Abstract
Pregnancy in women with hypertrophic cardiomyopathy is not well described. In this retrospective study, we analyzed data on pregnant women with hypertrophic cardiomyopathy who were under follow-up care in the cardiology department of a tertiary care hospital. We reviewed data on all women registered in the hypertrophic cardiomyopathy cohort and those who attended the cardio-obstetric clinic and delivered between January 2010 and June 2019. From these 2 groups, we identified 7 pregnant women with hypertrophic cardiomyopathy who delivered during this period. These 7 women (mean [SD] age, 25 [3.3] years) had a total of 15 pregnancies (range per woman,1–4). This was a high-risk cohort, as 7 (46.7%) pregnancies were in the modified World Health Organization class III. The mean (SD) left ventricular wall thickness was 19.71 (2.56) mm in all pregnancies. Two of the 7 women with left ventricular outflow tract obstruction developed severe symptoms in the third trimester; these improved soon after delivery. Eight pregnancies without obstruction were well tolerated. Two pregnancies occurred after successful alcohol septal ablation. Both remained asymptomatic throughout pregnancy. All women tolerated labor well. Adverse maternal outcomes, including death, were not seen in any patient. All women who became symptomatic during pregnancy had relief of symptoms after delivery. Most women remained asymptomatic or had mild symptoms during pregnancy. Of the women with left ventricular outflow tract obstruction, 28.6% had severe symptoms that improved after delivery. Pregnancy was well tolerated after successful alcohol septal ablation.
Keywords: Cardiomyopathy, hypertrophic; ventricular outflow tract obstruction; therapy; pregnancy
Hypertrophic cardiomyopathy is a genetic disorder that has an autosomal dominant mode of transmission. The prevalence of hypertrophic cardiomyopathy (HCM) in the general population is 1 in 500; therefore, a similar incidence in pregnant women is probable.1 There are only a few large studies published about pregnant women with HCM.2–4 Most have been small case series.5–9 We have previously reported that of 2,016 women registered in our cardio-obstetric clinic over a 22-year period, only 4 women had HCM.5–9 In this study, pregnancy was tolerated in all subgroups except those with restrictive physiology. These small numbers indicate that HCM is often not diagnosed because most women remain asymptomatic and do not seek medical attention. However, the increased use of echocardiography may help identify more patients with HCM.
Hypertrophic cardiomyopathy is usually well tolerated in pregnancy. Most patients fall in the class II group of the modified World Health Organization (WHO) maternal cardiovascular risk scheme.2 However, a subgroup of women with HCM may be at higher risk of adverse pregnancy outcomes. Women with severe left ventricular outflow tract obstruction (LVOTO), symptomatic arrhythmias, and moderate systolic left ventricular dysfunction are in the higher-risk class III group of the modified WHO scheme.2 In addition, the small minority of pregnant women with severe systolic left ventricular dysfunction or severe symptomatic LVOTO have a very high pregnancy risk. These women would be placed in the modified WHO class IV group in whom pregnancy is contraindicated.2 Women who have undergone alcohol septal ablation are not included in the WHO classification scheme, because of a lack of data.
A cohort of patients with HCM is maintained and patients followed up in our cardiology department for last 15 years. We report the clinical profile and outcomes of pregnancies in women with HCM in our hospital.
Patients and Methods
In this retrospective analysis, we collected clinical, imaging, management, and follow-up data on patients from the HCM registry maintained in the cardiology department. Obstetric history was also recorded. In addition, we obtained data on all women with HCM who were registered in the cardio-obstetric clinic and who delivered between January 2010 and June 2019. The diagnostic criterion for HCM was unexplained left ventricle hypertrophy of at least 13 mm or greater than 2 SDs as corrected for body surface area by echocardiography.1 Patients with any disorder that could produce that magnitude of left ventricular hypertrophy, including aortic stenosis and systemic hypertension, were excluded from our study. The study was approved by the Postgraduate Institute of Medical Education and Research ethics committee.
Clinical data, mode of delivery, weeks' gestation at delivery, and antenatal, intrapartum, and postpartum complications were noted. The modified WHO class, the severity of left ventricular hypertrophy, the presence of LVOTO, and previous procedures or arrhythmias were also noted. Significant and marked LVOTO were defined as gradients, either at rest or with provocation, of at least 30 mm Hg and at least 50 mm Hg, respectively.10
Results
We identified 7 women (mean [SD] age, 25 [3.3] years) who had HCM and who had a total of 15 pregnancies (range per woman, 1–4) during the study period. All 7 women were part of the cardiology department's HCM cohort, and 3 were registered in the cardio-obstetric clinic. The most common symptoms were exertional breathlessness and palpitations. Only 1 patient (patient 3) had a syncopal attack during pregnancy. Mean (SD) left ventricular wall thickness was 19.71 (2.56) mm. Left ventricular outflow tract obstruction was present in 7 pregnancies (Fig. 1), whereas nonobstructive HCM occurred during 8 pregnancies. Arrhythmias, including atrial fibrillation, were not recorded in any patient. Detailed clinical characteristics and pregnancy outcomes of all 7 patients are presented in Table I. All patients tolerated labor well with no aggravation of symptoms. One patient (patient 4) developed progressive breathlessness, chest pain, and palpitations (New York Heart Association class III) after her first pregnancy. She underwent successful alcohol septal ablation with abolition of the gradient 2 years after her first pregnancy (Fig. 2). The patient remained asymptomatic during 2 subsequent pregnancies. Only 1 patient (patient 6) had a restrictive filling pattern on echocardiography during pregnancy; however, the pattern was transient and was not seen on postdelivery echocardiograms.
Fig. 1.
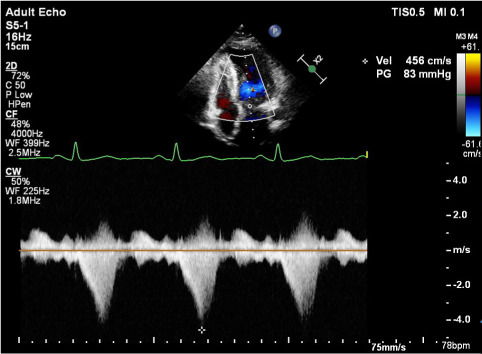
A continuous-wave Doppler echocardiogram from patient 2 reveals the characteristic dagger-shaped signal associated with left ventricular outflow tract obstruction.
TABLE I.
Patient Characteristics
| Age, y | NYHA class before pregnancy | Maximal LV wall thickness, mm | Modified WHO Class | Symptoms during pregnancy | LVOTO? | NYHA class during pregnancy | Drug(s) | Mode of delivery | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 22 | ||||||||
| First pregnancy | 26 | I | III | Asymptomatic | Present | I | Metoprolol | Uncomplicated LSCS at term forfetal distress | |
| Second pregnancy | 27 | I | III | Asymptomatic | Present | I | Metoprolol | Uncomplicated first trimester MTP | |
| Third pregnancy | 29 | I | III | Exertional palpitations at 12 weeks’ gestation | Present | II | Metoprolol | Missed abortion at 16 wk of gestation | |
| Patient 2 | 19 | ||||||||
| First pregnancy | 29 | I | III | Asymptomatic | Marked | I | Nil | Uncomplicated LSCS at term fornonprogress of labor | |
| Patient 3 | 24 | ||||||||
| First pregnancy | 19 | II | III | Progressive symptoms after 20 weeks’ gestation NYHA class IV by 34 wk | Marked | IV | Metoprolol, furosemide | FTNVD; labor well tolerated | |
| Patient 4 | 19 | ||||||||
| First pregnancy | 25 | II | III | Mild increase in breathlessness | Marked | II | Metoprolol | IUGR–LSCS for fetal distress at 34 wk | |
| Second pregnancy | 28 | I | Not classified | Asymptomatic after alcohol septal ablation | Absent | I | Nil | First-trimester MTP | |
| Third pregnancy | 29 | I | Not classified | Asymptomatic after alcohol septal ablation | Absent | I | Nil | Uncomplicated LSCS at term | |
| Patient 5 | 19 | ||||||||
| First pregnancy | 22 | II | II | Mild increase in breathlessness | Absent | II | Nil | FTNVD; labor well tolerated | |
| Second pregnancy | 25 | II | Mild increase in breathlessness | Absent | II | Nil | Uncomplicated LSCS at term forfetal distress | ||
| Patient 6 | 19 | ||||||||
| First pregnancy | 23 | I | II | Asymptomatic until 38 wk, breathlessness after IV fluid administration | Absent | IV | Furosemide | Uncomplicated LSCS at term forfetal distress | |
| Patient 7 | 16 | ||||||||
| First pregnancy | 20 | I | II | Mild breathlessness on exertion in post-partum period | Absent | I | Nil | FTNVD | |
| Second pregnancy | 21 | II | II | No aggravation of symptoms | Absent | II | Nil | Spontaneous abortion at 16 wk of gestation | |
| Third pregnancy | 25 | II | II | Mild increase in breathlessness in second and third trimesters | Absent | II | Furosemide | Uncomplicated LSCS at term for fetal distress | |
| Fourth pregnancy | 27 | II | III | LVOTO noted, worsening symptoms in third trimester | Marked | III | Metoprolol | Uncomplicated LSCS at term for IUGR and previous LSCS |
FTNVD = full-term normal vaginal delivery; IUGR = intrauterine growth restriction; IV = intravenous; LSCS = lower-segment cesarean section; LV = left ventricular; LVOTO = left ventricular outflow tract obstruction; MTP = medical termination of pregnancy; NYHA = New York Heart Association; WHO = World Health Organization
Fig. 2.
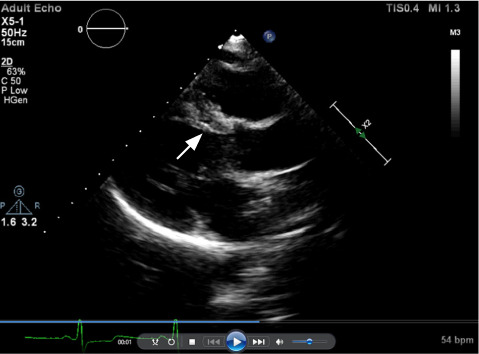
Transthoracic echocardiogram shows thinning of the basal septum (arrow) after successful alcohol septal ablation in patient 4.
Discussion
We have reported clinical and outcomes data on 15 pregnancies in 7 women over a 9-year period. Only 1 woman was diagnosed as having HCM during pregnancy, whereas 6 had been diagnosed before pregnancy. In our institute, 52,868 deliveries took place during this period. Assuming a prevalence of HCM of 1 in 500 in the general population, we would expect more than 100 of these women to have HCM.1 In our previous study, we showed that HCM often remained undiagnosed during pregnancy.5 This is because most pregnant women with HCM in the community either remain asymptomatic or have only mild symptoms that do not bring attention to the underlying condition.3,11 Our current findings show that underdiagnosis of HCM holds true in the current era. There was no overlap of patients between our 2 studies. Only women in whom key symptoms developed during pregnancy or who were previously diagnosed as having HCM come to medical attention. The true prevalence of HCM in pregnant women can be determined only if routine echocardiographic screening is performed in this group.
Our study population was a high-risk cohort, with 7 (46.7%) pregnancies falling in modified WHO class III. Most of the 7 patients tolerated pregnancy well; 3 patients were asymptomatic, and 2 had only mild symptoms. Two women had severe symptoms (New York Heart Association class III or IV) in the third trimester, but symptoms resolved for both of them within 1 week after delivery. Six of the 15 pregnancies (40%) were low risk according to the (modified WHO class II, and the women tolerated pregnancy well.
Maternal and fetal outcomes in previous studies of pregnancy in women with HCM are summarized in Table II.3–9,11 Although maternal deaths were rare, heart failure and arrhythmias were reported in approximately 30% of the pregnancies in some series.6,11 The larger studies lack detailed clinical profiles of patients in whom complications developed.3,4
TABLE II.
Previous Studies of Pregnancies in Women With Hypertrophic Cardiomyopathy
| Reference | No. of women and pregnancies | LVOTO; nonobstructive | Worsening NYHA class, No.(%) | Maternal complications | Fetal complications | Comments |
|---|---|---|---|---|---|---|
| Turner et al,9 1968 | 13 pregnancies in 9 women | 4 (30.8) | Nil | Nil | ||
| Autore et al,4 2002 | 100 women with 199 live births (only 40 women analyzed in detail) | 12/40 women (30%); 28/40 women (70%) | 3 (25); 3 (11) | SCD (n = 2); AF (n = 1) | Not mentioned | |
| Thaman et al,3 2003 | 271 pregnancies in 127 women | 10 women (7.9%) | 35/127 (27.6) | AF during labor (n = 1); postpartum pulmonary edema (n = 2) | IUFD (n = 3) | |
| Avila et al,6 2007 | 23 pregnancies in 23 women | 17 women (73.9%) | Heart failure (n = 7; 30.4%) (1 in second and 6 in third trimester); AF (n = 2; 8.7%); ischemic stroke (n = 1; 4.3%) | Preterm (n = 7 [26%]); IUGR (n = 3 [13%]); neonatal death (n = 1 [4.3%]) | ||
| Sikka et al,5 2014 | 5 pregnancies in 4 women | 3 pregnancies (60%); 2 pregnancies | Nil Restrictive physiology, n = 1 |
Nil Heart failure (n = 1) |
IUFD (n = 1) | Complications in 1 woman with restrictive physiology |
| Tanaka et al,8 2014 | 27 pregnancies in 23 women | 6 women (26.1%); 17 women (73.9%) | Cardiovascular events in 48.1% pregnancies, of which 76.5% were ventricular arrhythmias | Preterm (n = 7 [25.9%]) | High incidence of cardiovascular events (48.1%) | |
| Goland et al,11 2017 | 60 pregnancies | 25 pregnancies (41.7%) | Heart failure (n = 9 [15%]); ventricular arrhythmias (n = 6 [10%]); AF (n = 1 [1.7%]) | IUFD (n = 2 [3.3%]); preterm (n = 14 [24.6%]) | ||
| Billebeau et al,7 2018 | 28 pregnancies | SCD (n = 1 [3.6%]); heart failure (n = 2 [7.1%]) | IUFD (n = 2 [7.1%]); preterm (n = 4 [14.3%]) | |||
| Present series | 15 pregnancies in 7 women | 7 pregnancies (46.7%); 8 pregnancies (53.3%) | 4 (57.1); 5 (62.5) | Nil | IUGR (n = 2 [13.3%]); IUFD–nil | Both pregnancies after successful alcohol septal ablation were well tolerated. |
AF = atrial fibrillation; IUFD = intrauterine fetal death; IUGR = intrauterine growth restriction; LVOTO = left ventricular outflow tract obstruction; NYHA = New York Heart Association; SCD = sudden cardiac death
Left ventricular outflow tract obstruction is associated with a higher risk of pregnancy complications.2 In our study, LVOTO was present in 7 pregnancies. Patients were asymptomatic in 3 of the pregnancies, whereas mild exertional breathlessness occurred in 2. Severe symptoms developed in 2 pregnancies (28.6%) in the third trimester, but improved within 2 days of delivery. Pregnancy can have a variable effect on LVOTO. The increase in preload during pregnancy can potentially reduce the left ventricular outflow tract gradient, whereas increased contractility and reduction in afterload can increase the gradient.12,13 Overall, left ventricular out-flow tract gradients tend to increase in pregnancy.2 One woman in this study also developed LVOTO for the first time in her fourth pregnancy. This likely was caused by hemodynamic changes in pregnancy, because the gradient decreased from 103 mm Hg to 25 mm Hg after delivery. In other women with LVOTO in our study, the gradient did not decrease after delivery.
Left ventricular outflow tract obstruction was absent in 8 pregnancies. These patients tolerated pregnancy well. Only 1 woman had transient breathlessness at rest after intravenous fluid infusion. This patient had a restrictive filling pattern on echocardiography, which was not seen on postdelivery echocardiograms. The restrictive filling pattern was likely caused by plasma volume expansion and an increase in preload during pregnancy in the presence of diastolic dysfunction caused by HCM.14
One patient had 2 pregnancies after successful alcohol septal ablation. She was asymptomatic during pregnancy and in the postpartum period. Because of a paucity of data, patients who have undergone alcohol septal ablation have not been included in the modified WHO classification for maternal cardiovascular risk.2 This holds true even for risk stratification for sudden death. Patients who have undergone alcohol septal ablation or myectomy are also excluded from the HCM risk scores for sudden death.2,10
Labor is usually well tolerated in women with HCM. Only 9% of pregnant women develop transient aggravation of symptoms.3 Vaginal delivery is recommended for women with HCM.2 In our study, cesarean deliveries were conducted only for obstetric indications. All patients tolerated labor well. The only drugs used were metoprolol in 4 pregnancies and loop diuretics in 2. Adverse maternal outcomes, including death, were not seen in any patient. One pregnancy ended preterm. There were 2 spontaneous abortions, and 1 fetus had intrauterine growth retardation. All women who became symptomatic during pregnancy had relief of symptoms after delivery.
Conclusion
Hypertrophic cardiomyopathy commonly remains undiagnosed during pregnancy. Our findings show that most women were asymptomatic or had only mild symptoms. Of the women with LVOTO, 28.6% had severe symptoms that improved after delivery. No adverse maternal outcomes were noted. Pregnancies were well tolerated after successful alcohol septal ablation.
Funding Statement
Funding/Support: None
Footnotes
Conflict of Interest Disclosure: None
References
- 1.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary artery risk development in (young) adults. Circulation . 1995;92(4):785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 2.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J . 2014;35(39):2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 3.Thaman R, Varnava A, Hamid MS, Firoozi S, Sachdev B, Condon M et al. Pregnancy related complications in women with hypertrophic cardiomyopathy. Heart . 2003;89(7):752–756. doi: 10.1136/heart.89.7.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autore C, Conte MR, Piccininno M, Bernabò P, Bonfiglio G, Bruzzi P et al. Risk associated with pregnancy in hypertrophic cardiomyopathy. J Am Coll Cardiol . 2002;40(10):1864–1869. doi: 10.1016/s0735-1097(02)02495-6. [DOI] [PubMed] [Google Scholar]
- 5.Sikka P, Suri V, Aggarwal N, Chopra S, Bahl A, Vijayverghia R. Are we missing hypertrophic cardiomyopathy in pregnancy? Experience of a tertiary care hospital. J Clin Diagn Res . 2014;8(9):OC13–OC15. doi: 10.7860/JCDR/2014/9924.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avila WS, Amaral FM, Ramires JA, Rossi EG, Grinberg M, Bortolotto M et al. Influence of pregnancy on clinical course and fetal outcome of women with hypertrophic cardiomyopathy. Arq Bras Cardiol . 2007;88(4):480–485. doi: 10.1590/s0066-782x2007000400019. [DOI] [PubMed] [Google Scholar]
- 7.Billebeau G, Etienne M, Cheikh-Khelifa R, Vauthier-Brouzes D, Gandjbakhch E, Isnard R et al. Pregnancy in women with a cardiomyopathy: outcomes and predictors from a retrospective cohort. Arch Cardiovasc Dis . 2018;111(3):199–209. doi: 10.1016/j.acvd.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Kamiya C, Katsuragi S, Tanaka K, Miyoshi T, Tsuritani M et al. Cardiovascular events in pregnancy with hypertrophic cardiomyopathy. Circ J . 2014;78(10):2501–2506. doi: 10.1253/circj.cj-14-0541. [DOI] [PubMed] [Google Scholar]
- 9.Turner GM, Oakley CM, Dixon HG. Management of pregnancy complicated by hypertrophic obstructive cardiomyopathy. Br Med J . 1968;4(5626):281–284. doi: 10.1136/bmj.4.5626.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol . 2011;58(25):e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Goland S, van Hagen IM, Elbaz-Greener G, Elkayam U, Shotan A, Merz WM et al. Pregnancy in women with hypertrophic cardiomyopathy: data from the European Society of Cardiology initiated Registry of Pregnancy and Cardiac disease (ROPAC) Eur Heart J . 2017;38(35):2683–2690. doi: 10.1093/eurheartj/ehx189. [DOI] [PubMed] [Google Scholar]
- 12.Matlof HJ, Zener JC, Harrison DC. Idiopathic hypertrophic subaortic stenosis and heart block. Cycle-to-cycle variation as a function of alterations in preload and afterload. Am J Cardiol . 1973;32(5):719–722. doi: 10.1016/s0002-9149(73)80068-2. [DOI] [PubMed] [Google Scholar]
- 13.Hirota Y, Furubayashi K, Kaku K, Shimizu G, Kino M, Kawamura K et al. Hypertrophic nonobstructive cardiomyopathy: a precise assessment of hemodynamic characteristics and clinical implications. Am J Cardiol . 1982;50(5):990–997. doi: 10.1016/0002-9149(82)90407-6. [DOI] [PubMed] [Google Scholar]
- 14.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol . 1998;32(4):865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]


