Abstract
Background:
Methamphetamine use is increasing, and opioid use remains elevated in the US. Understanding interest in reducing/stopping substance use among people who inject drugs (PWID), as well as types of help wanted, can inform interventions.
Methods:
Data from the 2019 Washington State Syringe Exchange Survey were used in logistic regression analyses to assess if demographics, substance use, and concern about anxiety or depression were associated with interest in reducing/stopping substance use among people whose main drug was methamphetamine or opioids. Types of help wanted to reduce/stop use are reported.
Results:
Of 583 participants included, 76 % reported opioids were their main drug, of whom 82 % were interested in reducing/stopping their opioid use. 24 % reported methamphetamine as their main drug, of whom 46 % were interested in reducing/stopping their methamphetamine use. Among those whose main drug was an opioid, female gender (AOR:2.19, p = .023) and concern about depression (AOR:3.04, p = .002) were associated with interest in reducing/stopping opioid use. Among participants whose main drug was methamphetamine, being in jail in the past year and having an infection likely related to injection (e.g., abscess) in the past year were associated with over twice the odds of interest in reducing/stopping methamphetamine use (AOR:2.14, p = .056 and 2.43, p = .052, respectively); however, these findings were not significant. Several types of help to reduce/stop use were endorsed.
Conclusion:
There were high, though differing, levels of interest in reducing/stopping opioid or methamphetamine use and in a range of support services. PWID should be asked about interest in reducing/stopping use and provided appropriate support.
Keywords: Methamphetamine, Opioids, People who inject drugs, Syringe services programs, Substance use treatment
1. Introduction
Methamphetamine use is increasing and opioid use remains elevated in the United States (Centers for Disease Control and Prevention, 2020; The Lancet, 2018; United Nations Office on Drugs and Crime, 2013; US Department of Justice Drug Enforcement Administration, 2019). The National HIV Behavioral Surveillance (NHBS) survey among people who inject drugs (PWID) found that the proportion of respondents who had injected methamphetamine in the past year increased from 17 % in 2012 to 35 % in 2018, and the vast majority (90 %) reported past-year heroin injection both years (Centers for Disease Control and Prevention, 2012, 2018). Methamphetamine use and mortality in Washington State also have risen. Methamphetamine-involved deaths in Washington were approximately four times greater in 2018 than 2010 (Alcohol and Drug Abuse Institute, University of Washington, 2020a). Opioid-involved deaths remained high during this period. (Alcohol and Drug Abuse Institute, University of Washington, 2020b).
While there are no medications to treat methamphetamine use disorder currently approved by the US Food and Drug Administration (FDA), there is ongoing research of pharmacological agents (Coffin et al., 2019; Colfax et al., 2011; Kohno et al., 2018; Lee et al., 2018; Salehi et al., 2015; White, 2000). Other approaches for treating methamphetamine use disorder include behavioral interventions, such as 12-step programs, cognitive behavioral therapy, and contingency management (Courtney and Ray, 2014; Herrmann et al., 2017; Lee and Rawson, 2008; McPherson et al., 2018). In 2020, the Substance Abuse and Mental Health Services Administration (SAMHSA) amended its multibillion dollar State Opioid Response funding opportunity announcement to allow using funds to address stimulant misuse and use disorders, indicating the interest in and importance of this topic (Substance Abuse and Mental Health Services Administration, 2020).
Opioid use disorder treatment with methadone and buprenorphine has a strong evidence base for clinical effectiveness, improved functioning, reduced mortality, and cost savings (Clark et al., 2011; Connery, 2015; Mattick et al., 2009, 2014). However, these medications are under-utilized; fewer than 25 % of people appropriate for these medications receive them (Williams et al., 2019). Despite low utilization, an analysis of 2015 Washington State syringe service program (SSP) data indicates substantial interest in reducing or stopping use and in using medications for opioid use disorder (Frost et al., 2018).
This study aims to describe SSP clients in Washington State who report methamphetamine or an opioid as their main drug and to identify if sociodemographic characteristics, substance use, or concern about anxiety or depression are associated with an interest in reducing or stopping methamphetamine or opioid use. We also explore the types of help participants would want to reduce or stop using their main drug.
2. Materials and methods
2.1. Data source
We used data from the University of Washington (UW) Alcohol and Drug Abuse Institute (ADAI) 2019 Washington State Syringe Exchange Survey. This is a cross-sectional, biennial survey that began in 2015 (Banta-Green et al., 2016, 2018; Frost et al., 2018). Surveying was conducted between June and August 2019. Surveys were administered verbally, in-person, and in English by SSP personnel, ADAI staff, or Washington Department of Health staff. At sites in King County (where Seattle is located) most survey responses were entered by project staff into REDCap (Harris et al., 2009), which was then transmitted securely to ADAI. At all other sites, and when internet was unreliable in King County (e.g., during mobile delivery), paper surveys were completed on paper and sent to ADAI for data entry.
Twenty-one SSPs participated in the survey across 23 counties. (Map in supplemental materials.) The survey was an attempted census, thus all SSP participants were asked to participate, and if they declined and returned to the SSP during the survey period, were asked again. While an attempt was made to survey each participant, it was occasionally not possible to do this when personnel were busy with SSP activities. In order to not overburden SSP staff, survey non-response was not collected at most sites. The Washington State and UW Institutional Review Boards determined that data collection procedures and analyses were not human subjects’ research and did not require review. Participants received no financial compensation for survey completion, but were given candy.
2.2. Study population
This analysis was restricted to participants who reported that methamphetamine or an opioid (i.e., heroin, fentanyl, methadone, buprenorphine/Suboxone (likely obtained outside of substance use treatment, because people currently in treatment were excluded from the analyses, or other opiate medications) was their main drug, and that they were not currently receiving substance use disorder treatment. Participants who reported that their main drug was a goofball (i.e., heroin and methamphetamine mixed together) were excluded. Main drug was assessed by asking participants “Which of the drugs listed is your MAIN drug?”
Participants who reported “other” gender were excluded due to the small sample size. To focus our analysis on interest in reducing or stopping methamphetamine use, we further excluded participants who reported methamphetamine as their main drug but who used other stimulants in the past three months [i.e., cocaine, crack, or a speedball (cocaine and heroin mixed together)]. In order to include participants with the highest acuity, and therefore most likely to benefit from substance use disorder treatment, we also restricted the analyses to persons who reported that they used their main drug at least 5 of the last 7 days and injected it in the last 3 months.
2.3. Measurements
2.3.1. Outcome
The outcomes of interest were responses to the question “How interested are you in reducing or stopping your [stimulant or opioid] use?” We avoided using the word “treatment” in our outcome measurements because it has many connotations and may not accurately reflect an individual’s motivational level for behavior change. We created a binary variable that combined “very interested” and “somewhat interested” into “interested,” which was compared to respondents who reported that they were “not interested.”
2.3.2. Independent variables
Sociodemographic variables in this analysis included age, gender (male or female), race/ethnicity (white or not-white), being a man who has sex with men (MSM), having health insurance, rurality, housing status (unstable/homeless or permanent), and being in jail in the past year. Age was modeled continuously. MSM was defined as reporting male gender and having sex with any male partners during the past 12 months. Race was included as a binary variable due to most of the sample identifying as only white (75 %) and small sample sizes across the non-white racial categories. Health insurance was categorized as “public/government only” (i.e., Medicaid/Apple Health, Medicare, Veterans Affairs/military, or tribal health/Indian Health Service), “any private insurance,” or “other.” Rurality was defined by mapping respondent zip codes to the US Department of Agriculture Rural-Urban Commuting Area Codes (RUCA) four-level categorization of “urban core,” “suburban,” “large town,” or “small town” (Economic Research Service, 2016).
Concern about mental health was assessed by two questions “How concerned are you about depression?” and “How concerned are you about anxiety?” We used a binary measure that collapsed “very” and “somewhat” into “concerned,” which was compared to “not at all concerned” for each question. Sources of medical care in the past 12 months were categorized into “any ER,” “other sources of care” (e.g., doctor’s office/clinic/tribal clinic, medical hospital, and jail/prison), or “none.”
Substance use variables included overdose (any or none), acute adverse events related to methamphetamine use, years since initiating injecting (difference between current age and age when the participant first injected), number of injections on an average injecting day, frequency of injecting alone, and having a past-year infection that was likely related to injection (i.e., an abscess, skin infection such as cellulitis, blood clot or blood infection like sepsis, or endocarditis). Participants whose main drug was an opioid were asked how many times they had overdosed on opioids in the past year, which was defined as “when breathing slows down or stops and a person can’t be woken up.” Acute adverse events related to methamphetamine (sometimes called “overamping”) is challenging to measure due to varying clinical presentation. We assessed the presence of specific symptoms by asking participants if they had felt like they were “having a heart attack, stroke or seizure while on meth” or “losing your mind, manic, or psychotic while on meth” in the last three months. Frequency of injecting alone was measured by combining responses of “most of the time” and “always,” and comparing them to “some of the time” or “none.”.
Participants who reported that they were interested in reducing or stopping use of their main drug were asked “What types of help would you want if they were easy to get?” with a range of medication, counseling, and other services as response options.
2.3.3. Statistical analysis
We first report the proportion of participants interested, not sure, or not interested in reducing or stopping the use of their main drug. We then exclude participants who said that they were “not sure,” due to the limited interpretability of this answer. Among those who were interested or not interested in reducing or stopping their use, we conducted bivariate analyses to describe and compare participant characteristics across main drug. We then conducted bivariate analyses within each main drug group by interest in reducing or stopping use of their main drug across demographic characteristics, substance use variables, and level of concern about anxiety and depression. Comparisons of categorical variables were made using the Pearson chi-square test and Fisher’s exact test for expected cell counts less than five. Continuous variables were skewed, thus we compared medians with a Wilcoxon rank sum test. A p-value of less than 0.05 was considered statistically significant.
We then conducted a multivariable logistic regression analysis for each group defined by main drug. We first attempted to perform a log-binomial model considering the prevalence of the outcome, but the models did not both converge. Independent variables included in the multivariable analyses either had a p-value of less than 0.05 in the bivariate analyses or had been selected for inclusion a priori due to prior research indicating associations with seeking help to reduce methamphetamine or opioid use in other settings; variables chosen a priori included age, race, gender, and housing status (Corsi et al., 2009; Frost et al., 2018; Korte et al., 2011; Krawczyk et al., 2017; Maxwell, 2014; Nielsen et al., 2018; Palepu et al., 2010; Roth et al., 2015). The types of help participants reported wanting to receive, among those who said they were interested in reducing or stopping their methamphetamine or opioid use, are reported as frequencies. All analyses were performed in Stata 13 (StataCorp, 2013).
3. Results
3.1. Descriptive and bivariate analyses
There were 1269 respondents to the 2019 Washington State Syringe Exchange Survey. The selection criteria for these analyses are shown in Fig. 1. There were 583 participants who met the inclusion criteria, with 140 (24 %) reporting methamphetamine and 443 (76 %) reporting an opioid as their main drug. Among main opioid users, 438 (99 %) reported heroin as their main drug. Among participants who reported male or female gender and not being in drug treatment, 77 (8%) reported that a goofball (i.e., heroin and methamphetamine mixed together) was their main drug, of whom 58 (75 %) were from King County. Participants whose main drug was a goofball are not included in the remainder of the analysis.
Fig. 1.
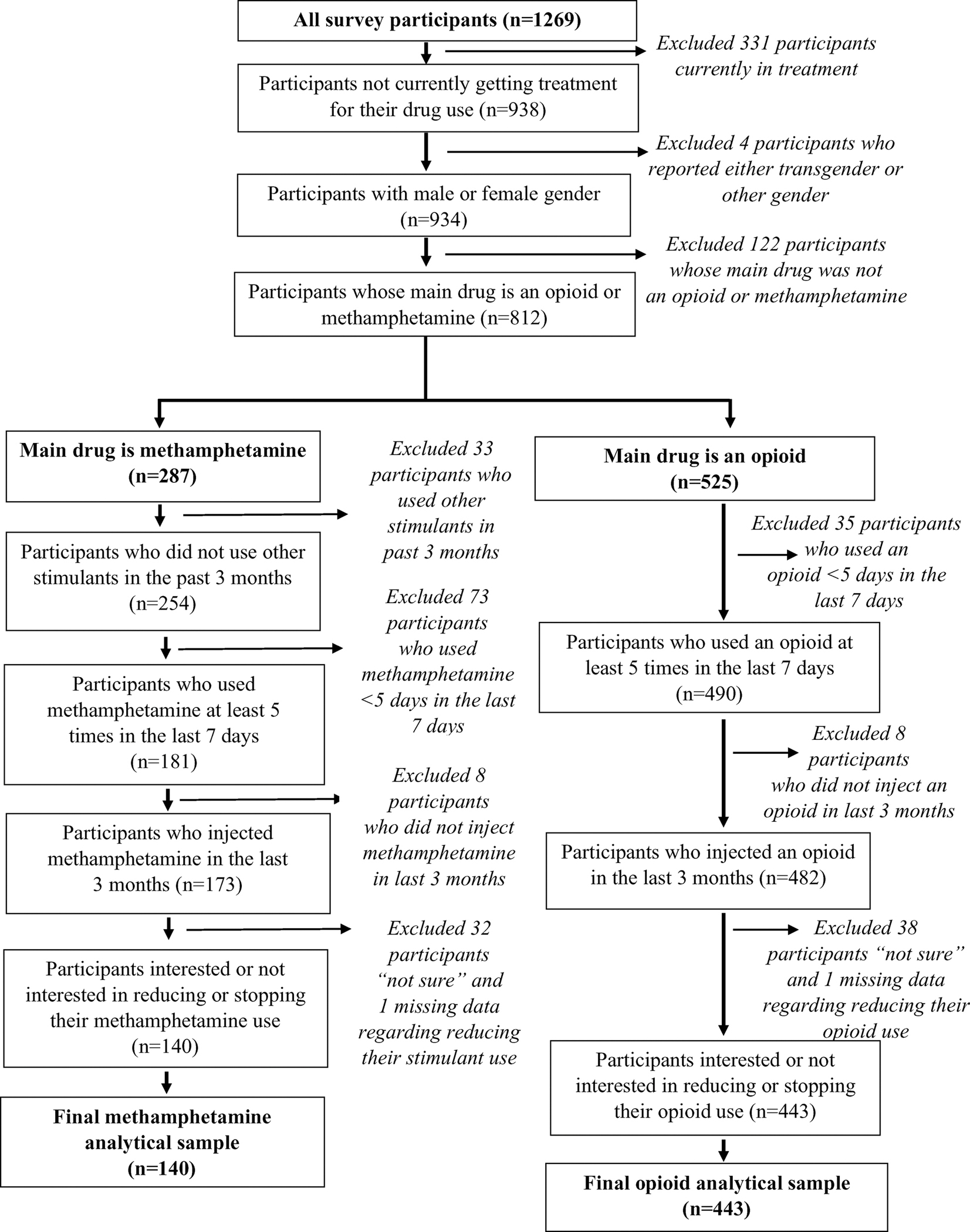
Flowchart of Sample Selection.
Slightly over half of the participants were male (55 %) and the median age was 35 years (IQR: 30–45). Most respondents were white (75 %), living in an urban setting (67 %), in unstable housing or homeless (70 %), and had health insurance (90 %). The median age of participants whose main drug was methamphetamine was significantly older than participants whose main drug was an opioid (39 vs 34 years old, p < .001) and a higher proportion were MSM (11 % vs 5%, p = .044). A smaller proportion of participants whose main drug was methamphetamine were concerned about anxiety (64 % vs 74 %, p = .023) or had an infection that was likely related to injection in the past 12 months (26 % vs 48 %, p < .001) compared to participants whose main drug was an opioid. (Table 1) Polysubstance use was common in both groups; however, a larger proportion of participants whose main drug was an opioid had used methamphetamine (83 %), compared to participants whose main drug was methamphetamine and reported using an opioid (29 %) in the past three months.
Table 1.
Participant Characteristics by Main Drug (n = 583) (Methamphetamine or Opioids).
| Characteristics† | Total (583) |
Methamphetamine (140) |
Opioids (443) |
p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
|
| |||||||
| Age | < .001 | ||||||
| Median (IQR) | 35 (30 – 45) | 39 (33 – 48.5) | 34 (29 – 43) | ||||
| Gender | .74 | ||||||
| Male | 322 | 55 % | 79 | 56% | 243 | 55 % | |
| Female | 261 | 45% | 61 | 44 % | 200 | 45% | |
| MSM | .044 | ||||||
| No | 301 | 93 % | 70 | 89% | 231 | 95 % | |
| Yes | 21 | 7% | 9 | 11 % | 12 | 5% | |
| Race | .054 | ||||||
| White | 439 | 75 % | 114 | 81% | 325 | 73% | |
| Non-White | 144 | 25 % | 26 | 19 % | 118 | 27% | |
| Insurance | .81 | ||||||
| Uninsured | 57 | 10 % | 13 | 9% | 44 | 10 % | |
| Insured | 524 | 90 % | 127 | 91% | 397 | 90 % | |
| Insurance type | .56 | ||||||
| Public/Government only | 495 | 94 % | 119 | 94 % | 376 | 95 % | |
| Any private insurance | 27 | 5% | 7 | 6% | 20 | 5% | |
| Other | 2 | 0% | 1 | 1% | 1 | 0% | |
| Urban/Rural | .67 | ||||||
| Urban Core | 388 | 67 % | 92 | 66% | 296 | 67 % | |
| Suburban | 36 | 6% | 9 | 6% | 27 | 6% | |
| Large Town | 127 | 22% | 29 | 21% | 98 | 22% | |
| Small Town/Rural | 30 | 5% | 10 | 7% | 20 | 5% | |
| Housing | .64 | ||||||
| Permanent | 174 | 30% | 44 | 31% | 130 | 29 % | |
| Unstable/Homeless | 409 | 70 % | 96 | 69% | 313 | 71 % | |
| Jail/Prison | .77 | ||||||
| Not in jail in last year | 356 | 61% | 84 | 60% | 272 | 61% | |
| In jail in last year | 227 | 39% | 56 | 40% | 171 | 39% | |
| Concerned about Depression | .25 | ||||||
| Not at all | 214 | 37% | 57 | 41% | 157 | 36 % | |
| Very/Somewhat | 366 | 63 % | 82 | 59% | 284 | 64 % | |
| Concerned about Anxiety | .023 | ||||||
| Not at all | 164 | 28% | 50 | 36 % | 114 | 26 % | |
| Very/Somewhat | 418 | 72 % | 90 | 64 % | 328 | 74 % | |
| Source of Medical Care in Last Year | .38 | ||||||
| Any ER | 304 | 53% | 73 | 53% | 231 | 53% | |
| Other source of care | 131 | 23% | 36 | 26 % | 95 | 22% | |
| None | 143 | 25 % | 29 | 21% | 114 | 26 % | |
| Length of Time Injecting (years) | 0.79 | ||||||
| Median (IQR) | 9 (4 – 18) | 9 (2.5 – 19.5) | 9 (4 – 17) | ||||
| Number of Injections per Day | < .001 | ||||||
| Median (IQR) | 3 (2 – 4) | 2 (2 – 4) | 3 (3 – 4) | ||||
| Injects Alone | .71 | ||||||
| Never | 159 | 27% | 38 | 27% | 121 | 28% | |
| Some of the time | 246 | 42% | 56 | 40% | 190 | 43% | |
| Most of the time or always | 175 | 30% | 46 | 33% | 129 | 29 % | |
| Infection likely related to injection in last 12 months * | < .001 | ||||||
| No | 331 | 57% | 103 | 74 % | 228 | 52% | |
| Yes | 250 | 43% | 36 | 26 % | 214 | 48 % | |
There were five missing values for source of care in the last year; three for concern about depression and injecting alone; two for insurance, RUCA, duration of infection, and infection likely related to injection; and one for age and concern about anxiety.
An infection likely related to injection was considered an abscess, skin infection (e.g., cellulitis), blood clot or blood infection (e.g., sepsis), or endocarditis.
When we included participants who reported that they were “not sure” about reducing or stopping use of their main drug, almost half (46 %) were interested in reducing or stopping their methamphetamine use, 36 % were not interested, and 19 % were “not sure.” The majority (82 %) were interested in reducing their opioid use, 10 % were not interested, and 8% were “not sure.” The demographic characteristics by interest in reducing or stopping main drug use by main drug, excluding those who reported they were “not sure,” are in Table 2.
Table 2.
Participant Characteristics and Substance Use Behaviors by Interest in Reducing or Stopping Methamphetamine or Opioid Use, Grouped by Main Drug (Methamphetamine or Opioid) (n = 583).
| Characteristics † | Methamphetamine | p value | Opioids | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Interested (79) | Not Interested (61) | Interested (395) | Not Interested (48) | |||||||
|
|
|
|
|
|||||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||
|
| ||||||||||
| Age * | .43 | .88 | ||||||||
| Median (IQR) | 39 (31 – 48) | 39 (34 – 49) | 34 (29 – 43) | 34.5 (27 – 43) | ||||||
| Gender | .89 | .018 | ||||||||
| Male | 45 | 57% | 34 | 43% | 209 | 86 % | 34 | 14% | ||
| Female | 34 | 56% | 27 | 44 % | 186 | 93 % | 14 | 7% | ||
| MSM * | .99 | .23 | ||||||||
| No | 40 | 57% | 30 | 43% | 200 | 87 % | 31 | 13% | ||
| Yes | 5 | 56% | 4 | 44 % | 9 | 75 % | 3 | 25 % | ||
| Race | .89 | .79 | ||||||||
| White | 64 | 56% | 50 | 44 % | 289 | 89% | 36 | 11 % | ||
| Non-White | 15 | 58% | 11 | 42% | 106 | 90 % | 12 | 10 % | ||
| Insurance | .70 | .26 | ||||||||
| Uninsured | 8 | 62% | 5 | 38% | 37 | 84% | 7 | 16% | ||
| Insured | 71 | 56% | 56 | 44 % | 356 | 90 % | 41 | 10 % | ||
| Insurance type | .83 | .11 | ||||||||
| Public/Government only | 67 | 56% | 52 | 44 % | 340 | 90 % | 36 | 10 % | ||
| Any private insurance | 3 | 43% | 4 | 57% | 15 | 75 % | 5 | 25 % | ||
| Other | 1 | 100 % | 0 | 0% | 1 | 100 % | 0 | 0% | ||
| Urban/Rural | .66 | .11 | ||||||||
| Urban Core | 53 | 58% | 39 | 42% | 264 | 89% | 32 | 11 % | ||
| Suburban | 5 | 56% | 4 | 44 % | 27 | 100 % | 0 | 0% | ||
| Large Town | 14 | 48 % | 15 | 52% | 86 | 88% | 12 | 12% | ||
| Small Town/Rural | 7 | 70 % | 3 | 30% | 16 | 80 % | 4 | 20 % | ||
| Housing | .50 | .98 | ||||||||
| Permanent | 23 | 52% | 21 | 48 % | 116 | 89% | 14 | 11 % | ||
| Unstable/Homeless | 56 | 58% | 40 | 42% | 279 | 89% | 34 | 11 % | ||
| Concerned about Depression | .083 | < .001 | ||||||||
| Not at all | 27 | 47% | 30 | 53% | 126 | 80 % | 31 | 20 % | ||
| Very/Somewhat | 51 | 62% | 31 | 38% | 267 | 94 % | 17 | 6% | ||
| Concerned about Anxiety * | .027 | < .001 | ||||||||
| Not at all | 22 | 44 % | 28 | 56% | 90 | 79 % | 24 | 21% | ||
| Very/Somewhat | 57 | 63 % | 33 | 37% | 304 | 93 % | 24 | 7% | ||
| Jail/Prison | .026 | .43 | ||||||||
| Not in jail in last year | 41 | 49 % | 43 | 51 % | 240 | 88% | 32 | 12% | ||
| In jail in last year | 38 | 68 % | 18 | 32% | 155 | 91% | 16 | 9% | ||
| Source of Medical Care in Last Year | .26 | .70 | ||||||||
| Any ER | 46 | 63 % | 27 | 37% | 204 | 88% | 27 | 12% | ||
| Other source of care | 18 | 50 % | 18 | 50 % | 84 | 88% | 11 | 12% | ||
| None | 14 | 48 % | 15 | 52% | 104 | 91% | 10 | 9% | ||
| Length of Time Injecting (years) | .14 | .42 | ||||||||
| Median (IQR) | 8 (2 – 17) | 12 (3 – 20) | 9 (4 – 17) | 8 (5 – 21) | ||||||
| Number of Injections per Day * | .41 | .57 | ||||||||
| Median (IQR) | 2 (2 – 4) | 2 (2 – 3) | 3 (3 – 4) | 3 (2.5 – 4.5) | ||||||
| Injects Alone | .41 | .09 | ||||||||
| Never | 18 | 47% | 20 | 53% | 114 | 94 % | 7 | 6% | ||
| Some of the time | 34 | 61% | 22 | 39% | 164 | 86 % | 26 | 14% | ||
| Most of the time or always | 27 | 59% | 19 | 41% | 114 | 88% | 15 | 12% | ||
| Overamp/Overdose | .023 | .37 | ||||||||
| No overamp in past 3 months/overdose in past year | 51 | 50 % | 50 | 50 % | 303 | 90 % | 34 | 10 % | ||
| Overamp in past 3 months/overdose in past year | 28 | 72 % | 11 | 28% | 92 | 87 % | 14 | 13% | ||
| Infection likely related to injection in last 12 months * | .03 | .59 | ||||||||
| No | 53 | 51 % | 50 | 49 % | 205 | 90 % | 23 | 10 % | ||
| Yes | 26 | 72 % | 10 | 28% | 189 | 88% | 25 | 12% | ||
There were five missing values for source of care in the last year; three for concern about depression and injecting alone; two for insurance, RUCA, duration of infection, and infection likely related to injection; and one for age and concern about anxiety.
These characteristics were significant (p < .05) comparing participants whose main drug was methamphetamine to participants whose main drug was an opioid.
Among participants whose main drug was methamphetamine, a larger proportion of participants who were in jail (68 % vs 49 %, p = .026) or reported an infection likely related to injection in the past year (72 % vs 51 %, p = .03) were interested in stopping or reducing their methamphetamine use. In addition, a higher proportion of those concerned about anxiety (63 % vs 44 %, p = .027) or who had an acute adverse event while using methamphetamine in the past three months reported interest in reducing their methamphetamine use (72 % vs 50 %, p = .023). Among participants whose main drug was an opioid, a higher proportion of women (93 % vs 86 %, p = .018) and participants who were concerned about depression or anxiety were interested in reducing or stopping their opioid use (94 % vs 80 %, p < .001 and 93 % vs 79 %, p < .001, respectively).
3.2. Multivariable regression
Among participants whose main drug was methamphetamine, being in jail in the past year and having an infection likely related to injection in the past year were associated with more than twice the odds of reporting interest in reducing methamphetamine use (AOR: 2.14, 95 % CI: 0.98–4.65, p = .056 and 2.43, 95 % CI: 0.99–5.96, p = .052 respectively); however, these results were not statistically significant (Table 3). Among participants whose main drug was an opioid, female gender was associated with more than twice the odds of interest in reducing or stopping opioid use (AOR: 2.19, 95 % CI: 1.11–4.29, p = .023) compared to male gender. Having a concern about depression was associated with three times the odds of interest in reducing opioid use (AOR: 3.04, 95 % CI: 1.48–6.22, p = .002) (Table 4).
Table 3.
Results of Multivariable Logistic Regression Analysis of Participant Characteristics Associated with Interest in Reducing or Stopping Methamphetamine Use among Syringe Services Program Participants Whose Main Drug was Methamphetamine (n = 139) †.
| Characteristics | AOR | 95 % CI | p-value |
|---|---|---|---|
|
| |||
| Increase in 10 years of age | 1.00 | 0.70 – 1.43 | .99 |
| Female gender | 0.85 | 0.96 – 1.04 | .68 |
| Nonwhite race | 1.31 | 0.51 – 3.41 | .58 |
| Unstable housing/homeless | 0.96 | 0.43 – 2.16 | .93 |
| Jail in past year | 2.14 | 0.98 – 4.65 | .056 |
| Concern about anxiety | 1.98 | 0.90 – 4.36 | .089 |
| Infection likely related to injection in the past year | 2.43 | 0.99 – 5.96 | .052 |
| Overamped in past year | 1.88 | 0.79 – 4.45 | .15 |
There was one missing value for infection likely related to injection in the past year.
Table 4.
Results of Multivariable Logistic Regression Analysis of Participant Characteristics Associated with Interest in Reducing or Stopping Opioid Use among Syringe Services Program Participants Whose Main Drug was an Opioid (n = 439) †.
| Characteristics | AOR | 95 % CI | p-value |
|---|---|---|---|
|
| |||
| Increase in 10 years of age | 1.00 | 0.73 – 1.36 | .98 |
| Female gender | 2.19 | 1.11 – 4.29 | .023 |
| Nonwhite race | 1.09 | 0.53 – 2.24 | .81 |
| Unstable housing/homeless | 0.82 | 0.41 – 1.63 | .57 |
| Concern about depression | 3.04 | 1.48 – 6.22 | .002 |
| Concern about anxiety | 1.89 | 0.93 – 3.83 | .078 |
There were two missing values for concern about depression, one missing value for age, and one missing value for concern about anxiety.
3.3. Type of help participants wanted
The types of help that participants wanted if they were “easy to get” among those who reported interest in reducing or stopping using their main drug are shown in Fig. 2. Among those whose main drug was methamphetamine and who were interested in reducing or stopping their methamphetamine use, the most common type of help wanted was “1:1 counseling/talking with someone” (49 %), followed by “medications that may help reduce stimulant use” (48 %). Among participants whose main drug was an opioid and who were interested in reducing or stopping their opioid use, the majority (71 %) reported “methadone, buprenorphine, [or] naltrexone” as their top type of help.
Fig. 2.
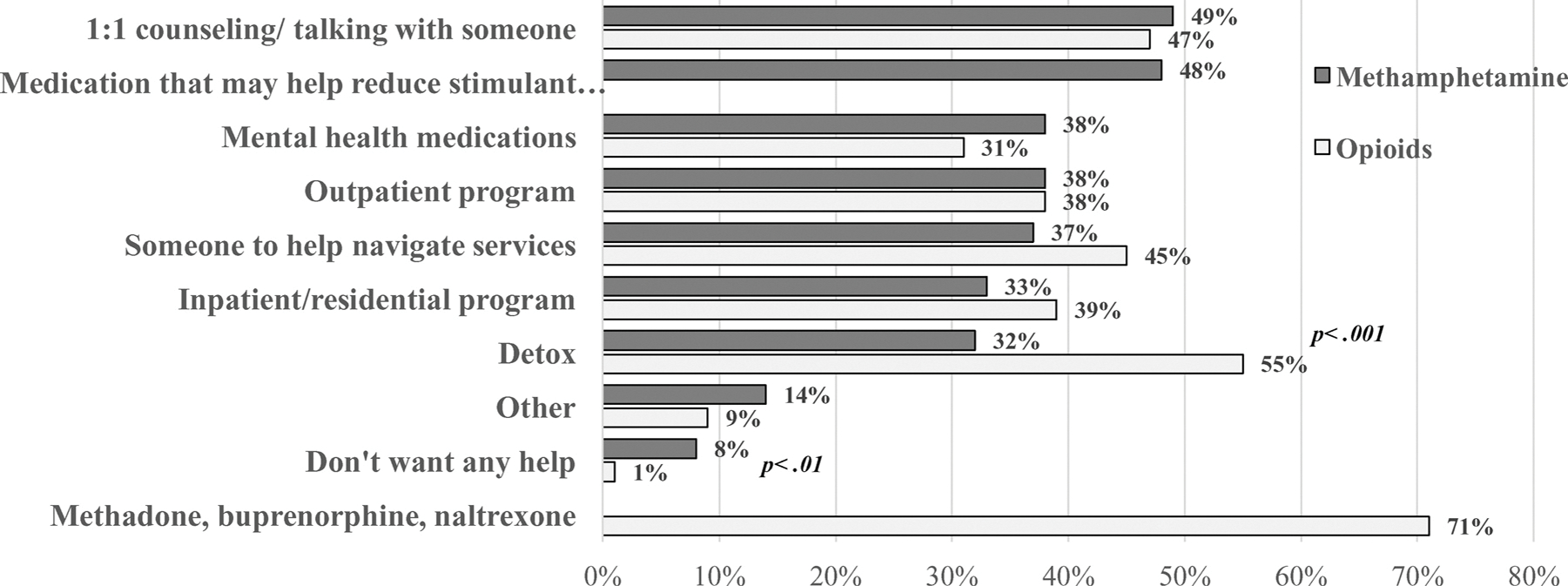
Among Participants who were Interested in Reducing or Stopping their Methamphetamine or Opioid Use, the Percent who Wanted Different Types of Help by Reported Main Drug (Methamphetamine or an Opioid) (n = 474).
4. Discussion
In this study, approximately half of respondents who reported that methamphetamine was their main drug were interested in reducing or stopping their methamphetamine use, and the substantial majority of those reporting opioids as their main drug were interested in reducing or stopping their opioid use. PWID should be asked about their interest in reducing or stopping their substance use and provided appropriate support.
Being in jail or having an infection likely related to injection were associated with interest in reducing methamphetamine use among participants whose main drug was methamphetamine. Referrals to support PWID to reduce their methamphetamine use should be provided in the jail system and in settings that treat infections among PWID, including SSPs.
Among participants whose main drug was methamphetamine and who were interested in reducing their use, almost half reported wanting medications that may reduce stimulant use. Ongoing research into pharmacological agents for methamphetamine use disorder should be a priority. The majority of those who reported opioids as their main drug wanted medications to help reduce or stop their opioid use. Considering the high level of interest in this modality among those actively using opioids who are not currently in substance use treament, and positive preliminary findings from low-threshold models, including SSPs, (Applewhite et al., 2020; Fox et al., 2015; Hood et al., 2019; Smith-Bernardin et al., 2018) health care providers, payers, and systems should continue to work to improve access to medications for opioid use disorder among PWID. Furthermore, several other types of help were desired by respondents; access to these resources should be increased for people who use methamphetamine or opioids.
Most participants were concerned about anxiety or depression, and for people whose main drug was an opioid, concern about depression was associated with three times the interest in reducing their opioid use. Furthermore, approximately one-third endorsed mental health medications as a type of help they would want to reduce their substance use. Depression has been shown to be positively associated with opioid use (Revadigar and Gupta, 2020; Saha et al., 2016; Sullivan, 2018), and anti-depressants have been studied as adjuvant therapy for opioid use disorder, with mixed results (Greenway et al., 2009; Pani et al., 2010). Anxiety is common among people who use methamphetamine (Darke et al., 2008; Zweben et al., 2004) and is a common symptom of acute withdrawal (McGregor et al., 2005; Zorick et al., 2010). In addition to services for reducing substance use, providers should ensure that people who use methamphetamine or, opioids have access to appropriate mental health care and medications for mental health disorders.
This study has several limitations. First, our study sample included PWID using SSP services in Washington State, and we cannot generalize these findings to other populations of PWID or non-injecting populations of people who use opioids and methamphetamine. Second, frequency of injecting methamphetamine or opioid use may be associated with interest in reducing or stopping use. While the survey asked about frequency of injecting any drug in the last week, we could not isolate the frequency of injecting specific drugs. Third, the survey included questions about stigmatized and illegal behaviors and there may have been social desirability bias. Fourth, our outcome combined interest in reducing and stopping substance use. People may have different levels of interest in reducing versus stopping use, and we were unable to assess for these differences. Fifth, our survey asked participants regarding their concern about anxiety or depression, and participants reporting concern may not have diagnoses of anxiety or depressive disorders. Finally, while we excluded 33 participants whose main drug was methamphetamine and who had used other stimulants in the past 3 months, it is possible that respondents were reporting interest in reducing other stimulant use (e.g., cocaine). However, recent use of other stimulants was not common; among those who reported using other stimulants in the past three months, most had not used them in the past week (87 % for cocaine, 80 % for speedball, and 67 % for crack cocaine).
Future research should assess interest in reducing or stopping methamphetamine and opioid use in other populations who are impacted by these substances, including people not using SSPs and individuals who smoke and snort methamphetamine or opioids and do not inject them. In addition, interest in reducing use among people who use both methamphetamine and heroin concurrently, including combined as a goofball, should be evaluated. Goofball use is increasing in Washington State, and there are unique challenges providing treatment for opioid use disorder among individuals who also use methamphetamine (Tsui et al., 2020). In our sample, only 8% reported a goofball as their main drug and we did not ask about interest in reducing goofball use, limiting our ability to describe interest among this group. Finally, considering the range of types of help desired for substance use reduction in this sample, access to these services for PWID should be ensured. SSPs may be an optimal location to increase this access, and future work should identify the best ways to incorporate SSPs in providing PWID with support to reduce their methamphetamine or opioid use.
5. Conclusions
We found a high level of interest in reducing or stopping opioid use, and a moderate level of interest in reducing or stopping methamphetamine use, among PWID who are engaged in SSP services across Washington State and whose main drug is an opioid or methamphetamine, respectively. Providers who engage with PWID should ask about their interest in reducing or stopping substance use and provide appropriate referrals and resources. SSPs may be ideal locations to engage PWID who are actively using substances and interested in reducing their substance use. Among PWID who use methamphetamine, referrals or service provision in jails and clinics that treat injection-related infections may be promising. Concerns about anxiety or depression are very common and associated with interest in reducing substance use. Providers should ensure that people who use methamphetamine or opioids have access to appropriate mental health care and medications for mental health disorders.
Supplementary Material
Acknowledgments
We thank the participating SEPs and their staff. We are grateful for the time and participation of the survey participants. We also thank the Washington State Department of Social and Health Services, Division of Behavioral Health and Recovery and NIH R34 DA 045620-01 for supporting this study.
Role of funding source
The funding sources for this study are The Washington State Health Care Authority, Division of Behavioral Health and Recovery and NIH R34 DA 045620-01. Neither funding source was involved in study design; the collection, analysis, and interpretation of the data; writing the report; or the decision to submit the article for publication.
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.drugalcdep.2020.108243.
References
- Alcohol and Drug Abuse Institute, University of Washington, 2020a. Methamphetamine Trends Across Washington State. http://adai.uw.edu/wadata/methamphetamine.htm.
- Alcohol and Drug Abuse Institute, University of Washington, 2020b. Opioids Trends Across Washington State. https://adai.uw.edu/WAdata/deaths.htm.
- Applewhite D, Regan S, Koenigs K, Mackin S, Schmidt C, Wakeman SE, 2020. Use of promethazine, gabapentin and clonidine in combination with opioids or opioid agonist therapies among individuals attending a syringe service program. Int. J. Drug Policy 79, 102752. [DOI] [PubMed] [Google Scholar]
- Banta-Green C, Kingston S, Ohta J, Taylor M, Sylla L, Tinsley J, Smith R, Couper F, Haruff R, Freng S, K VD, 2016. 2015 Drug Use Trends in King County Washington. Alcohol and Drug Abuse Institute, University of Washington. [Google Scholar]
- Banta-Green C, Newman A, Kingston S, 2018. Washington State Syringe Exhange Health Survey: 2017 Results. Alcohol and Drug Abuse Institute, University of Washington. [Google Scholar]
- Centers for Disease Control and Prevention, 2012. HIV Infection, Risk, Prevention, and Testing Behaviors Among Persons Who Inject Drugs: National HIV Behavioral Surveillance Injection Drug Use 20 US Cities. Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention, 2018. HIV Infection, Risk, Prevention, and Testing Behaviors Among Persons Who Inject Drugs: National HIV Behavioral Surveillance Injection Drug Use 23 US Cities. Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention, 2020. Opioid Overdose: Understanding the Epidemic. https://www.cdc.gov/drugoverdose/epidemic/index.html.
- Clark RE, Samnaliev M, Baxter JD, Leung GY, 2011. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff. 30, 1425–1433. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Hern J, Vittinghoff E, Walker JE, Matheson T, Santos D, Colfax G, Batki SL, 2019. Effects of Mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry 77, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colfax GN, Santos G-M, Das M, Santos DM, Matheson T, Gasper J, Shoptaw S, Vittinghoff E, 2011. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch. Gen. Psychiat. 68, 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery HS, 2015. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv. Rev. Psychiat. 23, 63–75. [DOI] [PubMed] [Google Scholar]
- Corsi KF, Kwiatkowski CF, Booth RE, 2009. Predictors of methamphetamine injection in out-of-Treatment IDUs. Subst. Use Misuse 44, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA, 2014. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 0, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economic Research Service, 2016. Documentation: 2010 Rural-Urban Commuting Area (RUCA) Codes. U.S. Department of Agriculture. [Google Scholar]
- Fox AD, Chamberlain A, Sohler NL, Frost T, Cunningham CO, 2015. Illicit buprenorphine use, interest in and access to buprenorphine treatment among syringe exchange participants. J. Subst. Abuse Treat. 48, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost MC, Williams EC, Kingston S, Banta-Green CJ, 2018. Interest in getting help to reduce or stop substance use among syringe exchange clients who use opioids. J. Addict. Med. 12, 428–434. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA, Group NBS, for the NBSG, 2009. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J. Clin. Endocrinol. Metab. 94, 4898–4906. [DOI] [PubMed] [Google Scholar]
- Harris Paul A., Thielke Robert, Payne RT, Jonathon Gonzales, Nathaniel Conde, Jose G, 2009. Research electronic data capture (REDCap) - A metadata-driven methodology and workfflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann ES, Matusiewicz AK, Stitzer ML, Higgins ST, Sigmon SC, Heil SH, 2017. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J. Subst. Abuse Treat. 72, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JE, Banta-Green CJ, Duchin JS, Breuner J, Dell W, Finegood B, Glick SN, Hamblin M, Holcomb S, Mosse D, Oliphant-Wells T, Shim MM, 2019. Engaging an unstably housed population with low-barrier buprenorphine treatment at a syringe services program: lessons learned from Seattle. Washington. Subst. Abus. 1–9. [DOI] [PubMed] [Google Scholar]
- Kohno M, McCready DL, H., Schwartz DL, Hoffman WF, Korthuis PT, 2018. A preliminary randomized clinical trial of naltrexone reduces striatal resting state functional connectivity in people with methamphetamine use disorder. Drug Alcohol Depend. 192, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte JE, Hiott FB, Brady KT, Malcolm RJ, See RE, 2011. Distinctive characteristics of methamphetamine users presenting at public clinics: steep rise in South Carolina, United States, 2000–2005. Drug Alcohol Depend. 115, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Feder KA, Fingerhood MI, Saloner B, 2017. Racial and ethnic differences in opioid agonist treatment for opioid use disorder in a U.S. National sample. Drug Alcohol Depend. 178, 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Rawson RA, 2008. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 27, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Jenner L, Harney A, Cameron J, 2018. Pharmacotherapy for amphetamine dependence: a systematic review. Drug Alcohol Depend. 191, 309–337. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2009. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009 (3), Cd002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev.(2), Cd002207. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, 2014. A new survey of methamphetamine users in treatment: who they are, why they like “meth,” and why they need additional services. Subst. Use Misuse 49, 639–644. [DOI] [PubMed] [Google Scholar]
- McPherson SM, Burduli E, Smith CL, Herron J, Oluwoye O, Hirchak K, Orr MF, McDonell MG, Roll JM, 2018. A review of contingency management for the treatment of substance-use disorders: adaptation for underserved populations, use of experimental technologies, and personalized optimization strategies. Subst. Abuse Rehabil. 9, 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Lintzeris N, Murnion B, Degenhardt L, Bruno R, Haber P, Johnson J, Hardy M, Ling S, Saddler C, Dunlop A, Demirkol A, Silsbury C, Phung N, Houseman J, Larance B, 2018. Understanding an emerging treatment population: protocol for and baseline characteristics of a prospective cohort of people receiving treatment for pharmaceutical opioid dependence. Drug Alcohol Rev. 37, 887–896. [DOI] [PubMed] [Google Scholar]
- Palepu A, Marshall BD, Lai C, Wood E, Kerr T, 2010. Addiction treatment and stable housing among a cohort of injection drug users. PLoS One 5 (7), e11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani PP, Vacca R, Trogu E, Amato L, Davoli M, 2010. Pharmacological treatment for depression during opioid agonist treatment for opioid dependence. Cochrane Database Syst. Rev. 9. [DOI] [PubMed] [Google Scholar]
- Revadigar N, Gupta V, 2020. Substance Induced Mood Disorders, StatPearls. StatPearls Publishing Copyright © 2020. StatPearls Publishing LLC., Treasure Island (FL). [PubMed] [Google Scholar]
- Roth AM, Armenta RA, Wagner KD, Roesch SC, Bluthenthal RN, Cuevas-Mota J, Garfein RS, 2015. Patterns of drug use, risky behavior, and health status among persons who inject drugs living in San Diego, California: a latent class analysis. Subst. Use Misuse 50, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, Grant BF, 2016. Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. J. Clin. Psychiatry 77, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Kheirabadi EA, Maracy GR, Sharbafchi MR, R. M, 2015. The effect of buprenorphine on methamphetamine cravings. J. Clin. Psychopharmacol. 35, 724–727. [DOI] [PubMed] [Google Scholar]
- Smith-Bernardin S, Rowe C, Behar E, Geier M, Washington S, Santos GM, Euren J, Martin J, Gleghorn A, Coffin PO, 2018. Low-threshold extended-release naltrexone for high utilizers of public services with severe alcohol use disorder: a pilot study. J. Subst. Abuse Treat. 85, 109–115. [DOI] [PubMed] [Google Scholar]
- StataCorp, 2013. Stata Statistical Software: Release 13. StataCorp LP, College Station, TX. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2020. State Opioid Response Grants: Funding Opportunity Announcment (FOA) Information. https://www.samhsa.gov/grants/grant-announcements/ti-20-012.
- Sullivan MD, 2018. Depression effects on long-term prescription opioid use, abuse, and addiction. Clin. J. Pain 34, 878–884. [DOI] [PubMed] [Google Scholar]
- The Lancet, 2018. Opioids and Methamphetamine: a Tale of Two Crises. pp. 391. [DOI] [PubMed]
- Tsui JI, Mayfield J, Speaker EC, Yakup S, Ries R, Funai H, Leroux BG, Merrill JO, 2020. Association between methamphetamine use and retention among patients with opioid use disorders treated with buprenorphine. J. Substance Abuse Treat. 109, 80–85. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, 2013. World Drug Report 2013.
- US Department of Justice Drug Enforcement Administration, 2019. 2019 National Drug Threat Assessment.
- White R, 2000. Dexamphetamine substitution in the treatment of amphetamine abuse: an initial investigation. Addiction 95, 229–238. [DOI] [PubMed] [Google Scholar]
- Williams AR, Nunes EV, Bisaga A, Levin FR, Olfson M, 2019. Development of a Cascade of Care for responding to the opioid epidemic. Am. J. Drug Alcohol Abuse 45, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


