Abstract
Protein phosphorylation is an important post‐transcriptional modification involving an extremely wide range of intracellular signaling transduction pathways, making it an important therapeutic target for disease intervention. At present, numerous drugs targeting protein phosphorylation have been developed for the treatment of various diseases including malignant tumors, neurological diseases, infectious diseases, and immune diseases. In this review article, we analyzed 303 small‐molecule protein phosphorylation kinase inhibitors (PKIs) registered and participated in clinical research obtained in a database named Protein Kinase Inhibitor Database (PKIDB), including 68 drugs approved by the Food and Drug Administration of the United States. Based on previous classifications of kinases, we divided these human protein phosphorylation kinases into eight groups and nearly 50 families, and delineated their main regulatory pathways, upstream and downstream targets. These groups include: protein kinase A, G, and C (AGC) and receptor guanylate cyclase (RGC) group, calmodulin‐dependent protein kinase (CaMK) group, CMGC [Cyclin‐dependent kinases (CDKs), Mitogen‐activated protein kinases (MAPKs), Glycogen synthase kinases (GSKs), and Cdc2‐like kinases (CLKs)] group, sterile (STE)‐MAPKs group, tyrosine kinases (TK) group, tyrosine kinase‐like (TKL) group, atypical group, and other groups. Different groups and families of inhibitors stimulate or inhibit others, forming an intricate molecular signaling regulatory network. This review takes newly developed new PKIs as breakthrough point, aiming to clarify the regulatory network and relationship of each pathway, as well as their roles in disease intervention, and provide a direction for future drug development.
Keywords: cell signaling, diseases, intervention therapy, protein kinases, protein phosphorylation
Human protein phosphorylation kinases were divided into eight groups and nearly 50 families, and delineated their main regulatory pathways, upstream and downstream targets. We analyzed more than 300 small‐molecule protein phosphorylation inhibitors listed by Food and Drug Administration or in clinical research for classification, generalization, and analysis. This review takes newly developed drugs as entry point, aiming to clarify the regulatory network and relationship of each pathway, as well as their roles in disease intervention, and provide a direction for future drug development.

1. INTRODUCTION
Protein phosphorylation is a common post‐transcriptional modification and plays an important role in intracellular signaling transduction. Protein phosphorylation refers to a process of transferring the phosphate group of adenosine triphosphate (ATP) to the amino acid residues (serine, threonine, tyrosine) of the substrate protein catalyzed by protein kinase, or binding to guanosine triphosphate (GTP) under the action of a signal. Protein phosphorylation is a common regulation method in organisms, and plays an important role in the process of cell signal transduction. 1 Protein phosphorylation is the most basic, common, and important mechanism for regulating and controlling protein activity and function. Phosphorylation of proteins can allosteric proteins, activate the corresponding protein activity, then form protein complexes with different proteins, and then further promote protein phosphorylation, which constitutes the basic mechanism of cell signal transduction. 2 Human protein kinases constitute about 1.7% of the total genes and mediate most signaling transduction in eukaryotic cells. In eukaryotes, there are more than 100,000 phosphorylation regulatory sites, involving many functions and influences such as cancer mutation, genetic variation, mRNA expression, DNA methylation, and molecular interaction. 3 The genetic alterations including mutation, overexpression, inhibition, and translocation are involved in cell signaling and development of diseases.
Because protein kinases are the most important components of intracellular signaling proteins, they also become important targets for drug intervention. Since the first approval of imatinib in 2001, small‐molecule kinase inhibitors have brought enormous benefits to patients in various therapeutic areas. 4 Currently, one‐third of the new drug development is focused on targeting protein phosphorylation kinases. 4 A database of management, annotation, and updating of protein kinase inhibitors (PKIs) in clinical trials, named Protein Kinase Inhibitor Database (PKIDB) (https://www.icoa.fr/pkidb/), provides the latest clinical trials on PKIs. 5 Up to date, there are 303 kinds of compounds under investigation. Up to the beginning of 2022, there are 68 small‐molecule PKIs approved by the Food and Drug Administration (FDA) of the United States. 6 Besides, there are many new types of PKIs that were under preclinical research. There are still some difficulties and challenges in the drug development process, such as high electrophilic reactivity, non‐specific cytotoxicity, and target cysteine mutation disadvantages relative to reversible kinase inhibitors that previously hindered the development of small‐molecule kinase inhibitors. 7
This review takes the PKIs that have been clinically used and are undergoing drug clinical trials as the entry point, and introduces the signaling pathways, regulatory pathways, mechanisms of action, and regulatory sites related to different groups of phosphorylated kinases (Table S1). Human protein kinases are divided into groups, families, and subfamilies. There are eight main groups 8 : (1) protein kinase A, G, and C (AGC) and receptor guanylate cyclase (RGC) group, (2) calmodulin‐dependent protein kinase (CaMK) group, (3) CMGC [Cyclin‐dependent kinases (CDKs), Mitogen‐activated protein kinases (MAPKs), Glycogen synthase kinases (GSKs), and Cdc2‐like kinases (CLKs)] group, (4) sterile (STE)‐MAPKs group, (5) tyrosine kinases (TK) group, (6) tyrosine kinase‐like (TKL) group, (7) atypical kinases group, and (8) other group. The characteristics, upstream and downstream regulation of PKI in different groups are introduced; PKI‐related diseases and intervention therapy are also introduced in this review.
2. KINASE GROUPS
2.1. AGC and RGC kinase group
Besides targeting AKT, some inhibitors target downstream signaling pathways mediated by AKT as will be discussed. One example is the nerve growth factor (NGF), pathway that activates AKT through its receptor, the TK receptor tropomyosin‐related kinases A (TrkA) 9 promoting neuronal survival and neurite outgrowth. The AGC kinase group consists of seven families, including protein kinase A (PKA), protein kinase B (PKB), protein kinase C (PKC), protein kinase G (PKG), phosphatidylinositol 2‐kinase (PI2K), phosphatidylinositol 3‐kinase (PI3K), rho‐associated coiled‐coil‐containing kinase (ROCK), and RGC families. The family members of the AGC kinase group have a hydrophobic motif sequence, which is composed of a universal motif, docked at the coevolutionary hydrophobic site in the core lobule of human protein kinase 10 (Figure 1).
FIGURE 1.
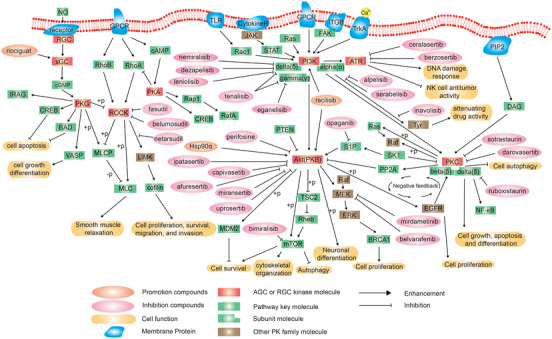
Schematic diagram of protein phosphorylation signaling pathway of protein kinase A, G, and C (AGC)‐receptor guanylate cyclase (RGC) group.
2.1.1. PKB family
PKB, which is known as AKT, acts as a downstream molecule of the PI3K. AKT regulates many cellular processes such as cell proliferation and survival, metabolism, tumor growth, and metastasis. 11 Neurotrophins, such as NGF, can activate methyl ethyl ketone (MEK)/extracellular signal‐regulated kinase (ERK)/AKT pathway to promote neuronal survival and neurite outgrowth by activating the TK receptor TrkA, for Ca2+‐dependent AKT phosphorylation. 9
AKT is involved in a variety of cellular programs, such as cell survival‐related glucose metabolism, cell apoptosis, cell proliferation, transcription, and cell migration. Thus, AKT is a good therapeutic target for diseases related to cell proliferation, glucose metabolism, and others.
AKT phosphorylation inhibitors are most commonly used in the treatment of cancers. Phosphatidylinositol‑4,5‑bisphosphate 3‑kinase catalytic subunit alpha (PIK3CA) is associated with hemangiosarcoma (HSA) and is phosphorylated by AKT, which enhances epidermal growth factor receptor (EGFR) signaling. 12 Alpelisib, a molecular drug targeting PIK3CA mutations, has significant antitumor affects in canine PIK3CA‐mutated HSA cell lines in animal experiments. 12 Multiple studies have demonstrated the synergistic antitumor activity using the combination of AKT pathway inhibitors, such as capivasertib, in breast cancer susceptibility genes (BRCA)‐related cancers. 13 Studies show that reduced AKT activity by AKT inhibitors is associated with reduced tumor cell proliferation 14 and multidrug resistance. 15 Several drugs of AKT inhibitor candidates such as afuresertib, ipatasertib, uprosertib, perifosine (KRX‐0401), and PHT‐427 have been studied. 14 Opaganib and sphingosine‐1‐phosphate (S1P) regulate AKT and affect cell proliferation, migration, colony formation, and apoptosis in metastatic melanoma. 16
AKT phosphorylation is involved in other diseases such as toluene diisocyanate (TDI)‐related asthma. Peng et al. 17 demonstrated by an animal asthma model that inhibition of AKT by MK2006 prevents TDI‐induced airway inflammation. Besides, one orally AKT inhibitor, ARO092, attenuates neutrophil–platelet interactions in sickle cell disease. 18 Nandan et al. 19 demonstrated that an AKT inhibitor, miransertib (ARQ092), enhanced mammalian target of rapamycin (mTOR)‐dependent autophagy in Leishmania‐infected macrophages.
Hsp90α promotes the AKT phosphorylation signaling pathway to stimulate the repair process of corneal injury. Thus, recombinant Hsp90α is expected to be a candidate drug for the treatment of corneal injury. 20 This exploits the pro‐proliferative effects of AKT agonists, which play an important role in the treatment of diseases requiring cell proliferation. PI3K/AKT signaling pathway activated by recilisib inhibits cardiomyocyte apoptosis during myocardial infarction. 21
There are no drugs of AKT inhibitor that are approved by the FDA, 6 but four drugs (afuresertib, capivasertib, ipatasertib, and uprosertib) are under clinical trials that can be found in the PKIDB. 5 These drugs are used in the treatment of spindle cell/sclerosing rhabdomyosarcoma and other diseases because of their good signal blocking and cell growth inhibitory activity. 22
2.1.2. PI3K family
PI3K is the upstream molecule of PI3K/AKT signaling pathway and has the same biological function as AKT. PI3K has three subunits including PI3Kα, PI3Kδ, and PI3Kγ.
PI3Kδ is a lipid kinase that phosphorylates the D‐3 position of the phosphatidylinositol ring. 23 The new highly selective PI3Kδ inhibitor, INCB050465 (Parsaclisib), was found to be a good drug with an excellent profile through in vivo pharmacodynamic and efficacy studies in animal experiments. 23 Other PI3Kδ inhibitors (leniolisib and seletalisib) were used for the therapy of the activated phosphoinositide 3‐kinase delta syndrome (APDS). 24 It has been demonstrated that an inhaled nemiralisib, a PI3Kδ inhibitor, was effective and safe for the treatment of chronic obstructive pulmonary disease (COPD). 25
PI3Kγ is another subunit and can be inhibited by eganelisib, which inhibits cell proliferation, self‐renewal, migration, and invasion in vitro. 26 There were some dual PI3K δ/γ inhibitors, for example tenalisib, whose phase I clinical trial was completed with acceptable safety up to 1200 mg twice daily with no dose limiting toxicity. 27 It is promising to become an important PI3K inhibitory antitumor drug, used for the treatment of many cancer patients such as relapsed/refractory T‐cell lymphoma. 28
However, several studies have shown that some PI3K pathway inhibition can negatively feedback activate TK receptor signaling, which then re‐blocks the pathway and reduces the therapeutic activity of the drug. 29 This is related to PI3Kα (encoded by PIK3CA), a p110α catalytic subunit, 30 which is inhibited by compounds such as alpelisib and serabelisib. 31
In the PKIDB or FDA approved drugs, there are nine drugs targeting PI3Kδ inhibition: dezapelisib, leniolisib, nemiralisib, parsaclisib, puquitinib, samotolisib, seletalisib, tenalisib, and umbralisib that are under clinical trials. 5 , 6 These drugs are used in the treatment of various diseases, including immunologic deficiency syndromes, disease susceptibility, genetic predisposition to disease, Epstein–Barr virus infections, COPD, asthma, eosinophilia, pneumonia, and respiratory tract diseases. 25
2.1.3. PKC family
PKC is an intracellular receptor for phorbol esters with tumor‐promoting activity and is considered a key player in carcinogenesis. 32 Studies have shown that the activation of PKC is essential to induce cell differentiation, proliferation, metastasis, and survival. 33 However, there are some studies which show that PKC also plays a role as a tumor suppressor, and suggest that future clinical work is likely to focus on restoring rather than inhibiting PKC pathway activity. 34
In the PKIDB or FDA approved drugs, there are four drugs targeting PKC inhibition 5 , 6 : ruboxistaurin, enzastaurin, sotrastaurin (AEB071), and darovasertib (IDE196). Ruboxistaurin is a PKCβ inhibitor, which has an activity for the treatment of diabetes, hypertension, kidney failure, and osteoarthritis. 35 Sotrastaurin is suggested to be involved in cytopathogenic effect and lymphocyte activation, and is used for the treatment of cancers such as uveal neoplasms, melanoma, urinary bladder neoplasms, esophageal neoplasms, and other diseases like cartilage diseases, insulin resistance, and diabetes mellitus. 36 , 37 Darovasertib is under a phase Ib clinical trial for uveal neoplasms treatment. 38
2.1.4. Ataxia telangiectasia and Rad3‐related family
Ataxia telangiectasia and Rad3‐related (ATR) kinase is one of the major kinases in the DNA damage response signaling pathway, which responds to replication stress as well as DNA damage caused by various genotoxic factors. 39 Many ATR inhibitors, such as ceralasertib, are reported to combining the inhibitory effect of DNA damage response and the effect on immune checkpoint blockade to boost antitumor activity of NK cells. 40 Berzosertib, an ATR inhibitor, is found to have an effect on cancer therapy and is settled in a multicenter, open‐label, randomized, phase II trial. 41 It has been used for the treatment of lung neoplasms, esophageal neoplasms, ovarian neoplasms, and osteosarcoma in the clinical trials. 42 , 43
2.1.5. ROCK family
ROCK can promote actin‐myosin‐mediated contractility through the phosphorylation process of various proteins. ROCK plays an important role in cell proliferation, differentiation, apoptosis, and oncogenic transformation. 10 They regulate many key cellular functions, including proliferation, exercise, and vitality. RhoA/ROCK signaling has been shown to be closely related to arterial hypertension, cardiovascular and renal remodeling, hypertensive nephropathy, and post‐transplant hypertension. 44
There are four drugs targeting ROCK in the PKIDB or FDA approved drugs: belumosudil, fasudil, netarsudil, and ripasudil. 5 , 6 Belumosudil (KD025) is a ROCK2 inhibitor, which modulates Th17/regulatory T‐cell balance and controls profibrotic pathways, which offers a novel approach for the treatment of chronic graft‐versus‐host disease (cGVHD) and systemic sclerosis. 45 , 46 Fasudil is an inhaled ROCK inhibitor, which affects the cascade of reactions involving various proinflammatory cytokine molecules, mainly for the treatment of cardiovascular diseases. 47 The clinical use of netasudil is limited to patients with glaucoma or ocular hypertension, and netasudil has a potential to affect Lin11, Isl‐1, and Mec‐3 kinase (LIMK) pathway for cancer treatment. 48 Ripasudil is used in glaucoma, ocular hypertension, blepharitis, conjunctival diseases macular edema, cataract, and retinopathy. 49 Additionally, ROCK can be considered as a key therapeutic target for the treatment of COVID‐19. 50
2.1.6. PKA family
PKA is regulated by cAMP and affects downstream cAMP response element‐binding (CREB) protein, forming the cAMP‐PKA‐CREB signaling pathway. It is known that the cAMP‐PKA‐CREB signaling pathway is related to osteoblasts, beta2‐adrenergic response, cell apoptosis, central nervous system (CNS) functional recovery, and upregulation of nuclear factor‐κB (NF‐κB). 51 It is demonstrated that some Chinese medicines, such as Zuogui Pill and Yougui Pill, regulate the PKA signaling pathway by improve cAMP levels and PKA expression. 52 Besides, it is demonstrated that liraglutide, a PKA inhibitor, has an effect on the mesenchymal stem cells for acute lung injury and acute respiratory distress syndrome. 53 But there are few drugs targeting PKA, most likely due their wide distribution in cells.
2.1.7. Receptor guanylate cyclase family
Two types of guanylate cyclase (GC) exist in mammals, which are distinguished by their location within the cell. Membrane‐bound guanylate cyclase (mGC) is the first type of GC‐coupled receptor. 15 The natriuretic peptides (NP) activate mGC. Seven subtypes of transmembrane mGC have been identified (GC‐A to GC‐G) in mice. 54 GC‐A has beneficial properties such as regulating blood pressure and natriuretic, which is activated by two types of NP: ANP and BNP. At present, GC‐A has become an essential target for treating cardiovascular diseases. 55 CNP activates GC‐B. In failing hearts, the CNP inhibits cardiac remodeling by activating the homologous receptor, GC‐B. 56 Intracellular soluble guanylate cyclase (sGC) is the second type of GC. In physiological processes, nitric oxide (NO) is diffused into the vessel lumen and vessel wall, activating sGC, which functions through the NO‐sGC‐cGMP signaling pathway. The NO‐sGC‐cGMP pathway is an essential regulator of myocardial function and vascular tone. 57
Since preclinical and clinical trials have been developed. GC has been viewed as an attractive target for pharmacological intervention. Riociguat is a stimulant of GC, which leads to relaxation of vascular smooth muscle. At present, riociguat has been approved for the treatment of two kinds of pulmonary hypertension [pulmonary arterial hypertension (PAH)/chronic thromboembolic pulmonary hypertension (CTEPH)]. 58 Versiciguat is a sGC stimulator that stimulates the production of cGMP and sGC independent of NO and enhances the effects of NO by stabilizing its binding to sGC. Versiciguat was recently approved in the USA to reduce the risk of heart failure in those with a 45% ejection fraction. 59 Praliciguat and olinciguat are newly developed sGC stimulators for the treatment of chronic heart failure. 59 , 60 Since severe renal diseases are mostly due to downregulation of NO‐sGC‐cGMP signal transduction, the current phase II and III clinical trials for vericiguat, praliciguat, and olinciguat are undertaken for the treatment of kidney diseases. 61 , 62 , 63 There is increasing evidence showing that this medication can effectively treat heart failure, kidney disease, and pulmonary hypertension and can be used as first‐line therapies.
2.2. CaMK group
CaMK group has only one family member. CaMK II, activated by the Ca2+/calmodulin complex, is a serine/threonine kinase of the CaMK group. 8 CaMK II responses to neurohormonal stimulation, promotes cardiac remodeling, and increases the risk of arrhythmia. 64 CaMK is regulated by the diacylglycerol (DAG)/PKC/PKD pathway. It regulates Ras, leading to the activation of the PI3K/AKT, as well as the MEK/ERK signaling pathways. 65 CaMK‐dependent activation of adenosine monophosphate (AMP)‐activated protein kinase (AMPK) is involved in mitotic phagocytosis, mitochondrial dynamics, and lysosomal biogenesis in microglia, which in turn produce cytotoxicity caused by mitotic phagocytosis and cathepsin B activation 66 (Figure 2).
FIGURE 2.
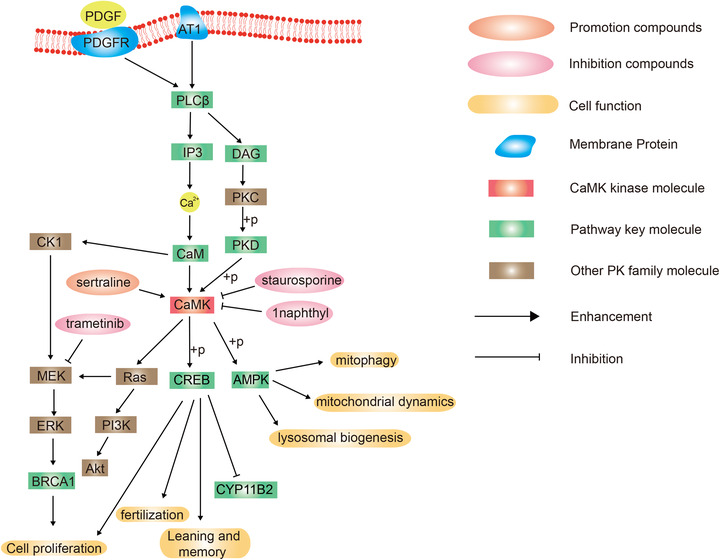
Schematic diagram of protein phosphorylation signaling pathway of calmodulin‐dependent protein kinase (CaMK) group.
Some CaMK inhibitors, such as staurosporine and 1naphthyl PP1, were under investigation for the treatment of schistosomiasis. 67 However, there is no CaMK inhibitor in a clinical trial in the PKIDB.
2.3. CMGC group
CMGC group contains seven main families including p38 mitogen‐activated protein kinases (p38 MAPKs), ERKs, c‐Jun N‐terminal kinases (JNKs), apoptosis signal‐regulating kinase 1 (ASK1), CLK2, CDKs, and GSK3. There are 31 drugs targeting the CMGC group in the PKIDB or in FDA approved drugs: eight in p38 MAPKs family, three in JNK family, five in ERK family, and 14 in CDKs family 5 (Figure 3).
FIGURE 3.

Schematic diagram of protein phosphorylation signaling pathway of the extracellular signal‐regulated kinase (ERK), P38, c‐Jun N‐terminal kinase (JNK), apoptosis signal‐regulating kinase (ASK), and Cdc2‐like kinase 2 (CLK2) families in CMGC (cyclin‐dependent kinases, mitogen‐activated protein kinases, glycogen synthase kinases, and Cdc2‐like kinases) group.
2.3.1. p38 MAPKs family
The p38 MAPK is an important signaling molecule, which responds to environmental and intracellular stress, and is related to cell proliferation, apoptosis, inflammation, metastasis, and angiogenesis (Figure 3). 68 , 69
There are eight drugs targeting the p38 MAPK family in the PKIDB or in FDA approved drugs 5 : acumapimod, dilmapimod, doramapimod, losmapimod, neflamapimod, pamapimod, ralimetinib, and talmapimod. These p38 MAPK inhibitors are often used in various inflammatory diseases. Acumapimod is an oral p38 MAPK inhibitor, which inhibits p38 MAPK‐regulated inflammatory response and is used for the treatment of COPD. 70 Dilmapimod is used for the prevention of acute respiratory distress syndrome and other organ damage in some large clinical trials. 71 BIRB796 (doramapimod), another p38 MAPK inhibitor, is used for the treatment of inflammatory bowel diseases. 72 In addition to their therapeutic effects in inflammatory diseases, some p38 MAPK inhibitors, such as neflamapimod, are used in the improvement of cognition and walking disorders. 73 Additionally, p38 MAPK is considered as a key therapeutic target for the treatment of COVID‐19. 50
2.3.2. ERKs family
ERK regulates the transcriptional activation of Elk‐1, activating transcription factor (ATF), Ap‐1, c‐fos, and c‐Jun in cells, and participates in various biological responses such as cell proliferation and differentiation, morphological maintenance, skeleton construction, apoptosis, and carcinogenesis. 74 , 75 ERKs are the key protein kinase nodes involved in Ras‐Raf‐MEK‐ERK‐MAP kinase signaling pathway, and involved in many biological processes including cell proliferation and apoptosis, immune response, nervous system function, and RNA synthesis and processing. 76
There are four drugs targeting ERKs family in the PKIDB or in FDA approved drugs 5 : dabrafenib, ravoxertinib, tomivosertib, and ulixertinib. Dabrafenib is widely used in the treatment of melanoma, skin neoplasms, brain neoplasms, lung cancer, thyroid cancer, and others because of its ERK inhibitory effect. 77 , 78 It is reported that IL4/IL4R signaling can activate the ERK pathway, affect osteoclast, and lead to osteolytic lesions, and can be inhibited by ravoxertinib. 79 Tomivoltinib is a highly potent and highly selective inhibitor of MNK1/2, which inhibits MNK‐eIF4E‐β‐catenin and increases the sensitivity of gastric cancer to chemotherapy. 80
2.3.3. JNKs family
JNK, also known as stress‐activated protein kinase (SAPK), plays an important role in a variety of biological and pathological processes including cell cycle, apoptosis infection, reproduction, and cellular stress. 81 , 82
There are three drugs targeting JNKs family in the PKIDB or in FDA approved drugs 5 : bentamapimod, pexmetinib, and tanzisertib. Bentamapimod is a specific c‐JNK inhibitor, which increases the sensitivity of many tumors to chemotherapeutic drugs. 83 For example, it increases the sensitivity of A2780 ovarian stem cells to carboplatin and paclitaxel, 84 and sensitizes glioma cells to temozolomide and vincristine. 85 Another JNKs inhibitor, pexmetinib, is used for the treatment of myelodysplastic syndromes and acute myeloid leukemia (AML). 86 Tanzisertib is a newly discovered c‐JNK inhibitor. It is related in fibrosis and is in clinical trials for the treatment of idiopathic pulmonary fibrosis. 87
2.3.4. ASK1 family
ASK1 kinase is the upstream molecule of p38 kinase and JNK kinase families. 88 It is regulated by tumor necrosis factor (TNF), transforming growth factor‐β (TGF‐β), Fas Ligand [FAS (Fas Cell Surface Death Receptor) is a protein coding gene's name] and more, and inhibited by the AKT kinase family. 89 ASK1 is a crucial cellular stress sensor, modulates diverse responses to oxidative and endoplasmic reticulum stress and calcium influx. 90 Although there are many drugs targeting its downstream p38 and JNK family, adverse reactions with serious side effects were observed. Therefore, inhibitors targeting ASK1 were proposed by many scholars to better the therapeutic effects. 90
Currently, inhibiting the ASK1 kinase family is expected to intervene diseases such as neoplasms, non‐alcoholic fatty liver disease, cerebral ischemia, Parkinson's disease, insulin resistance, diabetes, Alzheimer's disease, melanoma, and more. At present, no drug that specifically inhibits the ASK1 kinase family has been approved by the FDA, and no ASK1 kinase inhibitor has participated in human clinical trials. 5 Instead, some drugs, such as AKEX0011 88 and GS‐444217 91 are under basic experiments and have not yet received safety evaluation and clinical evaluation. Some scholars have also explored specific targeted inhibitory drugs through molecular docking and molecular dynamics. 92
2.3.5. CLK2 family
The CLK2 kinase family is regulated by the AKT and PI3K kinase families, and controls the ATR and the PKC families. 93 CLK2 is closely associated with many neurodegenerative diseases, metabolic diseases, and viral infection‐related diseases, and is considered as a potential drug target for these diseases. 94 Its specific inhibitory drugs are used in the experimental treatment of Alzheimer's disease, insulin resistance, and neoplasms, especially leukemia and triple‐negative breast cancer. 95 , 96 It is reported that the first CLK2 inhibitor, lorecivivint, has entered phase III clinical trials in recent years. 94 We look forward to the therapeutic prospects of CLK2 inhibitors.
2.3.6. CDKs family
The discovery of the CDKs and its regulation on cell cycle progression plays a key role in the treatment of malignant cancer. 97 CDKs are serine/threonine protein kinases that bind to proline and play a crucial role in controlling cell cycle progression in different aspects of transcriptional regulation. 98 Since the first report of using CDK4/6 inhibitors against cancer in 1990s, there have been thousands of reports on CDK inhibitors. CDK9 is a target enzyme for cancer therapy, but has limitations because of its adverse effects. 99
There are 17 drugs targeting CDKs family in the PKIDB or in FDA approved drugs 5 : abemaciclib, alvocidib, palbociclib, ribociclib, dinaciclib, fadraciclib, lerociclib, milciclib, riviciclib, trilaciclib, voruciclib, roniciclib, seliciclib, and zotiraciclib. Abemaciclib, palbociclib, and ribociclib were the first FDA approved CDKs inhibitors. They are mainly used for combined endocrine therapy for HR+/HER2 54 advanced or metastatic breast cancer in premenopausal/perimenopausal and postmenopausal women. 100 Alvocidib, as well as dinaciclib, seliciclib, SNS‐032, and RGB‐286638 are CDK9 targeted inhibitors, and are mainly used for the treatment of leukemia for reversing the progression of cytarabine resistance 101 , 102 (Figure 4).
FIGURE 4.

Schematic diagram of protein phosphorylation signaling pathway of the cyclin‐dependent kinases (CDKs) family in CMGC (cyclin‐dependent kinases, mitogen‐activated protein kinases, glycogen synthase kinases, and Cdc2‐like kinases) group.
2.3.7. GSK3 family
GSK3 is an evolutionarily highly conserved serine/threonine kinase, which participates in multiple signaling pathways and has more than 100 substrates. 103 GSK3 regulates signaling proteins, structural proteins, and transcription factors, and participates in the regulation of cell differentiation, proliferation, survival, and apoptosis. 104 Current research on GSK3 inhibitors is mainly focused on Alzheimer's disease. GSK3 is one of the key players in signaling pathways for normal brain function and a key molecular link between β‐amyloid (Aβ) and tau neurofibrillary tangles (NFTs). 105 Although GSK3 was considered a good target for drug intervention nearly 10 years ago, so far, no specific GSK3 inhibitor has been certified by the FDA. The main reason may be the side effects induced by GSK3. 106 Multiple clinical trials on GSK3 inhibitors are ongoing.
2.4. STE‐MAPKs group
2.4.1. Ste7/MAP2K family
The mitogen‐activated protein kinase kinase (MAPKK) is a classic molecule for signal transmission from cytoplasm to nucleus. Ste7 belongs to the MAPKK family of enzymes found in yeast, which participates in pheromone signaling and nutrient deprivation/invasive growth pathways. 107 Recent studies suggest the dual specificity of MAP2K in influencing the process of phosphorylation. 108 , 109
The MAPKKKs A‐Raf, B‐Raf, and C‐Raf (Raf‐1), the MAPKKs MEK1 and MEK2, and the MAPKs ERK1 and ERK2 constitute the mammalian ERK1/2 module. Signaling through the ERK pathway contributes to many cellular processes, including proliferation, differentiation, development, learning, and survival. 110 ERK1 and ERK2 can be activated by growth factors, such as fibroblast growth factor 2 (FGF2), which causes endometriosis by regulating the ERK signaling pathway 111 ; and vascular endothelial growth factor‐C (VEGF‐C), which promotes human mesenchymal stem cell migration via an ERK signaling pathway. 112 ERK is phosphorylated and activated by MEK. Given the role of the ERK pathway in cell proliferation and diseases, some MEK inhibitors have been developed and applied in the clinic. In 2015, both the FDA and European Medicines Agency approved cobometinib, a potent, selective oral MEK1/2 inhibitor that can be taken with the B‐rapidly accelerated fibrosarcoma (BRAF) inhibitor vemurafenib for the treatment of malignant melanoma. 113 Pimasertib is a selective MEK1/2 inhibitor in the stage of clinical experiment and shows good antitumor activity in melanoma and liver cancer. 114 , 115 , 116 Refametinib and selumetinib are in clinical trials and can be found in the PKIDB for the treatment of many cancers such as hepatocellular cancer, pediatric low‐grade glioma, non‐small‐cell lung cancer (NSCLC), and melanoma. 117 , 118 There are two kinds of MEK inhibitors, belvarafenib and mirdametinib that were under clinical trials for the treatment of malignant tumors such as melanoma. 14
2.4.2. Ste11/MAP3Ks family
Ste11/MAP3Ks are protein Ser/Thr kinases activated by extracellular stimuli through phosphorylation. 119 MAP3Ks phosphorylate both Ser/Thr residues of MAP2Ks’ activation loops. By phosphorylating signaling effectors and interacting specifically with proteins, the MAP3Ks activate MAP2K‐MAPK pathways in response to stimuli. 120
Rapidly accelerated fibrosarcoma (RAF) is a subfamily of MAP3Ks and a member of cytoplasmic serine/threonine kinases. RAF contributes to oncogenesis and critical physiological processes, including cell growth. The expression of C‐Raf is widespread, but that of A‐Raf and B‐Raf is limited to selected tissues. A‐Raf is primarily found in urogenital and gastrointestinal tissues. B‐Raf is mainly found in neural, testicular, splenic, and hematopoietic tissues. Raf molecules have distinct physiological functions in the development and progression of diseases, as shown by their expression patterns. 121 MEK/ERK pathway is activated when A‐Raf is active. The A‐Raf protein interacts with proteins located in different cellular compartments to control apoptosis, RNA catabolism, and GTPase activity. 122 Clinically, dominant‐activating mutations in B‐Raf have been associated with melanoma, colorectal cancer, breast cancer, and glioma. 123 , 124 C‐Raf represents the prototypical Raf family member, and is widely expressed. When C‐Raf is active, it activates MEK/ERK, resulting in increased resistance to apoptosis mediated by B‐cell CLL/Lymphoma 2 in the heart and liver cells. 125 More and more Raf kinase inhibitors have been developed and applied. Patients with metastatic colorectal cancer carrying the B‐Raf V600E mutation are benefited from a combination therapy of encorafenib, cetuximab, and binimetinib with significantly longer overall survival. 126 It is more effective to use MEK1/2 inhibitors in conjunction with B‐Raf inhibitors in treating melanoma, which has become the standard treatment. FDA approved the combinations of vemurafenib and cobimetanib, dabrafenib and trametinib, and encorafenib plus binimetinib for the treatment of BRAFV600E melanoma. 127
2.4.3. Ste20/MAP4K family
The MAP4Ks are members of the Ste20‐like family of serine/threonine kinases in mammals. MAP4Ks have been reported to activate JNK by activating the cascade of MAP3K‐MAP2K, including MAP4K1/hematopoietic progenitor kinase 1 (HPK1), MAP4K2/GCK, MAP4K3/GLK, MAP4K4/HGK, MAP4K5/KHS, and MAP4K6/MINK. 128 , 129
There has been evidence showing that several MAP4Ks are essential for human disease. Autoimmune diseases may occur by downregulating HPK1 or overexpressing GLK in T cells. 130 Type 2 diabetes and insulin resistance could result from the downregulation of HGK in T cells. The HPK1, GLK, and HGK genes play an essential role in tumor formation. 131 These findings suggest that MAP4Ks could be used as biomarkers and drug targets to treat these diseases.
The HPK1 inhibitor (CompK/Compound 1) inhibits the signaling pathways governing T‐cell receptors (TCRs) and B‐cell receptors through phosphorylation and ubiquitination of SLP‐76 and BLK, respectively. 132 , 133 It is suggested that blocking HPK1 kinase activity with inhibitors alone or with checkpoint inhibition may be a practical immunotherapy approach for cancer. HPK1 deficiency subverts the inhibition of antitumor immune responses and is associated with functional augmentation of antitumor T cells. By examining how MAP4Ks functioned in different types of cells, we may be able to understand human diseases and guide clinical diagnosis and treatment (Figure 5).
FIGURE 5.

Schematic diagram of protein phosphorylation signaling pathway of STE group.
2.5. Tyrosine kinases group
TKs can either be membrane bound coupled to receptors, usually growth factor receptors (RTK) or not (protein tyrosine kinase structure and function). Receptor tyrosine kinases (RTKs) are transmembrane glycoproteins that are activated by binding to their cognate ligands and relay extracellular signals to the cytoplasm by phosphorylating tyrosine residues on the receptor itself and downstream signaling proteins. The RTK family includes receptors for insulin and many growth factors, such as Trk, EGFR, FGF receptor (FGFR), VEGF, and feline mcdonough sarcoma (FMS)‐like tyrosine kinase 3 receptor (FLT3). In addition to RTKs, a large family of non‐receptor tyrosine kinases (NRTKs) exists, which includes Src‐family protein tyrosine kinases (SFK), Janus kinase (JAKs), and breakpoint cluster region‐Abelson fusion protein (BCR‐Abl), Bruton's tyrosine kinase (BTK) among others. NRTKs are components of a signaling cascade triggered by RTKs and other cell surface receptors, such as G‐protein‐coupled receptors and immune system receptors. The specific reactions catalyzed by protein tyrosine kinases are the transfer of ATP of γ‐phosphate to the hydroxyl group of tyrosine in the protein substrate (Figure 6).
FIGURE 6.

Schematic diagram of protein phosphorylation signaling pathway of tyrosine kinases (TK) group.
2.5.1. Trk family
The RTK family consists of Trks, and is present in three Trk subtypes: TrkA, TrkB as well as TrkC. 134 Every Trk subtype possesses a significant neurotrophic ligand. TrkA is mainly triggered by NGF, 135 and TrkB is more inclined to brain‐derived neurotrophic factor. 136 TrkC has a preference for neurotrophin‐3 (NT‐3). 137 Binding of specific neurotrophic ligands and their receptors can activate different downstream pathways such as PI3K, Ras/MAPK, and PLCγ, thereby promoting neuronal survival and cell differentiation. Pegcantratinib is a potent TRKA inhibitor with anti‐TRKB and TRKC activity. It is in the phase IIb clinical stage for treating inflammatory skin diseases, such as atopic dermatitis and psoriasis. 138 Next‐generation TRK inhibitors, such as selitrectinib, repotrectinib, and taletrectinib, are in early clinical stages and available in ongoing clinical trials. They have a compact macrocyclic structure that can bind to the ATP vesicle without being hindered by the space for TRK substitution. These drugs exhibited in vitro and in vivo resistance to NTRK wild‐type and mutant kinases. These inhibitors could help patients with solid tumors resistant to existing TRK inhibitors. 139
2.5.2. RET family
Rearranged during transfection (RET) is a TK receptor. 140 Typically, it interacts with subcellular ligands and exerts important effects in a multitude of cellular processes. RET is critical in the evolution of the central and peripheral nervous system. Mutations that activate or inhibit RET cause several aggressive illnesses, namely Hirschsprung disease and cancer. Ligand‐induced RET dimerization causes autophosphorylation of a wide range of tyrosine residues and contributes to the activation of intracellular signaling cascades, which impacts many cellular processes. 141 RET inhibitors, selpercatinib and pralsetinib, are in phase III clinical trials and approved for the therapeutic use in individuals with metastatic RET fusion‐positive NSCLC by comparing them with standard first‐line therapy to observe its efficacy in RET‐positive NSCLC patients. 142
2.5.3. EGFR family
EGFR is a TK, which participates in cellular processes and cancer progression. 143 EGFR, a classical transmembrane receptor, activates multiple downstream effectors and TKs by means of ligand‐induced dimerization, triggering a signaling cascade response. EGFR‐activated phosphorylation responses promote signaling cascades and are associated with embryogenesis and stem cell division. 144 The third‐generation EGFR‐tyrosine kinase inhibitors (TKI) that target T790M mutations include alflutinib, nazartinib, lazertinib, and more. Alflutinib is a newly developed third‐generation EGFR‐TKI that is currently in phase I/II studies. 144 Alflutinib is currently found to have clinical efficacy and acceptable toxicities in patients with advanced NSCLC with EGFR T790M mutations, including those with CNS metastases. Safety, clinical activity, and pharmacokinetics of alflutinib (AST2818) in patients with advanced NSCLC with EGFR T790M mutation. Nazatinib is in phase I/II and has been found to have a high safety profile with low skin toxicity and adverse effects such as maculopapular rash. 145 Lazertinib is used for the treatment of NSCLC. Lazertinib was approved for the first time for the treatment of EGFR T790M mutation‐positive patients with locally advanced or metastatic NSCLC who had previously received EGFR‐TKI therapy. 146
2.5.4. JAK family
The JAK family of TKs includes JAK1, JAK2, JAK3, and tyk2. 147 The JAK phosphorylates signal transducers and activators of transcription (STAT), dimerizes, and then translocates through the nuclear membrane to the nucleus to mediate the manifestation of the pertinent genes. This can be referred to as the JAK/STAT signaling pathway. In recent years, cytokine‐stimulated signaling pathway, known as the interleukin (IL)‐6 signaling pathway, was found. 148 The signaling pathway is associated with all kinds of body functions and is participating in a number of important biological processes, including cell proliferation, differentiation, apoptosis, and immune regulation. 149 At present, research on the JAK/STAT pathway associated with disease and drug innovation is concentrated in inflammatory and neoplastic diseases. JAK inhibitors as next‐generation targeted therapies for atopic dermatitis, such as delgocitinib, are used to treat atopic dermatitis in adults and children in Japan, but their safety, durability, and effectiveness still require extensive clinical trials. 150
2.5.5. VEGFR family
Due to the wide variety of TKs, among which VEGF, one of the main pro‐angiogenic factors, performing its function mostly by stimulating two TK receptors. Numerous reported studies have drawn attention to the critical role of VEGFR‐2 in tumorigenesis and tumor progression. 151 VEGFR‐2 activation is associated with phosphorylation of multiple downstream signals, including phosphoinositide 3‐kinase, PKB, p38 MAPK, and ERKs, which then activate endothelial cells. The VEGF/VEGFR‐2 signaling pathway is considered to be the most crucial element in promoting angiogenesis. It is potentially a therapeutic approach for angiogenesis‐related diseases, Alzheimer's disease, Parkinson's disease, and cancer. Hence, numerous VEGFR‐2 inhibitors have been clinically tested and/or validated for the therapeutic use in angiogenesis‐related diseases. 76 , 152 Brivanib, a selective dual‐RTK inhibitor, inhibits FGFR and VEGFRs. Li et al. 153 found that brivanib is an inhibitor of proliferation of microvascular endothelial cells. It could be a new therapeutic option for age‐related macular degeneration.
2.5.6. SFK family
SFK is associated with intracellular signaling cascade, 154 extracellular stimulation, and activation of various types of receptors including EGFR and VEGF receptors. These receptors translate stimuli into various intracellular signals by triggering SFK. SFK are activated to deliver signals to many pathways. Two of these pathways are associated with major cancer‐related signaling pathways: PI3K, MAPK, and NF‐κB pathway. 155 The SFKs have been regarded as molecular therapeutic targets. Bosutinib is a competitive inhibitor of Src and ABL TKs and has been shown to bind to the kinase structural domain BCR‐ABL1. 156 Bosutinib inhibits Src family Src, Lyn, and Hck kinases, as well as c‐Kit and platelet‐derived growth factor (PDGF) receptors. 157 Now it is used for the treatment of chronic granulocytic leukemia. 158
2.5.7. BCR‐Abl family
The protein Abl TK reacts with most of the proteins involved in the intracytoplasmic oncogenic pathway when fused to Bcr. Aberrant interactions between Bcr‐Abl oncoproteins favor dimerization or tetramerization, then autophosphorylation. This process causes an increase in the binding sites of phosphorylated tyrosine residues on Bcr‐Abl as well as the SH2 structural domain of other proteins. 159 Bcr‐Abl has been demonstrated to inhibit apoptosis and promote cell proliferation through the accumulation of genetic abnormalities. Ascitinib is an oral, small‐molecule selective metabolic inhibitor for the treatment of hematologic malignancies. 160 , 161 Currently in clinical trial phase I–III. Asciminib acts by selectively binding to the myristoyl pocket of ABL1, but is inactive in vitro against many other kinases as well as G‐protein‐coupled receptors, ion channels, transporters, and nuclear receptors, which may limit its potential for drug targeting. 160 Primary efficacy results from phase I and III trials demonstrate clinically meaningful efficacy and favorable safety profile of asciminib in patients with chronic myeloid leukemia (CML)‐chronic phase (a phase III, open‐label, randomized study of asciminib, a STAMP inhibitor, vs. bosutinib in CML after two or more prior TKIs).
2.5.8. FLT3 family
FLT3 is a family of RTKs. Its ligand combines with the extracellular domain to activate downstream signaling pathways, such as MAPK and PI3K/PKB signaling pathways that are responsible for hematopoietic cell survival, maturation, and proliferation. The FLT3 receptor is overexpressed in most acute leukemias. 162 First‐generation FLT3 inhibitors may lead to off‐target toxicity by inhibiting multiple downstream RTKs due to the lack of specificity of FLT3, while second‐generation FLT3 inhibitors can effectively target FLT3 with fewer off‐target effects. A phase III randomized controlled trial found quizartinib to have minimal cutaneous, pulmonary or gastrointestinal side effects and greater myelosuppressive effects than other FLT3 inhibitors. 162 Gilteritinib usually causes intestinal side effects, such as nausea and diarrhea, and increased bilirubin and transaminases are seen in some patients. Encephalopathy and pancreatitis may occur in severe cases (<1%–2%). Currently, gilteritinib is approved in the United States and Europe for the treatment of adult patients with relapsed or refractory FLT3 mutations in AML. 163
2.5.9. FGFR family
FGFRs belong to a member of RTK family that exerts a critical role in developing and adult cells. 164 A variety of cancers, such as uroepithelial, ovarian, and lung adenocarcinoma, are associated with dysregulation of FGFRs. FGFRs are currently regarded as potential pharmaceutical targets for the treatment of various cancers. 165 A variety of small‐molecule inhibitors have been developed against this family of kinases, and several of which are in clinical trials. Dysregulation of the FGFR TK family is associated with various types of cancers. Derazantinib is an ATP competitive multi‐kinase inhibitor with activity in the low nanomolar range against FGFR1, FGFR2, and FGFR3 kinases. 166 FGFR2 is the most sensitive kinase, followed by FGFR1. Drazotinib was found to significantly inhibit VEGFR2 phosphorylation through studies in human endothelial cells. Drazotinib significantly reduced tissue permeability and tumor functional vascular system according to animal studies. 166
2.5.10. BTK family
BTK is a family of the transient erythroblastopenia of childhood TKs. As a non‐receptor intracellular kinase, BTK has recently been found to be in certain tumor isoforms (e.g., multiple myeloma) and other regulating the interaction between malignant clones and the bone marrow microenvironment. 167 BTK is a key kinase involved in BCR signaling, FcR signaling, Toll‐like receptors (TLRs) signaling, and chemokine receptor signaling. By activating nuclear factor of activated T cells, NF‐κB, and MAPK pathways, it leads to cell proliferation and differentiation, antibody and cytokine production, and expression of co‐stimulatory molecules. 168 , 169 Tirabrutinib is a covalent BTK inhibitor used to treat B‐cell lymphoma and chronic lymphocytic leukemia. 170 Tirabrutinib was approved in Japan in March 2020 for the treatment of relapsed or refractory primary CNS lymphoma. A phase II clinical trial is ongoing to measure the safety and efficacy of tirabrutinib for the treatment of patients with refractory aspergillus. 171
2.6. Tyrosine kinase‐like group
TKL gene family is broadly known to exist in most eukaryotes and is engaged in many biological processes. It contains seven main families including the mixed‐lineage kinase (MLK), LIMK/TESK (LISK), IL‐1 receptor‐associated kinase (IRAK), Raf, receptor‐interacting protein kinase (RIPK), activin and TGF‐receptors (STR), and leucine‐rich repeat kinases (LRRK) (Figure 7).
FIGURE 7.

Schematic diagram of protein phosphorylation signaling pathway of tyrosine kinase‐like (TKL) group.
2.6.1. MLK family
MLKs belong to a member of MAP3K. 172 Common to all MLK members are the catalytic structural domains containing tagging sequences for serine/threonine (Ser/Thr) and tyrosine (Tyr) kinases, hence the name MLKs. Although MLKs have been demonstrated to be functional Ser/Thr kinases, none of the MLK members has been reported to be Tyr kinase. A possible partial or full member of MLK is functional Tyr kinases for very specific substrates that have not been identified. Another one is that the Tyr kinase activity of MLKs is mediated by specific stimuli; therefore, it is important to recognize these specific stimuli to determine their Tyr kinase activity. 173 MLK inhibitors have been proposed for a variety of diseases, including metastasis of cancer cells. 174 Although MLKs are known to be involved in tumorigenic pathways, it is anticipated that clinical application of MLK inhibitors may be challenging because of the existence of multiple downstream targets of MLKs.
2.6.2. LISK family
LIMK is a Ser/Thr kinase and LIMK1 and LIMK2 are the two major subtypes of LIMK. 175 In the presence of threonine 508 and threonine 505, LIMK1 and LIMK2 are phosphorylated by the upstream kinase ROCK1, tonic dystrophy kinase‐associated Cdc 42‐binding kinase, and p21‐activated kinase 1, 4, or 6. 176 Activation of kinase is controlled by phosphatases such as p21‐activated kinase 4 (PAK4). Activated LIMK1/2 persistently phosphorylates downstream target protein cofilin at serine 3, resulting in regulated remodeling of actin and microtubulin. 177 Regulation of LIMK2 localization and stability requires phosphorylation of aurora A and PKC at serine 283, threonine 494, and threonine 505. Thiazolylamide derivatives BMS‐3 and ‐5 can inhibit LIMK1 and LIMK 2 activity.
2.6.3. IRAK family
IRAK1, IRAK2, IRAK‐m, and IRAK4 constitute the IRAK family, which is involved in the activation of TLRs and IL‐1 receptors. 178 Members of IRAK play either a positive or a negative role in the regulation of inflammation, innate, and adaptive immunity. Accumulating evidence demonstrates that four members of the IRAK family receive activation signals from the junctional molecule MyD88, TIF, and could be auto‐phosphorylated. IRAK plays an essential part in the pathogenesis of inflammatory autoimmune diseases. Inhibition of IRAK has prospective therapeutic benefits. IRAK drugs/inhibitors have a long way to go because of the potential benefits of emerging drugs/inhibitors that target individual IRAKs. 179
2.6.4. Raf family
The function of RAS proteins is regulated by their post‐translational modifications, their membrane localization, as well as interactions with regulators and effectors. RAS proteins can be modified subtype specifically by distinct mechanisms of isoform specifics to facilitate translocation and plasma membrane localization. 180 Naporafenib is an ATP‐competitive inhibitor of B‐Raf and C‐Raf. It inhibits not only MAPK signaling activity but also mutated N‐ and Lysyl‐TRNA Synthetase (KRAS)‐driven signaling, and has shown efficacy in many MAPK‐driven human cancer cell lines and xenograft tumors with KRAS, Neuroblastoma RAS [Ras is a protein coding gene's name], and BRAF oncogenes. 181 Agerafenib eliminates activation of the ERK/MAPK pathway in neuroblastoma. Agerafenib was found to have good cytotoxicity in neuroblastoma mouse models, effectively inhibiting tumor growth and prolonging survival. 182
2.6.5. RIPK family
RIP kinases RIPK1 and RIPK3 are key cell fate enzymes that play multiple roles in cell death and inflammatory signaling pathways. 183 RIPK1 mediates NF‐κB activation, thereby enhancing the expression of several pro‐inflammatory cytokines. 184 During necroptosis, RIPK3 promotes stress‐mediated mitochondrial reactive oxygen species (ROS) generation. Necrosis of cervical cancer cells through phosphorylation of RIPK1, RIPK3, and MLKL, thereby triggering an antitumor immune response in cervical cancer. At present, no ripk1/ripk3/mlkl drugs are currently available for clinical treatment. 185
2.6.6. STRK family
The TGF‐β superfamily of cytokines plays key roles in the regulation of immune responses that can prevent or promote diseases such as allergies, autoimmunity and cancer. TGF‐β signaling pathway has a major effect in tumor progression, invasion, metastasis, and tumor immunity. Therefore, inhibiting TGF‐β signaling pathway is considered as a prospective anti‐cancer therapeutic strategy. 186 Vactosertib is a novel inhibitor of TGF‐β type I receptor. Vactosertib acts on the adenosine‐5‐triphosphate‐binding site of the TGF‐β type‐1 receptor thereby inhibiting the phosphorylation of Smad2 and Smad3 substrate proteins. 187 Currently, small‐molecule inhibitors like vactosertib and galunisertib are being clinically evaluated for the treatment of various cancers such as NSCLC, intestinal cancer, and pancreatic cancer.
2.6.7. LRRK family
LRRK is a serine/threonine kinase belonging to the ROCO protein superfamily. It possesses two homologs leucine‐rich repeat protein kinase, LRRK1 and LRRK2. On the conserved Thr/Ser residues, LRRK2 phosphorylates a subgroup of Rab proteins. 188 , 189 Pathogenic mutations in Parkinson's disease enhance LRRK2 protein kinase activity, which stimulates Rab protein phosphorylation. 190 Novel LRRK2 inhibitors of the aminoquinazoline structure as potential drugs for the therapy of Parkinson's disease. 191
2.7. Atypical group
2.7.1. ATP‐binding cassette transporter 1
ATP‐binding cassette transporter 1 (ABC1) protein kinase family belongs to the atypical protein kinase, which developed before the diversion of archaea and bacteria. ABC1 gene mutations are linked to respiratory defects in microbes and humans. 192 Complex Q, a complex of enzymes at the mitochondrial inner membrane stabilized by ABC1, is used in yeast and humans for ubiquinone biosynthesis. 193 Further study is needed to determine how ABC1 functions in the human body. Due to the paucity of research related to ABC1 protein kinase in human diseases, there are no relevant drugs on the market and no ongoing clinical trials.
2.7.2. Alpha kinase
Alpha kinases are an atypical class of protein kinase with a sequence that differs from that of conventional protein kinase families. 194 A wide variety of cellular processes are mediated by alpha kinases, including translation, Mg(2+) homeostasis, intracellular transport, migration, adhesion, and proliferation. 195 The eEF‐2K kinase is an atypical alpha kinase, which functions as a critical regulator of several cellular processes, and can lead to tumorigenesis by phosphorylating a component of eEF2. 196 , 197 The eEF2K is considered a therapeutic target in solid tumors due to its importance in tumorigenesis and tumor progression. 198 Since alpha kinase is an atypical alpha kinase, “classical” PKIs do not inhibit it, making small‐molecule inhibitor development difficult. There is no alpha kinase inhibitor that was approved by FDA, and no clinical trial was conducted based on the PKIDB.
2.7.3. Pyruvate dehydrogenase kinase
Pyruvate dehydrogenase kinase (PDHK) contributes to aerobic glycolysis by phosphorylating pyruvate dehydrogenase. 199 PDHK1, PDHK2, PDHK3, and PDHK4 are four distinct PDHK isoenzymes with a high sequence similarity. Pyruvate dehydrogenases (PDKs) are overexpressed in most cancer cells, causing abnormal glucose metabolism. So far, there are three kinds of PDHK inhibitors with different inhibition mechanisms, including PDH2 inhibitor (AED7545 and Nov3r), dichloroacetate (DCA), and N‐terminal ATP‐binding site inhibitor (radicicol). AED7545 is a novel PDHK small‐molecule inhibitor. A study on rats shows that PDHK inhibitor may be a drug to improve blood glucose control in type 2 diabetes. 200 Nov3r, another PDHK2 inhibitor, can regulate PDH activity and has been proved to have potential therapeutic value in diseases associated with ischemic retinal damage. 201 DCA, a potential next‐generation oral hypoglycemic drug, by stimulating PDH and increasing peripheral alanine and lactate oxidation, exerts a hypoglycemic effect. 202 Radicicol is an N‐terminal ATP‐binding site inhibitor, which is considered to have potential therapeutic value in cancer. 203 Although the number of reported PDHK inhibitors has increased over the years, there is no relevant drug on the market for this target.
2.7.4. Phosphatidylinositol‐3 kinase‐related kinases
Phosphatidylinositol‐3 kinase‐related kinases (PIKKs) are atypical serine/threonine kinases that are related to PI3K. These enzymes participate in a wide range of biological processes, including meiosis, recombination of V(D)J chromosomes, DNA damage detection and repair, and cell cycle progression or arrest. The PIKK family consists of ATR, ATM, DNA‐PKcs, mTOR, and hSMG, that are vital in cancer progression, autophagy, and survival following radiotherapy and chemotherapy. 204 , 205 , 206 Thus, targeting these PIKK kinases in cancer along with chemotherapy and radiation would be helpful for patients. Researchers have developed various PIKK inhibitors 207 and successfully made into preclinical studies as mono‐ or combinatorial therapies for various human cancers, including alpelisib, apitolisib, buparlisib, and copanlisib. 208 , 209 , 210 , 211
2.7.5. Right open reading frame kinases
Right open reading frame (RIO) kinases have an atypical fold like protein kinases, which occurs in an ancient group of proteins. There are at least four subfamilies in the RIO kinase family, namely Rio1, Rio2, Rio3, and RioB. RIO1 is an essential member of the RIO family of genes in Saccharomyces cerevisiae, which is critical to the proper progression of cell cycle and maintenance of chromosomes. 212 The FDA approved no RIO inhibitor, and no clinical trial was conducted in the PKIDB. The possible reason for this is that RIO kinase has potential as a therapeutic target, however, its biological function is unknown.
2.7.6. Transcription intermediary factor 1 kinases
In this family, three members, transcription intermediary factor 1 (TIF1)a, h, and g, are involved in transcriptional regulation. Since TIF1a has kinase activity, it is expected that the other TIF1 proteins also possess it due to their high level of conservation. It has been shown that TFIIEa, TAFII28, and TAFII55 can be phosphorylated in vivo. RING finger‐B boxes‐coiled coil (RBCC) and plant homeodomain finger and bromodomain are on one end of the protein, and a bromodomain on the other. It is unknown where the kinase motif is located. 213 In the PKIDB, there is no TIF1 inhibitor in clinical trial or FDA approval.
2.8. Other groups
2.8.1. CK1 family
CK1 kinase regulates mitotic checkpoint signaling transduction, and is involved in DNA repair, apoptosis, p53 pathway, protein translation, circadian rhythm, endocytosis, autophagy, immune response, inflammation, and other related processes. CK1 participates in the signaling transduction process of wingless/integrated (WNT), Hedgehog, NF‐κB, and Yap/Taz (Figure 8). 214 CK1 activates the WNT signaling pathway and promotes cell cycle and cell proliferation. CK1 is involved in the MEK/ERK/YAP/TAZ signaling pathway to affect focal adhesion formation and actin cytoskeleton rearrangement. 215 CK1 kinase primarily mediates the phosphorylation of microtubule constituent subunits, such as alpha, beta, and gamma tubulin, leading to the regulation of microtubule polymerization, stability, and spindle dynamics. 216 p53 is phosphorylated by CK1 and enhances the level of CK1. 217 Some CK1 inhibitors, such as PF‐670462, is being investigated in clinical trials for the treatment of different neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis (ALS). 218 , 219 , 220 However, there is no CK1 inhibitor approved by FDA, in PKIDB or in a clinical trial.
FIGURE 8.

Schematic diagram of protein phosphorylation signaling pathway of other groups.
2.8.2. CK1‐TTBK family
TTBK1 phosphorylates TDP‐43, a pathological hallmark of ALS, in cells and animal models. 221 TTBK is a kinase of tau tubulin, which is a pathological feature of many neurodegenerative diseases, and the level of tau protein correlates with the degree of cognitive impairment. 222 Besides, TTBK upregulates the level of integrin subunit beta 8 (ITGB8) and affects the proliferation, invasion, and promotes ferroptosis in glioma cells. 223 Although some TTBK inhibitors have been developed for investigation, there is no TTBK inhibitor approved by the FDA, in the PKIDB, or in a clinical trial.
2.8.3. CK1‐VRK family
In cancer cells, VRK activates TNFα/NF‐κB signaling by phosphorylating IKKβ, and regulates cell cycle and DNA damage response (Figure 8). 224 , 225 It was demonstrated that VRK2 is a specific therapeutic target, and its inhibitor combined with PD‐1 blockade may be effective in improving immunotherapy in patients with cancers. 226
2.8.4. DNA‐dependent protein kinase
DNA‐dependent protein kinase (DNA‐PK) is a type of kinase that phosphorylates nuclear DNA helicase II/RNA helicase A and hnRNP proteins in an RNA‐dependent manner. 227 The catalytic subunit of DNA‐PKs regulates the classical non‐homologous end joining (c‐NHEJ) pathway, which is an important repair mechanism for DNA double‐strand breaks (DSBs) related to DNA damage and cell survival. 228 In recent years, some DNA‐PK drugs have been researched and developed for the treatment of tumors, such as AZD‐7648, 229 peposertib, and others. Peposertib was reported as an oral DNA‐PK in 2020 and achieved satisfactory tumor‐inhibiting effects in animal experiments. 230 As a typical representative of DNA‐PK, peposertib can inhibit DNA double‐strand repair by inhibiting DSBs, enhancing the killing effect of DNA DSBs induced by radiotherapy and chemotherapy. 231
2.8.5. Immune checkpoint inhibitors
The use of immune checkpoint inhibitors (ICIs) has ushered an immune era of tumor treatment; however, the effective response rate is unsatisfactory for the cancers with low immunogenicity. The inhibition of some pathways can enhance the immune response of cells, such as ADP‐ribose polymerase inhibitor (PARPi), which can prevent single‐strand break DNA repair, activate antigen‐presenting cells, and upregulate tumor programmed death ligand‐1 (PD‐L1). 232
Prexasertib, rabusertib, and adavosertib are the latest ICIs that are in clinical trials. 233 Prexasertib has a good application value in ovarian neoplasms because there is evidence of cytotoxic T‐cell activation in the blood of patients when it is combined with PD‐L1 inhibitors. 234 Rabusertib showed a good synergistic activity in platinum‐resistant breast cancer cells. 235
2.8.6. Aurora kinase B
Aurora kinase B (AURKB) inhibitor is a kind of cell cycle inhibitors 236 and there are two AURKB inhibitors, named barasertib and danusertib, in PKIDB. 5 It is reported that barasertib, which is discovered in 2020, 237 can attenuate fibroblast activation and pulmonary fibrosis induced by TGF‐α. 238 Besides, danusertib is effective in the treatment of several tumors such as adenocarcinoma and hepatocellular carcinoma due to its effect on cell cycle arrest. 239 In addition to these two drugs, there is another one, ilorasertib, which has the inhibitory activity on AURKB and VEGF. 240
2.8.7. Multi‐kinase inhibitor
There are also many multi‐kinase inhibitors that achieve different effects depending on the kinase they inhibit. For example, ilorasertib can inhibit AURKB and VEGF; rigosertib can simultaneously inhibit CDK1, RAS, and MEK1 to affect cell cycle, cell proliferation, and survival. 241 , 242 , 243 Inhibitors with multiple targets can achieve better blocking effects, resulting in more effective therapeutic effects; but at the same time, there will also be a problem of increased side effects due to simultaneous blocking of multiple targets. 244
3. DISCUSSION
At present, including the earliest FDA approved imatinib, the vast majority of PKIs mainly focus on TK and TKL groups. Of the 303 drugs we discussed, more than half (63.4%) were concentrated in these two groups, and 18.2% of the drugs belonged to the AGC‐RGC group, 11.2% of the drugs belonged to the CMGC group, 2.3% of the drugs belonged to the STE‐MAPKs group, and 1.0% of the drugs belonged to the atypical group. The AGC group is represented by the cAMP‐dependent protein kinase PKA, the cGMP‐dependent protease PKG, and the calcium‐ and phospholipid‐dependent protein kinase PKC, and is characterized by activation by second messengers such as cAMP, cGMP, diacylglycerol, and Ca2+. 10 TKs can transfer γ‐phosphate on ATP to protein tyrosine residues, catalyze the phosphorylation of protein tyrosine residues of various substrates, and play an important role in cell growth, proliferation, and differentiation. 245 TKIs, on the other hand, further inhibit the growth and proliferation of tumor cells or other proliferative diseases, and promote apoptosis mainly by inhibiting cell signal transduction. 245 , 246 One of the advantages of TKI for antitumor is its anti‐angiogenesis effect. Because tumors are mostly rich in blood supply, TKI's inhibitory effect on neo‐angiogenesis can reduce unnecessary side effects. 247 Side effects are a constant problem in drug development. The known adverse events during TKI development include leukopenia, thrombocytopenia, anemia, neutropenia, hypertension, abnormal liver function, oral mucosal inflammation, fatigue, sensory nerve abnormalities, thyroid dysfunction, hypopigmentation, proteinuria, and gastrointestinal symptoms. 248 Many new TKIs have been improved in terms of enhancing targeted delivery, prolonging circulation time, improving targeted delivery efficiency, and reducing normal tissue damage to improve their effects. 249 The main focus of CMGC histone kinase drugs is the CDK, MAPK, and GSK3 families. 250 CDK mainly affects the cell cycle, MAPK is related to cell division, and GSK3 family is related to glycogen synthesis. 8 These pathways have effects on cell proliferation and active division, and are one of the main development directions of new PKI drugs. 251 Table 1 briefly summarized the protein kinase group, family, target, disease, and years approved by FDA in recent 5 years.
TABLE 1.
Proteins kinase inhibitor (PKI) drugs approved by Food and Drug Administration (FDA) in recent 5 years
| Approved years | Protein kinase | Group | Family | Targets | Disease |
|---|---|---|---|---|---|
| 2017 | Abemaciclib | CMGC | CDKs | CDK4/6 | Breast neoplasms, osteosarcoma |
| Neratinib | CMGC | CDKs | ErbB2/HER2 | Breast cancer | |
| Ribociclib | CMGC | CDKs | CDK4/6 | Breast cancer | |
| Brigatinib | TK | Raf | ALK | Non‐small‐cell lung cancer | |
| Midostaurin | TK | FLT3 | Flt3 | Acute myeloid leukemia | |
| Acalabrutinib | TKs | BTK | BTK | Chronic lymphocytic leukemia, mantle cell lymphoma | |
| 2018 | Netarsudil | AGC | ROCK | ROCK1/2 | Treat glaucoma |
| Binimetinib | STE‐MAPKs | Ste7/MAP2K | MEK1/2 | Metastatic colorectal cancer, advanced melanoma | |
| Dacomitinib | TK | EGFR | EGFR | Non‐small‐cell lung cancer | |
| Fostamatinib | TK | SYK | Syk | Immune thrombocytopenia | |
| Gilteritinib | TK | FLT3 | Flt3 | Acute myeloid leukemia | |
| Larotrectinib | TK | TRK | TRKA/B/C | Papillary thyroid cancer | |
| Lorlatinib | TK | ALK | ALK | Non‐small‐cell lung cancer | |
| R406 | TK | SYK | Syk | Leukemia, inflammation, glomerulonephritis | |
| Encorafenib | TKL | Raf | B‐Raf | Colorectal cancer, melanoma | |
| Baricitinib | TKs | JAK | JAK1/2 | Rheumatoid arthritis, moderate to severe atopic dermatitis | |
| 2019 | Erdafitinib | TK | FGFR | FGFR1/2/3/4 | Urothelial cancer |
| Fedratinib | TK | JAK | JAK2 | Myelofibrosis, myeloproliferative neoplasm | |
| Pexidartinib | TK | CSF | CSF1R | Tenosynovial giant cell tumors | |
| Upadacitinib | TK | JAK | JAK1 | Psoriatic arthritis, rheumatoid arthritis | |
| Zanubrutinib | TK | BTK | BTK | Waldenström macroglobulinemia, mantle cell lymphoma | |
| Entrectinib | TKL | Raf | TRKA/B/C, ROS1 | Non‐small‐cell lung cancer | |
| 2020 | Selumetinib | STE | Ste7/MAP2K | MEK1/2 | Neurofibromatosis type 1 |
| Capmatinib | TK | RET | c‐MET | Non‐small‐cell lung cancer, glioblastoma, liver cancer, malignant melanoma, breast cancer, colorectal cancer, head and neck cancer | |
| Pemigatinib | TK | FGFR | FGFR2 | Cholangiocarcinoma, myeloid/lymphoid neoplasms | |
| Pralsetinib | TK | RET | RET | Non‐small‐cell lung cancer, papillary thyroid cancer, and medullary thyroid carcinoma | |
| Ripretinib | TK | SFK | Kit, PDGFRα | Gastrointestinal stromal tumour | |
| Selpercatinib | TK | RET | RET | Non‐small‐cell lung cancer, thyroid cancer, and medullary thyroid cancer | |
| Tucatinib | TK | EGFR | ErbB2/HER2 | Breast cancer and colorectal cancer | |
| Avapritinib | TKs | SFK | PDGFRα | Systemic macrocytosis | |
| 2021 | Belumosudil | AGC | ROCK | ROCK1/2 | Chronic graft‐versus‐host disease |
| Umbralisib | AGC | PI3K | PI3K | Non‐Hodgkin lymphoma | |
| Trilaciclib | CMGC | CDK | CDK4/6 | Small‐cell lung cancer | |
| Asciminib | TK | BCR‐ABL | ABL | Chronic myeloid leukemia | |
| Infigratinib | TK | FGFR | FGFR | Cholangiocarcinoma | |
| Mobocertinib | TK | EGFR | EGFR | Non‐small‐cell lung cancer | |
| Tepmetko | TK | RET | MET | Non‐small‐cell lung cancer | |
| Sotorasib | TKL | RAF | KRAS | Non‐small‐cell lung cancer | |
| 2022 | Dabrafenib | TKL | RAF | BRAF | Mutated melanoma, non‐small‐cell lung cancer, mutated anaplastic thyroid cancer |
Abbreviations: AGC, protein kinase A, G, and C; ALK, anaplastic lymphoma kinase; BTK, Bruton's tyrosine kinase; BCR‐Abl, breakpoint cluster region‐Abelson fusion protein; CDK, cyclin‐dependent kinases; CMGC, cyclin‐dependent kinases, mitogen‐activated protein kinases, glycogen synthase kinases, and Cdc2‐like kinases; CSF, colony‐stimulating factor; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; FLT, FMS‐like tyrosine kinase; JAK, Janus kinase; MAPK, mitogen‐activated protein kinase; PDGF, platelet‐derived growth factor; ROCK, rho‐associated coiled‐coil‐containing kinase; SFK, Src‐family protein tyrosine kinase; SYK, spleen tyrosine kinase; TK, tyrosine kinase; TKL, tyrosine kinase‐like.
Source: FDA website https://www.fda.gov/
There are many studies, reports, and reviews that focus on protein phosphorylation, some provide updated PKI classification, and some provide good research depth and content. This review starts with the latest drugs under clinical trials or under clinical use, and focuses on their targets, which can reflect the frontier trends of intervention therapy targeting protein phosphorylation. In addition, the preliminary reasons for the groups and families with no or few new drugs are also analyzed, and suggestions and directions are provided for the development of drugs in this area. Because protein phosphorylation kinases are very complex, involving a lot of pathways, drugs, and diseases, this review cannot cover all the content, and there is no major improvement and innovation on the previous classification methods of protein kinases. In the following review, we will consider an improvement to the existing classification of protein phosphorylation kinases, and provide a novel framework for classification of protein kinases.
4. CONCLUSION
There are many kinds of human protein kinases that functionally promote or inhibit each other, constituting an extremely complex signaling transduction network and information regulation network in human beings. For these kinases, researchers have developed a variety of specific or non‐specific inhibitory drugs to achieve the purpose of disease treatment. The current status of PKIs development is that PKIs are mainly focused on antitumor treatment, and a few drugs are used for the treatment of Alzheimer's disease, immune diseases, proliferative diseases, and even COVID‐19. Now there are still some problems in the development of new PKIs, such as high electrophilic reactivity, non‐specific cytotoxicity, and target cysteine mutation disadvantages, which lead to some less therapeutic effects and unsatisfactory side effects. With the continuous efforts of scientists in recent years, new drugs with fewer side effects have been developed. We expect to see a bright future of drugs targeting protein phosphorylation to benefit patients.
AUTHOR CONTRIBUTIONS
Conceptualization: K.P., Z.‐S.C., and C.‐H.H. Literature review: K.P., W.W., J.‐X.Q., Z.‐D.S., L.H., Y.‐Y.M., Z.‐X.W., H.X., D.P., Z.‐S.C., and C.‐H.H. Writing—original draft preparation: K.P., W.W., and J.‐X.Q. Writing—review and editing: Z.‐S.C. and C.‐H.H. Supervision: Z.‐S.C. and C.‐H.H. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
Author Zhe‐Sheng Chen is an editorial board member of MedComm. Author Zhe‐Sheng Chen was not involved in the journal's review of, or decisions related to, this manuscript. The other authors declared no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Supporting information
Table S1: Proteins kinase inhibitor (PKI) drugs under clinical trials in Protein Kinase Inhibitor Database (PKIDB). Source: PKIDB website https://www.icoa.fr/pkidb/
ACKNOWLEDGMENTS
We would like to thank Uniwinsci Inc. for providing the high‐quality English editing service for this article.
Thanks to Adobe Illustrator CS6 (Version 16.0.0, 32bit) was used for all drawing tools in this article.
Pang K, Wang W, Qin J‐X, et al. Role of protein phosphorylation in cell signaling, disease, and the intervention therapy. MedComm. 2022;3:e175. 10.1002/mco2.175
Contributor Information
Zhe‐Sheng Chen, Email: chenz@stjohns.edu.
Cong‐Hui Han, Email: hanchdoctor@st.btbu.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Mori Y, Kawamura H, Sato T, et al. Identification and enzymatic analysis of an archaeal ATP‐dependent serine kinase from the hyperthermophilic archaeon Staphylothermus marinus . J Bacteriol. 2021;203(16):e0002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tavernier N, Sicheri F, Pintard L. Aurora A kinase activation: different means to different ends. J Cell Biol. 2021;220(9):e202106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo Y, Peng D, Zhou J, et al. iEKPD 2.0: an update with rich annotations for eukaryotic protein kinases, protein phosphatases and proteins containing phosphoprotein‐binding domains. Nucleic Acids Res. 2019;47(D1):D344‐D350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen P, Cross D, Janne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov. 2021;20(7):551‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bournez C, Carles F, Peyrat G, et al. Comparative assessment of protein kinase inhibitors in public databases and in PKIDB. Molecules. 2020;25(14):3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roskoski R Jr. Properties of FDA‐approved small molecule protein kinase inhibitors: a 2022 update. Pharmacol Res. 2022;175:106037. [DOI] [PubMed] [Google Scholar]
- 7. Lu X, Smaill JB, Patterson AV, Ding K. Discovery of cysteine‐targeting covalent protein kinase inhibitors. J Med Chem. 2022;65(1):58‐83. [DOI] [PubMed] [Google Scholar]
- 8. Giraud F, Pereira E, Anizon F, Moreau P. Recent advances in pain management: relevant protein kinases and their inhibitors. Molecules. 2021;26(9):2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Secondo A, Esposito A, Sirabella R, et al. Involvement of the Na+/Ca2+ exchanger isoform 1 (NCX1) in neuronal growth factor (NGF)‐induced neuronal differentiation through Ca2+‐dependent Akt phosphorylation. J Biol Chem. 2015;290(3):1319‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leroux AE, Schulze JO, Biondi RM. AGC kinases, mechanisms of regulation and innovative drug development. Semin Cancer Biol. 2018;48:1‐17. [DOI] [PubMed] [Google Scholar]
- 11. Basu A, Lambring CB. Akt isoforms: a family affair in breast cancer. Cancers. 2021;13(14):3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maeda M, Ochiai K, Michishita M, et al. In vitro anticancer effects of Alpelisib against PIK3CA mutated canine hemangiosarcoma cell lines. Oncol Rep. 2022;47(4):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yap TA, Kristeleit R, Michalarea V, et al. Phase I trial of the PARP inhibitor olaparib and AKT inhibitor capivasertib in patients with BRCA1/2‐ and non‐BRCA1/2‐mutant cancers. Cancer Discov. 2020;10(10):1528‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uko NE, Guner OF, Matesic DF, Bowen JP. Akt pathway inhibitors. Curr Top Med Chem. 2020;20(10):883‐900. [DOI] [PubMed] [Google Scholar]
- 15. Dove S. Mammalian nucleotidyl cyclases and their nucleotide binding sites. Handb Exp Pharmacol. 2017;238:49‐66. [DOI] [PubMed] [Google Scholar]
- 16. Grbcic P, Eichmann TO, Kraljevic Pavelic S, Sedic M. The sphingosine kinase 2 inhibitor ABC294640 restores the sensitivity of BRAFV600E mutant colon cancer cells to vemurafenib by reducing AKT‐mediated expression of nucleophosmin and translationally‐controlled tumour protein. Int J Mol Sci. 2021;22(19):10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng X, Huang M, Zhao W, et al. RAGE mediates airway inflammation via the HDAC1 pathway in a toluene diisocyanate‐induced murine asthma model. BMC Pulm Med. 2022;22(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim K, Li J, Barazia A, et al. ARQ 092, an orally‐available, selective AKT inhibitor, attenuates neutrophil–platelet interactions in sickle cell disease. Haematologica. 2017;102(2):246‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nandan D, Zhang N, Yu Y, et al. Miransertib (ARQ 092), an orally‐available, selective Akt inhibitor is effective against Leishmania. PloS One. 2018;13(11):e0206920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang M, Hu J, Qu J, et al. The therapeutic roles of recombinant Hsp90α on cornea epithelial injury. Invest Ophthalmol Vis Sci. 2022;63(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang J, Jiang R, Chu X, et al. Overexpression of microRNA‐23a‐5p induces myocardial infarction by promoting cardiomyocyte apoptosis through inhibited of PI3K/AKT signalling pathway. Cell Biochem Funct. 2020;38(8):1047‐1055. [DOI] [PubMed] [Google Scholar]
- 22. Choo F, Odintsov I, Nusser K, et al. Functional impact and targetability of PI3KCA, GNAS, and PTEN mutations in a spindle cell rhabdomyosarcoma with MYOD1 L122R mutation. Cold Spring Harb Mol Case Stud. 2022;8(1):a006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yue EW, Li YL, Douty B, et al. INCB050465 (Parsaclisib), a novel next‐generation inhibitor of phosphoinositide 3‐kinase delta (PI3Kdelta). ACS Med Chem Lett. 2019;10(11):1554‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jamee M, Moniri S, Zaki‐Dizaji M, et al. Clinical, immunological, and genetic features in patients with activated PI3Kdelta syndrome (APDS): a systematic review. Clin Rev Allergy Immunol. 2020;59(3):323‐333. [DOI] [PubMed] [Google Scholar]
- 25. Fahy WA, Homayoun‐Valiani F, Cahn A, et al. Nemiralisib in patients with an acute exacerbation of COPD: placebo‐controlled, dose‐ranging study. Int J Chron Obstruct Pulmon Dis. 2021;16:1637‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, Afify SM, Du J, et al. The efficacy of PI3Kgamma and EGFR inhibitors on the suppression of the characteristics of cancer stem cells. Sci Rep. 2022;12(1):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carlo‐Stella C, Delarue R, Scarfo L, et al. A first‐in‐human study of Tenalisib (RP6530), a dual PI3K delta/gamma inhibitor, in patients with relapsed/refractory hematologic malignancies: results from the European study. Clin Lymphoma Myeloma Leuk. 2020;20(2):78‐86. [DOI] [PubMed] [Google Scholar]
- 28. Huen A, Haverkos BM, Zain J, et al. Phase I/Ib study of Tenalisib (RP6530), a Dual PI3K δ/γ inhibitor in patients with relapsed/refractory T‐cell lymphoma. Cancers. 2020;12(8):2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song KW, Edgar KA, Hanan EJ, et al. RTK‐dependent inducible degradation of mutant PI3Kalpha drives GDC‐0077 (Inavolisib) efficacy. Cancer Discov. 2022;12(1):204‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vanhaesebroeck B, Burke JE. Precision targeting of mutant PI3Kα in cancer by selective degradation. Cancer Discov. 2022;12(1):20‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel CG, Rangachari L, Patti M, Griffin C, Shou Y, Venkatakrishnan K. Characterizing the sources of pharmacokinetic variability for TAK‐117 (Serabelisib), an investigational phosphoinositide 3‐kinase alpha inhibitor: a clinical biopharmaceutics study to inform development strategy. Clin Pharmacol Drug Dev. 2019;8(5):637‐646. [DOI] [PubMed] [Google Scholar]
- 32. Isakov N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin Cancer Biol. 2018;48:36‐52. [DOI] [PubMed] [Google Scholar]
- 33. Tyagi K, Roy A. Evaluating the current status of protein kinase C (PKC)‐protein kinase D (PKD) signalling axis as a novel therapeutic target in ovarian cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188496. [DOI] [PubMed] [Google Scholar]
- 34. Hsu AH, Lum MA, Shim KS, et al. Crosstalk between PKCalpha and PI3K/AKT signaling is tumor suppressive in the endometrium. Cell Rep. 2018;24(3):655‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao H, Gong L, Wu S, et al. The inhibition of protein kinase C beta contributes to the pathogenesis of preeclampsia by activating autophagy. EBioMedicine. 2020;56:102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan Y, Yangmei Z, Rongrong S, Xiaowu L, Youwei Z, Sun S. Sotrastaurin attenuates the stemness of gastric cancer cells by targeting PKCdelta. Biomed Pharmacother. 2019;117:109165. [DOI] [PubMed] [Google Scholar]
- 37. Bhattacharyya S, Feferman L, Tobacman JK. Distinct effects of Carrageenan and high‐fat consumption on the mechanisms of insulin resistance in nonobese and obese models of type 2 diabetes. J Diabetes Res. 2019;2019:9582714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shoushtari AN, Khan S, Komatsubara K, et al. A phase Ib study of Sotrastaurin, a PKC inhibitor, and Alpelisib, a PI3Kalpha inhibitor, in patients with metastatic uveal melanoma. Cancers. 2021;13(21):5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gorecki L, Andrs M, Rezacova M, Korabecny J. Discovery of ATR kinase inhibitor berzosertib (VX‐970, M6620): clinical candidate for cancer therapy. Pharmacol Ther. 2020;210:107518. [DOI] [PubMed] [Google Scholar]
- 40. Patin EC, Dillon MT, Nenclares P, et al. Harnessing radiotherapy‐induced NK‐cell activity by combining DNA damage‐response inhibition and immune checkpoint blockade. J Immunother Cancer. 2022;10(3):e004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Konstantinopoulos PA, Cheng SC, Wahner Hendrickson AE, et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum‐resistant high‐grade serous ovarian cancer: a multicentre, open‐label, randomised, phase 2 trial. Lancet Oncol. 2020;21(7):957‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Terranova N, Jansen M, Falk M, Hendriks BS. Population pharmacokinetics of ATR inhibitor berzosertib in phase I studies for different cancer types. Cancer Chemother Pharmacol. 2021;87(2):185‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Middleton MR, Dean E, Evans TRJ, et al. Phase 1 study of the ATR inhibitor berzosertib (formerly M6620, VX‐970) combined with gemcitabine +/‐ cisplatin in patients with advanced solid tumours. Br J Cancer. 2021;125(4):510‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seccia TM, Rigato M, Ravarotto V, Calo LA. ROCK (RhoA/Rho kinase) in cardiovascular‐renal pathophysiology: a review of new advancements. J Clin Med. 2020;9(5):1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graft‐versus‐host disease. J Clin Oncol. 2021;39(17):1888‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blair HA. Belumosudil: first approval. Drugs. 2021;81(14):1677‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amin F, Ahmed A, Feroz A, et al. An update on the association of protein kinases with cardiovascular diseases. Curr Pharm Des. 2019;25(2):174‐183. [DOI] [PubMed] [Google Scholar]
- 48. Lee MH, Kundu JK, Chae JI, Shim JH. Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch Pharm Res. 2019;42(6):481‐491. [DOI] [PubMed] [Google Scholar]
- 49. Testa V, Ferro Desideri L, Della Giustina P, Traverso CE, Iester M. An update on ripasudil for the treatment of glaucoma and ocular hypertension. Drugs Today. 2020;56(9):599‐608. [DOI] [PubMed] [Google Scholar]
- 50. Sonkar C, Doharey PK, Rathore AS, et al. Repurposing of gastric cancer drugs against COVID‐19. Comput Biol Med. 2021;137:104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Masjedi A, Hassannia H, Atyabi F, et al. Downregulation of A2AR by siRNA loaded PEG‐chitosan‐lactate nanoparticles restores the T cell mediated anti‐tumor responses through blockage of PKA/CREB signaling pathway. Int J Biol Macromol. 2019;133:436‐445. [DOI] [PubMed] [Google Scholar]
- 52. Li L, Fan X, Zhang XT, et al. The effects of Chinese medicines on cAMP/PKA signaling in central nervous system dysfunction. Brain Res Bull. 2017;132:109‐117. [DOI] [PubMed] [Google Scholar]
- 53. Yang X, Ma X, Don O, et al. Mesenchymal stem cells combined with liraglutide relieve acute lung injury through apoptotic signaling restrained by PKA/beta‐catenin. Stem Cell Res Ther. 2020;11(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kuhn M. Function and dysfunction of mammalian membrane guanylyl cyclase receptors: lessons from genetic mouse models and implications for human diseases. Handb Exp Pharmacol. 2009;191:47‐69. [DOI] [PubMed] [Google Scholar]
- 55. Sangaralingham SJ, Whig K, Peddibhotla S, et al. Discovery of small molecule guanylyl cyclase A receptor positive allosteric modulators. Proc Natl Acad Sci U S A. 2021;118(52):e2109386118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dickey DM, Dries DL, Margulies KB, Potter LR. Guanylyl cyclase (GC)‐A and GC‐B activities in ventricles and cardiomyocytes from failed and non‐failed human hearts: GC‐A is inactive in the failed cardiomyocyte. J Mol Cell Cardiol. 2012;52(3):727‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Breitenstein S, Roessig L, Sandner P, Lewis KS. Novel sGC stimulators and sGC activators for the treatment of heart failure. Handb Exp Pharmacol. 2017;243:225‐247. [DOI] [PubMed] [Google Scholar]
- 58. Sandner P, Zimmer DP, Milne GT, Follmann M, Hobbs A, Stasch JP. Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol. 2021;264:355‐394. [DOI] [PubMed] [Google Scholar]
- 59. Markham A, Duggan S. Vericiguat: first approval. Drugs. 2021;81(6):721‐726. [DOI] [PubMed] [Google Scholar]
- 60. Shea CM, Price GM, Liu G, et al. Soluble guanylate cyclase stimulator praliciguat attenuates inflammation, fibrosis, and end‐organ damage in the Dahl model of cardiorenal failure. Am J Physiol Renal Physiol. 2020;318(1):F148‐F159. [DOI] [PubMed] [Google Scholar]
- 61. Zimmer DP, Shea CM, Tobin JV, et al. Olinciguat, an oral sGC stimulator, exhibits diverse pharmacology across preclinical models of cardiovascular, metabolic, renal, and inflammatory disease. Front Pharmacol. 2020;11:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hanrahan JP, de Boer IH, Bakris GL, et al. Effects of the soluble guanylate cyclase stimulator praliciguat in diabetic kidney disease: a randomized placebo‐controlled clinical trial. Clin J Am Soc Nephrol. 2020;16(1):59‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beldhuis IE, Lam CSP, Testani JM, et al. Evidence‐based medical therapy in patients with heart failure with reduced ejection fraction and chronic kidney disease. Circulation. 2022;145(9):693‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hegyi B, Bers DM, Bossuyt J. CaMKII signaling in heart diseases: emerging role in diabetic cardiomyopathy. J Mol Cell Cardiol. 2019;127:246‐259. [DOI] [PubMed] [Google Scholar]
- 65. Hu G, He M, Ko WKW, Ye C, Hu Q, Wong AOL. IGFs potentiate TAC3‐induced SLalpha expression via upregulation of TACR3 expression in grass carp pituitary cells. Cells. 2019;8(8):887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sekar P, Huang DY, Hsieh SL, Chang SF, Lin WW. AMPK‐dependent and independent actions of P2×7 in regulation of mitochondrial and lysosomal functions in microglia. Cell Commun Signal. 2018;16(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nawaratna SSK, McManus DP, Gasser RB, et al. Use of kinase inhibitors against schistosomes to improve and broaden praziquantel efficacy. Parasitology. 2020;147(13):1488‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zeyen L, Seternes OM, Mikkola I. Crosstalk between p38 MAPK and GR signaling. Int J Mol Sci. 2022;23(6):3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pua LJW, Mai CW, Chung FF, et al. Functional roles of JNK and p38 MAPK signaling in nasopharyngeal carcinoma. Int J Mol Sci. 2022;23(3):1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Strambu IR, Kobalava ZD, Magnusson BP, MacKinnon A, Parkin JM. Phase II study of single/repeated doses of Acumapimod (BCT197) to treat acute exacerbations of COPD. Copd. 2019;16(5‐6):344‐353. [DOI] [PubMed] [Google Scholar]
- 71. Christie JD, Vaslef S, Chang PK, et al. A randomized dose‐escalation study of the safety and anti‐inflammatory activity of the p38 mitogen‐activated protein kinase inhibitor dilmapimod in severe trauma subjects at risk for acute respiratory distress syndrome. Crit Care Med. 2015;43(9):1859‐1869. [DOI] [PubMed] [Google Scholar]
- 72. Gardiner LJ, Carrieri AP, Bingham K, et al. Combining explainable machine learning, demographic and multi‐omic data to inform precision medicine strategies for inflammatory bowel disease. PloS One. 2022;17(2):e0263248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sacrez M, Lamothe M, Rauch L, Botzung A, Blanc F. Therapeutic trials and dementia with Lewy bodies: a systematic review of the literature. Geriatr Psychol Neuropsychiatr Vieil. 2021;19(3):289‐304. [DOI] [PubMed] [Google Scholar]
- 74. Hall PE, Schmid P. Emerging strategies for TNBC with early clinical data: new chemoimmunotherapy strategies. Breast Cancer Res Treat. 2022;193(1):21‐35. [DOI] [PubMed] [Google Scholar]
- 75. Niu Y, Ji H. Current developments in extracellular‐regulated protein kinase (ERK1/2) inhibitors. Drug Discov Today. 2022;27(5):1464‐1473. [DOI] [PubMed] [Google Scholar]
- 76. Roskoski R Jr. Targeting ERK1/2 protein‐serine/threonine kinases in human cancers. Pharmacol Res. 2019;142:151‐168. [DOI] [PubMed] [Google Scholar]
- 77. Truong TG, Kennedy LB, Patel SP. 25 years of adjuvant therapy in melanoma: a perspective on current approvals and insights into future directions. Curr Oncol Rep. 2022;24(4):533‐542. [DOI] [PubMed] [Google Scholar]
- 78. Iglesias P. Targeted therapies in the medical management of craniopharyngioma. Pituitary. 2022;25(3):383‐392. [DOI] [PubMed] [Google Scholar]
- 79. Jin Q, Yang H, Jing Z, et al. IL4/IL4R signaling promotes the osteolysis in metastatic bone of CRC through regulating the proliferation of osteoclast precursors. Mol Med. 2021;27(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang X, Liu Z, Yin X, Zeng Y, Guo G. Inhibition MNK‐eIF4E‐beta‐catenin preferentially sensitizes gastric cancer to chemotherapy. Fundam Clin Pharmacol. 2022;36(4):712‐720. [DOI] [PubMed] [Google Scholar]
- 81. Chen J, Ye C, Wan C, et al. The roles of c‐Jun N‐terminal kinase (JNK) in infectious diseases. Int J Mol Sci. 2021;22(17):9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tam SY, Law HK. JNK in tumor microenvironment: present findings and challenges in clinical translation. Cancers. 2021;13(9):2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kuramoto K, Yamamoto M, Suzuki S, et al. AS602801, an anti‐cancer stem cell drug candidate, suppresses gap‐junction communication between lung cancer stem cells and astrocytes. Anticancer Res. 2018;38(9):5093‐5099. [DOI] [PubMed] [Google Scholar]
- 84. Yamamoto M, Suzuki S, Togashi K, et al. AS602801 sensitizes ovarian cancer stem cells to paclitaxel by down‐regulating MDR1. Anticancer Res. 2019;39(2):609‐617. [DOI] [PubMed] [Google Scholar]
- 85. Zhang S, Gong Y, Wang H, et al. AS602801 sensitizes glioma cells to temozolomide and vincristine by blocking gap junction communication between glioma cells and astrocytes. J Cell Mol Med. 2021;25(8):4062‐4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bachegowda L, Morrone K, Winski SL, et al. Pexmetinib: a novel dual inhibitor of Tie2 and p38 MAPK with efficacy in preclinical models of myelodysplastic syndromes and acute myeloid leukemia. Cancer Res. 2016;76(16):4841‐4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nagy MA, Hilgraf R, Mortensen DS, et al. Discovery of the c‐Jun N‐terminal kinase inhibitor CC‐90001. J Med Chem. 2021;64(24):18193‐18208. [DOI] [PubMed] [Google Scholar]
- 88. Fan M, Xiao H, Song D, et al. A novel N‐arylpyridone compound alleviates the inflammatory and fibrotic reaction of silicosis by inhibiting the ask1‐p38 pathway and regulating macrophage polarization. Front Pharmacol. 2022;13:848435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Singh V, Huang E, Pathak V, Willard BB, Allende DS, Nagy LE. Phosphoproteomics identifies pathways underlying the role of receptor‐interaction protein kinase 3 in alcohol‐associated liver disease and uncovers apoptosis signal‐regulating kinase 1 as a target. Hepatol Commun. 2022;6(8):2022‐2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Obsilova V, Honzejkova K, Obsil T. Structural insights support targeting ASK1 kinase for therapeutic interventions. Int J Mol Sci. 2021;22(24):13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ogier JM, Gao Y, Dunne EM, et al. ASK1 is a novel molecular target for preventing aminoglycoside‐induced hair cell death. J Mol Med. 2022;100(5):797‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bhardwaj P, Biswas GP, Mahata N, Ghanta S, Bhunia B. Exploration of binding mechanism of triclosan towards cancer markers using molecular docking and molecular dynamics. Chemosphere. 2022;293:133550. [DOI] [PubMed] [Google Scholar]
- 93. Kanoh J, Yanagida M. Tel2: a common partner of PIK‐related kinases and a link between DNA checkpoint and nutritional response? Genes Cells. 2007;12(12):1301‐1304. [DOI] [PubMed] [Google Scholar]
- 94. Qin Z, Qin L, Feng X, Li Z, Bian J. Development of Cdc2‐like kinase 2 inhibitors: achievements and future directions. J Med Chem. 2021;64(18):13191‐13211. [DOI] [PubMed] [Google Scholar]
- 95. Labriere C, Lozach O, Blairvacq M, Meijer L, Guillou C. Further investigation of Paprotrain: towards the conception of selective and multi‐targeted CNS kinase inhibitors. Euro J Med Chem. 2016;124:920‐934. [DOI] [PubMed] [Google Scholar]
- 96. Riggs JR, Nagy M, Elsner J, et al. The discovery of a dual TTK protein kinase/CDC2‐like kinase (CLK2) inhibitor for the treatment of triple negative breast cancer initiated from a phenotypic screen. J Med Chem. 2017;60(21):8989‐9002. [DOI] [PubMed] [Google Scholar]
- 97. Qi J, Ouyang Z. Targeting CDK4/6 for anticancer therapy. Biomedicines. 2022;10(3):685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Adon T, Shanmugarajan D, Kumar HY. CDK4/6 inhibitors: a brief overview and prospective research directions. RSC Adv. 2021;11(47):29227‐29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Morales F, Giordano A. Overview of CDK9 as a target in cancer research. Cell Cycle. 2016;15(4):519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rascon K, Flajc G, De Angelis C, Liu X, Trivedi MV, Ekinci E. Ribociclib in HR+/HER2‐ advanced or metastatic breast cancer patients. Ann Pharmacother. 2019;53(5):501‐509. [DOI] [PubMed] [Google Scholar]
- 101. Zeidner JF, Lee DJ, Frattini M, et al. Phase I study of alvocidib followed by 7+3 (cytarabine + daunorubicin) in newly diagnosed acute myeloid leukemia. Clin Cancer Res. 2021;27(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 102. Zeidner JF, Karp JE. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leukemia Res. 2015;39(12):1312‐1318. [DOI] [PubMed] [Google Scholar]
- 103. Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase‐3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cloutier M, Kumar S, Buttigieg E, et al. Preventing erosion of X‐chromosome inactivation in human embryonic stem cells. Nat Commun. 2022;13(1):2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chauhan N, Paliwal S, Jain S, Verma K, Paliwal S, Sharma S. GSK‐3beta and its inhibitors in Alzheimer's disease: a recent update. Mini Rev Med Chem. 2022. [DOI] [PubMed] [Google Scholar]
- 106. Takahashi‐Yanaga F. Activator or inhibitor? GSK‐3 as a new drug target. Biochem Pharmacol. 2013;86(2):191‐199. [DOI] [PubMed] [Google Scholar]
- 107. Hurst JH, Dohlman HG. Dynamic ubiquitination of the mitogen‐activated protein kinase (MAPKK) Ste7 determines mitogen‐activated protein kinase (MAPK) specificity. J Biol Chem. 2013;288(26):18660‐18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Seternes OM, Kidger AM, Keyse SM. Dual‐specificity MAP kinase phosphatases in health and disease. Biochim Biophys Acta Mol Cell Res. 2019;1866(1):124‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual‐specificity protein phosphatases. Oncogene. 2007;26(22):3203‐3213. [DOI] [PubMed] [Google Scholar]
- 110. Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773(8):1213‐1226. [DOI] [PubMed] [Google Scholar]
- 111. Yu X, Wang Y, Tan X, Li M. Upregulation of fibroblast growth factor 2 contributes to endometriosis through SPRYs/DUSP6/ERK signaling pathway. Acta Histochem. 2021;123(5):151749. [DOI] [PubMed] [Google Scholar]
- 112. Ishii M, Takahashi M, Murakami J, Yanagisawa T, Nishimura M. Vascular endothelial growth factor‐C promotes human mesenchymal stem cell migration via an ERK‐and FAK‐dependent mechanism. Mol Cell Biochem. 2019;455(1‐2):185‐193. [DOI] [PubMed] [Google Scholar]
- 113. Indini A, Tondini CA, Mandalà M. Cobimetinib in malignant melanoma: how to MEK an impact on long‐term survival. Future Oncol. 2019;15(9):967‐977. [DOI] [PubMed] [Google Scholar]
- 114. Lebbe C, Dutriaux C, Lesimple T, et al. Pimasertib versus dacarbazine in patients with unresectable NRAS‐mutated cutaneous melanoma: phase II, randomized, controlled trial with crossover. Cancers. 2020;12(7):1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lebbé C, Italiano A, Houédé N, et al. Selective oral MEK1/2 inhibitor pimasertib in metastatic melanoma: antitumor activity in a phase I, dose‐escalation trial. Target Oncol. 2021;16(1):47‐57. [DOI] [PubMed] [Google Scholar]
- 116. Yamazaki K, Doi T, Ikeda M, et al. Phase I trial of pimasertib monotherapy in Japanese patients with solid tumors and those with hepatocellular carcinoma. Cancer Chemother Pharmacol. 2019;84(5):1027‐1037. [DOI] [PubMed] [Google Scholar]
- 117. Das M. Refametinib in RAS‐mutated hepatocellular cancer. Lancet Oncol. 2018;19(8):e389. [DOI] [PubMed] [Google Scholar]
- 118. Campagne O, Yeo KK, Fangusaro J, Stewart CF. Clinical pharmacokinetics and pharmacodynamics of selumetinib. Clin Pharmacol. 2021;60(3):283‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100‐3112. [DOI] [PubMed] [Google Scholar]
- 120. Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK‐activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wu PK, Becker A, Park JI. Growth inhibitory signaling of the Raf/MEK/ERK pathway. Int J Mol Sci. 2020;21(15):5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Terrell EM, Morrison DK. Ras‐mediated activation of the Raf family kinases. Cold Spring Harb Perspect Med. 2019;9(1):a033746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kohler M, Ehrenfeld S, Halbach S, et al. B‐Raf deficiency impairs tumor initiation and progression in a murine breast cancer model. Oncogene. 2019;38(8):1324‐1339. [DOI] [PubMed] [Google Scholar]
- 124. Chavda J, Bhatt H. Systemic review on B‐Raf(V600E) mutation as potential therapeutic target for the treatment of cancer. Eur J Med Chem. 2020;206:112675. [DOI] [PubMed] [Google Scholar]
- 125. Mikula M, Schreiber M, Husak Z, et al. Embryonic lethality and fetal liver apoptosis in mice lacking the c‐raf‐1 gene. EMBO J. 2001;20(8):1952‐1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Roviello G, D'Angelo A, Petrioli R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E‐mutated colorectal cancer. Transl Oncol. 2020;13(9):100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Roskoski R Jr. Targeting oncogenic Raf protein‐serine/threonine kinases in human cancers. Pharmacol Res. 2018;135:239‐258. [DOI] [PubMed] [Google Scholar]
- 128. Wu C, Whiteway M, Thomas DY, Leberer E. Molecular characterization of Ste20p, a potential mitogen‐activated protein or extracellular signal‐regulated kinase (MEK) kinase from Saccharomyces cerevisiae . J Biol Chem. 1995;270(27):15984‐15992. [DOI] [PubMed] [Google Scholar]
- 129. Chen W, Yazicioglu M, Cobb MH. Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J Biol Chem. 2004;279(12):11129‐11136. [DOI] [PubMed] [Google Scholar]
- 130. Chuang HC, Tan TH. MAP4K3/GLK in autoimmune disease, cancer and aging. J Biomed Sci. 2019;26(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chuang HC, Tan TH. MAP4K family kinases and DUSP family phosphatases in T‐cell signaling and systemic lupus erythematosus. Cells. 2019;8(11):1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. You D, Hillerman S, Locke G, et al. Enhanced antitumor immunity by a novel small molecule HPK1 inhibitor. J Immunother Cancer. 2021;9(1):e001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang Y, Zhang K, Georgiev P, et al. Pharmacological inhibition of hematopoietic progenitor kinase 1 positively regulates T‐cell function. PloS One. 2020;15(12):e0243145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bailey JJ, Jaworski C, Tung D, Wangler C, Wangler B, Schirrmacher R. Tropomyosin receptor kinase inhibitors: an updated patent review for 2016–2019. Expert Opin Ther Pat. 2020;30(5):325‐339. [DOI] [PubMed] [Google Scholar]
- 135. Lee S, Hwang C, Marini S, et al. NGF‐TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat Commun. 2021;12(1):4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Castren E, Monteggia LM. Brain‐derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. 2021;90(2):128‐136. [DOI] [PubMed] [Google Scholar]
- 137. Cui X, Jing J, Wu R, et al. Adipose tissue‐derived neurotrophic factor 3 regulates sympathetic innervation and thermogenesis in adipose tissue. Nat Commun. 2021;12(1):5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Danilenko M, Stamp E, Stocken DD, et al. Targeting tropomyosin receptor kinase in cutaneous CYLD defective tumors with pegcantratinib: the TRAC randomized clinical trial. JAMA Dermatol. 2018;154(8):913‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Harada G, Santini FC, Wilhelm C, Drilon A. NTRK fusions in lung cancer: from biology to therapy. Lung Cancer. 2021;161:108‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Saha D, Ryan KR, Lakkaniga NR, et al. Targeting rearranged during transfection in cancer: a perspective on small‐molecule inhibitors and their clinical development. J Med Chem. 2021;64(16):11747‐11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Jmaeff S, Sidorova Y, Nedev H, Saarma M, Saragovi HU. Small‐molecule agonists of the RET receptor tyrosine kinase activate biased trophic signals that are influenced by the presence of GFRa1 co‐receptors. J Biol Chem. 2020;295(19):6532‐6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cascetta P, Sforza V, Manzo A, et al. RET inhibitors in non‐small‐cell lung cancer. Cancers. 2021;13(17):4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Talukdar S, Emdad L, Das SK, Fisher PB. EGFR: an essential receptor tyrosine kinase‐regulator of cancer stem cells. Adv Cancer Res. 2020;147:161‐188. [DOI] [PubMed] [Google Scholar]
- 144. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rdgeneration EGFRTKI resistance in advanced nonsmall cell lung cancer (review). Int J Oncol. 2021;59(5):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tan DS, Leighl NB, Riely GJ, et al. Safety and efficacy of nazartinib (EGF816) in adults with EGFR‐mutant non‐small‐cell lung carcinoma: a multicentre, open‐label, phase 1 study. Lancet Respir Med. 2020;8(6):561‐572. [DOI] [PubMed] [Google Scholar]
- 146. Dhillon S. Lazertinib: first approval. Drugs. 2021;81(9):1107‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cartron AM, Nguyen TH, Roh YS, Kwatra MM, Kwatra SG. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46(5):820‐824. [DOI] [PubMed] [Google Scholar]
- 148. Montero P, Milara J, Roger I, Cortijo J. Role of JAK/STAT in interstitial lung diseases; molecular and cellular mechanisms. Int J Mol Sci. 2021;22(12):6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chen DY, Khan N, Close BJ, et al. SARS‐CoV‐2 disrupts proximal elements in the JAK‐STAT pathway. J Virol. 2021;95(19):e0086221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148(4):927‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Larionova I, Kazakova E, Gerashchenko T, Kzhyshkowska J. New angiogenic regulators produced by TAMs: perspective for targeting tumor angiogenesis. Cancers. 2021;13(13):3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Farghaly TA, Al‐Hasani WA, Abdulwahab HG. An updated patent review of VEGFR‐2 inhibitors (2017–present). Expert Opin Ther Pat. 2021;31(11):989‐1007. [DOI] [PubMed] [Google Scholar]
- 153. Li L, Zhu M, Wu W, et al. Brivanib, a multitargeted small‐molecule tyrosine kinase inhibitor, suppresses laser‐induced CNV in a mouse model of neovascular AMD. J Cell Physiol. 2020;235(2):1259‐1273. [DOI] [PubMed] [Google Scholar]
- 154. Berndt S, Liebscher I. New structural perspectives in G protein‐coupled receptor‐mediated Src family kinase activation. Int J Mol Sci. 2021;22(12):6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sen B, Johnson FM. Regulation of SRC family kinases in human cancers. J Signal Transduct. 2011;2011:865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gambacorti‐Passerini C, le Coutre P, Piazza R. The role of bosutinib in the treatment of chronic myeloid leukemia. Future Oncol. 2020;16(2):4395‐4408. [DOI] [PubMed] [Google Scholar]
- 157. Amsberg GK, Koschmieder S. Profile of bosutinib and its clinical potential in the treatment of chronic myeloid leukemia. Oncol Targets Ther. 2013;6:99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kazim N, Yen A. Evidence of off‐target effects of bosutinib that promote retinoic acid‐induced differentiation of non‐APL AML cells. Cell Cycle. 2021;20(24):2638‐2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Khajapeer KV, Baskaran R. Hsp90 inhibitors for the treatment of chronic myeloid leukemia. Leuk Res Treatment. 2015;2015:757694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Rea D, Hughes TP. Development of asciminib, a novel allosteric inhibitor of BCR‐ABL1. Crit Rev Oncol Hematol. 2022;171:103580. [DOI] [PubMed] [Google Scholar]
- 161. Deeks ED. Asciminib: first approval. Drugs. 2022;82(2):219‐226. [DOI] [PubMed] [Google Scholar]
- 162. Daver N, Venugopal S, Ravandi F. FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm. Blood Cancer J. 2021;11(5):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3‐mutated AML. N Engl J Med. 2019;381(18):1728‐1740. [DOI] [PubMed] [Google Scholar]
- 164. Mostafa GAE, Kadi AA, AlMasoud N, Attwa MW, Al‐Shakliah NS, AlRabiah H. LC‐MS/MS method for the quantification of the anti‐cancer agent infigratinib: application for estimation of metabolic stability in human liver microsomes. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1179:122806. [DOI] [PubMed] [Google Scholar]
- 165. Chioni AM, Grose RP. Biological significance and targeting of the FGFR axis in cancer. Cancers. 2021;13(22):5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Braun S, McSheehy P, Litherland K, et al. Derazantinib: an investigational drug for the treatment of cholangiocarcinoma. Expert Opin Investig Drugs. 2021;30(11):1071‐1080. [DOI] [PubMed] [Google Scholar]
- 167. Von Suskil M, Sultana KN, Elbezanti WO, et al. Bruton's tyrosine kinase targeting in multiple myeloma. Int J Mol Sci. 2021;22(11):5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton's tyrosine kinase in cancers: drug development advances. Leukemia. 2021;35(2):312‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Burger JA, Wiestner A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer. 2018;18(3):148‐167. [DOI] [PubMed] [Google Scholar]
- 170. Shirley M. Bruton tyrosine kinase inhibitors in B‐cell malignancies: their use and differential features. Target Oncol. 2022;17(1):69‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Dhillon S. Tirabrutinib: first approval. Drugs. 2020;80(8):835‐840. [DOI] [PubMed] [Google Scholar]
- 172. Gallo KA, Ellsworth E, Stoub H, Conrad SE. Therapeutic potential of targeting mixed lineage kinases in cancer and inflammation. Pharmacol Ther. 2020;207:107457. [DOI] [PubMed] [Google Scholar]
- 173. Rana A, Rana B, Mishra R, et al. Mixed lineage kinase‐c‐Jun N‐terminal kinase axis: a potential therapeutic target in cancer. Genes Cancer. 2013;4(9‐10):334‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Semba T, Wang X, Xie X, et al. Identification of the JNK‐active triple‐negative breast cancer cluster associated with an immunosuppressive tumor microenvironment. J Natl Cancer Inst. 2022;114(1):97‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chatterjee D, Preuss F, Dederer V, Knapp S, Mathea S. Structural aspects of LIMK regulation and pharmacology. Cells. 2022;11(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Prunier C, Prudent R, Kapur R, Sadoul K, Lafanechere L. LIM kinases: cofilin and beyond. Oncotarget. 2017;8(25):41749‐41763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mardilovich K, Baugh M, Crighton D, et al. LIM kinase inhibitors disrupt mitotic microtubule organization and impair tumor cell proliferation. Oncotarget. 2015;6(36):38469‐38486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Bagchi AK, Akolkar G, Mandal S, Ayyappan P, Yang X, Singal PK. Toll‐like receptor 2 dominance over Toll‐like receptor 4 in stressful conditions for its detrimental role in the heart. Am J Physiol Heart Circ Physiol. 2017;312(6):H1238‐H1247. [DOI] [PubMed] [Google Scholar]
- 179. Su LC, Xu WD, Huang AF. IRAK family in inflammatory autoimmune diseases. Autoimmun Rev. 2020;19(3):102461. [DOI] [PubMed] [Google Scholar]
- 180. Roskoski R Jr. Blockade of mutant RAS oncogenic signaling with a special emphasis on KRAS. Pharmacol Res. 2021;172:105806. [DOI] [PubMed] [Google Scholar]
- 181. Monaco KA, Delach S, Yuan J, et al. LXH254, a potent and selective ARAF‐sparing inhibitor of BRAF and CRAF for the treatment of MAPK‐driven tumors. Clin Cancer Res. 2021;27(7):2061‐2073. [DOI] [PubMed] [Google Scholar]
- 182. Li H, Yu Y, Zhao Y, et al. Small molecule inhibitor agerafenib effectively suppresses neuroblastoma tumor growth in mouse models via inhibiting ERK MAPK signaling. Cancer Lett. 2019;457:129‐141. [DOI] [PubMed] [Google Scholar]
- 183. Rucker AJ, Chan FK. Tumor‐intrinsic and immune modulatory roles of receptor‐interacting protein kinases. Trends Biochem Sci. 2022;47(4):342‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Taft J, Markson M, Legarda D, et al. Human TBK1 deficiency leads to autoinflammation driven by TNF‐induced cell death. Cell. 2021;184(17):4447‐4463. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mohanty S, Yadav P, Lakshminarayanan H, Sharma P, Vivekanandhan A, Karunagaran D. RETRA induces necroptosis in cervical cancer cells through RIPK1, RIPK3, MLKL and increased ROS production. Eur J Pharmacol. 2022;920:174840. [DOI] [PubMed] [Google Scholar]
- 186. Jung SY, Yug JS, Clarke JM, et al. Population pharmacokinetics of vactosertib, a new TGF‐beta receptor type Iota inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2020;85(1):173‐183. [DOI] [PubMed] [Google Scholar]
- 187. Jin CH, Krishnaiah M, Sreenu D, et al. Discovery of N‐((4‐([1,2,4]triazolo[1,5‐a]pyridin‐6‐yl)‐5‐(6‐methylpyridin‐2‐yl)‐1H‐imidazol‐2 ‐yl)methyl)‐2‐fluoroaniline (EW‐7197): a highly potent, selective, and orally bioavailable inhibitor of TGF‐beta type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem. 2014;57(10):4213‐4238. [DOI] [PubMed] [Google Scholar]
- 188. Steger M, Diez F, Dhekne HS, et al. Systematic proteomic analysis of LRRK2‐mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife. 2017;6:e31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Jeong GR, Jang EH, Bae JR, et al. Dysregulated phosphorylation of Rab GTPases by LRRK2 induces neurodegeneration. Mol Neurodegener. 2018;13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jeong GR, Lee BD. Pathological functions of LRRK2 in Parkinson's disease. Cells. 2020;9(12):2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sabnis RW. 2‐Aminoquinazolines as LRRK2 inhibitors for treating Parkinson's disease. ACS Med Chem Lett. 2022;13(5):775‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Espinoza‐Corral R, Lundquist PK. The plastoglobule‐localized protein AtABC1K6 is a Mn(2+)‐dependent kinase necessary for timely transition to reproductive growth. J Biol Chem. 2022;298(4):101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Stefely JA, Licitra F, Laredj L, et al. Cerebellar ataxia and coenzyme Q deficiency through loss of unorthodox kinase activity. Mol Cell. 2016;63(4):608‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Drennan D, Ryazanov AG. Alpha‐kinases: analysis of the family and comparison with conventional protein kinases. Prog Biophys Mol Biol. 2004;85(1):1‐32. [DOI] [PubMed] [Google Scholar]
- 195. Middelbeek J, Clark K, Venselaar H, Huynen MA, van Leeuwen FN. The alpha‐kinase family: an exceptional branch on the protein kinase tree. Cell Mol Life Sci. 2010;67(6):875‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Bircan HA, Gurbuz N, Pataer A, et al. Elongation factor‐2 kinase (eEF‐2K) expression is associated with poor patient survival and promotes proliferation, invasion and tumor growth of lung cancer. Lung Cancer. 2018;124:31‐39. [DOI] [PubMed] [Google Scholar]
- 197. Zhu S, Liao M, Tan H, et al. Inhibiting eukaryotic elongation factor 2 kinase: an update on pharmacological small‐molecule compounds in cancer. J Med Chem. 2021;64(13):8870‐8883. [DOI] [PubMed] [Google Scholar]
- 198. Temme L, Asquith CRM. eEF2K: an atypical kinase target for cancer. Nat Rev Drug Discov. 2021;20(8):577. [DOI] [PubMed] [Google Scholar]
- 199. Kwak CH, Lee JH, Kim EY, et al. Huzhangoside a suppresses tumor growth through inhibition of pyruvate dehydrogenase kinase activity. Cancers. 2019;11(5):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mayers RM, Butlin RJ, Kilgour E, et al. AZD7545, a novel inhibitor of pyruvate dehydrogenase kinase 2 (PDHK2), activates pyruvate dehydrogenase in vivo and improves blood glucose control in obese (fa/fa) Zucker rats. Biochem Soc Trans. 2003;31(Pt 6):1165‐1167. [DOI] [PubMed] [Google Scholar]
- 201. Sato K, Mochida S, Tomimoto D, et al. A pyruvate dehydrogenase kinase inhibitor prevents retinal cell death and improves energy metabolism in rat retinas after ischemia/reperfusion injury. Exp Eye Res. 2020;193:107997. [DOI] [PubMed] [Google Scholar]
- 202. Stacpoole PW, Greene YJ. Dichloroacetate. Diabetes Care. 1992;15(6):785‐791. [DOI] [PubMed] [Google Scholar]
- 203. Hadden MK, Lubbers DJ, Blagg BS. Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 N‐terminal ATP binding site. Curr Top Med Chem. 2006;6(11):1173‐1182. [DOI] [PubMed] [Google Scholar]
- 204. Angira D, Shaik A, Thiruvenkatam V. Structural and strategic landscape of PIKK protein family and their inhibitors: an overview. Front Biosci. 2020;25(8):1538‐1567. [DOI] [PubMed] [Google Scholar]
- 205. Izumi N, Yamashita A, Ohno S. Integrated regulation of PIKK‐mediated stress responses by AAA+ proteins RUVBL1 and RUVBL2. Nucleus. 2012;3(1):29‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Quek H, Lim YC, Lavin MF, Roberts TL. PIKKing a way to regulate inflammation. Immunol Cell Biol. 2018;96(1):8‐20. [DOI] [PubMed] [Google Scholar]
- 207. Shaik A, Kirubakaran S. Evolution of PIKK family kinase inhibitors: a new age cancer therapeutics. Front Biosci. 2020;25(8):1510‐1537. [DOI] [PubMed] [Google Scholar]
- 208. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA‐mutated, hormone receptor‐positive advanced breast cancer. N Engl J Med. 2019;380(20):1929‐1940. [DOI] [PubMed] [Google Scholar]
- 209. Jang DK, Lee YG, Chan Chae Y, et al. GDC‐0980 (apitolisib) treatment with gemcitabine and/or cisplatin synergistically reduces cholangiocarcinoma cell growth by suppressing the PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2020;529(4):1242‐1248. [DOI] [PubMed] [Google Scholar]
- 210. Xing J, Yang J, Gu Y, Yi J. Research update on the anticancer effects of buparlisib. Oncol Lett. 2021;21(4):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Magagnoli M, Carlo‐Stella C, Santoro A. Copanlisib for the treatment of adults with relapsed follicular lymphoma. Expert Rev Clin Pharmacol. 2020;13(8):813‐823. [DOI] [PubMed] [Google Scholar]
- 212. LaRonde‐LeBlanc N, Wlodawer A. A family portrait of the RIO kinases. J Biol Chem. 2005;280(45):37297‐37300. [DOI] [PubMed] [Google Scholar]
- 213. Fraser RA, Heard DJ, Adam S, et al. The putative cofactor TIF1alpha is a protein kinase that is hyperphosphorylated upon interaction with liganded nuclear receptors. J Biol Chem. 1998;273(26):16199‐16204. [DOI] [PubMed] [Google Scholar]
- 214. Janovska P, Normant E, Miskin H, Bryja V. Targeting casein kinase 1 (CK1) in hematological cancers. Int J Mol Sci. 2020;21(23):9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Li H, Raghunathan V, Stamer WD, Ganapathy PS, Herberg S. Extracellular matrix stiffness and TGFbeta2 regulate YAP/TAZ activity in human trabecular meshwork cells. Front Cell Dev Biol. 2022;10:844342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Roth A, Gihring A, Bischof J, Pan L, Oswald F, Knippschild U. CK1 is a druggable regulator of microtubule dynamics and microtubule‐associated processes. Cancers. 2022;14(5):1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Traub B, Roth A, Kornmann M, Knippschild U, Bischof J. Stress‐activated kinases as therapeutic targets in pancreatic cancer. World J Gastroenterol. 2021;27(30):4963‐4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Bolcato G, Cescon E, Pavan M, et al. A computational workflow for the identification of novel fragments acting as inhibitors of the activity of protein kinase CK1delta. Int J Mol Sci. 2021;22(18):9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Grieco I, Bissaro M, Tiz DB, et al. Developing novel classes of protein kinase CK1delta inhibitors by fusing [1,2,4]triazole with different bicyclic heteroaromatic systems. Euro J Med Chem. 2021;216:113331. [DOI] [PubMed] [Google Scholar]
- 220. Mahoney H, Peterson E, Justin H, et al. Inhibition of casein kinase 1 delta/epsilon improves cognitive performance in adult C57BL/6J mice. Sci Rep. 2021;11(1):4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Nozal V, Martinez‐Gonzalez L, Gomez‐Almeria M, et al. TDP‐43 modulation by Tau‐Tubulin kinase 1 inhibitors: a new avenue for future amyotrophic lateral sclerosis therapy. J Med Chem. 2022;65(2):1585‐1607. [DOI] [PubMed] [Google Scholar]
- 222. Dillon GM, Henderson JL, Bao C, et al. Acute inhibition of the CNS‐specific kinase TTBK1 significantly lowers tau phosphorylation at several disease relevant sites. PloS One. 2020;15(4):e0228771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zhang HY, Zhang BW, Zhang ZB, Deng QJ. Circular RNA TTBK2 regulates cell proliferation, invasion and ferroptosis via miR‐761/ITGB8 axis in glioma. Eur Rev Med Pharmacol Sci. 2020;24(5):2585‐2600. [DOI] [PubMed] [Google Scholar]
- 224. Chen J, Qiao K, Zhang C, et al. VRK2 activates TNFalpha/NF‐kappaB signaling by phosphorylating IKKbeta in pancreatic cancer. Int J Biol Sci. 2022;18(3):1288‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Carrion‐Marchante R, Frezza V, Salgado‐Figueroa A, Perez‐Morgado MI, Martin ME, Gonzalez VM. DNA aptamers against vaccinia‐related kinase (VRK) 1 block proliferation in MCF7 breast cancer cells. Pharmaceuticals. 2021;14(5):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Peled M, Tocheva AS, Adam K, Mor A. VRK2 inhibition synergizes with PD‐1 blockade to improve T cell responses. Immunol Lett. 2021;233:42‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Zhang S, Schlott B, Gorlach M, Grosse F. DNA‐dependent protein kinase (DNA‐PK) phosphorylates nuclear DNA helicase II/RNA helicase A and hnRNP proteins in an RNA‐dependent manner. Nucleic Acids Res. 2004;32(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Sudhanva MS, Hariharasudhan G, Jun S, et al. MicroRNA‐145 impairs classical non‐homologous end‐joining in response to ionizing radiation‐induced DNA double‐strand breaks via targeting DNA‐PKcs. Cells. 2022;11(9):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ding Z, Pan W, Xiao Y, Cheng B, Huang G, Chen J. Discovery of novel 7,8‐dihydropteridine‐6(5H)‐one‐based DNA‐PK inhibitors as potential anticancer agents via scaffold hopping strategy. Euro J Med Chem. 2022;237:114401. [DOI] [PubMed] [Google Scholar]
- 230. Zenke FT, Zimmermann A, Sirrenberg C, et al. Pharmacologic inhibitor of DNA‐PK, M3814, potentiates radiotherapy and regresses human tumors in mouse models. Mol Cancer Ther. 2020;19(5):1091‐1101. [DOI] [PubMed] [Google Scholar]
- 231. Liang S, Thomas SE, Chaplin AK, Hardwick SW, Chirgadze DY, Blundell TL. Structural insights into inhibitor regulation of the DNA repair protein DNA‐PKcs. Nature. 2022;601(7894):643‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Wanderley CWS, Correa TS, Scaranti M, Cunha FQ, Barroso‐Sousa R. Targeting PARP1 to enhance anticancer checkpoint immunotherapy response: rationale and clinical implications. Front Immunol. 2022;13:816642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Vincelette ND, Ding H, Huehls AM, et al. Effect of CHK1 inhibition on CPX‐351 cytotoxicity in vitro and ex vivo. Sci Rep. 2019;9(1):3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Do KT, Manuszak C, Thrash E, et al. Immune modulating activity of the CHK1 inhibitor prexasertib and anti‐PD‐L1 antibody LY3300054 in patients with high‐grade serous ovarian cancer and other solid tumors. Cancer Immunol Immunother. 2021;70(10):2991‐3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Nieto‐Jimenez C, Alcaraz‐Sanabria A, Martinez‐Canales S, et al. Checkpoint kinase 1 pharmacological inhibition synergizes with DNA‐damaging agents and overcomes platinum resistance in basal‐like breast cancer. Int J Mol Sci. 2020;21(23):9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Shaalan AK, Teshima THN, Tucker AS, Proctor GB. Inhibition of Aurora kinase B activity disrupts development and differentiation of salivary glands. Cell Death Discov. 2021;7(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Lakkaniga NR, Zhang L, Belachew B, Gunaganti N, Frett B, Li HY. Discovery of SP‐96, the first non‐ATP‐competitive Aurora kinase B inhibitor, for reduced myelosuppression. Euro J Med Chem. 2020;203:112589. [DOI] [PubMed] [Google Scholar]
- 238. Kasam RK, Ghandikota S, Soundararajan D, et al. Inhibition of Aurora kinase B attenuates fibroblast activation and pulmonary fibrosis. EMBO Mol Med. 2020;12(9):e12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Shen Z, Yin L, Zhou H, et al. Combined inhibition of AURKA and HSF1 suppresses proliferation and promotes apoptosis in hepatocellular carcinoma by activating endoplasmic reticulum stress. Cell Oncol. 2021;44(5):1035‐1049. [DOI] [PubMed] [Google Scholar]
- 240. Maitland ML, Piha‐Paul S, Falchook G, et al. Clinical pharmacodynamic/exposure characterisation of the multikinase inhibitor ilorasertib (ABT‐348) in a phase 1 dose‐escalation trial. Br J Cancer. 2018;118(8):1042‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Malacrida A, Cavaletti G, Miloso M. Rigosertib and cholangiocarcinoma: a cell cycle affair. Int J Mol Sci. 2021;23(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wang Y, Du P, Jiang D. Rigosertib inhibits MEK1‐ERK pathway and alleviates lipopolysaccharide‐induced sepsis. Immun Inflamm Dis. 2021;9(3):991‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Zhou X, Xiao Q, Fu D, et al. Efficacy of rigosertib, a small molecular RAS signaling disrupter for the treatment of KRAS‐mutant colorectal cancer. Cancer Biol Med. 2021;19(2):213‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Cawley JR, Stewart SD, Mochel JP, Veluvolu S, Khanna C, Fenger JM. Pharmacokinetic exposures associated with oral administration of sorafenib in dogs with spontaneous tumors. Front Vet Sci. 2022;9:888483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Saydam G, Ali R, Demir AM, et al. The effect of comorbidities on the choice of tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Int J Hematol Oncol. 2022;11(1):IJH38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Gubbi S, Koch CA, Klubo‐Gwiezdzinska J. Peptide receptor radionuclide therapy in thyroid cancer. Front Endocrinol. 2022;13:896287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Johnson M, Garassino MC, Mok T, Mitsudomi T. Treatment strategies and outcomes for patients with EGFR‐mutant non‐small cell lung cancer resistant to EGFR tyrosine kinase inhibitors: focus on novel therapies. Lung Cancer. 2022;170:41‐51. [DOI] [PubMed] [Google Scholar]
- 248. Lipton JH, Brümmendorf TH, Gambacorti‐Passerini C, Garcia‐Gutiérrez V, Deininger MW, Cortes JE. Long‐term safety review of tyrosine kinase inhibitors in chronic myeloid leukemia—what to look for when treatment‐free remission is not an option. Blood Rev. 2022:100968. [DOI] [PubMed] [Google Scholar]
- 249. Mou L, Tian X, Zhou B, et al. Improving outcomes of tyrosine kinase inhibitors in hepatocellular carcinoma: new data and ongoing trials. Front Oncol. 2021;11:752725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Pastor F, Shkreta L, Chabot B, Durantel D, Salvetti A. Interplay between CMGC kinases targeting SR proteins and viral replication: splicing and beyond. Front Microbiol. 2021;12:658721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Ghione S, Mabrouk N, Paul C, Bettaieb A, Plenchette S. Protein kinase inhibitor‐based cancer therapies: considering the potential of nitric oxide (NO) to improve cancer treatment. Biochem Pharmacol. 2020;176:113855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Proteins kinase inhibitor (PKI) drugs under clinical trials in Protein Kinase Inhibitor Database (PKIDB). Source: PKIDB website https://www.icoa.fr/pkidb/
Data Availability Statement
Not applicable.


