Abstract
Rationale:
The ongoing rise in opioid use disorder (OUD) has made it imperative to better model the individual variation within the human population that contributes to OUD vulnerability. Using animal models that capture such variation can be a useful tool. Individual variation in novelty-induced locomotion is predictive of substance use disorder (SUD) propensity. In this model, rats are characterized as high-responders (HR) or low-responders (LR) using a median split based on distance travelled during a locomotor test, and HR rats are generally found to exhibit a more SUD vulnerable behavioral phenotype.
Objectives:
The HR/LR model has commonly been used to assess behaviors in male rats using psychostimulants, with limited knowledge of the predictive efficacy of this model in females or the use of an opioid as the reward. In the current study, we assessed several behaviors across the different phases of drug addiction (heroin taking, refraining and seeking) in over 500 male and female heterogeneous stock rats run at two geographically separate locations. Rats were characterized as HRs or LRs within each sex for analysis.
Results:
Overall, females exhibit a more OUD vulnerable phenotype relative to males. Additionally, the HR/LR model was predictive of OUD-like behaviors in male, but not female rats. Furthermore, phenotypes did not differ in anxiety-related behaviors, reacquisition of heroin-taking or punished heroin-taking behavior in either sex.
Conclusions:
These results emphasize the importance of assessing females in models of individual variation in SUD and highlight limitations in using the HR/LR model to assess OUD propensity.
Keywords: individual variation, novelty-induced locomotion, high-responder, low-responder, heroin, addiction, vulnerable, relapse, sex differences
Introduction
The prevalence of opioid use disorder (OUD) has increased in the past two decades, with an over six-fold increase in opioid overdose deaths (WONDER 2020). The rise in OUD makes it imperative to gain a better understanding of the behavioral characteristics underlying opioid use vulnerability. A key barrier in assessing addiction liability is the substantial amount of individual variation within the human population that contributes to addiction vulnerability. Using animal models that inherently account for such variation in addiction-related behaviors is one approach that may improve capturing variability in human drug addiction, leading to more efficacious treatment options.
Separating outbred rats into high-responder (HR) and low-responder (LR) subgroups based on cumulative locomotor movements in a novel inescapable environment has been widely used to account for individual variation in addiction-related behaviors (Piazza et al. 1989). In this model, HRs more rapidly learn to self-administer nicotine (Suto et al. 2001), amphetamine (Piazza et al. 1989, Piazza et al. 1990, Piazza et al. 1991, Piazza et al. 1998, Klebaur et al. 2001, Cain et al. 2008), methamphetamine (Gancarz et al. 2011), and cocaine (Piazza et al. 2000, Mantsch et al. 2001, Ferris et al. 2013) relative to LRs. Additionally, HRs exhibit greater locomotor sensitization to repeated amphetamine (Hooks et al. 1992) and nicotine injections (Kayir et al. 2011), and greater motivation to take cocaine compared to LRs using a behavioral economics approach (O’Connor et al. 2021). Augmenting the potential translational value of the HR/LR model, novelty-induced locomotor behavior has been associated with increased vulnerability to addiction across several classes of drugs in humans (Wingo et al. 2016).
There are several applications of the HR/LR model that have yet to be fully explored. For example, previous studies have assessed differences between HR and LR rats using psychostimulants with only a few studies focused on opioids (Ambrosio et al. 1995, Lamarque et al. 2001, Xigeng et al. 2004, Swain et al. 2018, Chang et al. 2022). Also, with the exception of two studies, all work using this model have examined male rats only (Sell et al. 2005, Davis et al. 2008). Moreover, only a few studies have assessed whether HR/LR distinctions are reflected in other behaviors such as cue- or context-induced drug seeking, or compulsive drug taking in the presence of adverse consequences (Deroche-Gamonet et al. 2004, Belin et al. 2011, Flagel et al. 2016, Chang et al. 2022).
To further substantiate the use of the HR/LR model in capturing individual variation in addiction-related behaviors, we assessed the predictive validity of the model for OUD propensity using an outbred rat line: heterogeneous stock (HS) rats. HS rats were created from eight inbred strains and maintained in a way to minimize inbreeding (Hansen and Spuhler 1984, Solberg Woods and Palmer 2019). To best capture the genetic and phenotypic variability observed in humans, 507 male and female HS rat littermates shipped to two distinct laboratories were assessed for multiple behaviors that contribute to OUD liability; including heroin use, rewarded and unrewarded motivation to seek heroin, and learning to refrain from heroin seeking. Analgesic threshold and anxiety-like behavior were also assessed prior to heroin experience. Data were first assessed for behavioral differences between sexes, to which we found female rats exhibited a more vulnerable OUD behavioral phenotype compared to males. Next, data were analyzed within the scope of the HR/LR mode. In parallel with studies using psychostimulants, we hypothesized HR male and female rats would exhibit a more vulnerable OUD phenotype relative to LR rats. However, we found the HR/LR model successfully predicted OUD vulnerability in male rats, but had no predictability in females, emphasizing the necessity to account for sex differences in models of individual variation in addiction-related behaviors. HR and LR rats did not differ in reacquisition of heroin taking or punished heroin taking-behavior, cardinal features of OUD, regardless of sex, suggesting limits regarding the applicability of the HR/LR model in predicting OUD propensity.
Methods
All experimental procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee, and by the Italian Ministry of Health. Procedures abided by the National Institute of Health Guide for the Care and Use of Laboratory Animals and the Assessment and Accreditation of Laboratory Animals Care, as well as the European Community Council Directive for Care and Use of Laboratory Animals. The experimental timeline is shown in Fig. 1 with greater detail on each procedure in the following sections.
Fig. 1.
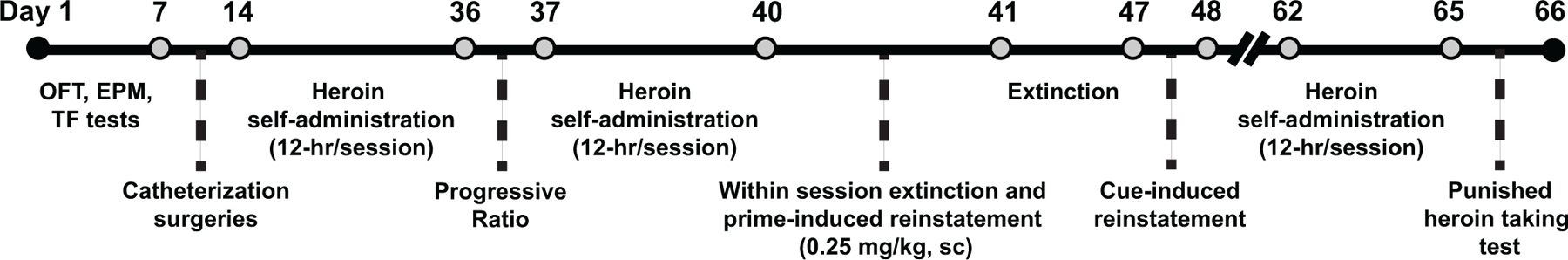
Experimental timeline and behavioral phenotype characterization. Rats underwent an open field test (OFT) in a novel inescapable environment, elevated-plus maze (EPM) and tail-flick test (TF) prior to catheterization surgeries. Animals were classified as either a high- or low-responder (HR/LR) based on novelty-induced locomotor behavior during the OFT. Next, heroin self-administration training (12 hr; 12 total sessions with 4/week) commenced followed by a progressive ratio test and additional self-administration training. A within session extinction and prime-induced reinstatement test (6 hr) occurred with a heroin prime (0.25 mg/kg, s.c.) administered 4 hr into the session. Rats then underwent extinction training (6 days) followed by a test for cue-induced reinstatement. A subset or rats underwent additional training approximately 3 weeks following heroin exposure. Rats underwent 3 days of heroin self-administration reacquisition followed by a punished heroin-taking test
Subjects
A total of 680 heterogeneous stock (N/NIH-HS) rats bred at Wake Forest University were used in these studies. Animals were shipped to either the Medical University of South Carolina (MUSC; USA) or the University of Camerino (UCAM; Italy) in batches of 40 (20 males and 20 females per site) at approximately 5 weeks of age. Upon arrival, animals were pair-housed and left undisturbed in a climate-controlled vivarium with a standard 12-hr light:dark cycle for 3 weeks prior to testing. Animals had ad libitum access to food and water over the course of training. All behavioral testing occurred during the dark cycle, between the hours of 18:00 and 6:00 h, with the exception of the locomotor test, elevated-plus maze and tail flick test which started at 8:00 h. To minimize site differences in behavioral output, all experimental procedures were standardized across the two sites. Of the 680 rats entering the study, a total of 100 rats were excluded from analyses due to death (surgery, n=21; illness, n=79), 14 rats were excluded due to technical issues regarding data collection, and an additional 59 were excluded as they underwent saline and not heroin self-administration training. Final analyses consisted of 507 rats (male, n=264 (MUSC, n=145; UCAM, n=119); females, n=243 (MUSC, n=132; UCAM, n=111)).
Drugs
Heroin hydrochloride supplied by the National Institute on Drug Abuse (Bethesda, MD) dissolved in 0.9% sterile saline was used in these studies.
Locomotor test
Following the acclimation period, rats underwent a 60-min locomotor test in a novel inescapable environment (i.e., open field test, OFT). Testing chambers were composed of clear Plexiglas within a metal frame (Omnitech Electronics, Columbus, OH; 16” L x 16” W x 12” H) with photocell beams that captured both lateral and vertical movements. All activity was recorded and analyzed using Versamax (Omnitech Electronics, Columbus, OH; version 1.80–0142). Ten animals, counterbalanced by sex, were run per day Monday-Thursday.
Elevated-plus maze
Approximately 1 hour following the completion of the OFT, rats underwent a 5-min elevated plus maze (EPM) test to assess anxiety-related behaviors. Testing apparatus were composed of black plexiglass (San Diego Instruments) and comprised of four arms (43.5” long and 4” wide) with two having enclosed walls along the arm (12” high walls; “closed” arms) and two without walls (“open” arms). The maze was elevated approximately 19.5” off the ground. The maze flooring was interchangeable based on rat color to optimize detection of each animal for analysis. ANY-maze behavioral tracking software (Stoelting, Wood Dale, IL; version 6.17) was used for automatic detection and quantification of the animal movement throughout the maze. To be considered in an arm, a minimum of 85% of the rat’s body had to be within it.
Tail flick test
A minimum of 1 hour after the EPM test, analgesic threshold for each rat was assessed using a tail flick (TF) test. The TF apparatus (Ugo Basile S.R.L., Gemonio, Italy) consisted of a flat platform with a mounted sensor that is irradiated by an infrared light beam below the platform to heat the rat’s tail. The light beam automatically turned off once the animal moved its tail, or after 10 seconds have passed, and the reaction time was indicated on the display screen. Fifteen minutes prior to the baseline session rats received an injection of saline (1 mg/kg, s.c.). One hour after baseline testing, rats received an injection of heroin (0.75 mg/kg heroin, s.c.) to assess potential changes in analgesic threshold with heroin present, and were tested 15 minutes later. Testing consisted of 4 trials, with the location on the rat’s tail being adjusted each subsequent trial by 1 cm to prevent tissue damage. Data from all 4 trials were averaged to compute the overall latency to remove tail from the sensor (i.e., reaction time). At the conclusion of testing, animals were returned to their home cage in the vivarium.
Estrous cycle identification
Following the tail flick test vaginal lumen samples were collected from a subset of female animals from MUSC (HR, n= 13; LR, n=23) to assess if estrous cycle phase affected distance travelled during the open field test and behavioral characterization (HR vs LR). Approximately 100 μl of sterile saline was gently flushed into the vagina and extracted using a sterile pipette tip. Samples will be stored at 4°C until being pipetted onto a glass slide and stained using a hematology stain to allow for accurate phase identification.
Heroin self-administration
Approximately 1 week after locomotor testing, rats underwent surgery for the implantation of an indwelling jugular catheter. Isoflurane anesthesia was used (5% induction, 2% maintenance), and an analgesic (Ketorolac, 2 mg/kg, s.c.; or Meloxicam, 0.5 mg/rat, s.c.) and an antibiotic (Cefazolin, 0.2 mg/kg, s.c.; or enrofloxacin, 1 mg/kg, i.v.) were administered post-operatively. Animals were given a minimum of three days of recovery prior to testing. All training occurred in standard behavioral testing chambers (Med Associated, St. Albans, VT). Chamber were outfitted with a house light and speaker on one wall, and two levers with lights above them on the opposite wall. During a session, presses on the active lever using a fixed-ratio 1 schedule of reinforcement resulted in presentation of a light and tone cues for 5-seconds and an infusion of heroin (20 µg/kg/100 µl infusion over 3 seconds). The house light turned off at the start of the infusion for 20-seconds to signal a timeout period whereupon additional presses on the active lever were recorded but without consequence. Throughout testing, presses on the inactive lever were recorded but without consequence. Sessions lasted 12 hr or until 300 infusions were earned. Training occurred Monday-Friday, with randomized one session off per week resulting in a total of four sessions/week. After 12 sessions were complete, rats underwent a progressive ratio test to assess motivation to continue taking heroin as the effort for an infusion increased. During this test, the number of active lever presses needed to receive an infusion of heroin exponentially increased after each infusion according to the following formula: (5 x e0.2n)-5 (Richardson and Roberts 1996). Sessions terminated after 12 hr or 1 hr of no earned infusions. Animals then underwent three more days of heroin self-administration training to re-establish heroin-taking behavior prior to additional testing.
Extinction training and reinstatement tests
Following heroin self-administration training, rats underwent a 6 hr within-session extinction-prime test. Rats were under extinction training conditions for the first 4 hr of testing whereupon presses on both the active and inactive lever were recorded but without consequence (i.e., no cue presentations or heroin infusion). Rats received a 0.25 mg/kg (s.c.) injection of heroin (Ma et al. 2014, Chen et al. 2016, de Guglielmo et al. 2017) with two hours left in the session and continued testing under extinction conditions (e.g., heroin-prime reinstatement). At the conclusion of this test, rats underwent daily 2 hr extinction training sessions for 6 consecutive days preceding a test for cue-induced reinstatement. During the 2 hr cue-induced reinstatement test, active lever presses resulted in cue presentation and pump activation, but no heroin infusion.
Punished heroin taking test
Following training and approximately 3 weeks after heroin experience, a subset of MUSC rats (males, n=15; females, n=14) underwent three additional days of heroin self-administration training to re-establish taking behavior. Chambers were then outfitted with a shock floor grid, and on the next day of training there was a 50% probability of foot shock delivery (0.40 mA, 0.5 seconds) with each infusion.
Data analysis and statistics
Once testing was complete, several behavioral measures were selected for analysis in order to best capture the different phases of drug addiction: heroin-taking, refraining and seeking behaviors. Heroin-taking behavior was comprised of the following: total heroin consumption (µg/kg) across the first 12 training sessions; escalation of heroin intake (µg/kg; average consumption days 1–3 subtracted from average days 10–12); break point achieved during the progressive ratio test. The break point was the number of active lever presses an animal was willing to expend in order to receive an infusion of heroin. Refraining, or withholding from seeking, behavior included active lever presses made during the first 2 hr of the within-session extinction-prime test (extinction burst) and the last day of extinction training (extinction day 6). Seeking behaviors included active lever presses made during the heroin-prime and cue-induced reinstatement tests.
Data was assessed for normal distribution using the Kolmogorov-Smirnov test. Raw data were first analyzed for sex differences using a non-parametric Mann-Whitney U test. Next, data were analyzed using a 2-way ANOVA, with site (MUSC vs UCAM) and sex (male vs female) as independent variables. Results showed several site and sex differences (see Table 1 and Online resource 1). Accordingly, all data were standardized using z-score transformation within site and sex and males and females were analyzed as independent groups. A 2-way repeated-measures ANOVA with sex (male vs female) and session (baseline vs test) was used to assess behavioral differences between sessions during the tail flick test. Animals were separated into high-responder and low-responder behavioral phenotypes using a median split based on OFT cumulative locomotor movements. Differences between phenotypes within each behavior, session for tail flick test, or locomotor distance during the OFT by estrous phase was evaluated using either a t-test (normally distributed) or Mann-Whitney U test (non-normally distributed). A Chi-square test was used to assess phenotype composition according to sex and site per cohort of animals, and between males and females when data were combined. Behavior during heroin reacquisition was analyzed using a repeated-measures ANOVA, and punishment training was assessed using a Mann-Whitey U test. Correlational analyses between variables for HR and LR rats by sex were evaluated using Spearman’s correlation coefficient. Analyses were performed using GraphPad Prism version 9.2.0 (San Diego, CA). Statistical significance was set to p<0.05 and when applicable post-hoc analyses were assessed using Bonferroni test to correct for multiple comparisons.
Table 1.
Raw data statistics for selected behavioral measures across training. Differences between site (MUSC vs UCAM) and sex (female vs male) were highly prevalent, resulting in all data being standardized via z-score transformation prior to additional analyses. There was a site by sex interaction for distance travelled during the open field test with post-hoc comparisons showing differences (p<0.05) between all groups with the exception of males run at MUSC and UCAM (p=0.99) and female and male rats run at MUSC (p=0.10). An interaction was also present for latency to remove tail from a noxious stimulus during the tail flick test session, however, all post-hoc comparisons were significant (p<0.05). No other interactions were present for the selected behaviors. Abbreviations: DF, degrees of freedom; F, F-test value; P, P-value
| Raw data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Site | Sex | Site * Sex | ||||||||
| DF | F | P | DF | F | P | DF | F | P | ||
| Distance travelled | 1,503 | 24.57 | <0.0001 | 1,503 | 75.90 | <0.0001 | 1,503 | 29.84 | <0.0001 | |
| EPM open arm | 1,502 | 34.67 | <0.0001 | 1,502 | 45.78 | <0.0001 | 1,502 | 2.04 | 0.15 | |
| Tail flick baseline | 1,503 | 32.63 | <0.0001 | 1,503 | 0.06 | 0.81 | 1,503 | 0.78 | 0.38 | |
| Tail flick test | 1,503 | 253.00 | <0.0001 | 1,503 | 34.37 | <0.0001 | 1,503 | 8.23 | 0.004 | |
| Escalation | 1,503 | 4.10 | 0.04 | 1,503 | 3.03 | 0.08 | 1,503 | 3.89 | 0.051 | |
| Consumption | 1,503 | 11.50 | 0.001 | 1,503 | 70.73 | <0.0001 | 1,503 | 0.03 | 0.86 | |
| Break point | 1,503 | 64.41 | <0.0001 | 1, 503 | 5.79 | 0.02 | 1,503 | 0.06 | 0.81 | |
| Extinction burst | 1,503 | 77.01 | <0.0001 | 1, 503 | 3.50 | 0.07 | 1,503 | 0.13 | 0.72 | |
| Prime reinstatement | 1,503 | 1.70 | 0.19 | 1,503 | 15.84 | <0.0001 | 1,503 | 3.13 | 0.08 | |
| Extinction day 6 | 1,503 | 12.43 | 0.001 | 1,503 | 5.72 | 0.02 | 1,503 | 2.95 | 0.09 | |
| Cued reinstatement | 1,503 | 23.27 | <0.0001 | 1,503 | 16.45 | <0.0001 | 1,503 | 2.22 | 0.14 | |
Results
Raw data
Behavioral differences between females and males for selected behaviors was first analyzed using the raw data. Females exhibited less anxiety-like behavioral relative to males in both the OFT (Mann-Whitney U= 20077, p<0.0001; Fig. 2a) and the EPM (Mann-Whitney U= 20978, p<0.0001; Fig. 2b). Sexes did not differ in analgesic threshold under baseline conditions (Mann-Whitney U= 31625, p=0.78; Fig. 2c). However, following administration of heroin, males showed a greater heroin-induced analgesic threshold relative to females (Mann-Whitney U= 23978, p<0.0001; Fig. 2d), suggesting differences in how an opioid affects pain processing in males and female rats.
Fig. 2.
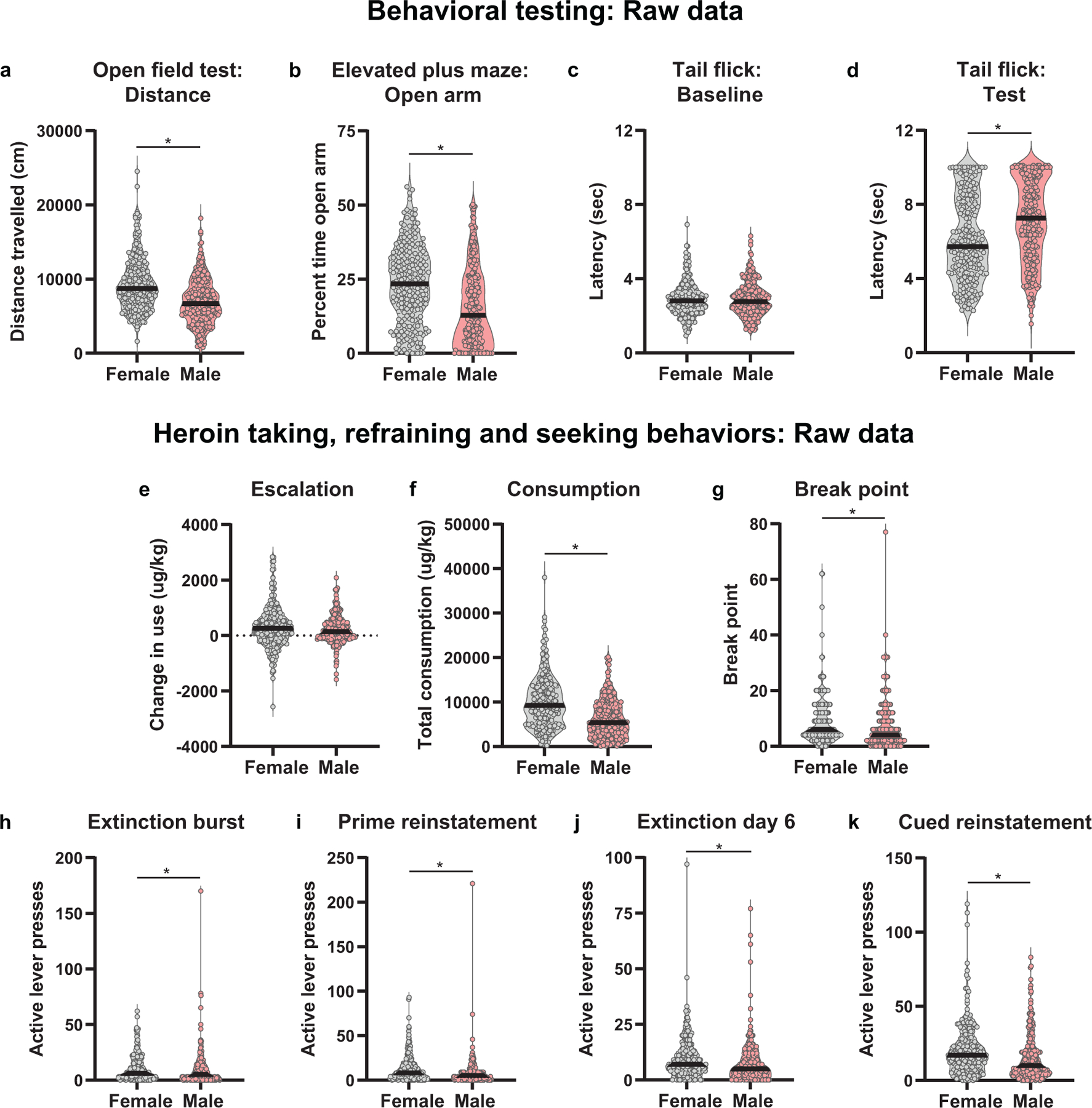
Raw data for behavioral testing, and heroin reinforced and non-reinforced behaviors in female and male rats. The black bar within each violin plot indicates the median value of the data. Prior to starting heroin self-administration training, (a) female rats travelled a greater distance during the open field test and (b) spent more time in the open arm of the elevated plus maze relative to male rats. (c) Sexes did not differ in analgesic threshold under baseline conditions (p=0.78), however, (d) males exhibited a greater heroin-induced analgesic threshold compared to females. Though male and female rats had similar levels of escalation of heroin intake across training, female rats showed potentiated (f) consumption of heroin across training, (g) motivation to work for an infusion of heroin (break point), refraining behavior at both the (h) start (extinction burst) and (j) end of extinction training (extinction day 6). Furthermore, female rats exhibited greater levels of both (i) heroin primed and (k) cue-induced reinstatement of heroin-seeking behavior relative to males. *p<0.05 (Males, n= 264; Females, n=243)
Several sex differences existed for both heroin reinforced and non-reinforced behaviors across measures of heroin taking, refraining and seeking. Compared to males, females showed augmented levels of heroin consumption (Mann-Whitney U= 19069, p<0.0001; Fig. 2f), motivation to work for an infusion of heroin (Mann-Whitney U= 25907, p=0.0002; Fig. 2g), refraining behavior both at the start (extinction burst; Mann-Whitney U= 26498, p=0.0007; Fig. 2h) of extinction training and at the end (extinction day 6; Mann-Whitney U= 26328, p=0.0005; Fig. 2j), as well as heroin-seeking behavior during the heroin-induced (Mann-Whitney U= 22939, p<0.0001; Fig. 2i) and cue-induced (Mann-Whitney U= 23988, p<0.0001; Fig. 2k) reinstatement tests. Females and males did not differ in the escalation of heroin intake across training (Mann-Whitney U= 29858, p=0.18; Fig. 2e), suggesting females start at and maintain a higher level of heroin consumption throughout training, but that escalation patterns between the two sexes are similar. These data suggest that females exhibit a more vulnerable OUD behavioral phenotype.
Locomotor test
Rats were designated as either high-responders (HR) or low-responders (LR) using a median split based on total distance travelled during the OFT creating two non-overlapping subpopulations of equal size. When sexes were combined for analysis, female rats predominated the HR group while males were more represented in the LR phenotype (x2(1, 507)= 13.54, p= 0.0002; Fig. 3a), suggesting female rats were more prone to higher levels of novelty-induced locomotor behavior. This finding, along with the substantial sex differences within the raw data reported above, resulted in sexes being analyzed separately and median splits for HR/LR characterization within sex (males: Mann-Whitney U= 0, p<0.0001, Fig. 3b; females: Mann-Whitney U= 0, p<0.0001, Fig. 3c). Following behavioral phenotype characterization, the composition of HR/LR rats for each cohort that underwent behavioral testing was assessed. For both male (MUSC males: x2(8, 145)= 11.85, p= 0.16; d, UCAM males: x2(7, 119)= 13.55, p= 0.06) and female (MUSC females: x2(8, 132)= 12.36, p= 0.14; b, UCAM females: x2(7, 111)= 9.17, p= 0.24) rats at both locations, there was no difference in HR/LR composition between the cohorts (Online resource 2). Estrous cycle phase was then assessed in a subset of female rats undergoing testing at MUSC following the open field test (Online resource 3). Both HR and LR phenotypes contained rats in the estrus and non-estrus phase, and phase did not affect distance travelled during the open field test (HR: t(11)= 0.50, p= 0.63; LR: t(21)= 0.92, p= 0.37), suggesting that circulating ovarian hormones did not affect behavioral characterization in female rats.
Fig. 3.
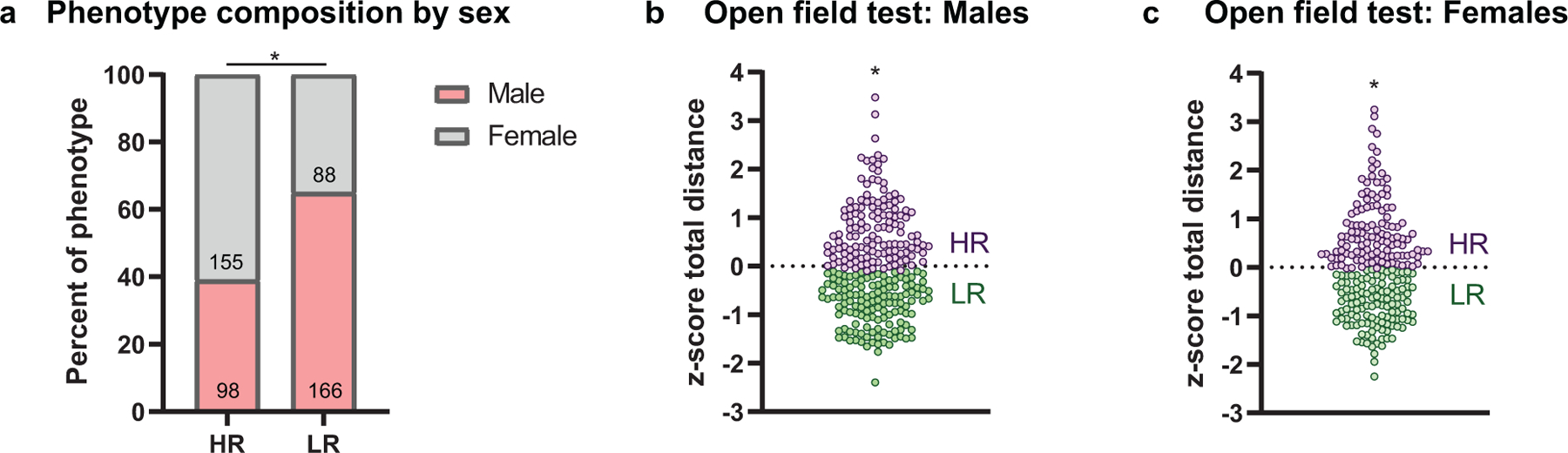
Behavioral phenotype characterization. (a) Phenotype composition when male and female rats are combined for analysis. The proportion of males and females differed within phenotypes, with a higher proportion of female rats characterized as high-responders, and males as low-responders. The n for each group is the number within each bar. (b-c) Phenotype composition when male and female rats are separated for analysis. (c) Male and (d) female HR and LR rats were characterized using a median split on z-scored total distance travelled during the locomotor test. *p<0.05 (Males: HR, n= 132; LR, n=132; Females: HR, n=121; LR, n=122)
Elevated-plus maze
Possible differences in anxiety-like behavior prior to heroin experience was assessed using the EPM test. Time spent in the open arms, an indicator of a less anxious phenotype, did not differ between HR or LR rats in either males (Mann-Whitney U= 8046, p=0.33, Fig. 4a) or females (Mann-Whitney U= 7146, p=0.67, Fig. 4b).
Fig. 4.
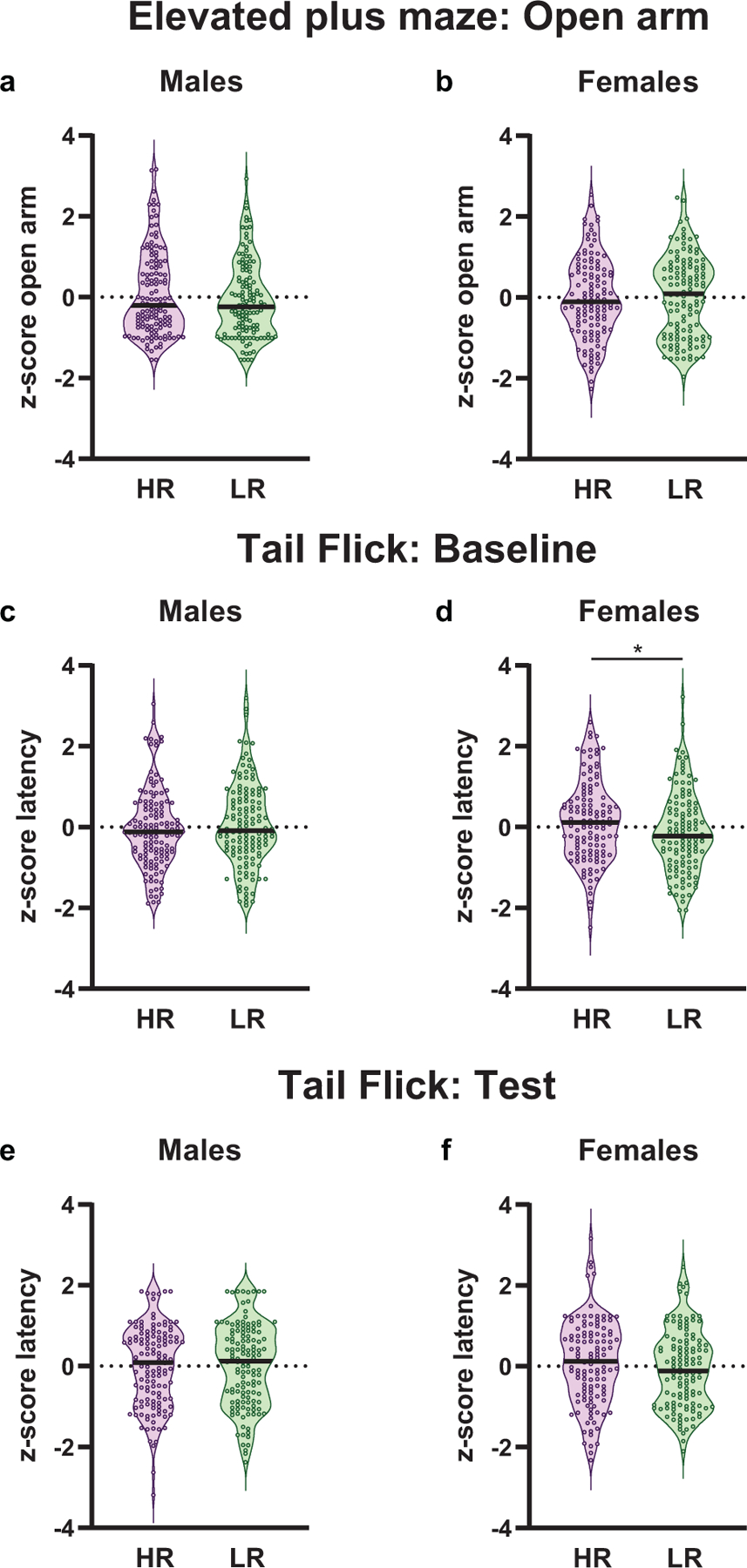
Behavior during the elevated-plus maze (EPM) and tail flick (TF) testing. The median value of the data is indicated by the black bar within the violin plot. All data represented as z-scores. HR and LR rats did not differ from one another in time spent in the open arms of the EPM in either (a) males (p=0.33) or (b) females (p=0.67). Possible phenotypic differences in analgesic threshold were assessed using a TF test. Male HR and LR rats did not differ in analgesic threshold either at (c) baseline (p=0.35), or (e) following an injection of heroin (p=0.75). (d) In contrast, HR female rats exhibit greater analgesic thresholds relative to LR rats under baseline conditions (p=0.03). (f) However, phenotypic differences are abolished following an injection of heroin (p=0.16). *p<0.05 (Males: HR, n= 132; LR, n=132; Females: HR, n=121; LR, n=122)
Tail flick test
Phenotypic differences in analgesic threshold was established using the TF test. As expected, all rats showed a greater latency to remove their tail from the noxious stimuli during the test session relative to the baseline session (sexes combined: F1,503= 1544.50, p<0.0001; males: F1,262= 1006.92, p<0.0001; females: F1,241= 574.18, p<0.0001) with no phenotypic differences present (sexes combined: F1,503= 0.14, p= 0.71; males: F1,262= 0.16, p= 0.69; females: F1,241= 0.73, p= 0.39). However, when analyzing sexes together, an interaction between sex and session was present (F1,503= 27.63, p<0.0001), with males showing a potentiated heroin-induced analgesic threshold relative to females (p<0.0001).
Data within each session were then assessed. Male HR and LR rats did not differ in latency to remove their tail from the noxious stimuli under baseline (i.e. saline injection; Mann-Whitney U= 8132, p=0.35, Fig. 4c) or testing (i.e. heroin injection; Mann-Whitney U= 8515, p=0.75, Fig. 4e) conditions. In contrast, female HR and LR rats differed under baseline conditions, with HR rats exhibiting a greater analgesic threshold compared to LR rats (t(241)= 2.14, p= 0.03; Fig. 4d). However, phenotypic differences were no longer present following an injection of heroin (Mann-Whitney U= 6609, p=0.16, Fig. 4f).
Capacity of HR/LR model in predicting heroin addiction-related behaviors in male and female rats
Akin to previous studies with psychostimulants (Piazza et al. 1989, Piazza et al. 1990, Piazza et al. 1991, Piazza et al. 1998, Piazza et al. 2000, Klebaur et al. 2001, Mantsch et al. 2001, Cain et al. 2008, Ferris et al. 2013), male HRs showed greater total heroin consumption across training relative to LRs (Mann-Whitney U= 7079, p= 0.01, Fig. 5a). Additionally, relative to male LRs, HRs exhibited greater motivation in heroin rewarded drug seeking in a progressive ratio task (Mann-Whitney U= 6764, p= 0.002, Fig. 5b) and greater cue-induced heroin seeking compared to LRs (Mann-Whitney U= 7403, p= 0.03, Fig. 5c). However, the HR/LR phenotype did not predict differences in escalation of heroin intake (Mann-Whitney U= 8291, p= 0.50, Fig. 5d), extinction burst (Mann-Whitney U= 7642, p= 0.08, Fig. 5e), extinction day 6 (Mann-Whitney U= 8059, p= 0.29, Fig. 5f), or heroin-prime reinstatement (Mann-Whitney U= 8690, p= 0.97, Fig. 5g). Together, these data suggest the HR/LR model successfully predicts some behaviors associated with OUD in male rats.
Fig. 5.
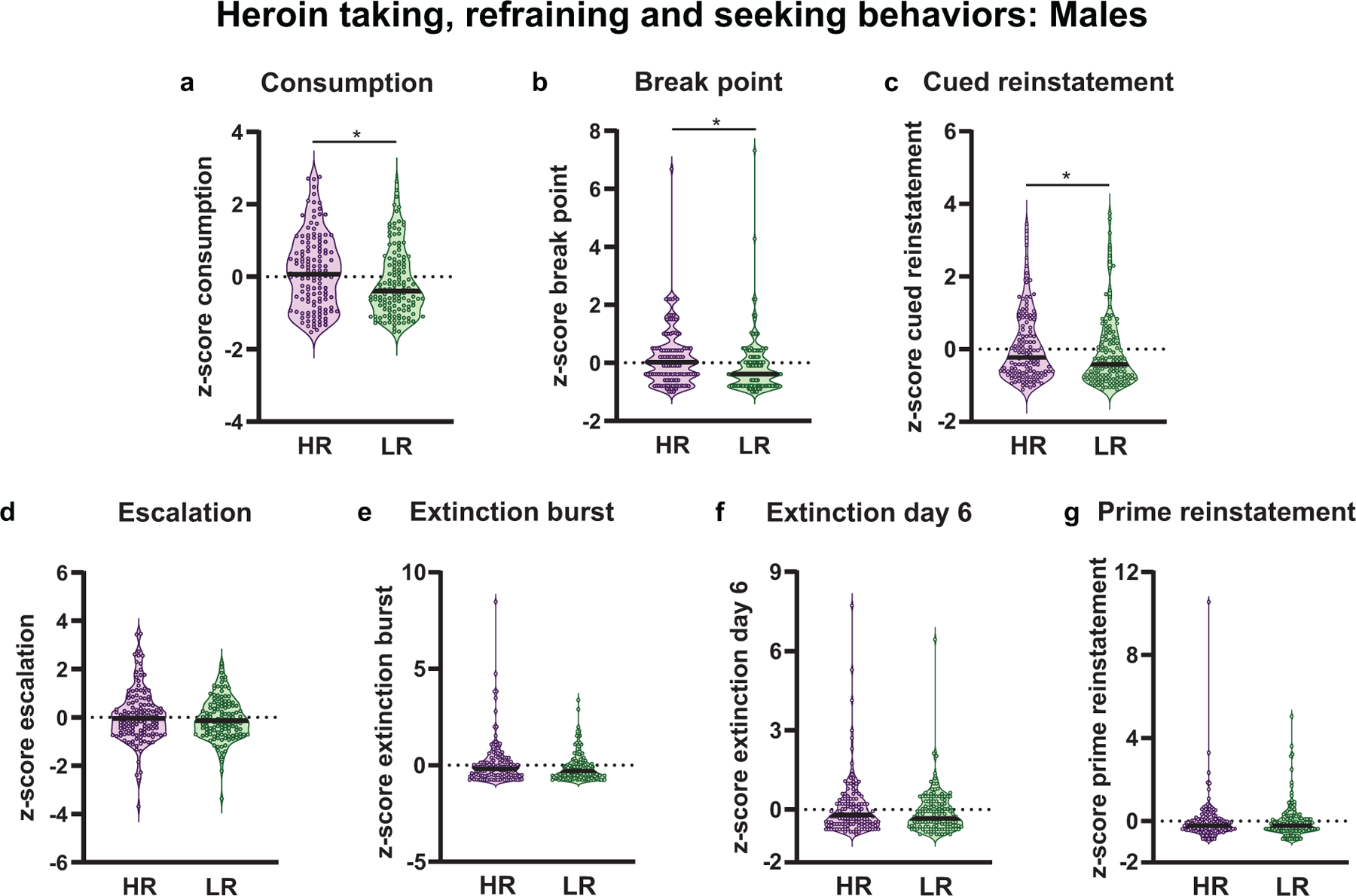
Heroin reinforced and non-reinforced behavior for male high- and low-responder (HR/LR) rats. Data represented as individual data point according to assigned phenotype. The black bar within each violin plot indicates the median value of the data set, and all data represented as z-scores. Male HRs showed (a) greater consumption of heroin across self-administration training, (b) as well as greater motivation to work for an infusion of heroin (break point) relative to LRs. (c) The two phenotypes also differed in non-reinforced reward seeking behavior, with HRs showing greater cue-induced reinstatement of heroin-seeking behavior compared to LRs. However, male HRs and LRs did not differ in (d) escalation of heroin intake, (e) refraining behavior at either the start (extinction burst) or (f) end of extinction training (extinction day 6), or (g) heroin primed reinstatement. *p<0.05 (Males: HR, n= 132; LR, n=132)
In females, HRs and LRs did not differ in any heroin reinforced behaviors (consumption: t(241)= 0.82, p= 0.42, Fig. 6a; break point: Mann-Whitney U= 7116, p= 0.63, Fig 6b; escalation: Mann-Whitney U= 6966, p= 0.045, Fig. 6d), extinction-related behaviors (extinction burst: Mann-Whitney U= 6477, p= 0.07, Fig. 6e; extinction day 6: Mann-Whitney U= 7125, p= 0.64, Fig. 6f) or reinstated heroin-seeking behaviors (prime reinstatement: Mann-Whitney U= 7289, p= 0.87, Fig. 6g; cued reinstatement: Mann-Whitney U= 7359, p= 0.97, Fig. 6c). These data show that in contrast to males, the novelty-induced locomotor trait is not predictive of OUD-associated behaviors in females.
Fig. 6.
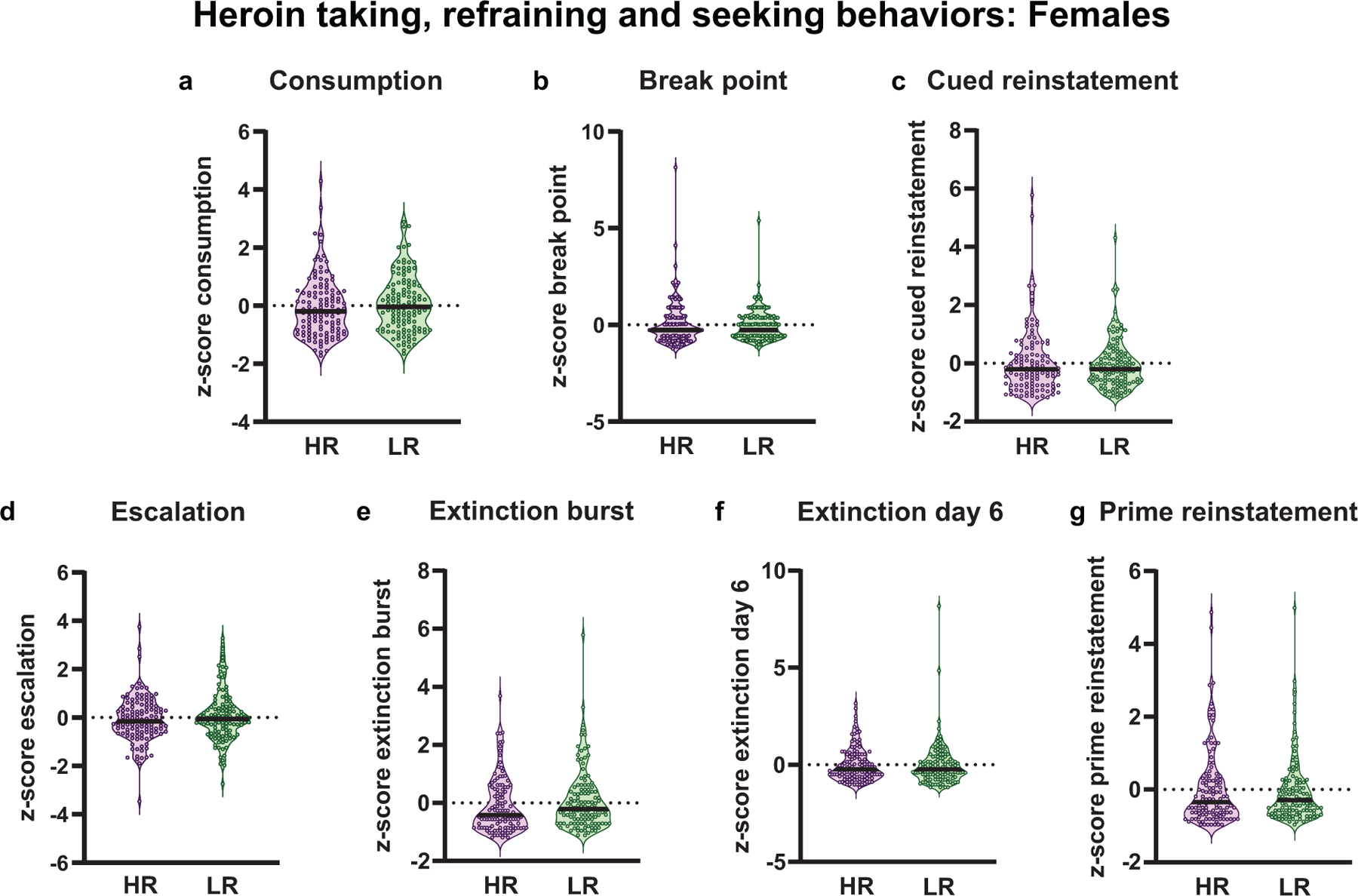
Heroin reinforced and non-reinforced behavior for female high- and low-responder (HR/LR) rats. Data represented as individual data point according to assigned phenotype. The black bar within each violin plot indicates the median value of the data set. All data represented as z-scores. In contrast to males, female HRs and LRs did not differ in (a) total heroin consumption across self-administration training, (b) motivation to work for an infusion (break point) or (c) cue-induced reinstatement of drug-seeking behavior. Akin to males, female HRs and LRS also did not differ in (d) escalation of heroin intake, (e) refraining behavior at the start (extinction burst) or (f) end (extinction day 6) of training, or (g) heroin primed reinstatement. (Females: HR, n=121; LR, n=122)
Correlational analyses between behavioral tests and measures of heroin taking, refraining and seeking behaviors showed a large proportion of significant relationships present within each phenotype for both sexes (Online Resource 4). However, the variance explained by these relationships were variable, making interpretation challenging. Furthermore, no significant correlations existed between the distance travelled during the OFT and any other measure for any group, suggesting that it is not the extent of novelty-induced locomotion that predicts performance in heroin addiction-related behaviors, but rather the overall behavioral phenotype designation. This suggests that novelty-induced locomotion interacts with variables in a non-linear manner when predicting heroin addiction vulnerability in male rats.
Heroin reacquisition and punished heroin-taking behavior
HR and LR rats exhibited potentiated heroin taking on day 1 of reacquisition, and then decreased consumption over training (Sexes combined: F1.53,42.78=27.39, p=0<0.0001, Fig. 7a; Males: F1.40,19.62=21.17, p<0.0001, Fig. 7b; Females: F1.43,17.20=9.67, p=0.003, Fig. 7c). However, phenotypes did not differ in reacquisition of heroin self-administration training following an abstinence period when sexes were analyzed together (F1,28=1.09, p=0.31; Fig. 7a), or separately (Males: F1,14=1.48, p=0.24, Fig. 7b; Females: F1,12=0.67, p=0.43, Fig. 7c). During the punished heroin taking test training, HR and LR rats equally consumed heroin when analyzed together (Mann-Whitney U= 100, p= 0.67; Fig. 7d) or separately (Males: Mann-Whitney U= 16.50, p=0.11, Fig. 7e; Females: Mann-Whitney U= 20.50, p=0.82, Fig. 7f). When data were analyzed by the number of infusions per hour, analyses showed no phenotypic effects (Sexes combined: F1,28=0.05, p=0.81; Males: F1,14=4.01, p=0.06; Females: F1,12=1.05, p=0.32). This analysis suggests time course of consumption also does not differ between HR and LR rats. Though there was a significant phenotype by hour interaction for males (F11,154=2.13, p=0.02), no significant post-hoc analyses were present. These data suggest that the HR/LR model does not capture differences in the reacquisition of heroin taking or continued heroin taking in the presence of an adverse stimuli, important features of substance use disorder (SUD).
Fig. 7.
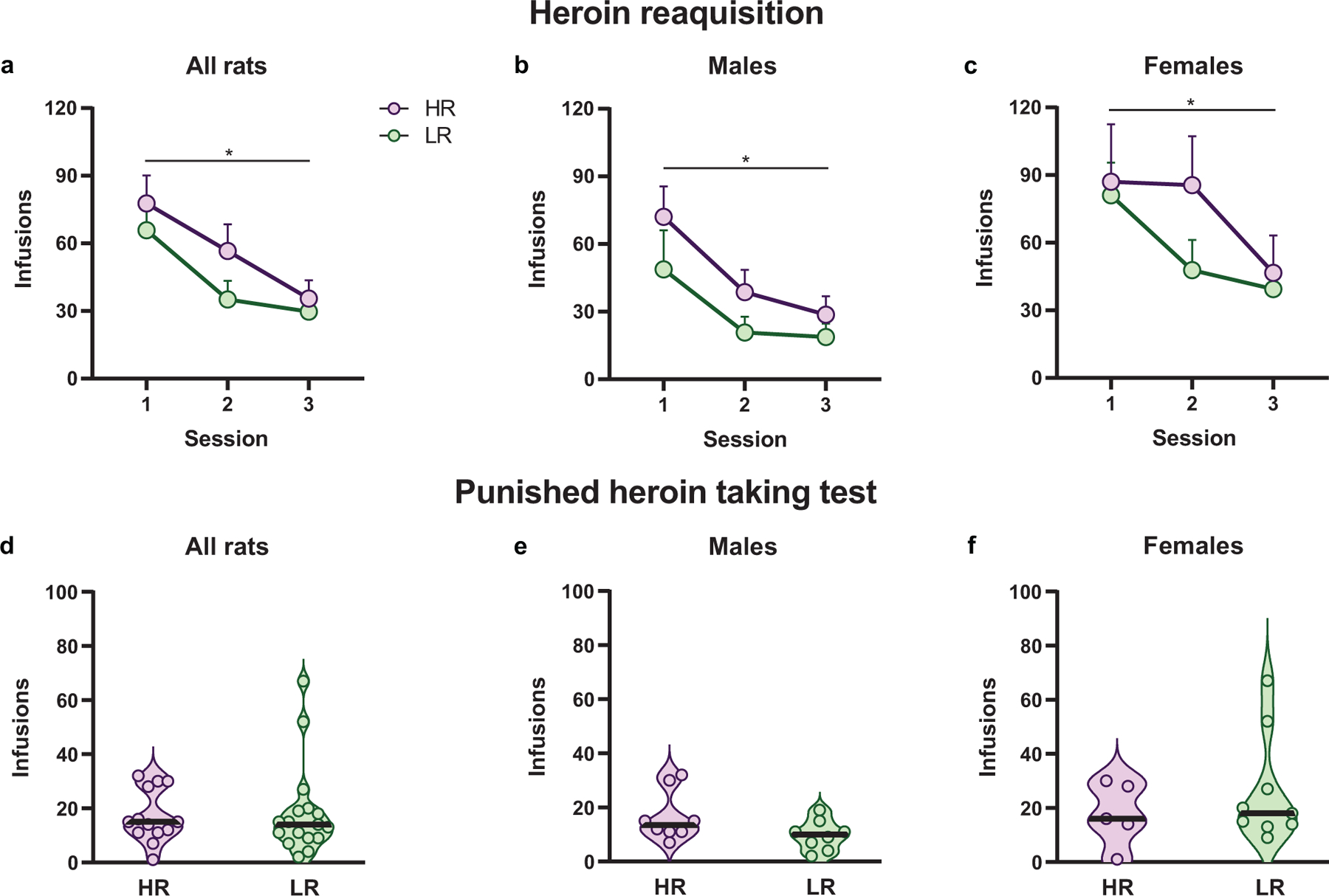
Heroin reacquisition training and punished heroin taking test for male and female rats in the high-responder/low-responder model. (a-c) Mean + SEM for infusions earned across three heroin self-administration reacquisition training sessions. High-responder/low-responder rats decreased infusions earned across training when (a) sexes were analyzed together (p<0.0001) as well as separately (b, Males: p<0.0001; c, Females: p=0.003). However, HR and LR rats did not differ from one another in any analyses suggesting the novelty-induced locomotor trait does not predict heroin reacquisition following a prolonged period of abstinence. During the punished heroin taking test, there was a 50% chance of foot shock delivery (0.40 mA, 0.5 seconds) with every infusion earned. Data represented as individual data point according to assigned phenotype. The black bar within the violin plot indicates the median value of the data set. HR and LR rats did not differ in heroin taking in the presence of the aversive foot shock delivery when sexes were analyzed together (d, p=0.67) and separately (e, Males: p=0.11; f, Females: p=0.82). *p<0.05 (Males, n= 16 (8 in each phenotype); Females, n= 14 (HR, n=5; LR, n=9))
Discussion
In an attempt to account for the extensive individual variation in addiction-related behaviors in humans, we employed the HR/LR model in outbred HS rats to assess how novelty-induced locomotor behavior predicted OUD vulnerability in male and female rats. Heterogeneous stock (HS) rats were used for these studies as they exhibit considerably more behavioral and genetic variation than commonly used laboratory inbred lines (Hansen and Spuhler 1984, Consortium et al. 2008, Johannesson et al. 2009, Solberg Woods and Palmer 2019), resulting in diversity more akin to the human population. HS rats have been used to model such variation in several disorders, including SUD-related behaviors (Wang et al. 2018, Hughson et al. 2019, Kallupi et al. 2020, Deal et al. 2021, King et al. 2021). Over 500 HS rat littermates underwent behavioral testing at two distinct locations (MUSC, Charleston, South Carolina, USA and UCAM, Camerino, Italy) in an effort to account for potential environmentally imposed epigenetic changes that may occur due to testing site. We assessed how multiple OUD behaviors across the phases of SUD (i.e., heroin taking, refraining and seeking) differed between male and female rats, and found female rats exhibit a greater OUD vulnerable phenotype relative to males. Next, rats were characterized as high or low locomotor responding in a novel open field test, a trait associated with SUD vulnerability (Piazza et al. 1989, Dellu et al. 1996). We showed that the HR/LR model successfully predicted OUD vulnerability only in male rats, with no predictability in female rats. Furthermore, phenotypes did not differ in heroin reacquisition or punished heroin-taking behavior, highlighting the theoretical limitations of this model for assessing OUD propensity.
Sex differences in anxiety, analgesic threshold and heroin OUD behaviors
Anxiety-related behaviors have been shown to differ between male and female rodents, and our results align with previous work showing female rats spend more time in the open arm of the EPM (Scholl et al. 2019, Knight et al. 2021) and exhibit greater locomotion during an OFT (Knight et al. 2021). These data infer females have less anxiety-like behaviors compared to males as measured using the EPM and OFT. In contrast, males appeared to be more sensitive to the analgesic effects of heroin, as latency to remove the tail from a noxious stimulus was higher in males than females. Clinically, opioids have been shown to be less efficacious in females than males (Cepeda and Carr 2003, Miller and Ernst 2004, Aubrun et al. 2005, Mogil and Bailey 2010), with females requiring higher doses to attain the same biological outcome (Cepeda and Carr 2003). This effect is mirrored in rodent models, with opioids producing a greater analgesic effect in male rats compared to female rats, and sexual dimorphisms in the engagement of pain processing pathways being implicated for this difference (for review see Averitt et al. 2019). Our results further support these findings and given the genetic and behavioral heterogeneity inherently captured in the HS rat line, future studies assessing sexual dimorphism in pain processing would benefit from using the HS rat.
Compared to males, we showed females exhibited a more vulnerable OUD phenotype for heroin taking, refraining and seeking behaviors. In humans, females stabilize at a higher drug dose and relapse more often than males across several classes of drugs, including opioids (Becker et al. 2017), and reach criteria for an OUD diagnosis at a faster rate (Hser et al. 1987). Work using rodent models have found that female rats consume more opioids (Lynch and Carroll 1999, Carroll et al. 2002, Cicero et al. 2003, Towers et al. 2019, George et al. 2021) and do so at a faster rate (Lynch and Carroll 1999, Carroll et al. 2002), and show more seeking behavior relative to males (Smethells et al. 2020, George et al. 2021, D’Ottavio et al. 2022). Our results support these findings, and also show that female rats are less able to refrain from non-reinforced drug seeking, and are more motivated to work for an infusion of heroin than males are. One explanation for these sex differences may be fluctuations in hormones as a result of different estrous cycle phases. Behaviors associated with drug use in humans are impacted by estrous cycle phase (Becker et al. 2017), and delivery of estradiol can potentiate opioid and cocaine self-administration in ovariectomized female rats (Becker et al. 2017). However, similar to female rats, male rats exhibit large variability in observed behaviors so while hormonal fluctuations during estrous cycle may contribute to differences within females, animals are behaving similarly between each sex.
In addition to sex differences, it is important to note that there were several site differences in the raw data prior to z-score transformation to standardize all data. It is likely that epigenetic factors differentially imposed at each site location affected subsequent behavior. All behavioral protocols were identical between sites and that the animals tested at each location were littermates, making this effect more impactful as we tightly controlled for experimental variance to the best of our ability. Thus, when undertaking experimental replications, particularly at a different site, it is important to consider how factors such as epigenetics can affect behavioral outcomes. To this end, future work is focusing on elucidating epigenetic differences in different brain regions in rats undergoing experimentation at MUSC versus UCAM.
Elevated plus maze and tail flick using HR/LR behavioral phenotype
HR and LR rats differ in several behavioral and endocrine measures associated with anxiety (for review see Clinton et al. 2021), as well as differential engagement of the hypothalamic-pituitary-adrenal axis (Piazza et al. 1991, Kabbaj et al. 2007). Work using outbred male Sprague-Dawley rats showed HR rats spend more time in the open arm of the EPM relative to LR rats, suggesting they exhibit a less anxious phenotype (Kabbaj et al. 2000). Here we show that male and female HS rats do not differ in anxiety-related measures as assessed by the EPM, suggesting that in a rat line capturing more genetic and behavioral variability akin to the human population, the HR/LR model is not efficacious for assessing the relationship between anxiety and addiction-related behaviors. Alternatively, these conflicting findings may be simply due to strain differences. We also evaluated analgesic threshold prior to heroin experience, and found no phenotypic difference in males. However, females HR rats were more resistant to a painful stimulus relative to LR rats. Behavior during this test did not predict any subsequent OUD-related behaviors, implying this phenotypic finding is not relevant to OUD, but rather may be a pertinent model for studying individual variation in the neurobiology of pain in a rodent model.
HR/LR behavioral phenotype in male HS rats
In an inbred rat line, novelty-induced locomotor behavior has been shown to correlate with morphine self-administration in male rats (Ambrosio et al. 1995). In contrast, this relationship does not appear to exist in an outbred rat model encompassing, presumably, more genetic variability (Swain et al. 2018). However, in this study, a much shorter behavioral paradigm, both in total duration and self-administration session length, was used in comparison to what was used in this manuscript, and rats were never characterized as HR or LR rats. Despite these contradictory findings, here we show that similar to, and in alignment with previous findings using psychostimulants (Piazza et al. 1989, Piazza et al. 1990, Piazza et al. 1991, Piazza et al. 1998, Piazza et al. 2000, Klebaur et al. 2001, Mantsch et al. 2001, Cain et al. 2008, Ferris et al. 2013, O’Connor et al. 2021), HRs consumed more heroin than LRs during heroin self-administration. Interestingly, the two phenotypes did not differ in escalation of intake, suggesting both groups were increasing at similar rates, but that HRs started at and maintained a higher rate of intake. This behavior has been observed in male HR rats during cocaine self-administration, with HRs consuming more drug when cost is low compared to LR rats (O’Connor et al. 2021). Relative to LRs, HRs also showed greater motivation to work for an infusion of heroin following self-administration training, implying these phenotypes differ not only in the acquisition of heroin-taking behavior, but also in more complex motivational behaviors like rewarded drug seeking.
We also show male HR rats exhibited greater cue-induced reinstatement of heroin-seeking behavior compared to LRs, a trait that has not been observed in outbred rats for any drug (Sutton et al. 2000, Deroche-Gamonet et al. 2004, Kuhn et al. 2021, Chang et al. 2022). However, the two phenotypes did not differ in heroin-prime reinstatement, suggesting HRs and LRs differ in discrete cue-reward motivated behaviors, but not in contextual or interoceptive cue motivated behaviors. One explanation is that HRs show greater cue-induced reinstatement of heroin-seeking behavior because they consume more heroin than LRs during self-administration, thus receiving more cue-reward pairings thereby producing a stronger association between the action, cue and reward. It is then possible that for heroin, but not for stimulants, the extent to which an individual acquires drug taking behaviors has long term effects on overall addiction liability in males, including relapse propensity, in the HR/LR model. An alternative explanation for the phenotypic difference in cue-induced reinstatement is variation in the motivational properties attributed to the reward-paired cues. However, the relationship between novelty-induced locomotor behavior and incentive salience attribution (assigning intense motivational value to reward-paired cues; for review see Flagel and Robinson 2017) has not been observed consistently in outbred rat lines, including HS rats, for cocaine (Robinson and Flagel 2009, Hughson et al. 2019, Kuhn et al. 2021) or an opioid (Chang et al. 2022). Lastly, our findings may not align with work using psychostimulants due to neurobiological differences imposed by drug choice (Shalev et al. 2002, Lenoir et al. 2012, De Pirro et al. 2018). Regardless, to further understand the phenotypic differences present during cue-induced reinstatement, future studies can include cue removal tests or devaluation procedures to assess the motivational value of the heroin-paired cues or standardize the number of infusions earned during daily self-administration sessions to clarify if differences in consumption affects cue-induced reinstatement behavior.
Contrary to our findings, recent work showed no HR/LR phenotypic differences in male rats during self-administration of remifentanil, a short acting opioid (Chang et al. 2022), or subsequent cued reinstatement. Differences are likely due to choice of opioid used, as remifentanil is quickly removed from circulation (half-life of 0.3–0.7 min, Crespo et al. 2005), whereas heroin remains in the bloodstream for substantially longer (half-life of 7.6 min, Gottas et al. 2013), with its active metabolites persisting even longer (morphine: half-life of 2–3 hr, 6-acetylmorphine: half-life of 22 min; Rook et al. 2006). It is plausible the duration of the interoceptive effects of the opioid affect HR/LR phenotypic differences in male rats, supporting the necessity to account for drug pharmacokinetics when assessing individual variation in OUD propensity. Alternatively, discrepancy in these findings may be due to the many methodological differences, or rat strain difference, between the current study and that by Chang and colleagues (Chang et al. 2022). For example, we employed long-access training sessions (12 hr versus <3 hr) and frequent brief abstinence periods during training, both of which likely impact the neurobiological mechanisms mediating drug taking and seeking behaviors (Grimm et al. 2002, Pickens et al. 2011, Purgianto et al. 2013).
The neurobiological mechanisms underlying differences in addiction-related behaviors in HR and LR rats is not well studied, however, differential adaptations of the mesolimbic dopamine system appear to contribute to phenotypic differences (for review see Norbury and Husain 2015). Work using outbred rats has shown that, relative to LR rats, HR rats show higher levels of dopamine cell firing in the ventral tegmental area (VTA) (Marinelli and White 2000) and dopamine release in the nucleus accumbens (NAc) (Chefer et al. 2003) under basal conditions. This enhanced NAc dopamine release is maintained after an injection of cocaine (Hooks et al. 1991, Chefer et al. 2003). Following cocaine self-administration, HRs exhibit alternations in nucleus accumbens dopamine uptake parameters (Ferris et al. 2013) and more persistent VTA cell firing rates (McCutcheon et al. 2009). Comparable to psychostimulants, opioids also induce changes in mesolimbic dopamine cell firing (for review see Pierce and Kumaresan 2006), thus it is plausible that similar dopaminergic mechanism may be underlying phenotypic differences for both stimulants and opioids in the HR/LR model. Non-dopaminergic processes also likely contribute toward phenotypic differences, such as differences in glutamatergic transmission (Kalivas 2009) and NAc tetrapartite synapse adaptations (for review see Kruyer et al. 2020). However, further experimentation is necessary in order to assess neurobiological differences in male HR and LR rats following an opioid and should evaluate both dopaminergic and non-dopaminergic processes.
HR/LR behavioral phenotype in female HS rats
While the HR/LR model had predictive validity for heroin addiction vulnerability in males, it did not for females. Though novelty-induced locomotion is not a predictive trait of OUD vulnerability in females, additional models of individual variation in SUD propensity should be employed to further understand sexual dimorphism of SUD predictive traits.
Punished heroin-taking behavior
Following several weeks of forced abstinence from heroin, HR and LR male and female rats did not differ in the reacquisition of heroin-taking behavior. These data suggest phenotypic differences, at least for male rats, in heroin self-administration is only present in the acquisition, and not long-term maintenance and compulsive taking of heroin. Phenotypes also did not differ in punished heroin-taking behavior for either sex. Compulsive drug taking in the presence of an adverse stimuli is an important feature of human SUD (Deroche-Gamonet et al. 2004, American Psychiatric Association 2013, Belin-Rauscent et al. 2016), and the lack of phenotypic differences in this assay expose limitations of the HR/LR phenotypes in modeling human OUD.
Conclusion
These results emphasize the advantages of accounting for both sex differences and individual variation in addiction-related behaviors when assessing heroin addiction vulnerability. We showed that relative to HS male rats, females show less anxiety-like behavior, lower levels of heroin-induced analgesia, and a more vulnerable OUD phenotype across several heroin taking, refraining and seeking measures. Next, we demonstrated that novelty-induced locomotion, a trait associated with human SUD vulnerability, is predictive of heroin addiction vulnerability in male, but not female, HS rats. These results highlight the necessity to assess sex differences in addiction-related behaviors and address the limitations associated with the HR/LR model when predicting OUD vulnerability in a heterogeneous population of rats.
Supplementary Material
Acknowledgements
The experiments were designed by BNK, NC, GH, RC and PWK, and conducted by BNK, NC, ADC, ATR, VL and RWN. Standardization of data was performed by CA and DC, and LCSW provided all heterogeneous stock rats used in these experiments. Data was analyzed by BNK, and BNK and PWK wrote the manuscript. We would like to thank Dr. Carmela Reichel for providing the equipment necessary to perform the punished heroin taking test.
Funding
Funding for these studies was provided by the National Institute on Drug Abuse branch of the National Institutes of Health U01DA45300 (awarded to PWK) and T32DA007288 (BNK).
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- (2020). Wide-ranging online data for epidemiologic research (WONDER) Atlanta, GA: CDC, National Center for Health Statistics. [Google Scholar]
- Ambrosio E, Goldberg SR and Elmer GI (1995) Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol 6(3): 229–237. [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders
- Aubrun F, Salvi N, Coriat P and Riou B (2005) Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology 103(1): 156–160 DOI: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- Averitt DL, Eidson LN, Doyle HH and Murphy AZ (2019) Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology 44(1): 155–165 DOI: 10.1038/s41386-018-0127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML and Reed BG (2017) Sex differences, gender and addiction. J Neurosci Res 95(1–2): 136–147 DOI: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin-Rauscent A, Fouyssac M, Bonci A and Belin D (2016) How Preclinical Models Evolved to Resemble the Diagnostic Criteria of Drug Addiction. Biol Psychiatry 79(1): 39–46 DOI: 10.1016/j.biopsych.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV and Deroche-Gamonet V (2011) High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36(3): 569–579 DOI: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Denehy ED and Bardo MT (2008) Individual differences in amphetamine self-administration: the role of the central nucleus of the amygdala. Neuropsychopharmacology 33(5): 1149–1161 DOI: 10.1038/sj.npp.1301478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC and Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 161(3): 304–313 DOI: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Cepeda MS and Carr DB (2003) Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg 97(5): 1464–1468 DOI: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- Chang SE, Krueger LD and Flagel SB (2022) Investigating individual differences in opioid-taking and opioid-seeking behavior in male rats. Psychopharmacology (Berl) DOI: 10.1007/s00213-021-06023-2. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I and Shippenberg TS (2003) Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci 23(7): 3076–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Xu WJ, Zhu HQ, Gao L, Lai MJ, Zhang FQ, Zhou WH and Liu HF (2016) Effects of histone deacetylase inhibitor sodium butyrate on heroin seeking behavior in the nucleus accumbens in rats. Brain Res 1652: 151–157 DOI: 10.1016/j.brainres.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC and Meyer ER (2003) Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 74(3): 541–549 DOI: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Shupe EA, Glover ME, Unroe KA, McCoy CR, Cohen JL and Kerman IA (2021) Modeling heritability of temperamental differences, stress reactivity, and risk for anxiety and depression: Relevance to research domain criteria (RDoC). Eur J Neurosci DOI: 10.1111/ejn.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium S, Saar K, Beck A, Bihoreau MT, Birney E, Brocklebank D, Chen Y, Cuppen E, Demonchy S, Dopazo J, Flicek P, Foglio M, Fujiyama A, Gut IG, Gauguier D, Guigo R, Guryev V, Heinig M, Hummel O, Jahn N, Klages S, Kren V, Kube M, Kuhl H, Kuramoto T, Kuroki Y, Lechner D, Lee YA, Lopez-Bigas N, Lathrop GM, Mashimo T, Medina I, Mott R, Patone G, Perrier-Cornet JA, Platzer M, Pravenec M, Reinhardt R, Sakaki Y, Schilhabel M, Schulz H, Serikawa T, Shikhagaie M, Tatsumoto S, Taudien S, Toyoda A, Voigt B, Zelenika D, Zimdahl H and Hubner N (2008) SNP and haplotype mapping for genetic analysis in the rat. Nat Genet 40(5): 560–566 DOI: 10.1038/ng.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Sturm K, Saria A and Zernig G (2005) Simultaneous intra-accumbens remifentanil and dopamine kinetics suggest that neither determines within-session operant responding. Psychopharmacology (Berl) 183(2): 201–209 DOI: 10.1007/s00213-005-0180-7. [DOI] [PubMed] [Google Scholar]
- D’Ottavio G, Reverte I, Ragozzino D, Meringolo M, Milella MS, Boix F, Venniro M, Badiani A and Caprioli D (2022) Increased heroin intake and relapse vulnerability in intermittent relative to continuous self-administration: Sex differences in rats. Br J Pharmacol DOI: 10.1111/bph.15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H and Becker JB (2008) The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav 90(3): 331–338 DOI: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G and Ciccocioppo R (2017) Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology (Berl) 234(2): 223–234 DOI: 10.1007/s00213-016-4452-1. [DOI] [PubMed] [Google Scholar]
- De Pirro S, Galati G, Pizzamiglio L and Badiani A (2018) The Affective and Neural Correlates of Heroin versus Cocaine Use in Addiction Are Influenced by Environmental Setting But in Opposite Directions. J Neurosci 38(22): 5182–5195 DOI: 10.1523/JNEUROSCI.0019-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal A, Cooper N, Kirse HA, Uneri A, Raab-Graham K, Weiner JL and Solberg Woods LC (2021) Early life stress induces hyperactivity but not increased anxiety-like behavior or ethanol drinking in outbred heterogeneous stock rats. Alcohol 91: 41–51 DOI: 10.1016/j.alcohol.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M and Simon H (1996) Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology 34(3): 136–145 DOI: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D and Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305(5686): 1014–1017 DOI: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Melchior JR, Roberts DC, Espana RA and Jones SR (2013) Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci 38(4): 2628–2636 DOI: 10.1111/ejn.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Chaudhury S, Waselus M, Kelly R, Sewani S, Clinton SM, Thompson RC, Watson SJ Jr. and Akil H (2016) Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. Proc Natl Acad Sci U S A 113(20): E2861–2870 DOI: 10.1073/pnas.1520491113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB and Robinson TE (2017) Neurobiological Basis of Individual Variation in Stimulus-Reward Learning. Curr Opin Behav Sci 13: 178–185 DOI: 10.1016/j.cobeha.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, San George MA, Ashrafioun L and Richards JB (2011) Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats. Behav Processes 86(2): 295–304 DOI: 10.1016/j.beproc.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George BE, Barth SH, Kuiper LB, Holleran KM, Lacy RT, Raab-Graham KF and Jones SR (2021) Enhanced heroin self-administration and distinct dopamine adaptations in female rats. Neuropsychopharmacology 46(10): 1724–1733 DOI: 10.1038/s41386-021-01035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottas A, Oiestad EL, Boix F, Vindenes V, Ripel A, Thaulow CH and Morland J (2013) Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br J Pharmacol 170(3): 546–556 DOI: 10.1111/bph.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y and Hope BT (2002) Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol 13(5–6): 379–388 DOI: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C and Spuhler K (1984) Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res 8(5): 477–479 DOI: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Liem BJ and Justice JB Jr. (1992) Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Pharmacol Biochem Behav 43(3): 815–823 DOI: 10.1016/0091-3057(92)90413-a. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB and Justice JB Jr. (1991) Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse 9(2): 121–128 DOI: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Hser YI, Anglin MD and Booth MW (1987) Sex differences in addict careers. 3. Addiction. Am J Drug Alcohol Abuse 13(3): 231–251 DOI: 10.3109/00952998709001512. [DOI] [PubMed] [Google Scholar]
- Hughson AR, Horvath AP, Holl K, Palmer AA, Solberg Woods LC, Robinson TE and Flagel SB (2019) Incentive salience attribution, “sensation-seeking” and “novelty-seeking” are independent traits in a large sample of male and female heterogeneous stock rats. Sci Rep 9(1): 2351 DOI: 10.1038/s41598-019-39519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson M, Lopez-Aumatell R, Stridh P, Diez M, Tuncel J, Blazquez G, Martinez-Membrives E, Canete T, Vicens-Costa E, Graham D, Copley RR, Hernandez-Pliego P, Beyeen AD, Ockinger J, Fernandez-Santamaria C, Gulko PS, Brenner M, Tobena A, Guitart-Masip M, Gimenez-Llort L, Dominiczak A, Holmdahl R, Gauguier D, Olsson T, Mott R, Valdar W, Redei EE, Fernandez-Teruel A and Flint J (2009) A resource for the simultaneous high-resolution mapping of multiple quantitative trait loci in rats: the NIH heterogeneous stock. Genome Res 19(1): 150–158 DOI: 10.1101/gr.081497.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR and Akil H (2000) Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci 20(18): 6983–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Morley-Fletcher S, Le Moal M and Maccari S (2007) Individual differences in the effects of chronic prazosin hydrochloride treatment on hippocampal mineralocorticoid and glucocorticoid receptors. Eur J Neurosci 25(11): 3312–3318 DOI: 10.1111/j.1460-9568.2007.05585.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10(8): 561–572 DOI: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Carrette LLG, Kononoff J, Solberg Woods LC, Palmer AA, Schweitzer P, George O and de Guglielmo G (2020) Nociceptin attenuates the escalation of oxycodone self-administration by normalizing CeA-GABA transmission in highly addicted rats. Proc Natl Acad Sci U S A 117(4): 2140–2148 DOI: 10.1073/pnas.1915143117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayir H, Goktalay G, Yavuz O and Uzbay TI (2011) Impact of baseline prepulse inhibition on nicotine-induced locomotor sensitization in rats. Behav Brain Res 216(1): 275–280 DOI: 10.1016/j.bbr.2010.08.004. [DOI] [PubMed] [Google Scholar]
- King CP, Tripi JA, Hughson AR, Horvath AP, Lamparelli AC, Holl KL, Chitre AS, Polesskaya O, Ishiwari K, Solberg Woods LC, Palmer AA, Robinson TE, Flagel SB and Meyer PJ (2021) Sensitivity to food and cocaine cues are independent traits in a large sample of heterogeneous stock rats. Sci Rep 11(1): 2223 DOI: 10.1038/s41598-020-80798-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM and Bardo MT (2001) Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol 12(4): 267–275. [DOI] [PubMed] [Google Scholar]
- Knight P, Chellian R, Wilson R, Behnood-Rod A, Panunzio S and Bruijnzeel AW (2021) Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol Biochem Behav 204: 173168 DOI: 10.1016/j.pbb.2021.173168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyer A, Chioma VC and Kalivas PW (2020) The Opioid-Addicted Tetrapartite Synapse. Biol Psychiatry 87(1): 34–43 DOI: 10.1016/j.biopsych.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn BN, Campus P, Klumpner MS, Chang SE, Iglesias AG and Flagel SB (2021) Inhibition of a cortico-thalamic circuit attenuates cue-induced reinstatement of drug-seeking behavior in “relapse prone” male rats. Psychopharmacology (Berl) DOI: 10.1007/s00213-021-05894-9. [DOI] [PubMed] [Google Scholar]
- Lamarque S, Taghzouti K and Simon H (2001) Chronic treatment with Delta(9)-tetrahydrocannabinol enhances the locomotor response to amphetamine and heroin. Implications for vulnerability to drug addiction. Neuropharmacology 41(1): 118–129 DOI: 10.1016/s0028-3908(01)00039-9. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Guillem K, Koob GF and Ahmed SH (2012) Drug specificity in extended access cocaine and heroin self-administration. Addict Biol 17(6): 964–976 DOI: 10.1111/j.1369-1600.2011.00385.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ and Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144(1): 77–82 DOI: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Ma B, Yue K, Chen L, Tian X, Ru Q, Gan Y, Wang D, Jin G and Li C (2014) L-stepholidine, a natural dopamine receptor D1 agonist and D2 antagonist, inhibits heroin-induced reinstatement. Neurosci Lett 559: 67–71 DOI: 10.1016/j.neulet.2013.10.066. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD and Kreek MJ (2001) Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 157(1): 31–39. [DOI] [PubMed] [Google Scholar]
- Marinelli M and White FJ (2000) Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci 20(23): 8876–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, White FJ and Marinelli M (2009) Individual differences in dopamine cell neuroadaptations following cocaine self-administration. Biol Psychiatry 66(8): 801–803 DOI: 10.1016/j.biopsych.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PL and Ernst AA (2004) Sex differences in analgesia: a randomized trial of mu versus kappa opioid agonists. South Med J 97(1): 35–41 DOI: 10.1097/01.smj.0000085743.68121.a9. [DOI] [PubMed] [Google Scholar]
- Mogil JS and Bailey AL (2010) Sex and gender differences in pain and analgesia. Prog Brain Res 186: 141–157 DOI: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- Norbury A and Husain M (2015) Sensation-seeking: Dopaminergic modulation and risk for psychopathology. Behav Brain Res 288: 79–93 DOI: 10.1016/j.bbr.2015.04.015. [DOI] [PubMed] [Google Scholar]
- O’Connor SL, Aston-Jones G and James MH (2021) The sensation seeking trait confers a dormant susceptibility to addiction that is revealed by intermittent cocaine self-administration in rats. Neuropharmacology 195: 108566 DOI: 10.1016/j.neuropharm.2021.108566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M and Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245(4925): 1511–1513 DOI: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M and Simon H (1990) Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 514(1): 22–26. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F and Le Moal M (2000) Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20(11): 4226–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Rouge-Pont F and Le Moal M (1998) Behavioral and biological factors associated with individual vulnerability to psychostimulant abuse. NIDA Res Monogr 169: 105–133. [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P and Simon H (1991) Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A 88(6): 2088–2092 DOI: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT and Shaham Y (2011) Neurobiology of the incubation of drug craving. Trends Neurosci 34(8): 411–420 DOI: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC and Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30(2): 215–238 DOI: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY and Wolf ME (2013) Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology 38(9): 1789–1797 DOI: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR and Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66(1): 1–11 DOI: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE and Flagel SB (2009) Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry 65(10): 869–873 DOI: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook EJ, Huitema AD, van den Brink W, van Ree JM and Beijnen JH (2006) Population pharmacokinetics of heroin and its major metabolites. Clin Pharmacokinet 45(4): 401–417 DOI: 10.2165/00003088-200645040-00005. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Afzal A, Fox LC, Watt MJ and Forster GL (2019) Sex differences in anxiety-like behaviors in rats. Physiol Behav 211: 112670 DOI: 10.1016/j.physbeh.2019.112670. [DOI] [PubMed] [Google Scholar]
- Sell SL, Dillon AM, Cunningham KA and Thomas ML (2005) Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats. Behav Brain Res 161(1): 69–74 DOI: 10.1016/j.bbr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW and Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54(1): 1–42 DOI: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Smethells JR, Greer A, Dougen B and Carroll ME (2020) Effects of voluntary exercise and sex on multiply-triggered heroin reinstatement in male and female rats. Psychopharmacology (Berl) 237(2): 453–463 DOI: 10.1007/s00213-019-05381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC and Palmer AA (2019) Using Heterogeneous Stocks for Fine-Mapping Genetically Complex Traits. Methods Mol Biol 2018: 233–247 DOI: 10.1007/978-1-4939-9581-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD and Vezina P (2001) Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 158(2): 175–180 DOI: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA and Self DW (2000) Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology 22(6): 626–641 DOI: 10.1016/S0893-133X(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Swain Y, Muelken P, LeSage MG, Gewirtz JC and Harris AC (2018) Locomotor activity does not predict individual differences in morphine self-administration in rats. Pharmacol Biochem Behav 166: 48–56 DOI: 10.1016/j.pbb.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers EB, Tunstall BJ, McCracken ML, Vendruscolo LF and Koob GF (2019) Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology 151: 189–194 DOI: 10.1016/j.neuropharm.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Han W, Chitre AS, Polesskaya O, Solberg Woods LC, Palmer AA and Chen H (2018) Social and anxiety-like behaviors contribute to nicotine self-administration in adolescent outbred rats. Sci Rep 8(1): 18069 DOI: 10.1038/s41598-018-36263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo T, Nesil T, Choi JS and Li MD (2016) Novelty Seeking and Drug Addiction in Humans and Animals: From Behavior to Molecules. J Neuroimmune Pharmacol 11(3): 456–470 DOI: 10.1007/s11481-015-9636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xigeng Z, Yonghui L, Xiaojing L, Lin X, Dongmei W, Jie L, Xiaoyan Y and Nan S (2004) Social crowding sensitizes high-responding rats to psychomotor-stimulant effects of morphine. Pharmacol Biochem Behav 79(2): 213–218 DOI: 10.1016/j.pbb.2004.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


