Abstract
Trophozoites of virulent Entamoeba histolytica transfected with the antisense gene encoding cysteine proteinase 5 (CP5) have only 10% of the CP activity but retain their cytopathic activity on mammalian monolayers. In the present study we found that the transfected trophozoites with low levels of CP activity were incapable of inducing the formation of liver lesions in hamsters.
Several cysteine proteinases (CP) with molecular masses in the range of 16 to 96 kDa have been detected in trophozoite extracts of pathogenic Entamoeba histolytica strains (9, 12, 15). CP are considered an important virulence factor in the pathogenesis of amebiasis and have been suggested to play a key role in tissue invasion, disruption of host tissues, and modulation of the cell-mediated immune response (4, 12–14). Bruchhaus and colleagues have identified up to six different CP genes which encode typical papain family proteinases with a high degree of conservation in all active site residues (3). One of them, CP5, was found to be associated with the trophozoite membranes and has been suggested to have a potential role in host tissue destruction. Interestingly, the gene encoding CP5 (cp5) is missing in the closely related but nonpathogenic species Entamoeba dispar (7). Recently, we have generated a transfectant of E. histolytica HM-1:IMSS in which the transcribed cp5 antisense RNA (E. histolytica HM-1:IMSS pSA8 transfectant) strongly reduces the expression of CP (2). In spite of their low levels of CP activity, the pSA8 transfectants are, however, not impaired in their ability to destroy tissue culture monolayers (cytopathic activity). On the other hand, pSA8 transfectants have significantly lower erythrophagocytosis activity than does the parental strain (2).
In this study we examined the ability of the E. histolytica HM-1:IMSS pSA8 transfectant to induce the formation of liver abscesses in hamsters. The level of CP in detergent lysates of pSA8 trophozoites grown in the presence of 60 μg of G418 per ml was determined, prior to intrahepatic inoculation into the hamsters, by monitoring the digestion of the fluorogenic substrate Z-Arg-Arg-pNA (8). The total CP activity in lysates of the pSA8 transfectant was approximately 10% of the level of CP activity determined in the control lysates of the pEhAct-Neo (1) transfectant. The generation times of the two transfected trophozoite cultures (pEhAct-Neo and pSA8) grown in TYI-S-33 medium (5) in the presence of 60 μg of G418 per ml and the nontransfected parental HM1:IMSS strain were 8 and 7 h, respectively. This difference is most likely due to some inhibitory effects of the neomycin derivative G418. Syrian golden hamsters (6 weeks old) were intrahepatically inoculated (18) with 106 trophozoites from the following cultures: (i) E. histolytica HM-1:IMSS pSA8 grown in the presence of 60 μg of G418 per ml, (ii) E. histolytica HM-1:IMSS pEhAct-Neo grown in the presence of 60 μg of G418 per ml, and (iii) E. histolytica HM-1:IMSS nontransfected trophozoites. Hamsters (eight in each group) were sacrificed 1 week after intrahepatic inoculation, and the formation of lesions was evaluated (Table 1). All eight hamsters injected with E. histolytica HM-1:IMSS trophozoites as well as those inoculated with the HM-1:IMSS pEhAct-Neo transfectant presented extensive necrotic lesions. The sizes of the lesions obtained with HM-1:IMSS trophozoites were larger (>2 cm) than those obtained with HM1:IMSS pEhAct-Neo (1 to 2 cm). The smaller size may be due to the above-mentioned difference in growth rates. Only one of the eight hamsters injected with the HM-1:IMSS pSA8 transfectant presented a small isolated lesion, whereas none of the others had any detectable hepathic lesions. A section of amoeba-infected liver was inoculated under sterile conditions in TYI-S-33 medium. For lesions obtained with the E. histolytica HM-1:IMSS trophozoites and the HM-1:IMSS pEhAct-Neo transfectant, a full trophozoite culture was obtained 24 h after inoculation into the TYI-S-33 medium in the absence of G418. For the small and isolated lesion obtained with the E. histolytica HM-1:IMSS pSA8 transfectant, it took 2 weeks to get a satisfactory culture of trophozoites in the absence of G418. Plasmid DNA was rescued from pSA8- and pEhAct-Neo-transfected trophozoites and introduced into Escherichia coli cells to yield ampicillin-resistant transfectants, showing that in both cases the plasmid was still present in trophozoites recovered from liver abscess (Fig. 1). As a control, we were also able to rescue pSA8 and pEhAct-Neo plasmid from transfected trophozoites initially grown in TYI-S-33 medium in the presence of 60 μg of G418 per ml and then cultivated for 2 weeks in TYI-S-33 medium in the absence of G418 (Fig. 1). Interestingly, lysates of pSA8-transfected amoebae that were recovered from the single abscess had 35% of the level of CP activity found in pEhAct-Neo-transfected trophozoites. This level of CP activity is slightly higher than the level of CP activity (10%) found in pSA8-transfected trophozoites before their passage through the livers of hamsters as demonstrated in this study as well as in our previous study (2). This result could be due to selection in the liver of a subpopulation of pSA8-transfected trophozoites that have slightly higher CP activity. The selection of amoeba clones with higher levels of CP activity by hamster liver passages was previously reported by Navarro-Garcia et al. (10).
TABLE 1.
Hamster liver abscess formation by transfected trophozoites
| Trophozoite source | No. of animals with abscess | Mean size of abscess (cm) |
|---|---|---|
| HM1:IMSS pEhAct-Neo | 8/8 | 1–2 |
| HM1:IMSS pSA8 | 1/8 | <0.2 |
| HM1:IMSS | 8/8 | >2 |
FIG. 1.
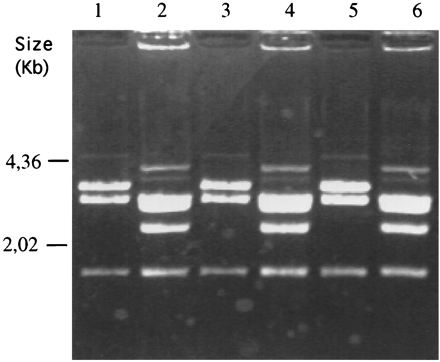
Restriction analysis of plasmid rescued in E. coli XL1-blue from pEhAct-Neo- and pSA8-transfected trophozoites isolated from liver abscess. Plasmid preparations were digested by restriction enzymes (XhoI-SalI) and size fractionated on agarose gels. Lanes: 1, plasmid pEhAct-Neo; 2, plasmid pSA8; 3, plasmid rescued from pEhAct-Neo transfectant isolated from liver abscess; 4, plasmid rescued from pSA8 transfectant isolated from liver abscess; 5, plasmid rescued from pEhAct-Neo transfectant cultivated in TYI-S-33 medium for 2 weeks in the absence of G418; and 6, plasmid rescued from pSA8 transfectant cultivated in TYI-S-33 medium for 2 weeks in the absence of G418.
Our results suggest an important role for amoeba CP in liver abscess formation in hamsters. This conclusion is in agreement with the results observed upon inhibition of CP by the specific inhibitor E-64 and reduction of liver abscess formation in SCID mice (17). Our present results clearly indicate that the in vitro cytopathic assay is not sufficiently indicative of ameobic virulence. In our previous study with tissue-cultured monolayers, we demonstrated that in contrast to intact trophozoites, lysates of the pSA8-transfected trophozoites had very poor cytolytic activity. These findings indicate that while intact trophozoite CP are not a main factor for destruction of monolayers, CP most likely play an important role in the mammalian liver where ameobae must battle the immunological defenses of the host (16). Based on the differences that we find between the in vitro and in vivo determinations of transfectant virulence, the presence and release of CP by intact and lysed ameoba are important in vivo components involved in tissue damage in the host. Other elements involved in ameobic virulence are resistance to complement and phagocytosis activity. It has been shown that after long-term cultivation in vitro pathogenic isolates were susceptible to lysis by the alternative pathway of complement (6). No significant difference was found in the susceptibility of pSA8 transfectants and that of the pEhAct-Neo control or untransfected strain HM-1:IMSS to complement. In all cases lysis was approximately ±70%. Another aspect that may also be of importance is the impaired rate of erythrophagocytosis of the pSA8 transfectants (2), which could also contribute to its inability to induce liver abscesses. However, in view of the conflicting reports about the correlation between the phatocytic activity of the trophozoites and their ability to induce liver abscesses (11, 19), more studies are needed for conclusions about the involvement of phagocytic activity in the formation of liver abscesses.
Acknowledgments
S. Ankri was supported by a Feinberg Graduate School postdoctoral fellowship (Weizmann Institute). This work was supported in part by the Leo and Julia Forchheimer Center for Molecular Genetics at the Weizmann Institute of Science and the Center for the Study of Emerging Diseases.
REFERENCES
- 1.Alon R, Bracha R, Mirelman D. Inhibition of expression of the lysine-rich 30 kDa surface antigen of Entamoeba dispar by the transcription of its antisense RNA. Mol Biochem Parasitol. 1997;90:193–201. doi: 10.1016/s0166-6851(97)00148-5. [DOI] [PubMed] [Google Scholar]
- 2.Ankri S, Stolarsky T, Mirelman D. Antisense inhibition of expression of cysteine proteinases in Entamoeba histolytica does not affect cytopathic or hemolytic activity but inhibits phagocytosis. Mol Microbiol. 1998;28:777–785. doi: 10.1046/j.1365-2958.1998.00837.x. [DOI] [PubMed] [Google Scholar]
- 3.Bruchhaus I, Jacobs T, Leippe M, Tannich E. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinases genes. Mol Microbiol. 1996;22:255–263. doi: 10.1046/j.1365-2958.1996.00111.x. [DOI] [PubMed] [Google Scholar]
- 4.Campell D, Chadee K. Survival strategies of Entamoeba histolytica: modulation of cell-mediated immune response. Parasitol Today. 1997;13:184–190. doi: 10.1016/s0169-4758(97)01022-3. [DOI] [PubMed] [Google Scholar]
- 5.Diamond L S, Harlow D R, Cunnick C C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 6.Hamelmann C, Foerster B, Burchard G D, Horstmann R D. Lysis of pathogenic and nonpathogenic Entamoeba histolytica by human complement: methodological analysis. Parasite Immunol. 1992;14:23–35. doi: 10.1111/j.1365-3024.1992.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs T, Bruchhaus I, Dandekar T, Tannich E, Leippe M. Isolation and molecular characterization of a surface-bound proteinase of Entamoeba histolytica. Mol Microbiol. 1998;27:269–276. doi: 10.1046/j.1365-2958.1998.00662.x. [DOI] [PubMed] [Google Scholar]
- 8.Leippe M. Spontaneous release of cysteine proteinases but not of pore-forming peptides by viable Entamoeba histolytica. Parasitology. 1995;111:569–574. doi: 10.1017/s0031182000077040. [DOI] [PubMed] [Google Scholar]
- 9.Luaces A L, Barrett A J. Affinity purification and biochemical characterization of histolysin, the major cysteine proteinase of Entamoeba histolytica. Biochem J. 1988;250:903–909. doi: 10.1042/bj2500903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro-Garcia F, Chavez-Duenas L, Tsutsumi V, Posadas del Rio F, Lopez-Revilla R. Entamoeba histolytica: increase of enterotoxicity and of 53- and 75-kDa cysteine proteinases in a clone of higher virulence. Exp Parasitol. 1995;80:361–372. doi: 10.1006/expr.1995.1048. [DOI] [PubMed] [Google Scholar]
- 11.Orozco E, Suarez M E, Sanchez T. Differences in adhesion, phagocytosis and virulence of clones from Entamoeba histolytica, strain HM1:IMSS. Int J Parasitol. 1985;15:655–660. doi: 10.1016/0020-7519(85)90012-8. [DOI] [PubMed] [Google Scholar]
- 12.Que X, Reed S L. The role of extracellular cysteine proteinases in pathogenesis of Entamoeba histolytica invasion. Parasitol Today. 1997;13:190–194. doi: 10.1016/s0169-4758(97)01043-0. [DOI] [PubMed] [Google Scholar]
- 13.Ravdin J I. Human infection by Entamoeba histolytica. In: Ravdin J I, editor. Amebiasis: human infection. New York, N.Y: John Wiley; 1988. pp. 166–176. [Google Scholar]
- 14.Reed S, Bouvier J, Pollack A S, Engel J C, Brown M, Hirata K, Que X, Eakin A, Hagblom P, Gillin F, et al. Cloning of a virulence factor of Entamoeba histolytica. J Clin Invest. 1993;91:1532–1540. doi: 10.1172/JCI116359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholze H, Frey S, Cejka Z, Bakker-Grunwald T. Evidence for the existence of both proteasomes and a novel high molecular weight peptidase in Entamoeba histolytica. J Biol Chem. 1996;271:6212–6216. doi: 10.1074/jbc.271.11.6212. [DOI] [PubMed] [Google Scholar]
- 16.Shibayama M, Campos-Rodriguez R, Ramirez-Rosales A, Flores-Romo L, Espinosa-Cantellano M, Martinez-Palomo A, Tsutsumi V. Entamoeba histolytica: liver invasion and abscess production by intraperitoneal inoculation of trophozoites in hamsters. Exp Parasitol. 1998;88:20–27. doi: 10.1006/expr.1998.4218. [DOI] [PubMed] [Google Scholar]
- 17.Stanley S L., Jr Role of the Entamoeba histolytica cysteine proteinase in amebic liver abscess formation in severe combined immunodeficient mice. Infect Immun. 1995;63:1587–1590. doi: 10.1128/iai.63.4.1587-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsutsumi V, Mena-López R, Anaya-Velázquez F, Martinez-Palomo A. Cellular bases of experimental amebic liver abscess formation. Am J Pathol. 1984;117:81–91. [PMC free article] [PubMed] [Google Scholar]
- 19.Tsutsumi V, Ramirez-Rosales A, Lanz-Mendoza H, Shibayama M, Chavez B, Rangel-Lopez E, Martinez-Palomo A. Entamoeba histolytica: erythrophagocytosis, collagenolysis, and liver abscess production as virulence markers. Trans R Soc Trop Med Hyg. 1992;86:170–172. doi: 10.1016/0035-9203(92)90555-q. [DOI] [PubMed] [Google Scholar]


