Abstract
We provide the first study of two siblings with a novel autosomal recessive LRP1-related syndrome identified by rapid genome sequencing and overlapping multiple genetic models. The patients presented with respiratory distress, congenital heart defects, hypotonia, dysmorphology, and unique findings, including corneal clouding and ascites. Both siblings had compound heterozygous damaging variants, c.11420G > C (p.Cys3807Ser) and c.12407T > G (p.Val4136Gly) in LRP1, in which segregation analysis helped dismiss additional variants of interest. LRP1 analysis using multiple human/mouse data sets reveals a correlation to patient phenotypes of Peters plus syndrome with additional severe cardiomyopathy and blood vessel development complications linked to neural crest cells.
Keywords: episodic respiratory distress, fetal ascites, hypertelorism, mild fetal ventriculomegaly, patent ductus arteriosus after premature birth, polyhydramnios, postductal coarctation of the aorta, small anterior fontanelle
INTRODUCTION
Identifying gene variants involved in early development has been challenging because of lethality, high number of potential variants, and slow turnaround time of clinical sequencing. Mainly driven by the Rady Children's Institute for Genomic Medicine, with a record of 16.5 h from sample collection to diagnosis using genome sequencing (GS) (Owen et al. 2021), rapid genomics is becoming a central tool in diagnosis within early childhood. Rapid GS (rGS) effectively impacts clinical care for pediatric patients in real time, specifically in the areas of medical and surgical management, palliative care, connecting patients with research opportunities, and family planning (Farnaes et al. 2018). Of the first 24 patients investigated with rGS by our site (Helen DeVos Children's Hospital), six were classified as having variants of uncertain significance (VUSs) (Beuschel et al. 2021), suggesting a higher percentage of uncertain classification than GS testing for undiagnosed disease analysis in older children (Bick et al. 2017). As rGS is used more frequently and broadly, this has increased the number of variants in genes not previously associated with human syndromes. In many cases, genes discovered have strong overlapping phenotypes studied within the animal modeling community (Prokop et al. 2018). This article presents two siblings with compound heterozygous LRP1 variants identified by rGS that correlated to the observations of Lrp1 knockout in the mouse. Although LRP1 has been linked to human disease such as Alzheimer's, as of 2022, LRP1 has not been linked to a human syndrome.
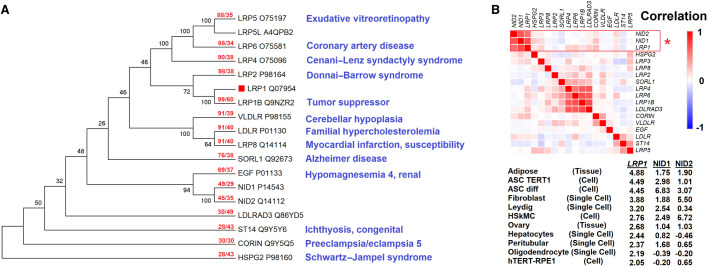
The low-density lipoprotein receptor ligated protein 1 (LRP1) is a low-density lipoprotein (LDL) receptor homologous to other genes like VLDLR, LDLR, and SORL1 (Fig. 1A). Many LRP-related genes have previously been connected to severe syndromes, including LRP2 in Donnai–Barrow syndrome (OMIM 222448), featuring facial/ocular anomalies, hearing loss, myopia, and proteinuria with kidney disfunction (Donnai and Barrow 1993); LRP4 in Cenani–Lenz syndactyly syndrome (OMIM #212780), featuring limb and kidney alterations (Li et al. 2010); and LRP5 in exudative vitreoretinopathy (OMIM #*603506) and osteoporosis–pseudoglioma syndrome (OMIM #259770), featuring altered osteoblast bone development and eye complications (Gong et al. 2001). Numerous LRP-related proteins have been shown to serve as receptors for Wnt signaling (Cong et al. 2004; Cadigan and Liu 2006; Li et al. 2010), including LRP1 (Terrand et al. 2009), establishing a central role of the LRP proteins in developmental biology. Although LRP1 is connected to many genes/proteins involved in disease biology and syndromes (Supplemental Fig. S1), no studies in humans have connected LRP1 to a syndrome. Relative to LRP-related genes, expression profiles cluster LRP1 near NID1 and NID2 (Fig. 1B); all three have no annotated developmental syndrome. Within this work, we present two siblings with matching phenotypes, in which the phenotypes for LRP1 are supported by human or mouse data.
Figure 1.
The low-density lipoprotein (LDL) receptor family and development. (A) Sequences with an E-value <1 × 10−20 based on BLAST of human LRP1 protein (red box). Sequences were aligned with the MUSCLE algorithm. The maximum likelihood tree shows the percent of 100 bootstrap analyses clustering at each node. The red text for each protein shows the length of coverage in BLAST hit followed by the percent identity relative to LRP1. Blue text is based on OMIM-annotated diseases for each gene. (B) Expression matrix for the genes of panel A using 200 data sets of tissues, cell lines, and single-cell annotations of the Human Protein Atlas. Correlations were calculated as 1 – Pearson's correlation. Below are the top samples for z-scores of LRP1, NID1, or NID2 expression levels.
RESULTS
Patient Phenotypes
The proband was a female born to a 31-yr-old, mother of European ancestry at 36 wk by repeat cesarean section after preterm premature rupture of membranes. Pregnancy was complicated by obesity, bipolar disorder, and insulin-dependent type-l diabetes. Prenatal ultrasounds showed ascites, cerebral ventriculomegaly, and polyhydramnios. These were confirmed by fetal magnetic resonance imaging (MRI) at 33 wk gestation. Prenatal karyotype and microarray performed on amniotic fluid were negative. Apgar scores (status of the newborn infant) were 5 (1 min) and 7 (5 min after birth), suggesting slight abnormality. Her birth weight was 3.24 kg (33rd centile), length was 47 cm (16th centile), and head circumference was 31 cm (<1st centile).
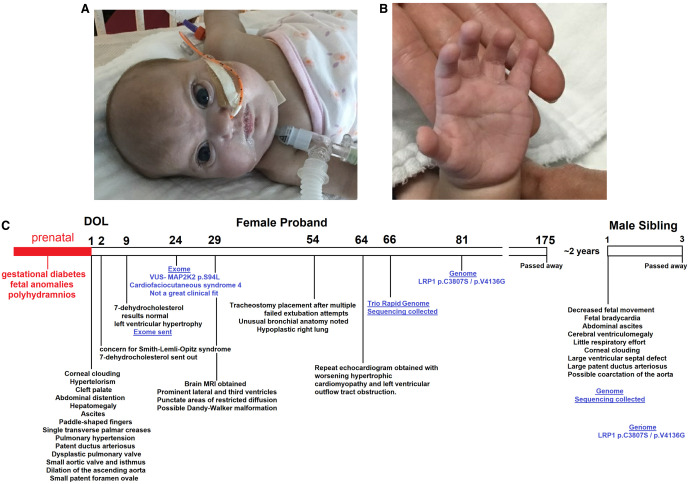
At birth, a cleft soft palate, hypertelorism, upslanting palpebral fissures, cloudy corneas, distended abdomen, paddle-shaped fingers, single palmar creases, and hypotonia were noted. She was placed on continuous positive airway pressure (CPAP) in the delivery room and progressed to intubation and eventual tracheostomy for respiratory distress and chronic lung disease. Initial echocardiogram revealed hypoplastic aortic valve, mild coarctation of the aorta, large patent ductus arteriosus, patent foramen ovale, and dysplastic pulmonary valve. Her condition progressed to pulmonary hypertension and severe hypertrophic cardiomyopathy. Brain MRI revealed mild third and lateral ventriculomegaly with a possible Dandy–Walker variant. She had stable hepatomegaly with ascites on abdominal ultrasound, with clinical resolution of ascites over time. Ophthalmology was consulted for her cloudy corneas, and she was diagnosed for bilateral glaucoma. Facial morphology was noted with palpebral fissures and enlarged pupils/irises (Fig. 2A), whereas hands were noted for paddle-shaped fingertips (Fig. 2B). She died at five months of age secondary to cardiac arrest. A time line of the clinical course is provided in Figure 2C.
Figure 2.
Patient morphology and time line. Facial (A) and hand (B) morphology of the proband. (C) Time line of both probands, including the genomics identified in blue. (DOL) Day of life.
The younger male sibling of the proband was born at 33 wk, 1 d (mother at 33-yr-old) via cesarean section because of decreased fetal movement and fetal bradycardia. Prenatal ultrasounds were notable for fetal abdominal ascites, bilateral cerebral ventriculomegaly, flat facial profile with a square-shaped forehead, and two-vessel umbilical cord. Polyhydramnios was present at 22 + 6 wk ultrasound consistent with nonimmune hydrops; amniotic fluid volume was normal by 33-wk scan. Genetic testing was deferred until after delivery per parents’ request. The mother presented at 33 wk gestation with decreased fetal movement for 3 d, a nonreactive nonstress test (NST), and fetal bradycardia prompting emergent cesarean section under general anesthesia. The baby was initially born with no respiratory effort. He required positive pressure ventilation (PPV), cardiopulmonary resuscitation (including three doses of epinephrine), paracentesis, intubation, and surfactant administration in the delivery room. Apgar scores were 0, 0, 3 at 1, 5, and 10 min, respectively, suggesting a poor birth condition. Birthweight was 2.610 kg (94th centile), length 44 cm (59th centile), head circumference 31.7cm (83rd centile) for gestational age. At birth, he was noted to be large for gestational age with a generously sized anterior fontanelle, bilateral corneal opacities, low-set ears, abdominal ascites, single umbilical artery, undescended and nonpalpable bilateral testes, possible rocker-bottom feet, global hypotonia, and no spontaneous movement.
Because of respiratory failure, he was placed on mechanical ventilation. Ventilation was significantly hindered by severe restriction of lung expansion by abdominal ascites requiring paracentesis in the delivery room. An echocardiogram on day of life 0 showed a large ventricular septal defect, large patent ductus arteriosus, and high suspicion for coarctation of the aorta. Hemodynamic stability was achieved with the addition of dopamine and hydrocortisone. Although initially presenting with poor perfusion, he later became plethoric, thrombocytopenic, and coagulopathic without active bleeding. He was also noted to have a right-sided closed humeral greenstick fracture without history of birth trauma. Ultrasound revealed bilateral hydronephrosis and proximal hydroureter with grade 3 dilation of the collecting system bilaterally. He remained oliguric. He was profoundly hypotonic and remained mostly unresponsive, with occasional minimal response to painful stimuli. Cranial ultrasound confirmed severe bilateral ventriculomegaly and possible third ventricle dilation. Parents opted for comfort care on day of life 2; he was extubated and expired shortly after. The overlap of phenotypes between the two siblings at various stages is listed in Table 1.
Table 1.
Overlapping phenotypes between the two siblings
| Classification | Phenotypes |
|---|---|
| Prenatal | Polyhydramnios, cerebral ventriculomegaly, fetal ascites |
| Facial | Hypertelorism, marked under orbital creases, corneal clouding, large anterior fontanelle, low-set ears |
| Pulmonary | Severe respiratory distress requiring intubation |
| Cardiac | Large patent ductus arteriosus, coarctation of the aorta |
| Abdomen | Profound ascites |
| Hand and feet | Paddle-shaped fingers and toes |
| Neurologic | Cerebral ventriculomegaly |
Genome Sequencing
rGS was performed for both children. Blood samples were sent to Rady Children's Institute of Genomic Medicine (RCIGM) for both parents and siblings. Trio-based genome sequence analysis revealed the same compound heterozygous LRP1 (gene of uncertain significance, GUS) variants in both siblings, classified as VUSs following American College of Medical Genetics and Genomics (ACMG) classification guidelines (Table 2). The first variant, c.11420G > C, (p.Cys3807Ser) was inherited maternally. The second variant, c.12407T > G, (p.Val4136Gly) was inherited paternally. Further analysis of variants between the siblings did not reveal any additional variants that met RCIGM reporting criteria.
Table 2.
Variant table
| Gene | Variant | ClinVar | Zygosity | Variant classification | Inheritance | Patient |
|---|---|---|---|---|---|---|
| LRP1 | c.11420G > C, (p.Cys3807Ser) | SCV002569952 | Heterozygous | VUS | Maternal | 1 and 2 |
| LRP1 | c.12407T > G (p.Val4136Gly) | SCV002569951 | Heterozygous | VUS | Paternal | 1 and 2 |
LRP1 Variant Analysis
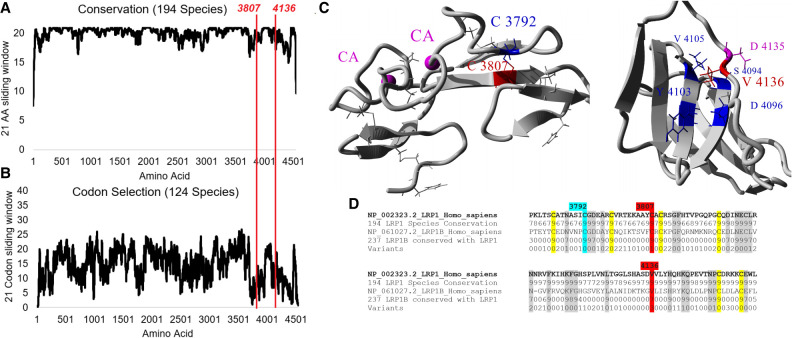
Evolutionary analysis of amino acid conservation (Fig. 3A) and codon selection (Fig. 3B) reveal the majority of LRP1 to be highly conserved, including the regions of the genomic variants p.Cys3807 and p.Val4136 (red lines). The p.Cys3807 amino acid forms a disulfide bond with p.Cys3792 (Fig. 3C) and is conserved throughout LRP1 and the closely related LRP1B (Fig. 3D), highlighting the disulfide bond's importance throughout evolution. The variant p.Cys3807Ser is predicted to be damaging in PolyPhen-2, deleterious in PROVEAN, Class C65 in Align-GVGD, has a codon score of 1.5 (out of 2, with 1.1 standard deviations above the mean of codon selection for any LRP1 codon), is 100% conserved throughout LRP1 and LRP1B sequences (431 total sequences), and is annotated in UniProt to form a disulfide bond. p.Val4136 is part of a hydrophobic core (Fig. 3C) and is conserved throughout LRP1 and LRP1B (Fig. 3D). The variant p.Val4136Gly is predicted deleterious in PROVEAN, damaging in SIFT, Class C35 in Align-GVGD, under weak selection but with 99% of species for LRP1 conserved as a V, 100% conserved as a hydrophobic amino acid, and has 61% conservation in LRP1B for V. Neither variant has been observed in gnomAD.
Figure 3.
LRP1 variant characterization. Amino acid conservation from 194 LRP1 protein sequences (A) or codon selection scores from 124 open reading frame sequences of LRP1 (B). Each is shown as a sliding window of additive conservation of 21 amino acids/codons, and the two variants from patients are marked with a red line. (C) Protein models for Cys3807 and Val4136 (red). (D) Sequence alignments of LRP1 and LRP1B for amino acids around 3807 and 4136 (red). The 194 protein sequences were determined for conservation of each site (1 to 9, with 9 being >90% conserved), and 237 sequences of LRP1B were used to determine conservation with LRP1 (0 being not conserved with LRP1 and 1 to 9 based on conservation, with 9 being >90% conserved). Cysteines involved in disulfide bonds and conserved are highlighted in yellow, and other amino acids conserved with LRP1B are highlighted in gray.
As of July 2021, there have been 1978 annotated LRP1 variants from gnomAD, TOPMed, Geno2MP, and ClinVar. Below are several noted variants with relevant connected phenotypes. The homozygous p.Lys1245Arg variant observed in a patient with keratosis pilaris atrophicans (Klar et al. 2015) is conserved as a K in 100% of sequences for LRP1 and LRP1B. It is likely that the Arg at this position does not fully replace Lys, but the functional conservation of the polar basic amino acid maintains most of the protein function to yield a subset of patient phenotypes. Within Geno2MP, an individual homozygous for p.Arg1993Trp is annotated with “abnormality of the cardiovascular system” (HP:0001626) and “abnormality of the cerebral vasculature” (HP:0100659), consistent with the siblings described here. Multiple heterozygous individuals with this same variant have an additional annotation of “hypoplastic left-heart syndrome” (HP:0004383) and “retinitis pigmentosa” (HP:0000510). Patients with p.Thr780Ile are also enriched for “hypoplastic left-heart syndrome” (HP:0004383). Annotated compound heterozygous damaging variants for LRP1 are observed in ClinVar for one individual (p.Leu526Val/p.Ser4520Cys) with ventricular septal defect and tricuspid atresia (ClinVar VCV000816915/VCV000816916). Two patients with compound heterozygous variants (p.Val315Ala/p.Arg1734His/p.Ser2907Lue/ p.Ala3634Thr) from a cardiomyopathy-specific paper by Jin et al. (2017) have also been observed. Additional work needs to be done to compare details of phenotypes within patients with LRP1 variants.
Lrp1 Mouse Phenotypes
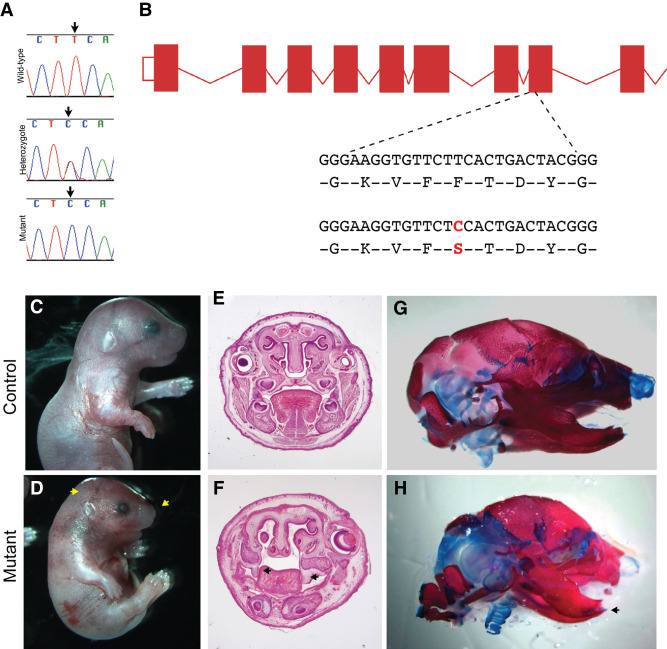
Mouse genetic editing can link genes to phenotypes, as assessed for Lrp1-related genetic changes (Supplemental Table S1). As part of the Bench to Bassinet Program (Lauer and Skarlatos 2010), chemically induced variants were screened for congenital heart disease. In that work, a homozygous Lrp1 variant (Lrp1b2b1554Clo, p.Cys4232Arg; http://www.informatics.jax.org/allele/MGI:5437079) was identified to drive embryonic lethality through pulmonary stenosis, atrioventricular septal defect, and pronounced abdominal wall complications. An ENU (N-ethyl-N-nitrosourea) mutational screen has also identified a craniofacial defect variant in mouse Lrp1 (c.Thr1019Cys, p.Phe340Ser) that results in abnormal head shape, short snout, smaller size, hemorrhage, body wall abnormalities, altered cleft palate, and short mandible (Fig. 4).
Figure 4.
ENU(N-ethyl-N-nitrosourea) mutation in Lrp1 results in craniofacial abnormalities in mice. (A,B) A perinatal recessive ENU screen for novel genes involved craniofacial defects identified a mutant line with a c.Thr1019Cys transition in Lrp1 resulting in an p.F340S amino acid change, which disrupts the conserved YWTD motif in the extracellular domain. (C,D) Gross morphology of the ENU mutant, showing an abnormal head shape and short snout (arrow), smaller size, hemorrhage, and body wall abnormalities. (E,F) Coronal sections of E18.5 mutant and control heads showing cleft secondary palate in mutants. Arrowheads indicate unelevated palate shelves. (G,H) Alizarin red/alcian blue staining showing overall abnormal skull shape and markedly short mandible (arrow).
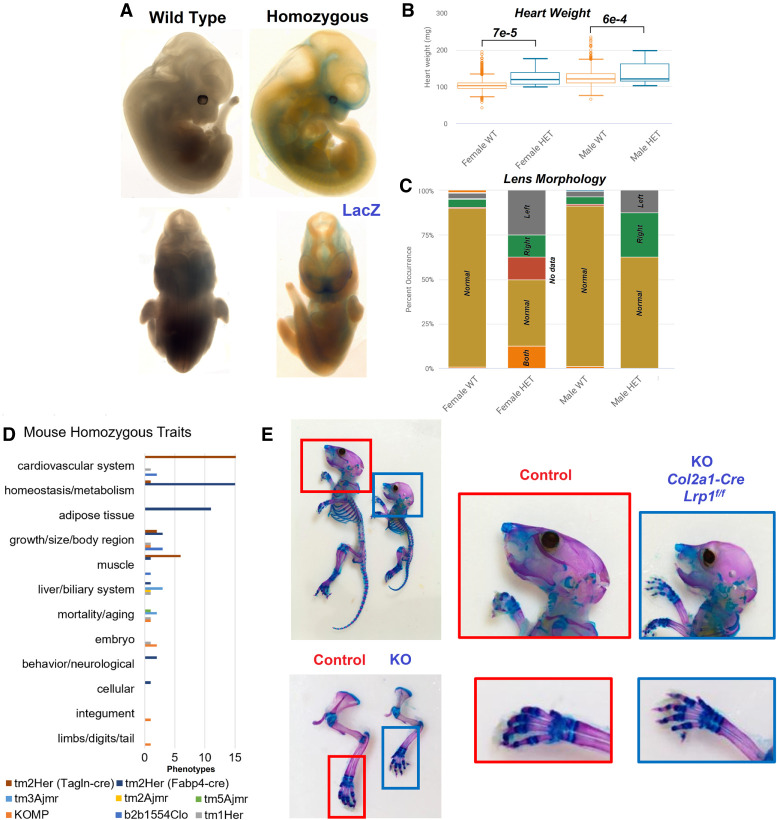
A homozygous knockout of Lrp1 in mice (replacing the gene with LacZ to track expression, Lrp1tm1.1(KOMP)Wtsi) was generated as part of the International Mouse Phenotyping Consortium (IMPC) (Meehan et al. 2017). These animals have preweaning lethality, altered heart development, pallor (pigment dysfunction), and abnormal limb morphology (Supplemental Table S2). Staining of embryo shows pronounced expression in bone, head, and abdominal areas (Fig. 5A), which are consistent with parts of our patients’ phenotypes.
Figure 5.
Mouse phenotypes for Lrp1 alterations. (A) Embryo imaging of wild-type or Lrp1 homozygous knockout, with blue representing the LacZ replacement of Lrp1. Data is from the International Mouse Phenotyping Consortium (IMPC; www.mousephenotype.org/data/genes/MGI:96828). (B,C) Heterozygous Lrp1 knockout causing significant differences in male and female heart weight (B) and lens morphology (C). (D) All phenotypes observed within the Mouse Genome Informatics (MGI) database (Supplemental Table S1) fit within the summary terms for the modified mouse strains. (E) Skeleton images of Col2a1 conditional Lrp1-knockout with zoomed images of wild type (red) and homozygous (blue) animals.
Three additional homozygous knockout strains (Lrp1tm1Her, Lrp1tm3Ajmr, Lrp1tm5Ajmr) also show embryonic lethality with growth retardation and altered liver morphology (Supplemental Table S1). Heterozygous knockout of Lrp1 can survive, but show cardiovascular alterations (Supplemental Table S3), heart weight changes (Fig. 5B), and eye dysmorphology (Fig. 5C). The eye changes in IMPC were noted for lens morphology changes in both left and right eyes of at least one male and female, but without complete penetrance and just short of significance (https://www.mousephenotype.org/data/charts?accession=MGI:96828&allele_accession_id=MGI:5494466&zygosity=heterozygote¶meter_stable_id=IMPC_EYE_016_001&pipeline_stable_id=JAX_001&procedure_stable_id=IMPC_EYE_001¶meter_stable_id=IMPC_EYE_016_001). Cardiovascular system dysfunction is the most annotated complication of the global and tissue-specific knockout mice generated (Fig. 5D). The Tagln-cre-driven smooth muscle conditional knockout of Lrp1 shows pronounced alterations of cardiovascular valves and results in dilated cardiomyopathy (Basford et al. 2013). A bone or cartilage conditional knockout for Lrp1 has been lacking within mouse databases or literature. The Yang lab at Van Andel Institute generated a Col2a1-driven Lrp1 knockout, which results in altered facial morphology and shortened bones, including fingers (Fig. 5E). The mouse phenotypes for Lrp1 genetic alterations thus provide a remarkable overlap to phenotypes observed within our patients.
LRP1 Human Expression Insights
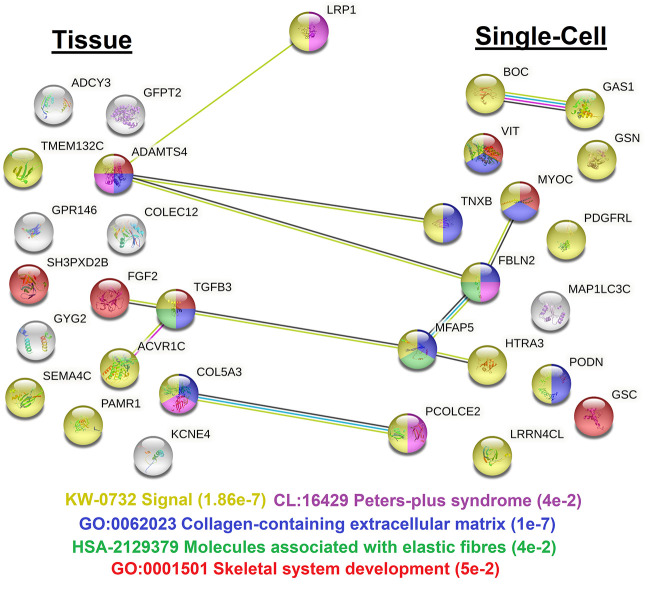
LRP1 is expressed in most human adult tissues, with high variability, elevation in fibroblasts, and no significant differences between males and females (Supplemental Fig. S2A). Of the 12 known isoforms of the gene, two are found expressed that result in proteins, with the LRP1-201 isoform (codes for a 4544-amino-acid, full-length protein) found most expressed (Supplemental Fig. S2B). The Human Protein Atlas maintains a list of genes that follow similar correlations to LRP1 gene expression at the tissue and single-cell levels (Fig. 6; Supplemental Table S4). Analysis of this gene list through OMIM reveals nine genes with known phenotypes, including SH3PXD2B with Frank–ter Haar syndrome (dermatocardioskeletal), MFAP5 with aortic aneurysm, GSC with short stature, TGFB3 with ventricular dysplasia, MYOC with glaucoma, GSN with amyloidosis, and TNXB with Ehlers–Danlos syndrome, classic-like. These phenotypes share some overlap with our patients’ features.
Figure 6.
STRING analysis of the top correlating genes based on expression with LRP1. Correlated genes were identified from either human tissue analysis or single-cell analysis. Lines represent known connections from text mining or experimental interactions. The colors of the nodes are based on the phenotypes listed at the bottom, with false discovery rate (FDR) values listed.
The similarly expressed genes are enriched for signaling cascades, specifically collagen-containing extracellular matrix and elastic fibers. In addition, we see genes enriched for skeletal system development and Peters plus syndrome. Peters plus syndrome is an autosomal recessive disorder caused by biallelic variants in B3GLCT with pronounced cloudy corneas, clefting, short limb dwarfism, and congenital heart malformation commonly linked to alternating neural crest development (Maillette de Buy Wenniger-Prick and Hennekam 2002), strongly overlapping the individuals described here.
Many of the phenotypes observed within the patients and public data sets, including Peters plus syndrome overlap, involve neural crest cell (NCC)-derived tissues such as vascular smooth muscle and cartilage of heart valves, face, and digits. We utilized transcriptomics of public data sets of NCCs to further our insights. A total of 197 neural crest cell RNA-seq data sets were downloaded from the National Center for Biotechnology Information (NCBI) sequence read archive (SRA) and processed, representing diverse insights from 19 separate studies (Supplemental Fig. S3A). The majority of LRP1 expression seen from these data sets was from transcript 201 (Supplemental Fig. S3B), representing the full isoform of the LRP1 protein. Few other LRP homologous proteins were expressed in the neural crest cells, including no LRP1B (Supplemental Fig. S3C). Stratifying expression data sets based on those with >1 standard deviation and those <1 for LRP1, 90 genes are higher in LRP1 enriched data sets and 127 genes lower (Supplemental Fig. S3D). Of these significant genes, there is enrichment for terms (Supplemental Fig. S3D) such as dilated cardiomyopathy (PCOLCE, COL1A1, TWIST1, SOX9, LOXL1, COL1A2, COL6A2, COL3A1, FBN1, BGN, COL5A1, COL4A1, POSTN, LOXL2), craniofacial development (PRRX1, TBX2, TWIST1, SOX9, SIX1, SIX2, ALX1, FOXC2, ALX4, DDR2, ALX3, FOXD3, PRRX2, HES3, MAB21L1, FOXC1, SOX10, SP8), and blood vessel development (FAP, COL1A1, PRRX1, TBX2, LRP1, SIX1, PDGFRA, PDGFRB, LOXL1, ACKR3, SFRP2, COL1A2, COL3A1, FOXC2, FN1, COL4A2, COL5A1, COL4A1, FOXC1, LOXL2, GPC3, ACTA2, GPR124, TGFBI, GREM1).
Human LRP1-Related Phenotypes
The artery and vascular smooth muscle connection of LRP1 and phenotypes are further supported based on common variants and migraine associations. The variant rs11172113 is the top signal for a significant expression quantitative trait loci (eQTL) of LRP1 in both aorta and tibial arteries (Supplemental Fig. S4A,B). The peak of the linkage disequilibrium block lies overtop the LRP1 gene (Supplemental Fig. S4C). Variant rs11172113 is found with a minor allele frequency (MAF) > 0.2 in all gnomAD populations except South Asian, with the highest enrichment in Latino/Admixed Americans (MAF = 0.5). The variant is the lead single-nucleotide polymorphism (SNP) in many migraine, headache, or headache treatment genome/phenome-wide association studies (GWASs/PheWASs; Supplemental Table S5). In most of those studies, this allele has one of the top five significant P-values of all sites within the genome. Similar to our patients, this variant is also associated with lung function and artery phenotypes (Supplemental Table S5). There are 33 SNPs with an R2 > 0.5, but only two are >0.8 R2 (Supplemental Fig. S4D). The lead variant, rs11172113, is located within a highly conserved intronic segment with enhancer annotation and multiple ENCODE-based transcription factor binding events (Supplemental Fig. S5).
Although the effect size in arteries for this allele is small relative to the effect of the missense patient variants, the eQTL and GWASs/PheWASs associations point to similar traits associated with disruption of human LRP1 as observed in the siblings. These also suggest that some individuals will carry modifying alleles that may result in phenotypic variability because of changes in LRP1 expression levels that can compensate or exacerbate missense variant associated phenotypes, which requires future analyses.
DISCUSSION
Complete knockout of mouse Lrp1 results in peri-implantation lethality through dysregulation of the uPA-PAI-1 complex internalization, altering migrating cells (Herz et al. 1992). Mice with a homozygous missense mutation in Lrp1 at p.Cys4232Arg survive to mid-gestation but die because of cardiac defects (Li et al. 2015). These mice exhibit atrioventricular septal defects with alteration in outflow tract septation and persistent truncus arteriosus (Lin et al. 2020). The mice die around E14.5–15.5 with 100% penetrant congenital heart defects (Lin et al. 2020). The IMPC's phenotyping of LRP1 knockout mice reveals homozygous preweaning lethality, altered heart development, pallor (pigment dysfunction), and abnormal limb morphology, with heterozygous knockout mice also having altered cardiovascular and eye phenotypes (Meehan et al. 2017). Additional ENU mutational phenotyping and collagen-specific knockout reveals altered bone and facial development, as shown here for the first time. These phenotypes align with the LRP1-related syndrome of the two individuals described above. Finally, recent work in LRP1 corneal protein interaction networks has shown evidence of the involvement in corneal transparency and structure (Mogensen et al. 2022), supporting our observations.
The missense variant LRP1 protein in cardiac NCCs is retained in the endoplasmic reticulum resulting in decreased cell motility/migration and altered Wnt signaling (Lin et al. 2020). Human LRP1 is critical for multiple NCC derivatives, including smooth muscle cells (Lehti et al. 2009; Zucker et al. 2019), adipocytes (Masson et al. 2009; Konaniah et al. 2017), chondrocytes (Kawata et al. 2012), osteocytes (Dennis et al. 2020), melanocytes (Cheng et al. 2008), and Schwann cells (Mantuano et al. 2010). Multiple NCC-derived cells have been connected to LRP1 role in amyloid-β uptake and Alzheimer's disease (Kanekiyo et al. 2012; Cheung et al. 2014). LRP1B represents the most similar paralog of LRP1. Although LRP1B is connected to cancer risks (Langbein et al. 2002), it is not redundant with LRP1, as shown in the Figure 1 expression, and is not developmentally lethal in knockout mice (Marschang et al. 2004).
Homozygous variants within LRP1 have been identified as potentially pathogenic for a family with multiple individuals for keratosis pilaris atrophicans based on exome sequencing (Klar et al. 2015). The p.Lys1245Arg variant identified in patients with keratosis pilaris has functional conservation. It does not show the more severe cardiac/bone phenotypes connected to knockout or damaging missense variants of Lrp1 mouse models, suggesting the change does not reflect a full loss of function within the LRP1 protein like the mouse p.Cys4232Arg or the patients described in this study. Findings within our two siblings presented here build support for cardiac/NCC-derived mouse model findings translating to a human syndrome.
There is ongoing work to identify more patients with LRP1-associated variants to define the phenotypic variability, with multiple patients worldwide presenting similar to our patients. Yet, it is already becoming clear from these initial communications and the keratosis pilaris atrophicans patients that there will be broad phenotypic variability for LRP1 variants. We speculate that expression-associated variants such as rs11172113 may likely impact phenotypes when compounded with missense changes. It may be possible that autosomal dominant phenotypes could be observed as homozygous for rs11172113 and having a single heterozygous rare variant. Compound heterozygous changes, such as in our patient, will also likely have more severe phenotypes compounded with heterozygous or homozygous rs11172113 alleles. Further complicating insights for LRP1 are the many chemicals such as BPA, copper, tobacco, and estradiol that can modulate the expression of LRP1 (Supplemental Table S6).
This work shows that the human LRP1 gene variants are associated with pulmonary, cardiac, vascular, and dysmorphic features in an autosomal recessive form similar to the studied mouse models. Subtle LRP1 eQTLs further support these phenotypes in adult vascular tissues that colocalize with GWASs/PheWASs of vascular dysfunction. Like in the mouse, the human NCCs with elevated LRP1 have genes highly associated with traits present in the presented siblings. As with nearly all the LRP-related genes having human syndromes, we provide the first proof of LRP1-associated human autosomal recessive disorder.
METHODS
Sequencing of samples was performed at the CLIA/CAP-certified Rady Children's Institute of Genomic Medicine as previously described (James et al. 2021), with sample collection reviewed and approved by the Spectrum Health or Bronson Institutional Review Board (IRB). The top variants were confirmed with Sanger sequencing. Protein modeling and evolutionary analysis were performed using our published sequence-to-structure-to-function workflow (Prokop et al. 2017). Human variants were extracted on 7/5/2021 for gnomAD (v.2.1.1 nonTOPMed, 135,743 individuals), Bravo (TOPMed; 132,345 individuals), Geno2MP (19,151 individuals), and ClinVar. Mouse phenotypes were extracted from the MGI database (Bult et al. 2008).
Col2a1-Cre and Lrp1 floxed mice were obtained from the Jackson Laboratory and maintained on the C57BL/6 background. The animal maintenance and procedures were approved by the Institutional Animal Care and Use Committee of the Van Andel Research Institute. P14 male mice were used. Skin and internal organs of euthanized mice were removed, and the remaining tissue was fixed in 95% ethanol overnight, followed by incubation in acetone for 24 h. The skeletons were stained with alcian blue (0.015% in 20% acetic acid and 80% ethanol) for 48 h, incubated with 2% KOH for another 48 h, and subsequently stained with alizarin red S (0.005% in 1% KOH) overnight. The stained skeletons were cleared in 1% KOH/20% glycerol solution for 2–3 d and stored in 95% ethanol/glycerol solution (1:1).
Expression analysis of human LRP1 was pulled from GTEx (GTEx Consortium 2020) or Human Protein Atlas (Uhlen et al. 2010). The SRA was searched for “Neural Crest” or “Smooth Muscle,” and all deposited human RNA data sets with paired-end, fastq files available, and >10 million matched reads were downloaded with the SRA toolkit. The reads were quasi-aligned to the Gencode38 (Frankish et al. 2019) transcriptome map or RefSeq genes (Prokop et al. 2020) using salmon_0.14.1 (Patro et al. 2017). Mapped transcripts per million (TPM) were processed through NetworkAnalyst3.0 (Zhou et al. 2019) using Limma (Ritchie et al. 2015) statistical analysis of LRP1 grouped expression. The eQTL data was extracted from GTEx (GTEx Consortium 2020) and assessed using Open Targets Genetics (Ghoussaini et al. 2021), RegulomeDB (Boyle et al. 2012), or the UCSC genome browser (Navarro Gonzalez et al. 2021).
ADDITIONAL INFORMATION
Data Deposition and Access
Raw data for the evolution and all genomic variant analysis can be found at https://doi.org/10.6084/m9.figshare.14920380.v1. Raw data for neural crest RNA-seq can be found at https://doi.org/10.6084/m9.figshare.14920644.v1 and the smooth muscle RNA-seq at https://doi.org/10.6084/m9.figshare.14991444.v1. All images are available in high resolution at https://doi.org/10.6084/m9.figshare.20510904.v1. Interpreted variants have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV002569951 and SCV002569952.
Ethics Statement
Patients were consented according to approved protocols reviewed by Spectrum Health and Bronson IRBs. Sequencing was performed according to approved IRB at Rady Children's Institute of Genomics Medicine. All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) at either the Jackson Laboratory or Van Andel Institute.
Author Contributions
P.R.M., C.P.B., and J.W.P. designed and oversaw experiments. P.R.M., A.E., D.L., J.Z., L.Z.R., S.R., and J.W.P contributed to interpretation of data. P.R.M. and A.L. treated patients and formed clinical descriptions. L.G., K.W., and M.S.W. analyzed rapid genome sequencing. S.A.M., T.Y., and D.L. generated or analyzed mouse data. P.R.M., A.E., and J.W.P. generated figures and wrote the manuscript. All authors approved of the final manuscript.
Funding
This work was supported by the Helen DeVos Children's Hospital Foundation, Gerber Foundation, National Institutes of Health (K01ES025435 to J.W.P.), and Michigan State University.
Competing Interest Statement
The authors have declared no competing interest.
Referees
Lance P. Doucette
Anonymous
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Basford JE, Koch S, Anjak A, Singh VP, Krause EG, Robbins N, Weintraub NL, Hui DY, Rubinstein J. 2013. Smooth muscle LDL receptor-related protein-1 deletion induces aortic insufficiency and promotes vascular cardiomyopathy in mice. PLoS ONE 8: e82026. 10.1371/journal.pone.0082026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschel J, Geyer H, Rich M, Leimanis M, Kampfschulte A, VanSickle E, Rajasekaran S, Bupp C. 2021. Leveraging rapid genome sequencing to alter care plans for pediatric patients in a community hospital setting in the United States. J Pediatr 239: 235–239. 10.1016/j.jpeds.2021.08.010 [DOI] [PubMed] [Google Scholar]
- Bick D, Fraser PC, Gutzeit MF, Harris JM, Hambuch TM, Helbling DC, Jacob HJ, Kersten JN, Leuthner SR, May T, et al. 2017. Successful application of whole genome sequencing in a medical genetics clinic. J Pediatr Genet 6: 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, et al. 2012. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22: 1790–1797. 10.1101/gr.137323.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult CJ, Eppig JT, Kadin JA, Richardson JE, Blake JA, Mouse Genome Database Group. 2008. The Mouse Genome Database (MGD): mouse biology and model systems. Nucl Acids Res 36: D724–D728. 10.1093/nar/gkm961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. 2006. Wnt signaling: complexity at the surface. J Cell Sci 119: 395–402. 10.1242/jcs.02826 [DOI] [PubMed] [Google Scholar]
- Cheng C-F, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, et al. 2008. Transforming growth factor α (TGFα)-stimulated secretion of HSP90α: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFβ-rich environment during wound healing. Mol Cell Biol 28: 3344–3358. 10.1128/MCB.01287-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Goh YT, Zhang J, Wu C, Guccione E. 2014. Modeling cerebrovascular pathophysiology in amyloid-β metabolism using neural-crest-derived smooth muscle cells. Cell Rep 9: 391–401. 10.1016/j.celrep.2014.08.065 [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. 2004. Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115. 10.1242/dev.01318 [DOI] [PubMed] [Google Scholar]
- Dennis EP, Edwards SM, Jackson RM, Hartley CL, Tsompani D, Capulli M, Teti A, Boot-Handford RP, Young DA, Piróg KA, et al. 2020. CRELD2 is a novel LRP1 chaperone that regulates noncanonical WNT signaling in skeletal development. J Bone Miner Res 35: 1452–1469. 10.1002/jbmr.4010 [DOI] [PubMed] [Google Scholar]
- Donnai D, Barrow M. 1993. Diaphragmatic hernia, exomphalos, absent corpus callosum, hypertelorism, myopia, and sensorineural deafness: a newly recognized autosomal recessive disorder? Am J Med Genet 47: 679–682. 10.1002/ajmg.1320470518 [DOI] [PubMed] [Google Scholar]
- Farnaes L, Hildreth A, Sweeney NM, Clark MM, Chowdhury S, Nahas S, Cakici JA, Benson W, Kaplan RH, Kronick R, et al. 2018. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genomic Med 3: 10. 10.1038/s41525-018-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankish A, Diekhans M, Ferreira A-M, Johnson R, Jungreis I, Loveland J, Mudge JM, Sisu C, Wright J, Armstrong J, et al. 2019. GENCODE reference annotation for the human and mouse genomes. Nucl Acids Res 47: D766–D773. 10.1093/nar/gky955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoussaini M, Mountjoy E, Carmona M, Peat G, Schmidt EM, Hercules A, Fumis L, Miranda A, Carvalho-Silva D, Buniello A, et al. 2021. Open Targets Genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucl Acids Res 49: D1311–D1320. 10.1093/nar/gkaa840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513–523. 10.1016/S0092-8674(01)00571-2 [DOI] [PubMed] [Google Scholar]
- GTEx Consortium. 2020. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369: 1318–1330. 10.1126/science.aaz1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Clouthier DE, Hammer RE. 1992. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71: 411–421. 10.1016/0092-8674(92)90511-A [DOI] [PubMed] [Google Scholar]
- James KN, Lau M, Shayan K, Lenberg J, Mardach R, Ignacio R, Halbach J, Choi L, Kumar S, Ellsworth KA. 2021. Expanding the genotypic spectrum of ACTG2-related visceral myopathy. Cold Spring Harb Mol Case Stud 7: a006085. 10.1101/mcs.a006085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, Zeng X, Qi H, Chang W, Sierant MC, et al. 2017. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet 49: 1593–1601. 10.1038/ng.3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Liu C-C, Shinohara M, Li J, Bu G. 2012. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer's amyloid-β. J Neurosci 32: 16458–16465. 10.1523/JNEUROSCI.3987-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata K, Kubota S, Eguchi T, Aoyama E, Moritani NH, Kondo S, Nishida T, Takigawa M. 2012. Role of LRP1 in transport of CCN2 protein in chondrocytes. J Cell Sci 125: 2965–2972. [DOI] [PubMed] [Google Scholar]
- Klar J, Schuster J, Khan TN, Jameel M, Mäbert K, Forsberg L, Baig SA, Baig SM, Dahl N. 2015. Whole exome sequencing identifies LRP1 as a pathogenic gene in autosomal recessive keratosis pilaris atrophicans. J Med Genet 52: 599–606. 10.1136/jmedgenet-2014-102931 [DOI] [PubMed] [Google Scholar]
- Konaniah ES, Kuhel DG, Basford JE, Weintraub NL, Hui DY. 2017. Deficiency of LRP1 in mature adipocytes promotes diet-induced inflammation and atherosclerosis-brief report. Arterioscler Thromb Vasc Biol 37: 1046–1049. 10.1161/ATVBAHA.117.309414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein S, Szakacs O, Wilhelm M, Sukosd F, Weber S, Jauch A, Lopez Beltran A, Alken P, Kälble T, Kovacs G. 2002. Alteration of the LRP1B gene region is associated with high grade of urothelial cancer. Lab Investig J Tech Methods Pathol 82: 639–643. 10.1038/labinvest.3780458 [DOI] [PubMed] [Google Scholar]
- Lauer MS, Skarlatos S. 2010. Translational research for cardiovascular diseases at the National Heart, Lung, and Blood Institute: moving from bench to bedside and from bedside to community. Circulation 121: 929–933. 10.1161/CIRCULATIONAHA.109.917948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J. 2009. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci 122: 126–135. 10.1242/jcs.035279 [DOI] [PubMed] [Google Scholar]
- Li Y, Pawlik B, Elcioglu N, Aglan M, Kayserili H, Yigit G, Percin F, Goodman F, Nürnberg G, Cenani A, et al. 2010. LRP4 mutations alter Wnt/β-catenin signaling and cause limb and kidney malformations in Cenani–Lenz syndrome. Am J Hum Genet 86: 696–706. 10.1016/j.ajhg.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ, Lemke K, Chen Y, Chatterjee B, Devine W, Damerla RR, et al. 2015. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 521: 520–524. 10.1038/nature14269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J-HI, Feinstein TN, Jha A, McCleary JT, Xu J, Arrigo AB, Rong G, Maclay LM, Ridge T, Xu X, et al. 2020. Mutation of LRP1 in cardiac neural crest cells causes congenital heart defects by perturbing outflow lengthening. Commun Biol 3: 312. 10.1038/s42003-020-1035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillette de Buy Wenniger-Prick LJ, Hennekam RC. 2002. The Peters’ plus syndrome: a review. Ann Genet 45: 97–103. 10.1016/S0003-3995(02)01120-6 [DOI] [PubMed] [Google Scholar]
- Mantuano E, Jo M, Gonias SL, Campana WM. 2010. Low density lipoprotein receptor-related protein (LRP1) regulates Rac1 and RhoA reciprocally to control Schwann cell adhesion and migration. J Biol Chem 285: 14259–14266. 10.1074/jbc.M109.085126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschang P, Brich J, Weeber EJ, Sweatt JD, Shelton JM, Richardson JA, Hammer RE, Herz J. 2004. Normal development and fertility of knockout mice lacking the tumor suppressor gene LRP1b suggest functional compensation by LRP1. Mol Cell Biol 24: 3782–3793. 10.1128/MCB.24.9.3782-3793.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson O, Chavey C, Dray C, Meulle A, Daviaud D, Quilliot D, Muller C, Valet P, Liaudet-Coopman E. 2009. LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue. PLoS ONE 4: e7422. 10.1371/journal.pone.0007422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan TF, Conte N, West DB, Jacobsen JO, Mason J, Warren J, Chen C-K, Tudose I, Relac M, Matthews P, et al. 2017. Disease model discovery from 3,328 gene knockouts by the International Mouse Phenotyping Consortium. Nat Genet 49: 1231–1238. 10.1038/ng.3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen EH, Poulsen ET, Thøgersen IB, Yamamoto K, Brüel A, Enghild JJ. 2022. The low-density lipoprotein receptor-related protein 1 (LRP1) interactome in the human cornea. Exp Eye Res 219: 109081. 10.1016/j.exer.2022.109081 [DOI] [PubMed] [Google Scholar]
- Navarro Gonzalez J, Zweig AS, Speir ML, Schmelter D, Rosenbloom KR, Raney BJ, Powell CC, Nassar LR, Maulding ND, Lee CM, et al. 2021. The UCSC Genome Browser database: 2021 update. Nucl Acids Res 49: D1046–D1057. 10.1093/nar/gkaa1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Niemi A-K, Dimmock DP, Speziale M, Nespeca M, Chau KK, Van Der Kraan L, Wright MS, Hansen C, Veeraraghavan N, et al. 2021. Rapid sequencing-based diagnosis of thiamine metabolism dysfunction syndrome. N Engl J Med 384: 2159–2161. 10.1056/NEJMc2100365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. 2017. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14: 417–419. 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop JW, Lazar J, Crapitto G, Smith DC, Worthey EA, Jacob HJ. 2017. Molecular modeling in the age of clinical genomics, the enterprise of the next generation. J Mol Model 23: 75. 10.1007/s00894-017-3258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop JW, May T, Strong K, Bilinovich SM, Bupp C, Rajasekaran S, Worthey EA, Lazar J. 2018. Genome sequencing in the clinic: the past, present, and future of genomic medicine. Physiol Genomics 50: 563–579. 10.1152/physiolgenomics.00046.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop JW, Shankar R, Gupta R, Leimanis ML, Nedveck D, Uhl K, Chen B, Hartog NL, Van Veen J, Sisco JS, et al. 2020. Virus-induced genetics revealed by multidimensional precision medicine transcriptional workflow applicable to COVID-19. Physiol Genomics 52: 255–268. 10.1152/physiolgenomics.00045.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucl Acids Res 43: e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, Zurhove K, Haffner P, Philippe C, Woldt E, et al. 2009. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem 284: 381–388. 10.1074/jbc.M806538200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, et al. 2010. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 28: 1248–1250. 10.1038/nbt1210-1248 [DOI] [PubMed] [Google Scholar]
- Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. 2019. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucl Acids Res 47: W234–W241. 10.1093/nar/gkz240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker MM, Wujak L, Gungl A, Didiasova M, Kosanovic D, Petrovic A, Klepetko W, Schermuly RT, Kwapiszewska G, Schaefer L, et al. 2019. LRP1 promotes synthetic phenotype of pulmonary artery smooth muscle cells in pulmonary hypertension. Biochim Biophys Acta Mol Basis Dis 1865: 1604–1616. 10.1016/j.bbadis.2019.03.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data for the evolution and all genomic variant analysis can be found at https://doi.org/10.6084/m9.figshare.14920380.v1. Raw data for neural crest RNA-seq can be found at https://doi.org/10.6084/m9.figshare.14920644.v1 and the smooth muscle RNA-seq at https://doi.org/10.6084/m9.figshare.14991444.v1. All images are available in high resolution at https://doi.org/10.6084/m9.figshare.20510904.v1. Interpreted variants have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV002569951 and SCV002569952.