Dear Editor-in-Chief,
Since December 2019, the world is confronting the devastating effects of the Coronavirus Disease 2019 (COVID-19) pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Apart from pulmonary disease, COVID-19 has also been linked with various neurological complications, such as stroke and transverse myelitis [1]. In the setting of SARS-CoV-2 infection, acute cerebral ischemic stroke has been attributed to factors such as immune-mediated hypercoagulability [2], whereas spinal cord dysfunction mainly involves demyelination [1]. Although cases of spinal stroke associated with SARS-CoV-2 infection are described in the literature [1, 3–7], analogous cases with preceding COVID-19 vaccination are lacking.
Case report
A 59-year-old Caucasian male was admitted to our Neurology Department due to acute-onset right lower limb weakness accompanied by abdominal pain, progressing to paraplegia within approximately 10 h. His symptoms commenced 20 days after receiving BNT162b2 mRNA COVID-19 vaccine. Nasopharyngeal swab PCR for SARS-CoV-2 was negative. His medical history was otherwise unremarkable. Neither vascular risk factors nor physical exertion or back hyperflexion/extension movements before symptoms onset were mentioned.
Upon admission, he was hemodynamically stable, afebrile, and without signs of meningeal irritation. Motor examination revealed paraplegia (MRC grade 0/5), whereas lower limb tendon reflexes were absent and plantar responses were neutral bilaterally. Bladder and bowel control was also lost. Complete loss of light touch and pinprick sensation below T7 level was noted. In contrast, lower limb vibration sensation and proprioception were intact. Higher cortical, cerebellar, upper limb, and cranial nerve examination was normal.
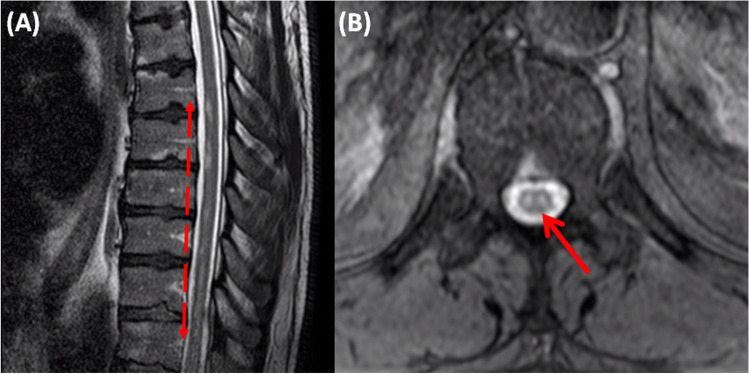
Urgent thoracic spinal cord MRI was performed and showed non-contiguous foci of anteriorly located T2 hyperintensities extending from T6 level to conus medullaris, whereas axial T2 images revealed characteristic “owl eyes sign” (Fig. 1). There was no evidence of compressive myelopathy or vertebral body infarction. Spinal DWI sequences or angiography were not performed.
Fig. 1.
Sagittal (A) and axial (B) T2W MRI images demonstrating anteriorly located hyperintensities extending from T6 level to conus medullaris and bilaterally symmetric circular high signal foci in the anterior horn cells of the spinal cord, known as owl-eyes or snake-eyes or fried-egg sign, respectively
Cerebrospinal fluid (CSF) analysis including white blood cell count and differential, red blood cell count, protein, glucose, IgG index, oligoclonal bands, Gram stain, bacterial culture, varicella-zoster virus, Epstein–Barr virus, and cytomegalovirus PCRs was unremarkable. Anti-myelin oligodendrocyte glycoprotein and anti-aquaporin-4 testing was also normal.
Taking into account (a) the lack of clinical history pointing toward traumatic myelopathy, (b) timing to reach neurological nadir (< 12 h), (c) absence of MRI findings suggestive of compressive myelopathy, (d) typical MRI (“owl eyes sign” on T2 axial and longitudinally extensive lesion on T2 sagittal images), and (e) non-inflammatory CSF findings, our patient fulfilled probable spontaneous spinal cord ischemia diagnostic criteria proposed by Zalewski et al. [8].
Thoracoabdominal and retroperitoneal CT scans excluded the presence of aortic aneurysm. Ischemic stroke work-up including transthoracic heart ultrasound, lipid profile, and systematic autoimmune and neoplastic disease laboratory investigations were normal. On coagulation panel, elevation of both fibrinogen (540 ng/ml) and D-dimers (849 ng/ml) was noted, whereas factors V, VII, and VIII and protein C and S levels were normal. The patient was discharged on antiplatelet therapy to a rehabilitation facility with grade B American Spinal Injury Association (ASIA) impairment scale. On 8-month follow-up, ASIA impairment scale was improved to grade C.
Discussion
Spinal cord infarcts are rare, representing less than 1% of total stroke cases, with incidence estimated at 3.1 per 100,000 person-years. Nevertheless, they constitute a major cause of morbidity among survivors [3]. In a manner similar to cerebral ischemia, the underlying pathophysiology for spinal stroke encompasses cardioembolism, large-vessel atherosclerosis, and small vessel occlusion [4]. Spinal cord ischemia is classified as iatrogenic or spontaneous. As for the first category, it is most frequently the result of thoracic aortic aneurysm repair, whereas other procedures such as spinal decompression, epidural injections, and angiography may also be related to spinal cord infarction as well. Mechanisms of spontaneous ischemia include arterial dissection; atherosclerosis; cardiac, aortic, or fibrocartilaginous embolism; hypercoagulability; and systematic hypotension [6, 7]. Spinal stroke is a frequently misdiagnosed entity, with a vast differential diagnosis including mechanical compression, neoplasms, vascular malformations, and inflammatory and infectious processes. Apart from general medical care and addressing the underlying cause, treatment is mainly based on secondary prevention with antiplatelets and regulation of atherosclerotic risk factors, whereas thrombolytic treatment is sporadically reported in a few cases [1, 6].
To date, spinal cord infarction has been described in association with COVID-19 infection. The first case was reported by Eissa et al. [3]. In accordance with our patient, the cases of Sampogna et al. [4] and Amalia et al. [7] comprise ischemia involving the lower thoracic spinal cord. Conversely, cervical spinal cord infarction was reported by Bax et al. and Kahan et al. [5, 6], whereas Eissa et al. [3] describe a case of vertebral artery thrombosis with concomitant posterior circulation stroke. In the case of Bax et al. [6], spinal cord ischemia preceded the initiation of COVID infection symptoms, in contrast to the remainder studies, where spinal stroke occurred 1 to 3 weeks after the flu-like symptoms [3–5, 7]. Of note, Khedr et al. [1] reported an equivocal case of a young female with acute cervico-thoracic myelopathy following COVID-19 pneumonia initially attributed to transverse myelitis. Anterior spinal artery occlusion was also included in differential diagnosis, given the localization of signal abnormalities on MRI imaging.
Whether COVID-19 vaccination induces thrombosis directly or merely accentuates the hypercoagulable condition in predisposed individuals remains controversial [2]. In our case, increased levels of fibrinogen and D-dimers reflect hypercoagulable state as an underlying ischemic mechanism. Similar findings were observed in the cases of Bax et al. [6] and Amalia et al. [7]. We therefore hypothesize that in our patient, vaccination may have precipitated the thrombotic process, ultimately leading to spinal stroke. Thrombotic events after COVID vaccination exposure are primarily associated with the ChAdOx1 nCoV-19 vaccine and the postulated mechanism is similar to that of heparin-induced thrombocytopenia, with involvement of anti-platelet factor 4 antibodies [2]. Conversely, in the case of mRNA-based vaccines, such as BNT162b2 mRNA COVID-19 vaccine, the presumed mechanism involves translation of a spike protein that enhances platelet aggregation and activation of the alternative complement pathway [9]. To the best of our knowledge, this is the first reported case of spinal cord ischemia post COVID-19 vaccination. Since the spectrum of neurological sequelae in relation to COVID-19 infection and vaccination is continuously expanding, increased awareness among clinicians will aid in accurate evaluation of prevalence and conduction of larger-scale studies providing insight into pathogenetic mechanisms.
Data availability
Data are available on reasonable request.
Declarations
Informed consent
Informed consent was obtained from the patient.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khedr EM, Karim AA, Soliman RK. Case report: Acute spinal cord myelopathy in patients with COVID-19. Front Neurol. 2020;11:610648. doi: 10.3389/fneur.2020.610648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campello E, Simion C, Bulato C, Radu CM, Gavasso S, Sartorello F, Saggiorato G, Zerbinati P, Fadin M, Spiezia L, Simioni P. Absence of hypercoagulability after nCoV-19 vaccination: an observational pilot study. Thromb Res. 2021;205:24–28. doi: 10.1016/j.thromres.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eissa M, Abdelhady M, Alqatami H, Salem K, Own A, El Beltagi AH. Spinal cord infarction in a 41-year-old male patient with COVID-19. Neuroradiol J. 2021;34(3):245–248. doi: 10.1177/1971400921988925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampogna G, Tessitore N, Bianconi T, Leo A, Zarbo M, Montanari E, Spinelli M. Spinal cord dysfunction after COVID-19 infection. Spinal Cord Ser Cases. 2020;6(1):92. doi: 10.1038/s41394-020-00341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahan J, Gibson CJ, Strauss SB, Bronstein M, Winchell RJ, Barie PS, Segal AZ. Cervical spinal cord infarction associated with coronavirus infectious disease (COVID)-19. J ClinNeurosci. 2021;87:89–91. doi: 10.1016/j.jocn.2021.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bax F, Gigli GL, Iaiza F, Valente M. Spontaneous spinal cord ischemia during COVID-19 infection. J Neurol. 2021;268(11):4000–4001. doi: 10.1007/s00415-021-10574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amalia L. Hypercoagulable state induced spinal cord stroke after coronavirus disease 19 infection. J Blood Med. 2021;12:1057–1060. doi: 10.2147/JBM.S329449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zalewski NL. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76(1):56–63. doi: 10.1001/jamaneurol.2018.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi Y. Cerebral venous sinus thrombosis after BNT162b2 mRNA COVID-19 vaccination. Cureus. 2021;13(10):e18775. doi: 10.7759/cureus.18775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.