Abstract
Multinucleated giant cells (MGC), a characteristic feature of tuberculous granulomas, form by fusion of monocytes or macrophages, but little is known about the mechanism of the fusion process itself. Several studies report an indirect effect of mycobacteria, i.e., induction of a soluble lymphocyte-derived fusion factor following stimulation by mycobacteria or mycobacterial products. The aim of our study was to determine whether contact with mycobacteria can induce MGC formation from human monocytes in vitro. Stimulation of monocytes with Mycobacterium bovis bacillus Calmette-Guérin (BCG) in combination with cytokine-containing supernatants of herpesvirus saimiri-transformed human T-cell clones (T-SN) led to MGC formation with fusion rates of about 27%. In contrast, stimulation with one component alone induced only low fusion rates of up to 10%. Heat-killed BCG in combination with T-SN induced monocyte fusion to the same extent as live mycobacteria. BCG culture supernatant, BCG lysate, or inert particles in combination with T-SN did not induce MGC formation. Experiments using transwell plates containing a semipermeable membrane revealed that induction of the fusion process is dependent on direct contact of monocytes and mycobacteria. MGC formation induced by BCG plus T-SN could be inhibited by addition of monoclonal antibodies to gamma interferon (but not tumor necrosis factor alpha) as well as to the β chain (CD18) of β2-integrins. These results demonstrate that contact with mycobacteria in combination with cytokine-containing supernatants is able to induce human monocytes to form MGC and that membrane-bound molecules of mycobacteria and monocytes are involved in the fusion process.
Multinucleated giant cell (MGC) formation is a common histopathologic feature of various granulomatous diseases (including tuberculosis, leprosy, schistosomiasis, and sarcoidosis) and of foreign body reactions. The presence of MGC within the tuberculous granuloma was first described in detail by Langhans in 1868 (27).
MGC originate from fusion of monocytes, but the precise mechanism of their formation and the contribution of these cells to the pathogenesis of tuberculosis are still poorly understood. MGC can be generated in vitro in quite different ways by stimulating cells of the monocyte/macrophage lineage with cytokines (13–16, 30, 31, 36, 37, 62), lectins (6, 57), conditioned media (1, 26, 39, 47, 52), or monoclonal antibodies (MAbs) (29, 43, 55). It is not clear which of these in vitro models reflects most precisely the generation of MGC in vivo. In particular, it is not known whether mycobacteria contribute directly to MGC formation of human monocytes during a mycobacterial infection. Several studies with cells from different species reported an indirect effect of mycobacteria, i.e., induction of a soluble lymphocyte-derived fusion factor following stimulation by mycobacteria or mycobacterial products (20, 46, 47, 61). In mice with Pneumocystis carinii pneumonia, however, MGC formation can occur independently of lymphocytes and their soluble products (22). As far as induction of MGC formation by mycobacteria is concerned, it was shown recently that swine microglia infected with Mycobacterium bovis or Mycobacterium tuberculosis form MGC in vitro (45). To our knowledge, direct induction of MGC formation by mycobacteria in the human system has not been reported.
It is remarkable that many authors who investigated the interactions of mycobacteria and human monocytes/macrophages do not mention the occurrence of MGC. In most studies on MGC formation, macrophages (from humans or other species) were used. However, there is evidence that monocytes newly arriving at the site of infection play a key role in MGC formation (5, 21, 35, 53). Furthermore, recent investigations by our group have shown that the in vitro fusion capacity of human monocytes following stimulation with cytokine-containing supernatants is gradually lost during monocyte-to-macrophage maturation (40). For this reason, we used human peripheral blood monocytes for our studies on the role of mycobacteria in MGC formation.
The effects of cytokines and anti-cytokine MAbs on MGC formation have been investigated in many studies. Both in vivo and in vitro experiments suggest a role for gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) in MGC and granuloma formation, although results have been somewhat conflicting. Among cytokines inducing fusion, IFN-γ appears to play a central role. IFN-γ has been reported to induce MGC formation directly (16, 42, 62) and to enhance fusion rates induced by other stimuli (1, 15, 37, 57). Antibodies against IFN-γ inhibit MGC formation in vitro (17, 39) as well as in vivo (3, 9). In several other studies, anti-IFN-γ antibodies had no effect on MGC formation (1, 30, 36, 37, 57), and even prevention of fusion by IFN-γ was reported (56, 60). Peterson et al. found that TNF-α contributes to mycobacterium-induced fusion of swine microglia (45). In contrast, TNF-α did not induce MGC formation with murine (60) or human (15, 37, 39, 57) monocytes/macrophages. Antibodies to TNF-α have been reported to inhibit the formation of MGC (23, 57) and of granulomas (10, 23, 25). However, in another study, anti-TNF-α MAb had no effect on cell fusion (37).
Since contact between cells is a prerequisite for fusion, surface molecules and especially adhesion molecules of cells undergoing fusion must be important for MGC formation. Inhibition of cell aggregation and/or fusion by antibodies against the α and/or β chain of leukocyte function-associated antigen 1 was found in various systems (16, 24, 30, 32, 39, 45, 55). Furthermore, it was demonstrated that the CD14 antigen is involved in mycobacterium-induced cell fusion in swine (45).
In this report, we show that contact with mycobacteria directly induces the fusion of human monocytes in vitro and that also in this system both cytokines and monocyte surface molecules are involved in MGC formation.
MATERIALS AND METHODS
Isolation of monocytes.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy adult volunteers (members of laboratory staff) by density gradient centrifugation with Ficoll-Paque (Pharmacia, Uppsala, Sweden). PBMC were washed twice with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 (HyClone, Cramlington, England) supplemented with 2 mM l-glutamine (PAA Laboratories, Linz, Austria) and 10% fetal calf serum (PAA Laboratories) (referred to as standard medium). After addition of 3% human serum, PBMC were plated on plastic petri dishes (Greiner, Kremsmünster, Austria) which had been previously coated with 2% gelatin (Merck, Darmstadt, Germany) and autologous plasma (19). Following incubation for 40 min at 37°C, nonadherent cells were removed by repeated vigorous washings with warm standard medium. Adherent monocytes were detached by incubation with 5 mM EDTA solution (1:1 mixture of 10 mM EDTA in PBS with standard medium) for 10 to 15 min at 37°C. Cells were washed twice, resuspended in standard medium, and plated on 96-well cell culture plates (Costar, Cambridge, Mass.) at a density of 3 × 104/well. All cultures were done without antibiotics. Preparations always contained >90% (and usually >95%) monocytes as revealed by fluorescence-activated cell sorter (FACS) analysis (monocytes were defined by their typical forward and side scatter characteristics and staining with CD14 MAb). Freshly isolated monocytes were >95% viable (assessed by their ability to exclude trypan blue dye).
Mycobacteria.
M. bovis bacillus Calmette-Guérin (BCG, strain Chicago, ATCC [American Type Culture Collection] 27289), BCG lysate (without membranes), and supernatant of BCG cultures (BCG-SN) were a kind gift from I. Flesch, Department of Immunology, University of Ulm, Ulm, Germany. Culture of BCG in Dubos broth and production of culture supernatant and BCG lysate are described in reference 50. The bacteria were stored in Dubos broth in aliquots of 2 × 107 at −80°C. Aliquots were thawed, washed once in sterile cold PBS, and resuspended in 10 ml of standard medium. Following vigorous vortexing and passaging of the bacterial suspension several times through a syringe equipped with a 25-gauge needle to disperse clumps of bacteria, the microorganisms were added to the monocytes in appropriate numbers (see below). For selected experiments, BCG mycobacteria were heat killed by autoclaving (125°C, 20 min). For short-time storage, aliquots of mycobacteria were kept at −20°C until use. Viability of the mycobacteria was confirmed by culture in BBL MGIT (Becton Dickinson; kindly provided by M. Fille, Federal Public Health Laboratory, Innsbruck, Austria). In some experiments, M. tuberculosis H37Ra (desiccated, nonviable; also supplied by I. Flesch) was used instead of BCG. These microorganisms were reconstituted in standard medium at 10 mg/ml. After vortexing, large clumps were allowed to settle out over 15 min; the upper bacterial suspension was passed through a syringe as described above and used for stimulation of monocytes (see below).
T-cell supernatants.
Herpesvirus saimiri-transformed human T-cell clones were kindly provided by H. Fickenscher (Institute of Clinical and Molecular Virology, University of Erlangen-Nürnberg, Erlangen, Germany). These cells demonstrate an activated, mature T-cell phenotype and do not release infectious virus but produce several cytokines (including IFN-γ and TNF-α) (12). We used supernatants from two different clones, HLA132 (CD4+) and XP1220 (CD8+). Since these supernatants did not differ in fusion-inducing capacity, hereafter they are collectively referred to as T-SN. The cells were grown in 45% RPMI 1640 and 45% CG medium (Vitromex, Vilshofen, Germany) supplemented with 10% fetal calf serum and 20 U of interleukin-2 (IL-2; Boehringer, Mannheim, Germany) per ml. Supernatants were harvested twice a week, centrifuged at 300 × g, passed through a 0.22-μm-pore-size filter (Minisart NML; Sartorius AG, Göttingen, Germany), and stored at −20°C until use. Since it was possible to culture the T-cell clones in standard medium without IL-2 for several weeks, supernatants of these cultures were tested as a control for the effects of CG medium and IL-2 on MGC formation.
Induction of MGC formation with mycobacteria in combination with T-SN or IFN-γ.
Monocytes were incubated with viable BCG (0.4/cell) and T-SN (50%). In control experiments, cells were cultured with either BCG or T-SN alone. After 3 days, medium was removed, and plates were stained with Giemsa (Sigma, Munich, Germany) and evaluated microscopically (TELAVAL 31; Zeiss, Jena, Germany). The fusion rate (or fusion index) of monocytes was calculated by determining the number of nuclei within MGC (more than two nuclei per cell) in a given area per total number of nuclei in that same area: fusion rate (%) = (number of nuclei within MGC/total number of nuclei counted) × 100. Between 300 and 400 nuclei from selected representative fields were counted for each preparation.
Alternatively, T-SN was replaced by IFN-γ (125 to 1,000 U/ml; a kind gift from J. Brzoska, Bioferon, Laupheim, Germany), TNF-α (2 to 2,000 U/ml; Pepro Tech, London, England), or IFN-γ plus TNF-α (500 and 2 to 2,000 U/ml, respectively). To rule out that lymphocytes remaining in the purified monocyte population have a significant influence on MGC formation, we readded 20% autologous lymphocytes to the cultures.
In some experiments, heat-killed BCG or M. tuberculosis H37Ra (nonviable) was used instead of viable BCG. For M. tuberculosis, a suspension was prepared as described above. Starting with 50 μl/well, serial 1:2 dilutions were tested alone and in combination with T-SN (50%) in order to determine optimal concentrations for induction of MGC formation. The highest fusion rates were obtained with a suspension diluted 1:64.
BCG lysate and BCG-SN (50) were applied at final dilutions of 1:6 to 1:100 and 1:4 to 1:64, respectively. Purified protein derivative (PPD), a kind gift from C. Schmidt (Sero-Merieux, Vienna, Austria), was used at concentrations of 1 to 2,000 μg/ml. All of these stimuli were tested alone as well as in combination with T-SN (50%). For control experiments, latex beads (Fluoresbrite; 2.23-μm-diameter pore size; Polyscience Inc., Warrington, Pa.) were added to the monocytes at a final ratio of 10, 5, 2.5, or 1 bead per cell. Latex beads were used either unopsonized or opsonized (50% human serum, 37°C, 30 min). Again, the particles were tested alone as well as in combination with T-SN (50%). In selected experiments, viability of MGC was assessed by the ability to exclude trypan blue dye and was found to be ≥85%.
For the specific staining of intracellular mycobacteria, MGC were generated in Lab-Tek chamber slides (Nunc, Roskilde, Denmark), using 1.2 × 105 monocytes per chamber and BCG plus T-SN referred to hereafter as BCG + T-SN) in standard concentrations. After 3 days, mycobacteria were stained with carbol fuchsin, and nuclei of MGC were counterstained with malachite green (both from Merck).
Induction of MGC formation with conditioned medium of BCG- or ConA-stimulated PBMC.
PBMC were isolated as described above, resuspended in standard medium, and plated on 24-well cell culture plates (Costar) at a concentration of 2 × 106/ml. After addition of BCG (0.5/cell) or concanavalin A (ConA; 16 μg/ml; Sigma), cultures were incubated for 3 days. Finally cells were removed, and supernatants (BCG-SN and ConA-SN) were sterile filtered (0.22-μm-pore-size filter) and added to monocytes at a final concentration of 50%. After incubation for 3 days, the fusion index was determined as described above.
Transwell cultures.
To separate cells from mycobacteria, incubations were done in 24-well cell culture plates with inserts containing a semipermeable membrane porous to solutes but not to bacteria (10-mm-diameter tissue culture inserts with Anopore membrane [pore size, 0.02 μm]; Nunc). Monocytes were cultured at a density of 4 × 105 per well; 1.6 × 105 mycobacteria (i.e., 0.4 BCG/cell) were added to the insert. T-SN was added to both compartments at a final concentration of 50%. Total volume was 800 μl, with 400 μl in each compartment. For control experiments, cells were cultured with either T-SN or BCG alone or with both components without separation.
Alternatively, monocytes in the well were stimulated with BCG + T-SN at standard concentrations, whereas monocytes in the insert (2 × 105) were cultured with T-SN alone. This experiment was performed in order to determine whether cells cocultured with BCG release a soluble factor which could then exert an effect on monocytes cultured in the other compartment separated from BCG.
All cultures were incubated for 3 days, and the fusion index was determined as described above.
Blocking experiments with MAbs.
Anti-IFN-γ MAb GZ4 (immunoglobulin G1; [IgG1]; a kind gift from G. Adolf, Bender, Vienna, Austria), anti-TNF-α MAb 1825.12 (IgG1; R&D Systems, Wiesbaden, Germany), anti-LFA-1β MAb TS1/18 (IgG1; ATCC) or CD14 MAb MY4 (IgG2b; Coulter, Krefeld, Germany) was added to monocyte cultures simultaneously with the fusion-inducing stimuli (antibody concentrations are indicated in the figures). To remove sodium azide from the antibody sample, CD14 MAb was dialyzed against PBS, using dialysis cassettes (Slide-A-Lyzer; Pierce, Rockford, Ill.). Control experiments were done with MAb of the same isotype (for TIB 191, IgG1; for TIB 109, IgG2b; ATCC).
Statistical analysis.
Data are expressed as mean ± standard error of the mean (SEM) of the indicated number of experiments. Analysis of variance was applied for comparison of grouped variables. Significant differences (P < 0.01) between groups were analyzed by unpaired Student’s t test.
RESULTS
Mycobacteria in combination with soluble factors are able to induce MGC formation in vitro.
Fusion rates induced by stimulation of monocytes with BCG, T-SN, or BCG-SN alone were very low and depended on the blood donor from whom the cells were derived. BCG alone generally led to fusion rates of up to 5%. However, with cells from one-third of the donors, BCG alone induced no fusion at all. Likewise, stimulation with T-SN or BCG-SN alone generally led to fusion rates below 10%. Only in a few experiments with T-SN were fusion rates between 10 and 20% obtained. In contrast to stimulation with BCG or T-SN alone, the combination of BCG + T-SN yielded fusion rates of about 27% (26.8% ± 1.7%; range, 15 to 37%) with cells of all donors tested (Fig. 1 and 2). After 1 day of incubation, only a few MGC had developed. Fusion rates increased during the following days, reaching maximal values on day 3. Addition of autologous lymphocytes did not increase fusion rates. Typical examples of differently stimulated monocytes after 3 days of culture are shown in Fig. 1.
FIG. 1.
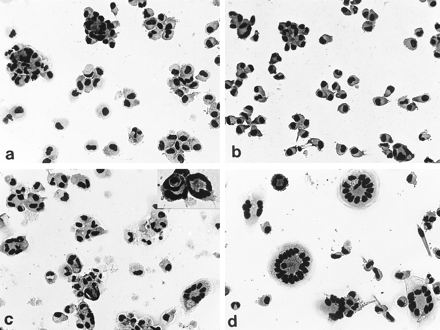
Typical examples of MGC formation after 3 days of culture. Monocytes were stimulated with BCG (0.4/cell; a), T-SN (50%) (b), BCG–T-SN (c); or ConA-SN (50%) (d). Stimulation of cells in different fusion systems leads to very different fusion rates (in the examples shown, about 4% [a]), 10% [b], 30% [c], and 70% [d]). The inset in Fig. 1c exemplifies that phagocytosis of whole cells by other cells might contribute to MGC formation. Giemsa staining; magnification, ×50 (×75 for inset).
FIG. 2.
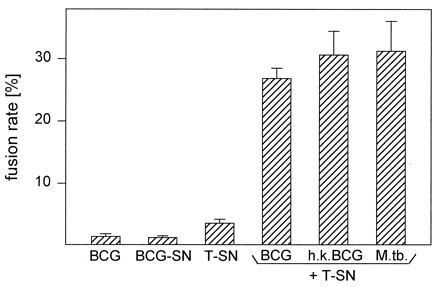
Stimulation of human monocytes with mycobacteria and T-SN in combination leads to more than additive fusion rates compared to stimulation with one component alone. Monocytes were cultured for 3 days with viable BCG (0.4/cell), BCG-SN (50%), or T-SN (50%) alone or with viable BCG, heat-killed (h.k.) BCG (0.4/cell), or M. tuberculosis H37Ra (M.tb.; 1:64) in combination with T-SN. Results represent mean ± SEM (n = 4 to 18). Fusion rates induced by stimuli in combination were significantly different from those obtained with BCG or T-SN alone (P < 0.01).
In preliminary experiments, monocytes were cultured with 0.01 to 100 BCG/cell in order to determine the BCG/cell ratio optimal for fusion and cell viability. With BCG/cell ratios of 0.3 to 1.2, we obtained similar fusion rates of about 30% (Fig. 3). However, with increasing amounts of BCG, mycobacteria had a toxic effect on monocytes of some donors. BCG/cell ratios of >1 led to extensive cell loss in all experiments (>1 for about half of all cells; >2 for about two-thirds of all cells). This effect was not influenced by T-SN, since cell loss observed with these BCG numbers occurred upon stimulation with BCG alone and in combination with T-SN. With BCG/cell ratios of <0.1, fusion rates declined rapidly. Thus, we used 0.4 BCG/cell for subsequent experiments.
FIG. 3.
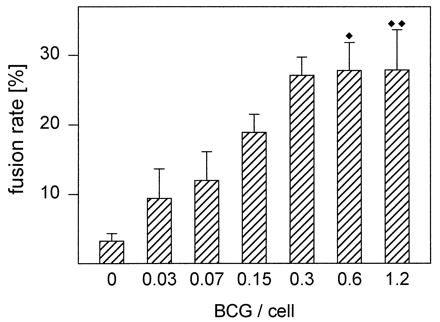
A relatively low BCG/cell ratio is optimal for MGC formation. Monocytes were cultured with indicated BCG numbers per cell and T-SN (50%) for 3 days. Results represent mean ± SEM (n = 4 to 10). ⧫, cell loss in 2 of 10 experiments; ⧫⧫, cell loss in 4 of 10 experiments.
Since it was not possible to disperse all clumps of bacteria, aggregates of different sizes were present in the BCG preparation. Specific staining of the mycobacteria with carbol fuchsin revealed that after 2 h of incubation, most BCG aggregates were surrounded by monocytes but not yet internalized. In contrast to solitary mycobacteria, phagocytosis of larger aggregates required longer time periods. Thus, it was not possible to separate the process of cell fusion from that of phagocytosis. After 3 days, almost all MGC contained BCG (Fig. 4). Since T-SN alone induced cell fusion to a certain extent, the rare MGC containing no mycobacteria might be ascribed to the stimulating effect of the T-SN.
FIG. 4.
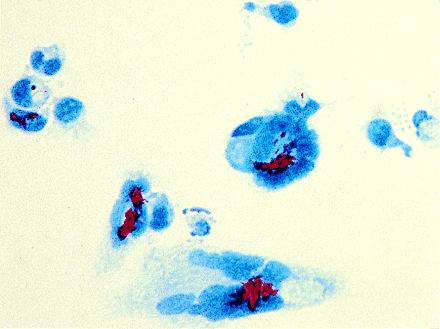
MGC containing mycobacteria. Monocytes (1.2 × 105) were cultured with BCG (0.4/cell) and T-SN (50%) in Lab-Tek chamber slides. After 3 days, mycobacteria were stained with carbol fuchsin and nuclei of MGC were counterstained with malachite green. Magnification, ×100.
In cultures treated with BCG + T-SN (but not with ConA-SN), cells with special features could be observed. Occasionally a cell (with one or more nuclei) was enclosed by another cell (which also could have one or more nuclei). A typical example is shown in the inset in Fig. 1c. After staining with Giemsa, the space between phagocytosed and phagocytosing cell often remained visible. In some cases, the membrane pseudopodia of the phagocytosing cell were about to fuse, whereas in other cases they had already fused.
For comparison, monocytes were cultured with ConA-SN. MGC induced in this fusion system were often larger and contained more nuclei (Fig. 1). However, stimulation of cells with ConA-SN led to very different results, depending on the blood donor. Fusion rates ranged from less than 10% to more than 90%. Furthermore, the fusion-inducing capacities of different ConA-SN preparations were very variable. For blocking experiments with MAb (see below), ConA-SN with high fusion-inducing capacity (fusion rates of ≥60%) was used. Phagocytosis per se was not sufficient for MGC formation induced by BCG + T-SN, since both unopsonized and opsonized latex beads, which were readily phagocytosed, did not lead to fusion rates beyond control values (neither alone nor in combination with T-SN; data not shown).
Heat-killed BCG in combination with T-SN could induce monocyte fusion to the same extent as viable BCG + T-SN. Moreover, comparable fusion rates could be induced by stimulation of monocytes with optimal concentrations of nonviable M. tuberculosis H37Ra + T-SN (Fig. 2). In contrast, neither BCG lysate nor supernatant of BCG cultures was able to substitute for intact mycobacteria. Similarly, monocytes from most donors could not be induced to form MGC by stimulation with PPD + T-SN. Only with cells of three of eight donors did PPD at concentrations of 250 or 500 μg/ml in combination with T-SN induce higher fusion rates than T-SN alone. PPD alone had no effect. At PPD concentrations above 500 μg/ml, viability of monocytes decreased and cell loss occurred (data not shown).
We then tested whether IFN-γ and TNF-α, two cytokines to which a role in MGC formation is attributed, were able to substitute for T-SN. With cells from 3 of 13 donors, IFN-γ in combination with BCG induced MGC formation, but only to a certain extent. Maximal fusion rates were about half as high as those achieved with BCG + T-SN. With IFN-γ alone, MGC were observed only occasionally and fusion rates never exceeded 5%. With TNF-α alone as well as in combination with BCG, only a few MGC developed even at TNF-α concentrations of up to 2,000 U/ml. Furthermore, IFN-γ and TNF-α in combination could not substitute for T-SN (data not shown).
IL-2 was present in the medium used for culture of T-cell clones but is probably not involved in induction of fusion for the following reasons. T-cell medium itself did not induce MGC formation, and there was no difference between the fusion-inducing capacity of normal T-SN and supernatant obtained from T-cell clones cultured in standard medium (not shown).
Direct contact of monocytes and BCG is necessary for induction of fusion.
To determine whether direct contact of monocytes and BCG is necessary for MGC formation, experiments with transwell plates containing a semipermeable membrane were performed. In cultures in which cells and mycobacteria were separated, fusion rates did not exceed those achieved with T-SN alone (Fig. 5). When monocytes were present in both compartments, only cells cocultured with BCG formed MGC. Monocytes in the other compartment remained largely mononuclear, with fusion rates not exceeding those in control wells stimulated with T-SN alone (not shown). Culture of cells on the membrane of the insert did not affect the ability of monocytes to fuse, since in control experiments cells cocultured with BCG in the insert readily formed MGC.
FIG. 5.
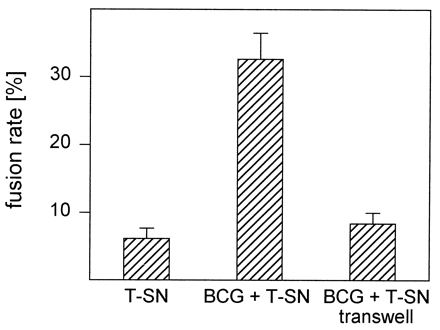
MGC formation induced by BCG in combination with T-SN requires direct contact of monocytes and mycobacteria. Monocytes were stimulated with BCG (0.4/cell) and T-SN (50%) in cell culture plates containing transwell inserts. Monocytes and BCG were either cultured in the same compartment or separated by the semipermeable membrane of the insert. Results represent mean ± SEM (n = 10). The fusion rates obtained with these two culture conditions differed significantly (P < 0.01).
Effects of MAb against monocyte surface molecules on MGC formation.
A CD18 MAb inhibited both BCG + T-SN- and ConA-SN-induced MGC formation efficiently (Fig. 6). Nevertheless, some small MGC with three or four nuclei were observed even at high antibody concentrations. In the case of BCG + T-SN-induced fusion, higher antibody concentrations were necessary to decrease fusion rates significantly, although ConA-SN induced higher fusion rates than BCG + T-SN (Fig. 6). This MAb also inhibited monocyte aggregation. In contrast, an antibody to the CD14 antigen did not inhibit MGC formation induced by either BCG + T-SN or ConA-SN. Control antibodies of the same isotypes did not exert any effect.
FIG. 6.
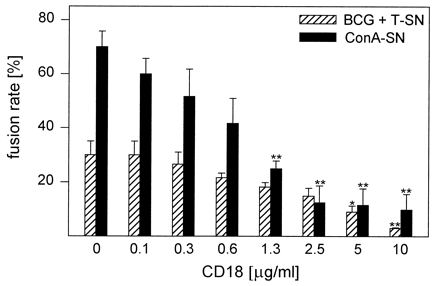
Influence of CD18 MAb on MGC formation. Monocytes were cultured for 3 days with BCG (0.4/cell) and T-SN (50%) or ConA-SN (50%). CD18 MAb was added simultaneously in the concentrations indicated. Results represent mean ± SEM (n = 3). The asterisks indicate statistically significant differences between cultures with and without antibody (∗, P < 0.05; ∗∗, P < 0.01).
Effects of MAb against IFN-γ or TNF-α on MGC formation.
A MAb against IFN-γ blocked MGC formation induced by BCG + T-SN or by ConA-SN in a dose-dependent manner (Fig. 7). As observed for CD18 antibody, the anti-IFN-γ MAb did not block MGC formation completely and less antibody was sufficient to inhibit ConA-SN-induced fusion significantly compared to BCG + T-SN-induced fusion.
FIG. 7.
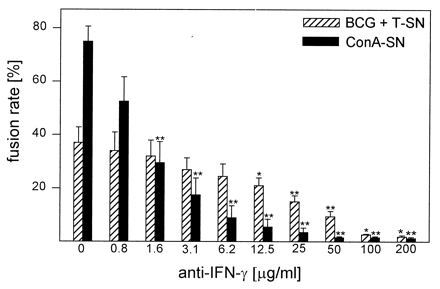
Influence of MAb against IFN-γ on MGC formation. Monocytes were cultured for 3 days with BCG (0.4/cell) and T-SN (50%) or with ConA-SN (50%). Anti-IFN-γ MAb was added simultaneously in the concentrations indicated. Results represent mean ± SEM (n = 2 to 5). The asterisks indicate statistically significant differences between cultures with and without antibody (∗, P < 0.05; ∗∗, P < 0.01).
MGC formation caused by ConA-SN was dose dependently reduced by a MAb against TNF-α. At 10 μg/ml, this antibody reduced fusion rates to about half-maximal values (Fig. 8). Higher antibody concentrations did not lead to further inhibition (data not shown). In contrast to anti-IFN-γ MAb, relatively low amounts of anti-TNF-α MAb (10 ng/ml) still inhibited MGC formation significantly. Unexpectedly, the same MAb did not inhibit fusion induced by BCG + T-SN but, on the contrary, enhanced fusion rates by about 20% (Fig. 8). This effect was independent on the presence of BCG, since anti-TNF-α MAb enhanced fusion rates caused by T-SN alone to a similar extent (data not shown). Control antibody of the same isotype had no effect.
FIG. 8.
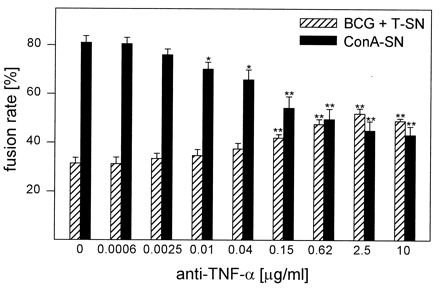
Influence of MAb against TNF-α on MGC formation. Monocytes were cultured for 3 days with BCG (0.4/cell) and T-SN (50%) or ConA-SN (50%). Anti-TNF-α MAb was added simultaneously in the concentrations indicated. Results represent mean ± SEM (n = 4 to 5). The asterisks indicate statistically significant differences between cultures with and without antibody (∗, P < 0.05; ∗∗, P < 0.01).
DISCUSSION
Although the occurrence of MGC was first recognized in the tuberculous granuloma, little is known about direct induction of MGC formation by mycobacteria. Most studies have focused on an indirect mechanism mediated by leukocyte-derived soluble factors. Only recently was the development of MGC from swine microglia cells in response to M. bovis reported (45). To our knowledge, the present study is the first to show that fusion of human monocytes in vitro requires direct contact with mycobacteria.
Whereas monocytes from some (one-third) of the donors did not fuse following stimulation with BCG alone, the combination of mycobacteria and cytokine-containing supernatants from T-cell clones induced cells from all donors to form MGC. Fusion rates were lower than those obtained by stimulation of monocytes with ConA-SN. However, using the combination of T-SN and BCG as stimulating agents, the wide variability of fusion rates with ConA-SN (caused by different fusion capacity of various ConA-SN and distinct readiness of monocytes from different donors to fuse) was overcome.
Experiments with transwell cultures revealed that direct contact of monocytes and mycobacteria is necessary to induce formation of MGC. BCG-SN and BCG lysate (containing no intact membranes) were not able to induce MGC formation (neither alone nor in combination with T-SN). Stimulation with PPD led to variable and inconsistent results. This mixture of mycobacterial proteins contains more or less degraded proteins of various origins (2). Since all of these mycobacterium-derived soluble factors are not sufficient for induction of fusion, probably molecules located within intact mycobacterial membranes are necessary to induce MGC formation. Whether membrane preparations of mycobacteria are able to contribute to induction of fusion is an interesting question that should be clarified in future studies. This hypothesis is supported by our observation that heat-killed BCG and nonviable M. tuberculosis induced fusion rates comparable to those caused by viable BCG. Similar observations were reported by Peterson et al. (45). In addition, this group demonstrated by transmission electron microscopy that MGC derived from swine microglia by stimulation with M. bovis contained intracellular bacilli. Also in our fusion system, BCG were found to be located within MGC, suggesting that phagocytosis of the bacilli might be involved in the fusion process. However, phagocytosis per se is not sufficient for MGC formation since neither unopsonized nor opsonized latex beads (alone or in combination with T-SN) were able to induce fusion. Furthermore, many pathogens are taken up by monocytes without leading to cell fusion, indicating that in addition to phagocytosis, distinctive features of the involved pathogen are essential for MGC formation.
There is good evidence that IFN-γ is one of the soluble factors involved in MGC formation induced by mycobacteria. First, anti-IFN-γ MAb dose dependently inhibited fusion induced by BCG + T-SN. However, relatively high amounts of antibody (>10 μg/ml) were necessary to reduce fusion rates significantly. This is somewhat surprising because ConA-SN-induced MGC formation was inhibited by a far lower amount of antibody (comparable to quantities needed to block other biological functions of IFN-γ). Second, with cells from some donors, IFN-γ substituted for the T-SN to a certain extent. At present we have no explanation for this donor variability. Nevertheless, these results indicate involvement of IFN-γ in cell fusion induced by BCG + T-SN and are in line with findings from other groups. IFN-γ alone (16, 42, 62) and in combination with other factors (1, 15, 37, 57) has been widely used to generate MGC in vitro. Furthermore, antibodies against IFN-γ have been shown to inhibit MGC formation in vivo (3, 9) as well as in vitro (17, 39). Surprisingly, in several other studies anti-IFN-γ MAb did not inhibit MGC formation (1, 30, 36, 37, 57), and even prevention of fusion by IFN-γ was reported (56, 60). The reason for these conflicting results is not known.
Evidence for the involvement of TNF-α in MGC or granuloma formation comes from in vivo (10, 25) as well as in vitro (23, 51, 57) studies and from investigations of biopsy material from tuberculosis patients with histopathologic methods (41). On the other hand, TNF-α failed to induce formation of multinucleated cells in other studies both in the murine system (60) and in the human system (15, 37, 39, 57). It was reported that stimulation of swine microglia in vitro with TNF-α or M. bovis induced the formation of similar numbers of MGC (45). In contrast, in our system using human monocytes, TNF-α alone or in combination with BCG or BCG + IFN-γ caused the formation of only occasional MGC. Comparison of these results is difficult, because Peterson et al. determined MGC numbers but not the fusion index (45). Since MAbs to swine TNF-α were not available to them, they could not perform blocking experiments. In our present study, an anti-TNF-α MAb did not inhibit MGC formation induced by BCG + T-SN but, on the contrary, enhanced fusion rates. However, this effect was independent of the presence of mycobacteria, since the anti-TNF-α MAb also enhanced fusion rates caused by T-SN alone. In another study, an anti-TNF-α MAb had no effect on MGC formation induced by IL-4 plus granulocyte-macrophage colony-stimulating factor (37). In contrast, ConA-SN-induced fusion rates were reduced to half-maximal values by the anti-TNF-α MAb in our study. Two other groups found anti-TNF-α MAbs to inhibit MGC or granuloma formation induced by Candida albicans or ConA in vitro (23, 57). At present we have no explanation for the differing effects of anti-TNF-α MAbs in the two fusion systems that we used. However, these results underline the distinctness of MGC formation induced by BCG + T-SN or ConA-SN under otherwise identical conditions.
It is not clear which additional cytokines are involved in MGC formation. For several reasons it could be excluded that IL-2, which was added to the T-cell medium as a growth factor, was responsible for induction of fusion. First, T-cell medium itself had no fusion-inducing capacity. Second, IL-2 alone or in combination with other factors (IFN-γ and BCG) (reference 39 and unpublished results) did not induce MGC formation. This observation is in accordance with results from others (37, 57). Third, T-SN from T-cell clones cultured in standard medium (without IL-2) had the same fusion-inducing capacity.
The inhibition of BCG + T-SN-induced MGC formation by CD18 antibody suggests that monocyte surface molecules containing the CD18 antigen (β2-integrins) are involved in the fusion process. One possibility is that these antibodies inhibit attachment and/or phagocytosis of the mycobacteria. A role in both complement-dependent (49) and complement-independent (11, 54) phagocytosis of mycobacteria has been reported for the β2-integrin CD11b/CD18 (complement receptor type 3). However, CD18 antibody also inhibited ConA-SN-induced MGC formation (a fusion system which does not involve phagocytosis of microorganisms). Furthermore, this MAb also strongly inhibited the aggregation of monocytes in both systems. Therefore, it is conceivable that antibodies to the common β chain of integrins influence the capacity of the monocytes to migrate and to establish cell-cell contact. The involvement of β2-integrins in monocyte migration on proteins of the extracellular matrix (44) and in the process of extravasation (34) has been demonstrated. It has been shown that a MAb to CD14 antigen inhibits the fusion of swine microglia (45). In our system, the same antibody did not influence fusion rates. This discrepancy might be due to differences in culture conditions, species, and/or cell types.
In both fusion systems, a few MGC developed during the first day of culture, but to achieve maximal fusion rates, an incubation period of 3 days was necessary. In contrast, Peterson et al. observed MGC as early as 2 h after the beginning of culture and found maximal MGC numbers after 18 h of incubation (45). This is exceptionally rapid, since in most fusion systems more than 24 h (and in most cases several days) of culture is required to obtain maximal fusion rates. Only in a few studies were incubation periods of less than 24 h sufficient to induce high fusion rates. Interestingly, in these investigations, very different stimuli, including supernatants from BCG-sensitized cells (20), ConA-SN (52), and antimacrophage serum (48), were used to induce fusion.
Relatively low BCG/cell ratios were optimal for MGC formation. The possibility exists that higher BCG/cell ratios would lead to higher fusion rates. However, in our system, which required prolonged culture of monocytes with mycobacteria, BCG/cell ratios of >1 led to extensive cell loss. Peterson et al. (45) used 5 and 50 mycobacteria/cell, respectively, but these experiments were finished after 18 h. In two other investigations, in which long incubation periods were necessary, only low mycobacterium/cell ratios were applied (33, 38). A recent study on stimulation of dendritic cells with BCG showed that cell viability decreased dramatically if BCG/cell ratios of >1.5 were used (58). In vivo, the cytotoxicity of mycobacteria leads to a relatively high rate of monocyte and lymphocyte turnover in the tuberculous granuloma (18).
The BCG preparations used in this study contained some aggregated bacteria which could not be dispersed. The presence of these aggregates and the prolonged exposure of monocytes to mycobacteria may reflect the in vivo situation in a granuloma. Longer time periods could be necessary to phagocytose these aggregates compared to single mycobacteria. In vivo granulomatous lesions form at sites where antigenic material persists for longer time periods. These granulomas serve to enclose pathogens and to prevent further dissemination. An alternative explanation could be that several phagocytes are necessary to ingest larger aggregates of bacteria. As a consequence, simultaneously attempted phagocytosis may lead to MGC formation. A similar concept was first proposed by Chambers (7). It is also conceivable that more than one mechanism contributes to MGC formation in vitro as well as in vivo. The observation that not only are the mycobacteria phagocytosed but sometimes whole cells are internalized by other cells, leading to the formation of multinucleated cells, could be of importance. Several similar observations have been reported (8, 35, 52, 59). It is conceivable that in vivo phagocytosis of one (probably infected) cell by another cell can lead to MGC formation.
Byrd reported mycobacterial cords growing throughout monocyte aggregates and suggested that MGC might limit the cell-to-cell spread of virulent mycobacteria by their nuclei forming a barrier (4). Furthermore, it was reported recently that intracellular mycobacteria within phagocytic vacuoles are more sensitive to H2O2-induced killing than extracellular bacilli and that extracellular mycobacteria were less sensitive to H2O2 treatment in medium containing monocyte lysates than in cell-free culture medium (28). Medium containing cell lysates may resemble the in vivo situation in granulomas containing necrotic cell debris. In this context, cell fusion leading to MGC formation could be the attempt of the host to retain full capacity to kill the pathogens and to prevent the release of viable bacilli from infected cells in order to hinder further dissemination of the infection.
Previous investigations by our group demonstrated that the capacity for cell fusion is gradually lost during monocyte-to-macrophage maturation in vitro. However, monocytes are able to fuse with other monocytes as well as with macrophages (40). It has been shown that monocytes have a greater capacity than macrophages to kill intracellular microorganisms, including mycobacteria (38, 63). Thus, fusion of monocytes newly arriving at the site of infection with infected monocytes or macrophages might be of benefit for the host.
In conclusion, contact with mycobacteria in combination with cytokine-containing supernatants directly induces human monocytes to form MGC in vitro. Apart from soluble factors, membrane-bound molecules both from mycobacteria and from monocytes appear to be involved in this fusion process. The role of MGC in an in vivo granuloma might be to retain mycobacteria inside cells which are able to kill the pathogens efficiently and prevent their dissemination beyond the local site of infection.
ACKNOWLEDGMENTS
We are grateful to I. Flesch for providing microorganisms and expertise on the handling of mycobacteria and to G. Adolf, J. Brzoska, H. Fickenscher, M. Fille, and C. Schmidt for supplying anti-IFN-γ MAb, IFN-γ, T-cell clones, media for culture of mycobacteria, and PPD, respectively. We also thank G. Mayr for performing FACS analyses, K. Mair for technical assistance with some experiments, A. Sarti for helpful discussions, D. Fuchs for statistical calculations, and N. Romani for critical reading of the manuscript.
This work was supported in part by the Austrian Science Fund (project P09581-MED) and by the Jubiläumsfonds of the Austrian National Bank (project 6656).
REFERENCES
- 1.Abe E, Ishimi Y, Jin C H, Hong M H, Sato T, Suda T. Granulocyte-macrophage colony-stimulating factor is a major macrophage fusion factor present in conditioned medium of concanavalin A-stimulated spleen cell cultures. J Immunol. 1991;147:1810–1815. [PubMed] [Google Scholar]
- 2.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 3.Belosevic M, Finbloom D S, van der Meide P H, Slayter M V, Nacy C A. Administration of monoclonal anti-IFN-γ antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–274. [PubMed] [Google Scholar]
- 4.Byrd T F. Cord formation as a potential mechanism for cell to cell spread of virulent Mycobacterium tuberculosis: limitation of cell to cell spread by IFN gamma/IL-3-induced multinucleated giant cell formation. Clin Infect Dis Suppl. 1994;19:564a. . (Abstract.) [Google Scholar]
- 5.Chambers T J. Failure of altered macrophage surface to lead to the formation of polykaryons. J Pathol. 1976;122:185–189. doi: 10.1002/path.1711220402. [DOI] [PubMed] [Google Scholar]
- 6.Chambers T J. Fusion of hamster macrophages induced by lectins. J Pathol. 1977;123:53–61. doi: 10.1002/path.1711230107. [DOI] [PubMed] [Google Scholar]
- 7.Chambers T J. Fusion of macrophages following simultaneous attempted phagocytosis of glutaraldehyde-fixed red cells. J Pathol. 1977;122:71–80. doi: 10.1002/path.1711220204. [DOI] [PubMed] [Google Scholar]
- 8.Chambers T J. The mechanism of fusion of hamster macrophages induced by antimacrophage serum. J Pathol. 1977;122:163–173. doi: 10.1002/path.1711220308. [DOI] [PubMed] [Google Scholar]
- 9.Chensue S W, Terebuh P D, Warmington K S, Hershey S D, Evanoff H L, Kunkel S L, Higashi G I. Role of IL-4 and IFN-γ in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992;148:900–906. [PubMed] [Google Scholar]
- 10.Chensue S W, Warmington K, Ruth J, Lincoln P, Kuo M, Kunkel S L. Cytokine response during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Am J Pathol. 1994;145:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 11.Cywes C, Godenir N L, Hoppe H C, Scholle R R, Steyn L M, Kirsch R E, Ehlers M R W. Nonopsonic binding of Mycobacterium tuberculosis to human complement receptor type 3 expressed in Chinese hamster ovary cells. Infect Immun. 1996;64:5373–5383. doi: 10.1128/iai.64.12.5373-5383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Carli M, Berthold S, Fickenscher H, Muller-Fleckenstein I, D’Elios M M, Gao Q, Biagiotti R, Giudizi M G, Kalden J R, Fleckenstein B, Romagnani S, Del Prete G. Immortalization with Herpesvirus saimiri modulates the cytokine secretion profile of established Th1 and Th2 human T cell clones. J Immunol. 1993;151:5022–5030. [PubMed] [Google Scholar]
- 13.DeFife K M, Jenney C R, McNally A K, Colton E, Anderson J M. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158:3385–3390. [PubMed] [Google Scholar]
- 14.Dugast C, Gaudin A, Toujas L. Generation of multinucleated giant cells by culture of monocyte-derived macrophages with IL-4. J Leukoc Biol. 1997;61:517–521. doi: 10.1002/jlb.61.4.517. [DOI] [PubMed] [Google Scholar]
- 15.Enelow R I, Sullivan G W, Carper H T, Mandell G L. Induction of multinucleated giant cell formation from in vitro culture of human monocytes with interleukin-3 and interferon-γ: comparison with other stimulating factors. Am J Respir Cell Mol Biol. 1992;6:57–62. doi: 10.1165/ajrcmb/6.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Fais S, Burgio V L, Silvestri M, Capobianchi M R, Pacchiarotti A, Pallone F. Multinucleated giant cells generation induced by interferon-γ. Changes in the expression and distribution of the intercellular adhesion molecule-1 during macrophages fusion and multinucleated giant cell formation. Lab Investig. 1994;71:737–744. [PubMed] [Google Scholar]
- 17.Fais S, Pallone F. Inability of normal human intestinal macrophages to form multinucleated giant cells in response to cytokines. Gut. 1995;37:798–801. doi: 10.1136/gut.37.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton M J, Vermeulen M W. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freundlich B, Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983;62:31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- 20.Galindo B, Lazdins J, Castillo R. Fusion of normal rabbit alveolar macrophages induced by supernatant fluids from BCG-sensitized lymph node cells after elicitation by antigen. Infect Immun. 1974;9:212–216. doi: 10.1128/iai.9.2.212-216.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillman T, Wright L J. Probable in vivo origin of multi-nucleated giant cells from circulating mononuclears. Nature. 1966;209:263–265. doi: 10.1038/209263a0. [DOI] [PubMed] [Google Scholar]
- 22.Hanano R, Reifenberg K, Kaufmann S H E. T- and B-lymphocyte-independent formation of alveolar macrophage-derived multinucleated giant cells in murine Pneumocystis carinii pneumonia. Infect Immun. 1996;64:2821–2823. doi: 10.1128/iai.64.7.2821-2823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinemann D E H, Peters J H, Gahr M. A human in vitro granuloma model using heat killed Candida albicans cells immobilized on plastic culture wells. Scand J Immunol. 1997;45:596–604. doi: 10.1046/j.1365-3083.1997.d01-435.x. [DOI] [PubMed] [Google Scholar]
- 24.Kazazi F, Chang J, Lopez A, Vadas M, Cunningham A L. Interleukin 4 and human immunodeficiency virus stimulate LFA-1-ICAM-1-mediated aggregation of monocytes and subsequent giant cell formation. J Gen Virol. 1994;75:2795–2802. doi: 10.1099/0022-1317-75-10-2795. [DOI] [PubMed] [Google Scholar]
- 25.Kindler V, Sappino A, Grau G E, Piguet P, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 26.Kreipe H, Radzun H J, Rudolph P, Barth J, Hansmann M, Heidorn K, Parwaresch M R. Multinucleated giant cells generated in vitro. Terminally differentiated macrophages with down-regulated c-fms expression. Am J Pathol. 1988;130:232–243. [PMC free article] [PubMed] [Google Scholar]
- 27.Langhans T. Ueber Riesenzellen mit wandständigen Kernen in Tuberkeln und die fibröse Form des Tuberkels. Virchows Arch Pathol Anat. 1868;42:382–404. [Google Scholar]
- 28.Laochumroonvorapong P, Paul S, Manca C, Freedman V H, Kaplan G. Mycobacterial growth and sensitivity to H2O2 killing in human monocytes in vitro. Infect Immun. 1997;65:4850–4857. doi: 10.1128/iai.65.11.4850-4857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarus D, Yamin M, McCarthy K, Schneeberger E E, Kradin R. Anti-RMA, a murine monoclonal antibody, activates rat macrophages. II. Induction of DNA synthesis and formation of multinucleated giant cells. Am J Respir Cell Mol Biol. 1990;3:103–111. doi: 10.1165/ajrcmb/3.2.103. [DOI] [PubMed] [Google Scholar]
- 30.Lee T T, Martin F C, Merrill J E. Lymphokine induction of rat microglia multinucleated giant cell formation. Glia. 1993;8:51–61. doi: 10.1002/glia.440080107. [DOI] [PubMed] [Google Scholar]
- 31.Lemaire I L, Yang H, Lauzon W, Gendron N. M-CSF and GM-CSF promote alveolar macrophage differentiation into multinucleated giant cells with distinct phenotypes. J Leukoc Biol. 1996;60:509–518. doi: 10.1002/jlb.60.4.509. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z X, Noguchi M, Hiwatashi N, Toyota T. Monocyte aggregation and multinucleated giant-cell formation in vitro in Crohn’s disease. The effect of cell adhesion molecules. Scand J Gastroenterol. 1996;31:706–710. doi: 10.3109/00365529609009154. [DOI] [PubMed] [Google Scholar]
- 33.López Ramírez G M, Rom W N, Ciotoli C, Talbot A, Martiniuk F, Cronstein B, Reibman J. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect Immun. 1994;62:2515–2520. doi: 10.1128/iai.62.6.2515-2520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luscinskas F W, Kansas G S, Ding H, Pizcueta P, Schleiffenbaum B E, Tedder T F, Gimbrone M A., Jr Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mariano M, Spector W G. The formation and properties of macrophage polykaryons (inflammatory giant cells) J Pathol. 1974;113:1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- 36.McInnes A, Rennick D M. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988;167:598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNally A K, Anderson J M. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 38.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Möst J, Neumayer H P, Dierich M P. Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-γ and expression of LFA-1. Eur J Immunol. 1990;20:1661–1667. doi: 10.1002/eji.1830200807. [DOI] [PubMed] [Google Scholar]
- 40.Möst J, Spötl L, Mayr G, Gasser A, Sarti A, Dierich M P. Formation of multinucleated giant cells in vitro is dependent on the stage of monocyte to macrophage maturation. Blood. 1997;89:662–671. [PubMed] [Google Scholar]
- 41.Myatt N, Coghill G, Morrison K, Jones D, Cree I A. Detection of tumour necrosis factor α in sarcoidosis and tuberculosis granulomas using in situ hybridisation. J Clin Pathol. 1994;47:423–426. doi: 10.1136/jcp.47.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagasawa H, Miyaura C, Abe E, Suda T, Horiguchi M, Suda T. Fusion and activation of human alveolar macrophages induced by recombinant interferon-γ and their suppression by dexamethasone. Am Rev Respir Dis. 1987;136:916–921. doi: 10.1164/ajrccm/136.4.916. [DOI] [PubMed] [Google Scholar]
- 43.Orentas R J, Reinlib L, Hildreth J E K. Anti-class II MHC antibody induces multinucleated giant cell formation from peripheral blood monocytes. J Leukoc Biol. 1992;51:199–209. doi: 10.1002/jlb.51.3.199. [DOI] [PubMed] [Google Scholar]
- 44.Penberthy T W, Jiang Y, Luscinskas F W, Graves D T. MCP-1-stimulated monocytes preferentially utilize β2-integrins to migrate on laminin and fibronectin. Am J Physiol. 1995;269:C60–C68. doi: 10.1152/ajpcell.1995.269.1.C60. [DOI] [PubMed] [Google Scholar]
- 45.Peterson P K, Gekker G, Hu S, Anderson W R, Teichert M, Chao C C, Molitor T W. Multinucleated giant cell formation of swine microglia induced by Mycobacterium bovis. J Infect Dis. 1996;173:1194–1201. doi: 10.1093/infdis/173.5.1194. [DOI] [PubMed] [Google Scholar]
- 46.Poste G. The tumoricidal properties of inflammatory tissue macrophages and multinucleated giant cells. Am J Pathol. 1979;96:595–610. [PMC free article] [PubMed] [Google Scholar]
- 47.Postlethwaite A E, Jackson B K, Beachey E H, Kang A H. Formation of multinucleated giant cells from human monocyte precursors. Mediation by a soluble protein from antigen- and mitogen-stimulated lymphocytes. J Exp Med. 1982;155:168–178. doi: 10.1084/jem.155.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ptak W, Porwit-Bòbr Z, Chlap Z. Transformation of hamster macrophages into giant cells with antimacrophage serum. Nature. 1970;225:655–657. doi: 10.1038/225655a0. [DOI] [PubMed] [Google Scholar]
- 49.Schlesinger L S, Bellinger Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 50.Schoel B, Kaufmann S H E. Influence of mycobacterial virulence and culture condition on γ/δ T cell activation. Microb Pathog. 1998;24:197–201. doi: 10.1006/mpat.1997.0187. [DOI] [PubMed] [Google Scholar]
- 51.Shikama Y, Kobayashi K, Kasahara K, Kaga S, Hashimoto M, Yoneya I, Hosoda S, Soejima K, Ide H, Takahashi T. Granuloma formation by artificial microparticles in vitro. Macrophages and monokines play a critical role in granuloma formation. Am J Pathol. 1989;134:1189–1199. [PMC free article] [PubMed] [Google Scholar]
- 52.Sone S, Bucana C, Hoyer L C, Fidler I J. Kinetics and ultrastructural studies of the induction of rat alveolar macrophage fusion by mediators released from mitogen-stimulated lymphocytes. Am J Pathol. 1981;103:234–246. [PMC free article] [PubMed] [Google Scholar]
- 53.Spector W G, Lykke A W J. The cellular evolution of inflammatory granulomata. J Pathol Bacteriol. 1966;92:163–177. doi: 10.1002/path.1700920117. [DOI] [PubMed] [Google Scholar]
- 54.Stokes R W, Haidl I D, Jefferies W A, Speert D P. Mycobacteria-macrophage interactions. Macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1993;151:7067–7076. [PubMed] [Google Scholar]
- 55.Tabata N, Ito M, Shimokata K, Suga S, Ohgimoto S, Tsurudome M, Kawano M, Matsumura H, Komada H, Nishio M, Ito Y. Expression of fusion regulatory proteins (FRPs) on human peripheral blood monocytes. Induction of homotypic cell aggregation and formation of multinucleated giant cells by anti-FRP-1 monoclonal antibodies. J Immunol. 1994;153:3256–3266. [PubMed] [Google Scholar]
- 56.Takahashi N, Mundy G R, Roodman G D. Recombinant human interferon-γ inhibits formation of human osteoclast-like cells. J Immunol. 1986;137:3544–3549. [PubMed] [Google Scholar]
- 57.Takashima T, Ohnishi K, Tsuyuguchi I, Kishimoto S. Differential regulation of formation of multinucleated giant cells from concanavalin A-stimulated human blood monocytes by IFN-γ and IL-4. J Immunol. 1993;150:3002–3010. [PubMed] [Google Scholar]
- 58.Thurnher M, Ramoner R, Gastl G, Radmayr C, Böck G, Herold M, Klocker H, Bartsch G. Bacillus Calmette-Guérin mycobacteria stimulate human blood dendritic cells. Int J Cancer. 1997;70:128–134. doi: 10.1002/(sici)1097-0215(19970106)70:1<128::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 59.Vignery A, Niven-Fairchild T, Ingbar D H, Caplan M. Polarized distribution of Na+, K+-ATPase in giant cells elicited in vivo and in vitro. J Histochem Cytochem. 1989;37:1265–1271. doi: 10.1177/37.8.2546991. [DOI] [PubMed] [Google Scholar]
- 60.Vignery A, Niven-Fairchild T, Shepard M H. Recombinant murine interferon-γ inhibits the fusion of mouse alveolar macrophages in vitro but stimulates the formation of osteoclastlike cells on implanted syngeneic bone particles in mice in vivo. J Bone Miner Res. 1990;5:637–644. doi: 10.1002/jbmr.5650050613. [DOI] [PubMed] [Google Scholar]
- 61.Warfel A H. Macrophage fusion and multinucleated giant cell formation, surface morphology. Exp Mol Pathol. 1978;28:163–176. doi: 10.1016/0014-4800(78)90049-7. [DOI] [PubMed] [Google Scholar]
- 62.Weinberg J B, Hobbs M M, Misukonis M A. Recombinant human γ-interferon induces human monocyte polykaryon formation. Proc Natl Acad Sci USA. 1984;81:4554–4557. doi: 10.1073/pnas.81.14.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson C B, Tsai V, Remington J S. Failure to trigger the oxidative metabolic burst by normal macrophages. Possible mechanism for survival of intracellular pathogens. J Exp Med. 1980;151:328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]


