Abstract
A 67-year-old man with a history of chest radiotherapy and severe aortic valve stenosis with calcification of the ascending aortic wall underwent implantation of an apicoaortic conduit from the left ventricular apex to the descending aorta. Eight years later, he presented with progressive exertional dyspnea. Imaging revealed severe native aortic valve insufficiency and calcification, with worsening left ventricular function. We decided to leave the apicoaortic conduit intact and perform transcatheter aortic valve replacement with a balloon-expandable prosthesis. Despite concerns that eliminating the obstruction across the native left ventricular outflow tract might decrease conduit flow and eventually cause graft thrombosis and peripheral embolization, we elected to move forward after a multidisciplinary discussion. The procedure resulted in angiographically and qualitatively similar forward flow across the newly implanted prosthesis and the existing apicoaortic conduit, with no hemodynamic or electrical dysfunction. The patient was discharged from the hospital the next day. At the 1-month follow-up visit, the patient felt well and reported marked functional improvement, with minimal symptoms during moderate to heavy exertion. The stroke volume index across the new bioprosthetic valve was low (13 mL/m2 at 1 mo and 18 mL/m2 at 1 y), suggesting that a substantial amount of blood was still exiting the ventricle through the left ventricle-to-aorta conduit. This report offers some guidance for treating patients with existing apicoaortic conduits and suggests that transcatheter aortic valve replacement is safe and effective if native aortic valve insufficiency develops.
Keywords: Aortic valve insufficiency, aortic regurgitation, left ventricle to aorta conduit, aortic valve stenosis, transcatheter aortic valve replacement
Before the advent of transcatheter aortic valve replacement (TAVR)—now a well-established alternative to surgical replacement of severely stenotic aortic valves (AVs)—patients with severe AV disease and problematic anatomic features (eg, previous sternotomy, previous chest radiation therapy, severe calcification of the ascending aorta) were difficult to treat surgically. In the early 1960s, a technique was developed to bypass the AV and a severely calcified aortic root and arch by implanting a conduit from the left ventricular (LV) apex to the aorta.1 This apicoaortic conduit included a bioprosthetic valve to prevent regurgitation of blood into the LV. Although successful, the technique fell into disuse after TAVR was introduced. Nevertheless, it remains unclear how best to treat patients with an apicoaortic conduit if further valvular or conduit dysfunction develops.2 We present the case of a patient with an LV-to-aorta conduit and a dysfunctional AV treated for significant insufficiency. We found no mention of this clinical scenario in existing treatment.
Case Report
A 67-year-old man presented at our clinic with worsening heart failure symptoms, most notably marked dyspnea on exertion, 8 years after undergoing implantation of a conduit from the LV apex to the mid descending aorta. He was found to have new LV dysfunction, quantified as an LV ejection fraction (LVEF) of 45%. The patient was prescribed guideline-directed medical therapy for heart failure and intermediate reduced LVEF. However, at successive follow-up visits over the next year, his symptoms worsened.
Medical History
The patient had undergone chest radiation therapy in 1981 to treat a mediastinal tumor and had aortocoronary bypass surgery in 1995 to treat coronary artery disease. In 2010, he was diagnosed with severe aortic stenosis and severe calcification of the ascending aortic wall, which made open AV replacement a prohibitively high-risk procedure. Instead, the patient underwent left thoracotomy and insertion of a conduit from the LV apex to the mid descending aorta; the conduit contained a 20-mm bioprosthetic valve. A postoperative echocardiogram showed normal LV function. The patient recovered well from the surgery and was able to return to his usual daily activities.
Current Presentation
At the current presentation, the differential diagnosis for the patient's worsening dyspnea included severe symptomatic regurgitation of the native AV that caused LV dysfunction, apicoaortic conduit flow obstruction, and coronary artery bypass graft stenosis. Pulmonary causes of dyspnea also were considered.
Echocardiograms obtained during a follow-up visit revealed severe native AV regurgitation, a reduction in LVEF to between 35% and 39%, and an increase in the LV end-diastolic volume index to 99 mL/m2. Pulsed-wave Doppler echocardiograms indicated a stroke volume index (SVi) across the native AV of 39 mL/m2. The native AV was calcified, consistent with previous severe aortic stenosis. Flow across the apicoaortic conduit could not be measured reliably, because of limitations in angulation and image acquisition.
A computed tomographic angiogram of the chest showed a vascular anatomy favorable for TAVR and the apicoaortic conduit's anatomy (Fig. 1). An Agatston score of 6,000 Hounsfield units calculated for the native AV confirmed severe calcification. Coronary angiograms showed patent coronary artery bypass grafts and no significant new native coronary artery stenosis. During the coronary angiographic procedure, a Swan-Ganz catheter deployed inside the apicoaortic conduit revealed no pressure gradient across the prosthetic valve.
Fig. 1.
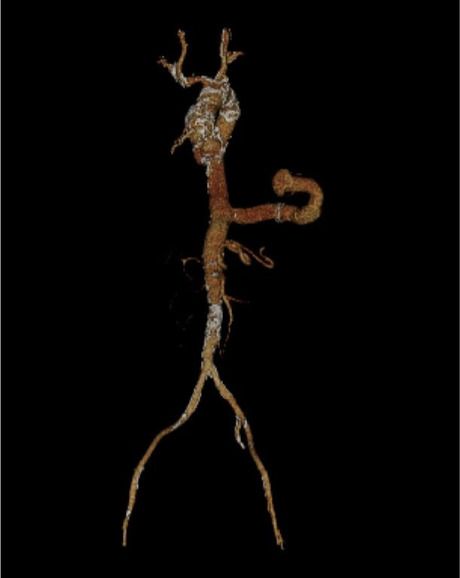
Three-dimensional, reconstructed computed tomographic angiogram reveals the anatomy of the apicoaortic conduit, with inflow through the left ventricle and outflow through the mid descending aorta. Delineating the conduit's anatomy in this way is crucial in planning for transcatheter aortic valve replacement.
Management
A multidisciplinary team discussed the best course of treatment for this patient, given concerns about the potential hemodynamic and thrombotic ramifications of eliminating native valve disease in the presence of an intact valved conduit. The team included a cardiothoracic surgeon, a heart failure specialist, a cardiac imaging specialist, and 2 interventional cardiologists. In consultation with the patient, we decided to leave the apicoaortic conduit intact and perform TAVR through a femoral approach. An aortogram of the aortic root confirmed severe aortic regurgitation (Fig. 2). A balloon-expandable 26-mm Edwards Sapien 3 AV prosthesis (Edwards Lifesciences Corp) was then implanted. A subsequent left ventriculogram showed forward flow across both the newly implanted prosthesis and the existing apicoaortic conduit (Fig. 3). No hemodynamic or electrical dysfunction developed during or after the operation. The patient was discharged from the hospital the day after TAVR and was prescribed an empiric regimen of warfarin.
Fig. 2.
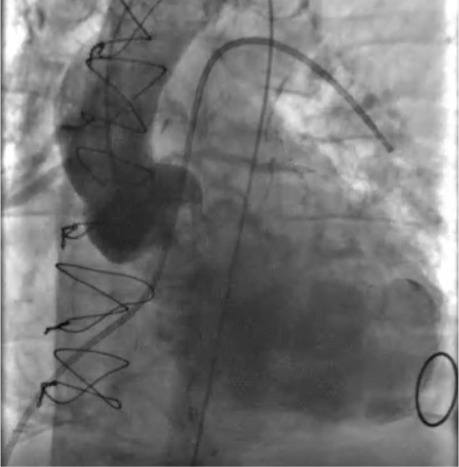
Left aortogram obtained before transcatheter aortic valve replacement (TAVR) shows severe aortic regurgitation and moderately reduced global left ventricular (LV) function. A Swan-Ganz catheter is shown in the “wedged” position. In patients with reduced LV function, evaluating both right-sided and left-sided hemodynamics before TAVR can facilitate planning for possible mechanical circulatory support or cardiovascular surgical backup and clarify the patient's risk-benefit profile.
Supplemental motion image is available for Figure 2.
Fig. 3.
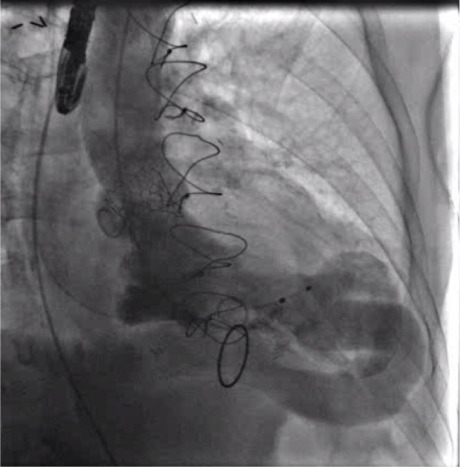
Left ventriculogram obtained immediately after transcatheter aortic valve replacement shows forward systolic flow through the apicoaortic conduit and through the newly deployed prosthetic aortic valve. Note also the forward diastolic flow through the conduit and lack of any substantial aortic regurgitation. Forward systolic flow is mainly through the conduit, which, therefore, is likely to remain patent. Given the sufficient forward flow through the conduit, we saw no need for endovascular plugging or surgical closure of the conduit.
Supplemental motion image is available for Figure 3.
Follow-Up
At the first follow-up visit 1 month after TAVR, the patient felt well and reported marked improvement in functional status, with minimal symptoms during moderate to heavy exertion. An echocardiogram revealed an LVEF of 45% to 49% and normal function of the newly implanted bioprosthetic AV, with a mean systolic gradient of 4 mm Hg and a dimensionless index of 0.39. The LV end-diastolic volume index had decreased to 82 mL/m2. Of note, the SVi across the new bioprosthetic valve was 13 mL/m2. Because we expected this value to be higher in an asymptomatic patient with normal functional capacity, we inferred that a significant amount of blood was still exiting the LV through the apicoaortic conduit.
At the second follow-up visit 1 year after TAVR, the patient continued to feel well, with only mild dyspnea during moderate exertion. Repeated echocardiograms showed further improvement in his LVEF to 50% to 55%, no substantial change in LV size, and normal bioprosthetic AV function, with a mean systolic gradient of 4 mm Hg and a dimensionless index of 0.41. The SVi across the aortic bioprosthesis remained low at 18 mL/m2, suggesting that the apicoaortic conduit remained patent.
Discussion
Our complex approach required multidisciplinary discussion and input to safely treat a dysfunctional, significantly insufficient AV in this patient with an existing apicoaortic conduit. There was concern that eliminating the obstruction across the native LV outflow tract might decrease flow across the conduit and eventually cause graft thrombosis and peripheral embolization. Endovascular plugging or surgical closure of the conduit during TAVR was considered. However, because the patient already had some forward flow across the native AV and the conduit remained patent, we opted for simplicity and performed TAVR only. We were prepared to initiate extracorporeal membrane oxygenation with mechanical support had cardiovascular collapse occurred. Forward flow across the patient's AV decreased after TAVR, as indicated by the SVi measured echocardiographically. This suggests that the patient's clinical improvement and favorable structural LV changes were probably related to the elimination of aortic regurgitation in an LV with limited functional reserve. Therefore, we think most of the patient's LV blood volume was probably being ejected through the conduit rather than through the new bioprosthetic AV.
The notable lack of change in forward flow across the LV outflow tract between the patient's 1-month and 1-year postoperative follow-up visits implies that thrombosis of the conduit and subsequent peripheral embolization after TAVR are unlikely. Still, the optimal duration of anticoagulation in our patient is unknown. We plan to continue anticoagulation indefinitely unless bleeding complications arise.
An important consideration, should our patient's valved apicoaortic conduit become dysfunctional, is to anticipate next steps. Stenosis of the conduit valve should not present a problem, given that TAVR resulted in unobstructed flow across the native AV. However, if the conduit valve were to degenerate and cause severe regurgitation, the patient could once again experience negative consequences of LV volume loading, in which case the simplest solution would be to occlude the valved conduit endovascularly. It might be feasible to place a new percutaneous valve inside the graft; however, this probably would not be needed and so would also avoid the unnecessary risk posed by implanting a large-bore device.
Conclusion
Now that TAVR is an established therapy for AV disease, the likelihood of having to implant an apicoaortic conduit is small. Nonetheless, as this case shows, the development of substantial native AV insufficiency in patients with existing apicoaortic conduits can be safely and effectively treated with TAVR.
Supplementary Material
Acknowledgments
Jeanie F. Woodruff, BS, ELS, of the Scientific Publications and Grants Department at the Texas Heart Institute, contributed to the editing of the manuscript.
Funding Statement
Funding/Support: None
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Renzulli A, Gregorio R, De Feo M, Ismeno G, Covino FE, Cotrufo M. Long-term results of apico-aortic valved conduit for severe idiopathic hypertrophic subaortic stenosis. Tex Heart Inst J . 2000;27(1):24–28. [PMC free article] [PubMed] [Google Scholar]
- 2.Inohara T, Vemulapalli S, Kohsaka S et al. Appropriateness of transcatheter aortic valve replacement: insight from the OCEAN-TAVI registry. Circ Cardiovasc Qual Outcomes . 2020;13(4):e006146. doi: 10.1161/CIRCOUTCOMES.119.006146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


