Abstract
Soluble CD14 (sCD14) mediates the response to lipopolysaccharide (LPS) in cells lacking membrane-bound CD14. We determined sCD14 concentrations in the sera of 38 periodontitis patients and 25 healthy controls by enzyme-linked immunosorbent assay. The sCD14 levels in the sera of patients with periodontitis were significantly higher than those of healthy subjects and decreased after treatment. Enhanced levels of sCD14 in serum may contribute to the host response to LPS in periodontitis. Furthermore, we showed in vitro that addition of LPS enhanced the release of sCD14 by monoblastic U937 cells treated with 1α,25-dihydroxyvitamin D3. Thus, increased sCD14 levels in periodontitis patients may be due to chronic exposure to LPS.
Periodontitis, which is a major cause of tooth loss, is characterized by chronic inflammatory diseases caused by gram-negative bacteria (32). The interaction of lipopolysaccharide (LPS) from gram-negative bacteria with host cells initiates the secretion of cytokines and the expression of cell adhesion molecules in gingival tissue (10, 28, 29), leading to loss of the alveolar bone and connective tissue supporting the teeth in periodontitis. The CD14 molecule, which is expressed primarily on macrophages, reportedly mediates LPS-induced cell activation via binding of LPS (30, 33). A soluble form of CD14 (sCD14) lacking the glycosylphosphatidylinositol anchor is also present in serum (3, 4). Recent reports have demonstrated that sCD14 participates in LPS-induced activation of endothelial or epithelial cells that normally do not express membrane-bound CD14 (1, 2, 7, 8, 11, 22, 24). In addition, in a previous study, we revealed that sCD14 mediates LPS-induced intercellular adhesion molecule 1 expression in cultured human gingival fibroblasts (9). Thus, sCD14 may be important in the regulation of inflammatory and immunological responses in periodontitis. The present study is the first to investigate concentrations of sCD14 in the sera of patients with periodontitis.
We examined 38 patients with either adult periodontitis (AP; n = 20; age range, 42 to 65 years) or early-onset periodontitis (EOP; n = 18; age range, 16 to 41 years). A serum sample was obtained from the median cubital vein of each patient during the initial examination. Twenty-five periodontally healthy donors (age range, 20 to 34 years) served as controls. None of the subjects had any history of systemic diseases. The following clinical evaluations of each patient were performed: (i) measurement of probing pocket depth, (ii) determination of the number of residual teeth, and (iii) determination of the amount of bone loss. By using the method of Schei et al. (25), alveolar bone resorption was measured on dental X-ray films. All of the periodontitis patients had periodontal pocket depths of greater than 5 mm involving at least four teeth and radiographic evidence of extensive bone resorption. To examine the effects of periodontal treatment on the sCD14 concentration in serum, we collected sera after active treatment from 16 of the 38 patients (9 with AP and 7 with EOP). These patients received oral hygiene instruction, scaling, and root planing. Subsequently, the patients were reevaluated as to the necessity of periodontal surgery. Twelve of the 16 patients received periodontal surgery. After the end of active treatment, the patients were reexamined. The treatment period ranged from 5 months to 5 years and 9 months (mean, 1 year and 11 months). In addition, we obtained serum samples from 5 of the 16 patients (1 with AP and 4 with EOP) at the reevaluation prior to periodontal surgery. All five of the patients received periodontal surgery.
The concentration of CD14 was measured by a sandwich enzyme-linked immunosorbent assay using two monoclonal antibodies (MAb) against different epitopes of sCD14 (IBL, Hamburg, Germany) in accordance with the manufacturer’s instructions. In brief, serum specimens were diluted 1:101 and incubated in duplicate for 2 h in a 96-well plate precoated with the anti-CD14 MAb. The plate was incubated for 1 h with the other anti-CD14 MAb conjugated with peroxidase. Substrate was then added, and A450 was measured by using a microtiter plate reader. The intra- and interassay coefficients of variation were 4.7 and 6.9%, respectively. Patient groups and controls were compared by one-way analysis of variance. For a comparison between pre- and posttreatment values, a paired t test was used. Correlations were assessed by using Spearman’s correlation coefficient analysis. Comparisons of sCD14 release by U937 cells were carried out by using an unpaired t test.
As shown in Fig. 1, the sCD14 concentration in serum was significantly higher in patients with periodontitis than in the healthy controls (3.22 versus 2.65 mg/liter; P < 0.01). When patients were classified as either AP or EOP, both groups showed significantly elevated sCD14 concentrations compared to the healthy controls (P < 0.01). There was no significant difference between AP and EOP. The levels of sCD14 in the control subjects were similar to those previously reported in control populations (6, 13, 15, 17–19, 31). No correlation between the levels of sCD14 and the clinical variations of the patients was demonstrated (data not shown).
FIG. 1.
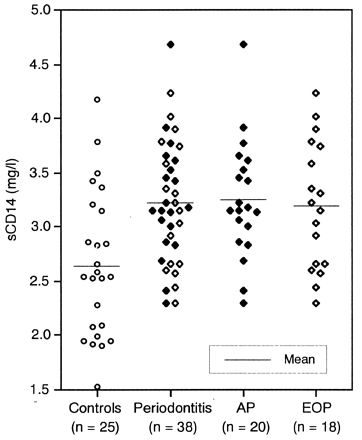
sCD14 concentrations in sera of patients with periodontitis and of healthy controls.
We then examined pre- and posttreatment sCD14 concentrations in the patients with periodontitis. The number of teeth with a probing pocket depth of more than 5 mm was significantly lower posttreatment than pretreatment (20.1 versus 2.7 teeth; P < 0.01), indicating clinical improvement. The sCD14 concentrations in serum following treatment were significantly lower than pretreatment levels (3.20 versus 2.67 mg/liter; P < 0.01) (Fig. 2). At the level of the individual, 14 showed a decline in the sCD14 concentration and 2 showed an increase after treatment. The sCD14 concentrations of the patients after treatment were comparable to those of the healthy controls. Furthermore, we determined the sCD14 concentrations at the time of reevaluation prior to periodontal surgery in 5 of the 16 patients. All of the concentrations were lower than the pretreatment levels and higher than the posttreatment levels (Fig. 3).
FIG. 2.
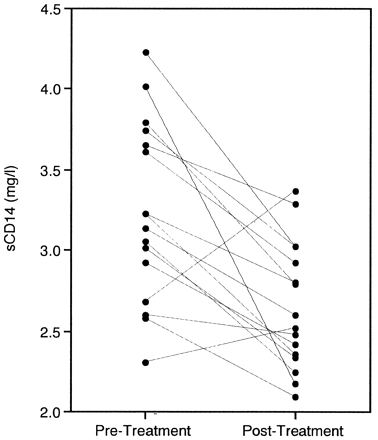
Change in sCD14 concentrations after periodontal treatment.
FIG. 3.
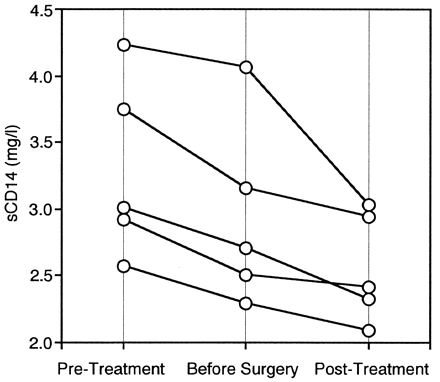
Change in sCD14 concentrations during periodontal treatment.
To elucidate the mechanism responsible for the enhancement of sCD14 levels in periodontitis patients, we further examined the effect of LPS on sCD14 release in 1α,25-dihydroxyvitamin D3 (VitD3)-treated U937 cells, which were reported previously to differentiate into monocytes and macrophages and to express CD14 on their surface (9, 12, 20). U937 cells were originally obtained from the American Type Culture Collection (Manassas, Va.). The cells were maintained in RPMI 1640 medium (GIBCO Laboratories, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal calf serum (GIBCO), 100 U of penicillin per ml, and 100 mg of streptomycin per ml in a humidified atmosphere of 5% CO2 at 37°C. Cells were pretreated with 100 nM VitD3 (Biomol Research Laboratories, Plymouth Meeting, Pa.) at a concentration of 2 × 105 cells/ml for 24 h and then cultured for the specified times with medium containing LPS from Escherichia coli O55:B5 (Difco Laboratories, Detroit, Mich.). Concentrations of sCD14 in culture supernatants were determined by the above-mentioned sandwich enzyme-linked immunosorbent assay. As shown in Fig. 4, sCD14 concentrations in supernatants of U937 cells treated with VitD3 were continuously elevated during culture. Incubation with 0.1- or 100-ng/ml LPS resulted in a rise in sCD14 release which was statistically significant at 3 days compared with the control (P < 0.05).
FIG. 4.
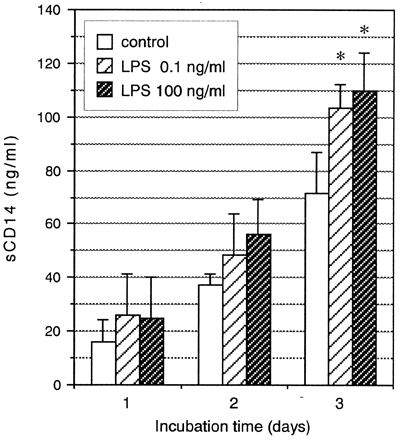
Effects of LPS on sCD14 release by U937 cells treated with VitD3. Data are expressed as means ± standard deviations of three separate experiments. ∗, significant increase (P < 0.05) compared with the culture without LPS (control) at selected time points.
We demonstrated that patients with periodontitis have elevated sCD14 concentrations in their serum. Recent studies suggest that sCD14 enables LPS to trigger responses by cells lacking cell surface CD14 (1, 2, 7–9, 11, 22, 24). We thus speculate that increased sCD14 in serum may contribute to heightened LPS responsiveness of CD14-negative cells, such as endothelial cells and fibroblasts, in periodontitis. Since no correlation was found between the levels of sCD14 and the clinical parameters of patients, it does not seem to be a suitable diagnostic marker of periodontitis. However, the majority of patients showed a decline in sCD14 following treatment. The sCD14 concentrations decreased as the number of steps of treatment increased. Thus, sCD14 may be a useful complementary marker for monitoring each patient with periodontitis.
Serum sCD14 in periodontitis patients may originate from peripheral monocytes and macrophage in the gingival tissues. sCD14 is spontaneously released by monocytes and macrophages (14, 18) and constantly present in the serum of healthy persons (6, 13, 15, 17–19, 31). The elevated sCD14 concentrations in periodontitis patient serum could be due to reduced sCD14 clearance or, alternatively, to enhanced liberation. The former is unlikely, because there may be no relationship between urinary output and periodontitis. The latter probably occurs during periodontitis. Consistent with other reports (14, 18), we showed that LPS is a potent stimulator of sCD14 release from monocytes and macrophages. LPS of dental plaque has been shown to penetrate the gingiva (26). Furthermore, bacteremia increases with increasing severity of gingival inflammation (27), suggesting that periodontal infection leads to systemic exposure to gram-negative bacteria, LPS, and other bacterial products. Therefore, elevated LPS concentrations in the gingiva and bloodstream may account for increased CD14 release in vivo.
Several studies have demonstrated that the concentration of sCD14 in serum is elevated under pathological conditions, such as sepsis, AIDS, malaria, or systemic lupus erythematosus (6, 13, 15, 17–19, 31). Most of these are systemic diseases. Periodontitis is known to be a long-standing chronic disease within a relatively small area of the body. That local periodontal infection upregulates the systemic sCD14 concentration in serum was an unexpected finding of the present study. Recent progress in bacterial classification and the realization that certain organisms are normally found only in the oral cavity have opened the way for a more accurate assessment of the risk of dental focal infection. Periodontal diseases are increasingly implicated in a variety of systemic disorders (5, 16, 23). A recent report suggests that periodontal diseases represent a previously unrecognized and clinically significant risk factor for preterm low birth weight (21). Thus, periodontal infections, which serve as reservoirs for gram-negative organisms, appear to pose a threat to systemic organs via transient bacteremia (27) or systemic changes such as an elevated level of sCD14 in serum.
Acknowledgments
We thank Y. Hsu for her technical assistance.
This work was supported by a grant from the Ministry of Education, Science and Culture of Japan. J.H. was a recipient of research fellowships from the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Arditi M, Zhou J, Dorio R, Rong G W, Goyert S M, Kim K S. Endotoxin-mediated endothelial cell injury and activation: role of soluble CD14. Infect Immun. 1993;61:3149–3156. doi: 10.1128/iai.61.8.3149-3156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arditi M, Zhou J, Torres M, Durden D L, Stins M, Kim K S. Lipopolysaccharide stimulates the tyrosine phosphorylation of mitogen-activated protein kinases p44, p42, and p41 in vascular endothelial cells in a soluble CD14-dependent manner. Role of protein tyrosine phosphorylation in lipopolysaccharide-induced stimulation of endothelial cells. J Immunol. 1995;155:3994–4003. [PubMed] [Google Scholar]
- 3.Bazil V, Baudys M, Hilgert I, Stefanova I, Low M G, Zbrozek J, Horejsi V. Structure relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 4.Bazil V, Horejsi V, Baudys M, Kristofova H, Strominger J, Kostka W, Hilgert I. Biochemical characterization of soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 5.Beck J, Gorcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 6.Burgmann H, Winkler S, Locker G J, Presterl E, Laczika K, Staudinger T, Knapp S, Thalhammer F, Wenisch C, Zedwitz L K, Frass M, Graninger W. Increased serum concentration of soluble CD14 is a prognostic marker in gram-positive sepsis. Clin Immunol Immunopathol. 1996;80:307–310. doi: 10.1006/clin.1996.0128. [DOI] [PubMed] [Google Scholar]
- 7.Camussi G, Mariano F, Biancone L, De M A, Bussolati B, Montrucchio G, Tobias P S. Lipopolysaccharide binding protein and CD14 modulate the synthesis of platelet-activating factor by human monocytes and mesangial and endothelial cells stimulated with lipopolysaccharide. J Immunol. 1995;155:316–324. [PubMed] [Google Scholar]
- 8.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi J, Masaka T, Saito I, Ishikawa I. Soluble CD14 mediates lipopolysaccharide-induced intercellular adhesion molecule 1 expression in cultured human gingival fibroblasts. Infect Immun. 1996;64:4946–4951. doi: 10.1128/iai.64.12.4946-4951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi J, Saito I, Ishikawa I, Miyasaka N. Effects of cytokines and periodontopathic bacteria on the leukocyte function-associated antigen 1/intercellular adhesion molecule 1 pathway in gingival fibroblasts in adult periodontitis. Infect Immun. 1994;62:5205–5212. doi: 10.1128/iai.62.12.5205-5212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haziot A, Rong G W, Silver J, Goyert S M. Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J Immunol. 1993;151:1500–1507. [PubMed] [Google Scholar]
- 12.Koehler M, Goorha R, Kitchingman G R, Ayers G D, Mirro J J. A monoclonal antibody to a novel surface antigen, MKW, blocks the antiproliferative and differentiation effects of granulocyte-macrophage colony-stimulating factor and vitamin D3. Blood. 1992;80:367–373. [PubMed] [Google Scholar]
- 13.Kruger C, Schutt C, Obertacke U, Joka T, Muller F E, Knoller J, Koller M, Konig W, Schonfeld W. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991;85:297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landmann R, Knopf H P, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landmann R, Zimmerli W, Sansano S, Link S, Hahn A, Glauser M P, Calandra T. Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J Infect Dis. 1995;171:639–644. doi: 10.1093/infdis/171.3.639. [DOI] [PubMed] [Google Scholar]
- 16.Loesche W J, Lopatin D E. Interactions between periodontal disease, medical diseases and immunity in the older individual. Periodontol 2000. 1998;16:80–105. doi: 10.1111/j.1600-0757.1998.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 17.Nockher W A, Bergmann L, Scherberich J E. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol. 1994;98:369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nockher W A, Scherberich J E. Monocyte cell-surface CD14 expression and soluble CD14 antigen in hemodialysis: evidence for chronic exposure to LPS. Kidney Int. 1995;48:1469–1476. doi: 10.1038/ki.1995.436. [DOI] [PubMed] [Google Scholar]
- 19.Nockher W A, Wigand R, Schoeppe W, Scherberich J E. Elevated levels of soluble CD14 in serum of patients with systemic lupus erythematosus. Clin Exp Immunol. 1994;96:15–19. doi: 10.1111/j.1365-2249.1994.tb06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberg F, Botling J, Nilsson K. Functional antagonism between vitamin D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J Immunol. 1993;150:3487–3495. [PubMed] [Google Scholar]
- 21.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 22.Pugin J, Schurer M C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rams T E, Slots J. Systemic manifestations of oral infections. In: Slots J, Taubman M A, editors. Contemporary oral microbiology and immunology. St. Louis, Mo: Mosby-Year Book, Inc.; 1992. pp. 500–510. [Google Scholar]
- 24.Read M A, Cordle S R, Veach R A, Carlisle C D, Hawiger J. Cell-free pool of CD14 mediates activation of transcription factor NF-kappa B by lipopolysaccharide in human endothelial cells. Proc Natl Acad Sci USA. 1993;90:9887–9891. doi: 10.1073/pnas.90.21.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schei O, Waerhaug J, Lovdal A, Arno A. Alveolar bone loss as related to oral hygiene and age. J Periodontol. 1959;30:7–16. [Google Scholar]
- 26.Schwartz J, Stinson F L, Parker R B. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972;43:270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- 27.Silver J G, Martin A W, McBride B C. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. J Clin Periodontol. 1977;4:92–99. doi: 10.1111/j.1600-051x.1977.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 28.Takada H, Mihara J, Morisaki I. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura M, Tokuda M, Nagaoka S, Takada H. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun. 1992;60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 31.Wenisch C, Wenisch H, Parschalk B, Vanijanonta S, Burgmann H, Exner M, Zedwitz L K, Thalhammer F, Georgopoulos A, Graninger W, Looareesuwan S. Elevated levels of soluble CD14 in serum of patients with acute Plasmodium falciparum malaria. Clin Exp Immunol. 1996;105:74–78. doi: 10.1046/j.1365-2249.1996.d01-723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams R C. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 33.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]


