Abstract
Background
Hereditary angioedema (HAE) is a serious and potentially life‐threatening condition that causes acute attacks of swelling, pain and reduced quality of life. People with Type I HAE (approximately 80% of all HAE cases) have insufficient amounts of C1 esterase inhibitor (C1‐INH) protein; people with Type II HAE (approximately 20% of all cases) may have normal C1‐INH concentrations, but, due to genetic mutations, these do not function properly. A few people, predominantly females, experience HAE despite having normal C1‐INH levels and C1‐INH function (rare Type III HAE). Several new drugs have been developed to treat acute attacks and prevent recurrence of attacks. There is currently no systematic review and meta‐analysis that included all preventive medications for HAE.
Objectives
To assess the benefits and harms of interventions for the long‐term prevention of HAE attacks in people with Type I, Type II or Type III HAE.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 3 August 2021.
Selection criteria
We included randomised controlled trials in children or adults with HAE that used medications to prevent HAE attacks. The comparators could be placebo or active comparator, or both; approved and experimental drug trials were eligible for inclusion. There were no restrictions on dose, frequency or intensity of treatment. The minimum length of four weeks of treatment was required for inclusion; this criterion excluded the acute treatment of HAE attacks.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. HAE attacks (number of attacks per person, per population) and change in number of HAE attacks; 2. mortality and 3. serious adverse events (e.g. hepatic dysfunction, hepatic toxicity and deleterious changes in blood tests). Our secondary outcomes were 4. quality of life; 5. severity of breakthrough attacks; 6. disability and 7. adverse events (e.g. weight gain, mild psychological changes and body hair). We used GRADE to assess certainty of evidence for each outcome.
Main results
We identified 15 studies (912 participants) that met the inclusion criteria. The studies included people with Type I and II HAE. The studies investigated avoralstat, berotralstat, subcutaneous C1‐INH, plasma‐derived C1‐INH, nanofiltered C1‐INH, recombinant human C1‐INH, danazol, and lanadelumab for the prevention of HAE attacks. We did not find any studies on the use of tranexamic acid for prevention of HAE attacks.
All drugs except avoralstat reduced the number of HAE attacks compared with placebo. For breakthrough attacks that occurred despite prophylactic treatment, intravenous and subcutaneous forms of C1‐INH and lanadelumab reduced attack severity. It is not known whether other drugs have a similar effect, as the severity of breakthrough attacks in people taking drugs other than C1‐INH and lanadelumab was not reported.
For quality of life, avoralstat, berotralstat, C1‐INH (all forms) and lanadelumab increased quality of life compared with placebo; there were no data for danazol. Four studies reported on changes in disability during treatment with C1‐INH, berotralstat and lanadelumab; all three drugs decreased disability compared with placebo.
Adverse events, including serious adverse events, did not occur at a rate higher than placebo. However, serious adverse event data and other adverse event data were not available for danazol, which prevented us from drawing conclusions about the absolute or relative safety of this drug. No deaths were reported in the included studies.
The analysis was limited by the small number of studies, the small number of participants in each study and the lack of data on older drugs, therefore the certainty of the evidence is low. Given the rarity of HAE, it is not surprising that drugs were rarely directly compared, which does not allow conclusions on the comparative efficacy of the various drugs for people with HAE.
Finally, we did not identify any studies that included people with Type III HAE. Therefore, we cannot draw any conclusions about the efficacy or safety of any drug in people with this form of HAE.
Authors' conclusions
The available data suggest that berotralstat, C1‐INH (subcutaneous, plasma‐derived, nanofiltered and recombinant), danazol and lanadelumab are effective in lowering the risk or incidence (or both) of HAE attacks. In addition, C1‐INH and lanadelumab decrease the severity of breakthrough attacks (data for other drugs were not available). Avoralstat, berotralstat, C1‐INH (all forms) and lanadelumab increase quality of life and do not increase the risk of adverse events, including serious adverse events. It is possible that danazol, subcutaneous C1‐INH and recombinant human C1‐INH are more effective than berotralstat and lanadelumab in reducing the risk of breakthrough attacks, but the small number of studies and the small size of the studies means that the certainty of the evidence is low. This and the lack of head‐to‐head trials prevented us from drawing firm conclusions on the relative efficacy of the drugs.
Keywords: Adult; Child; Female; Humans; Male; Administration, Intravenous; Angioedemas, Hereditary; Angioedemas, Hereditary/chemically induced; Angioedemas, Hereditary/drug therapy; Angioedemas, Hereditary/prevention & control; Complement C1 Inhibitor Protein; Complement C1 Inhibitor Protein/adverse effects; Complement C1 Inhibitor Protein/therapeutic use; Danazol; Danazol/therapeutic use; Quality of Life; Treatment Outcome
Plain language summary
Drug treatments for the prevention of attacks of hereditary angioedema
What is hereditary angioedema and how is it treated?
Hereditary angioedema (HAE) is a serious and potentially life‐threatening condition that causes acute (sudden onset) attacks of swelling, pain and reduced quality of life. Several new medicines have been developed to treat acute attacks and prevent attacks from occurring. Some medicines are taken by mouth, whereas others are injected under the skin, or given by a vein directly into the blood.
The medicines currently given for preventing HAE attacks are human C1 esterase inhibitor (often abbreviated as C1‐INH), berotralstat, lanadelumab, tranexamic acid, and danazol. In addition, we found a further medicine (avoralstat) that is currently being studied for its ability to prevent HAE attacks.
What did we want to find out?
We investigated whether these medicines reduce the number of HAE attacks, and if any attacks that do occur are less severe than they would otherwise be. We also looked at whether people taking the medicines experienced a better quality of life, and whether the medicines caused unwanted side effects.
What did we do?
We searched medical databases for clinical studies in children or adults with HAE that compared medications to prevent HAE attacks with placebo (a pretend treatment) or another medicine.
What did we find?
We found 15 studies with 912 participants. All medicines except avoralstat reduced the number of HAE attacks, and even when attacks did occur, they were less severe for C1‐INH and lanadelumab (there were no results for the other medicines). We found that most medicines improved the quality of life of the people with HAE and were generally safe as they did not increase the number of serious and less serious side effects.
We found no studies that tested tranexamic acid, and only one study tested danazol. There were also no studies that compared one medicine directly with another. This means that we cannot say for sure whether one medicine is better than another.
Conclusions
C1‐INH, berotralstat, lanadelumab and danazol appear to reduce the risk of HAE attacks and increase the quality of life in people with HAE. The medicines do not seem to result in an increase in side effects.
What are the limitations of the evidence?
Our findings are limited by the small number of studies and the small number of participants in each study. Therefore, our confidence in these findings is low.
How up to date is this evidence?
The evidence is current to 3 August 2021.
Summary of findings
Summary of findings 1. Avoralstat compared with placebo for preventing hereditary angioedema attacks.
| Avoralstat compared with placebo for preventing HAE attacks | ||||||
|
Patient or population: children or adults with Types I or II HAE Settings: outpatient setting Intervention: avoralstat Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo |
Risk with avoralstat |
|||||
|
Risk of HAE attacks (during follow‐up) |
Study population | RR 0.99 (0.92 to 1.06) | 110 (1) | ⊕⊕⊕⊝ Moderatea | — | |
| 1000 per 1000 | 990 per 1000 (920 to 1000) | |||||
|
Change in number of HAE attacks (per week) |
Study population | — | 134 (2) |
⊕⊕⊝⊝ Lowb | — | |
| The mean number of HAE attacks per week ranged across control groups from 0.59 to 1.27 | The mean number of HAE attacks per week in the intervention groups was 0.10 lower (0.37 lower to 0.18 higher) | |||||
|
Mortality (during follow‐up) |
Study population | N/A | N/A | N/A | No deaths reported. | |
| N/A | N/A | |||||
|
Serious adverse events (during follow‐up) |
Study population | RR 0.33 (0.01 to 7.80) | 24 (1) | ⊕⊝⊝⊝ Very lowc | — | |
| 40 per 1000 | 13 per 1000 (0 to 312) | |||||
|
Quality of life
Angioedema Quality of Life scale (lower score is better) (during follow‐up) |
Study population | — | 93 (2) |
⊕⊕⊕⊝ Moderatea | — | |
| The mean change in quality of life ranged across control groups from −0.6 to −12.14 points | The mean change in quality of life in the intervention groups was 6.78 points lower (11.61 lower to 1.95 lower) | |||||
|
Disability (any validated scale) (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
|
Adverse events (during follow‐up) |
Study population | RR 0.85 (0.62 to 1.16) | 24 (1) |
⊕⊕⊝⊝ Lowb | — | |
| 830 per 1000 | 706 per 1000 (515 to 963) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAE: hereditary angioedema; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision. bDowngraded one level each for imprecision and inconsistency. cDowngraded two levels for imprecision and one level for indirectness.
Summary of findings 2. Berotralstat compared with placebo or active control for preventing hereditary angioedema attacks.
| Berotralstat compared with placebo or active control for preventing HAE attacks | ||||||
|
Patient or population: children or adults with Types I or II HAE Settings: outpatient setting Intervention: berotralstat Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with berotralstat | |||||
|
Risk of HAE attacks (during follow‐up) |
Study population | RR 0.63 (0.39 to 1.00) | 37 (1) |
⊕⊕⊝⊝ Lowa | — | |
| 910 per 1000 | 573 per 1000 (355 to 910) | |||||
|
Change in number of HAE attacks (per week) |
Study population | — | 130 (3) |
⊕⊕⊝⊝ Lowa | — | |
| The number of HAE attacks per week ranged across control groups from 0.55 to 0.95 | The number of HAE attacks per week in the intervention groups was 0.39 attacks lower (0.74 lower to 0.05 lower) | |||||
|
Mortality (during follow‐up) |
Study population | N/A | N/A | N/A | No deaths reported. | |
| N/A | N/A | |||||
|
Serious adverse events (during follow‐up) |
Study population | RR 0.77 (0.02 to 24.03) | 128 (3) | ⊕⊕⊝⊝ Lowa | — | |
| 45 per 1000 | 35 per 1000 (1 to 1000) | |||||
| Quality of life Angioedema Quality of Life scale (lower score is better) (during follow‐up) | Study population | — | 130 (3) |
⊕⊕⊕⊝ Moderateb | — | |
| The mean change in quality of life ranged across control groups from 3.18 points to −9.69 points | The mean change in quality of life in the intervention group was 15.28 points lower (29.42 lower to 1.14 lower) | |||||
|
Disability
Standardised mean difference (lower is better) (during follow‐up) |
Study population | — | 50 (2) |
⊕⊕⊝⊝ Lowa | — | |
| The mean change in disability ranged across control groups from 1.51 to −1.95 | The mean change in disability in the intervention groups was 1.01 units lower (1.62 lower to 0.40 lower) | |||||
| Adverse events (during follow‐up) | Study population | RR 1.03 (0.88 to 1.22) | 128 (3) |
⊕⊕⊕⊝ Moderateb | — | |
| 761 per 1000 | 784 per 1000 (670 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAE: hereditary angioedema; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision. bDowngraded one level for imprecision.
Summary of findings 3. C1 esterase inhibitor compared with placebo or active control for preventing hereditary angioedema attacks.
| C1‐INH compared with placebo or active control for preventing HAE attacks | ||||||
|
Patient or population: children or adults with Types I or II HAE Settings: outpatient setting Intervention: C1‐INH(SC) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with C1‐INH(SC) | |||||
|
Risk of HAE attacks (during follow‐up) |
Study population | RR 0.29 (0.16 to 0.50) | 43 (1) | ⊕⊕⊝⊝ Lowa | — | |
| 810 per 1000 | 24 per 1000 (0 to 162) | |||||
|
Change in number of HAE attacks (per week) |
Study population | — | 45 (1) | ⊕⊕⊝⊝ Lowa | — | |
| The mean number of HAE attacks per week in the control group was 0.93 | The mean number of HAE attacks per week in the intervention group was 0.81 lower (0.98 lower to 0.64 lower) | |||||
|
Mortality (during follow‐up) |
Study population | N/A | N/A | N/A | No deaths reported | |
| N/A | N/A | |||||
|
Serious adverse events (during follow‐up) |
Study population | RR 0.34 (0.01 to 8.14) | 44 (1) | ⊕⊝⊝⊝ Very lowb | — | |
| 23 per 1000 | 8 per 1000 (0 to 187) | |||||
|
Quality of life
standardised mean difference (lower is better) (during follow‐up) |
Study population | — | 36 (1) |
⊕⊕⊝⊝ Lowa | — | |
| The mean change in quality of life in the control group was −0.87 units | The mean change in quality of life in the intervention groups was 0.29 units lower (0.76 lower to 0.18 higher) | |||||
|
Disability (any validated scale) (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
|
Adverse events (during follow‐up) |
Study population | RR 1.03 (0.84 to 1.27) | 44 (1) |
⊕⊕⊕⊝ Moderatec | — | |
| 663 per 1000 | 683 per 1000 (557 to 842) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). C1‐INH(SC): subcutaneous C1 esterase inhibitor; CI: confidence interval; HAE: hereditary angioedema; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision. bDowngraded three levels for imprecision. cDowngraded one level for imprecision.
Summary of findings 4. Plasma‐derived C1 esterase inhibitor compared with placebo or active control for preventing hereditary angioedema attacks.
| pdC1‐INH compared with placebo or active control for preventing HAE attacks | ||||||
|
Patient or population: children or adults with Types I or II HAE Settings: outpatient setting Intervention: pdC1‐INH Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with pdC1‐INH | |||||
|
Risk of HAE attacks (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported | |
| N/A | N/A | |||||
|
Change in number of HAE attacks (per week) |
Study population | — | 71 (1) |
⊕⊕⊝⊝ Lowa |
— | |
| The number of HAE attacks per week in the control group was 0.9 | The number of HAE attacks per week in the intervention group was 0.53 attacks lower (0.58 lower to 0.48 lower) | |||||
|
Mortality (during follow‐up) |
Study population | N/A | N/A | N/A | No deaths reported | |
| N/A | N/A | |||||
|
Serious adverse events (during follow‐up) |
Study population | RR 0.54 (0.09 to 3.10) | 71 (1) | ⊕⊝⊝⊝ Very lowb | — | |
| 53 per 1000 | 29 per 1000 (5 to 164) | |||||
|
Quality of life
Angioedema Quality of Life Score
(lower score is better) (during follow‐up) |
Study population | — | 31 (1) |
⊕⊕⊝⊝ Lowa | — | |
| The mean change in quality of life in the control group was −6.86 | The mean change in quality of life in the intervention group was 3.49 points lower (10.86 lower to 3.88 higher) | |||||
|
Disability
(any validated scale) (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
| Adverse events (during follow‐up) | Study population | RR 1.05 (0.78 to 1.42) | 71 (1) |
⊕⊕⊝⊝ Lowa | — | |
| 561 per 1000 | 589 per 1000 (438 to 797) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAE: hereditary angioedema; N/A: not applicable; pdC1‐INH: plasma‐derived C1 esterase inhibitor; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision. bDowngraded three levels for imprecision.
Summary of findings 5. Nanofiltered C1 esterase inhibitor compared with placebo or active control for preventing hereditary angioedema attacks.
| C1‐INH‐nf compared with placebo or active control for preventing HAE attacks | ||||||
|
Patient or population: children or adults with Types I or II HAE Settings: outpatient setting Intervention: C1‐INH‐nf Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with C1‐INH‐nf | |||||
|
Risk of HAE attacks (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
|
Change in number of HAE attacks (per week) |
Study population | — | 22 (1) |
⊕⊝⊝⊝ Very lowa |
— | |
| The mean number of HAE attacks per week in the control group was 1.06 | The mean number of HAE attacks per week in the intervention group was 0.53 lower (0.78 lower to 0.28 attacks per week lower) | |||||
|
Mortality (during follow‐up) |
Study population | N/A | N/A | N/A | No deaths reported. | |
| N/A | N/A | |||||
|
Serious adverse events (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
|
Quality of life
standardised mean difference (lower is better) (during follow‐up) |
Study population | — | 16 (1) |
⊕⊝⊝⊝ Very lowa | — | |
| The mean change in quality of life in the control group was 4.85 units | The mean change in quality of life in the intervention group was 0.91 units lower (1.64 lower to 0.18 lower) | |||||
|
Disability standardised mean difference (lower is better) (during follow‐up) |
Study population | — | 16 (1) |
⊕⊝⊝⊝ Very lowa | — | |
| The mean change in disability in the control group was −0.71 | The mean change in disability in the intervention group was 0.84 units lower (1.57 lower to 0.12 lower) | |||||
|
Adverse events (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). C1‐INH‐nf: nanofiltered C1 esterase inhibitor; CI: confidence interval; HAE: hereditary angioedema; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels for imprecision.
Summary of findings 6. Recombinant human C1 esterase inhibitor compared with placebo or active control for preventing hereditary angioedema attacks.
| rhC1‐INH compared with placebo or active control for preventing HAE attacks | ||||||
|
Patient or population: children or adults with Types I or II HAE Settings: outpatient setting Intervention: rhC1‐INH Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with rhC1‐INH | |||||
|
Risk of HAE attacks (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
|
Change in number of HAE attacks (per week) |
Study population | — | 32 (1) |
⊕⊝⊝⊝ Very lowa |
— | |
| The number of HAE attacks in the control group was 1.8 per week | The number of HAE attacks per week in the intervention groups was 0.92 attacks lower (1.31 lower to 0.53 lower) | |||||
|
Mortality (during follow‐up) |
Study population | N/A | N/A | N/A | No deaths reported. | |
| N/A | N/A | |||||
|
Serious adverse events (during follow‐up) |
Study population | RR 1.50 (0.06 to 34.66) | 29 (1) |
⊕⊝⊝⊝ Very lowa | No events reported in the placebo group, 1 event reported in the rhC1‐INH group. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Quality of life standardised mean difference (during follow‐up) | Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
| Disability (any validated scale) (during follow‐up) | Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
| Adverse events (during follow‐up) | Study population | RR 1.39 (0.71 to 2.70) | 29 (1) |
⊕⊕⊝⊝ Lowb |
— | |
| 286 per 1000 | 398 per 1000 (203 to 772) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAE: hereditary angioedema; N/A: not applicable; rhC1‐INH: recombinant human C1 esterase inhibitor; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels for imprecision. bDowngraded two levels for imprecision.
Summary of findings 7. Lanadelumab compared with placebo or active control for preventing hereditary angioedema attacks.
| Lanadelumab compared with placebo or active control for preventing HAE attacks | ||||||
|
Patient or population: children or adults with Types I or II HAE Settings: outpatient setting Intervention: lanadelumab Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with lanadelumab | |||||
|
Risk of HAE attacks (during follow‐up) |
Study population | N/A | N/A | N/A | Outcome not reported. | |
| N/A | N/A | |||||
|
Change in number of HAE attacks (per week) |
Study population | — | 83 (2) |
⊕⊕⊝⊝ Lowa |
— | |
| The number of HAE attacks per week ranged across control groups from 0.37 to 0.49 | The number of HAE attacks per week in the intervention groups was 0.41 attacks lower (0.48 lower to 0.35 lower) | |||||
|
Mortality (during follow‐up) |
Study population | N/A | N/A | N/A | No deaths reported. | |
| N/A | N/A | |||||
|
Serious adverse events (during follow‐up) |
Study population | RR 0.88 (0.08 to 10.39) | 162 (2) |
⊕⊕⊝⊝ Lowa | — | |
| 24 per 1000 | 73 per 1000 (7 to 765) | |||||
| Quality of life standardised mean difference (lower is better) (during follow‐up) | Study population | — | 68 (1) |
⊕⊕⊝⊝ Lowa | — | |
| The mean change in quality of life in the control group was −4.72 points | The mean change in quality of life in the intervention group was 0.91 units lower (1.43 lower to 0.40 lower) | |||||
| Disability Standardised mean difference (lower is better) (during follow‐up) | Study population | — | 64 (1) |
⊕⊕⊝⊝ Lowa | — | |
| The mean change in disability in the control group was −5.42 | The mean change in disability in the intervention group was 1.38 units lower (1.94 lower to 0.82 lower) | |||||
| Adverse events (during follow‐up) | Study population | RR 1.07 (0.77 to 1.47) | 158 (2) |
⊕⊕⊝⊝ Lowa | ||
| 1000 per 1000 | 840 per 1000 (710 to 980) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAE: hereditary angioedema; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision.
Background
Description of the condition
Hereditary angioedema (HAE) is a rare but serious condition that is characterised by random, recurrent attacks of swelling (angioedema). An attack is often heralded by a transient, non‐itchy rash called erythema marginatum (Zeerleder 2016). There may be prodromal symptoms (symptoms indicating onset), such as fatigue and feeling generally unwell before swelling occurs. At first, the swelling is typically painless and not itchy; however, it can become extremely painful and disabling. The swelling may affect the face and upper airway, intestinal mucosa, genitals and the extremities. Attacks peak at around 24 hours after onset and can last several days. Swelling of the airway is life‐threatening, as it can result in death by asphyxiation. Intestinal swelling causes abdominal pain and may be accompanied by nausea, vomiting and diarrhoea; signs and symptoms may present similar to acute bowel obstruction. A swelling attack may cause major fluid shifts, which may result in hypotension and shock. HAE attacks may be triggered by the following: 1. physical triggers such as surgery, injury or infection (Frank 1976); 2. pharmacological triggers such as oestrogens (Frank 1979), and angiotensin‐converting enzyme (ACE) inhibitors (Agostoni 1999); and 3. psychological factors such as stress or anxiety (Zotter 2014). However, in many cases, no precipitating factor can be identified. It is not known how many HAE attacks occur spontaneously, that is, do not have any precipitating factor.
HAE affects approximately one in every 50,000 to 150,000 people (Roche 2005; Zuraw 2008), and follows an autosomal‐dominant pattern of inheritance in most people (Germenis 2016). Compared with more common causes of angioedema, such as allergies and ACE inhibitor medications, HAE is rare and diagnosis is frequently missed or delayed. A misdiagnosis can be fatal, as swelling of the upper airway as a result of HAE does not respond to medications routinely used for allergic swelling, such as adrenaline, corticosteroids or antihistamines. HAE should be considered when a patient presents with recurrent, isolated angioedema without urticaria and with a family history of similar attacks (Henao 2016; Maurer 2018). However, 25% of people with HAE will not have a positive family history, as the condition often arises from a somatic mutation in the SERPING1 gene. Untreated, HAE has a mortality rate of 15% to 33% (Bork 2018). It is unclear what the mortality rate is for people who are treated for HAE.
Most HAE attacks are associated with increased levels of bradykinin, a potent vasodilator. Binding of bradykinin to the bradykinin 2 (B2) receptor on blood vessels results in fluid extravasation and tissue swelling. Bradykinin is a low molecular weight peptide that is formed when kininogen is cleaved by the protease kallikrein. Active kallikrein is generated by a cleavage event that processes prekallikrein, which involves coagulation factor XII, another serum protease. The proteolytic activity of kallikrein is regulated by the C1 esterase inhibitor (C1‐INH), a serine protease inhibitor that is encoded by the SERPING1 gene. People with Type I HAE (approximately 80% of all HAE cases) have insufficient amounts of C1‐INH; people with Type II HAE (approximately 20% of all cases) may have normal C1‐INH concentrations, but mutations in the SERPING1 gene result in C1‐INH variants that can no longer control kallikrein (Germenis 2016). A few people, predominantly females, have HAE despite having normal C1‐INH levels and C1‐INH function (US HAE Association 2018). These rare Type III or HAE nC1‐INH cases are often associated with mutations in the F12 gene. The consequences of these mutations are poorly understood but are believed to affect the factor XII‐mediated processing of prekallikrein. Finally, since 2018, next‐generation sequencing has allowed the identification of mutations in five additional genes in people with HAE but who have normal C1‐INH levels and function: ANGPT1 (angiopoietin‐1), PLG (plasminogen), KNG1 (kininogen), MYOF (myoferlin) and HS3ST6 (heparan sulphate‐glucosamine 3‐O‐sulfotransferase 6) (Bafunno 2018; Bork 2018; Lopes Veronez 2021). There are currently several abbreviations used for the HAE types. We have provided a table for reference (Table 8).
1. Hereditary angioedema nomenclature.
| Abbreviation used in this review | Other abbreviations used in the literature |
| Type I HAE | Type 1 HAE; HAE type 1; C1‐INH‐HAE (type 1) |
| Type II HAE | Type 2 HAE; HAE type 2; C1‐INH‐HAE (type 2) |
| Type III HAE | Type 3 HAE; HAE type 3; C1‐INH‐HAE (type 3) |
C1‐INH: C1 esterase inhibitor; HAE: hereditary angioedema.
The clinical diagnosis of angioedema should be followed by laboratory testing for both complement component 4 (C4) concentrations and C1‐INH concentration and function. Two estimations at different time points are recommended. The combination of low C4 and low C1‐INH function has a 98% specificity for HAE caused by C1‐INH deficiency (Gompels 2002; Tarzi 2007). Routine genetic testing is not usually performed but is indicated in HAE cases where people have normal C1‐INH, and is occasionally used for prompt diagnosis in the neonate.
Description of the intervention
Treatments for the prevention of HAE attacks act through the supplementation of insufficient concentrations of C1‐INH, or by providing functional inhibitor proteins in the case of subfunctional C1‐INH. Functional C1‐INH can be provided either in the form of a concentrate prepared from plasma or as a recombinant protein (Johnson 2018; Longhurst 2018). Both are administered as intravenous infusion or, more recently, as a subcutaneous injection. Traditionally, tranexamic acid and attenuated androgens have been the most commonly used pharmacological agents for the prophylaxis of HAE and are still the only forms of prophylaxis available in some countries. Tranexamic acid, an antifibrinolytic drug, interferes with the functions of plasminogen and plasmin; however, the mechanism of action in HAE is not well understood (Wintenberger 2014). Where other treatments are not available, tranexamic has been favoured in children because of a better adverse‐effect profile than attenuated androgens despite that its efficacy is considered modest (Frank 2016). Attenuated androgens, most commonly danazol, have been used for many years as an oral prophylactic medication in HAE. It is available in capsules of varying doses and is taken by mouth (FDA 2011). Newer preventive approaches target kallikrein. The first of these to reach clinical practice is lanadelumab, a human monoclonal antibody targeting plasma kallikrein that is given subcutaneously (Banerji 2017; Banerji 2018). Another is the oral kallikrein inhibitor berotralstat (Orladeyo) (Chen 2017). Several other molecules are being tested in clinical trials. These include a monoclonal anti‐FXII antibody (Cao 2015), and an oral plasma kallikrein inhibitor, avoralstat (OPuS‐1; OPuS‐2).
The large number of different C1‐INH products can cause confusion. Table 9 lists the drugs, their respective brand names and their routes of administration.
2. Drugs for the prevention of hereditary angioedema attacks.
| Drug | Brand | Route of administration |
| Avoralstat | Not approved | Oral |
| Berotralstat | Orladeyo | Oral |
| C1‐INH(SC) | Haegarda | SC |
| C1‐INH‐nf | Cinryze | Intravenous |
| pdC1‐INH | Berinert | Intravenous |
| rhC1‐INH | Ruconest | Intravenous |
| Danazol | Danocrine/Cyclomen | Oral |
| Lanadelumab | Takhzyro | SC |
| Tranexamic acid | Lysteda | Oral |
C1‐INH: C1 esterase inhibitor; C1‐INH‐nf: nanofiltered C1 esterase inhibitor; C1‐INH(SC): subcutaneous C1 esterase inhibitor; HAE: hereditary angioedema; pdC1‐INH: plasma‐derived C1 esterase inhibitor; rhC1‐INH: recombinant human C1 esterase inhibitor.
Interventions for the treatment of acute HAE attacks, such as C1‐INH concentrates for acute use (e.g. nanofiltered C1 esterase inhibitor (C1‐INH‐nf) (Cinryze), recombinant human C1 esterase inhibitor (rhC1‐INH) (Ruconest), icatibant (Firazyr) and ecallantide (Kalbitor)) will be covered in a separate Cochrane Review (Frese 2019).
How the intervention might work
Treatment with recombinant human C1‐INH (rhC1‐INH) and plasma‐derived C1‐INH (pdC1‐INH) concentrates supplies functional inhibitor proteins in sufficient amounts to improve C1‐INH activity levels and ideally restores normal inhibitor activity in people with a C1‐INH deficiency (e.g. in cases with insufficient C1‐INH plasma levels or with non‐functional C1‐INH variants). The therapeutic effect of danazol is not fully understood; it may promote C4 and C1‐INH synthesis, it may cause a minor increase in C1 concentrations (thus improving the complement system) or it may prevent C1‐INH breakdown (Fabiani 1990). Lanadelumab inhibits the kallikrein protease by blocking its substrate binding site (Kenniston 2014), which prevents the cleavage of high molecular weight kininogen into kininogen and bradykinin. Thus, lanadelumab can be used to control the production of excess bradykinin and, therefore, the subsequent development of acute HAE attacks (Banerji 2017; Banerji 2018). In summary, C1‐INH, danazol, lanadelumab, tranexamic acid and berotralstat prevent attacks by restoring normal C1‐INH activity or by inhibiting kallikrein.
Why it is important to do this review
Although HAE is rare, it is highly debilitating, may cause death, and is associated with high personal and economic burdens (Lumry 2018; Wilson 2010). The lives of people affected by this condition are disrupted by the apparently random nature of swelling attacks. HAE attacks can be very painful and are often associated with temporary disfigurement and severe morbidity (Longhurst 2016). Oedema of the upper airway in particular is life‐threatening. Thus, severe acute HAE attacks often result in presentations to the emergency department and, occasionally, in admission to hospital. Even with management at home, individuals may need several days away from school or work for recovery. Any effective preventive treatment for HAE should reduce the number of swelling attacks, improve the quality of life for people with HAE and prevent death. There are several options for the prevention of HAE attacks, but there is no systematic review of these treatments, and we currently do not know whether all preventive HAE treatments are equally effective and safe. This review presents the available evidence on the safety and efficacy of interventions for the long‐term prevention of HAE attacks, allowing evidence‐based decision‐making for health practitioners and patients.
Objectives
To assess the benefits and harms of interventions for the long‐term prevention of HAE attacks in people with Type I, Type II or Type III HAE.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials investigating interventions for the long‐term prevention of HAE attacks. We included blinded and open‐label trials. We excluded studies investigating interventions for the treatment of acute HAE attacks, as these are covered in another Cochrane Review (Frese 2019).
Types of participants
We included studies involving children or adults with Type I, Type II or Type III HAE (HAE nC1‐INH) who were treated for the prevention of HAE attacks. We defined Type I HAE as HAE caused by insufficient amounts of C1‐INH; Type II HAE as HAE presenting with sufficient amounts of C1‐INH, but subfunctional or non‐functional C1‐INH; and Type III HAE as HAE with normal C1‐INH concentrations and function (US HAE Association 2018). If the justification for designating the type of HAE is not specifically given, we accepted the diagnosis stated by the study authors.
Types of interventions
We included any intervention that had been tested for the prevention of HAE attacks, including concentrated C1‐INH (either derived from blood or produced as a recombinant protein), as well as the drugs danazol, tranexamic acid, berotralstat and lanadelumab. There were no restrictions on dose, frequency or intensity of treatment. The minimum length of treatment was four weeks; this criterion excluded the acute treatment of HAE attacks. Furthermore, we included only studies that compared interventions with placebo or any active comparator, or both.
Types of outcome measures
For all outcomes, we included the time points reported by individual studies, as long as they were not reporting on the treatment of an acute attack. Clinically relevant time study durations were four weeks or longer. The studies did not report their data at different time points, therefore we used the reported time point for each study in our analyses. Many studies reported data as mean number of events per week or mean number of events per month. In order to combine these data, we converted all 'per month' data to 'per week'.
Primary outcomes
HAE attacks (number of attacks per person, per population) and change in number of HAE attacks
Mortality
Serious adverse events, such as hepatic dysfunction, hepatic toxicity and deleterious changes in blood tests (e.g. glucose tolerance, thyroid hormones, lipids, lipoproteins)
Secondary outcomes
Quality of life (measured by any validated measure, such as Angioedema Quality of Life Questionnaire (AE‐QoL), Health‐Related Quality of Life Questionnaire for HAE (HAEQoL), 12‐Item Short Form Health Survey (SF‐12))
Severity of breakthrough attacks as reported by individual studies
Disability (measured by any validated measure, such as Work Productivity and Activity Impairment Questionnaire). This includes any outcome that measures changes in the ability of people to attend and function well in the workplace and in recreational activities
Adverse events, such as weight gain, mild psychological changes (irritability, nervousness, mood changes), increased body hair, gastrointestinal health, nausea, vomiting and flushing
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions:
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 3 August 2021);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 7) via the Cochrane Register of Studies Online (CRSO);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (1946 onwards) (searched 3 August 2021);
Embase Ovid (from 1974 onwards) (searched 3 August 2021);
CINAHL EBSCO (from 1982 onwards) (searched 3 August 2021).
The Information Specialist searched the following trials registries on 3 August 2021:
ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
The Information Specialist modelled search strategies for other databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2021). Search strategies for all databases are provided in Appendix 1.
Searching other resources
We searched grey literature for evidence of studies that have not been published in peer‐reviewed journals, but did not find any unpublished studies. We had no need to contact manufacturers of pharmaceutical drugs for unpublished trials, as all such trials are registered in clinical trials databases. We also checked references of included studies for relevant publications.
Data collection and analysis
Selection of studies
Two review authors (MF, NB) independently assessed each study for inclusion based on the inclusion criteria. We resolved any disagreements by consensus or discussion (or both) with a third review author (KM). We illustrated the study selection process in a PRISMA diagram (Liberati 2009). We listed all articles excluded after full‐text assessment in the Characteristics of excluded studies table along with the reason for their exclusion.
Data extraction and management
One review author (KM) extracted relevant data into a spreadsheet that was checked by another review author (ES). We resolved any disagreements by consensus.
We collected the following information for each included study: study design; exclusions postrandomisation; losses to follow‐up; duration of study; unit of randomisation; country and setting; number of participants; age and sex of participants; participant inclusion and exclusion criteria; intervention and control group sample sizes, type, dose and duration of intervention; outcomes (as specified in the Types of outcome measures section), funding source and declarations of interest declared by study authors.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies using the Cochrane RoB 1 tool. This tool involves assessing the risk of selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias (Higgins 2017). Two review authors (KM, NB) independently assessed the risk of bias, and we resolved disagreements by consensus or by reference to a third review author (MF).
Measures of treatment effect
We calculated and reported dichotomous outcome measures, such as number of attacks, mortality, serious adverse events and adverse events, using risk ratios (RRs) with the associated 95% confidence intervals (CIs). We calculated and reported continuous outcome measures for change in number of attacks, quality of life and disability scores using the mean difference (MD) and the associated 95% CIs. If the included studies used different scales, we calculated a standardised mean difference (SMD) instead. Where the included studies reported only CIs or standard errors (SE), we converted these to standard deviations (SD) using the Review Manager 5 calculator (Review Manager 2014). We based our calculations on an intention‐to‐treat (ITT) approach.
Unit of analysis issues
Our unit of analysis was the participant. We report on outcomes at a participant level.
Due to the small number of studies available for analysis, we combined cross‐over and parallel studies in all analyses. To mitigate the heterogeneity that could result from this, we used a random‐effects analysis for the analyses. The cross‐over studies did not involve a washout period, however we did not consider this to cause carryover effects, as C1‐INH has a mean functional half‐life of approximately 39 hours (Kunschak 1998), danazol has a mean elimination half‐life of approximately nine hours and avoralstat has a terminal half‐life that ranges from 12 hours to 31 hours. As such, the drugs are not expected to have carryover effects into the second cross‐over period.
The inclusion of cross‐over trials with parallel trials in a meta‐analysis can give rise to a unit of analysis error. That is, the CIs around the effect sizes may be too large, giving a study too little weight in an analysis. However, given the paucity of studies available to us, and the conservative nature of the error, we considered the unit of analysis error to be of less significance than the resulting loss of information from excluding the studies.
Dealing with missing data
Where measurements of variance (summary data) were missing, we imputed those values by taking the mean of the variance of other studies reporting on the same outcome using the same methodology. We did this for all studies with missing SDs/SEs. We also intended to undertake sensitivity analysis by removing studies with significant amounts of missing data (20% or more in a single outcome). As no study had high attrition, we did not perform this sensitivity analysis. We compared the rates of missing data between groups to determine if there was an imbalance between the groups. When it was possible, we carried out analyses using the ITT principle. We used per‐protocol data if ITT data were not available.
Assessment of heterogeneity
In first instance, we assessed the forest plots for each outcome to ensure there was overlap of the CIs of effect estimates. If no overlap existed, we planned to further assess the causes of heterogeneity.
We assessed heterogeneity using Chi2 and I2 statistic, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). In the context of the Chi² test, we used a P value of 0.10 or less to indicate significant heterogeneity. For our assessment of the significance of heterogeneity as measured using the I2 statistic, we took direction and size of effect into consideration and used the following guidance for interpretation, provided in Higgins 2021:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When the I² statistic was in an area of overlap between two categories (e.g. between 50% and 60%), we considered differences in participants and interventions among the trials contributing data to the analysis (Higgins 2021).
Assessment of reporting biases
We assessed reporting bias by creating a funnel plot using Review Manager 5 (Review Manager 2014). Because funnel plots are not informative where there are fewer than 10 studies (Higgins 2021), we only undertook funnel plot analysis for outcomes containing 10 studies or more.
Data synthesis
We undertook meta‐analysis of data from included studies using a fixed‐effect model where possible. If factors in the trials clearly indicated that variance between studies were likely to be due to factors other than chance, we used a random‐effects model. We also used the random‐effects model in analyses where we combined parallel and cross‐over studies.
Subgroup analysis and investigation of heterogeneity
We intended to undertake subgroup analyses for all outcomes, as follows:
type of HAE (Type I HAE versus Type II HAE versus Type III HAE);
baseline number of attacks (per week, per month, per year);
different drugs;
drug dose and drug frequency;
age (children versus adolescents versus adults versus older people). Children were defined as aged 0 to 10 years, adolescents as 11 to 17 years, adults as 18 to 64 years, and older people as 65 years and above;
sex (men versus women);
comorbidities;
concomitant medication versus no concomitant medication.
Unfortunately, the studies did not report data for most of the subgroups mentioned above. We could only undertake subgroup analyses by dose. The baseline number of attacks was usually reported; however, the range of attacks per year (one to 56) within studies meant that a subgroup analysis by baseline attack numbers would have been meaningless.
Sensitivity analysis
We intended to explore the impact of trials at high risk of performance and detection bias on the magnitude or direction of the overall effect by excluding from the analysis trials at high risk of bias. We defined studies to be at high risk of bias if we assessed the performance or detection bias at high risk of bias. However, most studies were at low risk of bias, and it was not meaningful to perform this sensitivity analysis.
We also intended to undertake sensitivity analyses in which we removed studies with significant amounts of missing data (20% or more in a single outcome). However, no study had high attrition and therefore this analysis was unnecessary.
We intended to look at the funding sources of clinical trials and undertake a sensitivity analysis by funding source, but pharmaceutical companies funded 14 trials.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables, which list key outcomes along with a degree of certainty according to the GRADE criteria (GRADE 2004; GRADEpro GDT). Reported outcomes were the efficacy (risk of HAE attacks, change in HAE attacks, mortality, quality of life, disability) and safety (serious adverse events, adverse events) of interventions for the prevention of HAE attacks, as listed in Types of outcome measures. We assessed and reported on the certainty of the evidence for each outcome. We graded the certainty as high, moderate, low or very low, based on the criteria of risk of bias, inconsistency, indirectness, imprecision and publication bias (GRADE 2004). We intended to report on the outcomes for each type of HAE (I, II and III) in separate summary of findings tables, but no studies included people with Type III HAE, and no included study differentiated between Type I and II HAE.
Results
Description of studies
Results of the search
The literature search revealed 3011 citations and we identified two additional records through other sources, of which 788 were duplicates (Figure 1). After screening titles and abstracts of the remaining 2225 records, we excluded further 1980 records. We obtained the remaining articles as full texts. After application of the inclusion and exclusion criteria, we excluded 36 studies (37 records) with reasons, two studies (two records) were ongoing and one study (one record) was awaiting classification, which resulted in the inclusion of 121 records or clinical trial registry entries that reported on 15 clinical trials (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; COMPACT; COMPACT extension; Gelfand 1976; HELP; NCT01005888; NCT01756157; NCT02052141; NCT02247739; OPuS‐1; OPuS‐2; SAHARA).
1.
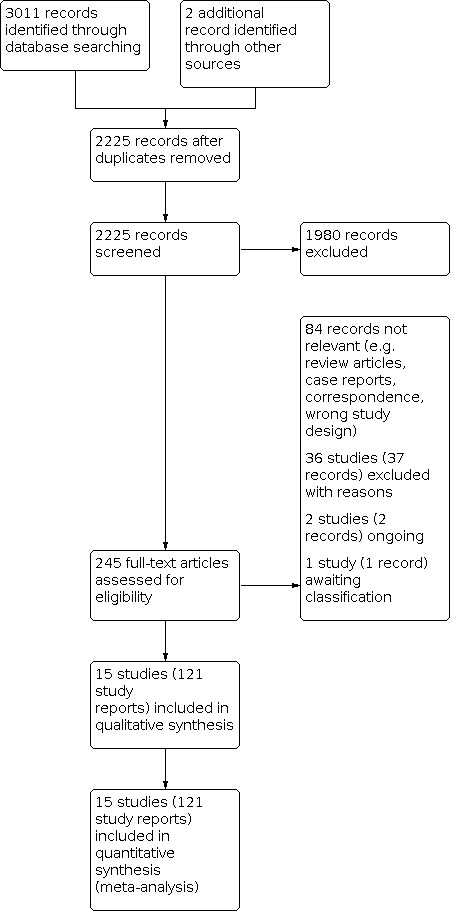
Study flow diagram.
Included studies
All 15 included studies were randomised, and most used placebo as the control (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; COMPACT; Gelfand 1976; HELP; NCT01005888; NCT02247739; OPuS‐1; OPuS‐2; SAHARA). Four studies directly compared different doses of the same medication (COMPACT; COMPACT extension; NCT01756157; NCT02052141), but no study compared one medication directly against another medication (head‐to‐head trials). Several studies compared several doses with placebo (APeX‐2; APeX‐J; Banerji 2017; COMPACT; HELP).
Seven were parallel studies (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; COMPACT extension; HELP; OPuS‐2), and seven were cross‐over studies (COMPACT; Gelfand 1976; NCT01005888; NCT01756157; NCT02052141; NCT02247739; OPuS‐1). SAHARA was a partial cross‐over study whereby 60/75 participants crossed over after 14 weeks and 15/75 participants had continuous plasma‐derived C1‐INH (pdC1‐INH) liquid treatment for 28 weeks to assess long‐term safety. We only included data from the first 14 weeks of the SAHARA study.
Two studies reported the efficacy and safety of avoralstat (OPuS‐1; OPuS‐2); three studies reported berotralstat (APeX‐1; APeX‐2; APeX‐J); six studies reported C1‐INH in various forms (C1‐INH‐nf: NCT01005888; NCT02052141; subcutaneous: COMPACT; COMPACT extension; pdC1‐INH: SAHARA; rhC1‐INH: NCT02247739); two studies reported lanadelumab (Banerji 2017; HELP); and one study reported danazol (Gelfand 1976). NCT01756157 compared human C1‐INH 2000 IU added to recombinant human hyaluronidase (rhH) 48,000 U with a lower dose of the same combination (C1‐INH 1000 IU plus rhH 24,000 U).
Only one study reported on children specifically (NCT02052141). None of the studies that included both children and adults presented results by age (COMPACT; COMPACT extension; NCT01756157; NCT02247739).
All studies included people with Type I and II HAE (thus no study included people with Type III HAE). No study presented data separately by HAE type.
The 15 studies included 912 participants ranging from nine participants (Gelfand 1976) to 126 participants (HELP).
Despite being an autosomal‐dominant condition, women tend to have more attacks and more severe attacks; this was reflected in the studies. Overall, the mean female representation in the studies was 69.3%, ranging from 56.0% to 87.5%. The mean body mass index (BMI) of people in the studies was 27.4. The range of BMI was not frequently reported, but in those studies that did report BMI range, the BMI varied from 18.6 to 49.5.
See Characteristics of included studies table for details of the included studies.
Excluded studies
We excluded 36 studies (37 records) (Aabom 2015; Aberer 2017; Agostoni 1978a; Agostoni 1978b; Agostoni 1980a; Agostoni 1983; Aygören‐Pürsün 2013; Baker 2013; Bernstein 2019; Birjmohun 2008; Blohmé 1972; Bork 2008; Bork 2011; Bork 2017; Busse 2017; Chyung 2014; Cicardi 1997; Davis‐Lorton 2016; Drouet 2008; EudraCT 2009‐010736‐18; EudraCT 2010‐019670‐32; Farkas 2010; Farkas 2013; Füst 2011; Hofstra 2012; NCT01108848; NCT01467947; NCT01576523; NCT01760343; Sharma 2009; Sweet 1980; Szegedi 2008; Széplaki 2005; Wang 2017; Waytes 1996Zotter 2013). Reasons for exclusion were generally wrong study design and wrong population.
See Characteristics of excluded studies table for reasons of the excluded studies.
Studies awaiting classification
One study is awaiting classification as we were unable to obtain a copy of the report (Zhang 1990). See Characteristics of studies awaiting classification table for more information.
Ongoing studies
We identified two ongoing studies (NCT03712228; NCT04656418). See Characteristics of ongoing studies table for more information.
Risk of bias in included studies
We assessed the risk of bias in the included studies over five domains: selection bias, performance bias, attrition bias, reporting bias and other potential sources of bias. See Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Eight studies did not report on method of randomisation and were at unclear risk of selection bias (APeX‐1; COMPACT extension; Gelfand 1976; NCT01005888; NCT01756157; NCT02052141; OPuS‐1; OPuS‐2). Banerji 2017 was at high risk of selection bias because allocation was sequential. The remaining studies were at low risk of selection bias as they used a computer‐generated system (APeX‐2), a web‐based randomisation system (HELP), or interactive response system to generate random sequence (APeX‐J; COMPACT; NCT02247739; SAHARA).
Allocation concealment
Nine studies did not report on concealment of allocation and were at unclear risk of selection bias (APeX‐1; Banerji 2017; COMPACT extension; Gelfand 1976; NCT01005888; NCT01756157; NCT02052141; OPuS‐1; OPuS‐2). The remaining studies were at low risk of selection bias, as they used computer or interactive‐based systems to ensure that allocation could not be predicted (APeX‐2; APeX‐J; COMPACT; HELP; NCT02247739; SAHARA).
Blinding
All but one study blinded the participants to their allocation. The exception was the COMPACT extension trial, an open‐label extension of the double‐blind COMPACT trial. We judged the COMPACT extension to be at high risk of performance bias. The remaining studies were at low risk of performance bias (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; COMPACT; Gelfand 1976; HELP; NCT01005888; NCT01756157; NCT02052141; NCT02247739; OPuS‐1; OPuS‐2; SAHARA).
Six studies reported that outcome assessors were blinded to study allocation and were at low risk of detection bias (APeX‐2; APeX‐J; HELP; NCT02247739; OPuS‐2; SAHARA). Both NCT02052141 and COMPACT extension stated that outcome assessors were not blinded and were at high risk of detection bias. The remaining studies were at unclear risk of detection bias (APeX‐1; Banerji 2017; COMPACT; Gelfand 1976; NCT01005888; NCT01756157; OPuS‐1).
Incomplete outcome data
All studies were at low risk of attrition bias. In some cases, this was because there was no attrition (APeX‐1; Banerji 2017; Gelfand 1976; NCT02052141; OPuS‐1); in other cases, attrition was low and evenly spread across groups or studies used an ITT analysis or both (APeX‐2; APeX‐J; COMPACT; COMPACT extension; HELP; NCT01005888; NCT01756157; NCT02247739; OPuS‐2; SAHARA).
Selective reporting
We compared each of the included studies with its published protocol. Thirteen studies reported results for all outcomes defined in the respective protocols (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; COMPACT; COMPACT extension; HELP; NCT01005888; NCT01756157; NCT02052141; NCT02247739; OPuS‐2; SAHARA). Gelfand 1976 did not publish a protocol and was at unclear risk of reporting bias. We judged OPuS‐1 at high risk of reporting bias.
Other potential sources of bias
The rare nature of the condition meant that many studies were very small. Small studies tend to overestimate treatment effects, so this should be taken into consideration.
We judged all included studies at low risk of other bias as we identified no other sources of bias. We considered the potential for cross‐over studies to bias outcomes due to carryover effects; however, the drugs used in cross‐over studies had very short half‐lives, and were, therefore, unlikely to have an impact on the second period of the cross‐over trial. Despite the potential for a unit of analysis error, we decided to combine data from cross‐over and parallel studies in order to maintain the maximum information.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Primary outcomes
Risk of hereditary angioedema attacks
Five studies comparing intervention with placebo reported on risk of HAE attacks (APeX‐1; COMPACT; Gelfand 1976; OPuS‐1; OPuS‐2). All interventions except avoralstat decreased the risk of HAE attacks; however, there were few studies for each drug (Figure 4). At approved doses, C1‐INH compared with placebo showed fewer HAE attacks than berotralstat (Analysis 1.2) (COMPACT). The RR for C1‐INH versus placebo was 0.29 (95% CI 0.16 to 0.50, 1 study, 85 participants; P < 0.001) and for berotralstat versus placebo was 0.63 (95% CI 0.39 to 1.00; 1 study, 37 participants; P = 0.05).
4.

Risk of hereditary angioedema attacks by drug (approved doses only).
1.2. Analysis.

Comparison 1: Risk of hereditary angioedema (HAE) attacks, Outcome 2: Risk of HAE attacks by drug (approved doses only)
Gelfand 1976 enroled nine participants in a study comparing danazol with placebo. The same nine people were randomised each 28‐day period to receive either danazol 200 mg capsules three times a day or placebo capsules three times a day for 28 days, for a total of 93 courses. During the 46 courses in which participants were taking danazol, there was only one HAE attack. In contrast, during the 47 courses of placebo, there were 44 HAE attacks. This was a reduction in attack rate from 93.6% to 2.2% (Gelfand 1976).
Several trials directly compared different doses of the same medication to one another (COMPACT; COMPACT extension; NCT01756157; NCT02052141). All of these trials compared different doses of C1‐INH. One trial reported the number of attacks as the rate of attacks (COMPACT extension); this trial showed no clear difference between the two doses of C1‐INH (RR 0.85, 95% CI 0.60 to 1.21; 1 study, 126 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Risk of hereditary angioedema (HAE) attacks, Outcome 6: Risk of HAE attacks (C1‐INH) – head‐to‐head trials
Eleven studies reported on change in number of HAE attacks per week (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; HELP; COMPACT; OPuS‐1; OPuS‐2; NCT01005888; NCT02247739; SAHARA). For OPuS‐1, we derived the SDs from the mean, the number of participants and the 95% CI of the intervention and control groups. APeX‐1, APeX‐2, APeX‐J, and OPuS‐2 did not report SDs. For these studies, we imputed the SDs by taking the mean of all SDs in the remaining studies.
When drugs were analysed for their ability to reduce the number of attacks per week, subcutaneous C1‐INH (C1‐INH(SC)) (COMPACT) and rhC1‐INH (NCT02247739) were the most effective drugs (i.e. they caused the largest reductions in weekly attack rates) (Figure 5; Analysis 2.2). Avoralstat was no more effective than placebo, whereas berotralstat reduced the number of weekly attacks by an average of 0.39 (95% CI −0.74 to −0.05; 3 studies, 130 participants). C1‐INH(SC) reduced attacks by 0.81 per week (95% CI −0.98 to −0.64; 1 study, 90 participants), while nanofiltered C1‐INH reduced attacks by 0.53 (95% CI −0.78 to −0.28; 1 study, 44 participants) and pdC1‐INH by 0.53 per week (95% CI −0.58 to −0.48; 1 study, 113 participants). Recombinant human C1‐INH reduced weekly attacks by 0.92 (95% CI −1.31 to −0.53; 1 study, 96 participants) and lanadelumab reduced attacks by 0.41 (95% CI −0.48 to −0.35; 2 studies, 83 participants). The mean placebo risk of weekly attacks across all studies was 0.90 (range 0.37 to 1.80).
5.

Change in number of hereditary angioedema attacks per week by drug (approved doses only).
2.2. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 2: Change in number of HAE attacks per week by drug (approved doses only)
We compared the 95% CIs of the meta‐analysis subgroups. Where the CIs did not overlap, we took this as indirect evidence of a significant difference between the interventions. Such indirect comparison of these results suggests that C1‐INH(SC) may be superior to avoralstat, berotralstat, pdC1‐INH and lanadelumab. Similarly, indirect evidence suggests that rhC1‐INH may be superior to avoralstat, berotralstat and lanadelumab.
Three trials compared different doses of C1‐INH with one another for their relative ability to reduce the number of attacks per week (Analysis 2.7) (COMPACT; NCT01756157; NCT02052141). Although all three trials individually found no reductions in attacks in response to higher doses, a meta‐analysis of the three studies combined revealed fewer attacks with a higher dose (MD −0.15, 95% CI −0.27 to −0.02; 3 studies, 153 participants).‐
2.7. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 7: Change in number of HAE attacks per week (C1‐INH) – head‐to‐head trials
Two studies reported on children and adolescents (HELP; NCT02052141). NCT02052141 enroled children aged six to 11 years and gave them either C1‐INH‐nf 1000 IU or 500 IU twice per week. Twelve participants took part in the trial. There was no clear difference in the number of attacks per week with the higher dose (MD −0.12, 95% CI −0.36 to 0.12; Analysis 2.8). The HELP trial group published a post‐hoc analysis of its adolescent participants (age 12 to 17 years) in the double‐blind phase of the study (Analysis 2.8). Five participants received lanadelumab 300 mg (three participants every four weeks, two participants every two weeks) and four participants received placebo. There was no clear difference between lanadelumab and placebo in the number of attacks per week (MD −0.14, 95% CI −0.38 to 0.10). The number of participants is currently too low to make definitive statements.
2.8. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 8: Change in number of HAE attacks per week (children and adolescents)
Mortality
There were no deaths in any study; therefore analyses were not possible.
Serious adverse events
We analysed the number of people with serious adverse events (SAEs) in each arm of studies that reported this outcome. Serious adverse events were rare (Figure 6). None of the placebo‐controlled studies reported a risk of SAEs that was different from placebo (Analysis 3.1) (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; NCT01005888; HELP; COMPACT; OPuS‐1; NCT02247739; SAHARA). No SAEs occurred in either group in NCT01005888. We intended to report on individual SAEs, but there were so few events that this would not be meaningful. The two studies that compared different doses of the same medication with one another did not reveal clear differences in the occurrence of SAEs (Analysis 3.2) (COMPACT; COMPACT extension).
6.

Risk of serious adverse events compared with placebo by drug.
3.1. Analysis.

Comparison 3: Serious adverse events, Outcome 1: Risk of serious adverse events compared with placebo by drug
3.2. Analysis.

Comparison 3: Serious adverse events, Outcome 2: Risk of serious adverse events – head‐to‐head trials
Secondary outcomes
Quality of life
The most common measure of quality of life for people with HAE is the Angioedema Quality of Life (AE‐QoL) scale. This scale has been validated and is used to measure the change in the disease state from the patient's perspective (Weller 2016). The minimum clinically important difference (MCID) for the AE‐QoL total score is a reduction of 6 points (Weller 2016). Meta‐analysis of the studies that measured the AE‐QoL revealed a clinically significant improvement in quality of life for three of the four drugs (avoralstat (OPuS‐1; OPuS‐2), berotralstat (APeX‐1; APeX‐2; APeX‐J), and lanadelumab (HELP); Analysis 4.1). Avoralstat reduced the AE‐QoL by 6.78 points (95% CI −11.61 to −1.95; 2 studies, 117 participants). Berotralstat reduced the AE‐QoL by an average of 15.28 points (95% CI −29.42 to −1.14; 3 studies, 130 participants), and lanadelumab reduced the AE‐QoL by 14.91 points (95% CI −21.89 to −7.92, 1 study, 117 participants), more than twice the cut‐off for clinical significance. In the SAHARA trial, there was no clear difference with pdC1‐INH in the first cross‐over period (MD −3.49, 95% CI −10.86 to 3.88; 1 study, 60 participants), but in the second period there was an improvement in quality of life (MD −16.87, 95% CI −22.79 to −10.95; 1 study, 53 participants) (Figure 7).
4.1. Analysis.

Comparison 4: Change in quality of life, Outcome 1: Change in Angioedema Quality of Life (AE‐QoL) Questionnaire scores
7.

Change in Angioedema Quality of Life (AE‐QoL) Questionnaire scores.
The COMPACT study reported on quality of life using the European Quality of Life Five Dimension (EQ‐5D) scale. This scale was reported as Health State Values and on the Visual Analogue Scale (VAS). The VAS is easier to interpret, and so we used this scale in our analysis. Overall, C1‐INH(SC) resulted in an increase in quality of life compared with placebo (MD 8.90, 95% CI 2.87 to 14.93; Analysis 4.2).
4.2. Analysis.

Comparison 4: Change in quality of life, Outcome 2: Difference in EQ‐5D scale compared with placebo
Finally, NCT01005888 reported on quality of life using the 36‐item Short Form (SF‐36 scale). Compared with placebo, C1‐INH‐nf increased quality of life (MD 9.04, 95% CI 2.32 to 15.76; 1 study, 32 participants; Analysis 4.3). No MCID has been established for this scale in people with HAE. Using the "half SD approach" to estimating an MCID (Norman 2003), this would suggest that an MCID for this scale may be approximately 5 points. Based on this approach, C1‐INF‐nf may increase quality of life to a clinically meaningful degree.
4.3. Analysis.

Comparison 4: Change in quality of life, Outcome 3: Change in SF‐36 compared with placebo
When we combined all reported quality of life measures using an SMD analysis, all drugs for which quality of life measurements were reported (avoralstat, berotralstat, C1‐INH (all forms), lanadelumab) led to an improvement in quality of life compared with placebo (Analysis 4.4; Figure 8): avoralstat resulted in an SMD of −0.48 (95% CI −0.84 to −0.11; 2 studies, 117 participants), berotralstat resulted in an SMD of −0.86 (95% CI −1.67 to −0.05; 3 studies, 130 participants), C1‐INH (including COMPACT; NCT01005888; SAHARA) resulted in an SMD of −0.39 (95% CI −0.75 to −0.04; 3 studies, 162 participants) and lanadelumab resulted in an SMD of −0.91 (95% CI −1.43 to −0.40; 1 study, 68 participants). Taking 0.5 units as a measure of clinical significance (Cohen 1988), both berotralstat and lanadelumab treatments resulted in clinically important improvements in quality of life.
4.4. Analysis.

Comparison 4: Change in quality of life, Outcome 4: Change in quality of life (all scales) by drug
8.

Change in quality of life (all scales) by drug.
The two studies comparing different doses of the C1‐INH(SC) with one another found no clear differences between the doses (Analysis 4.5) (COMPACT extension; NCT01756157).
4.5. Analysis.

Comparison 4: Change in quality of life, Outcome 5: Change in AE‐QoL total score – head‐to‐head trials
Severity of breakthrough attacks
Continuous outcomes
Only two studies reported severity of breakthrough attacks on a continuous scale (COMPACT; NCT01005888); both studies used C1‐INH as the intervention and placebo as the control. The COMPACT trial reported breakthrough attack severity for 40 IU/kg and 60 IU/kg; both doses were superior to placebo, with reductions in severity of around 0.3 points on a 0 to 3 scale (representing no symptoms ('0'); mild symptoms ('1'); moderate symptoms ('2'); or severe symptoms ('3')). NCT01005888 compared C1‐INH‐nf 1000 IU to placebo, and also showed a reduction in attack severity compared with placebo (Analysis 5.1).
5.1. Analysis.

Comparison 5: Difference in HAE attack severity, Outcome 1: Difference in HAE attack severity by dose (C1‐INH)
In the three studies that compared different doses of the same medication with one another, there were no clear differences in attack severity between any comparisons, and a meta‐analysis of the three studies did not change this result (MD −0.35, 95% CI −1.08 to 0.38; 3 studies, 154 participants; Analysis 5.2) (COMPACT; NCT01756157; NCT02052141).
5.2. Analysis.

Comparison 5: Difference in HAE attack severity, Outcome 2: Difference in HAE attack severity – head‐to‐head trials (C1‐INH)
Dichotomous outcomes
Three studies used a 0 to 3 scale to report the severity of breakthrough attacks (COMPACT; HELP; SAHARA), but presented data as the incidence of mild, moderate, or severe attacks, or no attacks. The risk of a severe breakthrough attack was reduced in people taking C1‐INH or lanadelumab; both drugs reduced this risk by around 80% (Analysis 5.3). The chance that a participant would have no symptoms (i.e. the participant did not have any attacks) was higher during treatment with C1‐INH or lanadelumab (percentages of participants who did not experience an attack: 39% with C1‐INH versus 9% with placebo; 44% with lanadelumab versus 2% with placebo; Analysis 5.6). Indirect comparison of these drugs can be made by examining both the magnitude of the placebo‐controlled differences and the test for subgroup differences. By this method, C1‐INH and lanadelumab appeared to be equally effective for this outcome.
5.3. Analysis.

Comparison 5: Difference in HAE attack severity, Outcome 3: Risk of a severe HAE attack by drug compared with placebo
5.6. Analysis.

Comparison 5: Difference in HAE attack severity, Outcome 6: Risk of no HAE attacks compared with placebo
Disability
Five trials reported on changes in disability (APeX‐1; APeX‐J; COMPACT; HELP; NCT01005888) (Figure 9). During C1‐INH(SC) treatment, the COMPACT study reported a placebo‐controlled change in activity impairment (measured using the Work Productivity and Activity Impairment scale) of −20.01 points (95% CI −30.86 to −9.27), but as this study did not report the individual changes, these findings were not included in the meta‐analysis. NCT01005888 reported on changes in the SF‐36 Physical Functioning component during treatment with C1‐INH‐nf. The APeX‐1 and APeX‐J (both berotralstat) and HELP (lanadelumab) trials reported changes in the Physical Functioning subscale of the AE‐QoL scale.
9.

Change in disability compared with placebo by drug (approved doses only) (standardised mean difference).
C1‐INH‐nf treatment resulted in an improvement in disability during the trial period (SF‐36 Physical Functioning component summary: MD 6.80 points, 95% CI 1.36 to 12.24; 1 study, 32 participants; Analysis 6.1) (NCT01005888). There is no MCID established for this component summary, but in children and adults with other conditions, the MCID was 10 points (Brigden 2018; Wyrwich 2005). This suggests that for the SF‐36 physical component summary, the change in disability may not be clinically meaningful.
6.1. Analysis.

Comparison 6: Disability, Outcome 1: Change in disability compared with placebo by drug (approved doses only) – SF‐36 scale
Meta‐analysis of the berotralstat trials revealed a reduction in physical impairment in the AE‐QoL Physical Functioning subscale favouring berotralstat (MD −22.5 points, 95% CI −34.91 to −10.08; 2 studies, 50 participants; Analysis 6.2) (APeX‐1; APeX‐J). The HELP trial revealed a reduction in physical impairment using the AE‐QoL Physical Functioning subscale favouring lanadelumab (MD −30.55 points, 95% CI −37.55 to −23.55; 1 study, 64 participants; Analysis 6.2). The MCID for the AE‐QoL Physical Functioning subscale has not been established, but taking the "half standard deviation" approach suggested by Norman 2003, this would suggest a difference of approximately 11.3 points (weighted mean of SDs in APeX‐1 and APeX‐J divided by two) and 10.9 points for HELP. By this measure, the placebo‐controlled change in quality of life for people receiving berotralstat was nearly double and for lanadelumab three times the cut‐off for an MCID.
6.2. Analysis.

Comparison 6: Disability, Outcome 2: Change in disability compared with placebo by drug (approved doses only) – AE‐QoL physical functioning subscale
When analysed as SMDs, all three drugs showed 'large' effect sizes, as defined by Cohen 1988, compared with placebo (C1‐INH‐nf: −0.84; berotralstat: −1.01; lanadelumab: −1.38). Tests for subgroup differences showed no clear differences between the subgroups (Analysis 6.3).
6.3. Analysis.

Comparison 6: Disability, Outcome 3: Change in disability compared with placebo by drug (approved doses only) (standardised mean difference)
Adverse events
Nine placebo‐controlled trials reported the incidence of adverse events (APeX‐1; APeX‐2; APeX‐J; Banerji 2017; COMPACT; HELP; NCT02247739; OPuS‐1; SAHARA) (Figure 10). None of the drugs tested in these trials caused a clear increase in the risk of adverse events (Analysis 7.1). Of the four studies that compared different doses of the same drug, none reported clear differences between doses (RR 1.02, 95% CI 0.96 to 1.09; 4 studies, 333 participants; Analysis 7.2) (COMPACT; COMPACT extension; NCT01756157; NCT02052141).
10.

Risk of any adverse event compared with placebo by drug.
7.1. Analysis.

Comparison 7: Risk of any adverse event compared with control, Outcome 1: Risk of any adverse event compared with placebo by drug
7.2. Analysis.

Comparison 7: Risk of any adverse event compared with control, Outcome 2: Risk of any adverse event – head‐to‐head trials
Publication bias
We assessed publication bias using a funnel plot analysis for outcomes with at least 10 included study arms (Figure 11; Figure 12). Visual inspection of the funnel plot of studies reporting on the number of HAE attacks per week suggested that it is possible that some studies of lower methodological quality with negative results may be missing (Figure 11). This slight skewing of the funnel plot was not evident in the plot of adverse events (Figure 12).
11.

Change in number of hereditary angioedema attacks per week by drug (approved doses only).
12.

Risk of any adverse event compared with placebo by drug.
Discussion
Summary of main results
Risk of hereditary angioedema attacks
For people with HAE, the number of attacks they experience is the most important outcome. Most studies reported the risk of HAE attacks during prophylactic treatment as the primary trial outcome. At doses approved by the US Food and Drug Administration, all drugs except avoralstat reduced the incidence of breakthrough attacks (berotralstat was borderline significant, P = 0.05) (Analysis 1.2). C1‐INH(SC) reduced attack rates from 81% to 23% and danazol from 94% to 2%. In contrast, taking berotralstat only reduced the risk from 91% to 57%. This result should be confirmed in other, preferably head‐to‐head studies. Nevertheless, the current findings suggest that berotralstat is only moderately effective in preventing HAE attacks when compared to placebo.
Similarly, when examined as a change in the mean number of HAE attacks per week, avoralstat showed no clear difference compared with placebo, whereas all other drugs reduced the number of attacks (Analysis 2.2). However, there were differences between subgroups. For example, the MD between berotralstat and placebo was smaller than the difference between C1‐INH(SC) and placebo, and rhC1‐INH versus placebo. Similarly, the difference between lanadelumab and placebo was smaller than the difference between C1‐INH(SC) and placebo, and rhC1‐INH versus placebo. However, our confidence in this analysis was limited by the small number of studies, with very few participants in each study.
The study comparing two doses of a single drug (C1‐INH) with one another found no clear differences between the doses (Analysis 1.6).
Quality of life
Another important outcome for people with HAE is quality of life. When measured using the validated AE‐QoL Questionnaire, both avoralstat and berotralstat lowered the AE‐QoL score compared with placebo (Analysis 4.1). Importantly, the MCID for the AE‐QoL is −6 points; therefore, avoralstat (MD 6.78 points) and berotralstat (MD −15.28 points) were effective in improving quality of life to a clinically significant degree.
Interestingly, avoralstat did not reduce the number of attacks in the OPuS‐1 and OPuS‐2 studies, but nevertheless improved quality of life (Analysis 4.4). Although the study authors described this as "intriguing" they failed to ask the study participants to explain the basis for the perceived improvement in the quality of life.
Severity of breakthrough attacks
Studies reported the reduction in severity of breakthrough attacks in two different ways. Some studies measured the severity of breakthrough attacks using a continuous scale (Analysis 5.1). The reported data show that C1‐INH(SC) was effective in reducing the mean severity of attacks compared with placebo. When measured on a 0 to 3 scale, (representing no symptoms ('0'); mild symptoms ('1'); moderate symptoms ('2'); or severe symptoms ('3')), C1‐INH(SC) reduced the severity of attacks compared with placebo (40 IU/kg twice per week: by 0.26, 60 IU/kg twice per week: by 0.30: 1000 IU twice per week: by 0.60 points). Unfortunately, no other studies reported on the change in severity using a continuous scale, so comparison between different drugs was not possible.
Other studies reported breakthrough attack severity as the percentage of people experiencing no symptoms, or a mild, moderate, or severe attack, compared with placebo (Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5). Compared with placebo, C1‐INH reduced the risk of a severe breakthrough attack by 73%. The overall RR for all C1‐INH drugs combined, compared with placebo, was 0.27 (95% CI 0.14 to 0.52); lanadelumab reduced the risk of a severe breakthrough attack to a similar degree (RR 0.22, 95% CI 0.05 to 0.88).
5.4. Analysis.

Comparison 5: Difference in HAE attack severity, Outcome 4: Risk of a moderate HAE attack compared with placebo
5.5. Analysis.

Comparison 5: Difference in HAE attack severity, Outcome 5: Risk of a mild HAE attack compared with placebo
The risk of having no symptoms also supported the use of HAE treatments. C1‐INH increased the risk of having no symptoms (RR 4.37, 95% CI 2.24 to 8.55). The RR for lanadelumab versus placebo was much higher, but based on a single, small study (RR 18.22, 95% CI 2.51 to 132.15). The test for subgroup differences showed no clear differences between the subgroups.
Disability
Studies reported change in disability for three drugs (berotralstat, C1‐INH‐nf, and lanadelumab).
Using the SF‐36 Physical Functioning component summary as a measure of disability, C1‐INH‐nf improved disability compared with placebo in one small study (MD 6.80 points, 95% CI 1.36 to 12.24). No MCID has been established for this subscale in people with HAE, but one study in children (Brigden 2018) and one study in people with asthma (Wyrwich 2005) found the MCID in these populations to be −10 points. If this were true also for HAE, then this difference would be statistically, but not clinically significant. The SMD for this analysis was −0.84, which corresponds to a 'large' effect, according to Cohen 1988. Therefore, it is likely that this difference is clinically relevant.
Two small studies comparing berotralstat with placebo used the Physical Functioning component of the AE‐QoL as a measure of disability. Meta‐analysis of the two studies demonstrated a reduction in disability of 22.5 points (95% CI −34.91 to −10.08). There is currently no established MCID for the subscales of the AE‐QoL. However, the SMD for this comparison was −1.01, which is a 'large' difference according to Cohen 1988. Therefore, it is likely that this difference is also clinically relevant.
The HELP trial revealed a reduction in physical impairment of 30.55 points in the AE‐QoL Physical Functioning subscale with lanadelumab versus placebo (95% CI −37.55 to −23.55; 1 study, 64 participants; Analysis 6.2). The SMD for this comparison was −1.38, which is a 'large' difference according to Cohen 1988. Therefore, it is likely that this difference is clinically relevant.
Adverse events
None of the studies reported a clear difference in serious adverse events (Analysis 3.1) or any adverse event (Analysis 7.1). Therefore, all drugs that were investigated in this review appear to be safe compared with placebo. There were also no clear differences in serious adverse events (Analysis 3.2) or any adverse event (Analysis 7.2) in trials comparing different doses of the same drug.
As there were no deaths in any of the studies, we could not perform analyses with mortality as an outcome.
Overall completeness and applicability of evidence
Originally, we had planned a several subgroup analyses. For example, we hoped to analyse data by age, sex, type of HAE, and BMI of the participants, by the region in which the work was done, by route of administration, etc. (Beard 2019). However, only a small number of studies met the inclusion criteria. In addition, the small number of people with HAE means that even in multicentre, multinational trials, no single study enroled more than 150 participants. In fact, the studies that focussed on children often had to pool studies to increase numbers for a statistically meaningful analysis.
For this reason, it is difficult to know how applicable the results of this analysis are for an individual. We cannot be sure if the drugs are more or less effective in men versus women, children versus adults and Type I versus Type II HAE. Furthermore, although we carried out a thorough search, we did not find any studies in people with Type III HAE.
In addition, all the studies on people with HAE were performed in Western countries. HAE is present in countries worldwide. Given that the available evidence did not allow us to determine if racial, social or other factors alter our results, we must state this as a limitation of the data.
The lack of head‐to‐head trials for different drugs is also a limitation of our study. Although we found that some studies tested different doses of the same drug, not a single identified trial tested two different drugs in the same population. Therefore, we cannot state with confidence that one drug is superior to another. However, indirect evidence (i.e. comparing the effect sizes or RRs between drugs and placebo) gave some indications that C1‐INH may be more effective than both lanadelumab and berotralstat in reducing the number of breakthrough HAE attacks per week. Furthermore, the two single studies that compared C1‐INH(SC) (COMPACT) and danazol (Gelfand 1976) with placebo suggest that both drugs may be superior to berotralstat in preventing the risk of having a breakthrough attack. However, as stated above, the indirect comparisons are hypothesis‐generating only, and should be tested with network meta‐analysis or head‐to‐head trials, or both.
Tranexamic acid and danazol are two older drugs that are taken orally. They have long been used for the prophylaxis of HAE attacks. However, the 2020 Position Paper of the Australasian Society of Clinical Immunology and Allergy stated that the drugs have "significant problems, lack of efficacy or side effects" (ASCIA 2020). However, we identified only one randomised controlled trial using danazol (Gelfand 1976), and none using tranexamic acid. Therefore, we believe there to be no high‐certainty evidence to suggest that these drugs are ineffective or unsafe. In fact, a single, double‐blind, controlled trial comparing danazol with placebo found a very large reduction in the risk of HAE attacks. However, this small trial (nine participants undertaking 47 placebo courses and 44 danazol courses) has never been replicated, used the highest dose approved by the US FDA, and included no safety data (Gelfand 1976). One non‐randomised study looked at adverse events resulting from long‐term prophylaxis with the attenuated androgens, danazol and stanozolol, and compared these adverse events with rates in people with HAE who had never received either drug (Cicardi 1997). The participants had been taking the attenuated androgens for a median of 125.5 months (range: 22 to 273 months). Cicardi 1997 found a dose‐related incidence of menstrual irregularities in 50% of premenopausal women, and a dose‐related increase in bodyweight in 28% of all participants. Hypertension was also more prevalent in people taking danazol than untreated controls. A conference abstract also described a case‐control study comparing women taking danazol versus untreated women (Zotter 2013). They found that in the 31 participants taking danazol for HAE, hirsutism was experienced by 13 women, weight gain by 12, menstrual disturbances by seven, and diaphoresis by seven. Despite this, the women rated their satisfaction with danazol treatment at 8.47 out of 10. There were no adverse changes for total cholesterol, triglycerides, low‐density lipoprotein, very low‐density lipoprotein, lipoprotein(a) and liver function. However, the study design (cohort study) precludes assigning causation to any of these outcomes. Furthermore, the participants in Cicardi 1997 who received danazol experienced two or fewer breakthrough attacks per year; so, for some patients, the reduction in attacks may be a reasonable trade‐off for increased adverse effects, particularly in men or postmenopausal women, and in people with low bodyweight and hypotension. Indeed, Zotter 2013 stated that the adverse events had not led to the discontinuation of danazol therapy in any participant.
Quality of the evidence
Most of the studies were of good methodological quality. The majority of trials had published a protocol prior to the start of the trial, were double‐blind, placebo‐controlled and reported all relevant results. The rarity of the disease means that the trials were relatively small, and there were rarely multiple trials for a drug. This made it impossible to assign comparative efficacies to drugs to treat HAE.
Almost all studies were funded by pharmaceutical companies. Although this does not necessarily mean that the results are biased in favour of the drug, we would have more confidence in the results if studies with corroborating results were published that had been funded by organisations without a financial interest in the outcome. We did not downgrade the certainty of the evidence for this reason, as the authors of the studies carried out clinical trials for several companies and several HAE drugs. Therefore, we believe the likelihood of bias for one drug, but not another, is unlikely.
Furthermore, most studies in this analysis did not report their method of randomisation or whether allocation was concealed. This leaves open the possibility that enrolment staff could anticipate the allocation of a particular patient, which can lead to biased outcomes. Similarly, around half of the studies had unclear blinding of outcome assessors, which could also have compromised the outcomes. We decided not to downgrade the certainty of evidence for these reasons, because several studies were cross‐over in design, which overcomes the potential of allocation bias, and the studies were performed at centralised facilities that use standardised methods.
The summary of findings tables reveal a generally low degree of certainty around our results (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7). The studies were downgraded mostly due to imprecision (few studies, small study sizes and few events).
We found moderate‐certainty evidence that avoralstat does not reduce the risk of HAE attacks compared with placebo, and low‐certainty evidence it does not reduce the number of attacks compared with placebo, but also moderate‐certainty evidence that it improves quality of life. We found low‐certainty evidence that avoralstat does not increase the risk of adverse events, and very‐low certainty evidence that it does not increase the risk of serious adverse events (Table 1).
Low‐certainty evidence suggests that berotralstat reduces the risk and number of HAE attacks per week and moderate‐certainty evidence that it increases quality of life. Moderate‐certainty evidence suggests that berotralstat does not increase the risk of adverse events, and low‐certainty evidence suggests that it does not increase the risk of serious adverse events (Table 2).
We found low‐certainty evidence that C1‐INH(SC) lowers the risk of HAE attacks and low‐certainty evidence that it does not increase quality of life. We also found moderate‐certainty evidence that C1‐INH(SC) does not increase the risk of adverse events, and very low‐certainty evidence that it does not increase the risk of serious adverse events (Table 3).
Low‐certainty evidence suggests that pdC1‐INH reduces the number of HAE attacks per week, and low‐certainty evidence that it does not increase quality of life. We found low‐certainty evidence that pdC1‐INH does not increase the risk of adverse events, and very low‐certainty evidence that it does not increase serious adverse events (Table 4).
Very low‐certainty evidence suggests that C1‐INH‐nf reduces the number of HAE attacks per week, increases quality of life, and reduces disability (Table 5). Similarly, very low‐certainty evidence suggests that rhC1‐INH reduces the number of HAE attacks per week (Table 6), and does not increase the risk of serious adverse events, and low‐certainty evidence that it does not increase the risk of adverse events.
Finally, low‐certainty evidence suggests that lanadelumab reduces the number of HAE attacks per week and increases quality of life. Low‐certainty evidence suggests that lanadelumab lowers the risk of adverse events, but does not change the risk of serious adverse events (Table 7).
Potential biases in the review process
Given the paucity of studies for our analysis, we made the decision to deviate from our a priori protocol (Beard 2019). We identified several studies that were clearly intended to be prophylaxis trials, but did not meet our inclusion criterion of six weeks in duration. We decided that, on balance, the benefits gained by the additional information by including the studies of a four‐week duration outweighed the risk of bias that this deviation engenders.
In order to compensate for a lack of data, we imputed several SDs using the mean SDs of similar studies. We intended to undertake sensitivity analyses removing these studies, but the paucity of information remaining after such removal resulted in meaningless data.
Agreements and disagreements with other studies or reviews
To our knowledge, there is no other meta‐analysis on this topic. The individual studies found, for the most part, similar results to our meta‐analysis, often because our subgroups contained only one or a very few trials.
Authors' conclusions
Implications for practice.
Our data show that there is an evidence base for the use of avoralstat, C1 esterase inhibitor (C1‐INH; in various forms), lanadelumab and danazol in preventing hereditary angioedema (HAE) attacks. We were unable to find any studies of the use of tranexamic acid in preventing attacks. Current data show that avoralstat is ineffective in preventing attacks, whereas the other drugs (C1‐INH, danazol, lanadelumab, berotralstat) demonstrated efficacy. All drugs for which data were available (avoralstat, berotralstat, C1‐INH (in all forms), and lanadelumab), do not appear to increase the risk of adverse events including serious adverse events. However, the implications for practice resulting from our analysis are limited. There are insufficient studies available to draw firm conclusions about the absolute or relative efficacy of any drug compared with placebo or an active comparator. It is possible that danazol, subcutaneous C1‐INH and recombinant human C1‐INH are more effective than berotralstat and lanadelumab in reducing the risk of breakthrough attacks, but the small number of studies and the small size of the studies means that the certainty of the evidence is low. This and the lack of head‐to‐head trials prevented us from drawing firm conclusions on the relative efficacy of the drugs. No studies were available in people with Type III HAE, and as such, we can provide no conclusions about the efficacy or safety (or both) of interventions for this HAE type.
Implications for research.
This analysis has highlighted the need for further investigation of all drugs for the prevention of HAE. We did not find a single trial that compared any drug with another drug. Both patients and clinicians need to know which drug is most effective and safest; the current data do not allow us to draw firm conclusions about the relative efficacy or safety of the various drugs available to patients. Furthermore, studies are needed in people with Type III HAE, and in populations of differing genetic and cultural backgrounds.
We are cognisant of the fact that the rarity of HAE makes such trials difficult and expensive, and the rarity of the condition makes manufacturers less willing to invest in the conditions. We therefore hope that a national or international funding agency will see the urgent requirement for clarity in this area, and assist with sufficient funding to provide more certainty for both patients and their doctors.
History
Protocol first published: Issue 8, 2019
Notes
Parts of the Methods section of the protocol were based on a standard template established by Cochrane Vascular.
Acknowledgements
The review authors are grateful to the Cochrane Vascular editorial team for help and support in publishing the review. Their unwavering support and expertise have significantly improved our review and we are extremely grateful for their help.
The review authors, and the Cochrane Vascular editorial base, are grateful to the following peer reviewers for their time and comments. We are also grateful to the peer reviewers who have opted to remain anonymous:
Prof Marcel Levi, University College London, UK;
Brian Duncan, USA.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW (date of most recent search: 3 August 2021) |
Angioedema* OR HAE AND INREGISTER | August 2019: 1 May 2020: 0 August 2021: 0 |
| CENTRAL via CRSO (date of most recent search: 3 August 2021) |
#1 MESH DESCRIPTOR Angioedemas, Hereditary EXPLODE ALL TREES 77 #2 (angioedema ADJ3 hereditary):TI,AB,KY 29 #3 HAE:TI,AB,KY 259 #4 #1 OR #2 OR #3 283 #5 MESH DESCRIPTOR Antibodies, Monoclonal EXPLODE ALL TREES 10335 #6 MESH DESCRIPTOR Bradykinin B2 Receptor Antagonists EXPLODE ALL TREES 13 #7 MESH DESCRIPTOR Complement C1 Inactivator Proteins EXPLODE ALL TREES 83 #8 MESH DESCRIPTOR Complement C1 Inhibitor Protein EXPLODE ALL TREES 59 #9 MESH DESCRIPTOR DANAZOL EXPLODE ALL TREES 197 #10 MESH DESCRIPTOR KALLIKREINS EXPLODE ALL TREES 1385 #11 MESH DESCRIPTOR Recombinant Proteins EXPLODE ALL TREES 8176 #12 MESH DESCRIPTOR Tranexamic Acid EXPLODE ALL TREES 830 #13 (attenuated androgens):TI,AB,KY 4 #14 BCX7353:TI,AB,KY 21 #15 Berinert:TI,AB,KY 25 #16 (bradykinin B2 receptor antagonists):TI,AB,KY 13 #17 (C1 esterase inhibitors):TI,AB,KY 0 #18 C1INH:TI,AB,KY 38 #19 C1‐INH:TI,AB,KY 115 #20 Cinryze*:TI,AB,KY 21 #21 (Complement C1*):TI,AB,KY 111 #22 (concentrated C1 esterase inhibitors):TI,AB,KY 0 #23 Danazol:TI,AB,KY 405 #24 Ecallantide:TI,AB,KY 39 #25 FFP:TI,AB,KY 400 #26 Firazyr:TI,AB,KY 7 #27 (fresh frozen plasma):TI,AB,KY 696 #28 Icatibant:TI,AB,KY 84 #29 Kalbitor:TI,AB,KY 0 #30 (kallikrein inhibit*):TI,AB,KY 113 #31 Lanadelumab:TI,AB,KY 28 #32 (monoclonal antibody):TI,AB,KY 6661 #33 (monoclonal anti‐f XII antibody):TI,AB,KY 0 #34 (nanofiltered C1 inhibitor):TI,AB,KY 10 #35 rhC1INH:TI,AB,KY 31 #36 rhC1‐INH:TI,AB,KY 5 #37 Ruconest:TI,AB,KY 5 #38 SDP:TI,AB,KY 73 #39 (tranexamic acid):TI,AB,KY 2225 #40 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 28198 #41 #4 AND #40 238 |
August 2019: 238 May 2020: 115 August 2021: 75 |
| ClinicalTrials.gov (date of most recent search: 3 August 2021) |
Angioedemas, Hereditary OR HAE | Antibodies, Monoclonal OR Bradykinin B2 Receptor Antagonists OR Complement C1 Inactivator Proteins OR Complement C1 Inhibitor Protein OR DANAZOL OR KALLIKREINS OR Recombinant Proteins OR Tranexamic Acid OR attenuated androgens | August 2019: 61 May 2020: 2 August 2021: 11 |
| ICTRP Search Portal (ICTRP portal not available May 2020) (date of most recent search: 3 August 2021) |
Angioedemas, Hereditary OR HAE | Antibodies, Monoclonal OR Bradykinin B2 Receptor Antagonists OR Complement C1 Inactivator Proteins OR Complement C1 Inhibitor Protein OR DANAZOL OR KALLIKREINS OR Recombinant Proteins OR Tranexamic Acid OR attenuated androgens | August 2019: 207 May 2020: n/a August 2021: 15 |
| MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) 1946 to present (date of most recent search: 3 August 2021) |
1 exp Angioedemas, Hereditary/ 2 (angioedema adj3 hereditary).ti,ab. 3 HAE.ti,ab. 4 or/1‐3 5 exp Antibodies, Monoclonal/ 6 exp Bradykinin B2 Receptor Antagonists/ 7 exp Complement C1 Inactivator Proteins/ 8 exp Complement C1 Inhibitor Protein/ 9 exp DANAZOL/ 10 exp KALLIKREINS/ 11 exp Recombinant Proteins/tu [Therapeutic Use] 12 exp Tranexamic Acid/ 13 attenuated androgens.ti,ab. 14 BCX7353.ti,ab. 15 Berinert.ti,ab. 16 bradykinin B2 receptor antagonists.ti,ab. 17 C1 esterase inhibitors.ti,ab. 18 C1INH.ti,ab. 19 C1‐INH.ti,ab. 20 Cinryze*.ti,ab. 21 Complement C1*.ti,ab. 22 concentrated C1 esterase inhibitors.ti,ab. 23 Danazol.ti,ab. 24 Ecallantide.ti,ab. 25 FFP.ti,ab. 26 Firazyr.ti,ab. 27 fresh frozen plasma.ti,ab. 28 Icatibant.ti,ab. 29 Kalbitor.ti,ab. 30 kallikrein inhibit*.ti,ab. 31 Lanadelumab.ti,ab. 32 monoclonal antibody.ti,ab. 33 monoclonal anti‐f XII antibody.ti,ab. 34 nanofiltered C1 inhibitor.ti,ab. 35 rhC1INH.ti,ab. 36 rhC1‐INH.ti,ab. 37 Ruconest.ti,ab. 38 SDP.ti,ab. 39 tranexamic acid.ti,ab. 40 or/5‐39 41 4 and 40 42 randomized controlled trial.pt. 43 controlled clinical trial.pt. 44 randomized.ab. 45 placebo.ab. 46 drug therapy.fs. 47 randomly.ab. 48 trial.ab. 49 groups.ab. 50 or/42‐49 51 exp animals/ not humans.sh. 52 50 not 51 53 41 and 52 |
August 2019: 713 May 2020: 63 August 2021: 79 |
| Embase via Ovid (date of most recent search: 3 August 2021) |
1 exp angioneurotic edema/ 2 (angioedema adj3 hereditary).ti,ab. 3 HAE.ti,ab. 4 or/1‐3 5 exp monoclonal antibody/ 6 exp bradykinin B2 receptor antagonist/ 7 exp complement component C1s inhibitor/ 8 exp danazol/ 9 exp kallikrein/ 10 recombinant protein/ 11 exp tranexamic acid/ 12 attenuated androgens.ti,ab. 13 BCX7353.ti,ab. 14 Berinert.ti,ab. 15 bradykinin B2 receptor antagonists.ti,ab. 16 C1 esterase inhibitors.ti,ab. 17 C1INH.ti,ab. 18 C1‐INH.ti,ab. 19 Cinryze*.ti,ab. 20 Complement C1*.ti,ab. 21 concentrated C1 esterase inhibitors.ti,ab. 22 Danazol.ti,ab. 23 Ecallantide.ti,ab. 24 FFP.ti,ab. 25 Firazyr.ti,ab. 26 fresh frozen plasma.ti,ab. 27 Icatibant.ti,ab. 28 Kalbitor.ti,ab. 29 kallikrein inhibit*.ti,ab. 30 Lanadelumab.ti,ab. 31 monoclonal antibody.ti,ab. 32 monoclonal anti‐f XII antibody.ti,ab. 33 nanofiltered C1 inhibitor.ti,ab. 34 rhC1INH.ti,ab. 35 rhC1‐INH.ti,ab. 36 Ruconest.ti,ab. 37 SDP.ti,ab. 38 tranexamic acid.ti,ab. 39 or/5‐38 40 4 and 39 41 randomized controlled trial/ 42 controlled clinical trial/ 43 random$.ti,ab. 44 randomization/ 45 intermethod comparison/ 46 placebo.ti,ab. 47 (compare or compared or comparison).ti. 48 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 49 (open adj label).ti,ab. 50 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 51 double blind procedure/ 52 parallel group$1.ti,ab. 53 (crossover or cross over).ti,ab. 54 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 55 (assigned or allocated).ti,ab. 56 (controlled adj7 (study or design or trial)).ti,ab. 57 (volunteer or volunteers).ti,ab. 58 trial.ti. 59 or/41‐58 60 40 and 59 |
August 2019: 1018 May 2020: 173 August 2021: 146 |
| CINAHL (date of most recent search: 3 August 2021) |
S53 S37 AND S52 S52 S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 S51 MH "Random Assignment" S50 MH "Triple‐Blind Studies" S49 MH "Double‐Blind Studies" S48 MH "Single‐Blind Studies" S47 MH "Crossover Design" S46 MH "Factorial Design" S45 MH "Placebos" S44 MH "Clinical Trials" S43 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S42 TX crossover OR "cross‐over" S41 AB placebo* S40 TX random* S39 TX trial* S38 TX "latin square" S37 S4 AND S36 S36 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 S35 TX tranexamic acid S34 TX SDP S33 TX Ruconest S32 TX rhC1‐INH S31 TX rhC1INH S30 TX nanofiltered C1 inhibitor S29 TX monoclonal anti‐f XII antibody S28 TX monoclonal antibody S27 TX Lanadelumab S26 TX kallikrein inhibit* S25 TX Kalbitor S24 TX Icatibant S23 TX fresh frozen plasma S22 TX Firazyr S21 TX FFP S20 TX Ecallantide S19 TX Danazol S18 TX concentrated C1 esterase inhibitors S17 TX Complement C1* S16 TX Cinryze* S15 TX C1‐INH S14 TX C1INH S13 TX C1 esterase inhibitors S12 TX bradykinin B2 receptor antagonists S11 TX Berinert S10 TX BCX7353 S9 TX attenuated androgens S8 (MH "Tranexamic Acid") S7 (MH "Recombinant Proteins+") S6 (MH "Danazol") S5 (MH "Antibodies, Monoclonal+") S4 S1 OR S2 OR S3 S3 TX HAE S2 TX angioedema N3 hereditary S1 (MH "Angioedema") |
August 2019: 76 May 2020: 8 August 2021: 10 |
| TOTAL before deduplication | August 2019: 2314 May 2020: 361 August 2021: 336 |
|
| TOTAL after deduplication | August 2019: 1863 May 2020: 299 August 2021: 267 |
|
Data and analyses
Comparison 1. Risk of hereditary angioedema (HAE) attacks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Risk of HAE attacks by drug vs placebo | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Avoralstat (all doses) | 1 | 110 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.92, 1.06] |
| 1.1.2 Berotralstat (all doses) | 1 | 77 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.65, 1.15] |
| 1.1.3 C1‐INH(SC) (all doses) | 1 | 172 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.21, 0.44] |
| 1.2 Risk of HAE attacks by drug (approved doses only) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Avoralstat (not approved) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.06] |
| 1.2.2 Berotralstat | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.39, 1.00] |
| 1.2.3 C1‐INH(SC) | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.16, 0.50] |
| 1.3 Risk of HAE attacks by dose (avoralstat) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Avoralstat 300 mg | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 1.3.2 Avoralstat 500 mg | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.05] |
| 1.4 Risk of HAE attacks by dose (berotralstat) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Berotralstat 62.5 mg | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.84, 1.31] |
| 1.4.2 Berotralstat 125 mg | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.39, 1.00] |
| 1.4.3 Berotralstat 250 mg | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.66, 1.16] |
| 1.4.4 Berotralstat 350 mg | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 0.99] |
| 1.5 Risk of HAE attacks by dose (C1‐INH(SC)) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 C1‐INH(SC) 40 IU/kg | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.19, 0.51] |
| 1.5.2 C1‐INH(SC) 60 IU/kg | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.16, 0.50] |
| 1.6 Risk of HAE attacks (C1‐INH) – head‐to‐head trials | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.21] |
1.1. Analysis.

Comparison 1: Risk of hereditary angioedema (HAE) attacks, Outcome 1: Risk of HAE attacks by drug vs placebo
1.3. Analysis.

Comparison 1: Risk of hereditary angioedema (HAE) attacks, Outcome 3: Risk of HAE attacks by dose (avoralstat)
1.4. Analysis.

Comparison 1: Risk of hereditary angioedema (HAE) attacks, Outcome 4: Risk of HAE attacks by dose (berotralstat)
1.5. Analysis.

Comparison 1: Risk of hereditary angioedema (HAE) attacks, Outcome 5: Risk of HAE attacks by dose (C1‐INH(SC))
Comparison 2. Change in number of HAE attacks per week.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Change in number of HAE attacks per week by drug | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Avoralstat | 2 | 158 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.37, 0.18] |
| 2.1.2 Berotralstat | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.45, ‐0.17] |
| 2.1.3 C1‐INH(SC) | 1 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐0.98, ‐0.67] |
| 2.1.4 pdC1‐INH | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.58, ‐0.48] |
| 2.1.5 C1‐INH‐nf | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.28] |
| 2.1.6 rhC1‐INH | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.31, ‐0.53] |
| 2.1.7 lanadelumab | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.45, ‐0.32] |
| 2.2 Change in number of HAE attacks per week by drug (approved doses only) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Avoralstat (not approved) | 2 | 158 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.37, 0.18] |
| 2.2.2 Berotralstat | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.74, ‐0.05] |
| 2.2.3 C1‐INH(SC) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.98, ‐0.64] |
| 2.2.4 C1‐INH‐nf | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.28] |
| 2.2.5 pdC1‐INH (not approved) | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.58, ‐0.48] |
| 2.2.6 rhC1‐INH (not approved) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.31, ‐0.53] |
| 2.2.7 Lanadelumab | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.48, ‐0.35] |
| 2.3 Change in number of HAE attacks per week by dose (avoralstat) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 300 mg 3 times/day | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.10, 0.28] |
| 2.3.2 400 mg 3 times/day | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.75, ‐0.15] |
| 2.3.3 500 mg 3 times/day | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.19, 0.19] |
| 2.4 Change in number of HAE attacks per week by dose (berotralstat) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 < 100 mg | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 2.4.2 100–149 mg | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.74, 0.05] |
| 2.4.3 150 mg | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.37, ‐0.05] |
| 2.4.4 250 mg | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.65, ‐0.19] |
| 2.4.5 350 mg | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.64, ‐0.22] |
| 2.5 Change in number of HAE attacks per week by dose (C1‐INH – all forms) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 2000 IU twice per week | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.58, ‐0.48] |
| 2.5.2 1000 IU twice per week | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.28] |
| 2.5.3 60 IU/kg twice per week | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.98, ‐0.64] |
| 2.5.4 50 IU/kg twice per week | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.52, ‐0.70] |
| 2.5.5 50 IU/kg once per week | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.16, ‐0.26] |
| 2.5.6 40 IU/kg twice per week | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.58, ‐0.44] |
| 2.6 Change in number of HAE attacks per week by dose (lanadelumab) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 150 mg every 4 weeks | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.46, ‐0.28] |
| 2.6.2 300 mg every 4 weeks | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.45, ‐0.27] |
| 2.6.3 300 mg every 2 weeks | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.48, ‐0.35] |
| 2.7 Change in number of HAE attacks per week (C1‐INH) – head‐to‐head trials | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.7.1 C1‐INH high dose vs low dose (all forms) | 3 | 153 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.27, ‐0.02] |
| 2.8 Change in number of HAE attacks per week (children and adolescents) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 Children (C1‐INH‐nf 1000 IU vs 500 IU) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.36, 0.12] |
| 2.8.2 Adolescents (lanadelumab vs placebo) | 1 | 9 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.38, 0.10] |
2.1. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 1: Change in number of HAE attacks per week by drug
2.3. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 3: Change in number of HAE attacks per week by dose (avoralstat)
2.4. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 4: Change in number of HAE attacks per week by dose (berotralstat)
2.5. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 5: Change in number of HAE attacks per week by dose (C1‐INH – all forms)
2.6. Analysis.

Comparison 2: Change in number of HAE attacks per week, Outcome 6: Change in number of HAE attacks per week by dose (lanadelumab)
Comparison 3. Serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Risk of serious adverse events compared with placebo by drug | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Avoralstat | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.80] |
| 3.1.2 Berotralstat | 3 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.02, 24.03] |
| 3.1.3 C1‐INH‐nf | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.1.4 C1‐INH(SC) | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.14] |
| 3.1.5 pdC1‐INH | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.10] |
| 3.1.6 rhC1‐INH | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.06, 34.66] |
| 3.1.7 Lanadelumab | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.08, 10.39] |
| 3.2 Risk of serious adverse events – head‐to‐head trials | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Short‐term trials | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.96] |
| 3.2.2 Long‐term trials | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.32, 4.01] |
Comparison 4. Change in quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Change in Angioedema Quality of Life (AE‐QoL) Questionnaire scores | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Avoralstat | 2 | 117 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐11.61, ‐1.95] |
| 4.1.2 Berotralstat | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐15.28 [‐29.42, ‐1.14] |
| 4.1.3 pdC1‐INH | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐10.37 [‐23.47, 2.74] |
| 4.1.4 Lanadelumab | 1 | 117 | Mean Difference (IV, Random, 95% CI) | ‐14.91 [‐21.89, ‐7.92] |
| 4.2 Difference in EQ‐5D scale compared with placebo | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 8.90 [2.87, 14.93] |
| 4.3 Change in SF‐36 compared with placebo | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 9.04 [2.32, 15.76] |
| 4.4 Change in quality of life (all scales) by drug | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.4.1 Avoralstat | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.84, ‐0.11] |
| 4.4.2 Berotralstat | 3 | 130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.67, ‐0.05] |
| 4.4.3 C1‐INH (all forms) | 3 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.75, ‐0.04] |
| 4.4.4 Lanadelumab | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.43, ‐0.40] |
| 4.5 Change in AE‐QoL total score – head‐to‐head trials | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.5.1 C1‐INH(SC) + rh hyaluronidase | 1 | 67 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.29, 0.67] |
| 4.5.2 C1‐INH(SC) | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.20, 0.62] |
Comparison 5. Difference in HAE attack severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Difference in HAE attack severity by dose (C1‐INH) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 40 IU/kg twice per week | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.49, ‐0.03] |
| 5.1.2 60 IU/kg twice per week | 1 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.52, ‐0.08] |
| 5.1.3 1000 IU twice per week | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.99, ‐0.21] |
| 5.2 Difference in HAE attack severity – head‐to‐head trials (C1‐INH) | 3 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.08, 0.38] |
| 5.3 Risk of a severe HAE attack by drug compared with placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 C1‐INH | 2 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.14, 0.52] |
| 5.3.2 Lanadelumab | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.88] |
| 5.4 Risk of a moderate HAE attack compared with placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.4.1 C1‐INH | 2 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.83, 2.66] |
| 5.4.2 Lanadelumab | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.05] |
| 5.5 Risk of a mild HAE attack compared with placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.5.1 C1‐INH | 2 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [0.67, 14.11] |
| 5.5.2 Lanadelumab | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [0.50, 41.54] |
| 5.6 Risk of no HAE attacks compared with placebo | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.6.1 C1‐INH | 2 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 4.37 [2.24, 8.55] |
| 5.6.2 Lanadelumab | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 18.22 [2.51, 132.15] |
Comparison 6. Disability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Change in disability compared with placebo by drug (approved doses only) – SF‐36 scale | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 6.80 [1.36, 12.24] |
| 6.2 Change in disability compared with placebo by drug (approved doses only) – AE‐QoL physical functioning subscale | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.2.1 Berotralstat | 2 | 50 | Mean Difference (IV, Random, 95% CI) | ‐22.50 [‐34.91, ‐10.08] |
| 6.2.2 Lanadelumab | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐30.55 [‐37.55, ‐23.55] |
| 6.3 Change in disability compared with placebo by drug (approved doses only) (standardised mean difference) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.3.1 C1‐INH | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.57, ‐0.12] |
| 6.3.2 Berotralstat | 2 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.62, ‐0.40] |
| 6.3.3 Lanadelumab | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐1.94, ‐0.82] |
Comparison 7. Risk of any adverse event compared with control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Risk of any adverse event compared with placebo by drug | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1.1 Avoralstat | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.16] |
| 7.1.2 Berotralstat | 3 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.88, 1.22] |
| 7.1.3 C1‐INH(SC) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.84, 1.27] |
| 7.1.4 pdC1‐INH | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.42] |
| 7.1.5 rhC1‐INH | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.71, 2.70] |
| 7.1.6 Lanadelumab | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.77, 1.47] |
| 7.2 Risk of any adverse event – head‐to‐head trials | 4 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.09] |
| 7.2.1 Short‐term trials | 3 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 7.2.2 Long‐term trials | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
APeX‐1.
| Study characteristics | ||
| Methods |
Design: dose‐ranging, placebo‐controlled, double‐blind, parallel, randomised trial Exclusions postrandomisation: none reported Losses to follow‐up: 2 participants never received a trial regimen or entered any diary data and 3 discontinued the trial regimen owing to an AE or laboratory abnormality Duration of study: 1 year (trial initiated in August 2016, the last participant observation in August 2017) Unit of randomisation: participant |
|
| Participants |
Country: international (86 patients screened from 26 sites across Europe, Canada and Australia) Setting: outpatient Number: 77 participants underwent randomisation, 75 received berotralstat (BCX7353) or placebo, 72 completed the trial; 23 participants received placebo, 7 received berotralstat 62.5 mg, 14 received berotralstat 125 mg, 15 received berotralstat 250 mg, and 18 received berotralstat 350 mg Age (mean): 44.5 (SD 12.5) years Sex: male 29 (38.7%); female 46 (61.3%) Inclusion criteria: adults aged 18–70 years with Type I or II HAE with history of ≥ 2 angioedema attacks per month for 3 consecutive months within a 6‐month period Exclusion criteria: suspected to have C1 inhibitor resistance or using a C1 inhibitor, androgens, or tranexamic acid for prophylaxis of attacks within 7 days before screening |
|
| Interventions | Berotralstat 62.5 mg once daily Berotralstat 125 mg once daily Berotralstat 250 mg once daily Berotralstat 350 mg once daily Placebo once daily Treatment duration: 28 days |
|
| Outcomes | Number of attacks (overall, by location), change in AE‐QoL, AEs | |
| Funding | Sponsored by BioCryst Pharmaceuticals. | |
| Declarations of interest | Numerous conflicts of interest among study authors; see disclosure form at www.nejm.org/doi/suppl/10.1056/NEJMoa1716995/suppl_file/nejmoa1716995_disclosures.pdf | |
| Notes | Funded by BioCryst, but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported on. |
| Other bias | Low risk | None. |
APeX‐2.
| Study characteristics | ||
| Methods |
Design: phase 3, randomised, double‐blind, placebo‐controlled parallel‐group multicentre study Exclusions postrandomisation: none reported Losses to follow‐up: 12 participants discontinued treatment early (4 receiving berotralstat 110 mg; 3 receiving berotralstat 150 mg and 5 receiving placebo). In addition, 5 participants discontinued treatment because of a laboratory result abnormality or AE (3 receiving berotralstat 110 mg; 1 receiving berotralstat 150 mg and 1 receiving placebo), 4 discontinued treatment due to perceived lack of efficacy (1 receiving berotralstat 110 mg; 1 receiving berotralstat 150 mg and 2 receiving placebo), 2 withdrew consent (1 receiving berotralstat 150 mg and 2 receiving placebo), and 1 withdrew consent for other reasons (1 receiving placebo) Duration of study: 24 weeks Unit of randomisation: participant |
|
| Participants |
Country: 40 sites in 11 countries, including the US, Canada and Europe Setting: outpatient Number: children or adults aged ≥ 12 years with Type I or II HAE. 121 people were randomised; 120 received ≥ 1 dose of treatment (41 received berotralstat 110 mg, 40 received berotralstat 150 mg, and 39 received placebo) Age (mean): 41.6 (SD 15.2) years Sex: female 80 (66.1%); male 41 (33.9%) Inclusion criteria: people with HAE aged ≥ 12 years if living in the US and Canada and ≥ 18 years if living in Europe. People with a C1‐INH functional level between 50% and the assay LLN (74%) or a C4 value greater than the LLN could qualify for inclusion under additional alternative protocol‐specified criteria. Used a prospective run‐in period of up to 70 days to determine baseline attack rate. Patients with ≥ 2 distinct investigator‐confirmed HAE attacks requiring treatment or causing functional impairment in first 56 days of prospective run‐in period were eligible for enrolment. Exclusion criteria: used androgen or tranexamic acid prophylaxis within 28 days of screening or C1‐INH prophylaxis within 14 days of screening. |
|
| Interventions | Berotralstat 110 mg once daily Berotralstat 150 mg once daily Placebo once daily Treatment duration: 24 weeks |
|
| Outcomes | Rate of HAE attacks, AE‐QoL, days with HAE symptoms, responders, rescue medication use, treatment satisfaction | |
| Funding | Funded by BioCryst Pharmaceuticals. | |
| Declarations of interest | Quote: "B. Zuraw reports personal fees from Adverum Biotechnologies, Attune Pharmaceuticals, BioCryst Pharmaceuticals, CSL Behring, Intellia Therapeutics, Pharming, and Shire/Takeda; research grants and travel support from the United States Hereditary Angioedema Association; and a laboratory service agreement from Ionis Pharmaceuticals outside the submitted work. B. Zuraw also has a TSKA patent pending (licensee, US Hereditary Angioedema Association [US HAEA]). W. R. Lumry reports grants from BioCryst Pharmaceuticals during the conduct of the study; in addition, he is a member of the US HAEA Medical Advisory Board, and he reports the following: grants and personal fees from CSL Behring and Shire/Takeda; grants from Ionis Pharmaceuticals and KalVista; and personal fees from Adverum Biotechnologies, Intellia, and Pharming outside the submitted work. D. Johnston reports personal fees from BioCryst Pharmaceuticals, CSL Behring, Pharming, Regenxbio, and Takeda outside the submitted work. E. Aygören‐Pürsün reports grants and personal fees from BioCryst Pharmaceuticals and grants from CSL Behring and Shire during the conduct of the study. In addition, E. Aygören‐Pürsün reports grants and personal fees from BioCryst Pharmaceuticals, CSL Behring, and Shire/Takeda; grants from KalVista; and personal fees from Pharming outside the submitted work. A. Banerji reports grants from BioCryst Pharmaceuticals, as well as personal fees and advisory board fees from BioCryst Pharmaceuticals, CSL Behring, KalVista, Pharming, Pharvaris, and Takeda outside the submitted work. J. Best reports grants and personal fees from BioCryst Pharmaceuticals, CSL Behring, Ionis Pharmaceuticals, KalVista, Pharming, and Shire/Takeda; in addition, she is a member of the US HAEA Medical Advisory Board, and she reports grants and personal fees from AstraZeneca, Genentech, Novartis, and Sanofi Regeneron outside the submitted work. S. Christiansen reports personal fees from BioCryst Pharmaceuticals, CSL Behring, and Takeda advisory boards outside the submitted work. J. S. Jacobs reports contracted research support from BioCryst Pharmaceuticals during the conduct of the study, and personal fees from Pharming and personal fees and contracted research support from CSL Behring and Takeda outside the submitted work. K. V. Sitz reports personal fees and grants from BioCryst Pharmaceuticals during the conduct of the study, as well as grants from 3M, AstraZeneca, GlaxoSmithKline, Novartis, Pearl, and Watson outside the submitted work. R. Gower reports clinical research and advisory board payments from BioCryst Pharmaceuticals during the conduct of the study, as well as speaker, advisory board, and clinical research trial payments and consultant fees from CSL Behring and Shire/Takeda outside the submitted work; in addition, R. Gower reports advisory board and clinical research trial payments, as well as consultant fees from Pharming outside the submitted work. T. Kinaciyan reports clinical study conducting fees from BioCryst Pharmaceuticals during the conduct of the study, personal fees and clinical study conducting fees from Shire/Takeda, expert meetings fees from CSL Behring, and clinical study conducting fees from KalVista outside the submitted work. J. Hanzlíková reports clinical study conducting fees from and cooperation with BioCryst Pharmaceuticals, CSL Behring, and Takeda. J. T. Anderson reports personal fees and other support from BioCryst Pharmaceuticals during conduct of the study, reports personal fees, clinical research, and advisory board fees from BioCryst Pharmaceuticals during the conduct of the study, and clinical research fees, speaker fees, and advisory board fees from CSL Behring, Pharming, and Shire/Takeda outside the submitted work. W. Yang is a member of advisory boards for BioCryst Pharmaceuticals, CSL Behring, Merck, Novartis, Sanofi, and Shire/Takeda; in addition, he has received speaker fees for AstraZeneca, Merck, Novartis, and Shire/Takeda and has received research grants from Aimmune Therapeutics, ALK, AnaptysBio, AstraZeneca, BioCryst Pharmaceuticals, CSL Behring, DBV Technologies, Dermira, Genentech, GlaxoSmithKline, Glenmark, Pharming, Regeneron, Roche, Sanofi, and Shire/Takeda. R. Tachdjian reports speaker and advisory board fees from CSL Behring, Pharming, and Takeda, as well as research support from Ionis Pharmaceuticals. P. Busse reports grants and personal fees from BioCryst Pharmaceuticals, CSL Behring, Novartis, Pharming, ResTORbio, and Shire/Takeda; she also reports personal fees from AstraZeneca, CVS Health, Fresenius, GlaxoSmithKline; the Law Offices of Levin, Riback, Adelman, and Flangel; and Pearl Therapeutics and Vedderprice. In addition, she has received nonfinancial support from the US HAEA and the American Academy of Allergy, Asthma & Immunology outside the submitted work. T. Craig reports past research, consultant, and speaker fees from CSL Behring and Takeda; speaker and consultant fees from Pharming; and research and consultant fees from BioCryst Pharmaceuticals; in addition, he is a member of the US HAEA Medical Advisory Board. H. H. Li reports grants from BioCryst Pharmaceuticals during the conduct of the study and grants and personal fees from CSL Behring, Pharming, and Shire/Takeda outside the submitted work. H. Farkas reports grants and personal fees from CSL Behring, Pharming, and Shire/Takeda, as well as personal fees from BioCryst Pharmaceuticals and KalVista outside the submitted work. J. M. Best, D. Clemons, M. Cornpropst, S. Dobos, H. A. Iocca, D. Kargl, E. Nagy, S. Murray, P. Collis, and W. P. Sheridan are employees of BioCryst Pharmaceuticals. M. Maurer reports grants and personal fees from BioCryst Pharmaceuticals during the conduct of the study; grants and personal fees from CSL Behring, KalVista, Moxie, and Shire/Takeda; grants from Pharming; and personal fees from Pharvaris outside the submitted work. M. Riedl reports grants and personal fees from BioCryst Pharmaceuticals during the conduct of the study; grants and personal fees from CSL Behring, Pharming, and Shire/Takeda; grants from Ionis Pharmaceuticals; and personal fees from Adverum Biotechnologies, Attune, KalVista, and Pharvaris outside the submitted work; in addition, he is a member of the US HAEA Medical Advisory Board. The rest of the authors declare that they have no relevant conflicts of interest." | |
| Notes | Funded by BioCryst, but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Quote: "All patients, investigators, and site and sponsor personnel were blinded to treatment group allocation, except for sponsor or vendor staff responsible for the management of study drug supplies." Computer generation of randomisation prevented anticipation of allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All patients, investigators, and site and sponsor personnel were blinded to treatment group allocation, except for sponsor or vendor staff responsible for the management of study drug supplies." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All patients, investigators, and site and sponsor personnel were blinded to treatment group allocation, except for sponsor or vendor staff responsible for the management of study drug supplies." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was ITT. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported on. |
| Other bias | Low risk | None. |
APeX‐J.
| Study characteristics | ||
| Methods |
Design: phase 3, randomised, double‐blind, placebo‐controlled, parallel group trial Exclusions postrandomisation: none reported Losses to follow‐up: 1 participant in the placebo group discontinued treatment early due to a TEAE of urticaria Duration of study: 24 weeks (quote: "This study remains ongoing and data presented herein summarize the results of the 24‐week placebo‐controlled period only") Unit of randomisation: participant; randomisation stratified by baseline expert‐confirmed attack rate (≥ 2 attacks/month vs < 2 attacks/month) at time of randomisation |
|
| Participants |
Country: 11 sites in Japan Setting: outpatient Number: children or adults aged ≥ 12 years with Type I or II HAE. 19 people randomised to receive once‐daily treatment (6 received berotralstat 110 mg, 7 received berotralstat 150 mg and 6 received placebo) Age (mean): 42 (SD 13) years Sex: 3 male (16%); 16 female (84%) Inclusion criteria: aged ≥ 12 years with a clinical diagnosis of Type I or II HAE, defined as having a C1‐INH functional level < 50% and C4 level below the LLN reference range as assessed during screening period. Patients with C1‐INH functional level between 50% and the assay LLN (74%) or a C4 value above the LLN could qualify via alternative protocol‐specified criteria. Patients underwent a prospective run‐in period of 56 days to determine eligibility. Patients with ≥ 2 independent expert‐confirmed HAE attacks during the prospective run‐in period were eligible for enrolment. Exclusion criteria: used androgens or tranexamic acid for prophylaxis of angioedema attacks within the 28 days before the screening visit or had any planned initiation during study, or had used C1‐INH for prophylaxis of angioedema attacks within 14 days before screening or had any planned initiation during study. |
|
| Interventions | Berotralstat 110 mg once daily Berotralstat 150 mg once daily Placebo once daily Treatment duration: 24 weeks |
|
| Outcomes | Rate of HAE attacks, number of days with HAE symptoms, change in AE‐QoL, responders, AEs, SAEs | |
| Funding | Funded by BioCryst Pharmaceuticals, Inc, Durham, North Carolina, USA | |
| Declarations of interest | Quote: "DH reports speaker fees from CSL Behring, Kyowa Kirin, Otsuka, Shire, and Takeda outside the submitted work. GC, MC, SCM, SMD, EN, SVD, LR, JB, HI, PC, and WPS are employees of BioCryst Pharmaceuticals. GC, EN, LR, and WPS hold stock options in BioCryst Pharmaceuticals. MH reports personal fees from BioCryst Pharmaceuticals during the conduct of the study; personal fees from Shire/Takeda; and grants and personal fees from CSL Behring, outside the submitted work; and a grant of the Ministry of Health, Labour and Welfare of Japan. IO, YSuzuki, TF, KK, EM, SM, OI, YSasaki, and MT have nothing to disclose." | |
| Notes | Funded by BioCryst, but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomized 1:1:1 to berotralstat 110 mg, berotralstat 150 mg, or placebo into part 1 of the study via an interactive response system." |
| Allocation concealment (selection bias) | Low risk | Allocation was blinded from investigators, staff and participants; interactive response system prevents knowledge of the next allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Allocation was blinded from investigators, staff and participants. |
| Blinding of outcome assessment (detection bias) | Low risk | Allocation was blinded from investigators, staff and participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported on. |
| Other bias | Low risk | None. |
Banerji 2017.
| Study characteristics | ||
| Methods |
Design: randomised, multicentre, double‐blind, placebo‐controlled, ascending dose trial Exclusions postrandomisation: 1 participant was found after trial enrolment not to have met the criteria for HAE with C1‐INH deficiency (C1‐INH testing was not consistent with Type I or II HAE despite historical laboratory tests indicating otherwise) Losses to follow‐up: 1 participant prematurely discontinued the trial after 1 dose Duration of study: approximately 120 days from the time of enrolment Unit of randomisation: participant |
|
| Participants |
Country: 12 study sites planned for the US, Italy and Jordan Setting: outpatient Number: 37 people with HEA with C1‐INH deficiency were randomly assigned to 1 of 5 groups (4 received lanadelumab 30 mg, 4 received lanadelumab 100 mg, 5 received lanadelumab 300 mg, 11 received lanadelumab 400 mg, 13 received placebo) Age (mean): 39.9 (SD 13.8) years Sex: 23 female (62%); 14 male (38%) Inclusion criteria: aged ≥ 18 years and a documented diagnosis of Type I or II HAE with C1‐INH deficiency, with diagnosis based on meeting all the following criteria: clinical history consistent with HAE with C1‐INH deficiency, C1‐INH antigen or functional level < 40% of normal level (patients with C1 inhibitor antigen or functional level 40–50% of normal level could be enroled if they also had a C4 level below normal range and family history consistent with Type I or II HAE with C1‐INH deficiency), and an age at reported onset of first angioedema symptoms ≤ 30 years or family history consistent with Type I or II HAE with C1‐INH deficiency. Patients must have had ≥ 2 attacks of angioedema per year, with ≥ 1 attack in previous 6 months. Exclusion criteria: received long‐term prophylactic medications for HEA with C1‐INH deficiency in previous 90 days, used a C1‐INH within 7 days before trial enrolment, had participated in another investigational study in previous 90 days, or had exposure within previous 5 years to a monoclonal antibody or recombinant protein bearing a Fc domain. |
|
| Interventions | Lanadelumab 30 mg every 14 days Lanadelumab 100 mg every 14 days Lanadelumab 300 mg every 14 days Lanadelumab 400 mg every 14 days Placebo every 14 days Treatment duration: 50 days |
|
| Outcomes | Number of HAE attacks, withdrawals due to AEs, SAEs, all AEs, plasma concentration of lanadelumab, pharmacokinetics of lanadelumab | |
| Funding | Funded by Dyax | |
| Declarations of interest | Quote: "Dr. Banerji reports receiving fees for serving on an advisory board from Alnylam Pharmaceuticals and grant support from Shire, CSL Behring, and Dyax; Dr. Busse, receiving consulting fees from CSL Behring, Shire, and Dyax; Dr. Lumry, receiving consulting fees (paid to his institution) from ViroPharma (now Shire), Valeant Pharmaceuticals (now Salix Pharmaceuticals), BioCryst Pharmaceuticals, and CSL Behring, lecture fees from ViroPharma (now Shire) and CSL Behring, and grant support from ViroPharma (now Shire), BioCryst Pharmaceuticals, and CSL Behring; Dr. Jacobs, receiving lecture fees and fees for serving on advisory boards from Dyax and Shire and grant support from Shire, CSL Behring, and BioCryst Pharmaceuticals; Dr. Baker, receiving grant support from Dyax, Shire, Pharming Group, and CSL Behring; Dr. Bernstein, receiving consulting fees from Shire, CSL Behring, and Salix Pharmaceuticals, lecture fees from Shire and CSL Behring, and grant and travel support from Dyax, Shire, CSL Behring, and BioCryst Pharmaceuticals; Dr. Li, receiving consulting fees from Dyax, ViroPharma (now Shire), CSL Behring, and Salix Pharmaceuticals/Pharming Group, lecture fees from ViroPharma (now Shire) and CSL Behring, and travel and grant support from Dyax, ViroPharma (now Shire), CSL Behring, Salix Pharmaceuticals/Pharming Group, and BioCryst Pharmaceuticals; Dr. Cicardi, receiving fees for board membership from Dyax (now Shire), CSL Behring, Alnylam Pharmaceuticals, and the Swedish Orphan Biovitrum, lecture fees from Dyax (now Shire), CSL Behring, and the Swedish Orphan Biovitrum, fees for the development of educational presentations from Dyax (now Shire), and grant support from Shire; Dr. Riedl, receiving consulting fees from Shire, Dyax, CSL Behring, BioCryst Pharmaceuticals, Salix Pharmaceuticals, Ionis Pharmaceuticals, Global Blood Therapeutics, and Arrowhead Pharmaceuticals, lecture fees from Shire, Dyax, CSL Behring, and Salix Pharmaceuticals, and grant support from Shire, Dyax, CSL Behring, BioCryst Pharmaceuticals, Pharming Group, and Ionis Pharmaceuticals; Dr. Kushner, receiving consulting fees from Dyax; Dr. Stevens, receiving fees for serving on an advisory board from Relypsa, receiving consulting fees from Dyax, Shire, Intarcia Therapeutics, Arsanis, Forum Pharmaceuticals, and Seres Therapeutics, and being chief medical officer for Arsanis; Dr. Soo, Dr. Sexton, Dr. Kenniston, Mr. Faucette, and Dr. Biedenkapp, being employees of Dyax; and Mr. Iarrobino, Dr. TenHoor, Dr. Mensah, Dr. Chyung, and Dr. Adelman, being former employees of Dyax. In addition, Dr. Sexton, Dr. TenHoor, Dr. Kenniston, Mr. Faucette, and Dr. Adelman are named inventors on a pending patent related to assays for determining plasma kallikrein system biomarkers (WO/2015/061183); Dr. Kenniston is a named inventor on a pending patent related to anti‐plasma kallikrein antibodies (WO/2014/152232 and US 20160017055); Dr. Sexton is a named inventor on a patent related to plasma kallikrein binding proteins (20160102150); Dr. Sexton, Dr. TenHoor, Dr. Kenniston, Mr. Faucette, Dr. Chyung, and Dr. Adelman are named inventors on a pending patent related to the evaluation and treatment of bradykinin‐mediated disorders (20150362493 and WO/2014/113712); and Mr. Iarrobino, Dr. Sexton, Dr. TenHoor, Dr. Kenniston, Mr. Faucette, Dr. Biedenkapp, Dr. Chyung, and Dr. Adelman are named inventors on a pending patent related to plasma kallikrein binding proteins and uses thereof in treating hereditary angioedema (WO/2015/112578); all these patents are held by Dyax (now Shire). No other potential conflict of interest relevant to this article was reported." | |
| Notes | Study also included low‐dose study arms, 30 mg (4 participants) and 100 mg (4 participants), which were not used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation was sequential. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 37 participants completed the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported on. |
| Other bias | Low risk | None. |
COMPACT.
| Study characteristics | ||
| Methods |
Design: randomised, multinational, multicentre, double‐blind, placebo‐controlled, dose‐ranging cross‐over trial Exclusions postrandomisation: none reported Losses to follow‐up: 11 patients discontinued for various reasons. 3 AEs led to trial discontinuation: pulmonary embolism in 1 participant who received placebo, urticaria in 1 participant who received C1‐INH(SC) (CSL830) 60 IU, and an increase in liver aminotransferase levels in 1 participant who received C1‐INH(SC) 60 IU Duration of study: from December 2013 to October 2015 Unit of randomisation: participant |
|
| Participants |
Country: international Setting: outpatient Number: children and adults aged ≥ 12 years with Type I or II HAE. 90 people with C1‐INH HAE were randomised to 1 of 4 treatment sequences with 2 doses: 40 IU/kg (45 participants), 60 IU/kg (45 participants). Of the 90 people who underwent randomisation, 79 completed trial Age (mean): 39 (SD 14.9) years Sex: 30 male (33%); 60 female (67%) Inclusion criteria: capable of providing written informed consent/assent and willing and able to adhere to all protocol requirements, or the subject's parent(s) or legal representative(s) capable of providing written informed consent; male or female, aged ≥ 12 years at time of providing written informed consent/assent (as appropriate), with clinical diagnosis of Type I or II HAE C1‐INH deficiency; experienced ≥ 4 HAE attacks (requiring acute treatment or medical attention or causing functional impairment over a consecutive 2‐month period within 3 months prior to screening) as documented in the person's medical records; patients who have used oral medication for prophylaxis against HAE attacks (i.e. androgens, tranexamic acid, progestins) within 3 months of the screening visit should have a stable regimen (dose and administration) during 3 months prior to screening; patients using oral medications for prophylaxis were expected to continue their stable regimen throughout the study period* (After amendment of the study protocol, the inclusion criterion in the original protocol read: willing to cease any pre‐existing HAE prophylaxis (e.g. C1‐INH, androgens, antifibrinolytics) after informed consent was obtained and patient was assessed by the investigator to be able to be adequately treated pharmacologically on acute treatments of HAE attacks alone); investigator believed person was willing and able to adhere to all protocol requirements; assessed by investigator as able to appropriately store study medication and capable of being trained to administer study medication (by participant or caregiver) outside study centre setting. Exclusion criteria: diagnosis of HAE with normal C1‐INH or features consistent with acquired C1‐INH deficiency; history of arterial or venous thrombosis requiring anticoagulant therapy or current, clinically significant prothrombotic risk; known incurable malignancy at the time of screening; bodyweight < 40 kg at screening visit; any clinical condition that is likely to interfere with the evaluation of C1‐INH(SC) or study conduct; use of intravenous C1‐INH for routine prophylaxis against HAE attacks (i.e. administered every 3 or 4 days) within 3 months of screening visit or plans to use C1‐INH for routine prophylaxis against attacks during study. Use of intravenous C1‐INH for preprocedure prevention of attacks was permitted, not exceeding 1 dose prior to each procedure; assessed by investigator as having HAE unable to be adequately managed pharmacologically with on‐demand treatment, administered either independently or with assistance; clinically significant history of poor response to C1‐INH therapy for management of HAE; female of childbearing potential not using or unwilling to use a reliable method of contraception or not sexually abstinent during study or not surgically sterile; females who started taking or changed dose of any hormonal contraceptive regimen or hormone replacement therapy (i.e. oestrogen/progesterone‐containing products) within 3 months prior to screening visit; intention to become pregnant during study; pregnant or breastfeeding; participation in another interventional clinical study within 30 days prior to screening visit, or at any time during study; alcohol, drug or medication abuse within 1 year prior to screening visit; currently receiving a therapy not permitted in study; mental condition rendering the person (or their legally acceptable representative(s)) unable to understand the nature, scope, and possible consequences of the study; known or suspected hypersensitivity to investigational product or its excipients; previously randomly assigned to or participated in the run‐in period of current study; employee at study site or spouse/partner/relative of the investigator or subinvestigators; any issue that, in investigator's opinion, would render the person unsuitable for study participation. |
|
| Interventions | C1‐INH(SC) 40 IU/kg twice‐weekly C1‐INH(SC) 60 IU/kg twice‐weekly Placebo twice‐weekly Regimen: C1‐INH(SC) 40 IU/kg bodyweight self‐administered during first 16‐week treatment period followed by placebo for the second 16‐week treatment period or vice versa (i.e. placebo first and C1‐INH second); or C1‐INH 60 IU/kg followed by placebo or vice versa Treatment duration: 16 weeks per treatment or placebo period |
|
| Outcomes | Rate of HAE attacks, attack severity, HAE symptoms, rescue medication use, C1‐INH activity, AE, SAEs, withdrawals due to AEs, quality of life. | |
| Funding | Supported by CSL Behring. Sponsor was involved in study design, data analysis and decisions concerning submission of data for publication. | |
| Declarations of interest | Quote: "HHL received institutional support from CSL Behring for the conduct of this study, and travel expenses and/or consultancy fees and speaker’s honoraria from CSL Behring, Shire/Dyax/ViroPharma, and Salix/Pharming. TC is a speaker for CSL Behring, Grifols and Dyax/Shire. He performs research for BioCryst, Boehringer Ingelheim, CSL Behring, Genentech, GlaxoSmithKline, Grifols, Merck, Novartis, Pharming, Sanofi, and Shire. He has received consultancy fees and/or speaker’s honoraria from BioCryst, Bellrose, CSL Behring, Dyax, Merck, Novartis, Pharming Technologies, and Shire, and has received non‐financial support from CSL Behring, Shire, and Grifols. BZ reports grant support from the Department of Defense and consultancy fees from Alnylam, Arrowhead Pharmaceuticals, BioCryst Pharmaceuticals, Nektar, CSL Behring, and Shire, and led the Scientific Steering Committee for this study. HJL has received grant support from CSL Behring, consultancy fees, and speaker’s honoraria from CSL Behring, Pharming, and Shire, and travel support from CSL Behring. MC has received grants from Shire and personal fees from Alnylam, BioCryst Pharmaceuticals, CSL Behring, Dyax, KalVista Pharming Technologies, Shire, Sobi (Swedish Orphan Biovitrum), and ViroPharma. KB reports personal fees from CSL Behring and Shire, outside the submitted work. HB received consultancy fees and speaker’s honoraria from CSL Behring, Pharming, and Shire. WL reports grant support from BioCryst Pharmaceuticals, CSL Behring, and Shire/Viropharma/Dyax; consultancy fees paid to his institution from Adverum, BioCryst Pharmaceuticals, CSL Behring, Pharming Technologies, and Shire/Viropharma/Dyax; speaker’s fees from Pharming Technologies, Shire/Viropharma and CSL Behring; and non‐financial support from the US Hereditary Angioedema Association outside the submitted work. JB reports grant support and personal fees from BioCryst Pharmaceuticals, CSL Behring, and Shire, outside the submitted work. MM reports grant support and consultant/speaker’s fees from CSL Behring, Shire, Dyax, and Shire; and personal fees from Salix and Pharming Technologies, outside the submitted work. DL has served on the speaker’s bureau, as a consultant, on a steering committee, and as a clinical investigator for CSL Behring. MR has received research grants from BioCryst Pharmaceuticals, CSL Behring, Dyax, Pharming Technologies, and Shire; consultant fees from Adverum Biotechnologies, Alnylam Pharmaceuticals, BioCryst Pharmaceuticals, CSL Behring, Global Blood Therapeutics, Ionis Pharmaceuticals, KalVista Pharmaceuticals, Pharming Technologies, and Shire; speaker’s honoraria from CSL Behring, Shire, and Pharming; and is an uncompensated advisory board member for the US Hereditary Angioedema Association. TM, SP HF and IP are employees of CSL Behring." | |
| Notes | Funded by CSL Behring but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using an interactive response system. |
| Allocation concealment (selection bias) | Low risk | Interactive response system prevents knowledge of the next allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded to their allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% attrition but ITT analysis used. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported. |
| Other bias | Low risk | None. |
COMPACT extension.
| Study characteristics | ||
| Methods |
Design: multicentre, randomised, parallel, open‐label extension of COMPACT trial Exclusions postrandomisation: none reported Losses to follow‐up: of 15 (11.9%) participants who discontinued treatment before week 53, 9 (7.1%) discontinued during treatment period 1 (4/63 (6.3%) in C1‐INH(SC) 60 IU/kg group and 5/63 (7.9%) in C1‐INH(SC) 40 IU/kg group) and 6 (4.8%) participants discontinued during treatment period 2 (3 in each group). In addition, 1 (1.6%) participant from C1‐INH(SC) 60 IU/kg group discontinued during extension period. 4 AEs led to study discontinuation, including the unrelated SAE of myocardial infarction. 4 (3.2%) participants discontinued treatment because of pregnancy, having received a total exposure of 15–85 doses of C1‐INH(SC), inclusive of administrations throughout the first trimester until pregnancy was detected. Duration of study: treatment period 1: 24 weeks; treatment period 2: 28 weeks Unit of randomisation: participant |
|
| Participants |
Country: 32 hospitals across 11 countries (Australia, Canada, Czech Republic, Germany, Hungary, Israel, Italy, Romania, Spain, the UK, and the US) Setting: outpatient Number: 63 participants received C1‐INH(SC) 40 IU/kg and 63 participants received C1‐INH(SC) 60 IU/kg Age (mean): 40.5 (SD 15.6) years Sex: 50 male (40%); 76 female (60%) Inclusion criteria: participants who completed COMPACT and study treatment‐naive patients were eligible to enrol into the COMPACT extension study to receive ≥ 52 weeks of continuous therapy with C1‐INH(SC); aged ≥ 6 years with biochemically confirmed diagnosis of Type I (C1‐INH deficiency) or Type II (C1‐INH dysfunction) HAE, with C1‐INH functional levels < 50%; people with a history of experiencing frequent attacks (≥ 4 attacks within 2 consecutive months) before enrolment into the COMPACT programme were eligible; people using oral prophylactic medication were required to be on a stable regimen and willing to continue this regimen for the study duration. Exclusion criteria: any clinical conditions likely to interfere with evaluation of study drug, clinical history of poor response to C1‐INH therapy, and any patient whose HAE could not be adequately managed by on‐demand pharmacological treatment as assessed by investigator. |
|
| Interventions | C1‐INH 40 IU/kg self‐administered twice‐weekly C1‐INH 60 IU/kg self‐administered twice‐weekly Treatment duration: 1.5–2 years |
|
| Outcomes | Long‐term safety: serious adverse events, withdrawals due to adverse events, thromboembolic events, anaphylaxis, HAE attacks requiring hospitalisation, injection site reactions, other adverse events, rate of HAE attacks, rescue medication use, duration of attacks, number of symptomatic days. | |
| Funding | CSL Behring (Marburg, Germany) | |
| Declarations of interest | Quote: "T. Craig reports grant support from CSL Behring during the conduct of the study; is a speaker for CSL Behring, Dyax, Grifols, Pharming, and Shire; reports grant support from AstraZeneca, BioCryst, Boehringer Ingelheim, CSL Behring, Dyax, Genentech, GlaxoSmithKline, Grifols, Merck, Novartis, Pharming, Sanofi, and Shire; has received consultancy fees and/or speaker’s honoraria from BioCryst, Bellrose, CSL Behring, Dyax, Grifols, Merck, Novartis, Pharming Technologies, and Shire; has received travel support from CSL Behring, Pharming, and Shire; and has received nonfinancial support from CSL Behring, Shire, and Grifols. B. Zuraw reports grant support from the Department of Defense; reports consultancy fees from Adverum, Alnylam, Arrowhead Pharmaceuticals, BioCryst, Nektar, CSL Behring, and Shire; and has led the Scientific Steering Committee for this study. H. Longhurst has received grant support, personal fees, and nonfinancial support from CSL Behring during the conduct of the study; grant support from BioCryst and Shire; personal fees from Adverum, BioCryst, Pharming, and Shire; and travel support from CSL Behring and nonfinancial support from Pharming and Shire. M. Cicardi has received grants from Shire and personal fees from Alnylam, BioCryst, CSL Behring, Dyax, KalVista, Pharming Technologies, Shire, Sobi (Swedish Orphan Biovitrum), and ViroPharma. K. Bork reports speaker fees from CSL Behring and Shire, outside the submitted work. C. Grattan reports personal fees as chair of the COMPACT Data Safety Monitoring Board (DSMB) from CSL Behring during the conduct of the study. C. Katelaris has received honoraria as a speaker and advisory board chair for Novartis, Shire, and Sequirus; has received travel support from Shire; and is a Principal Investigator for trials conducted by CSL Behring. G. Sussman has received grant support and personal fees from Novartis and personal fees from Merck, CSL Behring, and Pfizer, outside the submitted work. P. K. Keith has received grant support from CSL Behring and Shire during the conduct of the study, and consulting and speaker’s honoraria from CSL Behring and Shire, outside the submitted work. W. Yang has served as an advisory board member for BioCryst Pharmaceuticals, CSL Behring, and Shire, and has received research and/or educational grants from BioCryst Pharmaceuticals, CSL Behring, Shire, and Pharming, outside of the submitted work. J. Hébert has been a Principal Investigator for CSL Behring clinical trials. P. Staubach‐Renz has been a clinical trial investigator for CSL Behring and has received grants and/or speaker/ consultant fees from AbbVie, Astellas, Celgene, CSL Behring, Genentech, Janssen, Karrer, LEO, Leti, Lilly, MSD, Novartis, Pfizer, Shire, Sobi (Swedish Orphan Biovitrum), UCB, and ViroPharma, outside the submitted work. I. Martinez‐Saguer has received grants and speaker/consultant fees and been a clinical trial investigator for BioCryst, CSL Behring, Sobi (Swedish Orphan Biovitrum), Shire, and ViroPharma. M. Magerl has received financial compensation from CSL Behring for the conduct of the study and has also received speaker/consultant fees from BioCryst, CSL Behring, Novartis, Shire, and Pharming Technologies. E. Aygören‐Pürsün has received grant support as a clinical trial investigator for this study and has received honoraria as a speaker/advisor and/or grant support/clinical trial investigator support from BioCryst, CSL Behring, KalVista, Pharming Technologies, Shire, and ViroPharma. H. Farkas received institutional support for a clinical trial for this study from CSL Behring; advisory board/consultancy fees and/or speaker’s honoraria from BioCryst, CSL Behring, Shire, and Sobi (Swedish Orphan Biovitrum); and travel support from CSL Behring. S. Neri reports educational grants and honoraria for advisory boards and symposia from CSL Behring, Shire, and ViroPharma and other support from Pharming, outside of the submitted work. A. Reshef reports grant support from CSL Behring during the conduct of the study and has received grant support from Pharming. I. Crisan reports institutional support from CSL Behring during the conduct of the study. T. Caballero reports institutional support from CSL Behring during the conduct of the study; personal fees from BioCryst, CSL Behring, GlaxoSmithKline, MSD, and Sobi; personal fees and other support from CSL Behring, Novartis, and Shire HGT; and research funding from the IdiPaz Program for Promoting Research Activities, outside the submitted work. M. L. Baeza reports institutional support from CSL Behring during the conduct of the study. H. Li received institutional support from CSL Behring for the conduct of this study; travel expenses and/or consultancy fees and speaker’s honoraria from BioCryst, CSL Behring, Shire, and Salix/Pharming; and institutional support for clinical trials from BioCryst, Pharming, and Shire. W. Lumry reports grant support from CSL Behring, Pharming, and Shire/Viropharma; consultancy fees/honorarium paid to his institution from Adverum, BioCryst Pharmaceuticals, CSL Behring, and Shire/Viropharma; travel support paid to his institution from CSL Behring and the US Hereditary Angioedema Association; and fees for participation in review activities paid to his institution from BioCryst during the conduct of the study. J. A. Bernstein reports grant support and personal fees from BioCryst, CSL Behring, and Shire, outside the submitted work. I. Hussain reports institutional support from CSL Behring during the conduct of the study. J. Anderson reports personal fees as a consultant and for speaker’s bureau participation from CSL Behring, Pharming, and Shire; and other clinical research support from BioCryst, CSL Behring, Dyax, and Shire, outside the submitted work. L. B. Schwartz reports grant support from CSL Behring during the conduct of the study and grant support from Dyax outside the submitted work. J. Jacobs reports grant support from CSL Behring during the conduct of the study; grant support from BioCryst, Dyax Corp, and Shire PLC; and honoraria and advisory fees from CSL Behring, Dyax Corp, Shire, Pharming, and Shire PLC, outside the submitted work. M. Manning reports grant support from BioCryst, CSL Behring, Dyax, and Shire; and personal fees from CSL Behring, Dyax, Pharming Technologies, Salix, and Shire, outside the submitted work. D. Levy has served on the speaker’s bureau, as a consultant, on a steering committee, and as a clinical investigator for CSL Behring. M. Riedl reports grant support from CSL Behring during the conduct of the study and has received research grants from BioCryst, CSL Behring, Dyax, Ionis Pharmaceuticals, Pharming Technologies, and Shire; has served as a consultant and/or speaker for Adverum Biotechnologies, Alnylam Pharmaceuticals, Arrowhead Pharmaceuticals, BioCryst, CSL Behring, Dyax, Global Blood Therapeutics, Ionis Pharmaceuticals, KalVista Pharmaceuticals, Pharming Technologies, Salix Pharmaceuticals, and Shire; and is an uncompensated advisory board member for the US Hereditary Angioedema Association, outside the submitted work. S. Christiansen reports receiving personal fees as an advisory board member from Bio‐Cryst, CSL Behring, and Shire, outside of the submitted work. H. Feuersenger, I. Pragst, S. Mycroft, D. Pawaskar, and I. Jacobs are employees of CSL Behring. I. Pragst has patents WO 2016/131958 A1 and WO 2018/037046 pending. D. Pawaskar has a patent pending. The rest of the authors declare that they have no relevant conflicts of interests." | |
| Notes | Funded by CSL Behring but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | .Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label extension. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label extension. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% attrition but evenly distributed and ITT analysis used. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported. |
| Other bias | Low risk | We identified no other sources of bias. |
Gelfand 1976.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, single‐centre, cross‐over trial Exclusions postrandomisation: none reported Losses to follow‐up: 2 participants did not complete their series of treatment courses because the main study was terminated Duration of study: 6–11 months Unit of randomisation: participant |
|
| Participants |
Country: US Setting: outpatient Number: 5 women (aged 25–38 years) and 4 men (aged 28–63 years) with HAE were selected. Each had an attack frequency ≥ 1 per month. Diagnosis established on basis of characteristic clinical history, low serum C4 and low C1‐INH activity Age (mean): 34.9 (SD 11.4) years Sex: 4 men (44.4%); 5 women (55.6%) Inclusion criteria: attack frequency ≥ 1 per month; diagnosis of HAE. Exclusion criteria: not stated. |
|
| Interventions | Danazol capsules 200 mg 3 times/day Placebo capsules 3 times/day 9 people were randomised each 28‐day period to receive either danazol or placebo capsules for 28 days, for a total of 93 courses (46 courses in which participants were taking danazol, 47 courses where the participants were taking placebo). |
|
| Outcomes | Rate of HAE attacks, functional C1‐INH, C4 concentrations | |
| Funding | Funded and carried out by NIH. | |
| Declarations of interest | No conflicting interest information provided. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation generation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether allocation concealment took place. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded to allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not published. |
| Other bias | Low risk | We identified no other sources of bias. |
HELP.
| Study characteristics | ||
| Methods |
Design: randomised, multinational, double‐blind, parallel, placebo‐controlled trial Exclusions postrandomisation: 1 patient was a screen failure after randomisation to group that received lanadelumab 150 mg every 4 weeks. This patient was not treated and was withdrawn from study. This patient was counted in randomised population but excluded from both ITT and safety populations Losses to follow‐up: 6 participants in each group did not complete study. 2 who received placebo withdrew from study due to TEAEs of tension headache and HAE attack, which were of moderate severity. 1 participant in lanadelumab 300 mg every 4 weeks group with metabolic syndrome, fatty liver and multiple concomitant suspect medications withdrew due to isolated, asymptomatic and transient elevation of alanine transaminase (140 U/L) and aspartate transaminase (143 U/L) classified as related and severe on day 139 Duration of study: randomisation between 3 March 2016 and 9 September 2016; last day of follow‐up 13 April 2017 Unit of randomisation: participant |
|
| Participants |
Country: 41 sites in Canada, Europe, Jordan and the US Setting: outpatient Number: 126 people randomised: subcutaneous lanadelumab 150 mg every 4 weeks (29 participantsa), 300 mg every 4 weeks (29 participants), 300 mg every 2 weeks (27 participants), or placebo (41 participants). 113 (90.4%) completed study aQuote: "One patient was determined to be a screen failure after randomization to the group that received 150mg of lanadelumab every 4 weeks. This patient was not treated and was withdrawn from the study. This patient was counted in the randomized population but was excluded from both the intent‐to‐treat and safety populations." Age (mean): 40.7 (SD 14.7) years Sex: 37 male (29.6%) and 88 female (70.4%) Inclusion criteria: aged ≥ 12 years at screening with confirmed diagnosis of Type I or II HAE with ≥ 1 investigator‐confirmed attack per 4 weeks. Exclusion criteria: concomitant diagnosis of another form of chronic recurrent angioedema, such as acquired angioedema, Type III HAE, idiopathic angioedema, or recurrent angioedema associated with urticaria; participated in a prior lanadelumab study; dosed with or exposure to an investigational device within 4 weeks of screening; exposed to angiotensin‐converting enzyme inhibitors or any oestrogen‐containing medications with systemic absorption (such as oral contraceptives or hormonal replacement therapy) within 4 weeks of screening; exposed to androgens (e.g. stanozolol, danazol, oxandrolone, methyltestosterone, or testosterone) within 2 weeks of entering run‐in period; used long‐term prophylactic therapy for HAE (C1‐INH, attenuated androgens or antifibrinolytics) within 2 weeks of entering run‐in period; used short‐term prophylactic therapy for HAE within 7 days of entering run‐in period (defined as C1‐INH, attenuated androgens or antifibrinolytics used to avoid angioedema complications from medically indicated procedures; any of the following liver function test abnormalities: alanine aminotransferase > 3 × upper limit of normal, or aspartate aminotransferase > 3 × upper limit of normal, or total bilirubin > 2 × upper limit of normal (unless the bilirubin elevation was a result of Gilbert's syndrome); pregnancy or breastfeeding; any condition that, in opinion of investigator or sponsor, could have compromised safety or compliance, precluded successful conduct of study or interfered with interpretation of results (e.g. history of substance abuse or dependence, significant pre‐existing illness or other major comorbidity that the investigator considered could have confounded interpretation of study results). |
|
| Interventions | Lanadelumab 150 mg every 4 weeks Lanadelumab 300 mg every 4 weeks Lanadelumab 300 mg every 2 weeks Placebo every 2 weeks Treatment period: 26 weeks |
|
| Outcomes | Rate of HAE attacks, severity of HAE attacks, use of rescue medication, hypersensitivity reactions, antidrug antibodies, health‐related quality of life, AEs, injection site reactions, SAEs, withdrawals due to AEs. | |
| Funding | Dyax Corp (now Shire Human Genetic Therapies). | |
| Declarations of interest | Quote: "Dr Banerji reports grants from Shire, and being on advisory boards for Alnylam, BioCryst, CSL Behring, Pharming, and Shire. Dr Riedl reports grants, scientific consulting fees, and speaking fees to his institution from BioCryst, CSL Behring, Pharming, and Shire; and scientific consulting fees to his institution from Adverum, Alnylam, Ionis, and Kalvista. Dr Bernstein reports grants and personal fees from CSL Behring, Pharming, and Shire; and personal fees from BioCryst. Dr Cicardi reports grants, membership on advisory boards, and speaker fees from Pharming and Shire; and advisory boards and speaker fees from Adverum, BioCryst, and CSL Behring. Dr Longhurst reports personal fees for consulting and travel support for scientific meetings from Shire; personal fees and nonfinancial and other support from BioCryst and CSL Behring; other support from Pharming; and personal fees from Kalvista. Dr Zuraw reports personal fees for consultations from Adverum, BioCryst, CSL Behring, and Shire; chair, advisory board membership, and grants from the US Hereditary Angioedema Association; and adjudication board membership with Genentech, Novartis, and Sanofi. Dr Busse reports consulting and research fees from Pharming, and Shire; consulting fees from CSL Behring, Global Life Sciences, Pearl Therapeutics, and Teva; and medico‐legal fees support from the Law Offices of Victoria Broussard. Dr Anderson reports personal fees and other support for clinical research and consultation from CSL Behring and Shire; personal fees and other support for consultation from Pharming; and clinical research fees from BioCryst. Dr Magerl reports clinical research fees, personal fees, and nonfinancial support for consultations from Shire; and personal fees and nonfinancial support for consultations from BioCryst, CSL Behring, and Pharming. Dr Martinez‐Saguer reports honoraria, research funding, travel grants from, serving as a consultant for, and being on the advisory boards of BioCryst, CSL Behring, Pharming, and Shire. Dr Davis‐Lorton reports being a principal investigator for Novartis and Shire; advisory boards for CSL Behring, Pharming, and Shire; and speaker bureaus for CSL Behring, Pharming, and Shire. Dr Li reports grants, personal fees, and nonfinancial support from CSL Behring, Pharming, and Shire. Dr Craig reports grants and personal fees for consultations, research, and speaking from CSL Behring and Shire; grants and personal fees for speaking from Grifols; grants and personal fees for research and consultations from BioCryst; and advisory medical board membership for the US Hereditary Angioedema Association. Dr Jacobs reports grants and personal fees from CSL Behring and Shire; and personal fees from Pharming. Dr Johnston reports personal fees for consultations and speaking from CSL Behring and Shire; and consulting fees from BioCryst and Pharming. Dr Shapiro reports personal fees for clinical research and speaking from Shire and research support from Dyax and BioCryst. Dr Yang reports being a consultant and a member of the advisory board for CSL Behring and Shire; an unrestrictive educational grants from CSL Behring, Novartis, and Shire; and research grants from Aimmune, AstraZeneca, BioCryst, CSL Behring, DBV Technologies, Dyax/Shire, Galderma, Genentech/ Roche, GlaxoSmithKline, Merck, Pfizer, Pharming, Sanofi‐Genzyme, and Shire; and membership on the Canadian Hereditary Networks Guideline Publication Committee, and medical advisor to Hereditary Angioedema Canada. Dr Lumry reports grants from BioCryst, CSL Behring, Pharming, and Shire; consulting fees to his institution from Adverum, BioCryst, CSL Behring, Pharming, and Shire; travel support from CSL Behring and Shire; fees to his institution for being on the advisory board for BioCryst, speakers bureau fees to his institution from Alk, Genentech, and Stallergenes/Geer; manuscript preparation fees to his institution from Pharmacy Times; development of educational presentations to his institution from Medscape/WebMD; and medical advisory board payment to his institution from the US Hereditary Angioedema Association. Dr Manning reports grants and personal fees from CSL Behring and Shire; advisory board member for Shire; grants from Dyax; speaker bureau fees from Pharming; and personal fees and advisory board member for Salix. Dr Schwartz reports consulting fees from Dyax, Helix, Sanofi‐Aventis, and ViroPharma; research support from CSL Behring, Dyax, and Merck; royalties from Virginia Commonwealth University Innovation Gateway; and payment to participate in the “Atopic Dermatitis in America” study from the Asthma and Allergy Foundation of America. Dr Soteres reports other income from Shire; being an investigator in the conduct of the study; and personal fees for consulting, advisory boards, and speaking from Shire. Dr Zaragoza‐Urdaz reports consulting fees from BioCryst, Shire, and ViroPharma; and lecture fees from Baxter, Dyax, Pharming, Shire, Teva, and ViroPharma. Dr Gierer reports research grants from Dyax, Genentech/Novartis, and Shire. Dr Smith reports investigator fees from Shire. Dr Tachdjian reports advisory board and speaking honoraria from Shire, CSL Behring, and Pharming; and research grants from Shire and CSL Behring. Dr Wedner reports grants from Shire. Dr Hebert reports investigator fees from and being on an advisory board of Shire and consultant fees from GlaxoSmithKline, Merck, Novartis, Teva, Shire, CSL Behring, and Sanofi. Dr Staubach reports fees for advisory board membership from AbbVie, Beiersdorf, Celgene, CSL Behring, Genentech, Janssen, Leti, MSD, Novartis, Octapharma, Sanofi, Shire, Sobi, and UCB; consulting fees from CSL Behring; grants from Novartis and Shire; speaker fees from AbbVie, Astellas, CSL Behring, Janssen, Leo, Leti, Lilly, MSD, Pfleger, Novartis, and Shire; and travel support from CSL Behring, Janssen, MSD, Novartis, and Pfizer. Dr Schranz reports being a full‐time employee of and owning stock/options in Shire. Ms Baptista reports being a full‐time employee of and owning stock and options in Shire. Dr Nothaft reports being a full‐time employee of and owning stock and options in Shire. Dr Maurer reports grants and personal fees for consultations and speaking from Shire; and grants and personal fees from BioCryst, Pharming, and Shire. No other disclosures were reported." | |
| Notes | Funded by Dyax Corp (now Shire Human Genetic Therapies) but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation system. |
| Allocation concealment (selection bias) | Low risk | Allocation system used by blinded study staff. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Even withdrawal across groups, and ITT analysis used. |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in protocol were reported. |
| Other bias | Low risk | We identified no other sources of bias. |
NCT01005888.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, cross‐over trial Exclusions postrandomisation: after completion of treatment, 3 participants (1 in C1‐INH group and 2 in placebo group) were judged by an independent, blinded expert to have had episodes that were not true attacks of HAE. Participants were then excluded from efficacy analysis Losses to follow‐up: during first period, 1 participants from each group withdrew, leaving 22 participants (11 in each group) who completed first period and then crossed over to other treatment for second period Duration of study: 2 × 12 weeks Unit of randomisation: participant |
|
| Participants |
Country: US Setting: outpatient Number: acute attack treatment trial: 68 participants (35 in C1‐INH group and 33 in placebo group); prophylaxis trial (11 in placebo with cross‐over to C1‐INH group and 11 in C1‐INH with cross‐over to placebo) Age (mean): 36.5 (SD 15.9) years Sex: 15 male (22.1%); 53 female (77.9%) Inclusion criteria: aged ≥ 6 years with confirmed diagnosis of HAE, including a low C4, normal C1q and a low antigenic or functional C1‐INH level or a mutation in the C1‐INH gene known to cause HAE. Exclusion criteria: low C1q level, history of a B‐cell cancer, presence of anti‐C1 inhibitor antibody, history of allergic reaction to C1‐INH or other blood or plasma products, pregnancy, and narcotic addiction. |
|
| Interventions | C1‐INH‐nf 1000 IU twice‐weekly Placebo (saline) twice‐weekly Treatment duration: 12 weeks then crossing over to other treatment |
|
| Outcomes | Rate of HAE attacks, attack severity, attack duration, use of rescue medication, functional C1 inhibitor, AEs | |
| Funding | Lev Pharmaceuticals (now owned by ViroPharma Biologics) | |
| Declarations of interest | Quote: "Dr. Zuraw reports receiving consulting fees from Lev Pharmaceuticals, CSL Behring, Jerini (now Shire), and Dyax; reimbursements for travel or accommodation expenses from Lev Pharmaceuticals, Shire, and Dyax; fees for serving on the speakers bureau of the Robert Michael Educational Institute; grant support from Lev Pharmaceuticals, Pharming, and Shire; and fees for providing expert testimony for Lev Pharmaceuticals; Dr. Busse, receiving consulting fees and reimbursements for travel or accommodation expenses from Lev Pharmaceuticals; grant support from Lev Pharmaceuticals and Shire Pharmaceuticals; fees for reviewing a patient file from Eichorn and Eichorn; payment for manuscript preparation from Innovative Strategic Communications; and fees for serving on the speakers bureaus of ViroPharma and the Robert Michael Educational Institute; Dr. White, receiving consulting fees from Lev Pharmaceuticals and Dyax; reimbursements for travel or accommodation expenses from Dyax; honoraria from Dyax; grant support from Lev Pharmaceuticals, Dyax, Shire, ViroPharma, and Pharming; and fees for serving on the speakers bureau of ViroPharma; Dr. Jacobs, receiving consulting fees from ViroPharma; reimbursements for travel or accommodation expenses from Lev Pharmaceuticals and ViroPharma; honoraria from ViroPharma; grant support from Lev Pharmaceuticals; and fees for serving on the speakers bureaus of ViroPharma and Lev Pharmaceuticals; Dr. Lumry, receiving consulting fees and honoraria from ViroPharma; consulting fees from Dyax and Shire; reimbursements for travel or accommodation expenses from ViroPharma, Dyax, and Shire; grant support from Lev Pharmaceuticals, Dyax, and Shire; and payment for development of educational presentations from Dyax; Dr. Baker, receiving grant support from Lev Pharmaceuticals, Shire, Dyax, and Pharming; Dr. Craig, receiving consulting fees from ViroPharma, Dyax, and CSL Behring; fees for participation in review activities and reimbursements for travel or accommodation expenses from ViroPharma; honoraria from ViroPharma, Dyax, and CSL Behring; grant support from Lev Pharmaceuticals, ViroPharma, Dyax, CSL Behring, Pharming, and Shire; and payment for development of educational presentations from Dyax, CSL Behring, and ViroPharma; Dr. Grant, receiving reimbursements for travel or accommodation expenses from Lev Pharmaceuticals and Dyax and grant support from Lev Pharmaceuticals, Dyax, and Shire; Dr. Hurewitz, receiving consulting fees from Shire; reimbursements for travel or accommodation expenses from ViroPharma, Shire, Dyax, and CSL Behring; honoraria from Shire; and grant support from Lev Pharmaceuticals, ViroPharma, Shire, Dyax, and CSL Behring; Dr. Bielory, receiving reimbursements for travel or accommodation expenses from Lev Pharmaceuticals and grant support from Lev Pharmaceuticals and STARx Clinical Research Center; Dr. Cartwright, receiving grant support and consulting fees or honoraria from Lev Pharmaceuticals and ViroPharma; Dr. Koleilat, receiving grant support from Lev Pharmaceuticals; Dr. Ryan, receiving grant support from Lev Pharmaceuticals; Dr. Schaefer, receiving grant support from Lev Pharmaceuticals and reimbursements for travel or accommodation expenses from ViroPharma; Dr. Manning, receiving reimbursements for travel or accommodation expenses and grant support from Lev Pharmaceuticals and ViroPharma; honoraria from ViroPharma; and payment for development of educational presentations from ViroPharma; Dr. Patel, receiving grant support from Lev Pharmaceuticals; Dr. Bernstein, receiving consulting fees from Dynova, Lantheus, and Flint Hills Resources; honoraria from AstraZeneca, Alcon Labs, Dyax, ViroPharma, and CSL Behring; fees for providing expert testimony on environmental and drug reaction cases; and grant support from Lev Pharmaceuticals, Dyax, ViroPharma, CSL Behring, Shire, Pharming, Dynova, and Flint Hills Resources; and being listed as a patent holder on a patent held by the University of Cincinnati for a biosensor to detect airborne chemicals from Biosensors; Dr. Friedman, receiving reimbursements for travel or accommodation expenses from ViroPharma and grant support from Lev Pharmaceuticals; Dr. Wilkinson, receiving fees for study visits from Lev Pharmaceuticals and grant support from Dyax; Dr. Tanner, receiving grant support from Lev Pharmaceuticals; Dr. Gunther, receiving grant support from Lev Pharmaceuticals; Dr. Levy, receiving grant support from Lev Pharmaceuticals and ViroPharma; consulting fees from CSL Behring, Alcon Labs, Dyax, Jerini, and Sepracor; reimbursements for travel or accommodation expenses from CSL Behring and Dyax; and payment for manuscript preparation from Cadent Communications; Dr. McClellan, receiving a grant from Lev Pharmaceuticals; Dr. Redhead, receiving a grant from Lev Pharmaceuticals; Dr. Guss, receiving grant support from Lev Pharmaceuticals; Dr. Heyman, receiving consulting fees from Lev Pharmaceuticals; Dr. Blumenstein, receiving consulting fees and reimbursements for travel or accommodation expenses from Lev Pharmaceuticals and ViroPharma; Dr. Kalfus, receiving consulting fees and reimbursements for travel or accommodation expenses from Lev Pharmaceuticals and ViroPharma; payment for development of educational presentations from ViroPharma; and stocks and stock options from Lev Pharmaceuticals; and being previously employed as vice president of medical affairs at Lev Pharmaceuticals; and Dr. Frank, receiving consulting fees from ViroPharma, CSL Behring, Shire, Dyax, and Pharming; fees for providing expert testimony for Lev Pharmaceuticals, Jerini, and CSL Behring; reimbursements for travel or accommodation expenses from ViroPharma; and grant support from Lev Pharmaceuticals and CSL Behring. No other potential conflict of interest relevant to this article was reported." | |
| Notes | Funded by Lev Pharmaceuticals but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% attrition, but evenly distributed and ITT analysis used. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported on. |
| Other bias | Low risk | We identified no other sources of bias. |
NCT01756157.
| Study characteristics | ||
| Methods |
Design: multicentre, randomised, double‐blind, cross‐over trial Exclusions postrandomisation: none reported Losses to follow‐up: 1 participant discontinued study drug (on day 8 after 3 doses) due to unwillingness to continue because of logistical issues with the home health agency; discontinued from study on day 44. 1 participant discontinued study drug (on day 72, which was day 11 of period 2, after 11 doses) due to poor compliance. 1 participant discontinued study drug (on day 1 after 1 dose) due to wanting to resume regular prophylactic treatment. 1 participant discontinued study drug (on day 8 after 3 doses) and, subsequently, was lost to follow‐up after early termination visit, and was discontinued from study on day 43. 1 participant had moderate extremity attacks on days 46 and 48, and severe gastrointestinal or abdominal attacks (or both) on day 60 (during washout), each attack required treatment with icatibant acetate; the participant also received acute treatment for angioedema attacks with multiple doses of danazol on day 50; participant discontinued study drug on day 67 (day 4 of period 2) after 18 doses, and had a moderate extremity attack on day 70, 3 days after last dose of study drug; during the 3 months before randomisation, this patient had had 6 attacks. 1 participant discontinued study drug and study (on day 1 after 1 dose) due to being lost to follow‐up Duration of study: 8 weeks and then crossed over for another 8 weeks Unit of randomisation: participant |
|
| Participants |
Country: 20 sites in the US and at 4 sites in Europe (France, Germany, Spain and Sweden) Setting: outpatient Number: 47 people were randomised: 23 received C1‐INH 1000 U with 24,000 U recombinant human hyaluronidase and then C1‐INH 2000 U with recombinant human hyaluronidase 48,000 U; 24 received C1‐INH 2000 U with recombinant human hyaluronidase 48,000 U and then C1‐INH 1000 U with recombinant human hyaluronidase 24,000 U. 22 completed both treatment periods Age mean: 39 (SD 14.6) years Sex: 14 male (30%); 33 female (70%) Inclusion criteria: children and adults aged ≥ 12 years with HAE and history of C1‐INH antigen level or functional C1‐INH level below normal. Exclusion criteria: had received C1‐INH therapy or blood products for treatment or prevention of an angioedema attack within 7 days before first dose of study medication, had angioedema attack signs or symptoms within 2 days before first dose of study medication, had been receiving prophylactic intravenous C1‐INH that exceeded the approved dosage of 1000 U every 3 or 4 days, or a combination of these; androgen therapy within 7 days of the first dose of study medication; diagnosis of acquired AE; history of hypercoagulability; allergies to C1‐INH or hyaluronidase; pregnancy. |
|
| Interventions | C1‐INH(SC) 1000 U with recombinant human hyaluronidase 24,000 U every 3–4 days C1‐INH(SC) 2000 U with recombinant human hyaluronidase 48,000 U every 3–4 days Treatment duration: 8 weeks then crossing over to other treatment |
|
| Outcomes | Rate of HAE attacks, attack severity, pharmacokinetics, immunogenicity, AEs, injection site reactions, AE‐QoL | |
| Funding | ViroPharma Incorporated, now part of the Shire Group of Companies | |
| Declarations of interest | Quote: "M.A. Riedl has received research grants from Dyax, Shire, ViroPharma (now part of the Shire Group of Companies), CSL Behring, BioCryst, and Santarus; consultant fees from Dyax, Shire, CSL Behring, Biocryst, and Isis; payments for lectures from Dyax, Shire, ViroPharma, and CSL Behring; and is a member of the medical advisory board of the US Hereditary Angioedema Association. W.R. Lumry has received consultant fees from Dyax, Shire, ViroPharma (now part of the Shire Group of Companies), CSL Behring, and BioCryst; research grants from Dyax, Shire, CSL Behring, and BioCryst; and payments for lectures from Dyax, Shire, and CSL Behring; and is a member of the medical advisory board of the US Hereditary Angioedema Association. H.H. Li has received consultant fees from Shire, ViroPharma (now part of the Shire Group of Companies), Pharming, Santarus, and Salix; research grants from Dyax, Shire, ViroPharma, CSL Behring, Pharming, Salix, and BioCryst; and payments for lectures from Dyax, Shire, ViroPharma, and CSL Behring; and is a member of the medical advisory board of the US Hereditary Angioedema Association. A. Banerji has received research grants and consultant fees from Dyax, Shire, ViroPharma (now part of the Shire Group of Companies), and CSL Behring; and is a member of the medical advisory board of the US Hereditary Angioedema Association. J.A. Bernstein has received consultant fees from Dyax, Shire, CSL Behring, and ViroPharma (now part of the Shire Group of Companies); research grants from BI, Forest, ViroPharma, CSL Behring, Dyax, Shire, Pharming, and Novartis; payment for lectures from Shire, Teva, Dyax, ViroPharma, and CSL Behring; payment for the development of educational presentations from Shire, ViroPharma, and Medscape; and is a member of the medical advisory board of the US Hereditary Angioedema Association. M. Bas has received consultant fees, research grants, and payment for lectures from Shire; and has also received research grants from ViroPharma (now part of the Shire Group of Companies) and Pharming. J. Björkander has participated in advisory boards for Baxter, Octapharma, and CSL Behring; received research grants from CSL Behring and Viro‐Pharma; and worked as consultant for Astra‐Zeneca, CSL Behring, Shire and Viro‐Pharma. M. Magerl has received consultant fees and payment for lectures from BioCryst, CSL Behring, Shire, Sobi, and ViroPharma (now part of the Shire Group of Companies); and has received research grants from ViroPharma. M. Maurer has received research grants support, consultant fees, and/or payment for lectures from BioCryst, CSL Behring, Dyax, Shire, and ViroPharma (now part of the Shire Group of Companies). K. Rockich is an employee of Shire (formerly Viropharma). H. Chen is an employee of Shire (formerly ViroPharma) and owns Shire stock/options. J. Schranz is an employee of Shire (formerly ViroPharma) and owns Shire stock/options." | |
| Notes | Funded by ViroPharma (now Shire) but study performed externally. Study terminated early by sponsor as a precaution related to the unexpected incidence and titre of non‐neutralising antibodies to recombinant human hyaluronidase in 45% of participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded to allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High attrition but evenly distributed and ITT analysis used. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported. |
| Other bias | Low risk | We identified no other sources of bias. |
NCT02052141.
| Study characteristics | ||
| Methods |
Design: randomised, single‐blind, cross‐over trial Exclusions postrandomisation: none Losses to follow‐up: none Duration of study: after 12‐week baseline observation period, participants received C1‐INH 500 U or 1000 U, twice‐weekly, for 12 weeks before crossing over to alternate dose for 12 weeks Unit of randomisation: participant |
|
| Participants |
Country: 10 sites in the US, EU, Mexico and Israel Setting: outpatient Number: children aged 6–11 years with Type I or II HAE. 12 children randomised and completed study. 5 to the C1‐INH‐nf 500/1000 IU treatment sequence and 7 to C1‐INH‐nf 1000/500 IU sequence Age (mean): 10 (SD not reported) years Sex: 5 boys (41.7%); 7 girls (58.3%) Inclusion criteria: aged ≥ 6 to < 12 years with confirmed HAE type I or II diagnosis, functional C1‐INH level < 50% of normal, mean ≥ 1.0 (≥ 2.0 in Germany) attacks/month of moderate or severe intensity or requiring acute treatment. Exclusion criteria: not provided. |
|
| Interventions | C1‐INH‐nf 500 IU twice‐weekly C1‐INH‐nf 1000 IU twice‐weekly Treatment duration: 12 weeks then crossing over to other treatment |
|
| Outcomes | Number of HAE attacks, attack severity, EuroQol 5‐dimensional descriptive system (youth version) | |
| Funding | Shire HGT, a Takeda company | |
| Declarations of interest | Quote: "Emel Aygören‐Pürsün has received honoraria, research funding, and/or travel grants from, and/or served as a consultant for, Adverum, BioCryst, CSL Behring, Pharming Technologies, KalVista Pharmaceuticals, and Shire. Daniel F. Soteres is a speaker and has participated in advisory boards for Shire. Sandra A. Nieto‐Martinez is a speaker for, has received honoraria from, and has participated in advisory boards for Shire, and has received travel grants from CSL Behring, Pharming Technologies, and Shire. Kraig W. Jacobson has participated in clinical trials for Shire. Dumitru Moldovan received research funding and travel grants from CSL Behring, Pharming Technologies, and Shire HGT and unrestricted educational grants from CSL Behring, Pharming Technologies, Shire HGT, and Swedish Orphan Biovitrum and served as a consultant for Pharming Technologies and Swedish Orphan Biovitrum. Inmaculada Martinez‐Saguer has received honoraria, research funding, and travel grants from BioCryst, CSL Behring, Pharming Technologies, and Shire and/or served as a consultant for these companies. Arthur Van Leerberghe, Yongqiang Tang, Peng Lu, and Moshe Vardi are fulltime employees of Shire, a Takeda company (Lexington, MA, USA). Jennifer Schranz was a full‐time employee of Shire, a Takeda company (Lexington, MA, USA), at the time of this study. Jim Christensen has indicated that he has no potential conflicts of interest to disclose." | |
| Notes | Funded by Shire HGT, but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Only patients and parents/caregivers were blinded to treatment sequence and dose." |
| Blinding of outcome assessment (detection bias) | High risk | Staff and investigators were not blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported. |
| Other bias | Low risk | We identified no other sources of bias. |
NCT02247739.
| Study characteristics | ||
| Methods |
Design: multicentre, randomised, double‐blind, placebo‐controlled, cross‐over trial Exclusions postrandomisation: none reported Losses to follow‐up: per‐protocol population comprised 23 participants after exclusion of 6 who withdrew during study, 2 patients who received plasma‐derived C1‐INH and 1 who received wrong treatment Duration of study: enroled between 28 December 2014 and 3 May 2016. Each treatment sequence consisted of 3 × 4‐week treatment periods separated by a 1‐week washout period before cross‐over Unit of randomisation: participant |
|
| Participants |
Country: 10 centres in Canada, the Czech Republic, Israel, Italy, Macedonia, Romania, Serbia, and the US Setting: outpatient Number: 32 people were randomised and 26 completed the study Age (mean): 45.9 (SD 14.5) years Sex: 6 male (19%); 26 female (81%) Inclusion criteria: aged ≥ 13 years with functional concentrations of C1 inhibitor < 50% of normal, and history of frequent attacks of HAE (≥ 4 attacks per month for ≥ 3 consecutive months before study initiation). Exclusion criteria: allergy to rabbits or a diagnosis of acquired angio‐oedema; pregnant or breastfeeding; receiving angiotensin‐converting enzyme inhibitors. |
|
| Interventions | Recombinant human C1‐INH 50 IU/kg twice per week Recombinant human C1‐INH 50 IU/kg once per week Placebo once per week Placebo twice per week Treatment duration: 4 weeks then crossing over to another treatment until all treatments were experienced |
|
| Outcomes | Rate of HAE attacks, functional C1‐INH concentrations in plasma, AEs, SAEs | |
| Funding | Pharming Healthcare (Berkeley Heights, New Jersey, USA) and Salix Pharmaceuticals (Bridgewater, New Jersey, USA) | |
| Declarations of interest | Quote: "MAR has received research grants from BioCryst Pharmaceuticals, CSL Behring, Ionis Pharmaceuticals, Pharming Technologies, and Shire; has served as a consultant for Adverum Biotechnologies, Alnylam Pharmaceuticals, Arrowhead Pharmaceuticals, BioCryst Pharmaceuticals, CSL Behring, Global Blood Therapeutics, Ionis Pharmaceuticals, KalVista Pharmaceuticals, Pharming Technologies, Salix Pharmaceuticals, and Shire; and has served on the speakers’ bureaus for CSL Behring, Pharming Technologies, Salix Pharmaceuticals, and Shire. VG‐P has served as principal investigator for clinical trials sponsored by Pharming Group. DM has received grants from Swedish Orphan Biovitrum, Pharming Technologies, Shire, and CSL Behring, and personal fees from Pharming Technologies, Shire, Swedish Orphan Biovitrum, and CSL Behring. JB is a researcher for BioCryst Pharmaceuticals, CSL Behring, Dyax, Pharming Technologies, and Shire; has served as a consultant for BioCryst Pharmaceuticals; and has served on the speakers’ bureau for Shire. WHY has served as a member of the national and international advisory boards for BioCryst Pharmaceuticals, CSL Behring, and Shire, and has received research or educational grants from BioCryst Pharmaceuticals, CSL Behring, Shire, and Pharming Technologies. BMG is an employee of Pharming Group. ARes has received research grants from Pharming Technologies. RH has received financial support and personal fees from Pharming Group. JRH and ARel are employees of Pharming Healthcare. MC has received grants from Shire and personal fees from Shire, CSL Behring, Pharming Technologies, BioCryst Pharmaceuticals, Alnylam, and KalVista. SA, RFL, and SK declare no competing interests." | |
| Notes | Funded by Pharming Technologies, but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response technology system used for randomisation. |
| Allocation concealment (selection bias) | Low risk | Allocation could not be anticipated because of use of interactive response technology system. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.5% attrition but evenly spread and some ITT data available. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported. |
| Other bias | Low risk | We identified no other sources of bias. |
OPuS‐1.
| Study characteristics | ||
| Methods |
Design: binational, randomised, double‐blind, placebo‐controlled trial, cross‐over design Exclusions postrandomisation: 2 participants discontinued after randomisation but before first dose of study drug (1 withdrew consent and 1 was considered by investigator to be unable to meet visit schedule requirements) Losses to follow‐up: none reported Duration of study: November 2013 to May 2014 Unit of randomisation: participant |
|
| Participants |
Country: Germany and the UK Setting: outpatient Number: 26 randomised (1 did not meet age eligibility criteria and 1 withdrew consent before randomisation). 24 completed study Age (mean): 42 (SD 11) years Sex: 9 male (37%); 15 female (63%) Inclusion criteria: men and non‐pregnant, non‐lactating women with HAE Type I or II; aged 18–65 years; body mass index 19–36 kg/m2; clinical diagnosis of HAE as documented by low C4 level and 1. low C1‐INH antigenic level, or 2. normal or increased C1‐INH antigenic level and a low C1‐INH functional level; able to provide documentation of a mean 1 HAE attack per week over ≥ 3 months demonstrated within past year. Exclusion criteria: use within the 7 days before screening or planned use through study of C1 inhibitor or tranexamic acid for prophylaxis of angioedema attacks; use within 30 days before screening or planned use through study of anabolic steroids for prophylaxis of angioedema attacks; concurrent use of anticoagulants, antiplatelet drugs, angiotensin‐converting enzyme inhibitors, oestrogen‐ or progestin‐containing contraceptive or non‐steroidal anti‐inflammatory drugs; prolonged activated partial thromboplastin time or prothrombin time at screening. |
|
| Interventions | Avoralstat 400 mg 3 times per day Placebo 3 times per day Treatment duration: 4 weeks for each period Washout period: 1 week |
|
| Outcomes | Rate of HAE attacks, AEs, AE‐QoL score | |
| Funding | Supported by awards HL107188 and HL095021 from the National Heart, Lung, and Blood Institute, NIH | |
| Declarations of interest | Quote: "E. Aygoren‐Pursun reports grants from Bio‐Cryst during the conduct of the study. J. Graff reports grants from BioCryst during the conduct of the study. I. Martinez‐Saguer reports grants from BioCryst during the conduct of the study and personal fees from CSL Behring, Shire, Viropharma, and SOBI Biovitrum outside the submitted work. W. Kreuz reports grants from BioCryst during the conduct of the study and personal fees from CSL Behring, Shire, Viropharma, and from SOBI Biovitrum outside the submitted work. H. Longhurst reports grants from BioCryst during the conduct of the study; grants, personal fees, and nonfinancial support from CSL Behring, Shire, and Viropharma; personal fees and nonfinancial support from SOBI Biovitrum; and personal fees from Dyax outside the submitted work. I. Nasr receives research funding from BioCryst. M. Bas reports grants from BioCryst during the conduct of the study and grants, personal fees, and nonfinancial support from CSL Behring, Shire, and Viropharma outside the submitted work. U. Straßen receives research funding from BioCryst. L. Fang receives research funding from BioCryst. M. Cornpropst, S. Dobo, and P. Collis report personal fees from BioCryst Pharmaceuticals during the conduct of the study and are employees of BioCryst. W. P. Sheridan reports personal fees from BioCryst Pharmaceuticals and is an employee of BioCryst. M. Maurer receives research support, honorarium, and travel support and serves as a consultant for BioCryst; serves as a consultant and receives payment for lectures from Shire and Viropharma; and reports grants from BioCryst during the conduct of the study, personal fees and nonfinancial support from Shire, CSL Behring, and Viropharma, and personal fees from SOBI Biovitrum outside the submitted work." | |
| Notes | Funded by BioCryst, but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | High risk | 2/4 stated outcomes in the protocol were not reported (AE‐QoL scores and AAS) |
| Other bias | Low risk | We identified no other sources of bias. |
OPuS‐2.
| Study characteristics | ||
| Methods |
Design: multicentre, randomised, double‐blind, placebo‐controlled parallel trial Exclusions postrandomisation: none reported Losses to follow‐up: 7 (6.4%) participants discontinued study drug prior to week 12. Reasons for study drug discontinuation included AEs (1 participant due to rash and 1 participant due to angioedema attack in avoralstat 500 mg group), lack of efficacy (1 participant each in the avoralstat 300 mg group and the placebo group), positive pregnancy test (1 participant in placebo group), protocol violation (1 participant in avoralstat 500 mg group), and study non‐compliance (1 participant in placebo group) Duration of study: December 2014 to January 2016 Unit of randomisation: participant |
|
| Participants |
Country: 46 centres in North America and Europe Setting: outpatient Number: 110 people randomised: 38 received avoralstat (BCX4161) 500 mg, 36 received avoralstat 300 mg and 36 received placebo. 103 (93.6%) participants completed study Age (mean): 41.2 (SD 13.3) years Sex: 25 men (22.7%); 85 women (77.3%) Inclusion criteria: aged ≥ 18 years; clinical diagnosis of Type I or II C1‐INH HAE as documented by either a low C1‐INH antigenic level (Type I HAE) or a normal C1‐INH antigenic level and a low C1‐INH functional level (Type II HAE); documentation of minimum angioedema attack rate of 2 per month, either by audit of medical record (≥ 2 angioedema attacks per month for 3 consecutive months within 6 months prior to screening) or a participant diary record of ≥ 4 unique angioedema attacks collected in a run‐in period of ≤ 2 months, with ≥ 1 attack occurring each month. Exclusion criteria: use of C1‐INH or tranexamic acid within 7 days prior to screening visit or expected use at any time during study; use of androgens within 30 days unless the person was receiving a stable dose of androgens ≥ 90 days prior to screening visit, met required angioedema attack frequency while on stable dose, and planned to remain on current dose of androgens during study. |
|
| Interventions | Avoralstat 500 mg orally 3 times/day Avoralstat 300 mg orally 3 times/day Placebo orally 3 times/day Treatment duration: 12 weeks |
|
| Outcomes | Rate of HAE attacks, AE‐QoL, AAS | |
| Funding | BioCryst Pharmaceuticals, Inc | |
| Declarations of interest | Quote: "This study was sponsored by BioCryst Pharmaceuticals, Inc (BioCryst), Durham, NC. Dr. Riedl reports grants from BioCryst during the conduct of the study; grants and personal fees from BioCryst, CSL Behring, Shire, and Pharming; and personal fees from Adverum, Alnylam, Ionis, and Kalvista outside the submitted work. Dr. Aygören‑Pürsün reports grants from BioCryst during the conduct of the study; personal fees and nonfinancial support from BioCryst; grants, personal fees, and nonfinancial support from CSL Behring; grants, personal fees, and nonfinancial support from Shire; and personal fees from Pharming and Adverum outside the submitted work. Dr Baker reports grants from BioCryst during the course of the study. Dr. Farkas reports personal fees from BioCryst during the conduct of the study, and personal fees from CSL Behring, Pharming, and Shire outside the submitted work. Dr. Anderson reports other from BioCryst during the conduct of the study, and personal fees from Shire, CSL Behring, and Pharming outside the submitted work. Dr. Bernstein reports grants and personal fees from BioCryst, Shire, CSL Behring, and Pharming during the conduct of the study. Dr. Bouillet reports personal fees and nonfinancial support from Shire; grants, personal fees, and nonfinancial support from Behring; personal fees and nonfinancial support from Pharming; grants, personal fees, and nonfinancial support from Novartis; nonfinancial support from GSK, nonfinancial support from Pfizer, and grants and nonfinancial support from LFB outside the submitted work. Dr, Busse received personal fees from CSL Behring; grants, personal fees, and other from Shire; personal fees and other from Pharming; personal fees from Pearl Therapeutics; personal fees from Teva; other from Law Offices of Victoria Broussard; and personal fees from Global Life Sciences outside the submitted work. Dr. Manning reports grants from BioCryst during the conduct of the study; grants and personal fees from Shire and CSL Behring, and Dyax; and personal fees from Pharming outside the submitted work. Dr. Magerl reports personal fees and nonfinancial support from Shire, Viropharma, CSL Behring, and BioCryst, and personal fees from Sobi outside the submitted work. Dr. Gompels reports other from Allergy Therapeutics; other from Bristol Myers; personal fees from Advisory board for BioCryst; and other from Viiv, Gilead, BMS, and Janssen outside the submitted work. Dr. Huissoon reports nonfinancial support from CSL limited and Shire Limited outside the submitted work. Dr. Longhurst reports grants and personal fees from BioCryst during the conduct of the study; grants and personal fees from CSL Behring; personal fees from Kalvista; personal fees from Pharming; personal fees from Adverum; and grants and personal fees from Shire outside the submitted work. Dr. Lumry reports consultancy fees from BioCryst during the conduct of the study; nonfinancial support from Medical Advisory Board of US HAEA; other from Shire/Viropharma, Pharming, Adverum, and CSL Behring; research grants from Shire/Viropharma and CSL Behring; and speakers bureau honoraria and travel support from Shire/Viropharma, CSL Behring, and Pharming outside the submitted work. Dr Ritchie reports grants from Bio‐Cryst during the conduct of the study. Dr. Shapiro reports investigator and speaker; consultant fees from Shire; and investigator fees from GreenCross. Dr. Soteres reports other from BioCryst during the conduct of the study and other from BioCryst; personal fees and other from Shire; personal fees from CSL Behring; and personal fees from Pharming outside the submitted work. Dr. Banerji reports research grants and other from BioCryst during the conduct of the study; research grants and other from Shire; and other from CSL, Alnylam, and Pharming outside the submitted work. Dr Cancian reports grants from BioCryst during the conduct of the study. Dr. Johnston reports grants and personal fees from BioCryst, during the conduct of the study; personal fees from Shire; personal fees from CSL Behring; personal fees from BioCryst; and personal fees from Pharming outside the submitted work. Dr. Craig reports grants and other from BioCryst, during the conduct of the study; other from CSL Behring; other from Shire; other from Grifols; other from Pharming; and other from HAE Association, outside the submitted work. Dr. Launay reports grants from BioCryst Pharmaceuticals; grants from Shire; and grants from CSL Behring, during the conduct of the study. Dr Li reports grants and nonfinancial support from BioCryst during the conduct of the study, and grants, personal fees, and nonfinancial support from Shire, CSL Behring, and Pharming outside the submitted work. Drs. Nickel and Schrijvers report other from BioCryst during the conduct of the study. Drs Offenberger, Rae, Triggiani, and Wedner report grants from BioCryst during the conduct of the study. S. Dobo, M. Cornpropst, D. Clemons, P. Collis, and W. Sheridan are employees of BioCryst. L. Fang reports consultancy fees from Bio‐Cryst. Dr. Maurer reports grants and personal fees from BioCryst during the conduct of the study, and grants and personal fees from BioCryst, Shire, Pharming, and CSL Behring outside the submitted work. There is no further conflict of interests to declare. The sponsor provided research grant support to all investigators. All authors had access to all of the data in the study and approved the final published manuscript. The corresponding author had final responsibility for the decision to submit for publication." | |
| Notes | Funded by BioCryst Pharmaceuticals but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation unclear. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rates were low and evenly distributed, and ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Most outcomes in protocol were reported on. EuroQol was missing, but AE‐QoL was present. |
| Other bias | Low risk | We identified no other sources of bias. |
SAHARA.
| Study characteristics | ||
| Methods |
Design: multicentre, randomised, double‐blind, placebo‐controlled, partial cross‐over trial Exclusions postrandomisation: none reported Losses to follow‐up: reasons for study discontinuation included patient withdrawal (9), AEs (4), physician decision (1), lost to follow‐up (in 1 participant who completed treatment; 1), and other (2). 1 participant receiving pdC1‐INH liquid experienced 2 TEAEs that led to treatment withdrawal (nausea and headache). Both events were considered treatment related and occurred within 24 hours of administration. 2 participants receiving placebo experienced 2 TEAEs leading to withdrawal (1 had cardiac arrest and 1 had an HAE attack). Neither considered treatment related Duration of study: screening began 17 December 2015, and last participant completed treatment on 24 July 2017 Unit of randomisation: participant |
|
| Participants |
Country: 33 sites in North America and Europe Setting: outpatient Number: 75 participants randomised (60 for cross‐over sequence (31 received pdC1‐INH liquid in period 1; 29 received placebo in period 1), and 15 for continuous pdC1‐INH liquid). 58 (77%) completed study Age (mean): 41.3 (SD 14.6) years Sex: 23 male (30.7%); 52 female (69.3%) Inclusion criteria: children and adults aged ≥ 12 years (≥ 18 years in Germany and Israel) with Type I or II HAE and functional C1‐INH level < 50% of normal; experienced ≥ 2 HAE attacks per month during 3 consecutive months. Exclusion criteria: adults receiving prophylactic intravenous C1‐INH at doses > 1000 IU every 3 or 4 days (or weekly dose > 2000 IU) or adolescents currently receiving C1‐INH for prophylaxis. |
|
| Interventions | pdC1‐INH 2000 IU: self‐administered, subcutaneous, fixed‐dose liquid twice‐weekly Placebo twice‐weekly Treatment duration: 2 × 14 weeks for cross‐over sequence or 1 × 28 week continuous treatment |
|
| Outcomes | Rate of HAE attacks, attack severity, AEs, SAEs, injection site reactions, pharmacokinetics, pharmacodynamics | |
| Funding | Shire Human Genetic Therapies, Inc, a Takeda company | |
| Declarations of interest | Quote: "W. R. Lumry has received consultant fees from Adverum, BioCryst, CSL Behring, Pharming, and Shire (a Takeda company); research grants from BioCryst, CSL Behring, Pharming, and Shire (a Takeda company); payments for lectures from CSL Behring, Pharming, and Shire (a Takeda company); and is a member of the Medical Advisory Board of the US Hereditary Angioedema Association. I. Martinez‐Saguer has received honoraria, research funding, and travel grants from BioCryst, CSL Behring, Pharming, and Shire (a Takeda company) and/or served as a consultant and/or participated in advisory boards for these companies. W. H. Yang is a consultant and member of the advisory board for CSL Behring and Shire (a Takeda company); has received unrestricted educational grants from AnaptysBio, BioCryst, CSL Behring, Novartis, and Shire (a Takeda company); and research grants from Aimmune, AstraZeneca, BioCryst, CSL Behring, DBV Technologies, Galderma, Genentech/Roche, GlaxoSmithKline, Merck, Pfizer, Pharming, Regeneron, Sanofi‐Genzyme, and Shire (a Takeda company). J. A. Bernstein has been a clinical investigator for BioCryst, CSL Behring, Pharming, and Shire (a Takeda company); a speaker for CSL Behring, Pharming, and Shire (a Takeda company); and a consultant for BioCryst, CSL Behring, Pharming, and Shire (a Takeda company). J. Jacobs has received research grants from 3M, Aimmune, AstraZeneca, CSL Behring, Genentech, Novartis, Sanofi, Shire (a Takeda company), and Teva; consulting fees from AstraZeneca, CSL Behring, Pharming, Regeneron, Shire (a Takeda company), and Teva; and speaker honoraria from Shire and Teva. D. Moldovan has served as clinical investigator for BioCryst, CSL Behring, Pharming, and Shire (a Takeda company); a consultant for CSL Behring, Octapharma, Pharming, and Shire (a Takeda company); and has received travel grants from CSL Behring, Pharming, Shire (a Takeda company), and Swedish Orphan Biovitrum. M. A. Riedl has received research grants from BioCryst, CSL Behring, Pharming, and Shire (a Takeda company); consulting fees from Adverum, Alnylam, BioCryst, CSL Behring, Ionis, Pharming, and Shire (a Takeda company); payments for lectures from CSL Behring, Pharming, and Shire (a Takeda company); and is a medical advisory board member of the US HAE Association. D. T. Johnston has served on advisory boards for CSL Behring, Pharming/Valeant, and Shire (a Takeda company); received speaker fees from Shire (a Takeda company); and has served as an investigator for BioCryst and Shire (a Takeda company). H. H. Li has been a clinical investigator and received grants and/or honoraria from BioCryst, CSL Behring, Pharming, and Shire (a Takeda company). Y. Tang and J. Schranz were full‐time employees of Shire (a Takeda company) at the time of this analysis. P. Lu and M. Vardi are full‐time employees of Shire (a Takeda company). H. Farkas has received honoraria and travel grants from BioCryst, CSL Behring, Pharming, Shire (a Takeda company), and Swedish Orphan Biovitrum and/or served as a consultant for these companies." | |
| Notes | Funded by Shire Human Genetic Therapies, but study performed externally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response technology used for randomisation. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed as interactive response technology used for randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High overall attrition (23%), but evenly spread and cross‐over design meant that the worst outcome had only 11.7% attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in protocol were reported. |
| Other bias | Low risk | We identified no other sources of bias. |
AAS: Angioedema Activity Score; AE‐QoL: Angioedema Quality of Life Questionnaire; AE: adverse event; C1‐INH: C1 esterase inhibitor; C1‐INH(SC): subcutaneous C1 esterase inhibitor; C4: complement component 4; EuroQol: instrument for measuring quality of life; HAE: hereditary angioedema; ITT: intention‐to‐treat; LLN: lower limit of normal; C1‐INH‐nf: nanofiltered C1 esterase inhibitor; NIH: National Institutes of Health; pdC1‐INH: plasma‐derived C1 esterase inhibitor; SAE: serious adverse event; SD: standard deviation; TEAE: treatment‐emergent adverse event.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aabom 2015 | Wrong study design – not an RCT. |
| Aberer 2017 | Wrong study design – not an RCT. |
| Agostoni 1978a | Wrong study design – not an RCT. |
| Agostoni 1978b | Wrong study design – not an RCT. |
| Agostoni 1980a | Wrong study design. |
| Agostoni 1983 | Wrong study design – not an RCT. |
| Aygören‐Pürsün 2013 | Wrong study design – retrospective analysis. |
| Baker 2013 | Wrong study design – not an RCT. |
| Bernstein 2019 | Wrong study design – indirect comparison of study data from included studies COMPACT and NCT01005888. |
| Birjmohun 2008 | Wrong study design – healthy control group. |
| Blohmé 1972 | Acute use, not preventive use. |
| Bork 2008 | Wrong study design – not an RCT. |
| Bork 2011 | Wrong study design – not an RCT. |
| Bork 2017 | Wrong study design – investigating discontinuation of potential trigger factors and drug therapies. |
| Busse 2017 | Wrong study design – results from the Berinert (C1‐INH) international registry. |
| Chyung 2014 | Wrong population – healthy people. |
| Cicardi 1997 | Wrong study design – not an RCT. |
| Davis‐Lorton 2016 | Wrong population. |
| Drouet 2008 | Wrong study design – not an RCT. |
| EudraCT 2009‐010736‐18 | Wrong study design – not an RCT. |
| EudraCT 2010‐019670‐32 | Wrong study design – not an RCT. |
| Farkas 2010 | Wrong study design – not an RCT. |
| Farkas 2013 | Wrong study design – not an RCT. |
| Füst 2011 | Wrong study design – not an RCT. |
| Hofstra 2012 | Wrong study design – prophylaxis study not an RCT. |
| NCT01108848 | Wrong study design – Berinert (C1‐INH) international registry |
| NCT01467947 | Wrong study design – not an RCT. |
| NCT01576523 | Wrong study design. |
| NCT01760343 | Wrong study design. |
| Sharma 2009 | Wrong outcomes – survey. |
| Sweet 1980 | Wrong study design – not an RCT. |
| Szegedi 2008 | Wrong study design – not an RCT. |
| Széplaki 2005 | Wrong study design – not an RCT. |
| Wang 2017 | Wrong study design: pooled analysis of included study NCT01005888 and excluded study Baker 2013. |
| Waytes 1996 | Wrong study design. |
| Zotter 2013 | Wrong study design – not an RCT. |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Zhang 1990.
| Methods | Unable to obtain copy of article |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
NCT03712228.
| Study name | A study to investigate CSL312 in subjects with hereditary angioedema (HAE) |
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre trial |
| Participants | Adults aged 18–65 years with C1‐INH HAE or FXII/PLG HAE |
| Interventions | CSL312 (factor XIIa antagonist monoclonal antibody) Placebo |
| Outcomes | Number of HAE attacks, attack severity, pharmacokinetics, AEs |
| Starting date | 29 October 2018 |
| Contact information | CSL Behring |
| Notes | Anticipated completion date: 14 October 2021 |
NCT04656418.
| Study name | CSL312 (Garadacimab) in the prevention of hereditary angioedema attacks |
| Methods | Multicentre, double‐blind, randomised, placebo‐controlled, parallel‐arm study |
| Participants | Adults and children aged ≥ 12 years with confirmed C1‐INH HAE, who have experienced ≥ 3 attacks during the 3 months before screening |
| Interventions | CSL312 (garadacimab) factor XIIa antagonist (monoclonal antibody) Placebo (buffer without active ingredient) |
| Outcomes | Time‐normalised number of HAE attacks during treatment period; change in HAE attack rate during treatment period compared to the run‐in period; time‐normalised number of attacks requiring on‐demand treatment; time‐normalised number of moderate or severe (or both) HAE attacks; time‐normalised number of HAE attacks at various time points during treatment period; percent change in time‐normalised number of HAE attacks between CSL312 and placebo; Subject's Global Assessment of Response to Therapy; number of participants with adverse events, adverse events of special interest, serious adverse events, CSL312‐induced anti‐CSL312 antibodies, clinically significant abnormalities in laboratory assessments; percent of participants with adverse events, adverse events of special interest, serious adverse events, CSL312‐induced anti‐CSL312 antibodies, clinically significant abnormalities in laboratory assessments Time frame: 6–8 months |
| Starting date | 13 January 2021 |
| Contact information | CSL Behring |
| Notes |
AE: adverse event; C1‐INH: normal C1 inhibitor; FXII: coagulation factor XII; HAE: hereditary angioedema; PLG: plasminogen gene.
Differences between protocol and review
Our initial inclusion criteria included a minimum study duration of six weeks. However, we found three studies that were of four weeks each in duration (APeX‐1; NCT02247739; OPuS‐1). Given the limited number of studies in our analysis, we decided to include these studies in our review.
Contributions of authors
NB drafted the protocol. For the full review, she obtained studies, selected studies, drafted the final review and will update the review.
MF drafted the protocol. For the full review, he obtained studies, selected studies, assessed the risk of bias, drafted the final review and will update the review.
ES extracted data, checked data, and will update the final review.
PM drafted the protocol. For the full review, he provided statistical advice.
CK drafted the protocol. For the full review, she interpreted data, drafted the final review and will update the review.
KM drafted the protocol. For the full review, she obtained studies, assessed the risk of bias, extracted data, entered data into Review Manager, analysed data, interpreted data, drafted the final review, and will update the review. She is the guarantor of the review.
Sources of support
Internal sources
-
University of Canberra Library, Australia
The library sourced and supplied full texts of articles for inclusion or exclusion
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
NB: none.
MF: none.
ES: none.
PM: none.
CK: has conducted original investigator‐led research in the field of hereditary angioedema (HAE) and has participated as a principal investigator in sponsored multinational randomised controlled trials (RCTs) of several therapies used for HAE management (acute and prophylactic therapies). She has participated actively in several global HAE meetings and has chaired advisory boards discussing HAE management in Australia. CK has declared that her institution has received payment for the conduct of multinational clinical trials from CSL Behring, KalVista Pharmaceuticals and BioCryst Pharmaceuticals Inc. She has received honoraria from CSL Behring and Takeda Pharmaceutical Company for serving on advisory boards and for lectures and presentations. She is a board member of HAE Australasia (patient organisation).
KM: has declared that she conducts comparative effectiveness work for TruDataRx Inc, which provides independent advice to employers based on the efficacy of pharmaceutical drugs. This company does not receive funds from the pharmaceutical industry or government.
New
References
References to studies included in this review
APeX‐1 {published data only}
- Aygören‑Pürsün E, Bygum A, Grivcheva‑Panovska V, Magerl M, Graff J, Steiner UC, et al. Oral plasma kallikrein inhibitor for prophylaxis in hereditary angioedema. New England Journal of Medicine 2018;379(4):352-62. [DOI] [PubMed] [Google Scholar]
- Bygum A, Panovska VG, Maurer M, Magerl M, Farkas H, Cicardi M, et al. Pharmacokinetic (PK) and pharmacodynamic (PD) effects of BCX7353 in patients with hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE): results from APeX-1 study. Allergy 2018;73(Suppl 105):723. [Google Scholar]
- Farkas H, Aygoren-Pursun E, Panovska VG, Huissoon A, Kinaciyan T, Murray S, et al. Relationship of target concentrations with efficacy: results from the APeX-1 study of Bcx7353. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S33. [Google Scholar]
- Magerl M, Rae W, Aygoren-Pursun E, Bygum A, Panovska VG, Steiner UC, et al. BCX7353 improves health-related quality of life in hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE): findings from the APeX-1 study. Allergy 2018;73(Suppl 105):724. [Google Scholar]
- Steiner UC, Huissoon A, Bygum A, Panovska VG, Gompels M, Cancian M, et al. Analysis of gastrointestinal (GI) symptoms in patients with hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE) treated with BCX7353: results from APeX-1 study. Allergy 2018;73(Suppl 105):724-5. [Google Scholar]
APeX‐2 {published data only}
- Anderson J, Gagnon R, Best J, Murray S, Iocca H, Sitz K. Reduction in attacks in hereditary angioedema (HAE) with berotralstat is consistent regardless of prior prophylactic treatment: a subgroup analysis of the phase 3 APeX-2 trial. Journal of Allergy and Clinical Immunology 2021;147(2):AB23. [DOI: 10.1016/j.jaci.2020.12.122] [DOI] [Google Scholar]
- Aygören‑Pürsün E, Johnston D, Banerji A, Bernstein JA, Yang WH, Fritz S, et al. Berotralstat (BCX7353) treatment demonstrates robust and durable reduction in the rate of hereditary angioedema (HAE) attacks over 48 weeks of the phase 3 APeX-2 study. European Journal of Allergy and Clinical Immunology 2020;75(Suppl 109):457-8. [DOI: 10.1111/all.14508] [DOI] [Google Scholar]
- Gower R, Busse P, Best J, Murray S, Iocca H, Kinaciyan T. Berotralstat reduces use of on-demand medication in hereditary angioedema (HAE) patients previously treated with prophylactic therapies. Journal of Allergy and Clinical Immunology 2021;147(2 Suppl):AB146. [Google Scholar]
- Jacobs J, Aygören-Pürsün E, Sitz K, Tachdjian R, Li H, Best J, et al. Berotralstat positively impact patient-reported satisfaction: results from the phase 3 APeX-2 trial. Annals of Allergy, Asthma & Immunology 2020;125(5 Suppl):S25. [Google Scholar]
- Johnston D, Banerji A, Riedl M, Soteres D, Berstein J, Best J, et al. Berotralstat improves patient-reported quality of life through 48 weeks in the phase 3 APeX-2 trial. Annals of Allergy, Asthma & Immunology 2020;125(5 Suppl):S22. [Google Scholar]
- Li HH, Best J, Murray S, Iocca H, Tachdijan R. Berotralstat consistently demonstrates reductions in attack frequency in hereditary angioedema (HAE) irrespective of baseline attack rate: subgroup analysis from the APeX-2 trial. Journal of Allergy and Clinical Immunology 2021;147(2 Suppl):AB22. [Google Scholar]
- Wedner H, Zuraw B, Anderson J, Craig T, Kiani S, Iocca H, et al. Berotralstat reduces attacks in patients with hereditary angioedema (HAE): APeX-2 trial 48 week results. Annals of Allergy, Asthma & Immunology 2020;125(5 Suppl):S14. [Google Scholar]
- Wedner JH, Aygören-Pürsün E, Bernstein J, Craig T, Gower R, Jacobs JS, et al. Randomized trial of the efficacy and safety of berotralstat (BCX7353) as an oral prophylactic therapy for hereditary angioedema: results of APeX-2 through 48 weeks (part 2). Journal of Allergy and Clinical Immunology. In Practice 2021;9(6):P2305-14.E4. [DOI] [PubMed] [Google Scholar]
- Zuraw B, Lumry WR, Johnston DT, Aygören-Pürsün E, Banerji A, Bernstein JA, et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled phase 3 trial. Journal of Allergy and Clinical Immunology 2021;148(1):164-72.e9. [DOI: 10.1016/j.jaci.2020.10.015] [DOI] [PubMed] [Google Scholar]
APeX‐J {published data only}
- Ohsawa I, Honda D, Suzuki Y, Fukuda T, Kohga K, Morita E, et al. Oral berotralstat for the prophylaxis of hereditary angioedema attacks in patients in Japan: a phase 3 randomized trial. Allergy 2021;76(6):1789-99. [DOI: 10.1111/all.14670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerji 2017 {published data only}
- Banerji A, Busse P, Shennak M, Lumry W, Davis‑Lorton M, Wedner HJ, et al. Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. New England Journal of Medicine 2017;376(8):717-28. [DOI] [PubMed] [Google Scholar]
- Banerji A, Busse P, Shennak M, Lumry W, Davis-Lorton M, Wedner J, et al. Interim analysis results of a phase 1b, multiple ascending dose study to evaluate DX-2930, a fully human monoclonal antibody inhibitor of plasma kallikrein in development for long-term prophylaxis of hereditary angioedema. Allergy 2015;70(S101):107. [Google Scholar]
- Busse P, Banerji A, Shennak M, Lumry W, Davis-Lorton M, Wedner H, et al. Dx-2930 in patients with hereditary angioedema: final results of a phase 1b study. Annals of Allergy, Asthma & Immunology 2015;115(5 Suppl):A31. [Google Scholar]
- Jacobs JS, Busse PJ, Banerji A, Shennak M, Lumry WR, Davis-Lorton MA, et al. Relationship between drug exposure and clinical response observed in the phase 1b study of DX-2930 in subjects with hereditary angioedema. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl):AB251. [Google Scholar]
- Lumry W, Cicardi M, Busse P, Banerji A, Shennak M, Davis-Lorton M, et al. Attack frequency, C1-INH function, and levels of cleaved kininogen do not influence the clinical response to DX-2930 in patients with hereditary angioedema. Annals of Allergy, Asthma & Immunology 2015;115(5 Suppl):A31. [Google Scholar]
COMPACT {published data only}
- Anderson J, Krishnarajah G, Craig T, Lumry W, Supina D, Feuersenger H, et al. Use of rescue medication in hereditary angioedema attacks and its relation to attack severity. Annals of Allergy, Asthma & Immunology 2017;119(5 Suppl 1):S40. [Google Scholar]
- Christiansen S, Zuraw B, Feuersenger H, Pragst I, Pawaskar D. C1-Esterase inhibitor (C1-INH) functional activity of subcutaneous C1-INH for the prevention of hereditary angioedema (HAE) attacks: pharmacokinetic comparison in adolescent and adult patients. Allergy 2018;73(Suppl 105):613-4. [Google Scholar]
- Cicardi M, Zuraw B, Craig T, Longhurst H, Feuersenger H, Jacobs I, et al. Onset of effect of prophylactic treatment with subcutaneous C1-esterase inhibitor (C1-INH [SC]) for prevention of hereditary angioedema (HAE) attacks: findings from the phase III compact study. Allergy 2018;73(Suppl 105):615-6. [Google Scholar]
- Craig T, Lumry W, Cicardi M, Zuraw B, Bernstein JA, Anderson J, et al. Treatment effect of switching from intravenous to subcutaneous C1-inhibitor for prevention of hereditary angioedema attacks: COMPACT subgroup findings. Journal of Allergy and Clinical Immunology. In Practice 2019;7(6):2035-8. [DOI] [PubMed] [Google Scholar]
- Craig T, Zuraw B, Cicardi M, Longhurst H, Chiao J, Feuersenger H, et al. Prevention of hereditary angioedema (HAE) attacks by anatomical location with subcutaneous C1-esterase inhibitor (C1-INH [SC]) treatment: results from the phase III COMPACT study. Allergy 2018;73(Suppl 105):615. [Google Scholar]
- Craig T, Zuraw B, Lumry W, Bernstein J, Cicardi M, Anderson J, et al. Preventive effect of subcutaneous C1 inhibitor in patients with very frequent attacks of hereditary angioedema. Annals of Allergy, Asthma & Immunology 2017;119(Suppl 5):S4. [Google Scholar]
- Gower R, Li HH, Levy DS, Jacobs I. Response to subcutaneous C1-esterase inhibitor (C1-INH [SC]), a prophylactic treatment for adolescents and adults with hereditary angioedema: results from the phase 3 compact trial. Journal of Adolescent Health 2018;62(2 Suppl):S5-6. [Google Scholar]
- Levy D, Caballero T, Hussain I, Reshef A, Anderson J, Baker J, et al. Long-term efficacy of subcutaneous C1 inhibitor in pediatric patients with hereditary angioedema. Pediatric Allergy, Immunology, and Pulmonology 2020;33(3):136-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DS, Chiao J, Feuersenger H, Craig T, Longhurst HJ, Cicardi M, et al. Prevention of attacks of hereditary angioedema (HAE) with subcutaneous C1-esterase inhibitor (C1-INH [SC]) by anatomical location: results from the phase III COMPACT study (NCT01912456). Journal of Allergy and Clinical Immunology 2018;141(2 Suppl):AB45. [Google Scholar]
- Levy DS, Craig T, Zuraw B, Cicardi M, Longhurst H, Feuersenger H, et al. Prophylaxis with subcutaneous C1-esterase inhibitor (C1-INH [SC]) reduces symptom days and severity of symptoms in patients with hereditary angioedema (HAE): results from the COMPACT study. Allergy 2018;73(Suppl 105):611-2. [Google Scholar]
- Li HH, Mycroft S, Christiansen S, Wood DN, Feuersenger H, Pawaskar D, et al. Subcutaneous C1-esterase inhibitor to prevent hereditary angioedema attacks: safety findings from the COMPACT trial. Allergy and Asthma Proceedings 2018;39(5):365-70. [DOI] [PubMed] [Google Scholar]
- Li HH, Riedl MA, Craig T, Longhurst HJ, Cicardi M, Zuraw BL, et al. Initiation of prophylactic treatment with subcutaneous C1-esterase inhibitor (C1-INH [SC]) for prevention of hereditary angioedema (HAE) attacks and onset of effect: findings from the phase III COMPACT study. Journal of Allergy and Clinical Immunology 2018;141(2 Suppl):AB45. [Google Scholar]
- Li HH, Zuraw B, Longhurst HJ, Cicardi M, Bork K, Baker J, et al. Subcutaneous C1 inhibitor for prevention of attacks of hereditary angioedema: additional outcomes and subgroup analysis of a placebo‑controlled randomized study. Allergy, Asthma and Clinical Immunology 2019;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhurst H, Cicardi M, Craig T, Bork K, Grattan C, Baker J, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. New England Journal of Medicine 2017;376(12):1131-40. [DOI] [PubMed] [Google Scholar]
- Longhurst HJ, Li HH, Riedl MA, Craig TJ, Reshef A, Farkas H, et al. Subcutaneous C1-esterase inhibitor [C1-INH(SC)] to prevent hereditary angioedema (HAE) attacks: subject and investigator assessments from the compact trial. Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB234. [Google Scholar]
- Lumry W, Bernstein J, Cicardi M, Zuraw B, Craig T, Caballero T, et al. Subcutaneous C1 inhibitor prophylaxis substantially reduces the need for rescue medications in the compact study. Annals of Allergy, Asthma & Immunology 2017;119(5 Suppl):S39. [Google Scholar]
- Lumry W, Craig TJ, Farkas H, Feuersenger H. Health-related quality of life (HRQoL) and changes in work productivity measures with subcutaneous C1-inhibitor [C1-INH(SC)] for the prevention of hereditary angioedema (HAE) attacks: findings from the compact study. Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB233. [Google Scholar]
- Lumry WR, Craig T, Cicardi M, Zuraw BL, Longhurst H, Farkas H, et al. Can routine prophylactic subcutaneous C1-inhibitor [C1-INH(SC)] alleviate psychological and physical disabilities caused by HAE? Findings from the COMPACT study (NCT01912456). Allergy, Asthma and Clinical Immunology 2017;13(Suppl 2):29. [Google Scholar]
- Lumry WR, Craig T, Zuraw B, Longhurst H, Baker J, Li HH, et al. Health-related quality of life with subcutaneous C1-Inhibitor for prevention of attacks of hereditary angioedema. Journal of Allergy and Clinical Immunology. In Practice 2018;6(5):1733-41. [DOI] [PubMed] [Google Scholar]
- Lumry WR, Craig TJ, Magerl MA, Farkas H, Lawo JP, Chiao J, et al. Long-term health-related quality of life in patients treated with subcutaneous C1-inhibitor replacement therapy for the prevention of hereditary angioedema attacks. Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB38. [Google Scholar]
- Magerl M, Bernstein JA, Banerji A, Li HH, Lumry WR, Maurer M, et al. Response to subcutaneous human C1-inhibitor for the prevention of angioedema attacks in patients with hereditary angioedema: subgroups based on prior intravenous C1-inhibitor use. Allergy 2015;70(S101):60. [Google Scholar]
- Manning M, Caballero T, Hussain I, Feuersenger H, Chiao J, Jacobs I, et al. Long-term efficacy of subcutaneous C1 inhibitor in pediatric patients with hereditary angioedema. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S31-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AS, Watson DJ, Feuersenger H, Pragst I, Mycroft S, Craig T. On-demand vs prophylactic subcutaneous C1-esterase inhibitor for the management of hereditary angioedema: a benefit-risk assessment of the COMPACT study data. Allergy 2018;73(Suppl 105):614-5. [Google Scholar]
- Riedl MA, Jacobs J, Li HH, Levy DS, Chiao J, Feuersenger H, et al. Efficacy of prophylaxis with subcutaneous C1-esterase inhibitor (C1-INH [SC]) in female patients with hereditary angioedema: subgroup analysis from the COMPACT study. Allergy 2018;73(Suppl 105):614. [Google Scholar]
- Riedl MA, Lumry WR, Li HH, Banerji A, Bernstein JA, Bas M, et al. Subcutaneous human C1-inhibitor with recombinant human hyaluronidase for the prevention of angioedema attacks in patients with hereditary angioedema: results of a randomized, double-blind, dose-ranging, crossover study. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB278. [Google Scholar]
- Tarzi M, Cicardi M, Zuraw B, Craig T, Longhurst H, Caballero T, et al. Prevention of hereditary angioedema (HAE) attacks with subcutaneous C1-INH (SC) preparation of CSL830 in the COMPACT study: effects on severity and attack location. Allergy 2017;72(Suppl 103):597. [Google Scholar]
- Weller K, Maurer M, Magerl M, Fridman M, Supina D, Schranz J. Subcutaneous human C1-inhibitor (C1-INH) with recombinant human hyaluronidase (rHuPH20) for the prevention of angioedema attacks in patients with hereditary angioedema (HAE): health-related quality of life (HRQoL) results from a randomized, double-blind, dose-ranging, crossover study. Allergy 2015;70(S101):253. [Google Scholar]
COMPACT extension {published data only}
- Bernstein JA, Schwartz L, Yang W, Baker J, Anderson J, Farkas H, et al. Long-term safety and efficacy of subcutaneous C1-inhibitor in older patients with hereditary angioedema. Annals of Allergy, Asthma & Immunology 2020;125(3):334-40. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Li H, Chiao J, Jacobs I. Comparison of 40 IU/kg and 60 IU/kg doses of subcutaneous C1-esterase inhibitor (C1-INH [SC]) for the prophylactic treatment of hereditary angioedema (HAE): efficacy results from a phase 3 trial. Allergy and Asthma Proceedings 2017;38(5):397. [Google Scholar]
- Craig T, Longhurst H, Cicardi M, Zuraw B. Safety and efficacy of long-term subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. Annals of Allergy, Asthma & Immunology 2018;121(Suppl 5):S34. [DOI] [PubMed] [Google Scholar]
- Craig T, Zuraw B, Longhurst H, Cicardi M, Bork K, Grattan C, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. Journal of Allergy and Clinical Immunology. In Practice 2019;7(6):1793-802.e2. [DOI] [PubMed] [Google Scholar]
- Katelaris C, Prusty S. Efficacy and safety of subcutaneous C1-inhibitor in Australian patients with hereditary angioedema. Internal Medicine Journal 2019;49(Suppl S4):27. [Google Scholar]
- Li H, Chiao J, Jacobs I. Comparison of the safety profiles of 40 IU/kg and 60 IU/kg doses of subcutaneous C1-esterase inhibitor (C1- INH [SC]) in the prophylactic treatment of hereditary angioedema (HAE): results from a phase 3 trial. Allergy and Asthma Proceedings 2017;38(5):397. [Google Scholar]
- Li HH, Craig TJ, Longhurst H, Cicardi M, Feuersenger H, Pragst I, et al. Long-term safety of subcutaneous C1-inhibitor in the prophylactic treatment of hereditary angioedema. Journal of Allergy and Clinical Immunology 2019;143(Suppl 2):AB39. [Google Scholar]
- Li HH, Feuersenger H, Chiao J, Machnig T, Jacobs I. Long-term treatment experience with subcutaneous C1-esterase inhibitor for the prevention of HAE attacks: 2-year efficacy results from the US compact open-label extension study. Allergy and Asthma Proceedings 2018;39(5):401. [Google Scholar]
- Longhurst H, Cicardi M, Zuraw B, Craig T, Caballero T, Farkas H, et al. Subcutaneous C1-INH (SC) preparation (CSL830) in the prevention of hereditary angioedema (HAE) attacks: first findings from the COMPACT extension study. Allergy 2017;72(Suppl 103):100-1. [Google Scholar]
- Lumry WR, Zuraw B, Cicardi M, Craig T, Anderson J, Banerji A, et al. Long-term health-related quality of life in patients treated with subcutaneous C1-inhibitor replacement therapy for the prevention of hereditary angioedema attacks: findings from the COMPACT open-label extension study. Orphanet Journal of Rare Diseases 2021;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuraw B, Craig T, Cicardi M, Longhurst H, Feuersenger H, Prusty S, Katelaris C. Long-term efficacy of subcutaneous C1-inhibitor in patients with hereditary angioedema and very high attack burden. Internal Medicine Journal 2019;49(Suppl S4):27. [Google Scholar]
Gelfand 1976 {published data only}
- Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol: reversal of clinical and biochemical abnormalities. New England Journal of Medicine 1976;295(26):1444-8. [DOI] [PubMed] [Google Scholar]
HELP {published data only}
- Banerji A, Riedl M, Bernstein J, Cicardi M, Longhurst H, Zuraw B, et al. Lanadelumab for prevention of attacks in hereditary angioedema: results from the phase 3 HELP study. Annals of Allergy, Asthma & Immunology 2017;119(5 Suppl):S5. [Google Scholar]
- Banerji A, Riedl M, Zuraw B, Lumry W, Lu P, Hao J, et al. Lanadelumab 300mg every 2 weeks effectively prevented hereditary angioedema attacks in the HELP study. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S5. [Google Scholar]
- Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA 2018;320(20):2108-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Lanadelumab for prevention of attacks in hereditary angioedema: results from the phase 3 HELP study. Swiss Medical Weekly 2018;148(Suppl 231):28S-9S. [Google Scholar]
- Bernstein JA, Banerji A, Schranz J, Nurse C, Riedl M, Lu P. Lanadelumab reduces high morbidity attacks in patients with hereditary angioedema (HAE): results from the phase 3 HELP study. Allergy 2018;73(Suppl 105):288-89. [Google Scholar]
- Busse PJ, Tachdjian R, Martinez-Saguer I, Lu P, Nurse C, Aygoren-Pursun E. Efficacy and safety of lanadelumab for prophylactic treatment in adolescents with hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB43. [Google Scholar]
- Cicardi M, Shennak M, Zaragoza-Urdaz RH, Wang Y, Lu P. Pharmacokinetics and pharmacodynamics of lanadelumab in patients with HAE in the phase 3 HELP study. Allergy 2018;73(Suppl 105):722. [Google Scholar]
- Craig T, Zaragoza-Urdas R, Anderson J, Li H, Paes K, Ren H, Juethner S. Response to lanadelumab is not affected by race and ethnicity: findings from phase 3 studies. Journal of Allergy and Clinical Immunology 2021;147(2 Suppl):AB21. [Google Scholar]
- Gagnon R, Cicardi M, Shennak M, Zaragoza-Urdaz R, Marier JF, Lu P, et al. Lanadelumab Inhibition of plasma kallikrein activity for effective HAE prophylaxis. Allergy, Asthma, and Clinical Immunology 2019;15(Suppl 1):A63. [Google Scholar]
- Hebert J, Banerji A, Bernstein JA, Busse PJ, Cicardi M, Davis-Lorton M, et al. Efficacy and safety of lanadelumab for prevention of hereditary angioedema attacks: results from the phase 3 HELP study. Allergy, Asthma and Clinical Immunology 2019;15(Suppl 1):A66. [Google Scholar]
- Jacobs JS, Bernstein J, Davis-Lorton M, Li HH, Soteres DF, Nurse C, et al. Increased response to higher dose lanadelumab in hereditary angioedema patients: exploratory findings from the HELP and HELP OLE studies. Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB38. [Google Scholar]
- Jain G, Sussman G, Lumry WR, Lu P, Lewis H, Maurer M. Lanadelumab improves health-related quality of life in patients with hereditary angioedema (HAE): findings from the HELP study. Allergy, Asthma and Clinical Immunology 2019;15(Suppl 1):10. [Google Scholar]
- Johnston D, Banerji A, Riedl M, Zuraw B, Lumry W, Bernstein J, et al. Lanadelumab safety and immunogenicity: results from the phase 3 HELP study. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S36. [Google Scholar]
- Johnston DT, Anderson JT, Schranz J, Nurse C, Cicardi M. Efficacy of lanadelumab in patients switching from long-term prophylaxis with C1-inhibitor (C1-INH): results from the phase 3 HELP Study. Journal of Allergy and Clinical Immunology 2018;141(2 Suppl):AB47. [Google Scholar]
- Longhurst H, Lumry W, Weller K, Magerl M, Lu P, Jain G, et al. Lanadelumab improves health-related quality of life in patients with hereditary angioedema (HAE): findings from the HELP study. Clinical and Experimental Allergy 2018;48(11):1524-5. [Google Scholar]
- Longhurst H, Lumry W, Weller K, Magerl M, Lu P, Jain G, et al. Lanadelumab improves health-related quality of life in patients with hereditary angioedema (HAE): findings from the HELP study. Clinical and Experimental Allergy 2018;48(11):OP.019. [Google Scholar]
- Lumry WR, Weller K, Magerl M, Banerji A, Longhurst HJ, Riedl MA, et al. Impact of lanadelumab on health-related quality of life inpatients with hereditary angioedema in the HELP study. Allergy 2021;76(4):1188-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumry WR, Weller K, Magerl M, Lu P, Jain G, Lewis H, et al. Lanadelumab markedly improves health-related quality of life in hereditary angioedema patients in the HELP study. Swiss Medical Weekly 2018;148(Suppl 231):28S. [Google Scholar]
- Lumry WR, Weller K, Magerl M, Schranz J, Jain G, Doll H, et al. Lanadelumab markedly improves health-related quality of life in hereditary angioedema patients in the HELP study. Journal of Allergy and Clinical Immunology 2018;141(2 Suppl):AB47. [Google Scholar]
- Maurer M, Davis-Lorton M, Schranz J, Hao J, Busse PJ. High responder rates in lanadelumab-treated patients in the phase 3 HELP study. Allergy 2018;73(Suppl 105):289-90. [Google Scholar]
- Maurer M, Gierer S, Hébert J, Hao J, Lu P, Banerji A. Lanadelumab is highly efficacious at steady-state in hereditary angioedema (HAE): results of the phase 3 HELP study. Allergy 2018;73(Suppl 105):289. [Google Scholar]
- Maurer M, Gierer S, Hebert J, Hao J, Lu P, Bruelle J, et al. Lanadelumab is highly efficacious at steady-state in hereditary angioedema (HAE): results of the phase 3 HELP study. Swiss Medical Weekly 2018;148(Suppl 231):36S. [Google Scholar]
- Riedl MA, Maurer M, Bernstein JA, Banerji A, Longhurst HJ, Li HH, et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy 2020;75(11):2879-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MA, Tachdjian R, Schranz J, Nurse C, Bernstein JA. Consistent lanadelumab treatment effect in patients with hereditary angioedema (HAE) regardless of baseline attack frequency in the phase 3 HELP study. Journal of Allergy and Clinical Immunology 2018;141(2 Suppl):AB47. [Google Scholar]
- Schmaier AH, Bauer KA, Cicardi M, Hebert J, Johnston DT, Busse PJ, et al. Effect of lanadelumab on coagulation parameters in patients with hereditary angioedema: findings from the phase 3 HELP study. Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB41. [Google Scholar]
- Sexton DJ, Brown NJ, Lumry WR, Gower RG, Hao J, Lu P, et al. Lanadelumab and cardiovascular risk: findings from the phase 3 HELP study. Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB45. [Google Scholar]
- Wang Y, Marier JF, Kassir N, Gosselin NH, Martin P. Exposure-response analyses of lanadelumab in patients with hereditary angioedema. Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB40. [Google Scholar]
- Yang WH, Anderson JT, Busse P, Cicardi M, Davis-Lorton M, Johnston DT, et al. Efficacy of lanadelumab in the phase 3 HELP study: exploratory analyses based on prior disease activity and prior use of C1-INH long term prophylaxis therapy. Allergy, Asthma and Clinical Immunology 2019;15(Suppl 1):A64. [Google Scholar]
- Zanichelli A, Hebert J, Soteres D, Hao J, Lu P, Busse P. Efficacy of lanadelumab in patients with HAE is independent of body mass index: findings from the HELP study. Allergy 2018;73(Suppl 105):722-3. [Google Scholar]
NCT01005888 {published data only}
- Bernstein J, Manning M, Li H, White MV, Baker J, Lumry WR, et al. Safety and efficacy of escalating doses of C1 esterase inhibitor [human] as prophylaxis in patients with hereditary angioedema (HAE). Annals of Allergy, Asthma & Immunology 2012;109(5):A29. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Li HH, Craig TJ, Longhurst HJ, Farkas H, Manning ME, et al. Placebo-controlled trials of C1-inhibitor (C1-INH) replacement therapy for routine prevention of attacks in patients with hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB234. [Google Scholar]
- Dayno J, Miller DP, Hautamaki E, Newcomer S, Fitts D, Lumry WR. Relationship between angioedema attacks and health-related quality of life outcomes in patients with hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2014;133(2 Suppl):AB33. [Google Scholar]
- Lumry W, Manning ME, Hurewitz DS, Davis-Lorton M, Fitts D, Kalfus IN, et al. Nanofiltered C1-esterase inhibitor for the acute management and prevention of hereditary angioedema attacks due to C1-inhibitor deficiency in children. Journal of Paediatrics 2013;162(5):1017-22.e1. [DOI] [PubMed] [Google Scholar]
- Lumry WR, Miller DP, Beusterien K, Hautamaki E, Fitts D, Dayno J. Health-related quality of life (HRQoL) in patients with hereditary angioedema (HAE) receiving nanofiltered C1 inhibitor (C1 INH-nf) for prophylaxis: results of a randomised, placebo-controlled, cross-over study. Allergy 2014;69(S99):491. [Google Scholar]
- Lumry WR, Miller DP, Beusterien K, Hautamaki E, Fitts D, Dayno J. Quality of life in patients with hereditary angioedema receiving nanofiltered C1 inhibitor for prophylaxis: results of a randomized, placebo controlled cross-over study. Annals of Allergy, Asthma & Immunology 2013;111(5 Suppl 1):A105. [Google Scholar]
- Lumry WR, Miller DP, Newcomer S, Fitts D, Dayno J. Quality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacks. Allergy and Asthma Proceedings 2014;35(5):371-6. [DOI] [PubMed] [Google Scholar]
- Riedl M, Zuraw B, Baker J, Hurewitz D, White M, Vegh A, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) for the prophylaxis of hereditary angioedema attacks. Allergy: European Journal of Allergy and Clinical Immunology 2011;94:420-1. [Google Scholar]
- Zuraw B, Baker J, Hurewitz D, White M, Vegh A, Bielory L, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (CinryzeTM) for the prophylaxis of hereditary angioedema (HAE) attacks. Annals of Allergy, Asthma & Immunology 2010;105(5 Suppl):A100. [Google Scholar]
- Zuraw BL, Busse PJ, White M, Jacobs J, Lumry W, Baker J, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. New England Journal of Medicine 2010;363(6):513-22. [DOI] [PubMed] [Google Scholar]
- Zuraw BL, Kalfus I. Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. American Journal of Medicine 2012;125(9):938.e1-e7. [DOI] [PubMed] [Google Scholar]
NCT01756157 {published data only}
- Lumry WR, Li HH, Magerl M, Maurer M, Bernstein JA, Riedl MA, et al. Pharmacokinetics of subcutaneous C1 esterase inhibitor (human) with recombinant human hyaluronidase for the prevention of angioedema attacks in patients with hereditary angioedema. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB192. [Google Scholar]
- Reshef A, Riedl M, Panovska VG, Moldovan D, Baker J, Yang WH, et al. Randomized, double-blind, placebo-controlled trial of recombinant human C1 inhibitor for prophylaxis of hereditary angioedema attacks. World Allergy Organization Journal 2017;10(Suppl 1):A106. [Google Scholar]
- Riedl M, Grivcheva-Panovska V, Moldovan D, Baker J, Yang WH, Reshef A, et al. Randomized, double-blind, placebo-controlled trial of recombinant human C1 inhibitor for prophylaxis of hereditary angioedema attacks. Allergy and Asthma Proceedings 2017;38(3):237. [Google Scholar]
- Riedl MA, Lumry WR, Li HH, Banerji A, Bernstein JA, Ba M, et al. Subcutaneous administration of human C1 inhibitor with recombinant human hyaluronidase in patients with hereditary angioedema. Allergy and Asthma Proceedings 2016;37(6):489-500. [DOI] [PubMed] [Google Scholar]
- Weller K, Maurer M, Fridman M, Supina D, Schranz J, Magerl M. Health-related quality of life with hereditary angioedema following prophylaxis with subcutaneous C1-inhibitor with recombinant hyaluronidase. Allergy and Asthma Proceedings 2017;38(2):143-51. [DOI] [PubMed] [Google Scholar]
NCT02052141 {published data only}
- Aygören-Pürsün E, Jacobson K, Moldovan D, Christensen J, Leerberghe A, Wang Y, et al. Safety and efficacy of a C1 inhibitor for the prevention of hereditary angioedema attacks in children: interim results of a phase 3 study. Allergy, Asthma and Clinical Immunology 2017;13(Suppl 2):O-14. [Google Scholar]
- Aygören-Pürsün E, Soteres DF, Nieto-Martinez SA, Christensen J, Jacobson KW, Moldovan D, et al. A randomized trial of human C1 inhibitor prophylaxis in children with hereditary angioedema. Pediatric Allergy and Immunology 2019;30(5):553-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas H, Aygoren-Pursun E, Martinez-Saguer I, Kessel A, Hao J, Lu P, et al. The use of a C1 esterase inhibitor concentrate to manage hereditary angioedema attacks in children. Allergy 2017;72(Suppl 103):101. [Google Scholar]
- Jacobson K, Soteres D, Nieto-Martinez S, Moldovan D, Martinez-Saguer I, Vardi M, et al. C1 inhibitor administration in pediatric patients with hereditary angioedema: patient compliance with intravenous therapy. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S36. [Google Scholar]
- Martinez-Saguer I, Soteres D, Leerberghe A, Herrera EM, Devercelli G, Vardi M, et al. Improved health-related quality of life in pediatric patients with hereditary angioedema (HAE): a phase 3 study of C1 inhibitor for attack prevention. Allergy 2018;73(Suppl 105):718. [Google Scholar]
- Soteres DF, Aygoren-Pursun E, Nieto-Martinez SA, Christensen J, Jacobson KW, Moldovan D, et al. Safety, efficacy, and pharmacokinetics of intravenous C1 esterase inhibitor (human) prophylaxis in children with hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB41. [Google Scholar]
NCT02247739 {published data only}
- Riedl MA, Grivcheva-Panovska V, Moldovan D, Baker J, Yang WH, Giannetti BM, et al. Recombinant human C1 esterase inhibitor for prophylaxis of hereditary angio-oedema: a phase 2, multicentre, randomised, double-blind, placebo-controlled crossover trial. Lancet 2017;390(10102):1595-602. [DOI] [PubMed] [Google Scholar]
- Yang WH, Li HH, Chase C, Moldovan D, Grivcheva-Panovska V, Harper JR, et al. Recombinant Human C1 Inhibitor (rHC1INH) Is efficacious and well tolerated as prophylaxis for prevention of hereditary angioedema (HAE) attacks: a randomized, phase 2 trial. Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB231. [Google Scholar]
OPuS‐1 {published data only}
- Aygören-Pürsün E, Magerl M, Graff J, Martinez-Saguer I, Kreuz W, Longhurst H, et al. Efficacy correlates with plasma levels in OPuS-1, a proof-of-concept study of oral kallikrein inhibitor BCX4161 as a prophylaxis against attacks of hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2015;135(Suppl 1):AB192. [Google Scholar]
- Aygören-Pürsün E, Magerl M, Graff J, Martinez-Saguer I, Kreuz W, Longhurst H, et al. Prophylaxis of hereditary angioedema attacks: a randomized trial of oral plasma kallikrein inhibition with avoralstat. Journal of Allergy and Clinical Immunology 2016;138(3):934-6.e5. [DOI] [PubMed] [Google Scholar]
- Magerl M, Aygören-Pürsün E, Graff J, Martinez-Saguer I, Kreuz W, Longhurst H, et al. BCX4161, an oral kallikrein inhibitor, showed significant benefits on reducing disease burden and improving quality of life in subjects with hereditary angioedema in the OPuS-1 study. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB278. [Google Scholar]
- Magerl M, Rae W, Aygoren-Pursun E, Bygum A, Panovska VG, Steiner UC, et al. Prophylactic therapy with BCX7353 improves anxiety and stress in hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE) patients. Allergy 2018;73(Suppl 105):414. [Google Scholar]
OPuS‐2 {published data only}
- Riedl MA, Aygören-Pürsün E, Baker J, Farkas H, Anderson J, Bernstein JA, et al. Evaluation of avoralstat, an oral kallikrein inhibitor, in a phase 3 hereditary angioedema prophylaxis trial: the OPuS-2 study. Allergy 2018;73(9):1871-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
SAHARA {published data only}
- Farkas H, Martinez-Saguer I, Yang WH, Johnston D, Tang T, Vardi M, et al. Reduction of attack severity with fixed-dose subcutaneous (SC) C1 inhibitor liquid in hereditary angioedema patients: results from the phase 3 SAHARA study. Allergy 2018;73(Suppl 105):720-1. [Google Scholar]
- Lumry W, Bernstein JA, Jacobs J, Yang WH, Moldovan D, Riedl MA, et al. Fixed-dose subcutaneous (SC) C1 inhibitor liquid for prophylactic treatment of hereditary angioedema attacks: results from the phase 3 SAHARA study. Allergy 2018;73(Suppl 105):287. [Google Scholar]
- Lumry W, Bernstein JA, Jacobs J, Yang WH, Moldovan D, Riedl MA, et al. Fixed-dose subcutaneous (SC) C1 inhibitor liquid for prophylactic treatment of hereditary angioedema attacks: results from the phase 3 SAHARA study. Swiss Medical Weekly 2018;148(Suppl 231):26S-7S. [Google Scholar]
- Lumry WR, Martinez-Saguer I, Yang WH, Bernstein JA, Jacobs J, Moldovan D, et al. Fixed-dose subcutaneous C1-inhibitor liquid for prophylactic treatment of C1-INH-HAE: SAHARA randomized study. Journal of Allergy and Clinical Immunology. In Practice 2019;7(5):1610-18.e4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aabom 2015 {published data only}
- Aabom A, Andersen KE, Perez-Fernandez E, Caballero T, Bygum A. Health-related quality of life in Danish patients with hereditary angioedema. Acta Dermato-venereologica 2015;95(2):225-6. [DOI] [PubMed] [Google Scholar]
Aberer 2017 {published data only}
- Aberer W, Maurer M, Bouillet L, Zanichelli A, Caballero T, Longhurst HJ, et al. Breakthrough attacks in patients with hereditary angioedema receiving long-term prophylaxis are responsive to icatibant: findings from the Icatibant Outcome Survey. Allergy, Asthma, and Clinical Immunology 2017;13:31. [DOI: 10.1186/s13223-017-0203-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agostoni 1978a {published data only}
- Agostoni A, Marasini B, Cicardi M, Martignoni GC. Intermittent therapy with danazol in hereditary angioedema. Lancet 1978;1(8061):453. [DOI] [PubMed] [Google Scholar]
Agostoni 1978b {published data only}
- Agostoni A, Marasini B, Cicardi M, Martignoni G, Uziel L, Pietrogrande M. Hepatic function and fibrinolysis in patients with hereditary angioedema undergoing long-term treatment with tranexamic acid. Allergy 1978;33(4):216-21. [DOI] [PubMed] [Google Scholar]
Agostoni 1980a {published data only}
- Agostoni A, Cicardi M, Martignoni GC, Bergamaschini L, Marasini B. Danazol and stanozolol in long-term prophylactic treatment of hereditary angioedema. Journal of Allergy and Clinical Immunology 1980;65(1):75-9. [DOI] [PubMed] [Google Scholar]
Agostoni 1983 {published data only}
- Agostoni A. The management of hereditary angioedema. Ricerca in Clinica e in Laboratorio 1983;13(1):55-9. [DOI] [PubMed] [Google Scholar]
Aygören‐Pürsün 2013 {published data only}
- Aygören-Pürsün E, Martinez Saguer I, Kreuz W, Klingebiel T, Schwabe D. Risk of angioedema following invasive or surgical procedures in HAE Type I and II – the natural history. Allergy 2013;68(8):1034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2013 {published data only}
- Baker JW, Craig TJ, Riedl MA, Banerji A, Fitts D, Kalfus IN, et al. Nanofiltered C1 esterase inhibitor (human) for hereditary angioedema attacks in pregnant women. Allergy and Asthma Proceedings 2013;34(2):162-9. [DOI] [PubMed] [Google Scholar]
Bernstein 2019 {published data only}
- Bernstein JA, Li HH, Craig TJ, Manning ME, Lawo JP, Machnig T, et al. Indirect comparison of intravenous vs. subcutaneous C1-inhibitor placebo-controlled trials for routine prevention of hereditary angioedema attacks. Allergy, Asthma, and Clinical Immunology 2019;15:13. [DOI: 10.1186/s13223-019-0328-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birjmohun 2008 {published data only}
- Birjmohun RS, Hovingh GK, Stroes ES, Hofstra JJ, Dallinga-Thie GM, Meijers JC, et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clinical Therapeutics 2008;30(12):2314-23. [DOI] [PubMed] [Google Scholar]
Blohmé 1972 {published data only}
- Blohmé G. Treatment of hereditary angioneurotic oedema with tranexamic acid. A random double-blind cross-over study. Acta Medica Scandinavica 1972;192:293-8. [DOI] [PubMed] [Google Scholar]
Bork 2008 {published data only}
- Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Annals of Allergy, Asthma & Immunology 2008;100(2):153-61. [DOI] [PubMed] [Google Scholar]
Bork 2011 {published data only}
- Bork K, Hardt J. Hereditary angioedema: long-term treatment with one or more injections of C1 inhibitor concentrate per week. International Archives of Allergy and Immunology 2011;154(1):81-8. [DOI] [PubMed] [Google Scholar]
Bork 2017 {published data only}
- Bork K, Wulff K, Witzke G, Hardt J. Treatment for hereditary angioedema with normal C1-INH and specific mutations in the F12 gene (HAE-FXII). Allergy 2017;72(2):320-4. [DOI] [PubMed] [Google Scholar]
Busse 2017 {published data only}
- Busse P, Baker J, Martinez-Saguer I, Bernstein JA, Craig T, Magerl M, et al. Safety of C1-inhibitor concentrate use for hereditary angioedema in pediatric patients. Journal of Allergy and Clinical Immunology. In Practice 2017;5(4):1142-5. [DOI] [PubMed] [Google Scholar]
Chyung 2014 {published data only}
- Chyung Y, Vince B, Iarrobino R, Sexton D, TenHoor C, Kenniston J, et al. Results of a phase 1a, single ascending dose study of DX-2930, a human monoclonal antibody inhibitor of plasma kallikrein under investigation for long term prophylaxis of hereditary angioedema. Allergy 2014;69(S99):581. [Google Scholar]
Cicardi 1997 {published data only}
- Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. Journal of Allergy and Clinical Immunology 1997;2:194-6. [DOI] [PubMed] [Google Scholar]
Davis‐Lorton 2016 {published data only}
- Davis-Lorton MA, Busse PJ, Banerji A, Shennak M, Lumry WR, Wedner HJ, et al. Pharmacodynamic effect of DX-2930 on plasma kallikrein in hereditary angioedema patients. Journal of Allergy and Clinical Immunology 2016;137(2):AB252. [Google Scholar]
Drouet 2008 {published data only}
- Drouet C, Desormeaux A, Robillard J, Ponard D, Bouillet L, Martin L, et al. Metallopeptidase activities in hereditary angioedema: effect of androgen prophylaxis on plasma aminopeptidase P. Journal of Allergy and Clinical Immunology 2008;121(2):429-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
EudraCT 2009‐010736‐18 {published data only}
- EudraCT 2009-010736-18. An open-label exploratory phase II study of the safety and prophylactic effect of a weekly 50 U/kg rC1INH treatment in asymptomatic patients with hereditary C1INH deficiency (HAE) – OPERA. clinicaltrialsregister.eu/ctr-search/trial/2009-010736-18/HU (first received 4 April 2009).
EudraCT 2010‐019670‐32 {published data only}
- EudraCT 2010-019670-32. Pharmacokinetics and safety of human pasteurised C1-inhibitor concentrate (Berinert/CE1145) in subjects with congenital C1-INH deficiency. clinicaltrialsregister.eu/ctr-search/trial/2010-019670-32/IT (first received 19 December 2012).
Farkas 2010 {published data only}
- Farkas H, Czaller I, Csuka D, Vas A, Valentin S, Varga L, et al. The effect of long-term danazol prophylaxis on liver function in hereditary angioedema – a longitudinal study. European Journal of Clinical Pharmacology 2010;66(4):419-26. [DOI] [PubMed] [Google Scholar]
Farkas 2013 {published data only}
- Farkas H, Csuka D, Zotter Z, Varga L, Fust G. Prophylactic therapy in children with hereditary angioedema. Journal of Allergy and Clinical Immunology 2013;131(2):579-82.e1-2. [DOI] [PubMed] [Google Scholar]
Füst 2011 {published data only}
- Füst G, Farkas H, Csuka D, Varga L, Bork K. Long-term efficacy of danazol treatment in hereditary angioedema. European Journal of Clinical Investigation 2011;41(3):256-62. [DOI] [PubMed] [Google Scholar]
Hofstra 2012 {published data only}
- Hofstra JJ, Kleine Budde I, Twuyver E, Choi G, Levi M, Leebeek FW, et al. Treatment of hereditary angioedema with nanofiltered C1-esterase inhibitor concentrate (Cetor®): multi-center phase II and III studies to assess pharmacokinetics, clinical efficacy and safety. Clinical Immunology (Orlando, Fla.) 2012;142(3):280-90. [DOI] [PubMed] [Google Scholar]
NCT01108848 {published data only}
- NCT01108848. Patient registry study of Berinert® in normal clinical practice. clinicaltrials.gov/show/NCT01108848 (first received 22 April 2010).
NCT01467947 {published data only}
- NCT01467947. Postmarketing immunogenicity study in HAE subjects treated with Berinert. clinicaltrials.gov/show/NCT01467947 (first received 9 November 2011).
NCT01576523 {published data only}
- NCT01576523. A study to evaluate the clinical pharmacology and safety of C1-esterase inhibitor administered by the subcutaneous route. clinicaltrials.gov/show/NCT01576523 (first received 12 April 2012).
- Zuraw BL, Cicardi M, Longhurst HJ, Bernstein JA, Li HH, Magerl M, et al. Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate. Allergy 2015;70(10):1319-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01760343 {published data only}
- NCT01760343. A study to evaluate the safety and pharmacokinetics of two formulations of C1-esterase inhibitor. clinicaltrials.gov/show/NCT01760343 (first received 4 January 2013).
Sharma 2009 {published data only}
- Sharma K, Levy RJ, Wasserman RL, Jacobson KW, Laudadio C, Craig TJ. Evaluation of prodromal symptoms concurrent with hereditary angioedema therapy. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl):S100. [Google Scholar]
Sweet 1980 {published data only}
- Sweet LC, Jackson CE, Yanari SS, Yott JB. Danazol therapy in hereditary angioedema. Henry Ford Hospital Medical Journal 1980;28(1):31-5. [PubMed] [Google Scholar]
Szegedi 2008 {published data only}
- Szegedi R, Széplaki G, Varga L, Prohaszka Z, Szeplaki Z, Karadi I, et al. Long-term danazol prophylaxis does not lead to increased carotid intima-media thickness in hereditary angioedema patients. Atherosclerosis 2008;198(1):184-91. [DOI] [PubMed] [Google Scholar]
Széplaki 2005 {published data only}
- Széplaki G, Varga L, Valentin S, Kleiber M, Karadi I, Romics L, et al. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema. Journal of Allergy and Clinical Immunology 2005;115(4):864-9. [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang Y, Martin P, Lu P, Schranz J. Pharmacokinetics (PK) and pharmacodynamics (PD) of c1 esterase inhibitor (c1-IHN) for prophylaxis of angioedema attacks in patients (PTS) with hereditary angioedema (HAE). Allergy 2017;72(Suppl 103):148. [Google Scholar]
Waytes 1996 {published data only}
- Waytes AT, Rosen FS, Frank MM. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. New England Journal of Medicine 1996;334(25):1630-4. [DOI] [PubMed] [Google Scholar]
Zotter 2013 {published data only}
- Zotter Z, Czaller I, Csuka D, Szabo E, Kohalmi KV, Varga L, et al. Adverse effects of danazol prophylaxis in female patients with hereditary angioedema due to C1-INH deficiency (HAE-C1-INH). Allergy: European Journal of Allergy and Clinical Immunology 2013;97:430. [Google Scholar]
References to studies awaiting assessment
Zhang 1990 {published data only}
- Zhang H. Long-term therapy of hereditary angioedema. Chung-Hua i Hsueh Tsa Chih 1990;70(4):211-3. [PubMed] [Google Scholar]
References to ongoing studies
NCT03712228 {published data only}
- NCT03712228. A study to investigate CSL312 in subjects with hereditary angioedema (HAE). clinicaltrials.gov/ct2/show/NCT03712228 (first received 19 October 2018).
NCT04656418 {published data only}
- NCT04656418. CSL312 (Garadacimab) in the prevention of hereditary angioedema attacks. clinicaltrials.gov/ct2/show/NCT04656418 (first received 7 December 2020).
Additional references
Agostoni 1999
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffre D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999;44:21-5. [DOI] [PubMed] [Google Scholar]
ASCIA 2020
- Australasian Society of Clinical Immunology and Allergy. Hereditary angioedema (HAE) position paper. allergy.org.au/images/docs/ASCIA_HP_Position_Paper_HAE_2020_Aug_Update.pdf (accessed 13 May 2021).
Bafunno 2018
- Bafunno V, Firinu D, D'Apolito M, Cordisco G, Loffredo S, Leccese A, et al. Mutation of the angiopoietin-1 gene (ANGPT1) associates with a new type of hereditary angioedema. Journal of Allergy and Clinical Immunology 2018;141(3):1009-17. [DOI] [PubMed] [Google Scholar]
Banerji 2018
- Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA 2018;320(20):2108-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bork 2018
- Bork K, Wulff K, Steinmüller-Magin L, Braenne I, Staubach-Renz P, Witzke G, et al. Hereditary angioedema with a mutation in the plasminogen gene. Allergy 2018;73(2):442-50. [DOI] [PubMed] [Google Scholar]
Brigden 2018
- Brigden A, Parslow RM, Gaunt D, Collin SM, Jones A, Crawley E. Defining the minimally clinically important difference of the SF-36 physical function subscale for paediatric CFS/ME: triangulation using three different methods. Health and Quality of Life Outcomes 2018;16:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2015
- Cao Z, Biondo M, Rayzman V, Hardy M, McDonald A, Busfield S, et al. Development and characterization of an anti-FXIIa monoclonal antibody for the treatment of hereditary angioedema. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB194. [Google Scholar]
Chen 2017
- Chen X, Kotian P, Wilson R, Parker CD, Babu YS. Preclinical characterization of BCX7353, an oral plasma kallikrein inhibitor, for the treatment of hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB230. [Google Scholar]
Cicardi 1997
- Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. Journal of Allergy and Clinical Immunology 1997;99:194-6. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. New York (NY): Taylor & Francis, 1988. [DOI: 10.4324/9780203771587] [ISBN: 9780203771587] [DOI] [Google Scholar]
Fabiani 1990
- Fabiani JE, Paulin P, Simkin G, Leoni J, Palombarani S, Squiquera L. Hereditary angioedema: therapeutic effect of danazol on C4 and C1 esterase inhibitors. Annals of Allergy 1990;64(4):388-92. [PubMed] [Google Scholar]
FDA 2011
- Food and Drug Administration. Danocrine label ID: 3061327. www.accessdata.fda.gov/drugsatfda_docs/label/2011/017557s033s039s040s041s042lbl.pdf (accessed 20 December 2018):1-9.
Frank 1976
- Frank MM, Gelfand JA, Atkinson JP. Hereditary angioedema: the clinical syndrome and its management. Annals of Internal Medicine 1976;84:580-93. [DOI] [PubMed] [Google Scholar]
Frank 1979
- Frank MM. Effect of sex hormones on the complement-related clinical disorder of hereditary angioedema. Arthritis and Rheumatism 1979;22:1295-99. [DOI] [PubMed] [Google Scholar]
Frank 2016
- Frank MM, Zuraw B, Banerji A, Bernstein JA, Craig T, Busse P, et al. Management of children with hereditary angioedema due to C1 inhibitor deficiency. Pediatrics 2016;138(5):e20160575. [DOI] [PubMed] [Google Scholar]
Frese 2019
- Frese M, Beard N, Mere P, Katelaris C, Mills K. Interventions for the treatment of acute hereditary angioedema attacks. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013402. [DOI: 10.1002/14651858.CD013402] [DOI] [PMC free article] [PubMed] [Google Scholar]
Germenis 2016
- Germenis AE, Speletas M. Genetics of hereditary angioedema revisited. Clinical Reviews in Allergy and Immunology 2016;51(2):170-82. [DOI] [PubMed] [Google Scholar]
Gompels 2002
- Gompels MM, Lock RJ, Morgan JE, Osborne J, Brown A, Virgo PF. A multicentre evaluation of the diagnostic efficiency of serological investigations for C1 inhibitor deficiency. Journal of Clinical Pathology 2002;55(2):145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 20 December 2018. Hamilton, ON: McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Henao 2016
- Henao MP, Kraschnewski JL, Kelbel T, Craig TJ. Diagnosis and screening of patients with hereditary angioedema in primary care. Therapeutics and Clinical Risk Management 2016;12:701-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Johnson 2018
- Johnson NM, Phillips MA. New treatments for hereditary angioedema. Skin Therapy Letters 2018;23(1):6-8. [PubMed] [Google Scholar]
Kenniston 2014
- Kenniston JA, Faucette RR, Martik D, Comeau SR, Lindberg AP, Kopacz KJ, et al. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. Journal of Biological Chemistry 2014;289(34):23596-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunschak 1998
- Kunschak M, Engl W, Maritsch F, Rosen FS, Eder G, Zerlauth G, et al. A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion 1998;38(6):540-9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Manheimer E, Glanville J. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longhurst 2016
- Longhurst H, Bygum A. The humanistic, societal and pharmaco-economic burden of angioedema. Clinical Reviews in Allergy and Immunology 2016;51(2):230-9. [DOI] [PubMed] [Google Scholar]
Longhurst 2018
- Longhurst H. Optimum use of acute treatments for hereditary angioedema: evidence-based expert consensus. Frontiers in Medicine 2018;4:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopes Veronez 2021
Lumry 2018
- Lumry WR. Hereditary angioedema: the economics of treatment of an orphan disease. Frontiers in Medicine 2018;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maurer 2018
- Maurer M, Magerl M, Ignacio A, Emel AP, Betschel S, Bork K, et al. The international WAO/EAACI guideline for the management of hereditary angioedema – the 2017 revision and update. World Allergy Organization Journal 2018;11:5. [Google Scholar]
Norman 2003
- Norman GR, Sloan JA, Wyrich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Medical Care 2003;41(5):582-92. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roche 2005
- Roche O, Blanch A, Caballero T, Sastre N, Callejo D, Lopez-Trascasa M. Hereditary angioedema due to C1 inhibitor deficiency: patient registry and approach to the prevalence in Spain. Annals of Allergy, Asthma & Immunology 2005;94(4):498-503. [DOI] [PubMed] [Google Scholar]
Tarzi 2007
- Tarzi MD, Hickey A, Förster T, Mohammadi M, Longhurst HJ. An evaluation of tests used for the diagnosis and monitoring of C1 inhibitor deficiency: normal serum C4 does not exclude hereditary angio-oedema. Clinical and Experimental Immunology 2007;149(3):513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
US HAE Association 2018
- US HAE Association. Diagnosing HAE. www.haea.org/diagnosis.php (accessed 11 November 2018).
Weller 2016
- Weller K, Magerl M, Peveling-Oberhag A, Martus P, Staubach P, Maurer M. The Angioedema Quality of Life Questionnaire (AE-QoL) – assessment of sensitivity to change and minimal clinically important difference. Allergy 2016;71(8):1203-9. [DOI] [PubMed] [Google Scholar]
Wilson 2010
- Wilson DA, Bork K, Shea EP, Rentz AM, Blaustein MB, Pullman WE. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Annals of Allergy, Asthma and Immunology 2010;104(4):314-20. [DOI] [PubMed] [Google Scholar]
Wintenberger 2014
- Wintenberger C, Boccon-Gibod I, Launay D, Fain O, Kanny G, Jeandel PY, et al. Tranexamic acid as maintenance treatment for non-histaminergic angioedema: analysis of efficacy and safety in 37 patients. Clinical and Experimental Immunology 2014;178(1):112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wyrwich 2005
- Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Services Research 2005;40(2):577-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeerleder 2016
- Zeerleder S, Levi M. Hereditary and acquired C1-inhibitor-dependent angioedema: from pathophysiology to treatment. Annals of Medicine 2016;48(4):256-67. [DOI] [PubMed] [Google Scholar]
Zotter 2014
- Zotter Z, Csuka D, Szabó E, Czaller I, Nébenführer Z, Temesszentandrási G, et al. The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency. Orphanet Journal of Rare Diseases 2014;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuraw 2008
- Zuraw BL. Hereditary angioedema. New England Journal of Medicine 2008;359:1027-36. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Beard 2019
- Beard N, Frese M, Mere P, Katelaris C, Mills K. Interventions for the long-term prevention of hereditary angioedema attacks. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013403. [DOI: 10.1002/14651858.CD013403] [DOI] [PMC free article] [PubMed] [Google Scholar]


