Abstract
Background
Primary mediastinal nonseminoma germ cell tumors (PMNSGCT) are a subgroup of nonseminoma germ cell tumors (GCT) with poor prognosis. In this study, PMNSGCT-specific genomic landscape was analyzed and correlated with clinical outcomes.
Methods
DNA was extracted and sequenced from 28 archival tumor tissue of patients with mediastinal GCT (3 seminoma and 25 nonseminoma). Overall survival (OS) and association with gene alterations were estimated using the Kaplan-Meier and univariate Cox regression methods.
Results
Three patients (11%) had a karyotype XXY, 17/28 (61%) tumor samples presented chromosome 12p amplification. Somatic mutations were detected in 19/28 (68%) samples. The most frequently mutated genes were: TP53 (13/28; 46%), KIT (5/28; 18%), and KRAS (5/28; 18%). Deleterious TP53 alterations were associated with significantly reduced overall survival (HR: 7.16; P = .012).
Conclusions
TP53 alterations are common in PMNSGCT and are associated with reduced overall survival, potentially underlying the poor sensitivity to chemotherapy observed in these patients.
Keywords: TP53, nonseminoma, germ cell tumors
Germ cell tumors (GCT) are divided into 2 groups: seminoma and nonseminoma, with primary mediastinal nonseminoma GCT having the most aggressive clinical behavior. This article describes the genomic landscape of primary mediastinal GCTs and whether selected genomic alterations are associated with poor prognosis.
Introduction
Germ cell tumors (GCT) are uncommon malignancies typically arising from the male gonad. GCT are usually divided into 2 groups: seminoma and nonseminoma. Approximately 5% of GCT originate outside of the gonad, and among these, 80% originate from the mediastinum. The prognostic allocation of metastatic GCT is historically built upon clinical and serological characteristics included in the International Germ Cell Cancer Cooperative Group (IGCCCG) classification.1 While the prognosis of primary mediastinal seminoma is similar to that of testicular seminoma, primary mediastinal nonseminoma GCT (PMNSGCT) have more aggressive clinical behavior and overall poorer prognosis than their gonadal nonseminoma counterpart.2 Indeed, the survival of these patients has remained largely unchanged for several decades despite the increasing utilization of more intense chemotherapy regimens, which have ultimately improved the survival of the IGCCCG poor prognosis cisplatin resistant gonadal nonseminoma patients.3,4 Primary gonadal GCT are characterized by a low tumor mutational burden (0.5-1 mutations/Mb). However, a relatively higher TMB and greater frequency of TP53 alterations have been described in cisplatin-resistant GCT.5-7 To date, few studies have analyzed the genomic features of PMNSGCT and no correlations between genomic alterations and clinical outcomes have been reported specifically in PMNSGCT. Our study aimed to further define the genomic landscape of primary mediastinal GCTs and test whether selected genomic alterations are associated with poor prognosis.
Material and Methods
From 2003 to 2017, we conducted a multi-institutional retrospective cohort study including 28 patients with primary mediastinal GCT treated at BC Cancer Centres in British Columbia (Canada) and Fondazione IRCCS Istituto Nazionale dei Tumori, Milan (Italy). Ten samples were obtained from chemotherapy-naïve patients, 18 were collected post-chemotherapy. Twenty-five samples were from the primary tumor while the remaining were from metastatic sites (Supplementary Table S1).
Formalin-fixed paraffin-embedded (FFPE) tumor tissue blocks were macro-dissected for DNA extraction by a specialized cancer pathologist (LF) at the Vancouver Prostate Centre. Tumor DNA was subject to targeted sequencing across the coding regions of 578 genes using the Roche SeqCap EZ Comprehensive Cancer Design panel. Mutations and copy number changes were identified based on an established computational approach.8 All de-identified targeted DNA sequencing data have been deposited in the European Genome-Phenome Archive (EGA) under the accession code EGAS00001006455. Statistical tests and data analyses were conducted in Python 3.8.5. Survival was estimated via the Kaplan-Meier method and hazard ratios were calculated via univariate Cox proportional hazards regression. Overall survival (OS) was defined as the time from initial diagnosis to death or last follow-up. Hypothesis tests were 2 tailed and used a 5% significance threshold (Supplementary Methods).
Results
Overall, we analyzed 3 seminoma and 25 nonseminoma. The most frequent histology among nonseminoma patients was mixed germ cell tumor (16/25). Pure yolk sac tumor and teratoma were observed in 5 and 4 patients, respectively. Among the seminoma patients, 2 had intermediate and one had IGCCCG good prognosis. Twenty-six out of 28 patients (92%) received systemic chemotherapy. Most patients achieved partial response (21/28; 75%) and only one patient had progressive disease as best response to chemotherapy. Complete response to chemotherapy was observed in 2 patients, both seminomas. 25/28 patients (89%) underwent surgical resection of the mediastinal mass. Two patients, both teratomas, underwent surgery without receiving chemotherapy. Among those who did not have surgery, 2 were seminomas who achieved complete response to chemotherapy, and one was a nonseminoma who had a partial response after chemotherapy and received subsequent radiation therapy. The median follow-up time was 9.2 years (range: 5-227 months). At the time of this analysis, 11 patients (39%), all with nonseminoma, had died from their disease. Complete patient characteristics are summarized in Table 1 and Supplementary Table S2.
Table 1.
Patient characteristics.
| Seminoma | Non-seminoma | Total | |
|---|---|---|---|
| Histology, % (n) | |||
| Pure seminoma | 10.7 (3) | 0 (0) | 10.7 (3) |
| Pure YST | 0 (0) | 17.8 (5) | 17.8 (5) |
| Pure mature teratoma | 0 (0) | 14.2 (4) | 14.2 (4) |
| Mixed GCT | 0 (0) | 57.1 (16) | 57.1 (16) |
| IGCCCG prognostic groups, % (n) | |||
| Good | 3.5 (1) | NA | 3.5 (1) |
| Intermediate | 7.1 (2) | NA | 7.1 (2) |
| Poor | NA | 89.2 (25) | 89.2 (25) |
| First-line chemotherapy, % (n) | |||
| BEP | 3.5 (1) | 71.4 (20) | 75 (21) |
| VIP | 3.5 (1) | 10.7 (3) | 14.2 (4) |
| EP | 3.5 (1) | 0 (0) | 3.5 (1) |
| Best response to chemotherapy, % (n) | |||
| CR | 7.1 (2) | 0 (0) | 7.1 (2) |
| PR | 0 (0) | 75 (21) | 75 (21) |
| SD | 0 (0) | 0 (0) | 0 (0) |
| PD | 0 (0) | 3.5 (1) | 3.5 (1) |
| Surgery, % (n) | 3.5 (1) | 85.7 (24) | 89.2 (25) |
| Patients status | |||
| Alive | 10.7 (3) | 50 (14) | 60.7 (17) |
| Deceased | 0 (0) | 39.2 (11) | 39.2 (11) |
Abbreviations: BEP, bleomycin, etoposide, cisplatin; CR, complete response; EP, etoposide, cisplatin; GCT, germ cell tumours; IGCCCG, International Germ Cell Consensus Classification; PD, progressive disease.; PR, partial response; SD, stable disease; VIP, etoposide, ifosfamide, cisplatin; YST, yolk sac tumors.
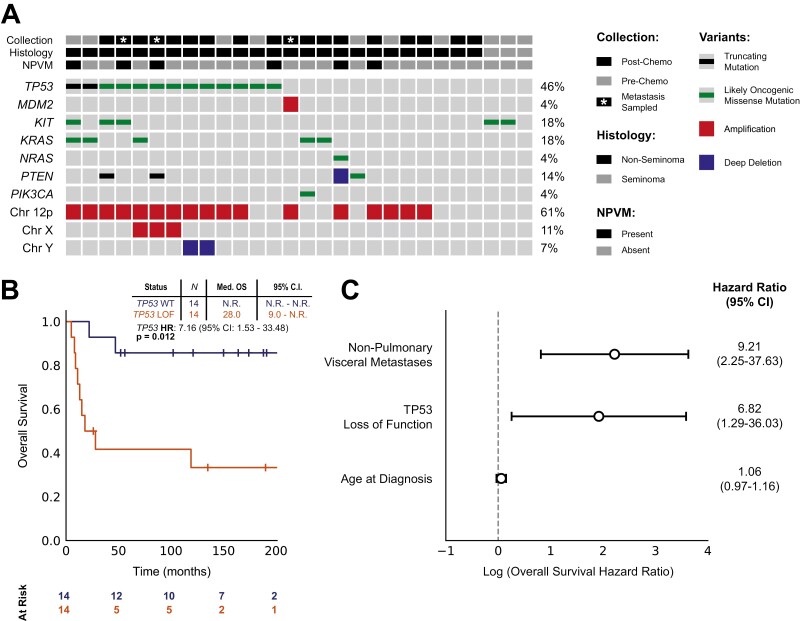
Since healthy normal tissue was unavailable from the majority of patients, we restricted our analyses to likely oncogenic events only (see Supplementary Methods). Using this criterion, somatic mutations were detected in 19/28 (68%) patients. The most frequently mutated genes were: TP53 (13/28; 46%), KIT (5/28; 18%), KRAS (5/28; 18%), PTEN (3/28; 11%), NRAS (1/28; 4%), and PIK3CA (1/28; 4%) (Fig. 1A; Supplementary Table S3). As described in primary gonadal disease, PMNSGCTs were markedly aneuploid.6 The majority of the tumor samples presented with large-scale copy number alterations. We identified 17/28 (61%) patients with amplification of chromosome 12p, 3/28 (11%) with amplification of chromosome X, and 2/28 (7%) with deletion of chromosome Y.9 We identified 3/27 (11%) patients with Klinefelter karyotype (XXY) by screening for heterozygous SNPs on the X chromosome (by contrast, in XY males all chromosome X polymorphisms are homozygous).
Figure 1.
Genomic alterations and association with survival outcomes. (A) Oncoprint diagram showing all likely oncogenic alterations detected across 28 PMNSGCT patients. (B) Kaplan-Meier curves showing overall survival (OS) in 28 PMNSGCT patients. OS was defined as the time from initial diagnosis to death or last follow-up. Stratification is based on the presence of any alteration predicted to impair TP53 function, including amplification of MDM2. (C) Forest plot showing a multivariate survival analysis of TP53 loss, presence of non-pulmonary visceral metastases (NPVM), and age at diagnosis. The x-axis represents a log-scaled hazard ratio for overall survival (OS).
Compared to the previously reported low frequency of TP53 alterations in primary gonadal GCT, we observed a markedly higher rate of TP53 alterations in PMNSGCT, consistently with previous findings.6,7,10 Notably, none of the 3 seminoma patients carried a TP53 alteration. We also identified one additional sample with focal amplification of the negative regulator of TP53, MDM2. Overall, we identified TP53 loss-of-function in 14/28 patients (50%), which rose to 56% when restricted to nonseminomas only.
TP53 aberrations were associated with significantly reduced OS in our cohort (HR: 7.16; P = .012; Fig. 1B). This remained significant after excluding the 3 seminoma patients for whom no TP53 alterations were detected (HR: 5.60; P = .029).
While all PMNSGCTs are defined as poor risk by the IGCCCG classification system, other characteristics have been associated with poorer prognosis including lung, brain, and liver metastases.11 The presence of non-pulmonary visceral metastases (NPVM) was isolated as an independent clinical prognostic factor among our patients (n = 6; HR: 6.27; P = .003). In multivariate analysis, both NPVM and TP53 loss-of-function were significantly associated with reduced OS (Fig. 1C). No significant difference in the rate TP53 alterations was observed between the pre- and post-chemotherapy samples (P = .71; Fisher’s exact test).
Discussion
Despite a limited sample size, we show that TP53 alterations occur at a higher rate in PMNSGCT than has been reported in gonadal primary disease or mediastinal seminoma and that these alterations are associated with marked reduction in OS. Meanwhile, due to the limitations of a targeted sequencing approach, rates of low-level 12p amplification may be underreported. Our findings suggest that underlying molecular abnormalities (ie, TP53) could explain the poor outcomes of PMNSGCT patients, most likely through poor sensitivity to cisplatin, as previously demonstrated in gonadal and extragonadal GCT.7 Further mechanistic studies are needed to explore the causality of cisplatin resistance in the presence of TP53 alterations in PMNSGCT.
Supplementary Material
Contributor Information
Jack V W Bacon, Vancouver Prostate Centre, Department of Urologic Sciences, University of British Columbia, British Columbia, Canada.
Patrizia Giannatempo, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei tumori, Milan, Italy.
Giovanna Cataldo, University “L. Vanvitelli”, Naples, Italy.
Ladan Fazli, Vancouver Prostate Centre, Department of Urologic Sciences, University of British Columbia, British Columbia, Canada.
Neetu Saxena, Vancouver Prostate Centre, Department of Urologic Sciences, University of British Columbia, British Columbia, Canada.
Guliz Ozgun, Vancouver Prostate Centre, Department of Urologic Sciences, University of British Columbia, British Columbia, Canada.
Maryam Soleimani, Department of Medical Oncology, BC Cancer, British Columbia, Canada.
Kim Chi, Department of Medical Oncology, BC Cancer, British Columbia, Canada.
Craig Nichols, Testicular Cancer Commons, Beaverton, OR, USA.
Andrea Necchi, Vita-Salute San Raffaele University; IRCCS San Raffaele Hospital, Milan, Italy.
Alexander W Wyatt, Vancouver Prostate Centre, Department of Urologic Sciences, University of British Columbia, British Columbia, Canada; Michael Smith Genome Sciences Centre, BC Cancer, Vancouver, British Columbia, Canada.
Christian K Kollmannsberger, Department of Medical Oncology, BC Cancer, British Columbia, Canada.
Lucia Nappi, Vancouver Prostate Centre, Department of Urologic Sciences, University of British Columbia, British Columbia, Canada; Department of Medical Oncology, BC Cancer, British Columbia, Canada.
Conflict of Interest
Maryam Soleimani: Bayer (C/A), AbbVie, Astellas, Bayer (RF), Pfizer, Ipsen (H); Alexander Wyatt: AstraZeneca, Merck, Janssen (C/A), ESSA Pharma (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Nichols CR. Mediastinal germ cell tumors. Clinical features and biologic correlates. Chest. 1991;99(2):472-479. [DOI] [PubMed] [Google Scholar]
- 2. Gillessen S, Sauvé N, Collette L, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG update consortium. J Clin Oncol. 2021;39(14):1563-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albany C, Adra N, Snavely AC, et al. Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors. Ann Oncol. 2018;29(2):341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University Experience. J Clin Oncol. 2017;35(10):1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cutcutache I, Suzuki Y, Tan IB, et al. Exome-wide sequencing shows low mutation rates and identifies novel mutated genes in seminomas. Eur Urol. 2015;68(1):77-83. [DOI] [PubMed] [Google Scholar]
- 6. Loveday C, Litchfield K, Proszek PZ, et al. Genomic landscape of platinum resistant and sensitive testicular cancers. Nat Commun. 2020;11(1):2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagrodia A, Lee BH, Lee W, et al. Genetic determinants of cisplatin resistance in patients with advanced germ cell tumors. J Clin Oncol. 2016;34(33):4000-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vandekerkhove G, Lavoie JM, Annala M, et al. Plasma ctDNA is a tumor tissue surrogate and enables clinical-genomic stratification of metastatic bladder cancer. Nat Commun. 2021; 12(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Litchfield K, Summersgill B, Yost S, et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat Commun. 2015;6(1):5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Necchi A, Bratslavsky G, Chung J, et al. Genomic features for therapeutic insights of chemotherapy-resistant, primary mediastinal nonseminomatous germ cell tumors and comparison with gonadal counterpart. Oncologist 2019;24(4):e142-e145. 10.1634/theoncologist.2018-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartmann JT, Nichols CR, Droz JP, et al. Prognostic variables for response and outcome in patients with extragonadal germ-cell tumors. Ann Oncol. 2002;13(7):1017-1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.