Abstract
Background
Maintaining functional status is among the most important patient-centered outcomes for older adults with cancer. This study investigated the association between comprehensive geriatric assessment (CGA) and progressive disease or decline of IADL-independence 1 year after chemotherapy, overall survival (OS), and premature termination of chemotherapy. CGA-based functional status and quality of life (QOL) 1 year after chemotherapy are also described.
Methods
This prospective cohort study involved patients aged ≥65 years treated with chemotherapy for any cancer type. CGA and the G8-screening tool were performed before and after the completion of chemotherapy. Analyses were adjusted for tumor type and treatment intent: (a) indolent hematological malignancies, (b) aggressive hematological malignancies, c) solid malignancies treated with curative intent, and (d) solid malignancies treated with palliative intent.
Results
All 291 included patients lived in The Netherlands; 193 (67.4%) lived fully independent prior to chemotherapy. The median age was 72 years; 164 (56.4%) were male. IADL independence, CGA-based functional status, and QOL were maintained in half of the patients 1 year after chemotherapy. An abnormal G8-score before chemotherapy was a higher risk for progressive disease or a decline of IADL-independence (OR 3.60, 95% CI, 1.98-6.54, P < .0001), prematurely terminated chemotherapy (OR 2.12, 95% CI, 1.24-3.65, P = .006), and shorter median OS (HR 1.71, 95% CI, 1.16-2.52, P = .007). The impact of an abnormal G8-score differed across tumor type (oncological or hematological) and treatment indication (adjuvant or palliative).
Conclusion
An abnormal G8 score before chemotherapy is associated with progressive disease and functional decline after chemotherapy and shorter median OS, especially in patients with solid malignancies.
Keywords: older adults, chemotherapy, cancer, G8, geriatric assessment, survival, functional decline
A maintained functional status is an important outcome for older adults with cancer. This article reports on the association between comprehensive geriatric assessment results and patient outcomes, focusing on instrumental activities of daily living (IADL) independence 1 year after chemotherapy.
Implications for Practice.
Our study shows that IADL independence, CGA-based functional status, and quality of life are maintained in approximately half of the older patients treated with chemotherapy. Patients with a low G8 score before treatment are at increased risk of prematurely stopping chemotherapy and suffering adverse outcomes. These patients experience more frequent loss of IADL functional status, while progressive disease occurs more and overall survival is lower. Screening patients older than 65 years, using the easy-to-use G8 score, provides valuable information that can help guide decision-making regarding chemotherapeutic treatment.
Introduction
Cancer is mainly a disease in the older adult, with patients >75 years of age currently compromising 33% of all new diagnoses in the Netherlands.1 It is expected that by 2035 older adults will represent 60% of all new cancer diagnoses due to the aging population.2 This results in increasing clinical challenges for physicians to choose the optimal treatment that offers an acceptable physical performance and quality of life while targeting disease control.
Most systemic antitumor treatments are also administered to older patients, although this may lead to comorbidity3 and loss of quality of life.4 The assessment of treatment risks before therapy is difficult in this patient category due to asymptomatic differences in physical reserve.5 Comprehensive geriatric assessment (CGA) is considered the gold standard to detect unidentified health problems in older cancer patients6,7 and it influenced therapy decision-making in multidisciplinary teams,8 resulting in improved communication between patient and physician about the optimal treatment.9
Despite the fact that guidelines currently recommend CGA-based interventions to improve clinical outcomes of older patients receiving cancer therapy,10,11 the use of CGA as standard assessment in routine patient care is limited outside clinical trials.12 An explanation for this is that the implementation of CGA in standard care is difficult due to the lack of institutional availability and limited resources.10 Nevertheless, several studies have shown that CGA is feasible in oncological care and it is increasingly incorporated in clinical trials.13,14 However, the majority of phase III cancer registration trials are still characterized by strictly defined tumor- and treatment settings and the inclusion of patients with a good ECOG performance score.15 These conditions are not always representative of the older oncological population in a peripheral hospital.16 Furthermore, oncologists and hematologists also perform their own clinical judgment to estimate treatment risks and to differentiate between fit and frail patients. Therefore, it is still an area of research on how CGA can add to standard patient assessments in oncological care. The prognostic value of CGA results in functional decline and well-established oncological parameters, such as survival, is unclear in populations of patients who are already deemed fit for chemotherapy by their treating oncologist.
Therefore, this observational study addresses this question. We investigated the association between CGA results and both functional and oncological outcomes in a heterogeneous older population as seen in daily practice and who were deemed fit for chemotherapy by their treating oncologist.
Patients and Methods
Study Design
This single-center prospective cohort study involved patients ≥65 years of age treated with chemotherapy with both curative and palliative intent. Patients were identified using lists of multidisciplinary tumor boards where therapeutic decisions were discussed. Patients were eligible for the study when they started any form of chemotherapy. Chemotherapy was defined as the use of at least 1 cytotoxic drug. Combination with other drugs, such as steroids and monoclonal antibodies was allowed. From 1 October 2013 until 1 January 2018, consecutive patients were included 2 months or less prior to the start of chemotherapy. The end of follow-up was 1 January 2020. The study was approved by the central review board (METC 2015_08, NL47663.101.15). All patients provided written informed consent prior to inclusion. The exclusion criteria were chemotherapy in the past 3 months prior to inclusion or a second malignancy. Included patients were assessed for the G8-score17 and CGA at inclusion, immediately after, and 1 year after completion of chemotherapy. Functional-, tumor-, treatment- and socio-demographic characteristics were prospectively collected.
The primary endpoint was designed to capture both disease control and preserved functional status. This was defined as the combination of the absence of progressive disease and the maintenance of the independence of instrumental activities of daily living (IADL) 1 year after chemotherapy. This endpoint was considered clinically relevant in older cancer patients since functional status and quality of life after treatment are of great importance in this population,18 but most of these patients also do not want to compromise on oncological efficacy once they decide to undergo chemotherapy. Disease progression was assessed using international RECIST criteria for each tumor type. The decline of IADL-independence was defined as a decline of >2 points immediately after chemotherapy or >1 point 1 year after chemotherapy19 on the scale of Lawton and Brody.20 This scale was scored from 0 to 8, with 0 being fully IADL-dependent and 8 being fully IADL-independent.
The secondary endpoints were premature termination of chemotherapy and overall survival (OS). Premature termination of chemotherapy was defined as not completing the preplanned number of chemotherapeutic cycles or as one of the following conditions in case of indefinite chemotherapy: 1. Termination of chemotherapy less than 3 months after the start. 2. Termination of chemotherapy due to any other reason than the progressive disease. The second aim of this study was to describe IADL independence, CGA results (as a marker of functional status), and quality of life 1 year after chemotherapy.
Assessment Tools
All patients underwent screening with the Geriatric 8 (G8) screening tool, which defines patients as high risk (G8 ≤ 14) or low risk (G8 > 14) for adverse outcomes on a scale from 0 to 20.17 Then, all study patients underwent CGA prior to chemotherapy, immediately after- and 1 year after chemotherapy and patients answered quality of life questionnaires (QLQ-C30).21 The CGA was performed by trained geriatric nurses. Geriatric interventions for impairments detected by CGA were not routinely conducted since CGA was not part of the routine care during the inclusion period of the study according to national guidelines back then. Impairments detected by CGA were communicated to the treating physician, after which geriatric consultation was the physician's choice.
The CGA consisted of: A Charlson comorbidity index,22 polypharmacy (≥5 medications) yes/no, activities of daily living (ADL),23 instrumental activities of daily living (IADL),20 a geriatric depression scale-15 (GDS-15),24 a Mini-Mental State Evaluation (MMSE)25 and a minimal nutritional assessment (MNA).26 Patients were classified as fit, vulnerable, or frail according to their CGA results. Fit patients had no or minimal comorbidity (Charlson comorbidity index ≤ 1 on a scale of 0-37), were fully ADL- and IADL-independent, had a good nutritional status (MNA > 23.5 on a scale of 0-30), no depressive symptoms (GDS-15 score ≤5 on a scale of 0-15), good cognition (MMSE ≥ 27 on a scale of 0-30) and no polypharmacy (number of medications ≤ 4). Patients were considered frail when they had one or more of the following symptoms: 1. Any ADL-dependence. 2. Multiple comorbidities (Charlson comorbidity index ≥ 3). 3. Impaired cognition (MMSE < 23). 4. Malnutrition (MNA ≤ 16). 5. Severe depressive symptoms (GDS-15 ≥ 10). Patients not fitting in one of these profiles were classified as vulnerable.27
Statistical Analyses
Continuous parameters were described as median + interquartile range (IQR) and categorical parameters as percentages. Cox proportional hazard analyses were used to determine the association between CGA results, G8-score, and OS. Binary logistic regression models were used to determine the association between CGA results or the G8-score and progressive disease or IADL decline 1 year after chemotherapy and preterm termination of chemotherapy. In these models, the endpoint of either progressive disease or IADL-decline 1 year after chemotherapy and premature termination of chemotherapy were the dependent variables. For the entire cohort, the prognostic impact of the CGA-score (fit, vulnerable, or frail) and the G8-score (abnormal vs. normal) was analyzed in separate models adjusted for age, tumor type and treatment intent. The variable "tumor type and treatment intent" consisted of the following categories: (1) (Neo)adjuvant chemotherapy for solid malignancies. (2) Palliative chemotherapy for solid malignancies. (3) Indolent hematological malignancies. (4) Aggressive hematological malignancies. The analyses were also performed in subgroups with only oncological or hematological patients. The analyses with only oncological patients were adjusted for age, tumor type (colorectal, other gastro-intestinal, breast/gynecological, or urogenital) and treatment intent (curative or palliative). The analyses with only hematological patients were adjusted for age and tumor type (low grade lymphoma/chronic leukemia, high grade lymphoma/acute leukemia, or multiple myeloma).
The EORTC QLQ-C30 questionnaire version 3.0 was used to assess the quality of life before and 1 year after chemotherapy. The questionnaire consisted of 5 functional scales (physical, role functioning, cognition, emotion, and social), 3 symptom scales (fatigue, pain, nausea, and/or vomiting), and 7 single-item scores (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial impact of disease or treatment, and global quality of life). All scores were transformed to a 0-100 scale using a linear transformation.21 Higher scores indicated a better performance on the functional scales. In contrast, higher scores indicated a worse level of symptoms on the symptom scales and single-item questions.
A P-value of ≤.05 was considered to be statistically significant. The proportional hazards assumption in the Cox proportional hazard models was assessed by including interaction effects of covariates and follow-up time in a Cox proportional hazard model with time-dependent covariates. All analyses were performed using SPSS version 24 (SPSS Inc., Chicago, IL).
Results
In total, 299 patients were identified between October 2013 and February 2018. Of these patients, 5 were excluded because they did not meet all of the inclusion criteria, 2 patients unexpectedly died before the start of chemotherapy, and one patient decided not to proceed with chemotherapy. The remaining 291 patients were deemed fit for chemotherapy by their treating physician and underwent chemotherapy accordingly. Characteristics of the study cohort are listed in Table 1. Of the 291 analyzed patients, 72 patients received (neo)adjuvant chemotherapy with curative intent for a solid malignancy, 49 patients received palliative chemotherapy for a solid malignancy, 115 patients were treated for an indolent hematological malignancy, and 55 patients for an aggressive hematological malignancy. Individual diagnoses within the group of indolent hematological malignancies were: Multiple myeloma (n = 51, 44.3%), chronic lymphatic leukemia (n = 26, 22.6%), low-grade B-cell lymphomas (n = 17, 14.8%), follicular lymphoma (n = 16, 13.9%), myelodysplastic syndrome (n = 3, 2.6%) and marginal zone lymphoma (n = 2, 1.7%). Individual diagnoses within the group of aggressive hematological malignancies were: Diffuse large B-cell lymphoma (n = 38, 69.1%), acute myeloid leukemia (n = 14, 25.5%), mantle cell lymphoma (n = 2, 3.6%) and Hodgkin lymphoma (n = 1, 1.8%). More details of all included tumor types and used chemotherapeutic regimens for each tumor type are described in Table 2. The median follow-up of the study was 34 months.
Table 1.
Patient characteristics (n = 291).
| Variable | n (%) |
|---|---|
| Age (years) | 72 (IQR 68-77) |
| 65-69 | 97 (33.3) |
| 70-74 | 82 (28.2) |
| 75-79 | 72 (24.7) |
| 80-84 | 31 (10.7) |
| ≥85 | 9 (3.1) |
| Male | 164 (56.4) |
| WHO performance score | |
| 0 | 81 (28.3) |
| 1 | 178 (62.2) |
| 2 | 22 (7.7) |
| 3 | 5 (1.7) |
| Unknown | 5 (1.7) |
| Social status | |
| With partner and fully independent | 151 (53.0) |
| Without partner and fully independent | 42 (14.4) |
| With partner and housekeeping aid | 47 (16.2) |
| Without partner, with housekeeping aid | 17 (5.8) |
| With partner and medical care at home | 16 (5.5) |
| Without partner, with medical care at home | 6 (2.1) |
| Necessarily living with other family | 5 (1.7) |
| Institutional health care | 1 (0.3) |
| Missing | 6 |
| Tumor type and treatment setting | |
| (Neo)adjuvant chemotherapy for solid malignancy | 72 (24.7) |
| Palliative chemotherapy for solid malignancy | 49 (16.8) |
| Indolent hematological malignancy | 115 (39.5) |
| Aggressive hematological malignancy | 55 (18.9) |
Table 2.
Specification of included tumor types and chemotherapeutic regimens.
| Tumor types | Number of patients | Chemotherapeutic regimen + duration of treatment |
|---|---|---|
| (Neo)adjuvant chemotherapy for solid malignancies | 72 (100%) | |
| Colorectal | 48 (66.7%) | CAPOX (n = 26) (median 17 weeks, IQR 5.8-24 weeks) Capecitabine monotherapya (n = 22) (median 5 weeks, IQR 4-8 weeks) |
| Breast | 11 (15.3%) | Anthracyclins + paclitaxel or docetaxel (n = 10) (median 15 weeks, IQR 15-23 weeks) Docetaxel + cyclophosphamide (6 weeks, n = 1) |
| Gastric cancer | 5 (6.9%) | ECX (n = 5) (median 7 weeks, IQR 3-10.5 weeks) |
| Pancreas | 4 (5.6%) | Gemcitabin + cisplatin (median 22 weeks, n = 3) Gemcitabin monotherapy (22 weeks, n = 1) |
| Bladder | 3 (4.2%) | Gemcitabin + cisplatin or carboplatin (median 9 weeks, n = 3) |
| Biliary tract cancer | 1 (1.4%) | Gemcitabin + cisplatin (10 weeks, n = 1) |
| Palliative chemotherapy for solid malignancies | 49 (100%) | |
| Colorectal | 29 (59.2%) | CAPOX/FOLFOXa (n = 18) (median 14 weeks, IQR 5.3-18 weeks) Capecitabine monotherapyb (n = 10) (median 13 weeks, IQR 7-31.3 weeks) FOLFIRI (16 weeks, n = 1) |
| Prostate | 7 (14.3%) | Docetaxel + prednisone (n = 7) (median 18 weeks, IQR 15-27 weeks) |
| Ovarian or endometrial cancer | 5 (10.2%) | Paclitaxel + carboplatin (n = 4) (median 18 weeks, IQR 9.8-20.3 weeks) Gemcitabin + carboplatin (21 weeks, n = 1) |
| Esophageal | 4 (8.2%) | Paclitaxel + carboplatin (n = 4) (median 13.5 weeks, IQR 9-5.8 weeks) |
| Breast | 2 (4.1%) | FAC (15 weeks, n = 1) Docetaxel + trastuzumab + pertuzumab (3 weeks, n = 1) |
| Pancreas | 1 (2.0%) | FOLFIRINOX (7 weeks, n = 1) |
| Biliary tract cancer | 1 (2.0%) | Gemcitabin + cisplatin (16 weeks, n = 1) |
| Indolent hematological malignancies | 115 | |
| Multiple myeloma | 51 (44.3%) | MPV or bortezomib-based (n = 28)c (median 25 weeks, IQR 16-43 weeks) Lenalidomide-/thalidomide- or pomalidomide-based (n = 12)d (median 28 weeks, IQR 7-45) Ixazomib- or carfilzomibe (n = 8) (median 36.5 weeks, IQR 35-42.3 weeks) Cyclophosphamide-based (n = 3) (13, 16, and 22 weeks) |
| Chronic- and hairy cell leukemia | 26 (22.6%) | Cyclophosphamide-based (n = 5)f (median 20 weeks, IQR 7-21 weeks) Chlorambucil-based (n = 9)g (median 21 weeks, IQR 4-22.5 weeks) 2-CDA (5 days, n = 4) R-bendamustine (1, 5, and 9 weeks, n = 3) Azacitidine (duration unknown, n = 3) R-DHAP (11 weeks, n = 1) Venetoclax (duration unknown, n = 1) |
| Lymphoplasmacytic-/small B-cell lymphoma | 17 (14.8%) | Cyclophosphamide-based (n = 14)h (median 21 weeks, IQR 17.8-22 weeks) R-bendamustine (20 & 21 weeks, n = 2) R-chlorambucil (68 weeks, n = 1) |
| Follicular lymphoma | 16 (13.9%) | Cyclophosphamide-based (n = 15)I (median 21 weeks, IQR 15-21 weeks) R-CHOP (21 weeks, n = 1) |
| Myelodysplastic syndrome | 3 (2.6%) | Azacitidine (36 weeks, n = 2) Daunorubicin + cytarabine (5 weeks, n = 1) |
| Marginal zone lymphoma | 2 (1.7%) | R-CVP (21 and 22 weeks, n = 2) |
| Aggressive haematological malignancies | 55 | |
| Diffuse large B-cell lymphoma | 38 (69.1%) | R-CHOP/R-CEOP (n = 37) (median 15 weeks, IQR 15-21 weeks) R-PECC (1 week, n = 1) |
| Acute myeloid leukemia | 14 (25.6%) | Daunorubicin + cytarabin (n = 10) (median 5 weeks, IQR 1.5-7 weeks) Azacitidine (n = 4) (median 52.5 weeks, IQR 11-66.3 weeks) |
| Mantle cell lymphoma | 2 (3.6%) | R-bendamustine (19 and 23 weeks, n = 2) |
| Hodgkin lymhoma | 1 (1.8%) | ABVD (38 weeks, n = 1) |
Combined with radiotherapy in 9 patients.
With or without bevacizumab.
With daratumumab (n = 1).
With daratumumab (n = 2).
All combined with lenalidomide or thalidomide.
R-CVP (n = 3) or with fludarabine (n = 2).
With rituximab (n = 6) or with lenalidomide and rituximab (n = 2) or with obinutuzumab (n = 1).
DRC (n= 12) or R-CVP (n= 2).
DRC (n= 1) R-CVP (n= 14).
Abbreviations: ABVD, adriamycine + bleomycine + vinblastine + dacarbazine; CAPOX/FOLFOX, capecitabine or fluorouracil + oxaliplatin; 2-CDA, 2-chlorodeoxyadenosine; DRC, dexamethasone + rituximab + cyclophosphamide; ECX, epirubicin + cisplatin + capecitabine; FAC, fluorouracil + doxorubicin + cyclophosphamide; FOLFIRI, 5-fluorouracil + irinotecan; IQR, interquartile range; MPV, melphalan + prednisone + bortezomib; R-CEOP, rituximab + cyclophosphamide + etoposide + vincristine + prednisone; R-CHOP, rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone; R-CVP, rituximab + cyclophosphamide + vincristine + prednisone; R-DHAP, rituximab + cisplatin + cytarabine + prednisone; R-PECC, rituximab + prednisone + etoposide + chlorambucil + lomustine.
Table 3 shows the prevalence of CGA-impairments (%) prior to chemotherapy in the study cohort per cancer type. For the entire cohort, 46 patients (15.8%) were classified as fit, 125 patients (43.0%) as vulnerable and 120 patients (41.2%) as frail. An abnormal G8 score (<15 points) was observed in 183 patients (62.9%).
Table 3.
CGA impairments before the start of chemotherapy.
| (Neo)adjuvant chemotherapy for solid malignancies n = 72 |
Palliative chemotherapy for solid malignancies n = 49 |
Indolent haematological malignancies n = 115 |
Aggressive haematological malignancies n = 55 |
|
|---|---|---|---|---|
| Abnormal G8 | 41 (56.9%) | 25 (51.0%) | 77 (67.0%) | 40 (72.7%) |
| CGA-result | ||||
| Fit | 16 (22.2%) | 7 (14.3%) | 14 (12.2%) | 9 (16.4%) |
| Vulnerable | 38 (52.8%) | 24 (49.0%) | 46 (40.0%) | 17 (30.9%) |
| Frail | 18 (25.0%) | 18 (36.7%) | 55 (47.8%) | 29 (52.7%) |
| Comorbidity present (CCI >2) |
4 (5.6%) | 5 (10.2%) | 21 (18.3%) | 12 (21.8%) |
| Any ADL-dependence | 13 (18.1%) | 11 (22.4%) | 31 (27.0%) | 18 (32.7%) |
| Any IADL-dependence | 27 (37.5%) | 23 (46.9%) | 53 (46.1%) | 19 (34.5%) |
| Cognitive impairment (MMSE ≤23) | 1 (1.4%) | 4 (8.2%) | 9 (7.8%) | 9 (16.4%) |
| Depressive symptoms (GDS ≥10) | 1 (1.4%) | 4 (8.2%) | 15 (13.0%) | 5 (9.1%) |
| Malnutrition (MNA <17) |
26 (36.1%) | 2 (4.1%) | 6 (5.2%) | 2 (3.6%) |
| Polypharmacy | 23 (31.9%) | 17 (34.7%) | 52 (45.2%) | 21 (38.2%) |
Association Between CGA Before Chemotherapy and Progressive Disease or IADL-Decline 1 Year After the Start of Chemotherapy.
After 1 year, follow-up regarding disease status and IADL decline was available in 266 of the 291 included patients (91.4%). The IADL status could not be assessed in the remaining 25 patients who were lost to follow up, although it was known that these patients were alive after 1 year. In 4 patients (16%), this was due to severe physical deterioration, in the other 21 patients the reason was unknown. Table 4 shows the prevalence of progressive disease or IADL decline within 1 year after the start of chemotherapy, median OS, and the prevalence of premature termination of chemotherapy per cancer type and according to CGA-result (fit, vulnerable, frail, and abnormal G8). The prevalence of either progressive disease or IADL decline 1 year after chemotherapy was 51.9%, and the median PFS was 25 months (IQR 8-48 months). In the first year after the start of chemotherapy, 117 patients (40.2%) were at least once admitted to the hospital and 28 patients (9.6%) 2 times or more.
Table 4.
Summary of clinical endpoints according to cancer type and CGA-results at baseline (before chemotherapy).
| (Neo)adjuvant chemotherapy for solid malignancies (n = 72) | Palliative chemotherapy for solid malignancies (n = 49) | Indolent haematological malignancies (n = 115) | Aggressive haematological malignancies (n = 55) | |
|---|---|---|---|---|
| Progressive disease and/or decline of IADL-independence (≥2 points) 1 year after chemotherapy (primary endpoint) | 27/72 (37.5%) | 35/49 (71.4%) | 42/115 (36.5%) | 22/55 (40%) |
| Unknown | 9/72 (12.5%) | 4/49 (8.2%) | 9/115 (7.8%) | 3/55 (5.5%) |
| Abnormal G8-score | 22/27 (81.5%) | 22/35 (62.9%) | 35/42 (83.3%) | 17/22 (77.3%) |
| Fit | 4/27 (14.8%) | 4/35 (11/4%) | 5/42 (11.9%) | 2/22 (9.1%) |
| Vulnerable | 11/27 (40.7%) | 19/35 (54.3%) | 18/42 (42.9%) | 7/22 (31.8%) |
| Frail | 12/27 (44.4%) | 12/35 (34.3%) | 19/42 (45.2%) | 13/22 (59.1%) |
| Median OS in months (IQR) | 44.5 (22-59) | 18 (12-29.5) | 34 (20-54) | 35 (16-52) |
| Unknown | 0 | 0 | 0 | 0 |
| Abnormal G8-score | 41 (13.5-60.5) | 13 (10.5-32.5) | 34 (18-52.5) | 35.5 (16.3-51) |
| Fit | 47.5 (20.3-63) | 19 (9-26) | 48 (21.3-59.8) | 48 (35-62) |
| Vulnerable | 47.5 (20.3-63) | 15.5 (12-23.8) | 35 (23.5-51.3) | 34 (17.5-51.5) |
| Frail | 39.5 (11.5-57.8) | 23.5 (12.5-38.3) | 33 (14-54) | 30 (8.5-51) |
| Preterm termination of chemotherapy | 29/72 (40.3%) | 26/49 (53.1%) | 48/115 (41.7%) | 16/55 (29.1%) |
| Unknown | 0 | 0 | 0 | 0 |
| Abnormal G8-score | 21/29 (72.4%) | 15/26 (57.7%) | 33/48 (68.8%) | 15/16 (93.8%) |
| Fit | 6/29 (20.7%) | 4/26 (15.4%) | 5/48 (10.4%) | 1/16 (6.3%) |
| Vulnerable | 17/29 (58.6%) | 13/26 (50%) | 17/48 (35.4%) | 4/16 (25%) |
| Frail | 6/29 (20.7%) | 9/26 (34.6%) | 26/48 (54.2%) | 11/16 (68.8%) |
| Deceased within 1 year after chemotherapy | 9/72 (12.5%) | 12/49 (24.5%) | 16/115 (13.9%) | 12/55 (21.8%) |
| Unknown | 0 | 0 | 0 | 0 |
| Abnormal G8-score | 8/9 (88.9%) | 7/12 (58.3%) | 12/16 (75%) | 9/12 (75%) |
| Fit | 1/9 (11.1%) | 2/12 (16.7%) | 2/16 (12.5%) | 1/12 (8.3%) |
| Vulnerable | 4/9 (44.4%) | 6/12 (50%) | 5/16 (31.3%) | 3/12 (25%) |
| Frail | 4/9 (44.4%) | 4/12 (33.3%) | 9/16 (56.3%) | 8/12 (66.7%) |
The prognostic value of an abnormal G8 score and CGA results after correction for age, tumor type, and treatment are reported in Table 5 (entire cohort), Supplementary Table 1 (oncological patients only), and Supplementary Table 2 (hematological patients only).
Table 5.
Association between CGA results and clinical endpoints.
| Progressive disease and/or decline of IADL-independence (≥2 points) 1 year after chemotherapy | Overall survival | Premature termination of chemotherapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | HR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.06 | 1.01-1.12 | .014 | 1.03 | 1.00-1.06 | .087 | 1.05 | 1.01-1.10 | .023 |
| Tumor type and treatment intent | <.001 | <.001 | .059 | ||||||
| Indolent hematological (n = 115) | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| (Neo)adjuvant chemotherapy for solid malignancies (n = 72) | 1.28 | 0.67-2.45 | .451 | 0.84 | 0.52-1.38 | .503 | 1.04 | 0.57-1.92 | .897 |
| Aggressive hematological (n = 55) | 1.04 | 0.52-2.06 | .919 | 1.08 | 0.66-1.78 | .748 | 0.54 | 0.27-1.08 | .081 |
| Palliative chemotherapy for solid malignancies (n = 49) | 5.61 | 2.49-12.64 | <.001 | 2.91 | 1.89-4.48 | <.001 | 1.69 | 0.85-3.34 | .135 |
| Abnormal G8 | 3.60 | 1.98-6.54 | <.001 | 1.71 | 1.16-2.52 | .007 | 2.12 | 1.24-3.65 | .006 |
| CGA | .149 | .352 | .595 | ||||||
| Fit | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Vulnerable | 1.55 | 0.72-3.33 | .261 | 1.39 | 0.81-2.39 | .236 | 1.22 | 0.59-2.50 | .593 |
| Frail | 2.14 | 0.98-4.67 | .056 | 1.49 | 0.87-2.57 | .15 | 1.44 | 0.69-3.00 | .326 |
Multivariable logistic regression and Cox proportional hazard models were performed with age and tumor type + treatment intent as independent variables, after which the G8 score and CGA results were added to this model in separate multivariable models.
An abnormal G8-score was independently associated with progressive disease or IADL decline 1 year after chemotherapy (OR 3.60, 95% CI, 1.98-6.54, P < .0001). This was mainly in patients with solid malignancies, possibly because of the toxicity of (neo)adjuvant chemotherapy (OR 4.12, 95% CI, 1.51-11.22, P = .006). No association was observed between CGA results and progressive disease or IADL decline 1 year after chemotherapy in the entire cohort (Table 5).
Association Between CGA-Results and Overall Survival
Median OS was 34 months (IQR 16-53 months) in the entire cohort. In the first year after the start of chemotherapy, 49 patients (16.8%) died. An abnormal G8 was associated with shorter median OS (HR 1.71, 95% CI, 1.16-2.52, P = .007), independently of age, tumor type, and treatment intent, while CGA results were not (Table 5). This was mainly observed in patients treated for solid malignancies (HR 1.66, 95% CI, 0.93-2.93), although the prognostic impact on OS did not reach statistical significance, possibly because of insufficient power for subgroup analysis. (Supplementary Table 1).
Association Between CGA-Results and Preterm Termination of Chemotherapy
Dose reductions of chemotherapy occurred in 89 patients (30.6%); of which, 36 patients (40.4%) were treated with curative intent and 53 patients (59.6%) were treated with palliative intent. The dose reductions in this study cohort included both upfront dose reductions at the start of therapy and dose reductions initiated during therapy. Dose delays of chemotherapy occurred in 61 patients (21.0%), of which 19 patients (31.1%) were treated with curative intent. Chemotherapy was prematurely terminated in 119 patients (41%). Factors associated with premature termination of chemotherapy were age (OR 1.05, 95% CI, 1.00-1.09, P = .023) and an abnormal G8-score (OR 2.12, 95% CI, 1.24-3.65, P = .006) (Table 5). The negative prognostic impact of an abnormal G8-score was only observed in the patients with solid malignancies (OR 2.34, 95% CI, 1.03-5.32, P = .043) (Supplementary Table 1) and not in the patients with hematological malignancies (OR 1.83, 95% CI 0.85-3.94, P = 0.121) (Supplementary Table 2).
Functional Outcomes 1 Year After Chemotherapy
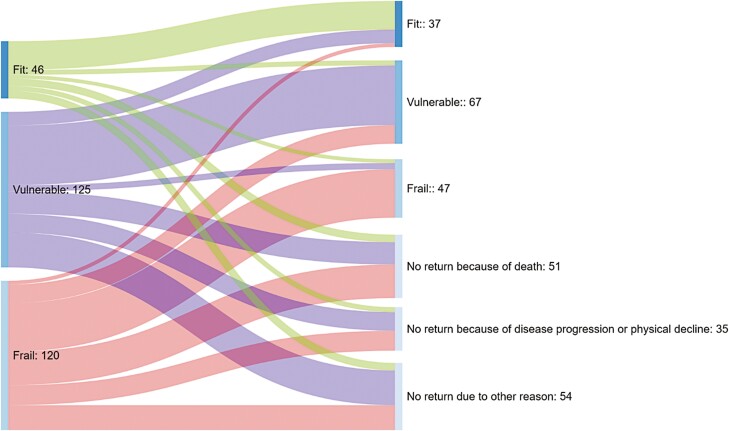
Almost half of the patients (n = 140, 48.1%) did not return for CGA 1 year after chemotherapy. This was mainly due to death or physical decline causing the follow-up CGA to be a too large burden (Fig. 1). CGA-based outcomes 1 year after chemotherapy of the patients who underwent follow-up CGA are described per cancer type in Table 6. In summary, most of the remaining patients (≥75%) maintained their pre-treatment CGA-based condition (fit, vulnerable, or frail), despite treatment with chemotherapy. Except for the patient group treated with palliative chemotherapy for a solid malignancy, <15% of the fit patients prior to chemotherapy became frail in the first year after chemotherapy, irrespective of cancer type. The level of IADL functioning after chemotherapy was maintained at 80% on average in fit patients, except in patients receiving palliative chemotherapy, in whom this percentage was 57.1% (Table 6).
Figure 1.
CGA-based condition before chemotherapy (left) and 1 year after chemotherapy (right).
Table 6.
Longitudinal CGA-results per cancer type.
| Pre-treatment CGA | Same level of ADL/IADL-functioning after 1 year | Fit after 1 year | Vulnerable after 1 year | Frail after 1 year |
|---|---|---|---|---|
| Fit | ||||
| Indolent haematological | 11 (78.6%) | 6 (75%) | 1 (12.5%) | 1 (12.5%) |
| Aggressive haematological | 8 (88.9%) | 6 (75%) | 1 (12.5%) | 1 (12.5%) |
| (Neo)adjuvant chemotherapy for solid malignancies | 13 (81.3%) | 9 (81.8%) | 1 (9.1%) | 1 (9.1%) |
| Palliative chemotherapy for solid malignancies | 4 (57.1%) | 2 (66.7%) | 1 (33.3%) | 0 (0%) |
| Vulnerable | ||||
| Indolent haematological | 31 (67.4%) | 7 (24.1%) | 21 (72.4%) | 1 (3.4%) |
| Aggressive haematological | 9 (52.9%) | 1 (10%) | 8 (80%) | 1 (10%) |
| (Neo)adjuvant chemotherapy for solid malignancies | 21 (55.3%) | 3 (16.7%) | 12 (66.7%) | 3 (16.7%) |
| Palliative chemotherapy for solid malignancies | 9 (37.5%) | 0 (0%) | 7 (100%) | 0 (0%) |
| Frail | ||||
| Indolent hematological | 31 (56.4%) | 2 (7.4%) | 9 (33.3%) | 16 (59.3%) |
| Aggressive hematological | 9 (31%) | 0 (0%) | 4 (28.6%) | 10 (71.4%) |
| (Neo)adjuvant chemotherapy for solid malignancies | 7 (38.9%) | 0 (0%) | 1 (12.5%) | 7 (87.5%) |
| Palliative chemotherapy for solid malignancies | 9 (50%) | 1 (12.5%) | 1 (12.5%) | 6 (75%) |
Quality of Life
Quality of life (QOL) assessment before chemotherapy was available in 283 patients (97.2%). A comparison with QOL 1 year after chemotherapy could be performed in 146 patients (51.6%). The other patients did not return the QOL questionnaires 1 year after chemotherapy and therefore, longitudinal QOL assessment was not possible in this group. In the majority of these patients, this was due to death, severe deterioration of the physical condition, or progressive disease (n = 85, 30%). In 14 patients (4.9%) it was not possible to study the impact of chemotherapy on quality of life because of premature termination of chemotherapy. In the remaining patients (n = 38, 13.4%), the reason for not returning the QOL questionnaires was unknown.
Before chemotherapy, self-reported QOL was high with functional scales all >75, meaning that patients regarded their physical function as >75% and with no or little impact on daily life. Mean scores on the symptom scales ranged from 0 to 22.2 on a scale from 0 to 100, where 0 was no complaints and 100 was very severe complaints. A mean score of 22.2 corresponded with very little impact on daily life. Mean overall QOL was 72.61 years after chemotherapy, the performance on functional scales and overall QOL was maintained and most patients experience fewer symptoms on the symptom scales.
Discussion
Our study showed that patients with an abnormal G8-score before chemotherapy were at higher risk of progressive disease or IADL-decline 1 year after treatment (OR 3.60, 95% CI 1.98-6.54). Also, these patients had shorter median OS (HR 1.71, 95% CI 1.16-2.52) and more often premature termination of chemotherapy (OR 2.12, 95% CI 1.24-3.65). This was mainly observed in patients treated (neo)adjuvant chemotherapy for solid malignancies. CGA classification (fit, vulnerable, or frail) before chemotherapy was not significantly associated with these clinical outcomes.
Systematic reviews in general show a negative prognostic impact of an abnormal G8 or CGA on OS and premature termination of chemotherapy28-30 Therefore, multiple studies presented at the ASCO 2020 investigated the prognostic impact of CGA-based interventions.31-33 These studies showed that CGA-based interventions resulted in a 10-20% reduction of chemotherapeutic toxicity31,32 and better quality of life.34 At the same time, OS was not diminished 6 months after chemotherapy compared to the patients without CGA-based dose reductions, indicating that oncological efficacy was not compromised.31,33 Our study adds to this knowledge by showing that the prognostic effect of G8-based geriatric impairments is dependent on tumor type and treatment setting. Our results suggest that especially patients treated with (neo)adjuvant chemotherapy benefit from the G8 screening as a diagnostic tool, which was also observed in another trial.35 Explanations for this remain speculative, but possibly lie in the high toxicity of adjuvant chemotherapeutic regimens. The threshold of administering highly toxic chemotherapy is lower than in other patient groups due to the desire to “cure” the patient, while a majority of these patients are treated without direct clinical benefit. As a result, older adults receiving chemotherapy with curative intent are especially in need of some form of CGA integrated with standard oncological care.
It must be noted that the integration of CGA in standard oncological care remains a clinical challenge because the prognostic impact differs according to tumor type, treatment intent (adjuvant or palliative), as shown by our study, and extensiveness of the assessment by the treating oncologist. Cooperation between oncologists and geriatricians differs across hospitals and countries, ranging from combined oncogeriatric outpatient departments to active participation of geriatricians in multidisciplinary tumor boards to no access to geriatric healthcare at all. Therefore, the actual additional value of CGA to standard oncological assessment needs to be further studied in prospective clinical trials across different treatment settings (adjuvant vs. palliative) and across different healthcare systems. Currently, a few prospective studies investigating this are underway.36-38
Studies reporting serial functional assessments and quality of life after completion of chemotherapy are very scarce.7,39-42 A functional decline after chemotherapy is reported in 33-41% of older patients with solid malignancies.43-45 In contrast to these studies, which selected patients with 1 or 2 distinct types of cancer, we report functional outcomes in a heterogeneous population of older patients with cancer, as seen in general practice. In our study, half of the patients demonstrated a good quality of life and functional performance 1 year after chemotherapy. One-third of the patients were deceased or had severe physical deterioration, hampering the measurements after 1 year. Approximately 25% of the patients did not fully recover in terms of IADL independence and CGA-based functional status, including the patient group classified as fit. Interestingly, the functional decline was least observed in frail patients treated with palliative chemotherapy for solid malignancies compared to frail patients in the other subgroups. The reason for this could not be determined in this study, but is possibly due to tumor response to palliative chemotherapy in combination with less toxic palliative treatment or an appropriate selection of patients for palliative chemotherapy by the oncologist independently of CGA.
Our study has some limitations. This was a single-center non-randomized observational study. As a result, the etiology of functional decline and shortened OS in patients with an abnormal G8 could not be assessed. The included patients were already deemed fit by their treating physician, which might have caused selection bias. Therefore, our results cannot be stretched to the entire population of older cancer patients. Additionally, one-third of the patients were under 70 years of age. These patients are not always considered old adults and this might have impacted the good baseline QOL- and functional results. A substantial amount of longitudinal CGA and QOL assessments were missing, which could not be avoided because of the observational design of this study, the dropout of patients, and the lack of implementation of CGA in standard care. Therefore, we decided to only describe the available longitudinal assessments. An analysis to determine any associations with pre-treatment CGA or other clinical factors was not performed.
The strengths of our study are that this study is among the first to report on a combined study endpoint of oncological efficacy and functional status, which are both important to older patients treated with chemotherapy. Our study provides insight into the long-term OS of older patients treated with chemotherapy with a median follow-up of almost 3 years. We also provide descriptions of functional and survival outcomes per tumor type and pretreatment CGA result. Hopefully, this will aid in the search for the exact role of CGA in oncological treatment. We also comment on functional decline 1 year after chemotherapy, which is firstly a good length for older patients treated with palliative intent and secondly, it is understandable that functional status might decline temporarily shortly after administering chemotherapy and possibly restores in the long term. As a result, our study gives insight into clinical and functional outcomes in a heterogeneous population of older adults with cancer patients and with multiple CGA deficits as seen in a general practice. These patients are often not included in large clinical trials.
Conclusion
IADL independence, CGA-based functional status, and quality of life are maintained in at least half of the older patients treated with chemotherapy across all different cancer types. Geriatric deficits prior to chemotherapy identified using the short and practical G8 score are associated with progressive disease or functional decline within 1 year after chemotherapy and shorter median OS in older patients treated with adjuvant chemotherapy for solid malignancies. Some form of CGA seems of additional value to the standard assessment by the oncologist in order to optimize the administration of chemotherapy to older patients.
Supplementary Material
Acknowledgments
We thank Marianne Wingelaar and Patricia Matthieu-van Welie for their participation in the patient inclusion and for conducting all geriatric assessments. This study was funded by ORAS (Oncological Research Albert Schweitzer hospital). The foundation had no involvement in the conduct of the study.
Contributor Information
Hánah N Rier, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands; Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Marieke C Meinardi, Department of Geriatric Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Joost van Rosmalen, Department of Biostatistics, Erasmus MC, Rotterdam, The Netherlands; Department of Epidemiology, Erasmus MC, Rotterdam, The Netherlands.
Peter E Westerweel, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Eva de Jongh, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Jos J E M Kitzen, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Joan van den Bosch, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Marija Trajkovic, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Mark-David Levin, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Funding
This study was funded by ORAS (Oncological Research Albert Schweitzer hospital). The foundation had no involvement in the conduct of the study.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Conception/design: H.N.R., M.C.M., P.E.W., M-D.L. Provision of study material or patients: H.N.R., M.C.M., P.E.W., J.J.E.M.K., J.v.d.B. Collection and/or assembly of data: H.N.R., M.C.M., P.E.W., J.J.E.M.K., J.v.d.B. Data analysis and interpretation: H.N.R., M.C.M., J.v.R., P.E.W., M-D.L. Manuscript writing: H.N.R., M.C.M., J.v.R., P.E.W., E.d.J. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. http://www.cijfersoverkanker.nl/nkr/index.
- 2. Pilleron S, Sarfati D, Janssen-Heijnen M, et al. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int J Cancer. 2019;144(1):49-58. 10.1002/ijc.31664. [DOI] [PubMed] [Google Scholar]
- 3. Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101(17):1206-1215. 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavdaniti M, Zyga S, Vlachou E, et al. Quality of life in elderly cancer patients undergoing chemotherapy. Adv Exp Med Biol. 2017;989:291-295. 10.1007/978-3-319-57348-9_27. [DOI] [PubMed] [Google Scholar]
- 5. Hamaker ME, Prins MC, Stauder R.. The relevance of a geriatric assessment for elderly patients with a haematological malignancy—a systematic review. Leuk Res. 2014;38(3):275-283. 10.1016/j.leukres.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 6. Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20(4):379-385. 10.1634/theoncologist.2014-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheepers ERM, Vondeling AM, Thielen N, et al. Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica. 2020;105(6):1484-1493. 10.3324/haematol.2019.245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamaker ME, Te Molder M, Thielen N, et al. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients—a systematic review. J Geriatr Oncol. 2018;9(5):430-440. 10.1016/j.jgo.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 9. Mohile SG, Epstein RM, Hurria A, et al. Communication with older patients with cancer using geriatric assessment: a cluster-randomized clinical trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2020;6(2):196-204. 10.1001/jamaoncol.2019.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadambi S, Loh KP, Dunne R, et al. Older adults with cancer and their caregivers—current landscape and future directions for clinical care. Nat Rev Clin Oncol. 2020;17(12 ):742-755. 10.1038/s41571-020-0421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for Geriatric oncology summary. J Oncol Pract. 2018;14(7):442-446. 10.1200/JOP.18.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKenzie GAG, Bullock AF, Greenley SL, et al. Implementation of geriatric assessment in oncology settings: a systematic realist review. J Geriatr Oncol. 2021;12(1):22-33. 10.1016/j.jgo.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 13. DuMontier C, Sedrak MS, Soo WK, et al. Arti Hurria and the progress in integrating the geriatric assessment into oncology: Young International Society of Geriatric Oncology review paper. J Geriatr Oncol. 2020;11(2):203-211. 10.1016/j.jgo.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290-1296. 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scher KS, Hurria A.. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30(17):2036-2038. 10.1200/JCO.2012.41.6727. [DOI] [PubMed] [Google Scholar]
- 16. Tack L, Lefebvre T, Lycke M, et al. Underrepresentation of vulnerable older patients with cancer in phase II and III oncology registration trials: a case–control study. J Geriatr Oncol 2020;11(2):320-326. 10.1016/j.jgo.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 17. Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166-2172. 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 18. Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061-1066. 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 19. Decoster L, Kenis C, Schallier D, et al. Geriatric assessment and functional decline in older patients with lung cancer. Lung. 2017;195(5):619-626. 10.1007/s00408-017-0025-2. [DOI] [PubMed] [Google Scholar]
- 20. Lawton MP, Brody EM.. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186. [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23. Mahoney FI, Barthel DW.. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61-65. [PubMed] [Google Scholar]
- 24. Arthur A, Jagger C, Lindesay J, et al. Using an annual over-75 health check to screen for depression: validation of the short Geriatric Depression Scale (GDS15) within general practice. Int J Geriatr Psychiatry. 1999;14(6):431-439. [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE.. McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26. Guigoz Y, Vellas B.. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;1:3-1–1.; discussion 11-2. [DOI] [PubMed] [Google Scholar]
- 27. Balducci L, Extermann M.. Management of cancer in the older person: a practical approach. Oncologist. 2000;5(3):224-237. 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 28. Kenis C, Decoster L, Van Puyvelde K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. 2014;32(1):19-26. 10.1200/JCO.2013.51.1345. [DOI] [PubMed] [Google Scholar]
- 29. Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One. 2014;9(12):e115060. 10.1371/journal.pone.0115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishii R, Ogawa T, Ohkoshi A, et al. Use of the Geriatric-8 screening tool to predict prognosis and complications in older adults with head and neck cancer: a prospective, observational study. J Geriatr Oncol. 2021;12(7):1039-1043. 10.1016/j.jgo.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 31. Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904. 10.1016/S0140-6736(21)01789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li D, Sun CL, Kim H, et al. Geriatric Assessment-Driven Intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158. 10.1001/jamaoncol.2021.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall PS, Swinson D, Cairns DA, et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869-877. 10.1001/jamaoncol.2021.0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.e19126.
- 35. Lund CM, Vistisen KK, Olsen AP, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO). Br J Cancer. 2021;124(12):1949-1958. 10.1038/s41416-021-01367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dolin TG, Mikkelsen M, Jakobsen HLet al. Geriatric assessment and intervention in older vulnerable patients undergoing surgery for colorectal cancer: a protocol for a randomised controlled trial (GEPOC trial). BMC Geriatr. 2021;21(1):88-021-02045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ditzel HM, Giger AW, Lund CM, et al. Predictive value of geriatric oncology screening and geriatric assessment in older patients with solid cancers: protocol for a Danish prospective cohort study (PROGNOSIS-G8). J Geriatr Oncol. 2021;12(8):1270-1276. 10.1016/j.jgo.2021.06.004. Epub 2021 Jun 25. [DOI] [PubMed] [Google Scholar]
- 38. Giger AW, Ditzel HM, Jorgensen TL, et al. Predictive value of geriatric oncology screening and geriatric assessment of older patients with cancer: a randomized clinical trial protocol (PROGNOSIS-RCT). J Geriatr Oncol. 2021;13(1):116-123. 10.1016/j.jgo.2021.07.005. Epub 2021 Aug 4. [DOI] [PubMed] [Google Scholar]
- 39. van Walree IC, Scheepers E, van Huis-Tanja L, et al. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol. 2019;10(6 ):847-858. 10.1016/j.jgo.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 40. Bruijnen CP, van Harten-Krouwel DG, Koldenhof JJ, et al. Predictive value of each geriatric assessment domain for older patients with cancer: a systematic review. J Geriatr Oncol. 2019;10(6):859-873. 10.1016/j.jgo.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 41. Decoster L, Kenis C, Naessens B, et al. Integrating geriatric assessment in the first line chemotherapy treatment in older patients with metastatic colorectal cancer: results of a prospective observational cohort study (AVAPLUS). J Geriatr Oncol. 2018;9(2):93-101. 10.1016/j.jgo.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 42. Puts MT, Tapscott B, Fitch M, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41(2):197-215. 10.1016/j.ctrv.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 43. Hurria A, Soto-Perez-de-Celis E, Allred JB, et al. Functional decline and resilience in older women receiving adjuvant chemotherapy for breast cancer. J Am Geriatr Soc. 2019;67(5):920-927. 10.1111/jgs.15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Abbema D, van Vuuren A, van den Berkmortel F, et al. Functional status decline in older patients with breast and colorectal cancer after cancer treatment: A prospective cohort study. J Geriatr Oncol. 2017;8(3):176-184. 10.1016/j.jgo.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 45. Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: a multicenter prospective study. J Geriatr Oncol. 2017;8(3):196-205. 10.1016/j.jgo.2017.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.