Abstract

Bacterially produced polyhydroxyalkanoates are valuable substitutes for petrochemical plastics, but their current production capacities are very scarce. Producing poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB-co-HV) from methane and odd-chain carbon fatty acids could make the production of this biodegradable polymer cost-effective. This study analyzes the main factors affecting methanotrophic growth and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) accumulation, simulating a pilot-scale process based on a double-stage approach. The effects of the nitrogen source and the oxygen partial pressure during a 20 day growth phase were studied; the cosubstrate concentration, the culture selected, and the methane partial pressure were investigated during the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production stage performed within 15 days under nutrient starvation. Methylocystis parvus OBBP and Methylosinus thricosporum OB3b reached the maximum growth productivities with ammonium as the nitrogen source and oxygen at high partial pressure. The simulation of the PHB-co-HV accumulation revealed that methanotrophs could better accumulate the polymer with low valeric acid concentrations. A methane-abundant gas stream (0.5 atm of methane) could increase process yields up to 0.32 kg m–3 d–1.
Keywords: biopolymers, eco-friendly materials, sustainable production, poly(3-hydroxybutyrate-co-3-hydroxyvalerate), process simulation, process optimization
Short abstract
A simulation study for optimizing the bioproduction of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from methane and valeric acid in pilot-scale bioreactors.
Introduction
Fossil-based plastics have largely spread in the last decades: their global production has been multiplied by a factor of 20 since the 1960s and is foreseen to double by 2036. Together with their production, plastic waste also has increased. Since fossil-based plastics are persistent and do not degrade in the environment, their overproduction has negatively impacted the environment, being today one of the leading causes of global pollution.1−3 Many effects have been observed on the food chain, human health, and ecosystem stability and as a result of plastic pollution of land, air, and water.2 Therefore, the scientific community is nowadays challenging the development of an effective alternative to petrochemical plastics, such as biopolymers.4,5 Among them, polyhydroxyalkanoates (PHAs) are very attractive as they are biodegradable and can be produced biologically by many bacteria and archaea as PHA granules inside of their cytoplasm when subjected to nutrient starvation.3,6−10 Anyway, according to the most recent reports on bioplastics, PHAs only represent 1.8% of the global production capacities because of the many factors that hinder their market spread, including the low productivities, the poor scalability of the process, the high market price, and the low quality of the most produced homopolymer, namely, poly(3-hydroxybutyrate) (PHB).8,11−13 For instance, some industries produce PHB for applications in packaging and drugs at a pilot scale using expensive substrates, which account for 40% of the final price and applying cost-intensive extraction techniques.11,14 In this context, it has been reported that the use of natural gas (i.e., methane) as a carbon source could lead to PHA accumulation in Type II methanotrophs, reducing the total cost by up to 30–35%.15,16 Anyway, although PHB can be compared to the commercial polypropylene (PP), it still presents many disadvantages: it is stiffer and more brittle, has a lower resistance to solvents, and a lower extension to break than PP.17−19 Nevertheless, the major obstacle in the applications of PHB is the narrow difference between its melting point (typically around 180 °C) and the temperature at which degradation begins (typically around 200 °C).15 These similar values can make polymer processing (e.g., applying the melt-extrusion technology) complex. Previous studies demonstrated that the mentioned disadvantages could be overcome when other building blocks are included in the molecular structure of the polymer.18 For example, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB-co-HV) is a thermoplastic polyester belonging to the PHA family and made of both 3-HV and 3-HB units.8,20 In the case of methanotrophic strains, the use of methane as the sole carbon source avoided the accumulation of 3-HV units, which can instead be promoted by the addition of odd-chain carbon fatty acids such as valeric, acetic, butyric, or propionic acid.21 In these conditions, some heterotrophic bacteria with nonspecific enzymatic activities can produce 3-hydroxyvalerate units, thus lowering the melting temperature (Tm), the glass-transition temperature, the degree of crystallinity, and the water permeability.8,22 The thermal and mechanical properties of PHB-co-HV make it more suitable for many applications, such as packaging and nanotechnology, among others.8 Many studies linked the thermal and mechanical properties of PHB-co-HV to the 3-HV fraction, which itself depends on the concentration of the cosubstrate.21,22 However, copolymers with similar 3-HV contents showed different characteristics, thus implying their dependence on additional factors such as the chemical composition distribution, the molecular structure of the polymer, the feeding strategy, and the carbon sources.7 For example, a prior study reported the accumulation of PHB-co-20%HV and PHB-co-40%HV from methane and volatile fatty acids, where the biopolymers exhibited molecular masses of 1.15 ± 0.11 × 106 and 9.34 ± 0.78 × 105 Da, respectively.22 Similarly, some authors obtained PHB-co-22%HV and PHB-co-37%HV, with Tm of 150 and 134 °C, respectively; the glass-transition temperatures computed were −2 and −6 °C for the lower and the higher fraction of 3-HV, respectively.23 On the other hand, higher melting temperatures were reported for PHB-co-15%HV (161 °C) and PHB-co-43%HV (170 °C) produced from methane, valerate, and n-pentanol, corresponding to degrees of crystallinity of 23 and 5%, respectively.15
The biodegradability of PHB-co-HV was also found to depend on the 3-HV fraction. Thus, the carbon conversion calculated for PHB-co-13%HV accounted for 96% and dropped to 83% for PHB-co-20%HV.24 Despite the promising characteristics of PHB-co-HV, only a few studies about the production of PHB-co-HV from methane and odd-chain carbon fatty acids are available in the literature. Moreover, the few efforts to develop innovative strategies to promote PHB-co-HV production might explain the limited capacities of PHA industrial producers. For instance, ICI company (UK) was reported to produce approximately 300 tons/year of PHB-co-HV; BASF (Germany) and Montesanto were recognized as pilot-plant PHB-co-HV producers, while a higher capacity of 2000 tons/year was reported for Zhejian Tian An (China).14
This study aims to comprehensively investigate the process for producing PHB-co-HV from methane and odd-chain carbon fatty acids (i.e., valeric acid). A simulation approach, which was previously proposed only for PHB25 and to investigate the effect of the reactor geometry and configuration, was applied in this study for the first time to the production of the enhanced poly(3-hydroxybutyrate-co-hydroxyvalerate). Moreover, the impact of the variation of the main operational parameters (type of biomass, gas composition, and cosubstrate concentration), which were only tested individually in prior experimental works, was assessed using the best geometry/configuration derived from our previous work.25 In particular, the parameters mentioned above were analyzed to simulate and correlate their effects, aiming to identify the limits and the most proper conditions for the optimal process performance on a larger scale.
Biopolymer Production Process Design and Simulation
PHB-co-HV Production Process Design
The PHB-co-HV production process from methane and valeric acid proposed in this manuscript is based on a two-stage strategy, which was reported to increase PHB-co-HV productivities,8 considering the following steps. First, a methanotrophic strain was grown for 20 days in a semicontinuous bioreactor investigating the effect of nitrogen source and oxygen partial pressure. Then, PHB-co-HV accumulation was induced within 15 days by depriving the culture medium of nitrogen and supplying methane and valeric acid as carbon sources. It is worth highlighting that the choice of the duration of the two stages was based on an experimental work by García-Pérez et al.26 The simulated process conditions, i.e., case studies investigated, are summarized in Figure 1. The production performances of Methylosinus thricosporum OB3b and Methylocystis parvus OBBP growing on ammonium (10 mM NH4Cl) and nitrate (NaNO3 10 mM) at several oxygen partial pressures (ppO2) in the range of 0.1–0.4 atm were investigated during case study 1 to determine the optimal growth conditions. Then, the biopolymer accumulation by pure and mixed cultures under a low concentration of valeric acid (LV = 100 ppm) was evaluated in case study 2. After that, the effect of the cosubstrate concentration was assessed by supplying low (LV = 100 ppm) and high (HV = 400 ppm) concentrations of valerate (case study 3). Finally, the optimal conditions, assessed during cases 1–3, were applied to case study 4 to investigate the effect of methane partial pressures (ppCH4) equal to 0.2 and 0.5 atm on M. thricosporum OB3b growth and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production capacity. Unless otherwise specified, the setup described above was used for case studies 1–4.
Figure 1.
Overview of the case studies for the two-step fermentation: methanotrophic growth (blue ■) and PHB-co-HV accumulation (red ■). The intervals studied were taken from previous experimental works.22,23,26−28
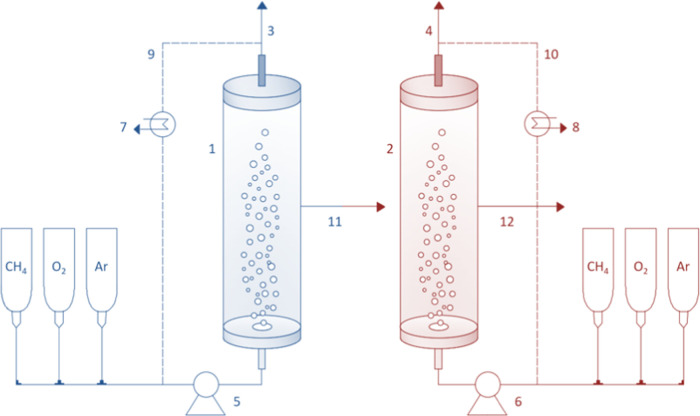
The process, sketched in Figure 2, was simulated using Aspen Plus and consisted of two 400 L bubble column bioreactors (D = 0.4 m; H = 3.2 m) working in series. The nonrandom two-liquid model was used to estimate the properties of the components involved in the process, among which the PHB-co-HV and the biomass were considered solid components. A temperature of 38 °C was considered. The biomass growth and PHB-co-HV accumulation reactions, detailed in the following paragraphs, were assumed zero-order reactions in agreement with the literature,29 thus meaning that the concentration of PHAs increases with a straight line until the external carbon source is depleted. The gas–liquid mass transfer coefficient was theoretically estimated based on the Frossling and Higbie equations, according to the procedure detailed in Amabile et al.25 Gas recycling was also incorporated into the system to recirculate a part of the gaseous streams leaving the reactors, as this configuration increases the performance of the system, according to our previous work.25
Figure 2.
Process simulation flowsheet: (1, blue ■) growth reactor; (2, red ■) accumulation reactor; (3–4) exhaust gas; (5–6) peristaltic pumps; (7–8) condensers; (9–10) recycling streams; (11) grown biomass; and (12) PHB-co-HV containing biomass.
A recycling rate (RR), i.e., the ratio of the internally recycled gas flow rate to the inlet fed gas flow rate, of 14 allowed one to obtain a virtual residence time (VRT) of 2 min when starting from an empty bed residence time (EBRT) of 30 min. The virtual residence time and the empty bed residence time are defined according to eqs 2 and 3, respectively
| 1 |
| 2 |
where Qfed (m3 min–1) is the fresh fed gas stream, Qrec (m3 min–1) is the recycled gas stream, and V (m3) is the volume of the reactor.
The gas flow rate was continuously bubbled into the reactors at a superficial gas velocity (Ug) under the limit for the transition from the homogeneous to the heterogeneous regime to avoid compromising the survival of the active biomass.30,31 The transition superficial gas velocity (Ut) was estimated according to Reilly’s eq 3.32
| 3 |
where Vsmall [m s–1] is the velocity of small bubbles and εt is the transition gas holdup, which can be estimated according to eqs 4 and 5, respectively
| 4 |
| 5 |
where ρg [kg m–3] is the gas density, σ [N m–1] is the surface tension, B is determined experimentally, and ρl [kg m–3] is the liquid density.
Scenarios Definition and Simulation Procedures
Case Study 1: Effect of N-Source and Oxygen Partial Pressure on the Growth of Type II Methanotrophs
Type II methanotrophs M. parvus OBBP and M. thricosporum OB3b (named S1 and S2, respectively) were grown in a 400 L bioreactor under several conditions to establish the optimal parameters for the cultivation and optimize the accumulation of PHB-co-HV. Nitrate (NaNO3) and ammonium (NH4Cl) (named N1 and N2, respectively) were used as N-sources. The reactions for the growth using the selected macronutrients (eqs 6 and 7) were taken from the experimental work by Rostkowski et al.27 and are based on an electron balance, which assumes that the sum of the electrons derived from methane to reduce oxygen (fe) and those used for the cell synthesis (fs) is equal to 1.
 |
6 |
 |
7 |
The effect of oxygen partial pressure when NO3– and NH4+ were employed during the growth of OBBP and OB3b strains was also assessed. Simulations were carried out at 0.1, 0.15, 0.2, 0.3, and 0.4 atm O2 (named P1–P5, with P1 and P5 corresponding to the lowest and the highest partial pressure, respectively). Argon was supplied as an inert gas to keep the total pressure constant at 1 atm. Table 1 shows the partition coefficient fs used for each case,27 the stoichiometric ratio of methane to biomass, the gas composition continuously bubbled into the reactor, the kinetic constants calculated according to the simplified zero-order reaction (kkin), and the gas–liquid mass transfer coefficient (kL). All of the input data reported were taken from the experimental works taken as reference, except for kL, which was estimated according to Amabile et al.25
Table 1. Case Study 1: Partition Coefficient fs, Methane-to-Biomass Stoichiometric Ratio, Gas Composition, Mass Transfer Coefficient (kL), and Kinetic Constant (kkin) (S1 = M. parvus OBBP; S2 = M. thricosporum OB3b; N1 = nitrate; N2 = ammonium; P1 = 0.1 atm; P2 = 0.15 atm; P3 = 0.2 atm; P4 = 0.3 atm; P5 = 0.4 atm).
| simulation run | fs | CH4/biomass[mol mol–1] | gas composition [atm] | kL[m s–1] | kkin[kmol m–3h–1] |
|---|---|---|---|---|---|
| S1N1P1 | 0.59 | 11.86 | CH4/O2/Ar = 0.04:0.1:0.86 | 3.12 × 10–4 | 8.4 × 10–5 |
| S2N1P1 | 0.69 | 10.14 | 3.16 × 10–4 | 9.8 × 10–5 | |
| S1N2P1 | 0.61 | 9.43 | 3.34 × 10–4 | 1.1 × 10–4 | |
| S2N2P1 | 0.43 | 13.4 | 3.27 × 10–4 | 7.5 × 10–5 | |
| S1N1P2 | 0.54 | 12.96 | CH4/O2/Ar = 0.04:0.15:0.81 | 3.13 × 10–4 | 7.8 × 10–5 |
| S2N1P2 | 0.66 | 10.6 | 3.16 × 10–4 | 9.4 × 10–5 | |
| S1N2P2 | 0.44 | 13.06 | 3.23 × 10–4 | 7.7 × 10–5 | |
| S2N2P2 | 0.41 | 14 | 3.22 × 10–4 | 7.3 × 10–5 | |
| S1N1P3 | 0.52 | 13.46 | CH4/O2/Ar = 0.04:0.2:0.76 | 3.13 × 10–4 | 7.5 × 10–5 |
| S2N1P3 | 0.69 | 10.14 | 3.17 × 10–4 | 9.8 × 10–5 | |
| S1N2P3 | 0.56 | 10.26 | 3.31 × 10–4 | 9.8 × 10–5 | |
| S2N2P3 | 0.66 | 8.7 | 3.38 × 10–4 | 1.2 × 10–5 | |
| S1N1P4 | 0.56 | 12.5 | CH4/O2/Ar = 0.04:0.3:0.67 | 3.14 × 10–4 | 8.1 × 10–5 |
| S2N1P4 | 0.62 | 11.3 | 3.15 × 10–4 | 9.0 × 10–5 | |
| S1N2P4 | 0.59 | 9.74 | 3.31 × 10–4 | 1.0 × 10–4 | |
| S2N12P4 | 0.5 | 11.5 | 3.27 × 10–4 | 8.7 × 10–5 | |
| S1N1P5 | 0.53 | 13.2 | CH4/O2/Ar = 0.04:0.4:0.56 | 3.13 × 10–4 | 7.5 × 10–5 |
| S2N1P5 | 0.64 | 10.9 | 3.16 × 10–4 | 9.4 × 10–5 | |
| S1N2P5 | 0.85 | 6.76 | 3.44 × 10–4 | 1.5 × 10–4 | |
| S2N2P5 | 0.81 | 7.1 | 3.48 × 10–4 | 1.4 × 10–4 |
Case Study 2: PHB-co-HV Production in Pure and a Mixed Methanotrophic Culture
The most proper condition for the growth of the methanotrophic strains was used for case study 2, in which the PHB-co-HV production from methane and valeric acid was evaluated by employing pure and mixed methanotrophic cultures. M. parvus OBBP, M. thricosporum OB3b, and a Methyclosystis-dominated enrichment (named S3) accumulated PHB-co-HV into a 400 L bubble column bioreactor. Methane at 4%v/v was fed as a carbon source into a gas stream containing oxygen and argon as well. Valeric acid at 100 ppm (LV) was provided as a cosubstrate in the culture medium to obtain around 20 mol % of the 3-HV monomer into the polymer.22,23 The reaction for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) accumulation (eq 8) was taken from a previous study and is based on an electron balance.27
| 8 |
Table 2 shows the partition coefficients for the three cultures studied, the stoichiometric ratios of methane to PHB-co-HV, the gas–liquid mass transfer coefficients computed (kL), and the kinetic constants (kkin) calculated according to the simplified zero-order reaction. All input data, except for the kL, were taken from prior experimental studies.
Table 2. Case Study 2 Simulation Plan: Partition Coefficients fs, Methane-to-PHB-co-HV Stoichiometric Ratios, Mass Transfer Coefficient (kL), and Kinetic Constant (kkin) (S1 = M. parvus OBBP; S2 = M. thricosporum OB3b; S3 = Methyclosystis-Dominated Enrichment; LV = 100 ppm Valeric Acid Concentration).
| simulation run | fs [−] | CH4/PHB-co-HV[mol mol–1] | kL[m s–1] | kkin[kmol m–3h–1] |
|---|---|---|---|---|
| S1LV | 0.66 | 6.8 | 3.48 × 10–4 | 1.54 × 10–4 |
| S2LV | 0.66 | 6.8 | 3.44 × 10–4 | 1.58 × 10–4 |
| S3LV | 0.65 | 6.9 | 3.33 × 10–4 | 1.06 × 10–4 |
Case Study 3: Effect of Cosubstrate Concentration on the Production of PHB-co-HV
The influence of the cosubstrate concentration on PHB-co-HV accumulation yields was assessed with regard to a mixed methane-utilizing culture dominated by the Methylocystis strain (76.4 ± 4%).22 The accumulation took place in a 400 L bubble column bioreactor, in which a gas stream containing methane at 4%v/v, oxygen, and argon was continuously fed. The culture medium was N-deprived, and valeric acid was supplied as a cosubstrate in the culture medium at concentrations of 100 ppm (LV) and 400 ppm (HV) to induce the accumulation of the 3-HV monomers. All of the input data taken from the literature (i.e., the partitioning coefficient used, the stoichiometric ratio of methane to PHB-co-HV, the kinetic coefficients) and the gas–liquid mass transfer coefficients (kL), which were estimated theoretically, are reported in Table 3.
Table 3. Case Study 3 Simulation Plan: Partition Coefficients fs, Methane-to-PHB-co-HV Stoichiometric Ratios, Mass Transfer Coefficient (kL), and Kinetic Constant (kkin) (S3 = Methyclosystis-Dominated Enrichment; LV = 100 ppm Valeric Acid Concentration; HV = 400 ppm Valeric Acid Concentration).
| simulation run | fs [−] | CH4/PHB-co-HV[mol mol–1] | kL[m s–1] | kkin[kmol m–3h–1] |
|---|---|---|---|---|
| S3LV | 0.65 ± 0.05 | 6.9 | 3.33 × 10–4 | 1.06 × 10–4 |
| S3HV | 0.55 ± 0.05 | 8.2 | 3.18 × 10–4 | 6.0 × 10–5 |
Case Study 4: Effect of Methane Partial Pressure on PHB-co-HV Production under Optimal Conditions
The effect of methane partial pressure on process performance was evaluated under the most proper operating conditions detected during the previous case studies. M. thricosporum OB3b was grown for 20 days on ammonium and accumulated PHB-co-HV for 15 days into a 400 L bubble column reactor working in the semicontinuous mode. The best-performing valeric acid concentration was provided in the culture medium to induce the accumulation of 3-HV units. Methane partial pressures of 0.2 and 0.5 atm were applied, and oxygen was provided to assure aerobic conditions at partial pressures of 0.2 and 0.33 atm, respectively. Argon was supplied as an inert gas to keep the total pressure constant. It is worth highlighting that the gas compositions were made to remain outside the range of explosiveness. The reactions considered for the growth and accumulation were previously reported (eqs 7 and 8), and the kinetic parameters, i.e., 1.1 × 10–4 and 1.9 × 10–4 kmol m–3 h–1 at 0.2 and 0.5 atm, respectively, were calculated according to previous studies in which the same gas compositions were used.23,28
Performance Indicators
The identification of the most promising scenario among those tested was based on the calculation and the comparison of some performance indicators. The productivities of biomass (p-BIO) and PHB-co-HV (p-PHB-co-HV) and the amount of biomass (BIO) at the end of the growth and accumulation cycles (after 20 and 15 days, respectively) were assessed, as well as the fraction of poly(3-hydroxybutyrrate-co-3-hydroxyvalerate) into the total suspended solids (%PHB-co-HV). Moreover, the production of biopolymers per unit of methane fed (sPHB-co-HV) was determined, and the methane-utilizing capacity (EC-CH4) was calculated according to the following equation (eq 9)11
| 9 |
where CCH4,in and CCH4,out are the methane concentrations in the inlet and outlet streams, Q is the total inlet gas flow rate, and V is the volume of the reactor.
Results and Discussion
Growth of Pure Type II Methanotrophs on NO3- and NH4+ at 0.1–0.4 atm O2
The growth of M. Parvus OBBP and M. thricosporum OB3b was studied at different oxygen partial pressures (0.1, 0.15, 0.2, 0.3, and 0.4 atm) using ammonium and nitrate as nitrogen sources (Case study 1). The results obtained, expressed in terms of the productivity of biomass, are reported for ammonium and nitrate in Figures 3 and 4, respectively. When ammonium was used as the N-source, the maximum productivities of 0.44 kg m–3 d–1 for OBBP and 0.39 kg m–3 d–1 for OB3b were obtained at 0.4 atm of O2, while the minimum was reached at 0.15 atm of O2 for both strains, with values of 0.12 kg m–3 d–1 for OBBP and 0.11 kg m–3 d–1 for OB3b (Figure 3). When nitrate was used as the N-source, the maximum productivities were reached at the lowest oxygen partial pressures: for OB3b, the highest value of p-BIO was at 0.2 atm O2 (0.17 kg m–3 d–1), while for OBBP, the peak was reached at 0.1 atm O2 (0.13 kg m–3 d–1) (Figure 4). The differences among the conditions tested were consistent with the experimental data reported by Rostkowski et al.,27 which measured the kinetic parameters for each condition evaluated, as well as the stoichiometric coefficients for the reaction calculation (fe and fs). The authors found that the maximum stoichiometric yields were fs = 0.69 and fs = 0.66 for OB3b, while those of OBBP were fs = 0.85 and fs = 0.50, using nitrate and ammonium, respectively. Even if the stoichiometric biomass production per unit of N-source (gBIO/gN) was higher when using nitrate, the growth rates obtained with ammonium, 1.2 × 10–4 kmol m–3 h–1 for OB3b and 1.5 × 10–4 kmol m–3 h–1 for OBBP, were higher than those achieved with nitrate, which resulted in 9.8 × 10–5 and 8.4 × 10–5 kmol m–3 h–1 for M. thricosporum OB3b and M. parvus OBBP, respectively. These results show that the effective biomass growth, and thus the actual yields of the process, is determined by the combination of the stoichiometric production (gBIO/gN) and the growth rate of the strain (μmax).33 The trends obtained in this work and in the prior experimental study27 could be linked to the growth kinetics of these Type II methanotrophs, which respond differently to the variations in oxygen concentrations. In this regard, Figure 3 shows that the p-BIO considerably varied when ammonium was used as the macronutrient. This could be related to the high level of reducing equivalents necessary for reducing hydroxylamine.27 On the contrary, when the strains were grown on nitrate, only a low variability of the data was observed (Figure 4).
Figure 3.
Effect of oxygen partial pressures on the growth of M. thricosporum OB3b and M. parvus OBBP in the presence of ammonium.
Figure 4.
Effect of oxygen partial pressures on the growth of M. thricosporum OB3b and M. parvus OBBP in the presence of nitrate.
Finally, ammonium seems to be the best-performing nitrogen source for the growth of M. parvus OBBP and M. thricosporum OB3b at higher oxygen partial pressures. Similar results have been reported in prior experimental works. Thus, a preliminary study analyzed the effect that ammonium and nitrate exert on several methanotrophic strains and found that the effective yields (as a combination of the stoichiometry and the growth rate) could be maximized by coupling the use of methane and ammonium during the process.33 However, other authors found that ammonia promoted a methane conversion of 80% in Methylocystis hirsuta, which was 100% in the presence of nitrate.12 These contradictory results suggest that the bacterial growth optimization is based not only on the nitrogen source and its concentration but also on the carbon source (e.g., methane as a pure gas or as a component of the biogas), the gas composition (e.g., methane concentration, CH4-to-O2 ratio), the methanotrophic strain (e.g., microorganisms belonging to the genera Methylocystis), and the operative conditions, e.g., temperature and pH among others.12,18,34
PHB-co-HV Accumulation in Pure and Mixed Cultures at Low and High Valerate Concentrations
The best condition for the growth of M. parvus OBBP and M. thricosporum OB3b (ammonium as the N-source at 10 mM, ppO2 = 0.4 atm) was used for accumulation of PHB-co-HV. First, the capacity of pure and mixed cultures to accumulate the polymer in the presence of low valerate concentrations at a methane partial pressure of 0.04 atm was assessed (Case study 2). Then, the effect of valeric acid on Methylocystis-dominated enrichment was studied under the same methane partial pressure (Case study 3). The amount of biomass conducted to the accumulation reactor is reported in Table 4 and was produced under optimal conditions identified during the previous test.
Table 4. Biomass Grown under Optimal Conditions Used for PHB-co-HV Accumulation.
| culture | p-BIO[kg m–3d–1] | BIO [kg] |
|---|---|---|
| M. thricosporum OB3b | 0.39 | 3.1 |
| M. parvus OBBP | 0.43 | 3.48 |
| Methylocystis-dominated enrichment | 0.27 | 2.15 |
The results of the simulations of PHB-co-HV accumulation are reported in Figure 5. The productivities of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) were nearly the same for both pure strains, with values of 0.22 kg m–3 d–1 for M. parvus OBBP and 0.23 kg m–3 d–1 for M. thricosporum OB3b, which correspond to 1.3 and 1.4 kg of PHB-co-HV produced after 15 days, respectively, in the 400 L fermentors. On the other hand, the mixed culture supplemented with 100 ppm of valerate supported the lowest value of p-PHB-co-HV 0.156 kg m–3 d–1 (0.93 kg after 15 days of accumulation). The methane utilization capacities were 62 g m–3 h–1 for the pure strains and 59 g m–3 h–1 for the mixed consortium. It is noteworthy that, when using a mixed consortium, despite the high selectivity of type II methanotrophs, a part of the consortium could be made of non-PHA-accumulating bacteria.2,22 This could explain the higher productivities obtained using the pure strains, where all cultures can accumulate PHA. However, it is important to point out that mixed cultures could be used at an industrial scale, where high sterility and control are not required.35
Figure 5.
Effect of valeric acid concentration on productivity of PHB-co-HV, molar fraction of 3-hydroxyvalerate, and PHB-co-HV content for pure and mixed cultures (LV = 100 ppm, HV = 400 ppm).
The evaluation of the strain capacity to accumulate PHB-co-HV was followed by the study of the effects that the valerate concentration has on the process yields. According to the experimental data reported by a previous study, the mixed methanotrophic strain was used for these simulations.22 The production of PHB-co-HV from methane and valerate under 100 and 400 ppm was investigated. The experimental results showed that the higher the valerate concentration, the lower the PHB-co-HV produced and the higher the molar fraction of HV accumulated in the cells.23 The results of these simulations showed that using a high valerate concentration could reduce the productivity of PHB-co-HV by up to 54%, ranging from 0.156 kg m–3 d–1 at 100 ppm to 0.072 kg m–3 d–1 (0.43 kg after 15 days of accumulation) at 400 ppm of valeric acid, in agreement with the literature data. Accordingly, the methane utilization capacity was reduced to 55 g m–3 h–1 when using 400 ppm of cosubstrate. It should be noted that under the highest concentration of valerate reported in the present work, the molar fraction of 3-HV can reach up to 40 mol % of the total polymer accumulated; however, only 22 mol % 3-HV was reported at lower concentrations (100 ppm)22,36 (Figure 5). The reduction of PHB-co-HV productivity, and the consequent reduction of the methane utilization capacity, could be related to a decrease in pH since higher amounts of valeric acid could be responsible for the acidification of the culture medium as a lower pH has been measured under these conditions.21
The PHB-co-HV content (%PHB-co-HV) (Figure 5) was calculated with respect to the total suspended solids and thus depended on the growth yields (Table 4). The maximum PHB-co-HV content was assessed for M. thricosporum OB3b and for the mixed consortium at 100 ppm of cosubstrate (30%w/w) (Figure 5). Lopez et al.21 obtained a maximum PHB-co-HV accumulation ability of 53 ± 9.8%w/w when working with M. hirsuta under an optimal cosubstrate concentration since a microbial activity inhibition was detected as low as 177 mg L–1 valeric acid.21 The accumulated contents previously experimented for a mixed culture were 43%w/w and 30%w/w under low and high valerate concentrations, respectively.22 The difference with respect to the results obtained in these simulations could be justified by the more favorable growth conditions used in this work (high oxygen partial pressure), which led to a higher concentration of biomass and reduced the fraction of polymer in the total suspended solids. Regarding the accumulation of PHB-co-HV by a mixed methanotrophic culture from methane and volatile fatty acids, a prior experimental study reported a maximum accumulation capacity of 10%w/w.19 It could be stated that, based on the results here obtained and previously reported from experimental studies, the potential for accumulating PHB-co-HV is strongly linked to the operating conditions rather than the strain used.
The production of PHB per unit of the substrate was almost constant for all cases at 0.6 g of PHB-co-HV gCH4–1, which was previously reported as the peak value,36 thus suggesting that the conditions here simulated could guarantee the optimization of process yields and substrate consumption.
A comparison between the simulation results and the data available in prior experimental works, in terms of %PHB-co-HV (wt %) and 3-HV mol %, shows that the values obtained in the present study are in the same range as those obtained experimentally (Table 5). It should be stressed that all of the data reported in the literature about PHB-co-HV production are referred to small lab-scale experiments, while larger volumes were simulated in this work. It is worth highlighting that no experimental data at pilot or large scales are available in the scientific literature.
Table 5. PHB-co-HV Production from Methane and Odd-Chain Carbon Fatty Acids Measured in Experimental Works and This Simulation Study Comparison.
| volume [L] | substrate | cosubstrate | cosubstrate concentration [ppm] | strain | PHB-co-HV[wt %] | 3-HV mol % | refs |
|---|---|---|---|---|---|---|---|
| 0.16 | methane | valeric acid | 100 | Methylocystis-dominate culture | 43 | 20 | Myung et al.22 |
| valeric acid | 400 | Methylocystis-dominate culture | 30 | 40 | |||
| 0.16 | methane | propionate | 100 | M. thricosporumOBBP | 32 ± 4 | 8 ± 2 | Myung et al.23 |
| valeric acid | 100 | M. thricosporumOBBP | 54 ± 3 | 22 ± 3 | |||
| valeric acid | 100 | M. parvusOB3b | 50 ± 4 | 20 ± 4 | |||
| 0.25 | methane | valeric acid | 50 | Methylocystis-dominated culture | 34 ± 3 | 25 ± 2 | Fergala et al.17 |
| valeric acid | 100 | Methylocystis-dominated culture | 47 ± 4 | 35 ± 3 | |||
| 0.16 | methane | propionate | 100 | Methylosinus-dominated culture | 3.5 | 22.6 | Luangthongkam et al.18 |
| valeric acid | 100 | Methylosinus-dominated culture | 14.1 | 65 | |||
| 0.25 | methane | proprionato | 100 | mixed culture | 2.6 | 70 | Luangthongkam et al.19 |
| valerato | 100 | mixed culture | 5.45 | 66 | |||
| valerate | 200 | mixed culture | 9.6 | 64 | |||
| proprionate | 100 | mixed culture | 1.42 | 84.6 | |||
| valerate | 100 | mixed culture | 3.16 | 68.8 | |||
| valerate | 200 | mixed culture | 8.4 | 64.8 | |||
| proprionate | 100 | mixed culture | 2.14 | 63.5 | |||
| not available | valerate | 100 | mixed culture | 3.73 | 76.2 | ||
| not available | valerate | 200 | mixed culture | 3.8 | 79 | ||
| methane | acetic acid | 100 | mixed culture | 2.4 | 0 | ||
| not available | acetic acid | 100 | mixed culture | 0.95 | 0 | ||
| methane | acetic and propionic acids | 100 + 100 | mixed culture | 1.9 | 18.7 | ||
| not available | acetic and propionic acids | 100 + 100 | mixed culture | 6.75 | 39.7 | ||
| 2.15 | biogas | acetic acid | 181 ± 16 | M. hirsuta | 52.3 ± 0.7 | 0 | López et al.21 |
| propionic acid | 123 ± 2 | M. hirsuta | 47.9 ± 0.7 | 2 | |||
| butyric acid | 130 ± 6 | M. hirsuta | 52.2 ± 2.1 | 0 | |||
| valeric acid | 181 ± 16 | M. hirsuta | 53.8 ± 0.8 | 25 | |||
| 400 | methane | valeric acid | 100 | M. thricosporumOB3b | 34 | ≈20 | this study |
| valeric acid | 100 | M. parvusOBBP | 28 | ≈20 | |||
| valeric acid | 100–400 | Mixed methane-utilizing culture | 30–17 | ≈20–40 |
PHB-co-HV Accumulation at High Methane Partial Pressures
M. thricosporum OB3b was selected for simulation test 4 to grow and accumulate PHB-co-HV under higher methane partial pressures of 0.2 and 0.5 atm (ppCH4), and oxygen was provided to assure aerobic conditions at partial pressures of 0.2 and 0.33 atm, respectively. The results, reported in Table 6, show that the process performance indicators, i.e., p-BIO, p-PHB-co-HV, EC-CH4, and %PHB-co-HV, increased with the partial pressure of CH4, thus suggesting a convenience of working with higher amounts of methane. In this context, it is important to highlight that the gas composition should be designed to remain outside the explosion range.
Table 6. PHB-co-HV Production from Methane and Odd-Chain Carbon Fatty Acids.
| pp CH4 [atm] | p-BIO[kg m–3d–1] | p-PHB-co-HV[kg m–3d–1] | EC-CH4[g m–3h–1] | PHB-co-HV [%] | sPHB-co-HV[kgPHB-co-HV kgCH4–1] |
|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.17 | 259 | 24 | 0.48 |
| 0.5 | 0.44 | 0.32 | 630 | 35 | 0.61 |
To the best of the authors’ knowledge, a similar study on the production of PHB-co-HV was not conducted before, either experimentally or as a simulation work. However, some experimental data about the effect of methane partial pressure on poly(3-hydroxybutyrate) (PHB) were reported by Zhang et al.28 They investigated the production of PHB from methane at the same ppCH4 used in this work and found that the accumulation capacity of methanotrophic bacteria increased with this parameter: 41.5%w/w PHB was obtained at 0.5 atm of CH4 and 0.33 atm of O2, while only 25%w/w PHB was obtained at methane and oxygen partial pressures of 0.2 atm and 0.2 atm, respectively. Moreover, Jiang et al.37 reported that an excess of carbon source helped the intracellular PHA accumulation of the activated sludge when the PHB production was carried out from the mixtures of volatile fatty acids. Although O2 and CH4 mass transfer coefficients are essentially equal, such as their Henry’s law constants are very similar, the reaction stoichiometry requires the theoretical ratio of 1.5 mol O2 per mol of CH4, and therefore, with the scenarios proposed, the process would be limited by oxygen; the simulation results, supported by the experimental findings, highlighted that PHAs are favored by the high availability of carbon, i.e., a high transfer rate of carbon in the liquid solution, which allows the consumption of oxygen. This achievement can be supported considering that methanotrophs prefer to grow in a condition where both oxygen and methane are completely consumed.38
Conclusions
In this work, the growth of methanotrophic cultures and the accumulation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) were investigated by simulating the influence of the main process parameters affecting the production yield, such as the type of culture, nitrogen source, oxygen and methane partial pressures, and cosubstrate concentration. The highest growth productivity was obtained for pure methanotrophic cultures at high oxygen partial pressure with ammonium as the N-source. However, mixed cultures might be more suitable on an industrial scale. The accumulation of PHB-co-HV by pure and mixed cultures grown under optimal conditions showed that the higher the cosubstrate concentrations, the higher the 3-HV fraction in the copolymer. Furthermore, using a methane-rich gas stream increases the PHB-co-HV production per unit of substrate fed, suggesting that the higher the methane partial pressure, the higher the PHB-co-HV yields. Finally, the production of biopolymers was favored by the high availability of carbon and limited by oxygen deficiency at low carbon contents.
Acknowledgments
This work was supported by the Ministry of Economic Development through the “Fondo per la Crescita Sostenibile – Sportello “Agrifood” PON I&C 2014-2020”, di cui al D.M. 5 marzo 2018 Capo III. Prog. n. F/200125/01-03/X45. The authors would like to thank VALERE “VAnviteLli pEr la RicErca” PROGRAMME by the University of Campania Luigi Vanvitelli. The Regional Government of Castilla y León and the EU-FEDER (CLU 2017-09, CL-EI-2021-07, and UIC 315) are also gratefully acknowledged.
Glossary
Abbreviations and Symbols
- 3-HB
3-hydroxybutyrate
- 3-HV
3-hydroxyvalerate
- CCH4
methane concentration [g m–3]
- D
reactor diameter [m]
- EBRT
empty bed residence time [min]
- EC-CH4
methane utilization capacity [g m–3h–1]
- fe, fs
partition coefficient
- H
reactor height [m]
- kkin
kinetic constant [kmol m–3h–1]
- kL
mass transfer coefficient [m s–1]
- p-BIO
biomass productivity [kg m–3d–1]
- PHAs
polyhydroxyvalkanoates
- PHB
poly(3-hydroxybutyrate)
- PHB-co-HV
poly(3-hydroxybutyrate-co-3-hydroxyvalerate)
- PP
polypropylene
- p-PHB-co-HV
PHB-co-HV productivity [kg m–3d–1]
- Qfed
gas flow rate [m3 h–1]
- Qrec
recycled gas flow rate [m3 h–1]
- RR
recycling rate
- sPHB-co-HV
PHB-co-HV productivity for unit of substrate [kgPHB-co-HV kgCH4–1]
- Tm
melting temperature [°C]
- Ug
superficial gas velocity [m s–1]
- Ut
transition superficial gas velocity [m s–1]
- V
reactor volume [m3]
- VRT
virtual residence time [min]
- Vsmall
small bubble velocity [m s–1]
- εt
transition gas holdup
- μmax
maximum growth rate [h–1]
- ρg
gas density [kg m–3]
- ρl
liquid density [kg m–3]
- σ
surface tension [N m–1]
The authors declare no competing financial interest.
References
- Nguyenhuynh T.; Yoon L. W.; Chow Y. H.; Chua A. S. M.; Wan L.; Hui Y.; Seak A.; Chua M. An Insight into Enrichment Strategies for Mixed Culture in Polyhydroxyalkanoate Production: Feedstocks, Operating Conditions and Inherent Challenges. Chem. Eng. J. 2021, 420, 130488 10.1016/j.cej.2021.130488. [DOI] [Google Scholar]
- Salem R.; Soliman M.; Fergala A.; Audette G. F.; Eldyasti A. Screening for Methane Utilizing Mixed Communities with High Polyhydroxybutyrate (Phb) Production Capacity Using Different Design Approaches. Polymers 2021, 13, 1579. 10.3390/polym13101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Rathour R.; Singh R.; Sun Y.; Pandey A.; Gnansounou E.; Lin K. A.; Tsang D. C. W.; Shekhar I. Bacterial Polyhydroxyalkanoates: Opportunities, Challenges, and Prospects. J. Clean. Prod. 2020, 263, 121500 10.1016/j.jclepro.2020.121500. [DOI] [Google Scholar]
- Ojumu T. V.; Yu J.; Solomon B. O. Production of Polyhydroxyalkanoates, a Bacterial Biodegradable Polymer. African J. Biotechnol. 2004, 3, 18–24. 10.5897/AJB2004.000-2004. [DOI] [Google Scholar]
- Chee J.; Yoga S.; Lau N.; Ling S.; Abed R. M. M. Bacterially Produced Polyhydroxyalkanoate (PHA): Converting Renewable Resources into Bioplastics. Curr. Res., Technol. Education Topics Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1395–1404. [Google Scholar]
- Zhou S. J.; Wang H. M.; Xiong S. J.; Sun J. M.; Wang Y. Y.; Yu S.; Sun Z.; Wen J. L.; Yuan T. Q. Technical Lignin Valorization in Biodegradable Polyester-Based Plastics (BPPs). ACS Sustainable Chem. Eng. 2021, 9, 12017–12042. 10.1021/acssuschemeng.1c03705. [DOI] [Google Scholar]
- Bossu J.; Angellier-Coussy H.; Totee C.; Matos M.; Reis M.; Guillard V. Effect of the Molecular Structure of Poly(3-Hydroxybutyrate- Co-3-Hydroxyvalerate) (P(3HB-3HV)) Produced from Mixed Bacterial Cultures on Its Crystallization and Mechanical Properties. Biomacromolecules 2020, 21, 4709–4723. 10.1021/acs.biomac.0c00826. [DOI] [PubMed] [Google Scholar]
- Policastro G.; Panico A.; Fabbricino M. Improving Biological Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Co-Polymer: A Critical Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 479–513. 10.1007/s11157-021-09575-z. [DOI] [Google Scholar]
- Mcgauran T.; Harris M.; Dunne N.; Smyth B. M.; Cunningham E. Production of Feather-Based Biopolymers as a Direct Alternative to Synthetic Plastics. ACS Sustainable Chem. Eng. 2022, 10, 486–494. 10.1021/acssuschemeng.1c06791. [DOI] [Google Scholar]
- Khosravi-Darani K.; Mokhtari Z. B.; Amai T.; Tanaka K. Microbial Production of Poly(Hydroxybutyrate) from C1 Carbon Sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. 10.1007/s00253-012-4649-0. [DOI] [PubMed] [Google Scholar]
- Rodríguez Y.; Firmino P. I. M.; Pérez V.; Lebrero R.; Muñoz R. Biogas Valorization via Continuous Polyhydroxybutyrate Production by Methylocystis Hirsuta in a Bubble Column Bioreactor. Waste Manag. 2020, 113, 395–403. 10.1016/j.wasman.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Rodríguez Y.; Firmino P. I. M.; Arnáiz E.; Lebrero R.; Muñoz R. Elucidating the Influence of Environmental Factors on Biogas-Based Polyhydroxybutyrate Production by Methylocystis Hirsuta CSC1. Sci. Total Environ. 2020, 706, 135136 10.1016/j.scitotenv.2019.135136. [DOI] [PubMed] [Google Scholar]
- Plastics Europe. Bioplastic Market Development Update 2020, 2020.
- Chen G.-Q.Industrial Production of PHA. In Microbiology Monographs, 2010; pp 121–132. [Google Scholar]
- Cal A. J.; Sikkema W. D.; Ponce M. I.; Franqui-Villanueva D.; Riiff T. J.; Orts W. J.; Pieja A. J.; Lee C. C. Methanotrophic Production of Polyhydroxybutyrate-Co-Hydroxyvalerate with High Hydroxyvalerate Content. Int. J. Biol. Macromol. 2016, 87, 302–307. 10.1016/j.ijbiomac.2016.02.056. [DOI] [PubMed] [Google Scholar]
- Liu L. Y.; Xie G. J.; Xing D. F.; Liu B. F.; Ding J.; Ren N. Q. Biological Conversion of Methane to Polyhydroxyalkanoates: Current Advances, Challenges, and Perspectives. Environ. Sci. Ecotechnol. 2020, 2, 2. 10.1016/j.ese.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergala A.; Alsayed A.; Khattab S.; Ramirez M.; Eldyasti A. Development of Methane-Utilizing Mixed Cultures for the Production of Polyhydroxyalkanoates (PHAs) from Anaerobic Digester Sludge. Environ. Sci. Technol. 2018, 52, 12376–12387. 10.1021/acs.est.8b04142. [DOI] [PubMed] [Google Scholar]
- Luangthongkam P.; Strong P. J.; Syed Mahamud S. N.; Evans P.; Jensen P.; Tyson G.; Laycock B.; Lant P. A.; Pratt S. The Effect of Methane and Odd-Chain Fatty Acids on 3-Hydroxybutyrate (3HB) and 3-Hydroxyvalerate (3HV) Synthesis by a Methylosinus-Dominated Mixed Culture. Bioresour. Bioprocess. 2019, 6, 50. 10.1186/s40643-019-0285-1. [DOI] [Google Scholar]
- Luangthongkam P.; Laycock B.; Evans P.; Lant P.; Pratt S. Thermophilic Production of Poly(3-Hydroxybutyrate-Co-3-Hydrovalerate) by a Mixed Methane-Utilizing Culture. New Biotechnol. 2019, 53, 49–56. 10.1016/j.nbt.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Akdoğan M.; Çelik E. Enhanced Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Biopolymer by Recombinant Bacillus Megaterium in Fed-Batch Bioreactors. Bioprocess Biosyst. Eng. 2021, 44, 403–416. 10.1007/s00449-020-02452-z. [DOI] [PubMed] [Google Scholar]
- López J. C.; Arnáiz E.; Merchán L.; Lebrero R.; Muñoz R. Biogas-Based Polyhydroxyalkanoates Production by Methylocystis Hirsuta: A Step Further in Anaerobic Digestion Biorefineries. Chem. Eng. J. 2018, 333, 529–536. 10.1016/j.cej.2017.09.185. [DOI] [Google Scholar]
- Myung J.; Galega W. M.; Van Nostrand J. D.; Yuan T.; Zhou J.; Criddle C. S. Long-Term Cultivation of a Stable Methylocystis-Dominated Methanotrophic Enrichment Enabling Tailored Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate). Bioresour. Technol. 2015, 198, 811–818. 10.1016/j.biortech.2015.09.094. [DOI] [PubMed] [Google Scholar]
- Myung J.; Flanagan J. C. A.; Waymouth R. M.; Criddle C. S. Methane or Methanol-Oxidation Dependent Synthesis of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Obligate Type II Methanotrophs. Process Biochem. 2016, 51, 561–567. 10.1016/j.procbio.2016.02.005. [DOI] [Google Scholar]
- Budwill K.; Fedorak P. M.; Page W. J. Methanogenic Degradation of Poly(3-Hydroxyalkanoates). Appl. Environ. Microbiol. 1992, 58, 1398–1401. 10.1128/aem.58.4.1398-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabile C.; Abate T.; Crescenzo C.; De Sabbarese S.; Migliaccio A.; Chianese S.; Musmarra D.; De Crescenzo C.; Sabbarese S.; Migliaccio A.; Chianese S.; Musmarra D. Poly(3-Hydroxybutyrate) Production from Methane in Bubble Column Bioreactors: Process Simulation and Design Optimization. New Biotechnol. 2022, 70, 39–48. 10.1016/j.nbt.2022.04.004. [DOI] [PubMed] [Google Scholar]
- García-Pérez T.; López J. C.; Passos F.; Lebrero R.; Revah S.; Muñoz R. Simultaneous Methane Abatement and PHB Production by Methylocystis Hirsuta in a Novel Gas-Recycling Bubble Column Bioreactor. Chem. Eng. J. 2018, 334, 691–697. 10.1016/j.cej.2017.10.106. [DOI] [Google Scholar]
- Rostkowski K. H.; Pfluger A. R.; Criddle C. S. Stoichiometry and Kinetics of the PHB-Producing Type II Methanotrophs Methylosinus Trichosporium OB3b and Methylocystis Parvus OBBP. Bioresour. Technol. 2013, 132, 71–77. 10.1016/j.biortech.2012.12.129. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Zhou J.; Wang X.; Zhang Y. Poly-β-Hydroxybutyrate Production by Methylosinus Trichosporium Ob3b at Different Gas-Phase Conditions. Iran. J. Biotechnol. 2019, 17, 10–16. 10.21859/ijb.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. 10.3390/fermentation4020030. [DOI] [Google Scholar]
- Rikmanis M.; Berzinš A.; Viesturs U. Excess Turbulence as a Cause of Turbohypobiosis in Cultivation of Microorganisms. Open Life Sci. 2007, 2, 481–501. 10.2478/s11535-007-0038-6. [DOI] [Google Scholar]
- Priede M. A.; Vangas J.; Viesturs U. E.; Bujalski W.; Tucker K. J.; Thomas C. R. Hydrodynamic, Physiological, and Morphological Characteristics of Fusarium Moniliforme in Geometrically Dissimilar Stirred Bioreactors. Biotechnol. Bioeng. 1995, 48, 266–277. 10.1002/bit.260480313. [DOI] [PubMed] [Google Scholar]
- Reilly I. G.; Scott D. S.; Debruijn T. J. W.; Macintyre D. The Role of Gas Phase Momentum in Determining Gas Holdup and Hydrodynamic Flow Regimes in Bubble Column Operations. Can. J. Chem. Eng. 1994, 72, 3–12. 10.1002/cjce.5450720102. [DOI] [Google Scholar]
- Tays C.; Guarnieri M. T.; Sauvageau D.; Stein L. Y. Combined Effects of Carbon and Nitrogen Source to Optimize Growth of Proteobacterial Methanotrophs. Front. Microbiol. 2018, 9, 2239. 10.3389/fmicb.2018.02239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez R.; Cantera S.; Bordel S.; García-Encina P. A.; Muñoz R. The Effect of Temperature during Culture Enrichment on Methanotrophic Polyhydroxyalkanoate Production. Int. Biodeterior. Biodegrad. 2019, 140, 144–151. 10.1016/j.ibiod.2019.04.004. [DOI] [Google Scholar]
- Shalin T.; Sindhu R.; Binod P.; Soccol C. R.; Pandey A. Mixed Cultures Fermentation for the Production of Poly- β- Hydroxybutyrate. Braz. Arch. Biol. Technol. 2014, 57, 644–652. 10.1590/S1516-89132013005000016. [DOI] [Google Scholar]
- García-Pérez T.; López J. C.; Passos F.; Lebrero R.; Revah S.; Muñoz R. Simultaneous Methane Abatement and PHB Production by Methylocystis Hirsuta in a Novel Gas-Recycling Bubble Column Bioreactor. Chem. Eng. J. 2018, 334, 691–697. 10.1016/j.cej.2017.10.106. [DOI] [Google Scholar]
- Jiang Y.; Chen Y.; Zheng X. Efficient Polyhydroxyalkanoates Production from a Waste-Activated Sludge Alkaline Fermentation Liquid by Activated Sludge Submitted to the Aerobic Feeding and Discharge Process. Environ. Sci. Technol. 2009, 43, 7734–7741. 10.1021/es9014458. [DOI] [PubMed] [Google Scholar]
- Amaral J. A.; Knowles R. Growth of Methanotrophs in Methane and Oxygen Counter Gradients. FEMS Microbiol. Lett. 1995, 126, 215–220. 10.1111/j.1574-6968.1995.tb07421.x. [DOI] [Google Scholar]