by an anti-CD44 monoclonal antibody and binding hyaluronic acid as the other adhesin (38). Moran recently reported that the adherence of H. pylori is a multistep process mediated by different adhesins to different sites in the gastric tissue (32). In the present study, SEM showed that E. corrodens 1073 adhered to the microvillus-like process on KB cells and that the infected KB cells exhibited fibrillar protrusions (Fig. 2). These observations corresponded to the findings that Actinobacillus actinomycetemcomitans attached to KB cells via microvilli (44) and that Porphyromonas gingivalis attached to KB cell membrane and to microvillus-like fibrillar protrusions (10). It has also been reported that these bacteria invade and spread to epithelial cells (10, 44), but it was not known whether E. corrodens 1073 invades epithelial cells. The question of E. corrodens invasion of epithelial cells is not answered by the present results.
The cells that line mucosal surfaces, in addition to being able to form a mechanical barrier, can provide signals that are essential for the initiation and amplification of inflammatory responses during the course of bacterial infection. Among these signals, many cytokines can be induced by bacterial infection, thereby contributing to the pathologic changes seen in inflammation. Chemokines are possible candidates as second signals following the contact of bacteria with the epithelium (45, 51), and the expression and secretion of these chemokines are considered early events that participate in the cascade of events leading to inflammation. In this study, we demonstrated that the exposure of E. corrodens 1073 to KB epithelial cells induces the protein secretion and mRNA expression of significant quantities of IL-6 and IL-8 and an extremely small quantity of TNF-α, but not of IL-1β (Fig. 3). Significant increases of IL-6- and IL-8-specific mRNAs were detected within 60 to 90 min by RT-PCR, and significant increases of IL-6 and IL-8 secreted proteins were detected within 0.5 and 4 h after the exposure of oral epithelial cells to E. corrodens 1073 compared to the levels in uninfected controls. This early expression of IL-6 after exposure to bacteria is consistent with the results reported by two groups: Hedges et al. (18) observed secretion of IL-6 in a bladder epithelial cell line as early as 2 h after stimulation with uropathogenic E. coli, and Weinstein et al. (50) demonstrated the secretion of IL-6 in intestinal epithelial cells as early as 90 min after stimulation with Salmonella typhi. Regarding IL-8 secretion, our result is consistent with reports that the secretion of IL-8 and other chemokines appears to be a later event in the interaction between bacteria and intestinal epithelial cells (13, 21). Aihara et al. reported that MKN45 cells started to express IL-8 mRNA rapidly (within 1 h) after the start of coculture with H. pylori (2). In the present ELISA after an 8-h bacterial exposure, the IL-8 and IL-6 protein levels were 13.5- and 8.3-fold higher than those in the nonexposed controls, respectively. At 26.5 h, the IL-8 protein level was 61.8-fold greater than the control level. The protein responses were time dependent. Our data show that the increase in IL-6 secretion was inversely correlated with the amount of bacteria added in the range from 107 to 108 CFU/ml in the suspension and that the increase in IL-8 secretion was positively correlated with the amount of bacteria added (data not shown). These results suggested that the IL-6 secretion may be increased more than the IL-8 secretion by a small amount of bacteria. Therefore, in the course of bacterial growth and epithelial cell-bacterium contact, the IL-6 secretion may be induced earlier than the IL-8 secretion. In addition, Rieder et al. and Crowe et al. reported that the optimal level of secretion of IL-8 occurs with a ratio of approximately 10 to 100 bacteria per epithelial cell (7, 39). Our present findings showed that the optimal level of secretion of IL-8 occurred at a ratio of approximately 100 to 1,000 bacteria per KB cell.
Darveau et al. reported that E. corrodens 23834 elicited an accumulation of IL-8 in the gingival epithelial cell culture supernatant after an 18-h incubation (8). Our results are in agreement with this finding. Also, we found that the exposure of KB cells to E. corrodens 23834 induces protein secretion and mRNA expression of significant quantities of IL-6 and IL-8 (data not shown). We are now testing other cell lines (e.g., HEp-2 cells) and normal gingival epithelial cells by using the same methods.
Kim et al. reported that the IL-8 mRNA expressed by HT-29 cells in response to E. histolytica trophozoites was downregulated in the presence of galactose, N-acetylgalactosamine, or N-acetyllactosamine (0.1 to 100 mM), which are known to bind the 170-kDa lectin of E. histolytica, and this was paralleled by a decreased IL-8 protein secretion (22). They also reported that the IL-8 protein level in the coculture group was lower than that in E. histolytica without lectins and that the addition of 1 mM GalNAc resulted in a reduction in the IL-8 transcript level of more than 60% in HT-29 cells. Kim et al. suggested that the galactose-specific lectin of E. histolytica trophozoites may interact with HT-29 cells to upregulate the cytokine expression. However, our present data showed that IL-8 and IL-6 secretion was inhibited by only about 20 to 30% in the presence of 50 mM GalNAc (data not shown), as was the attachment of E. corrodens 1073 to KB cells (Fig. 1). Moreover, the levels of IL-6 and IL-8 secretion in KB cells exposed to E. corrodens 612L, a strain showing no hemagglutination, which involves the interaction of EcLS on E. corrodens cells (12), were similar to those induced by the 1073 strain. These results suggested that E. corrodens 1073 may have other components that induce the proinflammatory cytokine expression. We recently cloned and expressed the porin-like major outer membrane protein which is one of the components of the EcLS complex in E. coli. Several reports have suggested that bacterial porin induces cytokines released from host cells (16, 48, 49). However, it is not known whether this porin-like outer membrane protein has GalNAc-specific lectin activity, because recombinant PorA was expressed as insoluble inclusion bodies. However, this porin-like protein may induce cytokine expression and not have lectin activity. These possibilities may be coincident with the present finding of a slight inhibition of cytokine (IL-6 and IL-8) secretion by GalNAc, and they are now under investigation.
We also examined whether direct contact of E. corrodens 1073 with KB cells is a prerequisite for the induction of cytokine expression. We demonstrated the induction of IL-8 at the levels of mRNA and protein secretion in 4-, 8-, and 21-h cultures in an in vitro separation assay (Fig. 7). These results suggest that the supernatants including the secreted products from E. corrodens 1073, except for the products that mediate the attachment of E. corrodens 1073 to KB cells, induce IL-6 and IL-8 expression and secretion. Generally, the observation that direct contact or adhesion of bacteria to epithelial cells is required for cytokine stimulation has been made with several other bacteria. Eckmann et al. (14) showed that Salmonella dublin, which remains inside phagosomal vacuoles, and Listeria monocytogenes, which enters the cytoplasm, stimulate intestinal and cervical epithelial cells to secrete IL-8 in response to bacterial entry. In addition, Rieder et al. and Aihara et al. reported that the direct contact of H. pylori with epithelial cells is a possible prerequisite for the stimulation of IL-8 secretion (2, 39). Kim et al. suggested that proinflammatory cytokine gene expression is induced by direct contact through galactose-N-acetylgalactosamine-specific lectin between E. histolytica trophozoites and the host’s colon epithelial cells (22). It was also suggested that only adherence to epithelial cells is required for the secretion of IL-8 in response to E. coli (1), Pseudomonas aeruginosa (9), H. pylori (19, 41), and L. monocytogenes (3). In contrast, our results showed that direct contact of E. corrodens 1073 with oral epithelial cells is not necessarily required for the stimulation of IL-6 and IL-8 secretion.
The question remains as to which components of the bacterial surface other than EcLS and those secreted from bacteria are important in the induction of cytokine production, especially that of IL-6 and IL-8, as a secondary signal in E. corrodens infection. Reddi et al. (36) and Meghji et al. (30) reported that surface-associated materials from E. corrodens, extracted by gentle stirring in 0.85% (wt/vol) saline, stimulated human gingival fibroblasts to release IL-6 and stimulated bone resorption at picomolar concentrations. However, our results show that secreted soluble components from E. corrodens induced IL-6 and IL-8 production. In our recent preliminary experiments, only cell-free culture medium, which was recovered after 9 h of incubation with E. corrodens in DMEM, significantly stimulated KB cells to secrete IL-6 and IL-8. We speculate that these secreted soluble components may be different from the surface-associated materials described above and that differences in the responsiveness of the human cell lines used for experiments may be responsible for the different results.
A question also remains as to whether E. corrodens induces autocrine cytokines, other than IL-1β and TNF-α, which upregulate IL-6 and IL-8 synthesis by the KB cell line. McGee et al. demonstrated that the proinflammatory cytokines IL-1β and transforming growth factor β could act synergistically to induce IL-6 secretion in a rat small intestinal epithelial cell line (IEC-6) (27–29). An in vitro assay with human gastric cancer cells showed that IL-8 expression is regulated via TNF-α-activated NF-κB, which binds to a specific NF-κB binding site located 70 bp upstream of exon 1 of the IL-8 gene (55). NF-κB is known to be a transcription factor which in its active form is indispensable for IL-8 gene transcription (33). Various types of inflammatory stimuli, such as IL-1 and TNF-α, induce NF-κB complex formation, culminating in IL-8 gene transcription. It is likely that the interaction with bacteria induces the activation of NF-κB (2). These issues, including the possibility of autocrine cytokines (e.g., transforming growth factor β) and the activation of NF-κB, are currently under investigation.
There is new evidence of the local involvement of some cytokines in the gingival tissues and gingival crevicular fluid in periodontal diseases (23). The most obvious cause is the large number of gram-negative periodontopathic bacteria present in the subgingival plaque (36). Since these bacteria do not invade the periodontium in any appreciable number, the stimulus for cytokine production may be their solubilized components, which can diffuse into the gingiva and associated periodontal tissues including epithelium. Our present results are in accord with this concept.
In future studies, we will try to identify the components secreted from E. corrodens 1073 which induce IL-6 and IL-8 production by KB cells and the receptors on the surfaces of KB cells for these secreted components. The identification of the components and their receptors may enable the selective blocking and inhibition of inflammatory periodontal disease caused by periodontopathic bacteria.
ACKNOWLEDGMENTS
We thank T. Okamoto (Hiroshima University School of Dentistry) for supplying KB cells; S. Murakami, Y. Kusumoto, and T. Nozaki (Osaka University Faculty of Dentistry) for supplying cytokine-specific primers and advice; and H. Azakami (Yamaguchi University) for advice.
This work was supported in part by a Grant-in Aid for Scientific Research (10771218) from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Agace W W, Hedges S R, Caska M, Svanberg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aihara M, Tsuchimoto D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, Inagawa K, Kikuchi M, Mukaida N, Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line. MKN45. Infect Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold R, Scheffer J, Konig B, Konig W. Effects of Listeria monocytogenes and Yersinia enterocolitica on cytokine gene expression and release from human polymorphonuclear granulocytes and epithelial (HEp-2) cells. Infect Immun. 1993;61:2545–2552. doi: 10.1128/iai.61.6.2545-2552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggiolini M, Walz A, Kunkel S L. Neutrophil-activating peptide-1/interleukin-8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C-K C, Wilson M E. Eikenella corrodens in non-oral infections: a review. J Periodontol. 1992;63:941–953. doi: 10.1902/jop.1992.63.12.941. [DOI] [PubMed] [Google Scholar]
- 6.Crawford A C R, Socransky S S, Smith E, Phillips R. Pathogenicity testing of oral isolates in gnotobiotic rats. J Dent Res. 1977;56:B120. [Google Scholar]
- 7.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Muller M, Sherman P, Patel J, Jin Y, Ernst P B. Expression of interleukin-8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 8.Darveau R P, Belton C M, Reife R A, Lamont R J. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMango E, Zar H J, Bryan R, Prince A. Diverse P. aeruginosa gene products stimulate respiratory epithelial cells to produce IL-8. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan M J, Nakao S, Skobe Z, Xie H. Interaction of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebisu S, Nakae H, Okada H. Coaggregation of Eikenella corrodens with oral bacteria mediated by bacterial lectin-like substance. Adv Dent Res. 1988;2:323–327. doi: 10.1177/08959374880020022101. [DOI] [PubMed] [Google Scholar]
- 12.Ebisu S, Okada H. Agglutination of human erythrocytes by Eikenella corrodens. FEMS Microbiol Lett. 1983;18:153–156. [Google Scholar]
- 13.Eckmann L, Jung H C, Scurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin-8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 14.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald J E, Kreutzer D L. Localization of interleukin-8 in human gingival tissues. Oral Microbiol Immunol. 1995;10:297–303. doi: 10.1111/j.1399-302x.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 16.Galdiero F, Cipollaro de L’Ero G, Bendetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geivelis M. Interleukin-6 levels in gingival crevicular fluid. J Periodontol. 1990;61:773–774. doi: 10.1902/jop.1993.64.10.980. [DOI] [PubMed] [Google Scholar]
- 18.Hedges S, Svensson M, Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992;60:1295–1301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, O’Tool P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jandinski J J, Stashenko P, Feder L S, Leung C C, Peros W J, Rynar J E, Deasy M J. Localization of interleukin-1β in human periodontal tissues. J Periodontol. 1991;62:36–43. doi: 10.1902/jop.1991.62.1.36. [DOI] [PubMed] [Google Scholar]
- 21.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J M, Jung H C, Jin D Z, Im K I, Song I S, Kim C Y. Cytokine genes are down-regulated when attachment of Entamoeba histolytica to HT-29 colon epithelial cells is prevented. Scand J Immunol. 1997;45:613–617. doi: 10.1046/j.1365-3083.1997.d01-442.x. [DOI] [PubMed] [Google Scholar]
- 23.Kjeldsen M, Holmstrup P, Bendtzen K. Marginal periodontitis and cytokines: a review of the literature. J Periodontol. 1993;64:1013–1022. doi: 10.1902/jop.1993.64.11.1013. [DOI] [PubMed] [Google Scholar]
- 24.Madianos P N, Papapanou P N, Sandros J. Porphyromonas gingivalis infection of oral epithelium inhibits neutrophil transepithelial migration. Infect Immun. 1997;65:3983–3990. doi: 10.1128/iai.65.10.3983-3990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masada M P, Persson R, Kenney J S, Lee S W, Page R C, Allison A C. Measurement of interleukin-1α and -1β in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. J Periodont Res. 1990;25:156–163. doi: 10.1111/j.1600-0765.1990.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsuki Y, Yamamoto T, Hara K. Localization of interleukin-1 (IL-1) mRNA expressing macrophages in human inflammed gingiva and IL-1 activity in gingival crevicular fluid. J Periodont Res. 1993;28:35–42. doi: 10.1111/j.1600-0765.1993.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 27.McGee D W, Beagley K W, Aicher W K, McGhee J R. Transforming growth factor-β enhances interleukin-6 secretion by intestinal epithelial cells. Immunology. 1992;77:7–12. [PMC free article] [PubMed] [Google Scholar]
- 28.McGee D W, Beagley K W, Aicher W K, McGhee J R. Transforming growth factor-β and Il-1β act in synergy to enhance IL-6 secretion by the intestinal epithelial cell line, IEC-6. J Immunol. 1993;151:970–978. [PubMed] [Google Scholar]
- 29.McGee D W, Elson C O, McGhee J R. Enhancing effect of cholera toxin on interleukin-6 secretion by IEC-6 intestinal epithelial cells: mode of action and augmenting effect of inflammatory cytokines. Infect Immun. 1993;61:4637–4644. doi: 10.1128/iai.61.11.4637-4644.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meghji S, Wilson M, Barber P, Henderson B. Bone resorbing activity of surface associated material from Actinobacillus actinomycetemcomitans and Eikenella corrodens. J Med Microbiol. 1994;41:197–203. doi: 10.1099/00222615-41-3-197. [DOI] [PubMed] [Google Scholar]
- 31.Miki Y, Ebisu S, Okada H. The adherence of Eikenella corrodens to guinea pig macrophages in the absence and presence of anti-bacteria antibodies. J Periodont Res. 1987;22:359–365. doi: 10.1111/j.1600-0765.1987.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 32.Moran A P. Pathogenic properties of Helicobacter pylori. Scand J Gastroentel Suppl. 1996;215:22–31. [PubMed] [Google Scholar]
- 33.Mukaida N, Okamoto S-I, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukocyte Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 34.Nakae H, Yumoto H, Matsuo T, Ebisu S. Mitogenic stimulation of murine B lymphocytes by N-acetyl-d-galactosamine specific bacterial lectin-like substance from Eikenella corrodens. FEMS Microbiol Lett. 1994;116:349–354. doi: 10.1111/j.1574-6968.1994.tb06726.x. [DOI] [PubMed] [Google Scholar]
- 35.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 36.Reddi K, Wilson M, Nair S, Poole S, Henderson B. Comparison of the pro-inflammatory cytokine-stimulating activity of the surface-associated proteins of periodontopathic bacteria. J Periodont Res. 1996;31:120–130. doi: 10.1111/j.1600-0765.1996.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 37.Reinhardt R A, Masada M P, Kaldahl W B, DuBois L M, Kornman K S, Choi J I, Kalkwarf K L, Allison A C. Gingival fluid IL-1 and IL-6 levels in refractory periodontitis. J Clin Periodontol. 1993;20:225–231. doi: 10.1111/j.1600-051x.1993.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 38.Renesto P, Sansonetti P J, Guillen N. Interaction between Entamoeba histolytica and intestinal epithelial cells involves a CD44 cross-reactive protein expressed on the parasite surface. Infect Immun. 1997;65:4330–4333. doi: 10.1128/iai.65.10.4330-4333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieder G, Hatz R A, Moran A P, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–3630. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossomando E F, Kennedy J E, Hadjimichael J. Tumor necrosis factor alpha in gingival crevicular fluid as a possible indicator of periodontal disease in humans. Arch Oral Biol. 1990;35:431–434. doi: 10.1016/0003-9969(90)90205-o. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S A, Tummuru M K R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slots J. The predominant cultivable microflora of advanced periodontitis. Scan J Dent Res. 1977;85:114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 43.Socransky S S. Microbiology of periodontal disease. Present status and future consideration. J Periodontol. 1977;48:497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- 44.Sreenivasan P K, Meyer D H, Fives-Taylor P M. Requirements for invasion of epithelial cells by Actinobacillus actinomycetemcomitans. Infect Immun. 1993;61:1239–1245. doi: 10.1128/iai.61.4.1239-1245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taub D D, Oppenheim J J. Review of the chemokine meeting “The Third International Symposium of Chemotactic Cytokines.”. Cytokine. 1993;5:175–179. doi: 10.1016/1043-4666(93)90001-l. [DOI] [PubMed] [Google Scholar]
- 46.Tokuda M, Duncan M, Cho M-I, Kuramitsu H K. Role of Porphyromonas gingivalis protease activity in colonization of oral surfaces. Infect Immun. 1996;64:4067–4073. doi: 10.1128/iai.64.10.4067-4073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tonetti M S, Imboden M A, Gerber L, Lang N P, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tufano M A, Rossano F, Cutalanotti P, Liguori G, Marinelli A, Baron A, Marinelli P. Properties of Yersinia enterocolitica porins: interference with biological functions of phagocytes, nitric oxide production and selective cytokine release. Inst Pasteur Res Microbiol. 1994;145:297–307. doi: 10.1016/0923-2508(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 49.Tufano M A, Rossano F, Catalanotti P, Liguori G, Capasso C, Ceccarelli M T, Marinelli P. Immunological activities of Helicobacter pylori porins. Infect Immun. 1994;62:1392–1399. doi: 10.1128/iai.62.4.1392-1399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinstein D L, O’Neill B L, Metcalf E S. Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infect Immun. 1997;65:395–404. doi: 10.1128/iai.65.2.395-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whivher J T, Evans S W. Cytokines in disease. Clin Chem. 1990;36:1269–1281. [PubMed] [Google Scholar]
- 52.Wilson M, Reddi K, Henderson B. Cytokine-inducing components of periodontopathic bacteria. J Periodont Res. 1996;31:393–407. doi: 10.1111/j.1600-0765.1996.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki Y, Ebisu S, Okada H. Eikenella corrodens adherence to human buccal epithelial cells. Infect Immun. 1981;31:21–27. doi: 10.1128/iai.31.1.21-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamazaki Y, Ebisu S, Okada H. Partial purification of a bacterial lectinlike substance from Eikenella corrodens. Infect Immun. 1988;56:191–196. doi: 10.1128/iai.56.1.191-196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasumoto K, Okamoto S-I, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor α and interferon γ synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 56.Yumoto H, Azakami H, Nakae H, Matsuo T, Ebisu S. Cloning, sequencing and expression of an Eikenella corrodens gene encoding a component protein of the lectin-like adhesin complex. Gene. 1996;183:115–121. doi: 10.1016/s0378-1119(96)00522-7. [DOI] [PubMed] [Google Scholar]