Summary
The evolution of streptophytes had a profound impact on life on Earth. They brought forth those photosynthetic eukaryotes that today dominate the macroscopic flora: the land plants (Embryophyta).1 There is convincing evidence that the unicellular/filamentous Zygnematophyceae—and not the morphologically more elaborate Coleochaetophyceae or Charophyceae—are the closest algal relatives of land plants.2, 3, 4, 5, 6 Despite the species richness (>4,000), wide distribution, and key evolutionary position of the zygnematophytes, their internal phylogeny remains largely unresolved.7,8 There are also putative zygnematophytes with interesting body plan modifications (e.g., filamentous growth) whose phylogenetic affiliations remain unknown. Here, we studied a filamentous green alga (strain MZCH580) from an Austrian peat bog with central or parietal chloroplasts that lack discernible pyrenoids. It represents Mougeotiopsis calospora PALLA, an enigmatic alga that was described more than 120 years ago9 but never subjected to molecular analyses. We generated transcriptomic data of M. calospora strain MZCH580 and conducted comprehensive phylogenomic analyses (326 nuclear loci) for 46 taxonomically diverse zygnematophytes. Strain MZCH580 falls in a deep-branching zygnematophycean clade together with some unicellular species and thus represents a formerly unknown zygnematophycean lineage with filamentous growth. Our well-supported phylogenomic tree lets us propose a new five-order system for the Zygnematophyceae and provides evidence for at least five independent origins of true filamentous growth in the closest algal relatives of land plants. This phylogeny provides a robust and comprehensive framework for performing comparative analyses and inferring the evolution of cellular traits and body plans in the closest relatives of land plants.
Keywords: conjugating green algae, conjugatophyceae, Charophyta, streptophyte algae, multicellularity, phylogenomics, plant terrestrialization, plant evolution
Graphical abstract
Highlights
-
•
Comprehensive phylogenomic analyses for 46 taxonomically diverse Zygnematophyceae
-
•
Five-order system for the Zygnematophyceae, the closest relatives of land plants
-
•
Filamentous and pyrenoid-lacking Mougeotiopsis sits in a deep clade of unicells
-
•
Evidence for at least five independent origins of true filamentous growth
Hess et al. use comprehensive phylogenomic analyses and present a five-order system for the Zygnematophyceae. They place the filamentous and pyrenoid-lacking Mougeotiopsis among unicellular zygnematophytes. Based on this framework, they propose at least five independent origins of true filamentous growth for the closest relatives of land plants.
Results and discussion
Morphology and phylogenetic position of a filamentous zygnematophyte without pyrenoids
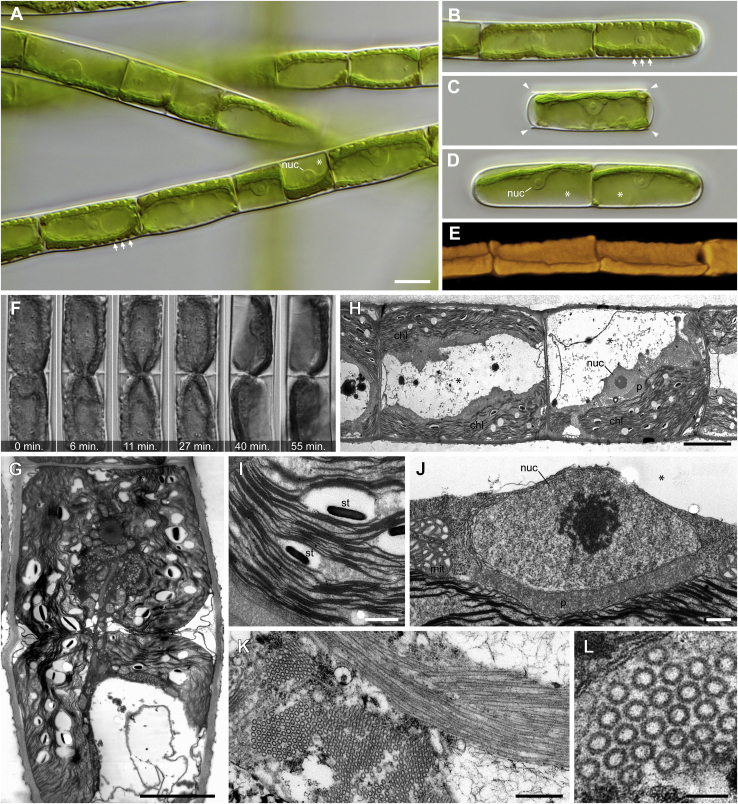
Strain MZCH580 forms unbranched filaments with smooth cell walls and rounded tips (Figures 1A and 1B). Infolded cross walls (“replicate walls”) or rhizoids known from some filamentous zygnematophytes10 were not observed in our cultures. The filaments of strain MZCH580 tend to fragment as the cultures age, but cells divide and grow back into new filaments when fresh medium is added (Figures 1C and 1D). Interphase cells are 10–15 μm wide (mean = 12 μm, n = 40) and 12–55 μm long (mean = 22 μm, n = 80), and usually contain a single chloroplast. The chloroplast lacks visible pyrenoids and has a variable shape ranging from an off-center straight plate (Figure 1D) to a more parietal morphology, like a channel or half-pipe (Figures 1A and 1B). The 3D reconstruction of confocal fluorescence data reveals a common intermediate morphology (Figure 1E). The lateral sides of half-pipe-shaped chloroplasts display clear indentations, which are rare in filamentous green algae with chloroplasts of similar morphology (Figures 1A and 1B, arrows)—Entransia fimbriata (Klebsormidiophyceae), for example, has fimbriate or lobed chloroplasts, but of much more irregular morphology.11 The nucleus is spherical (4–6 μm in diameter, n = 40) with a prominent central nucleolus (1–3 μm in diameter, n = 40), and always closely associated with the chloroplast (Figures 1A and 1D; nuc). Both chloroplast and nucleus are surrounded by a thin sheath of cytoplasm and opposed to or surrounded by a large vacuole (Figure 1D; asterisks).
Figure 1.
Morphology, cell division, and ultrastructure of Mougeotiopsis calospora strain MZCH580
(A) Filaments with cells of varying length; differential interference contrast (DIC). Note the indented chloroplast margins (arrows), the prominent nuclei (nuc) and the large vacuoles (asterisk).
(B) Filament with rounded tip; DIC.
(C) Single cell after fragmentation with cell wall remnants (arrowheads); DIC.
(D) Two-celled filament with smooth tips; DIC. Note the prominent nuclei (nuc) and the large vacuoles (asterisks).
(E) Three-dimensional reconstruction of the chloroplasts based on their autofluorescence; confocal microscopy.
(F) Time series of a dividing cell shows ingrowing cross wall; DIC.
(G) Ultrathin section through a dividing cell reveals the ingrowing cell wall (see plasma membrane) and the chloroplast in division.
(H) Ultrathin section through vegetative filament showing the position of the nucleus (nuc), peroxisome (p), chloroplasts (chl) and vacuoles (asterisks).
(I) Ultrathin section of starch grains (st) between the thylakoids of the chloroplast.
(J) Ultrathin section of the nucleus (nuc) with nucleolus, the large, elongate peroxisome (p), and mitochondria (mit). The vacuolar space is marked by the asterisk.
(K) Ultrathin section of bundled macrotubules in cross section (left) and longitudinal section (right).
(L) Detail of macrotubules in cross section.
Scale bars 10 μm in (A) (applies also for B–D); 5 μm in (G) and (H); 500 nm in (I)–(K); 100 nm in (L).
See also Figures S1, S2, and S4.
Cell division is intercalary and involves the centripetal formation of a cross wall (Figure 1F; Videos S1 and S2). We did not observe any phragmoplast-like structure as known from many streptophyte algae.12, 13, 14 Instead, ingrowing cell wall material seemed to pinch off the chloroplast (Figure 1F and Videos S1 and S2), which is corroborated on the ultrastructural level (Figure 1G). It appears that the chloroplast does not divide before the inset of cytokinesis, and that the cell division in strain MZCH580 largely depends on furrowing (cleavage, thus centripetal cell wall ingrowth). However, we cannot exclude the existence of a phragmoplast and our ultrastructural data of late stages of cytokinesis seem compatible with phragmoplast-like structures as known from many streptophyte algae, including other zygnematophytes (e.g., Spirogyra and Mougeotia12,13).
The movie shows that cytokinesis is dominated by centripetal cleavage. Note the rotating chloroplasts and the migrating nuclei. Imaging was performed at six frames per minute, shown as 10 FPS
A second movie to illustrate the different phenotypes of cell division; see description for Video S1.
Our ultrastructural data confirm that the chloroplasts of strain MZCH580 lack pyrenoids but contain numerous lentiform starch grains (up to ∼1 μm) interspersed between the thylakoids (Figures 1H and 1I). This is a very unusual chloroplast configuration. Pyrenoids are found in all other known zygnematophytes (and most green algae) and are considered important compartments for carbon concentration. That said, hornworts have frequently gained and lost pyrenoids—a phenomenon that does not correlate with atmospheric CO2 concentration or lifestyle changes.15 Mougeotiopsis appears to compensate for the lack of pyrenoid-based carbon concentration by an extremely high expression of homologs of ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit 2 (rbcS2) and rubisco activase (rca); in fact, with transcripts per million (TPM) values of 44002 and 15238, they were, respectively, the highest and fourth highest expressed transcript in the whole transcriptome. In contrast, in the transcriptomes of the pyrenoid-bearing alga Mougeotia sp. MZCH240, rbcs and rca homologs never ranged among the top 100 most abundant transcripts (see de Vries et al.16 and Fürst-Jansen et al.17). The ecophysiological consequences of the absence of pyrenoids in Mougeotiopsis are currently obscure.
Other noteworthy ultrastructural characteristics of strain MZCH580 are a giant peroxisome situated between the nucleus and the chloroplast (Figure 1J), and the occurrence of macrotubules (∼44 nm in diameter; 44.02 nm ± 2.4 nm, n = 446) in cells with incomplete cytokinesis likely promoted by environmental factors (Figures 1K, 1L, and S1); the occurrence of macrotubules has been described in land plant tissues—for example, in cells of root tips but with a distinct mean diameter18 (35 nm). A single peroxisome of similar localization was also reported for Klebsormidiophyceae such as Klebsormidium, Hormidiella, and Streptosarcina,19, 20, 21, 22 and the Zygnematophyceae Zygogonium,23 suggesting that this is a rather widespread character in streptophyte algae. However, the filamentous zygnematophytes Mougeotia, Spirogyra, and Zygnema contain numerous, much smaller peroxisomes, which do not exceed 1 μm in our TEM sections (Figure S2).
Based on taxonomic comparisons (see Table 1 and STAR Methods for details), we apply the name Mougeotiopsis calospora to strain MZCH580. However, as we did not observe any sexual processes (conjugation, flagellated gametes), zoospores, or aplanospores in our cultivated material, the suspected affinity to the zygnematophytes remained uncertain. While analysis of the rbcL gene (coding for the large chain of ribulose-1,5-bisphosphate carboxylase/oxygenase) placed strain MZCH580 within the streptophytes, a robust phylogenetic placement was not possible. To scrutinize the phylogenetic position of strain MZCH580, we generated RNA-seq data by Illumina sequencing and performed a de novo transcriptome assembly. The resulting transcriptome has a completeness of 96.3% (benchmarked universal 272 single-copy orthologs) and contains 52,188 predicted open reading frames (ORFs). We built a comprehensive multigene dataset of 326 conserved proteins (see STAR Methods) from streptophyte algae, land plants, and select chlorophyte algae as outgroup, with 84 taxa in total (see species and deposited data in STAR Methods). Our phylogenomic inferences with a sophisticated site-heterogeneous model of protein sequence evolution (LG+PMSF(C60)+F+Γ) resulted in a well-supported phylogeny, whose overall topology is in line with current knowledge about streptophyte evolution (cf. Figure S3 and One Thousand Plant Transcriptomes Initiative6). To scrutinize this, we performed an approximately unbiased (AU) test under the best-fit model LG+C60+F+Γ with 10,000 multiscale bootstrap replicates. Our dataset rejected the topology of the One Thousand Plant Transcriptomes Initiative6 (AU test p = 0.000). This, however, only concerned some relationships within Desmidiales, and neither their monophyletic arrangement nor any other aspect of the gross topology, thus also having no effect on any trait inferences below. Strain MZCH580 groups within the Zygnematophyceae with full nonparametric bootstrap support and forms a deep-branching lineage with the unicellular Serritaenia sp. (strain CCAC 0155) and “Mesotaenium endlicherianum” (strain SAG 12.97). Hence, strain MZCH580, referred to as Mougeotiopsis calospora hereafter, is clearly distinct from other filamentous genera (Mougeotia, Spirogyra, Zygnema, and Zygnemopsis), and represents a new lineage of zygnematophytes with filamentous growth.
Table 1.
Five-order taxonomy of the Zygnematophyceae
| Order Serritaeniales |
| S.Hess & J.de Vries ord. nov. Diagnosis: comprises unicells and filaments with smooth sidewalls, cells with axial or parietal chloroplasts, and simple cell walls (no pores and ornamentations), phylogenetically closely related to the type species (Serritaenia testaceovaginata; rbcL MW159377). Type:Serritaeniaceae S.Hess & J.de Vries fam. nov. Family Serritaeniaceae S.Hess & J.de Vries fam. nov. Diagnosis: with characteristics of order Serritaeniales; unicells and filaments with smooth sidewalls, cells with axial or parietal chloroplasts, and simple cell walls (no pores and ornamentations), embedded or not in the mucilage. Type:Serritaenia A.Busch & S.Hess 2021. Comment: Currently the Serritaeniales includes a single family, the Serritaeniaceae with the genera Serritaenia and Mougeotiopsis. |
| Order Zygnematales |
| Bessey emend. S.Hess & J.de Vries Emended description: Comprises unicells and unbranched and uniseriate filaments with smooth side walls, cells with stellate, plate- or ribbon-like chloroplasts and simple cell walls (no pores and ornamentations), phylogenetically closely related to strains SAG 698-1a (Genbank transcriptome shotgun assembly GFYA00000000). Type:Zygnema C.Agardh, 1817, nom. et typ. cons. Comment: Currently the Zygnematales includes a single family Zygnemataceae with the genera Cylindrocystis, Mesotaenium (current assumption, pending discovery of type species), Mougeotia, Zygnema, and Zygnemopsis. No culture is available from the type species of Zygnema. |
| Order Desmidiales |
| Bessey emend. S.Hess & J.de Vries Emended description: Comprises unicells and chain-like filaments. Cell walls and morphologies of diverse complexity, including the "placoderm desmids" with cell wall pores, ornamentations and clear isthmus, and species with smooth cell walls and without isthmus. Phylogenetically closely related to strain Desmidium aptogonum (RNA-seq ERX2100155). Type:Desmidium C.Agardh ex Ralfs, 1848. Comment: Currently the Desmidiales includes a single family Desmidiaceae with the genera Bambusina, Closterium, Cosmarium, Cosmocladium, Desmidium, Euastrum, Micrasterias, Netrium, Nucleotaenium, Onychonema, Penium, Phymatodocis, Planotaenium, Pleurotaenium, Staurastrum, Staurodesmus, Xanthidium, and more. No culture is available from the type species of Desmidium. |
| Order Spirogyrales |
| Clements emend. S.Hess & J.de Vries Emended description: Comprises filaments with smooth side walls, cells with one or more helical chloroplast and smooth cell walls without pores or ornamentation. Phylogenetically closely related to strain Spirogyra pratensis strain MZCH10213 (RNA-seq data: NCBI BioProject PRJNA543475, TSA GICF00000000). Type:Spirogyra Link, 1820, nom. cons. Comment: Currently the Spirogyrales includes only the genus Spirogyra. The closely related genus Sirogonium Kützing may also belong to this order, but this needs to be confirmed by phylogenomic studies. No culture is available from the type species of Spirogyra. The order Spirogyrales was originally validated by Clements (1909: 12); his description specified “Typically one-celled or filamentous algae, without zoospores; sexual reproduction by the conjugation of similar gametes; two fungous families.” No fungi are currently included in this order. |
Phylogenomics support a five-order taxonomy of the Zygnematophyceae
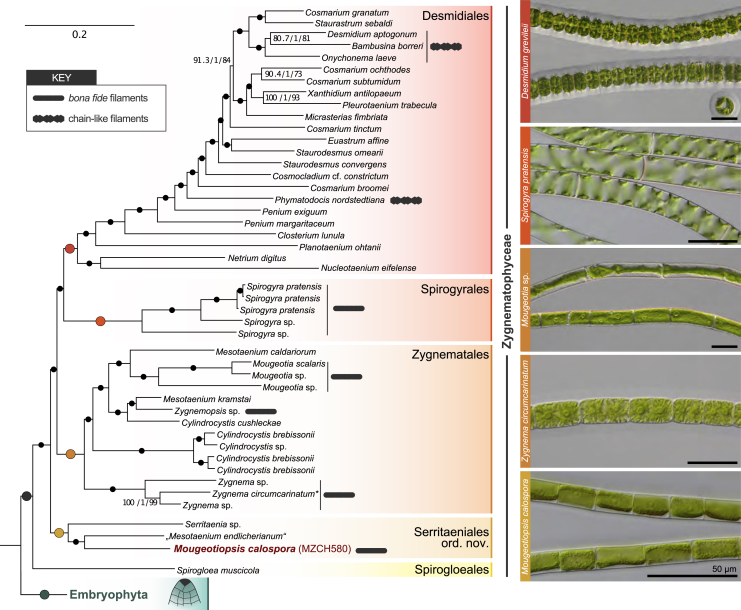
Previous phylogenies based on single (or few) marker genes have suggested that the traditional taxonomic separation into the two orders Desmidiales and Zygnematales does not reflect the evolutionary relationships of the Zygnematophyceae.7,8 Yet, the taxonomy of this important algal class remains unresolved, in part due to the lack of robust phylogenetic data. Our multigene phylogeny clearly demonstrates that the Zygnematales as previously defined (all filamentous members plus unicells that are not placoderm desmids) are paraphyletic. Instead, the Zygnematophyceae comprise at least five deep-branching clades that we feel can be treated at the level of orders (Figure 2).
Figure 2.
Position of strain MZCH580 in a well-resolved zygnematophycean phylogeny based on 326 genes
Section of the phylogenomic tree limited to zygnematophytes and embryophytes. Support values from three analyses (SH-aLRT/aBayes/nonparametric bootstrapping) are shown at the corresponding branches, except for branches with maximum support (marked by dots); large colored dots correspond to the (full) support recovered for the higher-order clades labeled on the right. The Zygnematophyceae comprise five deep-branching clades, which are here defined as orders. Gray symbols highlight zygnematophytes that form chain-like filaments (see micrograph of Desmidium) and bona fide filaments (see micrographs of Spirogyra, Mougeotia, Zygnema, and Mougeotiopsis); scale bars in all micrographs are 50 μm.
Scale bar for phylogeny is 0.2 expected substitutions per site. The entire phylogenomic tree with all streptophyte taxa is shown in Figure S3. Asterisk: a recent study by Feng et al.24 found that SAG698-1a might be Z. cylindricum instead of Z. circumcarinatum.
We introduce a new, phylogenomically informed five-order taxonomy of the Zygnematophyceae, by reinterpreting existing ordinal names and introducing a new order for Mougeotiopsis and its unicellular relatives (see Table 1). The Serritaeniales ord. nov. currently comprises the name-giving genus Serritaenia (unicells with a plate-like chloroplast and a mostly aerophytic life style25), the genome-sequenced strain SAG 12.97 (often referred to as “Mesotaenium endlicherianum”26; unicells with half-pipe-like chloroplasts and an aquatic lifestyle) and Mougeotiopsis calospora, strain MZCH580. Although these species differ markedly in growth form (unicells versus filaments), their chloroplasts are all characterized by indented or undulated margins25,26 that are otherwise rare in zygnematophytes. Yet, Mougeotiopsis calospora is the only known zygnematophyte that lacks pyrenoids.
Our data corroborate the position of the Spirogloeales, consisting of the unicellular Spirogloea muscicola (formerly Spirotaenia muscicola), as sister lineage to all other Zygnematophyceae.26 For the remaining part of the phylogenomic tree, we redefine three traditional orders. The Zygnematales are now limited to a morphologically diverse clade comprising unicellular zygnematophytes currently assigned to Cylindrocystis and Mesotaenium, plus three distinct branches of filamentous members (Mougeotia, Zygnema, and Zygnemopsis); the recovered topology demonstrates the polyphyly of the unicellular genera belonging to that order (Cylindrocystis and Mesotaenium), which require a taxonomic revision in the future. Chloroplasts of the Zygnematales are either stellate (Cylindrocystis, Zygnema, and Zygnemopsis) or ribbon/plate-like with smooth margins (Mesotaenium and Mougeotia).
The Spirogyra species with their characteristic helical chloroplasts form another, deep-branching clade, which is here defined as Spirogyrales Clements 1909 (Figure 2 and Table 1). This order was initially introduced to include algae of yellow-green appearance (including Spirogyra) and some fungal families.27 We limit the concept of the Spirogyrales to those zygnematophycean algae that form the sister clade of the Desmidiales in our phylogeny. The latter order mainly comprises symmetric unicells with a pronounced central constriction (isthmus) and ornamented cell walls. However, at the base of the clade containing these typical placoderm desmids are three genera (Netrium, Nucleotaenium, and Planotaenium), which display a much simpler morphology (no cell wall ornamentations and no isthmus) and were formerly classified with the Zygnematales (in the family Mesotaeniaceae).28 Interestingly, the same arrangement was previously recovered by combined analyses of three genes (nuclear SSU rRNA, rbcL, and chloroplast LSU rRNA),29 and is here confirmed by phylogenomics. It appears that the desmids with elaborate cell shapes and complex cell walls (e.g., Cosmarium, Penium, Micrasterias, and Xanthidium) descended from unicellular ancestors with a simpler structure. Hence, the genera Netrium, Nucleotaenium, and Planotaenium are here formally included in the order Desmidiales. The internal phylogeny and taxonomy of the Desmidiales, however, needs to be resolved by extended taxon sampling in the future, as many classically recognized desmid genera (e.g., Cosmarium, Penium, and Staurodesmus) are not monophyletic.
On the unicellularity of the ancestral zygnematophyte
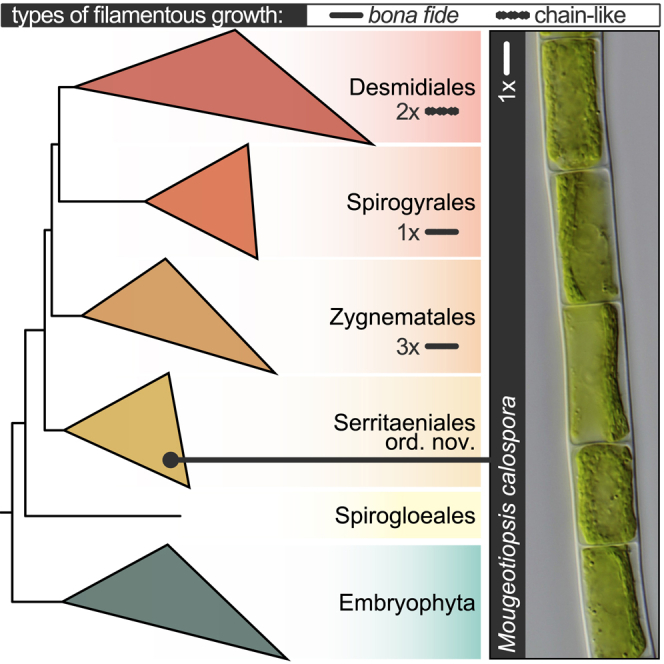
Our robust phylogenetic framework of the zygnematophytes now enables comparisons of species in an evolutionary context; thus assessment of evolutionary scenarios with great confidence are feasible. It is remarkable that the majority of zygnematophycean species are unicellular,30 as most of their streptophyte relatives (Embryophyta, Coleochaetophyceae, Charophyceae, Klebsormidiophyceae, and Chlorokybophyceae) display some kind of multicellularity, from sarcinoids to three-dimensional tissues.31 However, some zygnematophycean lineages exhibit more developmental complexity such as the formation of filaments, sometimes even with rhizoids or branched cells.31,32 Traditionally these filamentous members have been bundled in the family Zygnemataceae,28 but a close relationship of them was not recovered in previous phylogenies.7,8
Our fully supported phylogenomic tree reveals at least five separate lineages that contain true filaments, found in three orders (Figure 2): Spirogyra (Spirogyrales), Mougeotia, Zygnema, Zygnemopsis (all Zygnematales), and Mougeotiopsis (Serritaeniales). Other filamentous taxa (e.g., Temnogametum iztacalense and Zygogonium ericetorum) await genomic/transcriptomic sequencing and phylogenomic placement33,34 The cells of all these filamentous species have straight and relatively simple cell walls, no central constrictions, and display an intimate cell-cell contact (i.e., typical cross walls)—yet without plasmodesmata.35 At the same time, there are also filamentous desmids (e.g. Desmidium, Bambusina, Onychonema, and Phymatodocis36), which differ markedly from the aforementioned lineages in their cellular details and filament morphology (see also Hall et al.37). The cells of Desmidium, Bambusina, Onychonema, and Phymatodocis display the typical characters of desmid cells (e.g., central constriction and cell wall ornamentation) and rather appear as cell chains. Together with the fact that the filamentous desmids are nested within the unicellular desmids, it is conceivable that there are distinct types of filamentous growth in the Zygnematophyceae, which evolved independently; we account for this possibility in our analyses (see Figure 3 and below). The Zygnematophyceae as a whole are nested within a clade of mostly multicellular streptophytes, the Phragmoplastophyta, with the most morphologically elaborate (the Embryophyta) as sister clade. Previous studies have therefore noted that the streamlined body plans of extant zygnematophytes—down to unicellularity—might have arisen by reductive evolution from a morphologically more complex ancestor.5,38, 39, 40, 41 Based on our current phylogeny, it seems most parsimonious that the last common ancestor of the zygnematophytes was unicellular—thus having already experienced a reduction in its body plan. This scenario goes along with five independent origins of bona fide filaments; the alternative would require at least seven losses of multicellularity.
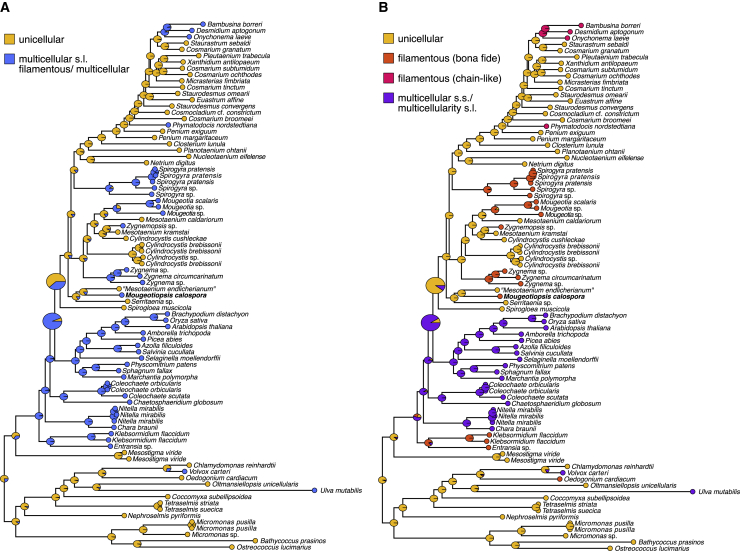
Figure 3.
Ancestral character state reconstruction for unicellular or multicellular (including filamentous) growth characters
(A and B) Growth types were coded as either a simplistic (A) two- and more nuanced (B) four- character state distributions to reflect different levels of complexity regarding the possibilities/hypotheses for the homology of growth types: yellow, unicellular; blue, multicellular sensu lato (including filamentous growth); orange, bona fide filamentous growth; pink, chain-like filaments (desmids); and purple, multicellular growth sensu stricto.
In an attempt to infer the body plan of the common ancestor of zygnematophytes, we performed ancestral character state reconstructions (ACSR) with various data coding strategies concerning the types of multicellularity (Figure 3). Irrespective of how the growth types were coded, a unicellular zygnematophyte ancestor was consistently inferred by our analyses, albeit with varying support (posterior probability [PP] = 0.58–0.93). Hence, we infer up to five tentative independent origins of true filamentous growth, and two additional independent origins of chain-like filaments (in the Desmidiales) (unicellular ancestors have PP = 0.80–1.00); under this scenario, the last common ancestor of the Zygnematophyceae and land plants was likely filamentous or multicellular (PP = 0.91–0.93), whereas the last common ancestor of Zygnematophyceae was likely unicellular (PP = 0.58–0.89). Given the effect of character coding in these analyses, we conclude that expanding our knowledge about the homology of the various types of multicellular and filamentous body plans in the green algae is essential.
Filamentous growth as observed in the Zygnematophyceae can be considered the least elaborate type of multicellularity.42 Yet, the cellular and molecular traits underpinning this growth type remain obscure. The multiple growth type transitions in the zygnematophytes are consistent with parallel evolution from a common molecular machinery, but the relative simplicity of filamentous growth renders convergent evolution equally plausible. The hypothetical unicellular lynchpin at the base of the Zygnematophyceae is an attractive hypothesis: it could explain why zygnematophytes lack plasmodesmata (e.g., Brunkard and Zambryski35), why the cross walls often look distinct from other streptophytes, and perhaps even why the group as such returned to a cleavage-like cell division mechanism (see Buschmann and Zachgo14). Future research on the different filamentous lineages will need to establish a deeper understanding of the molecular machinery underpinning their common morphology.
In addition, recent culture-based efforts to explore terrestrial zygnematophytes indicated a high diversity of unicellular lineages,43 which are not yet covered by genomic/transcriptomic sequencing and might change the evolutionary picture. Biased taxon sampling is indeed a serious problem for ACSR,44,45 and thus genomic sequencing of further zygnematophytes is an important task for the future. The fossil record for Zygnematophyceae is sparse. Several of the ordinal lineages of Zygnematophyceae are potentially several hundreds of millions of years old (estimations based on molecular clock results presented in Morris et al.46). Hence, important information might be obscured by extinction events and new discoveries of living or fossil taxa could easily lead to new interpretations. For now, our phylogenomic data demonstrate that the zygnematophytes comprise multiple transitions of their body plan, and also enable the selection of relevant species for comparative cell biological research.
Conclusion
The identification of the Zygnematophyceae as the sister lineage to land plants was surprising, in part because of their relatively simple body plans. The study of zygnematophycean trait evolution is a challenge because of their species richness, diverse morphologies, and unresolved phylogeny. We have provided a phylogenomic backbone and a congruent classification system for the closest algal relatives of land plants. Looking at algal growth types through the lens of phylogenomics reveals dynamic emergence and formation of filamentous and unicellular growth among the Zygnematophyceae—traits whose evolutionary history might also feature reductive evolution from a more complex ancestor of Zygnematophyceae and land plants.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| DNAse I | Thermo Fisher, Waltham, MA, USA | N/A |
| NEB mRNA stranded Library preparation kit | New England Biolabs, Beverly, MA, USA | N/A |
| Trizol | Thermo Fisher, Waltham, MA, USA | N/A |
| Deposited data | ||
| Alignment | This study | https://doi.org/10.5281/zenodo.6805950 |
| Amborella trichopoda genome | Amborella genome project47 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Atrichopoda |
| Arabidopsis thaliana genome TAIR V10 | TAIR48 | http://www.arabidopsis.org |
| Azolla filiculoides genome | Li et al.49 | https://www.fernbase.org |
| Bambusina borreri CCAC 0045 transcriptome, 1KP Code QWFV | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Bathycoccus prasinos genome | Moreau et al.51 | https://phycocosm.jgi.doe.gov/Batpra1/Batpra1.info.html |
| Brachypodium distachyon | The International Brachypodium Initiative52 | https://phytozome-next.jgi.doe.gov/info/Bdistachyon_v3_1 |
| Chaetophaeridium globosum SAG26.98 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Chara braunii S276 genome | Nishiyama et al.54 | https://bioinformatics.psb.ugent.be/orcae/overview/Chbra |
| Chlamydomonas reinhardtii genome v5.5 | Merchant et al.55; Blaby et al.56 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Creinhardtii |
| Closterium lunula M2156 transcriptome, 1KP Code DRFX | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Coccomyxa subellipsoidea genome v2.0 | Blanc et al.57 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_CsubellipsoideaC_169 |
| Coleochaete scutata SAG50.90 transcriptome | de Vries et al.58 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GFXZ00000000 |
| Coleochaete orbicularis transcriptome | Ju et al.59 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GBSL01&search=GBSL01000000&display=scaffolds |
| Coleochaete orbicularis transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Cosmarium broomei CCAC 0143 transcriptome, 1KP Code HIDG | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cosmarium granatum CCAC 0137 transcriptome, 1KP Code MNNM | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cosmarium ochthodes M1384 transcriptome, 1KP Code HJVM | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cosmarium subtumidum M3067 transcriptome, 1KP Code WDGV | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cosmarium tinctum M2301 transcriptome, 1KP Code BHBK | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cosmocladium cf. constrictum ASW 07118 transcriptome, 1KP Code RQFE | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cylindrocystis brebissonii M2213 transcriptome, 1KP Code YOXI | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cylindrocystis brebissonii M2853/M2213 transcriptome, 1KP Code YLBK | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cylindrocystis brebissonii M2853 transcriptome, 1KP Code RPGL | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cylindrocystis cushleckae M2158 transcriptome, 1KP Code JOJQ | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Cylindrocystis sp. M3015 transcriptome, 1KP Code VAZE | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Desmidium aptogonum ASW 07112 transcriptome, 1KP Code DFDS | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Entransia sp. transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Euastrum affine ASW 07012 transcriptome, 1KP Code GYRP | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Klebsormidium flaccidum UTEX 321 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Klebsormidium flaccidum SAG2307 transcriptome | de Vries et al.58 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GFXY00000000 |
| Marchantia polymorpha genome v3.1 | Bowman et al.60 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Mpolymorpha |
| Mesotaenium braunii (Serritaenia sp.) M2214 transcriptome, 1KP Code WSJO | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Mesotaenium caldariorum SAG 648-1 transcriptome, 1KP Code HKZW | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Mesotaenium kramstei UTEX LB 1025 transcriptome, 1KP Code NBYP | Carpenter et al.50 | http://www.onekp.com/public_data.html |
|
“Mesotaenium endlicherianum“ SAG12.97 transcriptome, 1KP Code WDCW |
Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Mesostigma viride CCAC 1140 transcriptome | Ju et al.59 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GBSK00000000 |
| Mesostigma viride NIES995 transcriptome | de Vries et al.58 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GFXX00000000 |
| Micrasterias fimbriata ASW 07026 transcriptome, 1KP Code MCHJ | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Micromonas pusilla genome v3.0 | Worden et al.61 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_MpusillaCCMP1545 |
| Micromonas sp. RCC299 genome v3.0 | Worden et al.61 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_MspRCC299 |
| Mougeotia scalaris SAG164.80 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Mougeotia sp. MZCH240 transcriptome | de Vries et al.16; Fürst-Jansen et al.17 |
https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GHUK00000000. |
| Mougeotiopsis calospora transcriptome assembly | This study | GenBank: GJZN00000000.1 |
| Mougeotiopsis calospora transcriptome reads | This study | Sequence Read Archive: SRR19751296 |
| Nephroselmis pyriformis CCMP 717 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Netrium digitus CCAC 0148 transcriptome, 1KP Code FFGR | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Nitella mirabilis transcriptome | Ju et al.59 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GBST01&search=GBST01000000&display=scaffolds |
| Nitella mirabilis transcriptomes of lower and upper tissues | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Nucleotaenium eifelense M3006 transcriptome, 1KP Code KMNX | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Oedogonium cardiacum UTEX LB40 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Oltmansiellopsis unicellularis SCCAP K-0250 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Onychonema laeve CCAC 0151 transcriptome, 1KP Code GGWH | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Oryza sativa Nipponbare genome v7.0 | Kawahara et al.62 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Osativa |
| Ostreococcus lucimarinus genome v2.0 | Palenik et al.63 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Olucimarinus |
| Penium exiguum CCAC 0142 transcriptome, 1KP Code YSQT | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Penium margaritaceum SAG22.82 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Phymatodocis nordstedtiana SVCK 327 transcriptome, 1KP Code RPQV | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Physcomitrium patens genome v3.3 | Lang et al.64 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Ppatens |
| Picea abies genome | Nystedt et al.65 | https://plantgenie.org/FTP?dir=Data%2FConGenIE%2FPicea_abies%2Fv1.0 |
| Planotaenium ohtanii M2697 transcriptome, 1KP Code SNOX | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Pleurotaenium trabecula CCAC 0163 transcriptome, 1KP Code MOYY | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Salvinia cucullata genome | Li et al.49 | https://www.fernbase.org |
| Selaginella moellendorffii genome | Banks et al.66 | https://phytozome-next.jgi.doe.gov/info/Smoellendorffii_v1_0 |
| Sphagnum fallax v0.5 genome | Obtained from Phytozome with permission | https://phytozome-next.jgi.doe.gov/info/Sfallax_v0_5 |
| Spirogloea muscicola CCAC 0214 transcriptome, 1KP Code TPHT | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Spirogyra pratensis MZCH10213 transcriptome | de Vries et al.16 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GICF00000000. |
| Spirogyra pratensis UTEX 921 transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Spirogyra pratensis UTEX 928 transcriptome | Ju et al.59 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GBSM01000000 |
| Spirogyra sp. M1810 transcriptome, 1KP Code HAOX | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Spirogyra sp. Transcriptome Au1 | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Staurastrum sebaldi M1129 transcriptome, 1KP Code ISHC | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Staurodesmus convergens M2558 transcriptome, 1KP Code WCQU | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Staurodesmus omearii M0751 transcriptome, 1KP Code RPRU | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Tetraselmis striata transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Tetraselmis suecica transcriptome | Cooper and Delwiche53 | https://figshare.com/articles/dataset/Green_algal_transcriptomes_for_phylogenetics_and_comparative_genomics/1604778 |
| Ulva mutabilis genome | De Clerck et al.67 | https://bioinformatics.psb.ugent.be/orcae/overview/Ulvmu |
| Volvox carteri genome v2.1 | Prochnik et al.68 | https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Vcarteri |
| Xanthidium antilopaeum M1229 transcriptome, 1KP Code GBGT | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Zygnema circumcarinatum SAG698-1a transcriptome | de Vries et al.58 | https://www.ncbi.nlm.nih.gov/Traces/wgs/wgsviewer.cgi?val=GFYA00000000 |
| Zygnema sp.-B M1384 transcriptome 1KP Code WGMD | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Zygnema sp. transcriptome 1KP Code FMRU | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Zygnemopsis sp. CCAP 699/1 transcriptome 1KP Code MFZO | Carpenter et al.50 | http://www.onekp.com/public_data.html |
| Experimental models: Organisms/strains | ||
| Mougeotiopsis calospora MZCH580 | Obtained from Microalgae and Zygnematophyceae Collection Hamburg (MZCH) | maintained at Microalgae and Zygnematophyceae Collection Hamburg (MZCH) |
| Mougeotia sp. MZCH240 | Obtained from Microalgae and Zygnematophyceae Collection Hamburg (MZCH) | maintained at Microalgae and Zygnematophyceae Collection Hamburg (MZCH) |
| Spirogyra pratensis MZCH10213 | Obtained from Microalgae and Zygnematophyceae Collection Hamburg (MZCH) | maintained at Microalgae and Zygnematophyceae Collection Hamburg (MZCH) |
| Zygnema circumcarinatum MZCH10230 | Obtained from Microalgae and Zygnematophyceae Collection Hamburg (MZCH) | maintained at Microalgae and Zygnematophyceae Collection Hamburg (MZCH) |
| Software and algorithms | ||
| BUSCO v.5.0.0 | Seppey et al.69 | https://busco.ezlab.org |
| FASTQC | Babraham Institute | www.bioinformatics.babraham.ac.uk/projects/fastqc |
| IQ-Tree v1.5.5 and v1.6.12 | Nguyen et al.70 | http://www.iqtree.org |
| MAFFT v7.310 | Katoh and Standley71 | https://mafft.cbrc.jp/alignment/software/ |
| Phytools | Revell72 | https://cran.r-project.org/web/packages/phytools/index.html |
| Posterior Mean Site Frequency Profiles | Wang et al.73 | Implemented in IQ-Tree http://www.iqtree.org |
| Re-routing method according to Yang 1995 | Yang74 | N/A |
| Trimal v1.4.rev15 | Capella-Gutierrez et al.75 | http://trimal.cgenomics.org |
| Transcdecoder v.5.5.0 | Brian J. Haas | https://github.com/TransDecoder/TransDecoder/releases |
| Trimmomatic v0.36 | Bolger et al.76 | http://www.usadellab.org/cms/?page=trimmomatic |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jan de Vries (devries.jan@uni-goettingen.de).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
RNA-seq data have been deposited at the NCBI under the BioProject accession PRJNA849386 and the Sequence Read Archive (SRA) under the accession SRR19751296; all data are publicly available as of the date of publication. Accession numbers are additionally listed in the key resources table.
-
•
A transcriptome assembly has been deposited at NCBI Transcriptome Shotgun Assembly Sequence Database (TSA) under the accession GJZN00000000. The version described in this paper is the first version, GJZN01000000. The assembly is publicly available as of the date of publication. The accession number is additionally listed in the key resources table. The alignment has been uploaded to Zenodo: https://doi.org/10.5281/zenodo.6805950
-
•
No original code was used; all computational analyses were performed with published tools and are cited in the STAR Methods section.
Experimental model and subject details
Algal strains
Mougeotiopsis calospora (strain MZCH580), Mougeotia sp. (MZCH240), Spirogyra pratensis (strain MZCH20213) and Zygnema circumcarinatum (MZCH10230) were obtained from the Microalgae and Zygnematophyceae Collection Hamburg (MZCH)77,78 and grown in WHM medium79 or Waris-H medium80 at 20°C and under full-spectrum fluorescent lamps or white LEDs (30-50 μmol photons m-2 s-1; 16h:8h light-dark cycle), if not stated otherwise in the experimental details (see below).
Method details
Rationale for the application of the name Mougeotiopsis calospora to strain MZCH580
In terms of its gross morphology, strain MZCH580 resembles members of the genera Klebsormidium (Klebsormidiophyceae), Ulothrix (Ulvophyceae) and Mougeotia (Zygnematophyceae), all of which form unbranched filaments and have plate-like or parietal plastids. However, the absence of pyrenoids in strain MZCH580 is a major distinguishing character, as algae from the three mentioned genera (and classes) typically have prominent pyrenoids surrounded by a sheath of starch grains. There are, however, two historical descriptions from the late 19th century that describe pyrenoid-lacking, filamentous green algae with plate like chloroplasts: Mougeotiopsis calospora Palla, 1894 and Mesogerron fluitans Brand, 1899. Mougeotiopsis is a putative zygnematophyte, as scalariform conjugation and the formation of zygospores was clearly documented.9 Instead, Mesogerron was only described on the basis of vegetative material, and first suspected to be related to Ulothrix (Ulvophyceae, Chlorophyta). Based on the marked resemblance in their vegetative characters (filament width of 15–18 μm, cell architecture, and chloroplast morphology), Mougeotiopsis and Mesogerron were later treated as heterotypic synonyms (Krieger, 194181). Strain MZCH580 matches both descriptions concerning the varying cell length (including cells that are shorter than wide), cell architecture (plastid-associated nucleus) and chloroplast morphology (plate-like to parietal with pronounced lateral indentations), but it has somewhat smaller cells (filament width of 10–15 μm). The morphological similarity, however, is compelling, and variation in filament width is known for many closely related strains or species of green algae. We were unable to locate the type material of Mougeotiopsis calospora, but studied original material of Mesogerron fluitans (collected by F. Brand in 1899 and provided by the Herbarium of the Academy of Natural Sciences of Philadelphia). The dried filaments of that species were morphologically similar to those of strain MZCH580, especially in the marked variation in cell length observed in the filaments (Figure S4). Amplification of genetic material from this sample did not work.
Rationale for establishing a new order, Serritaeniales ord. nov.
In our phylogeny, the branch in question comprises three distinct groups of organisms: Mougeotiopsis calospora (one strain known), the genus Serritaenia (several strains known25), and strain SAG 12.97, a unicellular zygnematophyte that is often referred to as “Mesotaenium endlicherianum”. Currently, there is only one existent ordinal name that is based on the mentioned taxon names, namely Mesotaeniales Fritsch. However, the phylogenetic position of the genus Mesotaenium is still uncertain, as the designation of strain SAG 12.97 is potentially based on misidentification. In the opinion of some authors (S.H. and A.B.), the morphology of SAG 12.97 does not conform with the description of the type species M. endlicherianum Nägeli. This problem was already recognized by other specialists for zygnematophycean algae who studied strain SAG 12.97.82,83 Hence, we are hesitant to reuse the name Mesotaeniales and instead introduce a new ordinal name based on the well-studied and credible genus Serritaenia. Descriptions of the zygnematophycean orders defined in this study are provided in Table 1.
Light microscopy, time-lapse photography, and confocal imaging
High-resolution imaging of Mougeotiopsis calospora was done with the Zeiss IM35 inverted microscope (Carl Zeiss, Oberkochen, Germany) equipped with the objective lens Planapochromat 63×/1.4, electronic flash, and the Canon EOS 6D digital single-lens reflex camera (Canon, Tokyo, Japan). Time lapse imaging was performed on a Leica DM5000B microscope (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany) controlled by the Micromanager software at six frames per minute, shown as 10 FPS. Color balance and contrast of micrographs were adjusted with Photoshop CS4 (Adobe Inc., CA, USA). Confocal laser scanning microscopy was done with a Leica TCS SPE system (SP5) and the Leica LCS software (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany). Chlorophyll was excited with a wavelength of 635 nm and the emission of 646–782 nm was recorded. Confocal z stacks were processed and converted to three-dimensional data with the image processing package Fiji.84
Transmission electron microscopy
Algal filaments were fixed with 2 % glutaraldehyde in 75 mM cacodylate buffer (pH 7.0) for 1 h at RT, rinsed with 75 mM cacodylate buffer, and postfixed with 1 % osmium tetroxide in 75 mM cacodylate buffer overnight at 4 °C. After rinsing in cacodylate buffer, the samples were dehydrated in a graded acetone series and embedded according to Spurr.85 The resulting TEM blocks were sectioned on an Ultracut E ultramicrotome (Leica-Reichert-Jung, Vienna, AU), stained with 2 % uranyl acetate and 2 % lead citrate. Sections were then examined with the LEO 906E transmission electron microscope (LEO, Oberkochen, Germany) and imaged with a MultiScan Typ 794 CCD camera and the Digital Micrograph 3.4.4 software (both Gatan Inc., Pleasanton, USA).
RNA isolation, sequencing and phylogenomics
For the isolation of total RNA, Mougeotiopsis calospora was grown on a modified freshwater F/2 medium86 with 1% agar at 22°C. An LED light source provided photosynthetically active radiance at 120 μmol photons∗m-2∗s-1 under a 12:12 h light/dark photocycle. Harvesting, RNA extraction and transcriptome sequencing was carried out as described by de Vries et al.16 In brief, filaments of a growing algal culture were harvested and directly transferred into Trizol (Thermo Fisher, Waltham, MA, USA). The algal sample was homogenized using a Tenbroek tissue homogenizer and all following steps were performed in accordance to the manufacturer’s instructions. To remove possible residual DNA, RNA samples were treated with DNAse I (Thermo Fisher). Adequate RNA quality was verified using a formamide agarose gel. Samples were shipped on dry ice to Genome Québec (Montreal, Canada), where additional RNA quantification and quality assessments were performed using a Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, USA). Library construction was performed using the NEB mRNA stranded Library preparation kit (New England Biolabs, Beverly, MA, USA). Sequencing of the libraries was carried out on the NovaSeq 6000 (Illumina), yielding 28188133 paired end reads of 101 base pairs in length. Quality of the reads was assessed using FastQC version 0.11.7. Reads were trimmed using Trimmomatic version 0.3676, applying settings for quality trimming and adapter removal (ILLUMINACLIP:Adapters.fa:2:30:10:2:TRUE HEADCROP:10 TRAILING:3 SLIDINGWINDOW:4:20 MINLEN:36). The transcriptome was assembled de novo with Trinity.87 Transcriptome completeness was assessed with BUSCO v.5.0.069 using the viridiplantae_odb10 database in the transcriptome mode. Open reading frames (ORFs) were predicted with Transdecoder v.5.5.0.
We downloaded 83 transcriptomes and genomes of Streptophyta and Chlorophyta (see key resources table). Using a previously constructed phylogenomic dataset, we searched the selected sequencing data for orthologs of the 351 highly conserved proteins.88 After alignment and trimming using MAFFT v7.31071 and trimal v1.4.rev15,75 careful inspection of single-protein phylogenies estimated with IQ-TREE v1.5.5 under the LG4X model was undertaken to remove contaminants and paralogs. Once the data set was refined, orthologs that were missing in over 50% of taxa were removed; that said, we retained all orthologs that were present in Mougeotiopsis (overwriting the aforementioned 50% filtering). We estimated a maximum likelihood phylogeny based on the concatenated alignment of a final set of 326 translated proteins (cumulative maximum of 115,424 sites; see alignment on Zenodo, https://doi.org/10.5281/zenodo.6805950;) the final set of proteins/protein-coding genes was: AAP, ABHD13, Actin, ADK2, AGB1, AGX, AKTIP, ALG11, ALIS1, AMP2B, AOAH, AP1S2, AP3M1, AP3S1, AP4M, AP4S1, APBLC, ar21, arf3, ARL6, ARP2, ARP3, arpc1, ARPC4, ATEHD2, ATG2, atp6, ATP6V0A1, ATP6V0D1, ATPDIL14, ATSAR2, Atub, BAT1, Btub, C16orf80, C22orf28, C3H4, calr, capz, CC1, CCDC113, CCDC37, CCDC40, CCDC65, cct-A, cct-B, cct-D, cct-E, cct-G, cct-N, cct-T, cct-Z, CDK5, CLAT, COP-beta, COPE, COPG2, COPS2, COPS6, COQ4-mito, CORO1C, crfg, CRNL1, CS, CTP, D2HGDH-mito, DCAF13, DHSA1, DHSB3, DHYS, DIMT1L, DNAI2, DNAJ, DNAL1, DNM, DPP3, DRG2, ECHM, EF2, EFG-mito, EFTUD1, EIF3B, EIF3C, EIF3I, EIF4A3, EIF4E, ERLIN1, ETFA, FA2H, FAH, FAM18B, FAM96B, FAM, fh, fibri, FOLD, fpps, FTSJ1, GAS8, GCST, gdi2, GDI, glcn, GLGB2, GMPP3, gnb2l, gnbpa, GNL2, grc5, GRWD1, GSS, Gtub, H2A, H2B, h3, h4, HDDC2, HGO, HM13, hmt1, HSP70C, hsp70mt, HSP90, HYOU1, if2b, if2g, if2p, if6, IFT46, IFT57, IFT88, IMB1, IMP4, ino1, IP5PD, IPO4, IPO5, KARS, KDELR2, l10a, l12e-D, LRRC48, mat, mcm-A, mcm-B, mcm-C, mcm-D, mcm-E, metap2, METTL1, MLST8, MMAA-mito, mra1, MTHFR, MTLPD2, MYG1, NAA15, NAE1, NAPA, ndf1, NDUFV2-mito, NFS1-mito, NMD3, NMT1, NOP5A, NSA2, nsf1-C, nsf1-E, nsf1-G, nsf1-H, nsf1-I, nsf1-J, nsf1-K, nsf1-L, nsf1-M, nsf2-A, nsf2-F, ODB2, ODBA, ODBB, ODO2A, ODPA2, ODPB, oplah, orf2, osgep, PABPC4, pace2-A, pace2B, Pace2C, pace5, PCY2, PELO, PGM2, PIK3C3, PLS3, PMM2, PMPCB, PPP2R3, PPP2R5C, PPX2, PR19A, PSD11, PSD7, psma-A, psma-B, psma-C, psma-E, psma-F, psma-G, psma-H, psma-J, psmb-K, psmb-L, psmb-M, psmb-N, PSMD12, PSMD6, psmd, PURA, PYGB, rac, rad23, Rad51A, ran, RBX1, rf1, rla2a, rla2b, RPAC1, RPF1, rpl11, rpl12, Rpl13A, Rpl13e, Rpl14e, Rpl15, rpl17, Rpl18, rpl19, rpl20, rpl21, Rpl24A, rpl26, rpl27, Rpl2, rpl30, rpl31, rpl32, rpl33, rpl35, Rpl3, rpl43, rpl44, Rpl4b, Rpl5, rpl6, Rpl7a, rpl9, RPN1B, rpo-A, rpo-B, rpo-C, RPPK, rppO, rps10, rps11, rps12, rps14, rps15, rps16, rps17, rps18, rps20, rps23, rps26, rps27, rps2, rps3, rps4, rps5, rps6, rps8, RPTOR, RRAGD, RRM1, s15a, s15p, sap40, SCO1-mito, SCSB, SEC23, SF3B2, SND1, SPTLC1, sra, srp54, STXBP1, suca, SYGM1, SYNJ, tfiid, TM9SF1, TMS, topo1, trs, UBA3, ubc, UBE12, UBE2J2, Ubq, VAPA, VARS, vata, vatb, vatc, vate, VBP1, VPS18, VPS26B, WBSCR22, WD66, wd, wrs, xpb, YKT6. This tree was used as a guide to infer the final phylogeny under the LG+PMSF(C60)+F+Γ model73 of evolution; this is in line with the results of ModelFinder,89 which determined from 144 protein models LG+F+I+G4 as best-fit model according to Bayesian Information Criterion. Bootstrap analysis was conducted with 100 nonparametric bootstrap replicates using this model.
Ancestral character state reconstruction
Ancestral character state reconstruction was performed with Phytools (Revell72), which implements Yang’s74 re-rooting method to infer marginal ancestral state estimates for the internal nodes in the tree (Figure 3). We performed two independent analyses assuming 2-, and 4-character states in order to understand the effect of character coding on the inferred ancestral character states. The 2-state model used (1) unicellular and (2) multicellular sensu lato (filamentous or multicellular); the 4-state model differentiated between (2) bona fide filamentous algae excluding desmids, (3) chain-like filamentous desmids, and (4) multicellular sensu stricto (embryophytes, Coleochaetophyceae, Charophyceae, Volvox, Ulva). All models assumed unordered states (equal rates of change).
Quantification and statistical analysis
For the quantification of the average diameter of macrotubules, 446 sections of macrotubules were examined with the LEO 906E transmission electron microscope (LEO, Oberkochen, Germany) and imaged with a MultiScan Typ 794 CCD camera; all 446 counts of the diameter were obtained with the Digital Micrograph 3.4.4 software (both Gatan Inc., Pleasanton, USA).
After inspection of single-protein phylogenies estimated with IQ-TREE v1.5.5 under the LG4X to remove contaminants and paralogs, the data set was refined: orthologs that were missing in over 50% of taxa were removed; that said, we retained all orthologs that were present in Mougeotiopsis (overwriting the 50% filtering). The final phylogeny was inferred under the LG+PMSF(C60)+F+Γ model73 of evolution; this is in line with the results of ModelFinder,89 which determined from 144 protein models LG+F+I+G4 as best-fit model according to Bayesian Information Criterion. Bootstrap analysis was conducted with 100 nonparametric bootstrap replicates using this model; approximate likelihood ratio test (SH-aLRT) was carried out with 1000 replicates and additionaly approximate Bayes (aBayes) test was carried out.
For the Approximately Unbiased test of the phylogenetic tree, we compared our phylogenomic hypothesis with that previously proposed by the One Thousand Plant Transcriptomes Initiative6 (main ASTRAL tree in Figure 2 based on 410 loci), which differed with ours in the relative position of a few species within Desmidiales. We performed an Approximately Unbiased test (AU test)90 under best-fit LG+C60+F+Γ model with 10,000 multiscale bootstrap replicates using IQ-TREE v.1.6.12.
Acknowledgments
This work was funded by the German Research Foundation grants 283693520 (Research Fellowship) and 417585753 (Emmy Noether Programme) both to S.H., grants 440231723 (VR 132/4-1) to J.d.V. and 440540015 (BU 2301/6-1) to H.B. within the framework of the Priority Programme “MAdLand – Molecular Adaptation to Land: Plant Evolution to Change” (SPP 2237), and grant 410739858 in the frame of the project CharMod to K.v.S.; J.d.V. further thanks the European Research Council for funding under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 852725; ERC-StG “TerreStriAL”). We thank Richard McCourt (Drexel University) and the Herbarium of the Academy of Natural Sciences of Philadelphia (PH) for destructive sampling of material from Mesogerron fluitans, and Elke Woelken (Universität Hamburg) for excellent support in electron microscopy.
Author contributions
Conceptualization, S.H., J.d.V.; investigation, S.H., S.K.W., A.B., I.I., S.d.V., H.B., K.v.S., J.d.V.; writing – original draft, S.H., I.I., and J.d.V.; writing – review and editing, all authors; visualization, S.H., A.B., I.I., and J.d.V.; and funding acquisition, S.H. and J.d.V.
Declaration of interests
The authors declare no competing interests.
Published: September 1, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2022.08.022.
Contributor Information
Sebastian Hess, Email: sebastian.hess@uni-koeln.de.
Jan de Vries, Email: devries.jan@uni-goettingen.de.
Supplemental information
References
- 1.Bar-On Y.M., Phillips R., Milo R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wodniok S., Brinkmann H., Glöckner G., Heidel A.J., Philippe H., Melkonian M., Becker B. Origin of land plants: Do conjugating green algae hold the key? BMC Evol. Biol. 2011;11:104. doi: 10.1186/1471-2148-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timme R.E., Bachvaroff T.R., Delwiche C.F. Broad Phylogenomic Sampling and the Sister Lineage of Land Plants. PLoS One. 2012;7:e29696. doi: 10.1371/journal.pone.0029696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruhfel B.R., Gitzendanner M.A., Soltis P.S., Soltis D.E., Burleigh J.G. From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol. Biol. 2014;14:23. doi: 10.1186/1471-2148-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wickett N.J., Mirarab S., Nguyen N., Warnow T., Carpenter E., Matasci N., Ayyampalayam S., Barker M.S., Burleigh J.G., Gitzendanner M.A., et al. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl. Acad. Sci. USA. 2014;111:E4859–E4868. doi: 10.1073/pnas.1323926111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.One Thousand Plant Transcriptomes Initiative One thousand plant transcriptomes and the phylogenomics of green plants. Nature. 2019;574:679–685. doi: 10.1038/s41586-019-1693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gontcharov A.A. Phylogeny and classification of Zygnematophyceae (Streptophyta): current state of affairs. Fottea. 2008;8:87–104. [Google Scholar]
- 8.Hall J.D., Karol K.G., McCourt R.M., Delwiche C.F. Phylogeny of the conjugating green algae based on chloroplast and mitochondrial nucleotide sequence data. J. Phycol. 2008;44:467–477. doi: 10.1111/j.1529-8817.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 9.Palla E. Ueber eine neue, pyrenoidlose Art und Gattung der Conjugaten. Ber. Deutsch. Bot. Ges. 1894;12:228–236. [Google Scholar]
- 10.Takano T., Higuchi S., Ikegaya H., Matsuzaki R., Kawachi M., Takahashi F., Nozaki H. Identification of 13 Spirogyra species (Zygnemataceae) by traits of sexual reproduction induced under laboratory culture conditions. Sci. Rep. 2019;9:7458. doi: 10.1038/s41598-019-43454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook M.E. Structure and asexual reproduction of the enigmatic charophycean green alga Entransia fimbriata (Klebsormidiales, Charophyceae) J. Phycol. 2004;40:424–431. [Google Scholar]
- 12.Fowke L.C., Pickett-Heaps J.D. Cell division in Spirogyra. II. Cytokinesis. J. Phycol. 1969;5:273–281. doi: 10.1111/j.1529-8817.1969.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 13.Pickett-Heaps J.D., Wetherbee R. Spindle function in the green alga Mougeotia: absence of anaphase A correlates with postmitotic nuclear migration. Cell Motil Cytoskeleton. 1987;7:68–77. [Google Scholar]
- 14.Buschmann H., Zachgo S. The evolution of cell division: from streptophyte algae to land plants. Trends Plant Sci. 2016;21:872–883. doi: 10.1016/j.tplants.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Villarreal J.C., Renner S.S. Hornwort pyrenoids, carbon-concentrating structures, evolved and were lost at least five times during the last 100 million years. Proc. Natl. Acad. Sci. USA. 2012;109:18873–18878. doi: 10.1073/pnas.1213498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries J., de Vries S., Curtis B.A., Zhou H., Penny S., Feussner K., Pinto D.M., Steinert M., Cohen A.M., von Schwartzenberg K., Archibald J.M. Heat stress response in the closest algal relatives of land plants reveals conserved stress signaling circuits. Plant J. 2020;103:1025–1048. doi: 10.1111/tpj.14782. [DOI] [PubMed] [Google Scholar]
- 17.Fürst-Jansen J.M.R., de Vries S., Lorenz M., von Schwartzenberg K., Archibald J.M., de Vries J. Submergence of the filamentous Zygnematophyceae Mougeotia induces differential gene expression patterns associated with core metabolism and photosynthesis. Protoplasma. 2022;259:1157–1174. doi: 10.1007/s00709-021-01730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komis G., Apostolakos P., Galatis B. Hyperosmotic Stress Induces Formation of Tubulin Macrotubules in Root-Tip Cells of Triticum turgidum: Their Probable Involvement in Protoplast Volume Control. Plant Cell Physiol. 2002;43:911–922. doi: 10.1093/pcp/pcf114. [DOI] [PubMed] [Google Scholar]
- 19.Stewart K.D., Floyd G.L., Mattox K.R., Davis M.E. Cytochemical demonstration of a single peroxisome in a filamentous green alga. J. Cell Biol. 1972;54:431–434. doi: 10.1083/jcb.54.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda M., Hashimoto H. Close association of centrosomes to the distal ends of the microbody during its growth, division and partitioning in the green alga Klebsormidium flaccidum. Protoplasma. 2007;231:127–135. doi: 10.1007/s00709-007-0267-6. [DOI] [PubMed] [Google Scholar]
- 21.Holzinger A., Lütz C., Karsten U. Desiccation stress causes structural and ultrastructural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an Alpine soil crust. J. Phycol. 2011;47:591–602. doi: 10.1111/j.1529-8817.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- 22.Mikhailyuk T., Lukešová A., Glaser K., Holzinger A., Obwegeser S., Nyporko S., Friedl T., Karsten U. New taxa of streptophyte algae (Streptophyta) from terrestrial habitats revealed using an integrative approach. Protist. 2018;169:406–431. doi: 10.1016/j.protis.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aigner S., Remias D., Karsten U., Holzinger A. Unusual phenolic compounds contribute to ecophysiological performance in the purple-colored green alga Zygogonium ericetorum (Zygnematophyceae, Streptophyta) from a high-alpine habitat. J. Phycol. 2013;49:648–660. doi: 10.1111/jpy.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng X., Holzinger A., Permann C., Anderson D., Yin Y. Characterization of two Zygnema strains (Zygnema circumcarinatum SAG 698-1a and SAG 698-1b) and a rapid method to estimate nuclear genome size of zygnematophycean green algae. Front. Plant Sci. 2021;12:610381. doi: 10.3389/fpls.2021.610381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busch A., Hess S. Sunscreen mucilage: a photoprotective adaptation found in terrestrial green algae (Zygnematophyceae) Eur. J. Phycol. 2022;57:107–124. [Google Scholar]
- 26.Cheng S., Xian W., Fu Y., Marin B., Keller J., Wu T., Sun W., Li X., Xu Y., Zhang Y., Wittek S., Reder T., Günther G., Gontcharov A., Wang S., Li L., Liu X., Wang J., Yang H., Xu X., Delaux P.M., Melkonian B., Wong G.K.S., Melkonian M. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell. 2019;179:1057–1067.e14. doi: 10.1016/j.cell.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Clements F.E. HW Wilson Company; 1909. The genera of fungi. [Google Scholar]
- 28.Guiry M.D. Taxonomy and nomenclature of the Conjugatophyceae (= Zygnematophyceae) ALGAE. 2013;28:1–29. [Google Scholar]
- 29.Gontcharov A.A., Melkonian M. Molecular phylogeny and revision of the genus Netrium (Zygnematophyceae, Streptophyta): Nucleotaenium gen. nov. J. Phycol. 2010;46:346–362. [Google Scholar]
- 30.Hall J.D., McCourt R.M. In: Handbook of the protists. Archibald J.M., Simpson A.G., Slamovits C.H., editors. Springer International Publishing; 2017. Zygnematophyceae; pp. 135–163. [Google Scholar]
- 31.Buschmann H. Into another dimension: how streptophyte algae gained morphological complexity. J. Exp. Bot. 2020;71:3279–3286. doi: 10.1093/jxb/eraa181. [DOI] [PubMed] [Google Scholar]
- 32.Ikegaya H., Sonobe S., Murakami K., Shimmen T. Rhizoid differentiation of Spirogyra is regulated by substratum. J. Plant Res. 2008;121:571–579. doi: 10.1007/s10265-008-0182-8. [DOI] [PubMed] [Google Scholar]
- 33.Garduño-Solórzano G., Martínez-García M., Scotta Hentschke G., Lopes G., Castelo Branco R., Vasconcelos V.M.O., Campos J.E., López-Cano R., Quintanar-Zúñiga R.E. The phylogenetic placement of Temnogametum (Zygnemataceae) and description of Temnogametum iztacalense sp. nov., from a tropical high mountain lake in Mexico. Eur. J. Phycol. 2021;56:159–173. [Google Scholar]
- 34.Stancheva R., Hall J.D., Herburger K., Lewis L.A., McCourt R.M., Sheath R.G., Holzinger A. Phylogenetic position of Zygogonium ericetorum (Zygnematophyceae, Charophyta) from a high alpine habitat and ultrastructural characterization of unusual aplanospores. J. Phycol. 2014;50:790–803. doi: 10.1111/jpy.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunkard J.O., Zambryski P.C. Plasmodesmata enable multicellularity: new insights into their evolution, biogenesis, and functions in development and immunity. Curr. Opin. Plant Biol. 2017;35:76–83. doi: 10.1016/j.pbi.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Andosch A., Höftberger M., Lütz C., Lütz-Meindl U. Subcellular sequestration and impact of heavy metals on the ultrastructure and physiology of the multicellular freshwater alga Desmidium swartzii. Int. J. Mol. Sci. 2015;16:10389–10410. doi: 10.3390/ijms160510389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall J.D., McCourt R.M., Delwiche C.F. Patterns of cell division in the filamentous Desmidiaceae, close green algal relatives of land plants. Am. J. Bot. 2008;95:643–654. doi: 10.3732/ajb.2007210. [DOI] [PubMed] [Google Scholar]
- 38.Harholt J., Moestrup Ø., Ulvskov P. Why Plants Were Terrestrial from the Beginning. Trends Plant Sci. 2016;21:96–101. doi: 10.1016/j.tplants.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Delwiche C.F., Cooper E.D. The evolutionary origin of a terrestrial flora. Curr. Biol. 2015;25:R899–R910. doi: 10.1016/j.cub.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 40.de Vries J., Archibald J.M. Plant evolution: landmarks on the path to terrestrial life. New Phytol. 2018;217:1428–1434. doi: 10.1111/nph.14975. [DOI] [PubMed] [Google Scholar]
- 41.Fürst-Jansen J.M.R., de Vries S., de Vries J. Evo-physio: on stress responses and the earliest land plants. J. Exp. Bot. 2020;71:3254–3269. doi: 10.1093/jxb/eraa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niklas K.J., Newman S.A. The origins of multicellular organisms. Evol. Dev. 2013;15:41–52. doi: 10.1111/ede.12013. [DOI] [PubMed] [Google Scholar]
- 43.Busch A., Hess S. A diverse group of underappreciated zygnematophytes deserves in-depth exploration. Applied Phycology. 2022:1–18. doi: 10.1080/26388081.2022.2081819. [DOI] [Google Scholar]
- 44.Heath T.A., Hedtke S.M., Hillis D.M. Taxon sampling and the accuracy of phylogenetic analyses. J. Syst. Evol. 2008;46:239–257. [Google Scholar]
- 45.Litsios G., Salamin N. Effects of Phylogenetic Signal on Ancestral State Reconstruction. Syst. Biol. 2012;61:533–538. doi: 10.1093/sysbio/syr124. [DOI] [PubMed] [Google Scholar]
- 46.Morris J.L., Puttick M.N., Clark J.W., Edwards D., Kenrick P., Pressel S., Wellman C.H., Yang Z., Schneider H., Donoghue P.C.J. The timescale of early plant evolution. Proc. Natl. Acad. Sci. USA. 2018;115:E2274–E2283. doi: 10.1073/pnas.1719588115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amborella Genome Project The Amborella genome and the evolution of flowering plants. Science. 2013;342:1241089. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- 48.Lamesch P., Berardini T.Z., Li D., Swarbreck D., Wilks C., Sasidharan R., Muller R., Dreher K., Alexander D.L., Garcia-Hernandez M., et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2012;40:D1202–D1210. doi: 10.1093/nar/gkr1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F.-W., Brouwer P., Carretero-Paulet L., Cheng S., de Vries J., Delaux P.-M., Eily A., Koppers N., Kuo L.-Y., Li Z., et al. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat. Plants. 2018;4:460–472. doi: 10.1038/s41477-018-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpenter E.J., Matasci N., Ayyampalayam S., Wu S., Sun J., Yu J., Jimenez Vieira F.R., Bowler C., Dorrell R.G., Gitzendanner M.A., et al. Access to RNA-sequencing data from 1, 173 plant species: The 1000 Plant transcriptomes initiative (1KP) GigaScience. 2019;8:giz126. doi: 10.1093/gigascience/giz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreau H., Verhelst B., Couloux A., Derelle E., Rombauts S., Grimsley N., Van Bel M., Poulain J., Katinka M., Hohmann-Marriott M.F., et al. Gene functionalities and genome structure in Bathycoccus prasinos reflect cellular specializations at the base of the green lineage. Genome Biol. 2012;13:R74. doi: 10.1186/gb-2012-13-8-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The International Brachypodium Initiative Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature. 2010;463:763–768. doi: 10.1038/nature08747. [DOI] [PubMed] [Google Scholar]
- 53.Cooper E., Delwiche C. Green algal transcriptomes for phylogenetics and comparative genomics. Figshare. 2016 doi: 10.6084/m9.figshare.1604778. [DOI] [Google Scholar]
- 54.Nishiyama T., Sakayama H., de Vries J., Buschmann H., Saint-Marcoux D., Ullrich K.K., Haas F.B., Vanderstraeten L., Becker D., Lang D., et al. The Chara genome: secondary complexity and implications for plant terrestrialization. Cell. 2018;174:448–464.e24. doi: 10.1016/j.cell.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 55.Merchant S.S., Prochnik S.E., Vallon O., Harris E.H., Karpowicz S.J., Witman G.B., Terry A., Salamov A., Fritz-Laylin L.K., Maréchal-Drouard L., et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blaby I.K., Blaby-Haas C.E., Tourasse N., Hom E.F.Y., Lopez D., Aksoy M., Grossman A., Umen J., Dutcher S., Porter M., et al. The Chlamydomonas genome project: a decade on. Trends Plant Sci. 2014;19:672–680. doi: 10.1016/j.tplants.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blanc G., Agarkova I., Grimwood J., Kuo A., Brueggeman A., Dunigan D.D., Gurnon J., Ladunga I., Lindquist E., Lucas S., et al. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol. 2012;13:R39. doi: 10.1186/gb-2012-13-5-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Vries J., Curtis B.A., Gould S.B., Archibald J.M. Embryophyte stress signaling evolved in the algal progenitors of land plants. Proc. Natl. Acad. Sci. USA. 2018;115:E3471–E3480. doi: 10.1073/pnas.1719230115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ju C., Van de Poel B., Cooper E.D., Thierer J.H., Gibbons T.R., Delwiche C.F., Chang C. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat. Plants. 2015;1:14004. doi: 10.1038/nplants.2014.4. [DOI] [PubMed] [Google Scholar]
- 60.Bowman J.L., Kohchi T., Yamato K.T., Jenkins J., Shu S., Ishizaki K., Yamaoka S., Nishihama R., Nakamura Y., Berger F., et al. Insights into Land Plant Evolution Garnered from the Marchantia polymorpha Genome. Cell. 2017;171:287–304.e15. doi: 10.1016/j.cell.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Worden A.Z., Lee J.H., Mock T., Rouzé P., Simmons M.P., Aerts A.L., Allen A.E., Cuvelier M.L., Derelle E., Everett M.V., et al. Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science. 2009;324:268–272. doi: 10.1126/science.1167222. [DOI] [PubMed] [Google Scholar]
- 62.Kawahara Y., de la Bastide M., Hamilton J.P., Kanamori H., McCombie W.R., Ouyang S., Schwartz D.C., Tanaka T., Wu J., Zhou S., et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palenik B., Grimwood J., Aerts A., Rouzé P., Salamov A., Putnam N., Dupont C., Jorgensen R., Derelle E., Rombauts S., et al. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc. Natl. Acad. Sci. USA. 2007;104:7705–7710. doi: 10.1073/pnas.0611046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lang D., Ullrich K.K., Murat F., Fuchs J., Jenkins J., Haas F.B., Piednoel M., Gundlach H., Van Bel M., Meyberg R., et al. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J. 2018;93:515–533. doi: 10.1111/tpj.13801. [DOI] [PubMed] [Google Scholar]
- 65.Nystedt B., Street N.R., Wetterbom A., Zuccolo A., Lin Y.-C., Scofield D.G., Vezzi F., Delhomme N., Giacomello S., Alexeyenko A., et al. The Norway spruce genome sequence and conifer genome evolution. Nature. 2013;497:579–584. doi: 10.1038/nature12211. [DOI] [PubMed] [Google Scholar]
- 66.Banks J.A., Nishiyama T., Hasebe M., Bowman J.L., Gribskov M., DePamphilis C., Albert V.A., Aono N., Aoyama T., Ambrose B.A., et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science. 2011;332:960–963. doi: 10.1126/science.1203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Clerck O., Kao S.-M., Bogaert K.A., Blomme J., Foflonker F., Kwantes M., Vancaester E., Vanderstraeten L., Aydogdu E., Boesger J., et al. Insights into the Evolution of Multicellularity from the Sea Lettuce Genome. Curr. Biol. 2018;28:2921–2933.e5. doi: 10.1016/j.cub.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 68.Prochnik S.E., Umen J., Nedelcu A.M., Hallmann A., Miller S.M., Nishii I., Ferris P., Kuo A., Mitros T., Fritz-Laylin L.K., et al. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science. 2010;329:223–226. doi: 10.1126/science.1188800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seppey M., Manni M., Zdobnov E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019;1962:227–245. doi: 10.1007/978-1-4939-9173-0_14. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katoh K., Standley D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Revell L.J. phytools: an R package for phylogenetic comparative biology (and other things): phytools: R package. Methods Ecol. Evol. 2012;3:217–223. [Google Scholar]
- 73.Wang H.-C., Minh B.Q., Susko E., Roger A.J. Modeling Site Heterogeneity with Posterior Mean Site Frequency Profiles Accelerates Accurate Phylogenomic Estimation. Syst. Biol. 2018;67:216–235. doi: 10.1093/sysbio/syx068. [DOI] [PubMed] [Google Scholar]
- 74.Yang Z., Kumar S., Nei M. A new method of inference of ancestral nucleotide and amino acid sequences. Genetics. 1995;141:1641–1650. doi: 10.1093/genetics/141.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von Schwartzenberg K., Bornfleth S., Lindner A.-C., Hanelt D. The Microalgae and Zygnematophyceae Collection Hamburg (MZCH) – living cultures for research on rare streptophytic algae. Algol. Stud. 2013;142:77–107. [Google Scholar]
- 78.Zhou H., von Schwartzenberg K. Zygnematophyceae: from living algae collections to the establishment of future models. J. Exp. Bot. 2020;71:3296–3304. doi: 10.1093/jxb/eraa091. [DOI] [PubMed] [Google Scholar]
- 79.Nichols H.W. In: Handbook of Phycological Methods. Stein J.R., editor. Cambridge University Press; London: 1973. Growth media – freshwater; pp. 16–17. [Google Scholar]
- 80.McFadden G.I., Melkonian M. Use of Hepes buffer for microalgal culture media and fixation for electron microscopy. Phycologia. 1986;25:551–557. [Google Scholar]
- 81.Krieger H., Kolkwitz R., Krieger H. In: Rabenhorst L., editor. Vol. 13. Becker & Erler; Leipzig: 1941. Zygnemales; pp. 1–499. (Kryptogamen-Flora von Deutschland und der Schweiz). [Google Scholar]
- 82.Gontcharov A.A., Marin B.A., Melkonian M.A. Molecular phylogeny of conjugating green algae (Zygnemophyceae, Streptophyta) inferred from SSU rDNA sequence comparisons. J. Mol. Evol. 2003;56:89–104. doi: 10.1007/s00239-002-2383-4. [DOI] [PubMed] [Google Scholar]
- 83.Gontcharov A.A., Marin B., Melkonian M. Are combined analyses better than single gene phylogenies? A case study using SSU rDNA and rbc L sequence comparisons in the Zygnematophyceae (Streptophyta) Mol. Biol. Evol. 2004;21:612–624. doi: 10.1093/molbev/msh052. [DOI] [PubMed] [Google Scholar]
- 84.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spurr A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 86.Guillard R.R.L. In: Culture of marine invertebrate animals. Smith W.L., Chanley M.H., editors. Plenum Book Publ. Corp; New York: 1975. – Culture of phytoplankton for feeding marine invertebrates; pp. 29–60. [Google Scholar]
- 87.Haas B.J., Papanicolaou A., Yassour M., Grabherr M., Blood P.D., Bowden J., Couger M.B., Eccles D., Li B., Lieber M., et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang S., Tice A.K., Spiegel F.W., Silberman J.D., Pánek T., Čepička I., Kostka M., Kosakyan A., Alcântara D.M.C., Roger A.J., et al. Between a Pod and a Hard Test: The Deep Evolution of Amoebae. Mol. Biol. Evol. 2017;34:2258–2270. doi: 10.1093/molbev/msx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002;51:492–508. doi: 10.1080/10635150290069913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The movie shows that cytokinesis is dominated by centripetal cleavage. Note the rotating chloroplasts and the migrating nuclei. Imaging was performed at six frames per minute, shown as 10 FPS
A second movie to illustrate the different phenotypes of cell division; see description for Video S1.
Data Availability Statement
-
•
RNA-seq data have been deposited at the NCBI under the BioProject accession PRJNA849386 and the Sequence Read Archive (SRA) under the accession SRR19751296; all data are publicly available as of the date of publication. Accession numbers are additionally listed in the key resources table.
-
•
A transcriptome assembly has been deposited at NCBI Transcriptome Shotgun Assembly Sequence Database (TSA) under the accession GJZN00000000. The version described in this paper is the first version, GJZN01000000. The assembly is publicly available as of the date of publication. The accession number is additionally listed in the key resources table. The alignment has been uploaded to Zenodo: https://doi.org/10.5281/zenodo.6805950
-
•
No original code was used; all computational analyses were performed with published tools and are cited in the STAR Methods section.