Abstract
Objectives:
Head and neck lymphatic malformations (HNLM) are caused by gain-of-function somatic mutations in PIK3CA. Acetylsalicylic acid (ASA/aspirin) is thought to limit growth in PIK3CA-mutated neoplasms through PI3K pathway suppression. We sought to determine if ASA could be beneficial for HNLM.
Methods:
Retrospective case series of patients (0–18 years) offered ASA (3–5 mg/kg/day) for HNLM treatment (2010–2018). Clinical and treatment characteristics, patient-reported symptom improvement, medication tolerance, compliance, and complications were recorded. Treatment response was determined by change in patient/caregiver-reported symptoms, or HNLM size [complete (resolved), partial (decreased), or stable].
Results:
Fifty-three patients were offered ASA, 23 (43%) accepted (median age 10 years, IQR 6–14). Compared to patients who declined, patients receiving ASA were more likely to have extensive malformations: ex-utero intrapartum treatment procedure, bilateral malformations, oral cavity location, ≥2 invasive treatments, or tracheotomy (p < 0.05). All patients with tissue available had PIK3CA mutations (13/23). Treatment indications included oral pain/blebs (12, 52%), recurrent pain/swelling (6, 26%), or sudden/persistent swelling (5, 22%). Treatment plan was commonly one 81 mg tablet daily (19, 83%) for 3–12 months (8, 42%). Therapeutic adherence was reported by 18 patients (78%). Symptoms improved in 18 patients [78%; decreased pain (9, 39%) and swelling (8, 35%)]. Treatment resulted in partial (14, 61%) or complete response (4, 17%). Three patients developed oral bleb bleeding, which resolved with medication discontinuation.
Conclusion:
ASA seems to be a well-tolerated, low-risk medication for HNLM treatment. This pilot study suggests that it often improves symptoms and reduces HNLM size. Further prospective, randomized studies are warranted to comprehensively assess indications, safety, and efficacy.
Level of evidence:
Level 4.
Keywords: Lymphatic malformation, Lymphatic abnormalities, Acetylsalicylic acid, PI3K, Treatment
1. Introduction
Management of head and neck lymphatic malformations (HNLM) in children is challenging. Observation, surgery and sclerotherapy are common treatments, but all have limitations based on the HNLM extent and degree of posttreatment persistence [1–5]. Lower stage malformations (De Serres I-III) that are localized and macrocystic can respond well to observation, surgery or sclerotherapy, but extensive, high stage, mixed macro-microcystic malformations often have unpredictable, incomplete treatment responses [3,4,6,7]. This leaves the HNLM patient with persistent dysfunction and untreated symptoms, such as sleep apnea, dysphagia, pain, bleeding and inflammation [4,6,7]. Recognition of the dilemma posed by incomplete and ineffective treatment of certain aspects of HNLM has led to further investigation into their etiology.
Lymphatic malformations (LM) consist of anomalous, dilated, lymphatic endothelial-lined spaces. DNA from affected tissue contains somatic gain-of-function mutations in phosphatidylinositol-4,5-bisphospate 3-kinase, catalytic subunit alpha (PIK3CA), a crucial component of the phosphatidylinositol 3-kinase (PI3K) pathway [8,9]. This pathway promotes cell growth and inhibits cell death (Fig. 1) [10]. Gain-of function PIK3CA mutations result in constitutive activation of the PI3K pathway producing overgrowth such as that seen in HNLM and other conditions [9,10].
Fig. 1.
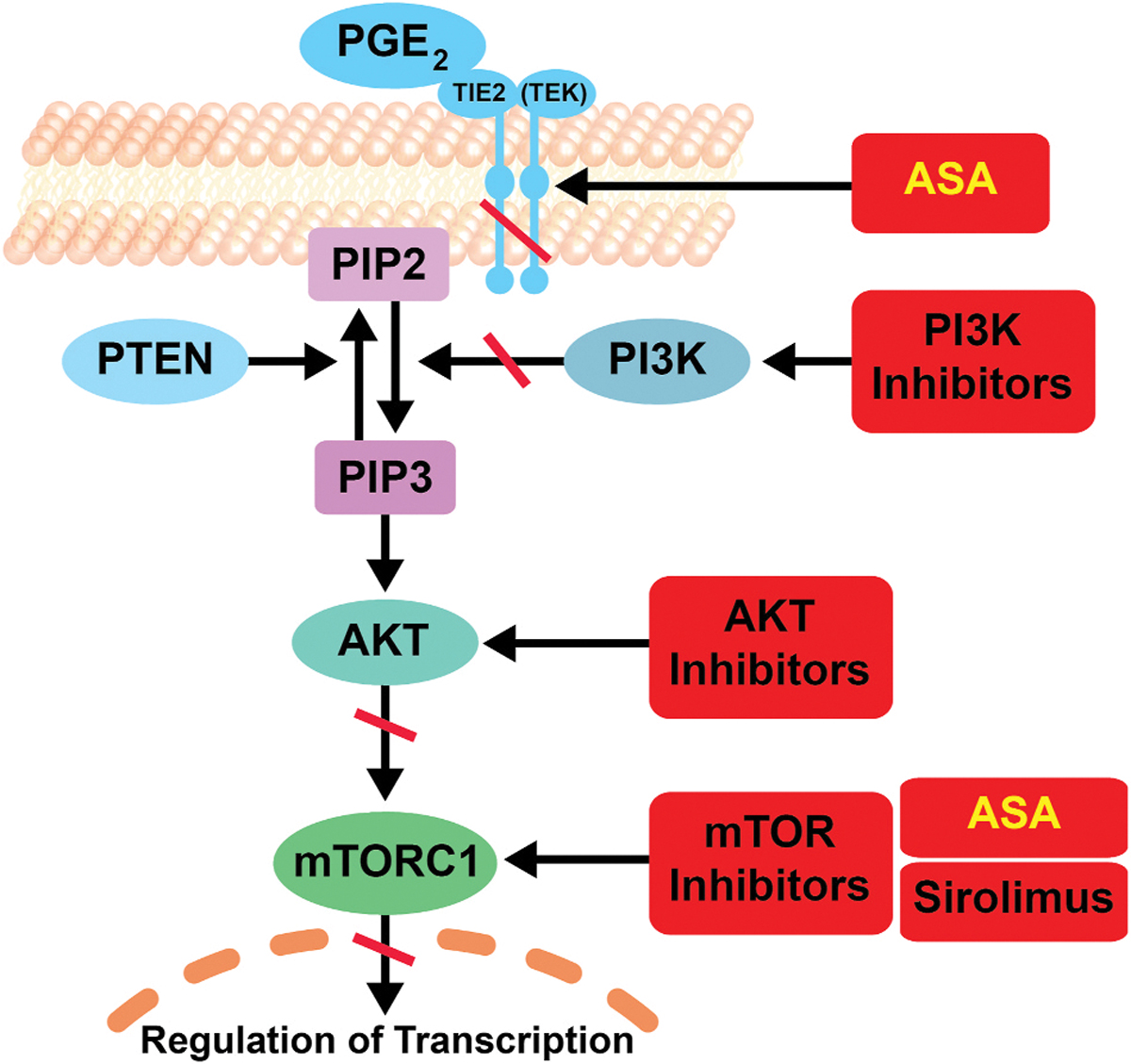
Proposed Mechanisms of Action of Acetylsalicylic Acid on the Phosphatidylinositol 3-kinase Pathway.
Lymphatic malformations are caused by somatic mutations in PIK3CA that lead to constitutive activation of the PI3K pathway. PI3K is a heterodimer comprised of the p85 regulatory subunit and p110 catalytic subunit, encoded by PIK3CA. The pathway is activated by signaling through the growth factor receptor tyrosine kinase on the cell membrane, which bind to and activate PI3K. PI3K is an enzyme that converts phosphatidylinositol-4,5- bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3) leading to the activation of AKT by phosphorylation. Then AKT, TSC1/2 and mTORC1 signaling mediate gene expression leading to the downstream effects of this pathway. Therapeutic targets in the pathway are illustrated in red.
Adapted from: Al-Olabi L, Polubothu S, Dowsett K et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest. 2018; 128 (11):5185.; Liao X, Lochhead P, Nishihara R et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012; 367 (17):1596–1606; Baron JA, Cole BF, Sandler RS et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003; 348 (10):891–899.; Din FV, Valanciute A, Houde VP et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012; 142 (7):1504–1515 e1503. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Downstream PI3K pathway suppression with sirolimus (rapamycin) has been used off-label in HNLM treatment (Fig. 1) [11]. Sirolimus inhibits mammalian target of rapamycin (mTOR), a component of the PI3K pathway, and has cytostatic, antiproliferative, and immunosuppressive properties [12–14]. While its exact mechanism of action in HNLM is unknown, Sirolimus is thought to reduce lymphangiogenesis and lymph leakage [11,13–15]. In HNLM, sirolimus treatment caused partial reduction in malformation size, improved mucosal/skin surface integrity, and reduced malformation pain, but significant adverse effects occurred in almost all patients [14,16]. Other agents that provide upstream PI3K pathway suppression are in use but have not been broadly applied to HNLM [17–20].
Acetylsalicylic acid (ASA or aspirin) is an anti-inflammatory medication and suppresses the PI3K pathway in two places: upstream through COX- 2/PGE-2 pathway inhibition, and downstream through reduction of mTOR signaling (Fig. 1) [21–23]. Epidemiologic and experimental studies report daily ASA use in patients with sporadic cancers and tumors containing PIK3CA mutations prevent new tumor growth, reduce tumor growth and metastasis and sometimes improves survival [24–27]. In children, low dose ASA is safely used as primary therapy in multiple pediatric diseases, such as maintenance dosing in Kawasaki disease [28], rheumatoid arthritis, and others [29–32]. It has been proposed as a prophylactic strategy in some hereditary cancer syndromes such as Lynch Syndrome and Constitutional Mismatch Repair Deficiency Syndrome [33–35]. The risk of Reye’s syndrome is limited to the use of ASA to treat acute febrile viral illnesses in children, and recent data suggest that susceptibility to Reye’s syndrome is determined by a detectable inherited metabolic disorder [36–38]. Given this evidence as well as the relatively small risk associated with ASA therapy, we hypothesized that ASA could be a beneficial low-risk targeted HNLM therapy. We describe our initial experience treating pediatric HNLM patients with ASA.
2. Materials and methods
A retrospective analysis was performed evaluating all HNLM patients who were offered off-label use of ASA for treatment (December 2010 to July 2018). The indication for treatment was having a symptomatic HNLM, manifested as oral pain or localized mucosal blebs; recurrent pain or swelling with upper respiratory infections (acute exacerbations); or sudden or persistent LM swelling. HNLM were diagnosed clinically, radiographically, and when available, histopathologically. Patients and/or caregivers were counselled regarding the genetic etiology of HNLM and available treatments, which included: observation, invasive therapy (i.e. surgery, sclerotherapy) or standard medical therapy (i.e. corticosteroids, antibiotics, sirolimus). Risks, benefits, alternatives and anticipated outcomes were discussed for each treatment. Additionally, HNLM patients were offered ASA therapy as an alternative to standard treatment, based on its anti-inflammatory effects and inhibition of the PI3K pathway [8]. Prior treatments did not influence the decision to offer ASA therapy. Patients with medical contraindications to ASA therapy, such as conditions that would predispose to bleeding, were not offered ASA treatment. ASA was prescribed following weight-based recommendations based on Kawasaki disease (3–5 mg/kg/day, rounded to half or whole 81 mg tablets). Duration of treatment was based on patients response and their willingness to continue the medicine. This study was approved by the Institutional Review Board at Seattle Children’s Hospital.
All patients offered ASA medical therapy for HNLM were included in the study and categorized by acceptance or refusal of ASA therapy. Demographic data collected included: clinical and previous treatment characteristics, sex, age at HNLM diagnosis, race/ethnicity, prenatal diagnosis, family history, history of ex-utero intrapartum treatment procedure (EXIT), De Serres Stage [39], HNLM site/laterality, presence of a tracheotomy, presence of lymphocytopenia, and known somatic PIK3CA mutation in lesion tissue when available from prior surgeries. Invasive treatment history included treatment type and number. For patients who declined ASA therapy, the treatment received after was recorded (observation, surgery, or sclerotherapy). Data obtained from the medical record and patient and family testimony specific to ASA therapy included: ASA dosage, duration of therapy, and medication adherence. Any adverse events that occurred during treatment with ASA were recorded. Medical treatment response was determined by parents and treating physician report, as well as evaluation of clinical photographs. The main outcome was treatment response determined by change in symptoms and size of the lesion as reported by the caregiver or the treating provider during the clinical exam, report on medical records, clinical photographs, and imaging when available. Response in size was categorized as complete, if there was no clinical evidence of HNLM, partial, if HNLM size reduction was noted, or stable, if no change in size was noted. Specific areas of improvement in symptoms as reported by the patient or their family attributed to ASA therapy were recorded, including changes in HNLM size/swelling, mucosal blebs, and lesional bleeding or pain. Patients were monitored as part of the standard of care with clinic visits every 3–6 months. Outcomes were assessed at the last clinic visit while on medication.
2.1. Statistical analysis
Demographic and clinical and treatment characteristics of patients who accepted ASA treatment were summarized descriptively and compared to patients who refused ASA therapy using Fisher’s exact test for categorical variables and Wilcoxon rank sums for continuous measures. Frequency and rate of patient-reported improvement, ASA adherence, and complications, as well as treatment response were reported for patients who accepted ASA treatment.
3. Results
Fifty-three patients were offered treatment with ASA of which 23 accepted and initiated therapy (43%, Table 1).
Table 1.
Description of Patients who were Offered Acetylsalicylic Acid Therapy.
| Characteristic | Accepted ASA n = 23 |
Declined ASA n = 30 |
p Value† | ||
|---|---|---|---|---|---|
| s | (%) | n | (%) | ||
|
| |||||
| EXIT | 4 | (17.4) | 0 | (0.0) | 0.033 |
| De Serres Stage | 0.004 | ||||
| Stage I | 3 | (13.0) | 15 | (50.0) | |
| Stage II | 11 | (47.8) | 11 | (36.7) | |
| Stage III | 3 | (13.0) | 4 | (13.3) | |
| Stage IV | 5 | (21.7) | 0 | (0.0) | |
| Stage V | 1 | (4.3) | 0 | (0.0) | |
| Site | 0.039 | ||||
| Oral Cavity | 7 | (30.4) | 2 | (6.7) | |
| Neck, Parotid and Parapharyngeal Space | 11 | (47.8) | 24 | (80.0) | |
| Midface and Upper Lip | 4 | (17.4) | 4 | (13.3) | |
| Orbit, Skull Base and Scalp | 1 | (4.3) | 0 | (0.0) | |
| Initial Treatment Observation | 6 | (26.1) | 13 | (43.3) | 0.054 |
| Primary Surgery | 6 | (26.1) | 13 | (43.3) | |
| Staged Surgery | 8 | (34.8) | 3 | (10.0) | |
| Primary Sclerotherapy | 1 | (4.3) | 1 | (3.3) | |
| Combined Sclerotherapy and Surgery | 2 | (8.7) | 0 | (0.0) | |
| Total Invasive Treatments | 0.015 | ||||
| None | 7 | (30.4) | 13 | (43.3) | |
| One | 3 | (13.0) | 12 | (40.0) | |
| 2–5 | 6 | (26.1) | 4 | (13.3) | |
| 6–10 | 3 | (13.0) | 1 | (3.3) | |
| >10 | 4 | (17.4) | 0 | (0.0) | |
| Tracheotomy | 6 | (26.1) | 0 | (0.0) | 0.004 |
Data for patients who accepted (n = 23) and declined (n = 30) acetylsalicylic acid treatment (N = 53).
Abbreviations: ASA: Acetylsalicylic Acid; EXIT: Ex-Utero Intrapartum Treatment Procedure.
p values calculated using Fisher’s exact.
3.1. Characteristics associated with willingness to trial ASA therapy
Patients willing to trial ASA therapy were more likely to have extensive/recalcitrant HNLM compared to those who declined ASA: history of EXIT procedure (p = 0.033), high De Serres stage (IV–V or bilateral distribution, p = 0.004), LM in the oral cavity (p = 0.039), treatment with staged surgeries (p = 0.054), ≥2 invasive treatments (p = 0.015), and tracheotomy (p = 0.004, Table 1). Additional clinical characteristics of our study population are described in the supplement (eTable 1).
3.2. ASA treatment plan
Medical treatment with ASA was initiated on 23 patients for painful mucosal LM related blebs (12, 52%), LM swelling or pain associated with respiratory tract inflammation (6, 26%), or for sudden or persistent neck swelling (5, 22%). The treatment plan was most frequently a single ASA tablet (81 mg) daily (19, 83%) for 3–12 months (8, 42%; Table 2). Eighteen patients reported adherence to treatment (78%). During therapy, of seven patients with LM involving their oral cavity, three patients had bleeding from oral cavity blebs, which resolved upon ASA discontinuation. Two patients were later restarted on ASA and did not have recurrence of bleeding.
Table 2.
Description of acetylsalicylic acid treatment plan.
| Characteristic | N | (%) |
|---|---|---|
|
| ||
| Dose | ||
| 0.5 baby aspirin† daily | 1 | (4) |
| 1 baby aspirin† daily | 19 | (83) |
| 1 baby aspirin† twice daily | 1 | (4) |
| 2 baby aspirin† daily | 2 | (9) |
| Duration | ||
| <3 Months | 7 | (37) |
| 3–12 Months | 8 | (42) |
| >12 Months | 4 | (21) |
| Unknown | 4 | (21) |
Data for patients who accepted Acetylsalicylic Acid Treatment (N = 23).
A “baby aspirin” is a 81 mg tablet of acetylsalicylic acid.
3.3. ASA treatment outcomes
There was improvement in the primary complaint that motivated initiation of ASA therapy for nine of twelve patients with oral blebs (75%), four of six patients with recurrent pain or swelling with respiratory tract inflammation (67%), and all five patients with sudden or persistent neck swelling (100%, Fig. 2A). Overall, 18 (78%) patients had improvement in their symptoms. These symptoms were swelling (14, 61%), pain (9, 39%), bleeding (4, 17%), and visible blebs (1, 4%). Illustrative cases of two patients with improvement in symptoms are presented in Fig. 2B. Due to the small number of patients treated, this study was underpowered to test associations between duration of therapy and treatment outcomes.
Fig. 2.
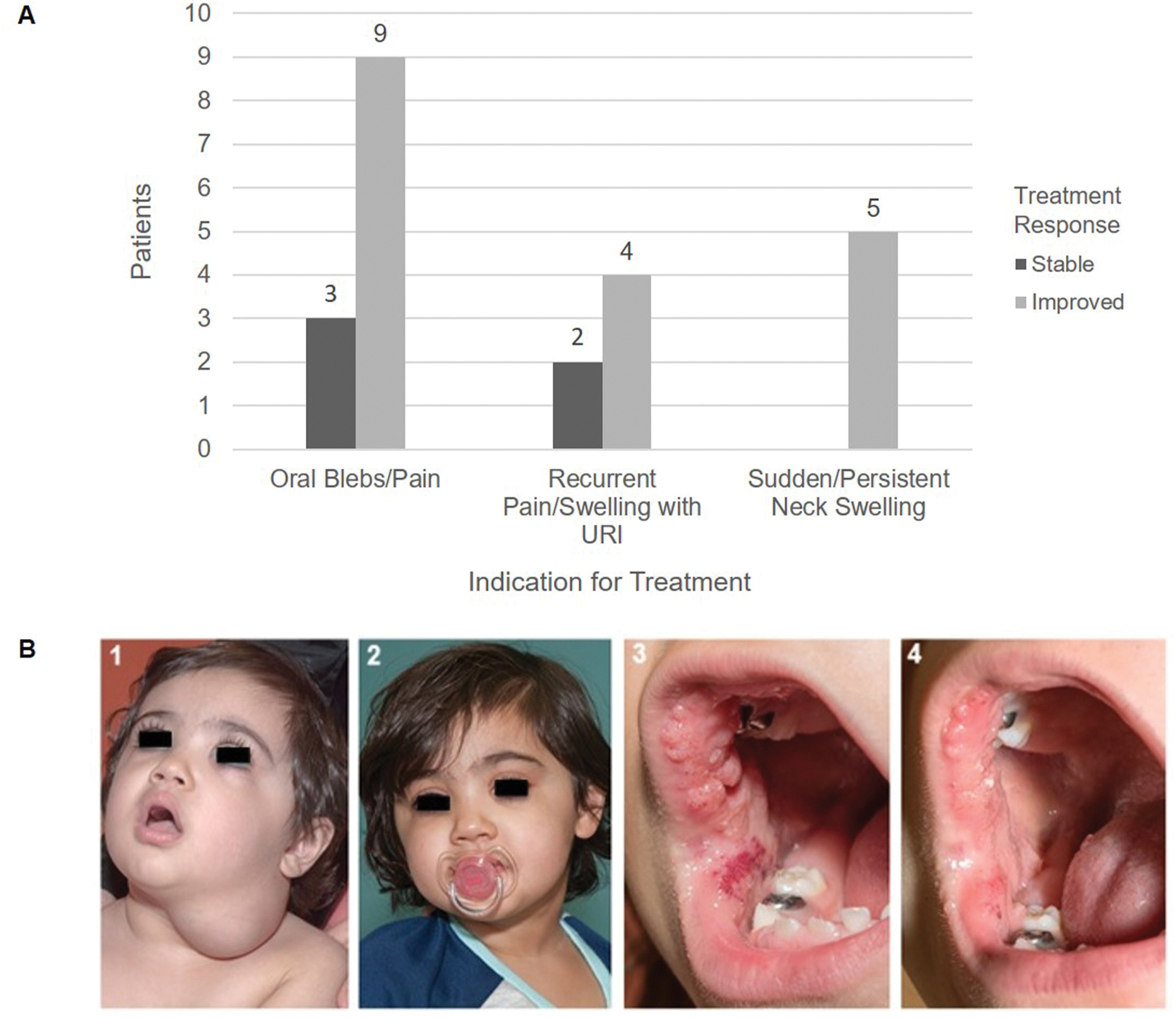
Response to Treatment with Acetylsalicylic Acid
(A) Primary Indication for Treatment with Acetylsalicylic Acid and Response at the Completion of Therapy: The primary complaint that motivated initiation of acetylsalicylic acid (ASA) therapy was recorded as the primary indication for treatment for each patient (x-axis). At the completion of therapy change in this symptom was recorded as stable, or improved. The histogram illustrates the number of patients who had improvement in their chief complaint using ASA and those who did not. No patients had worsening of their primary complaint during therapy.
(B) Clinical Improvement in Oral and Neck Lymphatic Malformations: (1): Two year old patient who presented with sudden neck swelling and limited range of motion. She was admitted for antibiotics. An MRI demonstrated an extensive stage III lymphatic malformation extending from the parotid to the mediastinum. At the first outpatient follow-up the mass was smaller and neck mobility was back to baseline (picture shown). She was started on ASA. (2): Patient at 6 months after initiating ASA with decreased size of the lesion. (3): Patient with life-long oral lesions that would become larger with an upper respiratory infection, and were sensitive to certain foods, but was otherwise asymptomatic. She has a PIK3CA p.E545K mutation. She was started on ASA at 6.5 years of age (picture shown). (4): Patient 4 months after initiation of ASA with decreased size and vascularity of the lesions.
The majority of patients had a partial response (14, 61%), four patients had resolution of their HNLM (17%), and none worsened. To ensure a genetically homogenous sample set we performed genetic testing when tissue was available. PIK3CA genotyping data was available for 13 patients and demonstrated somatic activating mutations in all patients tested. The genotypes are listed in Table 3. The majority of patients with hotspot mutations in PIK3CA showed benefit in terms of treatment response and improvement in symptoms (9, 69%). However, ASA was beneficial even in patients with HNLM who did not undergo genetic testing. Given the small sample size, statistical associations of genotype and treatment response were not tested [40].
Table 3.
Outcomes of acetylsalicylic acid treatment for head and neck lymphatic malformations by genotype.
| Treatment Response |
PIK3CA Genotype |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total N = 23 |
p.E542K n = 4 |
p.E545K n = 4 |
p.H1047R n = 4 |
p.Q546K n = 1 |
Unknown n = 10 |
|||||||
| N | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
|
| ||||||||||||
| Response in Size | ||||||||||||
| Complete | 4 | (17) | 1 | (25) | 1 | (25) | 0 | (0) | 0 | (0) | 2 | (20) |
| Partial | 14 | (61) | 0 | (0) | 3 | (75) | 4 | (100) | 0 | (0) | 7 | (70) |
| None | 5 | (22) | 3 | (75) | 0 | (0) | 0 | (0) | 1 | (100) | 1 | (10) |
| Improvement in Symptoms | 18 | (78) | 1 | (25) | 4 | (100) | 4 | (100) | 0 | (0) | 9 | (90) |
Treatment outcomes for patients who accepted Acetylsalicylic Acid (ASA) therapy (N = 23). Genotype data is presented for the 13 patients with tissue available for targeted PIK3CA sequencing. The mutations identified correspond to well known hotspot mutations, except for p, Q546K which is also an activating mutation although identified less frequently [40].
3.4. Treatment plan and outcomes for patients who declined ASA
Patients who declined ASA elected to continue observation (21, 70%) or have surgery (9, 30%). For patients observed, 5 low stage (I or II) lesions spontaneously regressed with complete response, 10 had a partial response, and 6 had no change. For patients treated surgically, 6 low stage lesions (I or II) were completely removed, and 3 had symptomatic malformation persistence. Treatment outcomes for patients who declined ASA therapy were not compared to those who accepted given the small sample size, retrospective nature of the study, lack of standardized follow-up and heterogeneity of patients, stage and treatments.
4. Discussion
This report on patient/family selected ASA therapy for HNLM treatment suggests that medical therapy could be an acceptable option for some patients, especially those with malformations that are extensive and in locations that were recalcitrant to prior invasive therapy. Patients were fully counseled about all HNLM management options, and 40% of patients chose to try ASA therapy. In this select patient series, ASA treatment seems to provide some degree of symptomatic and physical (i.e. lesion size reduction) benefit to 78% of patients. ASA therapy appears to be safe and well tolerated, with few reported side effects. Oral bleeding occurred in three of seven patients with oral cavity LM, but stopped with medication cessation.
The discovery of HNLM molecular genetic pathophysiology has furthered our understanding of their biology and can potentially explain the tissue overgrowth and persistence of these lesions, especially when they are extensive. Mosaic PIK3CA gain-of-function mutations in LM serve not only as diagnostic biomarkers but also identify a viable biologic therapeutic target (Fig. 1). The application of precision medicine principles and the emergence of targeted medical therapies to treat LM is a paradigm shift in the field of vascular anomalies. The role that medications will have in clinical practice is yet to be defined and will be informed by increased understanding of their efficacy in size reduction or improvements in symptoms or function, as we explored in this case series. Children who live with LM may experience chronic pain, mucosal blebs, ulceration, bleeding, infections, and sudden onset swelling. They may develop bony and soft tissue overgrowth, disfigurement and intermittent inflammation. In some instances, patients with HNLM have associated lymphocytopenia, coagulopathies, or compromise of their ability to breathe and feed, with significant impact on their quality of life. In other cases HNLM can exhibit spontaneous regression. The biology underlying the spectrum of severity in this disease is incompletely understood, but could be related to differential activation of the PI3K pathway, and thus medical therapies could provide benefit for children with LM [5,9,40–43]. We conceive that in small lesions ASA or other medications may serve to reduce the size, could induce or accelerate the process of spontaneous regression, and potentially result in resolution in some HNLM. In others, such as patients with extensive suprahyoid malformations with or without infrahyoid involvement, medical therapies could improve symptoms and function in a subset of patients in whom traditional treatments have been less successful [3,39,44].
Medical therapies that act at different levels of the PI3K pathway have been explored as treatment modalities for LM (Fig. 1). Sirolimus is the most commonly used agent in LM and other disorders of lymphangiogenesis [11,16]. Treatment outcomes for patients with head and neck involvement have been retrospectively examined in one case series on 19 patients with previously treated extensive symptomatic malformations who were either refractory to therapy or the family desired medical therapy prior to considering further procedures [16]. The authors report the improvement in lesion size (on imaging) was modest for 12/19 (63%, <20% reduction), moderate in 3/19 (16%, 20–50% reduction) and significant in 4/19 (21%, >50% reduction) patients. Improvements in symptoms were measured by reduction of mucosal blebs (14/14 patients) and decreased rates of infection (6/6 patients). However, 17/19 patients had one or several adverse reactions (headache, mouth sores, eczema, rash, acne, nausea, emesis, diarrhea, neutropenia, fatigue, irregular menses, alopecia, joint pain, cellulitis treated with oral or intravenous antibiotics, transaminitis, elevated cholesterol and triglycerides) with two patients having to stop treatment. No opportunistic infections were reported, but all patients received antibiotic prophylaxis for pneumocystis jiroveci [16]. Direct inhibition of other components of the PI3K pathway are being examined as medical therapies in children with mosaic activating PIK3CA mutations. These include the AKT inhibitor miransertib [45] (NCT03094832, NCT03317366) and the PIK3CA inhibitor alpeliisib (Fig. 1). Alpelisib (BYL719) showed reduction of PIK3CA-induced overgrowth and reversal of other physiologic alterations in these conditions [17]. Alpelisib is being offered to some PIIK3CA positive LM through compassionate use protocols (NCT03941782). These targeted therapies are not FDA approved for non-malignant PIK3CA induced conditions, but potentially could be used to treat LM. In the meantime ASA could be a reasonable medical treatment option for selected PIK3CA positive HNLM, as it has been helpful in some PIK3CA positive sporadic neoplasia [21–23]. Given that LM exhibit activation of the PI3K pathway, a medication that suppresses the pathway both upstream through COX-2/PGE-2 inhibition, and downstream through reduction of mTOR signaling could be effective for this disease (Fig. 1) [21–23]. Along with PI3K pathway suppression ASA could reduce inflammation which frequently causes HNLM symptoms that are often managed with antibiotics and corticosteroids. It is possioble that the some of the improvements seen in the study cohort were due to inflammation reduction [46–48]. Further studies are needed to clarify the mechanism for ASA’s effect on LM.
This study’s limitations include the retrospective nature, lack of randomization, patients self-selecting to each group, lack of validated patient or caregiver reported outcome measures, no systematic method to assess for medication adherence, and a small sample size. As an initial pilot study on a rare disease, data on 23 patients receiving a novel, low-cost, well-tolerated, FDA-approved medication that appears to be beneficial is encouraging. Further studies would be improved through randomization, blinding to medication vs. placebo, structured measurement of treatment outcomes, and stratification of the study cohort by disease severity.
5. Conclusion
Low dose ASA seems to be well-suited for treatment of some HNLM. Cited work shows that ASA therapy has an effect on PIK3CA-related tumors and their progression [21–27,49]. The biologic mechanism for this effect is being investigated but appears to be through inhibition of COX2, PGE2 and mTOR signaling (Fig. 1) [21,25,27,50]. ASA therapy is commonly used to treat a wide spectrum of pediatric conditions, has less toxicicity than other medical therapies in common use, and could be applied to HNLM that are also caused by activating PIK3CA mutations. We speculate that ASA’s therapeutic effect may occur via PI3K pathway suppression, but further research is needed to investigate this. This report provides initial data that suggests that ASA is a safe, well-tolerated medical therapy for HNLM. Although these results are encouraging, studies to substantiate these findings are needed. We hope that this initial study will provide testable parameters that support a prospective randomized treatment trial that examines the efficacy of ASA treatment for HNLM.
Supplementary Material
Acknowledgements
The authors would like to thank Eden Palmer for her assistance with preparation of the figures. Kaitlyn Zenner was supported by The National Heart, Lung, and Blood Institute (PI: Kaitlyn Zenner; F32 HL147398) and The National Institute on Deafness and Other Communication Disorders via the University of Washington Otolaryngology Research Training Program (PI: Dr. Jennifer Stone, PhD; T32 DC000018). Lymphatic malformation genotyping was supported by 1RO1 HL103996 from the National Heart, Lung, and Blood Institute (PI: William B. Dobyns).
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Financial disclosure statement
Dr. Randall Bly is co-founder and holds a financial interest of ownership equity with Edus Health, Inc and EigenHealth, Inc. He is Consultant and stock holder, Spiway, LLC. These are not related to this study. All other authors do not have information to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijporl.2021.110869.
References
- [1].Adams MT, Saltzman B, Perkins JA, Head and neck lymphatic malformation treatment: a systematic review, Otolaryngol. Head Neck Surg. 147 (2012) 627–639. [DOI] [PubMed] [Google Scholar]
- [2].Balakrishnan K, Menezes MD, Chen BS, Magit AE, Perkins JA, Primary surgery vs primary sclerotherapy for head and neck lymphatic malformations, JAMA Otolaryngol Head Neck Surg 140 (2014) 41–45. [DOI] [PubMed] [Google Scholar]
- [3].Bonilla-Velez J, Moore BP, Cleves MA, Richter GT, Surgical resection of macrocystic lymphatic malformations of the head and neck: short and long-term outcomes, Int. J. Pediatr. Otorhinolaryngol. 134 (2020) 110013. [DOI] [PubMed] [Google Scholar]
- [4].Richter GT, Friedman AB, Hemangiomas and vascular malformations: current theory and management, Int. J. Pediatr. 2012 (2012) 645678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perkins JA, Maniglia C, Magit A, Sidhu M, Manning SC, Chen EY, Clinical and radiographic findings in children with spontaneous lymphatic malformation regression, Otolaryngol. Head Neck Surg. 138 (2008) 772–777. [DOI] [PubMed] [Google Scholar]
- [6].Renton JP, Smith RJ, Current treatment paradigms in the management of lymphatic malformations, Laryngoscope 121 (2011) 56–59. [DOI] [PubMed] [Google Scholar]
- [7].Perkins JA, Manning SC, Tempero Rm, et al. , Lymphatic malformations: review of current treatment, Otolaryngol. Head Neck Surg. 142 (2010) 795–803, 803 e791. [DOI] [PubMed] [Google Scholar]
- [8].Wassef M, Blei F, Adams D, et al. , Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies, Pediatrics 136 (2015) e203–214. [DOI] [PubMed] [Google Scholar]
- [9].Luks VL, Kamitaki N, Vivero Mp, et al. , Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA, J. Pediatr. 166 (2015), 1048–1054 e1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Karakas B, Bachman KE, Park BH, Mutation of the PIK3CA oncogene in human cancers, Br. J. Canc. 94 (2006) 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adams DM, Trenor CC 3rd, Hammill AM, et al. , Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies, Pediatrics 137 (2016), e20153257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morris RE, Rapamycins: antifungal, antitumor, antiproliferative, and immunosuppressive macrolides, Transplant. Rev (1992) 39–87. [Google Scholar]
- [13].Sehgal SN, Sirolimus: its discovery, biological properties, and mechanism of action, Transplant. Proc. 35 (2003) 7S–14S. [DOI] [PubMed] [Google Scholar]
- [14].Wiegand S, Wichmann G, Dietz A, Treatment of lymphatic malformations with the mTOR inhibitor sirolimus: a systematic review, Lymphatic Res. Biol. 16 (2018) 330–339. [DOI] [PubMed] [Google Scholar]
- [15].MacFarland SP, Sullivan LM, States LJ, et al. , Management of refractory pediatric kaposiform hemangioendothelioma with sirolimus and aspirin, J. Pediatr. Hematol. Oncol. 40 (2018) e239–e242. [DOI] [PubMed] [Google Scholar]
- [16].Strychowsky JE, Rahbar R, O’Hare MJ, Irace AL, Padua H, Trenor CC 3rd, Sirolimus as treatment for 19 patients with refractory cervicofacial lymphatic malformation, Laryngoscope 128 (2018) 269–276. [DOI] [PubMed] [Google Scholar]
- [17].Venot Q, Blanc T, Rabia SHet al, Targeted therapy in patients with PIK3CA-related overgrowth syndrome, Nature 558 (2018) 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Parker VER, Keppler-Noreuil KM, Faivre L, et al. , Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum, Genet. Med. 21 (2019) 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Perkins JA, New frontiers in our understanding of lymphatic malformations of the head and neck: natural history and basic research, Otolaryngol. Clin 51 (2018) 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Padia R, Zenner K, Bly R, Bennett J, Bull C, Perkins J, Clinical application of molecular genetics in lymphatic malformations, Laryngoscope Investigative Otolaryngology 4 (2019) 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Din FV, Valanciute A, Houde Vp, et al. , Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells, Gastroenterology 142 (2012) 1504–1515 e1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaur J, Sanyal SN, PI3-kinase/Wnt association mediates COX-2/PGE(2) pathway to inhibit apoptosis in early stages of colon carcinogenesis: chemoprevention by diclofenac, Tumour Biol 31 (2010) 623–631. [DOI] [PubMed] [Google Scholar]
- [23].Uddin S, Ahmed M, Hussain A, et al. , Cyclooxygenase-2 inhibition inhibits PI3K/AKT kinase activity in epithelial ovarian cancer, Int. J. Canc 126 (2010) 382–394. [DOI] [PubMed] [Google Scholar]
- [24].Algra AM, Rothwell PM, Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials, Lancet Oncol. 13 (2012) 518–527. [DOI] [PubMed] [Google Scholar]
- [25].Baron JA, Cole BF, Sandler Rs, et al. , A randomized trial of aspirin to prevent colorectal adenomas, N. Engl. J. Med. 348 (2003) 891–899. [DOI] [PubMed] [Google Scholar]
- [26].Dube C, Rostom A, Lewin G, et al. , The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force, Ann. Intern. Med. 146 (2007) 365–375. [DOI] [PubMed] [Google Scholar]
- [27].Liao X, Lochhead P, Nishihara R, et al. , Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival, N. Engl. J. Med. 367 (2012) 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zheng X, Yue P, Liu L, et al. , Efficacy between low and high dose aspirin for the initial treatment of Kawasaki disease: current evidence based on a meta-analysis, PloS One 14 (2019), e0217274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Karimi M, Haghpanah S, Pishdad P, Zahedi Z, Parand S, Safaei S, Frequency of silent brain lesions and aspirin protection evaluation over 3 years follow-up in beta thalassemia patients, Ann. Hematol. 98 (2019) 2267–2271. [DOI] [PubMed] [Google Scholar]
- [30].Walsh S, Knofler R, Hahn G, et al. , Childhood primary large vessel CNS vasculitis: single-centre experience and review of the literature, Clin. Exp. Rheumatol. 35 (Suppl 103) (2017) 213–220. [PubMed] [Google Scholar]
- [31].Schmugge M, Speer O, Kroiss S, et al. , Monitoring aspirin therapy in children after interventional cardiac catheterization: laboratory measures, dose response, and clinical outcomes, Eur. J. Pediatr. 174 (2015) 933–941. [DOI] [PubMed] [Google Scholar]
- [32].Monagle P, Cochrane A, Roberts R, et al. , A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children, J. Am. Coll. Cardiol. 58 (2011) 645–651. [DOI] [PubMed] [Google Scholar]
- [33].Israels SJ, Michelson AD, Antiplatelet therapy in children, Thromb. Res. 118 (2006) 75–83. [DOI] [PubMed] [Google Scholar]
- [34].Wood LE, Tulloh RM, Kawasaki disease in children, Heart 95 (2009) 787–792. [DOI] [PubMed] [Google Scholar]
- [35].Leenders E, Westdorp H, Bruggemann RJ, et al. , Cancer prevention by aspirin in children with constitutional Mismatch Repair deficiency (CMMRD), Eur. J. Hum. Genet. 26 (2018) 1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Beutler AI, Chesnut GT, Mattingly JC, Jamieson B, Fpin’s Clinical Inquiries, Aspirin use in children for fever or viral syndromes, Am. Fam. Physician 80 (2009) 1472. [PubMed] [Google Scholar]
- [37].Schror K, Aspirin and Reye syndrome: a review of the evidence, Paediatr Drugs 9 (2007) 195–204. [DOI] [PubMed] [Google Scholar]
- [38].Food Drug Administration Hhs, Labeling for oral and rectal over-the-counter drug products containing aspirin and nonaspirin salicylates; Reye’s Syndrome warning. Final rule, Fed. Regist 68 (2003) 18861–18869. [PubMed] [Google Scholar]
- [39].de Serres LM, Sie KC, Richardson MA, Lymphatic malformations of the head and neck. A proposal for staging, Arch. Otolaryngol. Head Neck Surg. 121 (1995) 577–582. [DOI] [PubMed] [Google Scholar]
- [40].Zenner K, Cheng CV, Jensen DM, et al. , Genotype correlates with clinical severity in PIK3CA-associated lymphatic malformations, JCI Insight 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Balakrishnan K, Bauman N, Chun RH, et al. , Standardized outcome and reporting measures in pediatric head and neck lymphatic malformations, Otolaryngol. Head Neck Surg. 152 (2015) 948–953. [DOI] [PubMed] [Google Scholar]
- [42].Kirkham EM, Edwards TC, Weaver EM, Balakrishnan K, Perkins JA, The lymphatic malformation function (LMF) instrument, Otolaryngol. Head Neck Surg. 153 (2015) 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Padia R, Zenner K, Bly R, Bennett J, Bull C, Perkins J, Clinical application of molecular genetics in lymphatic malformations, Laryngoscope Investig Otolaryngol 4 (2019) 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bonilla-Velez Jw, K. B., Ganti S, Zenner K, et al. Active Observation as an Alternative to Invasive Treatments for Pediatric Head and Neck Lymphatic Malformations. [DOI] [PubMed]
- [45].Biesecker LG, Edwards M, O’Donnell S, et al. , Clinical report: one year of treatment of Proteus syndrome with miransertib (ARQ 092), Cold Spring Harb Mol Case Stud 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kim D, Benjamin L, Wysong A, Hovsepian D, Teng J, Treatment of complex periorbital venolymphatic malformation in a neonate with a combination therapy of sirolimus and prednisolone, Dermatol. Ther 28 (2015) 218–221. [DOI] [PubMed] [Google Scholar]
- [47].Harsha WJ, Crawford JV, Sorensen DM, An unusual case of adult airway obstruction from a lymphovenous malformation, Ear Nose Throat J. 87 (2008) 402–404. [PubMed] [Google Scholar]
- [48].Levy G, Clinical pharmacokinetics of aspirin, Pediatrics 62 (1978) 867–872. [PubMed] [Google Scholar]
- [49].Burn J, Gerdes AM, Macrae F, et al. , Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial, Lancet 378 (2011) 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Al-Olabi L, Polubothu S, Dowsett K, et al. , Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy, J. Clin. Invest. 128 (2018) 5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


