Abstract
Objective:
To evaluate endometrial cancer (EC) risk assessment and early detection strategies in high-risk populations, we designed a large, prospective cohort study of women undergoing endometrial biopsy to assess risk factors and collect novel biospecimens for future testing of emerging EC biomarkers. Here we report on the baseline findings of this study.
Methods:
Women aged ≥45 years were enrolled at the Mayo Clinic from February 2013 – June 2018. Risk factors included age, body mass index (BMI), smoking, oral contraceptive and hormone therapy use, and parity. We collected vaginal tampons, endometrial biopsies, and Tao brush samples. We estimated mutually-adjusted odds ratios (OR) and 95% confidence intervals (CI) using multinomial logistic regression; outcomes included EC, atypical hyperplasia, hyperplasia without atypia, disordered proliferative endometrium, and polyps, versus normal endometrium.
Results:
Subjects included 1,205 women with a mean age of 55 years; 55% were postmenopausal, and 90% had abnormal uterine bleeding. The prevalence of EC was 4.1% (n=49), predominantly diagnosed in postmenopausal women (85.7%). Tampons and Tao brushings were obtained from 99% and 68% of women, respectively. Age (OR 1.14, 95% CI 1.1– 1.2) and BMI (OR 1.39, 95% CI 1.1–1.7) were positively associated with EC; atypical hyperplasia (OR 1.07, 95% CI 1.0–1.1; OR 2.00, 95% CI 1.5–2.6, respectively), and polyps (OR 1.06, 95% CI 1.0–1.1; OR 1.17, 95% CI 1.0–1.3, respectively); hormone therapy use and smoking were inversely associated with EC (OR 0.42, 95%, 0.2–0.9; OR 0.43, 95% CI, 0.2–0.9, respectively). Parity and past oral contraception use were not associated with EC.
Conclusions:
Well-established EC risk factors may have less discriminatory accuracy in high-risk populations. Future analyses will integrate risk factor assessment with biomarker testing for EC detection.
Keywords: Endometrial cancer, endometrial hyperplasia, postmenopausal bleeding, risk factors, prospective cohort
Introduction
Endometrial cancer (EC) is the most common gynecologic cancer diagnosed in the U.S., with approximately 61,880 new cases and 12,160 deaths estimated for 2019.1 Unlike most cancers, EC incidence and mortality rates have been increasing worldwide2, 3, and these trends are projected to continue over the next decade.4 EC is most commonly diagnosed in postmenopausal women, with peak incidence rates occurring among those aged 60 to 70 years. Obesity is by far the strongest risk factor for EC; other major risk factors for EC are those presumptively related to cumulative lifetime estrogen exposure, including early age at menarche, older age at menopause, and nulliparity.5–7 Diabetes and metabolic syndrome, tamoxifen exposure, and family history of EC (particularly Lynch Syndrome) are also associated with increased risk of EC, whereas a history of oral contraceptive use and smoking have been shown to be protective against EC.6, 8–12
Most ECs are diagnosed at an early, localized stage, with a 5-year survival of approximately 95%. In contrast, the 5-year survival among women diagnosed with regional and distant stage EC is 70% and 18%, respectively.3, 13–15 The development of invasive EC is preceded by precancerous lesions which can manifest with abnormal uterine bleeding in premenopausal or perimenopausal women (AUB) or postmenopausal bleeding (PMB). Because PMB occurs in approximately 90% of postmenopausal EC, including early stages amenable to cure, a diagnostic and/or therapeutic window of opportunity exists for the early detection and treatment of pre-invasive or early invasive lesions16, 17 However, AUB and PMB are also common symptoms of benign uterine conditions, and only 5% of postmenopausal women undergoing initial diagnostic evaluation for PMB are diagnosed with endometrial cancer in the U.S.16
The increasing burden of EC underscores the need for improved risk assessment and minimally invasive and cost-effective diagnostic options in order to improve early detection without causing undue morbidity and cost in patients without cancer or pre-cancerous conditions.18 While epidemiologic risk prediction models for EC have been validated in population-based studies19, 20, it is not clear how they would perform in high-risk populations such as women with AUB or PMB. Similarly, promising molecular markers for early detection of EC measured from novel, non-invasive sampling devices such as vaginal tampons and Pap tests, require validation in clinically-relevant target populations. 21–23
To address this important gap, we designed a large, prospective clinical cohort study of women who present for diagnostic evaluation secondary to signs/symptoms common in EC. The study includes the collection of a novel biospecimen, the vaginal pool 21, 24, through the placement of an intravaginal tampon, as well as collection of material from the uterine lining using a Tao brush. Here we describe the design, methods, and baseline findings from this study, with emphasis on evaluating associations of epidemiologic risk factors among women with pre- or perimenopausal AUB and PMB and EC.
Materials and Methods
Study Population
Women presenting to the Mayo Clinic’s Division of Gynecology with a clinical indication for endometrial biopsy were prospectively enrolled from February 21, 2013 through June 25, 2018, with passive follow-up every 6 to 12 months. The current analysis includes data from the baseline visit only. Women who were eligible included those aged 45 years or older presenting with clinical signs and/or symptoms common in EC including, pre- or perimenopausal AUB, PMB, and/or abnormal pelvic ultrasound findings. AUB was defined as AUB occurring at age 45 years or older in women who were not in menopause. Women were also eligible if they had a diagnosis of Lynch syndrome and had not undergone risk reducing hysterectomy. Exclusion criteria included prior hysterectomy, prior pelvic radiation, endometrial sampling within the past 3 months, and current pregnancy. The majority of patients were non-Hispanic white (96%); very few identified as non-Hispanic black or Hispanic (1% respectively). This study was approved by the Mayo Clinic and National Cancer Institute Institutional Review Boards; written informed consent was obtained from all participants prior to study enrollment.
Clinical Evaluation
Women enrolled underwent clinical evaluation of the endometrium as determined by their care provider. Clinical testing could include any combination of the following: transvaginal ultrasound, office hysteroscopy, and office endometrial biopsy. If complete workup in the clinic was not feasible, women underwent assessment under anesthesia via hysteroscopy and dilation and curettage (D&C). If clinical indications existed, hysterectomy was performed.
Transvaginal Ultrasound.
Ultrasound was performed as clinically indicated and the interpretation performed by radiologists specialized in pelvic ultrasound interpretation. Abnormal ultrasound findings were defined as any of the following: the presence of an endometrial mass with or without evidence of myometrial invasion, papillary endometrial projections, suspected blood in the uterine cavity, cystic endometrial lesions, or endometrial debris among postmenopausal women. Endometrial stripe thickness was measured by the interpreting clinical radiologist and reported in millimeters (mm).
Hysteroscopy.
Office hysteroscopy using a flexible 3.1 mm diagnostic scope was performed as per the treating clinician’s discretion according to patient age, imaging findings, and clinical feasibility. If an endometrial polyp was found, polyps were resected using an intrauterine morcellator either in the office or under anesthesia in the operating room. If a hysteroscopy was indicated and not feasible to perform in the clinic, women underwent a hysteroscopy under anesthesia.
Endometrial Sampling.
All women enrolled in this study were anticipated to undergo diagnostic evaluation via endometrial sampling. This was performed as a clinic endometrial biopsy with a Pipelle (Pipelle® CooperSurgical, Turnbull, CT) or Endosampler device (MedGyn, Addison, IL) or as a D&C if clinic biopsy was not feasible. Pathology diagnoses were made clinically by gynecologic pathologists. Final pathology from the baseline visit on endometrial sampling was the criterion standard for endometrial pathology diagnosis among those who did not require a hysterectomy. Among women who underwent hysterectomy, the most severe pathology diagnosis on either the endometrial sampling or hysterectomy specimen from the baseline visit was considered the final study diagnosis.
Study Biospecimen Sampling Methods
An intravaginal tampon to collect the vaginal pool, the vaginal effluent from the female reproductive tract 21, 24, was obtained from each woman prior to their clinically indicated office procedures. After providing informed consent, women self-inserted a regular sized polyester-cotton blend tampon into the vagina until the time of clinical exam (approximately 30 minutes). The intravaginal tampon dwell time was recorded. After removal, the tampon was placed in a 50mL conical tube containing a sterile buffer and processed in the research laboratory as previously described 21.
An endometrial brushing was performed using a Tao brush (Cook® Medical, Bloomington, IN) either prior to or following endometrial biopsy. The brush end was removed and placed into a vial containing PreservCyt solution and transferred to the research laboratory where they were processed as previously described.25, 26
Outcome Definitions
Final baseline endometrial pathology diagnoses were classified as: Normal endometrium, endometrial polyp, disordered proliferative endometrium (DPEM), hyperplasia without atypia, hyperplasia with atypia, EC, or other benign histologic findings (e.g., endometritis, fibroids). If an endometrial biopsy was not clinically indicated or performed, the indication was recorded and final pathology was determined from the surgical specimen at baseline, if applicable. Inadequate or non-diagnostic biopsies were also recorded.
Endometrial Cancer Risk Factors and Clinical Characteristics
Baseline information on relevant clinical risk factors for EC including the indication for endometrial sampling, bleeding description, age, body mass index (BMI), hypertension, type II diabetes, and smoking history were abstracted from electronic medical records. Other epidemiologic risk factors including, tamoxifen exposure, current and former hormone replacement therapy (HRT) use, past oral contraception (OC) use, parity, and family history of EC were ascertained via patient interview. Among postmenopausal women, PMB was characterized as either initial episode of or recurrent bleeding. Among premenopausal women, AUB pattern was characterized as one of the following: Heavy menstrual bleeding, intermenstrual bleeding, menometrorrhagia, irregular menses, or other AUB.
Statistical Analysis
We summarized baseline characteristics for the study cohort, overall and by worst diagnosis, using descriptive statistics with Pearson’s chi-square test for categorical variables and one-way ANOVA tests for continuous variables. Among women diagnosed with EC, we summarized method of diagnosis (endometrial sampling and/or hysterectomy), FIGO stage, grade, histology, myometrial invasion, and tumor size, using descriptive statistics, stratified by menopausal status.
We evaluated epidemiologic EC risk factors using multinomial logistic regression analyses, mutually adjusted for age (continuous per 1-year increase), BMI (continuous, per 5kg/m2 increase), ever HRT use, past OC use, parity (parous vs. nulliparous), type II diabetes, hypertension, ever smoking, and family history of EC. We estimated adjusted odds ratios (OR) and 95% confidence intervals (CI) for the association of these risk factors with the full range of histologic outcomes including uterine polyps, DPEM, hyperplasia without atypia, atypical hyperplasia, and EC. For these analyses our reference group included women with normal or other benign histology findings as well as those without an indication for biopsy. We also evaluated associations of EC risk factors with combined endpoints of DPEM and hyperplasia without atypia as well as atypical hyperplasia and EC with the reference group including normal or other benign histology findings, no indication for biopsy, and uterine polyps. Women with inadequate biopsy results were excluded from all models. Results are presented for all women and separately for women with PMB. All analyses were performed in Stata (version 14). All statistical tests were two-sided with p<0.05 considered significant.
Results
Study Population
A total of 2,274 eligible patients presented for evaluation for EC at the Mayo Clinic between February 21, 2013 and June 25, 2018 (Table 1), of these, 1,210 women agreed and consented to participate, and 1,205 aged 45–86 years (mean 55.3 years) were included in the current study (Figure 1). A majority of women presented with PMB (47.1%) or AUB (42.7%), with most postmenopausal women reporting recurrent PMB (59.5%) and most pre- and perimenopausal women reporting menometrorrhagia (38.1%). Among the full cohort, the mean BMI was 30.2 kg/m2 (range 17.2 kg/m2 – 68.6 kg/m2) and the prevalence of hypertension and type II diabetes was 24.3% and 7%, respectively. Most women reported never using HRT (67.3%), never smoking (68.8%) and having one or more live births (83.5%).
Table 1.
Characteristics of 1,205 Women Enrolled in the Mayo Baseline Study by Worst Outcome*
| Total | Not Done† | Inadequate | Normal | Other ‡ | Polyps | DPEM | Hyperplasia without atypia | Atypical Hyperplasia | Endometrial Cancer | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total, n (%) | 1,205 (100.0) | 118 (9.8) | 46 (3.8) | 652 (54.1) | 23 (1.9) | 158 (13.1) | 109 (9.1) | 30 (2.5) | 20 (1.7) | 49 (4.1) |
| Mean Age ±SD | 55.3 ± 8.0 | 55.6 ± 8.6 | 58.0 ± 7.0 | 54.2 ± 7.4 | 52.6 ± 7.1 | 58.1 ± 9.1 | 52.1 ± 5.0 | 57.0 ± 7.9 | 57.8 ± 7.8 | 63.4 ± 8.9 |
| Race/Ethnicity | ||||||||||
| Non-Hispanic White | 1,125 (93.4) | 112 (94.9) | 43 (93.5) | 610 (93.6) | 22 (95.7) | 147 (93.0) | 101 (92.7) | 27 (90.0) | 19 (95.0) | 44 (89.9) |
| Non-Hispanic Black | 11 (0.9) | 1 (0.9) | 1 (2.2) | 7 (1.1) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Hispanic | 18 (1.5) | 1 (0.9) | 1 (2.2) | 12 (1.8) | 0 (0.0) | 3 (1.9) | 0 (0.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Asian/Hawaiian Pacific Islander | 12 (1.0) | 1 (0.9) | 1 (2.2) | 7 (1.1) | 1 (4.3) | 1 (0.6) | (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Other/Unknown | 39 (3.2) | 2 (2.5) | 0 (0.0) | 16 (2.5) | 0 (0.0) | 6 (3.8) | 8 (7.3) | 3 (10.0) | 0 (0.0) | 3 (6.1) |
| Menopausal Status, n (%)§ | ||||||||||
| Pre-menopausal | 544 (45.2) | 52 (44.1) | 3 (6.5) | 314 (47.8) | 16 (69.6) | 50 (32.7) | 81 (74.3) | 15 (50.0) | 6 (30.0) | 7 (14.3) |
| Post-menopausal | 661 (54.8) | 66 (55.9) | 43 (93.5) | 343 (52.2) | 7 (30.4) | 103 (67.3) | 28 (25.7) | 15 (50.0) | 14 (70.0) | 42 (85.7) |
| Abnormal Bleeding, n (%)§ | ||||||||||
| None | 123 (10.2) | 13 (11.0) | 11 (23.9) | 67 (10.2) | 1 (4.4) | 23 (15.0) | 4 (3.7) | 0 (0.0) | 1 (5.0) | 3 (6.1) |
| Postmenopausal Bleeding | 567 (47.1) | 60 (50.9) | 33 (71.7) | 291 (44.3) | 7 (30.4) | 81 (52.9) | 27 (24.8) | 15 (50.0) | 13 (65.0) | 40 (81.6) |
| Abnormal Uterine Bleeding | 515 (42.7) | 45 (38.1) | 2 (4.4) | 299 (45.5) | 15 (65.2) | 49 (32.0) | 78 (71.6) | 15 (50.0) | 6 (30.0) | 6 (12.2) |
| Bleeding Episode (postmenopausal), n (%)* | ||||||||||
| Recurrent | 323 (59.5) | 32 (54.2) | 14 (46.7) | 154 (56.0) | 3 (42.9) | 48 (60.8) | 19 (73.1) | 11 (73.3) | 12 (100.0) | 30 (75.0) |
| Initial | 220 (40.5) | 27 (45.8) | 16 (53.3) | 121 (44.0) | 4 (57.1) | 31 (39.2) | 7 (26.9) | 4 (26.7) | 0 (0.0) | 10 (25.0) |
| Abnormal Uterine Bleeding Type (premenopausal), n (%)‖ | ||||||||||
| Menorrhagia | 160 (31.1) | 13 (28.9) | 0 (0.0) | 106 (35.5) | 2 (13.3) | 12 (24.5) | 22 (28.2) | 3 (20.0) | 2 (33.3) | 0 (0.0) |
| Metrorrhagia | 110 (21.4) | 15 (33.3) | 1 (50.0) | 61 (20.4) | 4 (26.7) | 12 (24.5) | 11 (14.1) | 4 (26.7) | 0 (0.0) | 2 (33.3) |
| Menometrorrhagia | 196 (38.1) | 9 (20.0) | 1 (50.0) | 106 (35.5) | 6 (40.0) | 20 (40.8) | 39 (50.0) | 7 (46.7) | 4 (66.7) | 4 (66.7) |
| Anovulatory Bleeding | 25 (4.9) | 1 (2.2) | 0 (0.0) | 15 (5.0) | 0 (0.0) | 3 (6.1) | 5 (6.4) | 1 (6.7) | 0 (0.0) | 0 (0.0) |
| Other | 24 (4.7) | 7 (15.6) | 0 (0.0) | 11 (3.7) | 3 (20.0) | 2 (4.1) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Current Tamoxifen Use, n (%)‖ | ||||||||||
| No | 1,148 (95.3) | 118 (100.0) | 41 (89.1) | 619 (94.2) | 21 (91.3) | 145 (94.8) | 105 (96.3) | 30 (100.0) | 20 (100.0) | 49 (100.0) |
| Yes | 57 (4.7) | 0 (0.0) | 5 (10.9) | 38 (5.8) | 2 (8.7) | 8 (5.2) | 4 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hormone Replacement Therapy Use, n (%)‖ | ||||||||||
| Never | 811 (67.3) | 86 (72.9) | 29 (63.0) | 434 (66.1) | 12 (52.2) | 97 (63.4) | 84 (77.1) | 17 (56.7) | 15 (75.0) | 37 (75.5) |
| Former | 144 (12.0) | 11 (9.3) | 5 (10.9) | 75 (11.4) | 1 (4.4) | 29 (19.0) | 8 (7.3) | 4 (13.3) | 3 (15.) | 8 (16.3) |
| Current | 250 (20.8) | 21 (17.8) | 12 (26.1) | 148 (22.5) | 10 (43.5) | 27 (17.6) | 17 (15.6) | 9 (30.0) | 2 (10.0) | 4 (8.2) |
| Mean Body Mass Index ± SD§ | 30.2 ± 8.0 | 28.0 ± 6.4 | 30.3 ± 8.7 | 29.5 ± 7.7 | 28.9 ± 7.5 | 31.2 ± 8.1 | 30.8 ± 7.5 | 30.9 ± 8.0 | 41.9 ± 7.8 | 34.2 ± 10.4 |
| Hypertension, n (%)§ | ||||||||||
| No | 912 (75.7) | 87 (73.7) | 31 (67.4) | 526 (80.1) | 17 (73.9) | 114 (74.5) | 82 (75.2) | 19 (63.3) | 8 (40.0) | 28 (57.1) |
| Yes | 293 (24.3) | 31 (26.3) | 15 (32.6) | 131 (19.9) | 6 (26.1) | 39 (25.5) | 27 (24.8) | 11 (36.7) | 12 (60.0) | 21 (42.9) |
| Type II Diabetes, n (%)‖ | ||||||||||
| No | 1,121 (93.0) | 114 (96.6) | 39 (84.8) | 621 (94.5) | 21 (91.3) | 141 (92.2) | 103 (94.5) | 26 (86.7) | 16 (80.0) | 40 (81.6) |
| Yes | 84 (7.0) | 4 (3.4) | 7 (15.2) | 36 (5.5) | 2 (8.7) | 12 (7.8) | 6 (5.5) | 4 (13.3) | 4 (20.0) | 9 (18.4) |
| Smoking Status, n (%) | ||||||||||
| Never | 828 (68.8) | 71 (60.2) | 32 (71.1) | 463 (70.5) | 20 (87.0) | 93 (60.8) | 73 (67.6) | 23 (76.7) | 14 (70.0) | 39 (79.6) |
| Current | 81 (6.7) | 7 (5.9) | 4 (8.9) | 47 (7.2) | 0 (0.0) | 12 (7.8) | 8 (7.4) | 0 (0.0) | 1 (5.0) | 2 (4.1) |
| Former | 294 (24.4) | 40 (33.9) | 9 (20.0) | 147 (22.4) | 3 (13.0) | 48 (31.4) | 27 (25.0) | 7 (23.3) | 5 (25.0) | 8 (16.3) |
| Past Oral Contraception Use, n (%) | ||||||||||
| No | 315 (27.2) | 40 (34.8) | 7 (16.3) | 162 (25.5) | 5 (22.7) | 46 (30.7) | 23 (22.6) | 8 (28.6) | 6 (31.6) | 18 (40.9) |
| Yes | 843 (72.8) | 75 (65.2) | 36 (83.7) | 473 (74.5) | 17 (77.3) | 104 (69.3) | 79 (77.4) | 20 (71.4) | 13 (68.4) | 26 (59.1) |
| Parity, n (%)‖ | ||||||||||
| Nulliparous | 199 (16.5) | 29 (24.6) | 7 (15.2) | 89 (13.6) | 6 (26.1) | 33 (21.6) | 16 (14.8) | 5 (16.7) | 4 (20.0) | 10 (20.4) |
| Parous | 1,005 (83.5) | 89 (75.4) | 39 (84.8) | 556 (86.4) | 17 (73.9) | 120 (78.4) | 92 (85.2) | 25 (83.3) | 16 (80.0) | 39 (79.6) |
| Family History of EC, n (%) | ||||||||||
| No | 1,097 (91.0) | 105 (89.0) | 40 (87.0) | 595 (90.6) | 20 (87.0) | 141 (92.2) | 101 (92.7) | 30 (100.0) | 20 (100.0) | 45 (91.8) |
| Yes | 108 (9.0) | 13 (11.0) | 6 (13.0) | 62 (9.4) | 3 (13.0) | 12 (7.8) | 8 (7.3) | 0 (0.0) | 0 (0.0) | 4 (8.2) |
Worst outcome corresponds to the worst pathology of either the endometrial biopsy or hysterectomy specimen at baseline
Not clinically indicated
Other benign uterine conditions such as endometriosis, fibroids
p<0.0001
p<0.05
Abbreviations: DPEM, Disordered proliferative endometrium; SD, standard deviation; EC, endometrial cancer
Note: Values may not sum to 100% due to rounding
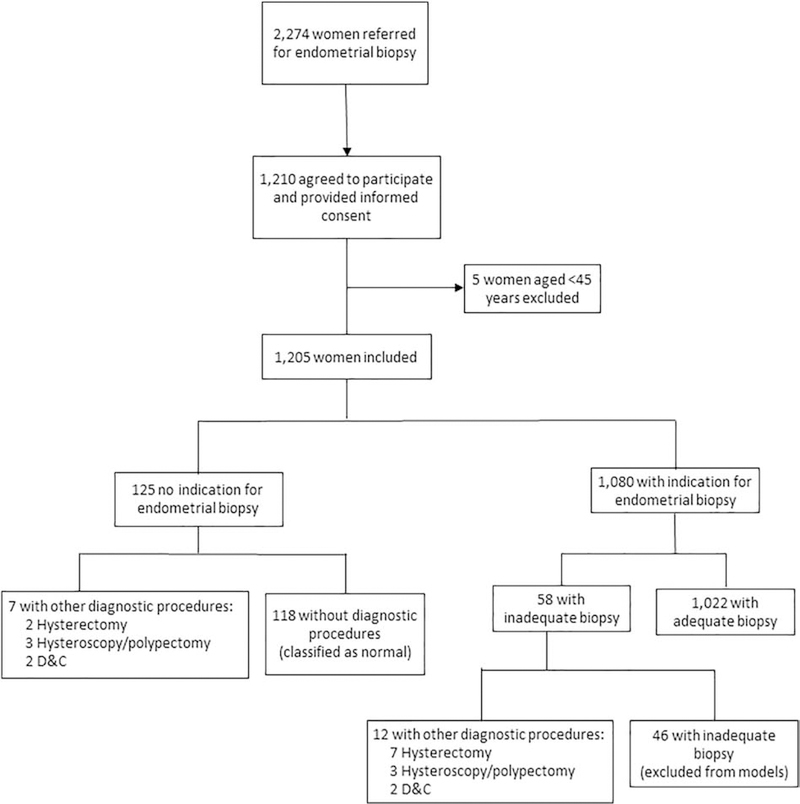
Figure 1.
Flow-Diagram of Study Population.
Biospecimen Collection
Overall, a total of 1,041 women had a histologically-confirmed diagnosis (Table 1). A total of 1,080 women had an endometrial biopsy performed, and in 1,022 of these sampling was adequate (Figure 1). Among the 58 women with inadequate biopsies, 12 underwent other surgical procedures in which a diagnostic tissue specimen was obtained. Of the 125 women without endometrial biopsy, 7 underwent surgery and in the remaining 118, biopsy was not clinically indicated based on age, ultrasound, and/or hysteroscopy results. Intravaginal tampon samples were obtained from 1,197 women (99.3%) prior to biopsy. Tao brush samples were obtained from 826 women (68.5%); the majority of women who did not have a Tao brush sample (n=371) had either a benign endometrial sampling (n=172, 46%) or inadequate/no sampling performed (n=140, 38%).
Histologic Outcomes
The overall prevalence of EC in the study cohort was 4.1% (n=49), with 7 cases occurring in pre- or perimenopausal women (14.3%) and 42 occurring in postmenopausal women (85.7%). Of the 42 cases in postmenopausal women, 40 had PMB (95.2%) and of the 7 cases in pre-or perimenopausal women, 6 had AUB (85.7%). A total of 36 cancers were diagnosed on both biopsy and hysterectomy specimens and 11 cases were diagnosed on hysterectomy only, with two women having benign and 9 having atypical hyperplasia diagnoses on the biopsy specimen. One woman had a biopsy diagnosis of EC and benign endometrium diagnosed from the hysterectomy specimen and one has not undergone hysterectomy (Table 2). Overall, most ECs were endometrioid histology (81.6%), low grade (84%; Grade 1 or 2) and diagnosed at an early stage (85.7%; Stage I). All 7 cases with non-endometrioid histology were diagnosed in postmenopausal women (Table 2). Other pathology diagnoses included normal endometrium (54.1%), polyps (13.1%), DPEM (9.1%), hyperplasia without atypia (2.5%), and atypical hyperplasia (1.7%) (Table 1).
Table 2.
Characteristics of Endometrial Cancers Overall and by Menopausal Status
| Total | Postmenopausal | Premenopausal | |
|---|---|---|---|
| Total | 49 (100.0) | 42 (85.7) | 7 (14.3) |
| Diagnostic Specimen | |||
| Biopsy only* | 2 (4.1) | 2 (4.8) | 0 (0.0) |
| Hysterectomy only† | 11 (22.4) | 8 (19.0) | 3 (42.9) |
| Biopsy and Hysterectomy | 36 (73.5) | 32 (76.2) | 4 (57.1) |
| Pathology Grade | |||
| FIGO Grade 1 | 27 (55.1) | 22 (52.4) | 5 (71.4) |
| FIGO Grade 2 | 14 (28.6) | 12 (28.6) | 2 (28.6) |
| FIGO Grade 3 | 6 (12.2) | 6 (14.3) | 0 (0.0) |
| Missing | 2 (4.1) | 2 (4.8) | 0 (0.0) |
| Pathology Stage | |||
| FIGO Stage IA | 35 (71.4) | 29 (69.1) | 6 (85.7) |
| FIGO Stage IB | 7 (14.3) | 7 (16.7) | 0 (0.0) |
| FIGO Stage IIIA | 1 (2.0) | 1 (2.4) | 0 (0.0) |
| FIGO Stage IIIC1/2 | 3 (6.1) | 2 (4.8) | 1 (14.3) |
| FIGO Stage IVB | 1 (2.0) | 1 (2.4) | 0 (0.0) |
| Missing | 2 (4.1) | 2 (4.8) | 0 (0.0) |
| Histology | |||
| Endometrioid | 40 (81.6) | 33 (78.6) | 7 (100.0) |
| Clear Cell | 2 (4.1) | 2 (4.8) | 0 (0.0) |
| Serous‡ | 3 (6.1) | 3 (7.1) | 0 (0.0) |
| Mixed Endometrioid/Mucinous | 1 (2.0) | 1 (2.4) | 0 (0.0) |
| Carcinosarcoma (MMMT) | 1 (2.0) | 1 (2.4) | 0 (0.0) |
| Missing | 2 (4.1) | 2 (4.8) | 0 (0.0) |
| Myometrial Invasion | |||
| No | 10 (20.4) | 7 (17.5) | 3 (42.9) |
| Yes | 36 (73.5) | 33 (82.5) | 3 (42.9) |
| Missing | 3 (4.1) | 2 (4.8) | 1 (14.3) |
| Tumor Size, Mean ± SD | 3.2 ± 1.6 | 3.3 ± 1.6 | 3.1 ± 1.6 |
Of the two cases diagnosed on biopsy only, one woman has not yet undergone surgery
Of the cases diagnosed by hysterectomy only, 2 had a normal biopsy and 9 had atypical hyperplasia diagnosed on biopsy
Includes one mixed clear cell and serous
Abbreviations: MMMT, malignant mixed Mullerian tumor; SD, standard deviation
Risk Factor Associations with EC, Atypical Hyperplasia, and Benign Histology Diagnoses
Among the whole cohort, older age (per each 1-year increase) and increasing BMI (per each 5kg/m2 increase) were associated with atypical hyperplasia (OR, 1.07, 95% CI, 1.0–1.1 and OR 2.00, 95% CI, 1.5–2.6, respectively) and EC (OR, 1.14, 95% CI, 1.1–1.2 and OR 1.39, 95% CI, 1.1–1.7, respectively) (Table 3). Ever HRT use and ever smoking were both associated with significantly decreased odds of EC compared to never HRT use and never smoking, respectively (OR 0.42, 95% CI, 0.2–0.9 and OR 0.43, 95% CI, 0.2–0.9, respectively), but not with atypical hyperplasia, although smoking showed a similar, but non-significant protective effect (OR, 0.57, 95% CI, 0.2–1.7). Other factors, such as past OC use, parity, type II diabetes, hypertension, and family history of EC were not significantly associated with EC or atypical hyperplasia in this cohort (Table 3; Figure 2A). We observed similar associations in analyses evaluating combined endpoints atypical hyperplasia and EC, although the protective effect of ever HRT use on the odds of EC and atypical hyperplasia was slightly attenuated compared to the association with EC alone (OR 0.55, 95% CI, 0.3–1.0; Supplemental Table 1).
Table 3.
Associations of Endometrial Cancer Risk Factors with Histologic Outcomes in all Women (n=1,159)*
| Polyps (n=158) | p-value | DPEM (n=109) | p-value | Hyperplasia without atypia (n=30) | p-value | Atypical Hyperplasia (n=20) | p-value | Endometrial Cancer (n=49) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (per 1 year increase) | 1.06 (1.0–1.1) | <0.0001 | 0.95 (0.9–1.0) | 0.006 | 1.03 (1.0–1.1) | 0.253 | 1.07 (1.0–1.1) | 0.045 | 1.14 (1.1–1.2) | <0.0001 |
| BMI (per 5kg/m2 increase) | 1.17 (1.0–1.3) | 0.011 | 1.12 (1.0–1.3) | 0.107 | 1.10 (0.9–1.4) | 0.446 | 2.00 (1.5–2.6) | <0.0001 | 1.39 (1.1–1.7) | 0.001 |
| HRT Use | ||||||||||
| Never (n=782) | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Ever (n=377) | 0.81 (0.5–1.2) | 0.320 | 0.75 (0.5–1.3) | 0.276 | 1.45 (0.6–3.3) | 0.369 | 1.00 (0.3–3.1) | 0.987 | 0.42 (0.2–0.9) | 0.022 |
| Past Oral Contraception Use | ||||||||||
| No (n=308) | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Yes (n=807) | 0.94 (0.6–1.4) | 0.773 | 1.30 (0.8–2.2) | 0.312 | 1.01 (0.4–2.4) | 0.980 | 0.88 (0.3–5.5) | 0.819 | 0.82 (0.4–1.6) | 0.573 |
| Parity | ||||||||||
| Nulliparous (n=192) | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Parous (n=966) | 0.58 (0.4–0.9) | 0.014 | 1.13 (0.6–2.0) | 0.674 | 0.87 (0.3–2.4) | 0.786 | 0.86 (0.3–2.9) | 0.806 | 0.60 (0.3–1.3) | 0.209 |
| Type II Diabetes | ||||||||||
| No (n=1,082) | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Yes (n=77) | 1.14 (0.6–2.3) | 0.713 | 0.88 (0.3–2.2) | 0.787 | 2.09 (0.6–7.0) | 0.235 | 1.63 (0.5–5.9) | 0.457 | 2.11 (0.9–5.2) | 0.107 |
| Hypertension | ||||||||||
| No (n=881) | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Yes (n=278) | 0.79 (0.5–1.2) | 0.303 | 1.18 (0.7–2.0) | 0.529 | 1.55 (0.6–3.7) | 0.328 | 1.59 (0.5–4.6) | 0.394 | 0.94 (0.5–1.9) | 0.870 |
| Smoking Status | ||||||||||
| Never (n=796) | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Ever (n=362) | 1.27 (0.9–1.8) | 0.196 | 1.12 (0.7–1.7) | 0.613 | 0.63 (0.3–1.5) | 0.301 | 0.57 (0.2–1.7) | 0.311 | 0.43 (0.2–0.9) | 0.030 |
| Family History of EC | ||||||||||
| No (n=1,057) | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Yes (n=102) | 0.70 (0.4–1.3) | 0.287 | 0.74 (0.3–1.6) | 0.449 | -- | -- | -- | -- | 0.71 (0.2–2.2) | 0.549 |
The reference group includes normal or other benign histology or no indication for biopsy, women with inadequate biopsy results are excluded (n=46)
Note: Two dashes (--) indicate the odds ratio, confidence intervals, and corresponding p-value could not be estimated from the model.
Abbreviations: DPEM, Disordered proliferative endometrium; BMI, body mass index; HRT; hormone replacement therapy; EC, endometrial cancer
Figure 2. Effect estimates for associations of endometrial cancer risk factors with histologic outcomes.
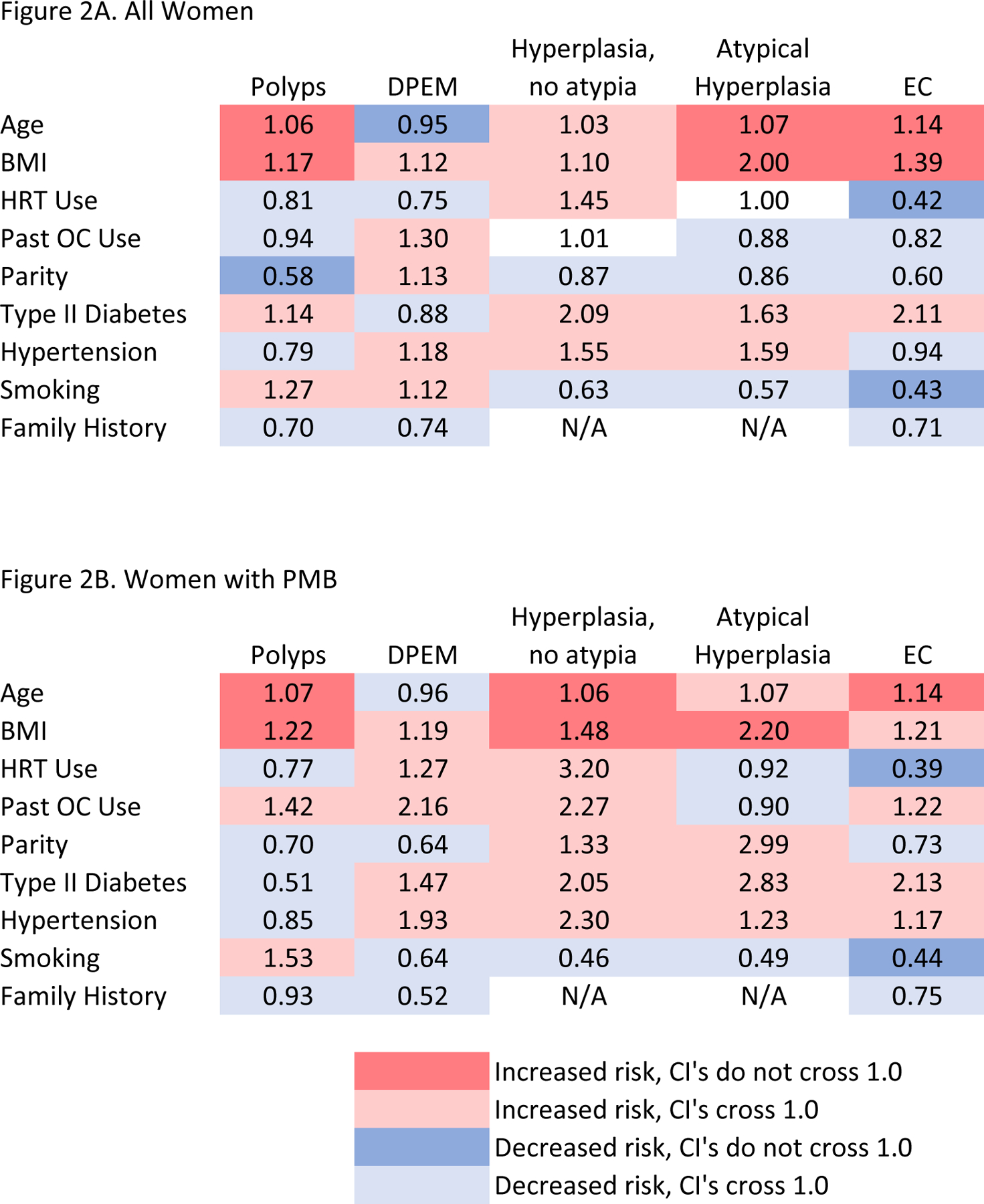
Figure 2A shows associations among all women and Figure 2B shows associations among women with postmenopausal bleeding. Red indicates positive associations for which the confidence intervals do not cross 1.0, whereas pink indicates positive associations for which the confidence intervals cross 1.0. Likewise, blue indicates inverse associations for which the confidence intervals do not cross 1.0, whereas light blue indicates inverse associations for which the confidence intervals cross 1.0. Abbreviations: DPEM, disordered proliferative endometrium; EC, endometrial cancer; BMI, body mass index; HRT, hormone replacement therapy; OC, oral contraceptive
With respect to benign endometrial histologic diagnoses, older age was significantly associated with increased odds of endometrial polyps (OR 1.06 per 1-year increase in age, 95% CI 1.0–1.1), and with decreased odds of DPEM (OR 0.95 per 1-year increase in age, 95% CI 0.9–1.0). Elevated BMI was significantly associated with increased odds of endometrial polyps (OR 1.17 per each 5kg/m2 increase, 95% CI 1.0–1.3), whereas parity was associated with decreased odds of endometrial polyps compared to nulliparity (OR 0.58, 95% CI, 0.4–0.9) (Table 3; Figure 2A). We observed similar associations in analyses evaluating combined endpoints of DPEM and hyperplasia without atypia (Supplemental Table 1).
Risk factor patterns among women with PMB were generally similar to those observed in the overall cohort (Table 4; Figure 2B). Of note, BMI became significantly associated with increased odds of hyperplasia without atypia (OR 1.48, 95% CI 1.0–2.1) and older age (OR, 1.06, 95% CI, 1.0–1.1) and ever HRT use (OR, 3.20, 95% CI, 0.9–11.4) became marginally significantly associated with increased odds of hyperplasia without atypia in women with PMB. Patterns were similar in analyses of combined endpoints, with the exception of type II diabetes becoming significantly associated with increased odds of atypical hyperplasia and EC (OR 2.64, 95% CI, 1.1–6.2) compared to not having diabetes. The effects of age, BMI, and ever HRT use were not significantly associated with odds of DPEM and hyperplasia without atypia combined (Supplemental Table 2).
Table 4.
Associations of Endometrial Cancer Risk Factors with Histologic Outcomes in Women with Postmenopausal Bleeding, n=534*
| Polyps (n=82) | p-value | DPEM (n=27) | p-value | Hyperplasia without atypia (n=15) | p-value | Atypical Hyperplasia (n=13) | p-value | Endometrial Cancer (n=40) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (per 1 year increase) | 1.07 (1.0–1.1) | <0.0001 | 0.96(0.9–1.0) | 0.293 | 1.06 (1.0–1.1) | 0.093 | 1.07 (1.0–1.2) | 0.149 | 1.14 (1.1–1.2) | <0.0001 |
| BMI (per 5kg/m2 increase) | 1.22 (1.0–1.4) | 0.020 | 1.19 (0.9–1.6) | 0.183 | 1.48 (1.0–2.1) | 0.030 | 2.20 (1.5–3.3) | <0.0001 | 1.21 (1.0–1.5) | 0.110 |
| HRT Use | ||||||||||
| Never (n=278) | Ref | Ref | Ref | Ref | Ref | |||||
| Ever (n=256) | 0.77 (0.5–1.3) | 0.345 | 1.27 (0.5–3.0) | 0.591 | 3.20 (0.9–11.4) | 0.073 | 0.92 (0.2–4.2) | 0.911 | 0.39(0.2–0.9) | 0.022 |
| Past Oral Contraception Use | ||||||||||
| No (n=153) | Ref | Ref | Ref | Ref | Ref | |||||
| Yes (n=352) | 1.42 (0.8–2.5) | 0.234 | 2.16 (0.7–6.8) | 0.188 | 2.27 (0.6–8.9) | 0.239 | 0.90 (0.2–3.3) | 0.872 | 1.22 (0.5–2.7) | 0.625 |
| Parity | ||||||||||
| Nulliparous (n=91) | Ref | Ref | Ref | Ref | Ref | |||||
| Parous (n=443) | 0.70 (0.4–1.3) | 0.278 | 0.64 (0.2–1.7) | 0.368 | 1.33 (0.3–6.6) | 0.726 | 2.99 (0.3–27.0) | 0.329 | 0.73 (0.3–1.8) | 0.501 |
| Type II Diabetes | ||||||||||
| No (n=490) | Ref | Ref | Ref | Ref | Ref | |||||
| Yes (n=44) | 0.51 (0.2–1.6) | 0.250 | 1.47 (0.4–5.9) | 0.589 | 2.05 (0.5–9.2) | 0.352 | 2.83 (0.7–11.8) | 0.153 | 2.13 (0.8–5.9) | 0.150 |
| Hypertension | ||||||||||
| No (n=382) | Ref | Ref | Ref | Ref | Ref | |||||
| Yes (n=152) | 0.85 (0.5–1.6) | 0.616 | 1.93 (0.8–5.0) | 0.176 | 2.30 (0.7–7.8) | 0.181 | 1.23 (0.3–4.9) | 0.764 | 1.17 (0.5–2.6) | 0.691 |
| Smoking Status | ||||||||||
| Never (n=355) | Ref | Ref | Ref | Ref | Ref | |||||
| Ever (n=179) | 1.53 (0.9–2.5) | 0.102 | 0.64 (0.3–1.6) | 0.353 | 0.46 (0.1–1.6) | 0.226 | 0.49 (0.1–1.9) | 0.302 | 0.44 (0.2–1.0) | 0.059 |
| Family History of EC | ||||||||||
| No (n=479) | Ref | Ref | Ref | Ref | Ref | |||||
| Yes (n=55) | 0.93 (0.4–2.1) | 0.862 | 0.52 (0.1–2.4) | 0.397 | -- | -- | -- | -- | 0.75 (0.2–2.5) | 0.631 |
The reference group includes normal or other benign histology or no indication for biopsy, women with inadequate biopsy results are excluded (n=33)
Note: Two dashes (--) indicate the odds ratio, confidence intervals, and corresponding p-value could not be estimated from the model.
Abbreviations: DPEM, Disordered proliferative endometrium; BMI, body mass index; HRT, hormone replacement therapy; EC, endometrial cancer
Discussion
The growing burden of EC and the impact of early diagnosis on improved cancer-specific survival underscore the imperative for developing effective early detection approaches. Currently population-based screening for EC does not exist, and the most common presenting symptoms, pre- or perimenopausal AUB or PMB, lack specificity for EC.16 This study was designed to evaluate clinical and epidemiologic risk factors for EC, and to collect novel biospecimens for future testing of emerging molecular markers of EC in a clinical cohort of women at elevated risk. 21, 26 Importantly, as opposed to most previous efforts, our study design enables the evaluation of EC risk assessment and early-detection approaches in a target population of women in whom these strategies would ultimately be applied; women presenting for evaluation prior to biopsy.
The overall prevalence of EC in our study population was 4.1% and varied by menopausal and bleeding status, with the highest prevalence among women with PMB (7.1%). Among pre- and perimenopausal women with AUB the most common abnormal finding was DPEM, whereas among women with PMB, the most common abnormal finding was uterine polyps. Interestingly, the prevalence of atypical hyperplasia was much lower than the prevalence of EC in our cohort. Approximately 30% of women with atypical hyperplasia are expected to progress to EC over 25 years, suggesting that the prevalence of atypical hyperplasia in the population should be higher than EC.27 It is conceivable that a subset of atypical hyperplasia does not present with abnormal bleeding, while almost all women with EC have abnormal bleeding. It is also plausible that a subset of atypical hyperplasias regress spontaneously or in response to pharmacologic therapy.28
Women with abnormal bleeding are an ideal group for EC early detection strategies, given their increased risk of being diagnosed with EC and the fact that most ECs occur in women with abnormal bleeding, including early-stage ECs.16 However, most epidemiologic risk prediction models19, 20 and molecular biomarkers for EC detection22, 23 have not been evaluated in this population. Unlike population-based studies, our study was enriched for women with pre- and perimenopausal AUB and PMB, including those with benign uterine conditions. Importantly, not all established EC risk factors showed associations with EC in our population. While some risk factors including older age, increasing BMI, and smoking (protective), were associated with EC as expected, the established strong protective risk factor OC use was not significantly associated with reduced risk of EC in our study cohort, but instead showed an increased odds ratio. While the association between OC use and EC is strong and undisputed11,12, approximately 70% of women in our study, including those with a pathologically unremarkable appearing endometrium, reported past OC use compared with 40–50% of women in other population-based cohort studies.29–31 Thus, the lack of an association between past OC use and EC in our study is likely secondary to higher OC use in our reference group compared to the general population. Further, we found that age, BMI, and nulliparity, were significantly associated with benign endometrial polyps, suggesting that shared associations between benign endometrial conditions and EC may limit the performance of established risk factors for prediction of EC in women with AUB/PMB. In the future, molecular markers may allow to better distinguish polyps, and other benign conditions, from EC among women presenting with AUB or PMB.
We observed a strong inverse relationship between ever HRT use and decreased odds of EC. This observation could have several explanations: In the general population, studies have shown that certain combined formulations of estrogen plus progestin hormone therapy are protective against EC.32 Further, HRT can cause irregular uterine bleeding, particularly within the first 6 months of use, which likely leads to an enrichment of our population for women with HRT-related uterine bleeding not related to EC. In support of that, in a recent review of the literature, we found that among women with PMB, the risk of EC was significantly lower in studies of women using HRT compared to studies that excluded these women, in line with our results.16
Strengths of this study include a large clinical population of women undergoing diagnostic evaluation for perimenopausal AUB and PMB, symptoms that are present in 90% of women diagnosed with EC, which allows us to evaluate current clinical practice and inform evidence-based clinical decision making. Follow-up of this study cohort is ongoing and will enable prospective evaluation of clinical gynecologic outcomes following the baseline endometrial pathology diagnoses at the time of enrollment. Currently, our understanding of the longitudinal sensitivity of endometrial biopsy and other diagnostic and sampling approaches is limited. The assessment of longitudinal associations of epidemiologic and clinical risk factors, long-term performance of diagnostic and management approaches at enrollment and in subsequent encounters, as well as testing of emerging molecular EC biomarkers will lead to further understanding of diagnostic procedures and early intervention and prevention opportunities. The limitations of this study include the fact that, despite a large sample size, the prevalence of EC and precursor lesions in this population was still relatively low, therefore, our power to detect statistically significant associations for some EC risk factors with smaller effect sizes was limited. However, we were adequately powered to detect associations with key risk factors like BMI and OC use. As enrollment is still ongoing, and follow-up of this population continues, the opportunity to accrue additional cases and study prospective outcomes and biomarkers exists. Additionally, while our study cohort reflects real-world clinical practice, the population was predominantly white, we did not have data on factors such as socioeconomic status, and results may not be generalizable to other, racially-diverse populations and/or clinical settings (e.g., primary care or Women’s Health clinics).
Conclusions
The growing incidence of EC supports the need to develop clinically-useful risk prediction models and early detection strategies. Findings from the present study suggest that models based on classic epidemiologic risk factors for EC may have more limited discriminatory accuracy in elevated risk populations with the ambiguous symptom of AUB or PMB, given the shared risk factors between EC and benign etiologies of AUB/PMB. Our prospective cohort study establishes a rich resource that will integrate clinical, epidemiologic, and biomarker data in a population of women at elevated risk for EC. Going forward, it will be important to evaluate the performance of established epidemiologic19, 20 and clinical EC risk prediction models33, as well as novel candidate EC biomarkers, in our study population.
Supplementary Material
Highlights:
This large clinical prospective cohort study evaluates risk factors for endometrial cancer and its precursors
Vaginal tampons, endometrial biopsies, and Tao brushes were collected for future testing of emerging biomarkers
Of the 1,205 participants, 90% had abnormal uterine bleeding and the prevalence of endometrial cancer was 4.1% (n=49)
Endometrial cancer and benign uterine conditions (e.g., polyps) shared risk factors such as age and body mass index
Parity and oral contraception use were not associated with endometrial cancer risk in this high-risk population
Acknowledgments:
This research was supported in part by the Mayo Clinic Specialized Program of Research Excellence (SPORE) in Ovarian Cancer, CA136393 from the National Institutes of Health; Mayo Clinic’s NCI Cancer Center Support Grant, P30 CA 15083; and the Intramural Research Program of the National Cancer Institute (Z01CP010124–21). We acknowledge Ann VanOosten for her assistance with study recruitment and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J Natl Cancer Inst 2017. [DOI] [PubMed]
- 3.Clarke MA, Devesa SS, Harvey S, Wentzensen N. Hysterectomy-corrected uterine corpus incidence trends and differences in relative survival reveal racial disparities and rising incidence of non-endometrioid cancers. In Press 2019. [DOI] [PMC free article] [PubMed]
- 4.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–21. [DOI] [PubMed] [Google Scholar]
- 5.Suarez AA, Felix AS, Cohn DE. Bokhman Redux: Endometrial cancer “types” in the 21st century. Gynecol Oncol 2017; 144: 243–9. [DOI] [PubMed] [Google Scholar]
- 6.Felix AS, Brinton LA. Cancer Progress and Priorities: Uterine Cancer. Cancer Epidemiol Biomarkers Prev 2018; 27: 985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B. Recent changes in endometrial cancer trends among menopausal-age U.S. women. Cancer Epidemiol 2013; 37: 374–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, Wolk A, Wentzensen N, Weiss NS, Webb PM, van den Brandt PA, van de Vijver K, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013; 31: 2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer 2018. [DOI] [PubMed]
- 10.Onstad MA, Schmandt RE, Lu KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J Clin Oncol 2016; 34: 4225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol 2015; 16: 1061–70. [DOI] [PubMed] [Google Scholar]
- 12.Felix AS, Yang HP, Bell DW, Sherman ME. Epidemiology of Endometrial Carcinoma: Etiologic Importance of Hormonal and Metabolic Influences. Adv Exp Med Biol 2017; 943: 3–46. [DOI] [PubMed] [Google Scholar]
- 13.Weiderpass E, Antoine J, Bray FI, Oh JK, Arbyn M. Trends in corpus uteri cancer mortality in member states of the European Union. Eur J Cancer 2014; 50: 1675–84. [DOI] [PubMed] [Google Scholar]
- 14.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006; 95 Suppl 1: S105–43. [DOI] [PubMed] [Google Scholar]
- 15.UK CR. Uterine cancer statistics, vol. 2018: Cancer Research UK. [Google Scholar]
- 16.Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA Intern Med 2018; 178: 1210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matteson KA, Robison K, Jacoby VL. Opportunities for Early Detection of Endometrial Cancer in Women With Postmenopausal Bleeding. JAMA Intern Med 2018; 178: 1222–3. [DOI] [PubMed] [Google Scholar]
- 18.Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, Boyd N, Pike J, Anglesio M, Kwon JS, Karnezis AN, Huntsman DG, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017; 123: 802–13. [DOI] [PubMed] [Google Scholar]
- 19.Pfeiffer RM, Park Y, Kreimer AR, Lacey JV Jr., Pee D, Greenlee RT, Buys SS, Hollenbeck A, Rosner B, Gail MH, Hartge P. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med 2013; 10: e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortner RT, Husing A, Kuhn T, Konar M, Overvad K, Tjonneland A, Hansen L, Boutron-Ruault MC, Severi G, Fournier A, Boeing H, Trichopoulou A, et al. Endometrial cancer risk prediction including serum-based biomarkers: results from the EPIC cohort. Int J Cancer 2017; 140: 1317–23. [DOI] [PubMed] [Google Scholar]
- 21.Bakkum-Gamez JN, Wentzensen N, Maurer MJ, Hawthorne KM, Voss JS, Kroneman TN, Famuyide AO, Clayton AC, Halling KC, Kerr SE, Cliby WA, Dowdy SC, et al. Detection of endometrial cancer via molecular analysis of DNA collected with vaginal tampons. Gynecol Oncol 2015; 137: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinde I, Bettegowda C, Wang Y, Wu J, Agrawal N, Shih Ie M, Kurman R, Dao F, Levine DA, Giuntoli R, Roden R, Eshleman JR, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med 2013; 5: 167ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Li L, Douville C, Cohen JD, Yen TT, Kinde I, Sundfelt K, Kjaer SK, Hruban RH, Shih IM, Wang TL, Kurman RJ, et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med 2018; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiegl H, Gattringer C, Widschwendter A, Schneitter A, Ramoni A, Sarlay D, Gaugg I, Goebel G, Muller HM, Mueller-Holzner E, Marth C, Widschwendter M. Methylated DNA collected by tampons--a new tool to detect endometrial cancer. Cancer Epidemiol Biomarkers Prev 2004; 13: 882–8. [PubMed] [Google Scholar]
- 25.Kipp BR, Medeiros F, Campion MB, Distad TJ, Peterson LM, Keeney GL, Halling KC, Clayton AC. Direct uterine sampling with the Tao brush sampler using a liquid-based preparation method for the detection of endometrial cancer and atypical hyperplasia: a feasibility study. Cancer 2008; 114: 228–35. [DOI] [PubMed] [Google Scholar]
- 26.Wentzensen N, Bakkum-Gamez JN, Killian JK, Sampson J, Guido R, Glass A, Adams L, Luhn P, Brinton LA, Rush B, d’Ambrosio L, Gunja M, et al. Discovery and validation of methylation markers for endometrial cancer. Int J Cancer 2014; 135: 1860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacey JV Jr., Sherman ME, Rush BB, Ronnett BM, Ioffe OB, Duggan MA, Glass AG, Richesson DA, Chatterjee N, Langholz B. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J Clin Oncol 2010; 28: 788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol 2012; 125: 263–70. [DOI] [PubMed] [Google Scholar]
- 29.Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the Associations Between Duration of Oral Contraceptive Use and Ovarian, Endometrial, Breast, and Colorectal Cancers. JAMA Oncol 2018; 4: 516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michels KA, Brinton LA, Pfeiffer RM, Trabert B. Oral Contraceptive Use and Risks of Cancer in the NIH-AARP Diet and Health Study. Am J Epidemiol 2018; 187: 1630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlton BM, Rich-Edwards JW, Colditz GA, Missmer SA, Rosner BA, Hankinson SE, Speizer FE, Michels KB. Oral contraceptive use and mortality after 36 years of follow-up in the Nurses’ Health Study: prospective cohort study. BMJ 2014; 349: g6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trabert B, Wentzensen N, Yang HP, Sherman ME, Hollenbeck AR, Park Y, Brinton LA. Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? Int J Cancer 2013; 132: 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burbos N, Musonda P, Duncan TJ, Crocker SG, Morris EP, Nieto JJ. Estimating the risk of endometrial cancer in symptomatic postmenopausal women: a novel clinical prediction model based on patients’ characteristics. Int J Gynecol Cancer 2011; 21: 500–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.