Abstract
Our previous work using 3T functional Magnetic Resonance Imaging (fMRI) parcellated the human dentate nuclei (DN), the primary output of the cerebellum, to three distinct functional zones each contributing uniquely to default-mode, salience-motor, and visual brain networks. In this perspective piece, we highlight the possibility to target specific functional territories within the cerebellum using non-invasive brain stimulation, potentially leading to the refinement of cerebellar-based therapeutics for precision psychiatry. Significant knowledge gap exists in our functional understanding of cerebellar systems. Intervening early, gauging severity of illness, developing intervention strategies and assessing treatment response, are all dependent on our understanding of the cerebello-cerebral networks underlying the pathology of psychotic disorders. A promising yet under-examined avenue for biomarker discovery is disruptions in cerebellar output circuitry. This is primarily because most 3T MRI studies in the past had to exclude cerebellum from the field of view due to limitations in spatiotemporal resolutions. Using recent technological advances in 7T MRI (e.g., parallel transmit head coils) to identify functional territories of the DN, with a focus on dentato-cerebello-thalamo-cortical (CTC) circuitry can lead to better characterization of brain-behavioral correlations and assessments of co-morbidities. Such an improved mechanistic understanding of psychiatric illnesses can reveal aspects of CTC circuitry that can aid in neuroprognosis, identification of subtypes, and generate testable hypothesis for future studies.
Keywords: cerebellum, psychotic disorder, functional connectivity, dentate nuclei, cerebello-thalamo-cortical circuitry
Introduction
Cerebellum's role in schizophrenia, led by Andreasen's work on the role of cerebello-thalamo-cortical (CTC) circuitry [“cognitive dysmetria” hypothesis (1, 2)], following Schmahmann's “dysmetria of thought” theory (3) is now widely acknowledged. Functional connectivity (FC) abnormalities have been reported in first-episode schizophrenia (4) and in individuals at clinical high risk (CHR) for psychosis (5–15). In spite of this mounting evidence, the FC of the dentate nuclei (DN), the primary “door-out” of the cerebellum is yet to be systematically investigated in psychotic disorders. The DN, clusters of neuronal bodies embedded in the white matter (WM) of the cerebellum (16), link the cerebellar cortex to the extracerebellar regions, and contributes to the modulation of many aspects of motor and non-motor behavior (17). However, significant knowledge gaps exist in our functional understanding of cerebellar systems (18) in imaging-based systems neuroscience. This can be attributed to spatiotemporal limitations in functional MRI, because prior to the advent of simultaneous multi-slice imaging (19), cerebellum was often excluded from the field of view. It remains to be established whether abnormalities in dentato-cerebellar functional connectivity (FC) precede the manifestation of symptoms in psychiatric diseases. Critically, we lack an effective predictive model of disease onset/progression that takes the dentato-CTC FC into account.
A recent study (20), acknowledged as a pivotal work (21), provides causal evidence for cerebellar dysfunction in schizophrenia. The basis of this advancement is on the premise that greater network-wide modulation could be achieved with cerebellar stimulation compared to cerebral cortical stimulation (22). A large and expanding body of evidence from tract tracing studies in rodents and monkeys (23–26) has revealed DN-thalamic and DN-cerebellar anatomical relations. To date, no study has comprehensively characterized dentato-CTC FC networks in humans. A further refined mechanistic picture underlying the development, regulation, and modulation of behaviors characterizing the pathophysiology in psychiatric and neurological diseases can be gained by utilizing a circuit level approach including the DN in cerebellar-focused investigations. In this perspective piece, we highlight some of the ways to go about gaining a refined mechanistic understanding of CTC circuity and improving our causal understanding of symptom amelioration in treatment strategies such as transcranial magnetic stimulation (TMS).
Probing dentato-CTC connectivity
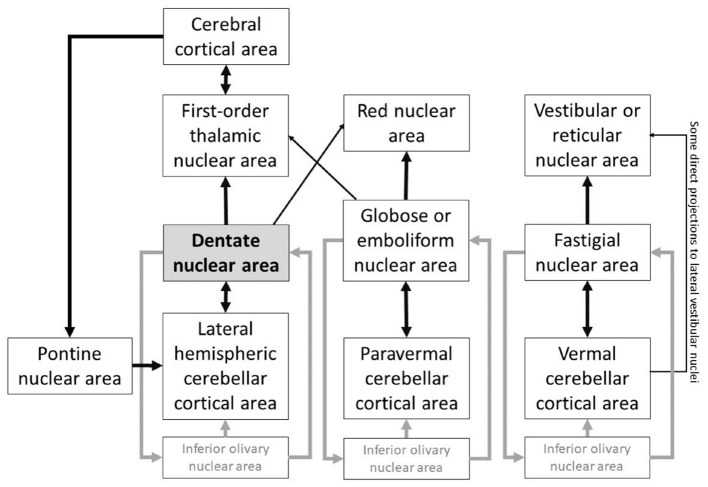
Heterogeneous FC arrangement of the cerebellar cortex with extracerebellar structures emerges from the backdrop of a homogenous cerebellar cortical cytoarchitecture. Anatomical connections between cerebellar cortex and extracerebellar territories engaged in cognition and affect form the basis of the neuroscience of cerebellar behavioral neurology and psychiatry (27). Improved understanding of the functional anatomy of DN provides a novel avenue to study CTC circuitry. The deep cerebellar nuclei have reciprocal connections with cerebellar cortical areas (28). The thalamic nuclei, that are anatomically linked with virtually all macroscale networks of brain organization, including dopaminergic pathways (29), receive connections from the DN and also have reciprocal projections with specific cerebral cortical areas (30). For example, DN stimulation modulates prefrontal dopamine (31), a neurotransmitter system implicated in working memory (32). Pontine nuclei receive connections from each cerebral cortical territory targeted by the DN (33), and serves as a “door-in” to the cerebellar cortex (34). These reverberating connections that link the dentate nuclei to the rest of the brain are part of the complex circuitry of the nuclei of the cerebellum (Figure 1), and establish the significance of the DN in cerebello-cerebral interactions.
Figure 1.
DN plays a central node in the cerebellar connections to other extracerebellar areas, and is part of a highly complex system of reverberating connections linking the nuclei of the cerebellum to the rest of the brain. Based on (28, 30, 34–40).
Characterization of functional territories in dentate nuclei in early psychosis
About one-third of individuals with clinical high-risk (CHR) for psychosis develop psychotic symptoms later on. For the reliable implementation of cerebellar-based therapeutics, a thorough understanding of dentate-cerebello-cerebral FC is imperative. DN, the largest and most lateral structure of the cerebellar nuclei system, receive projections from all aspects of the cerebellar cortex lateral to the paravermis (35). DN projects mainly to thalamus, connecting cerebellar cortex to thalamo-cortical projections, thus playing a central role in CTC circuitry. Multiple reverberating patterns exist in the connectivity between cerebellar cortex, cerebellar nuclei, and extracerebellar structures (Figure 1). These anatomical circuits establish DN as a central node in the cerebellar output circuitry, with functional specialization spanning the whole spectrum of primary, task-positive, and task-negative domains of brain function (41). The functional specialization in the DN echoes a similar set of macroscale divisions as that of the cerebral cortex. Default-mode processing [functional territory 1, FT1, in (41)] is the apex of the central axis of brain organization. Salience processing (FT2) is the cognitive opposite pole of default-mode processing, and is linked to sensorimotor control in the brain. Visual processing (FT3) is the unimodal opposite pole of sensorimotor function, and represents the third and last central component of human DN specialization. This continuous unimodal-to-transmodal view is not only a theoretical construct of cognitive science, but also an anatomical reality in the cerebral cortex (42).
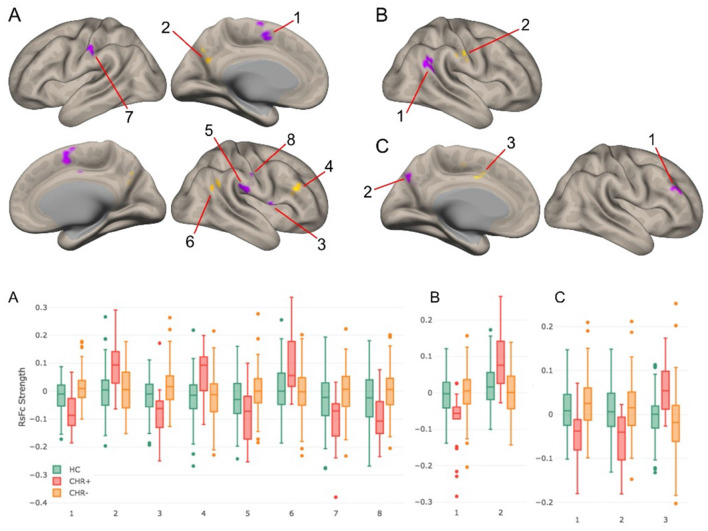
The functional parcellation of DN (41)can add precision to the selection of seed regions of interest in studies of psychotic disorders (43, 44). In Anteraper et al. (43), we analyzed 153 participants with CHR and 93 age-, sex-, and education-matched healthy controls (HC) in the Shanghai At Risk for Psychosis (SHARP) program. Twenty-three subjects converted to psychosis (CHR+) before the next clinical follow-up, a year later. There were no significant differences in baseline Structured Interview of Psychosis-risk Syndromes (SIPS) scores in CHR+ compared to those who did not develop psychosis (CHR-). While functional abnormalities were detected in all FTs of the DN, the DMN territory revealed more statistically significant differences compared to FT2 and FT3. Lack of anti-correlations between FT1 and DLPFC (cluster 4 in Figure 2) may indicate difficulties in executive control (43). Xie et al. (44) studied 92 patients and 86 controls, and reported that dentato-cerebello-cerebral FC abnormalities may contribute to schizophrenia symptoms and its pathophysiology.
Figure 2.
Top panel: RsFc results for CHR+ vs. CHR– contrast at a voxel-level height threshold of p < 0.005 (2-sided) and cluster size FDR corrected threshold of p < 0.05. (A–C) correspond to DMN, salience-motor, and visual functional territories of DN. Bottom panel: Bar plots for each of the significant clusters in the healthy control, CHR+, and CHR– groups. Notably, for each of these group contrasts, CHR– participants were not significantly different compared to healthy controls (43).
Improvement of our causal understanding of symptom amelioration in treatment strategies such as TMS
Precision medicine relies on the degree of our causal understanding of response to treatment. The prediction accuracy of disease status/sub-types can accelerate progress in this domain. Prediction of disease status prior to symptom manifestation (early detection) is another key component of this. Specific examples of how functional changes in brain network organization can be used for prediction has been reported previously in the context of conversion to psychosis (45). When combined with longitudinal behavioral measures, FC measures are emerging as promising biomarkers to understand vulnerability to predict clinical outcome in the prodromal stage of schizophrenia (46). Since the cerebellum is capable of operating in a compensatory role to restore function in response to insult, building on and leveraging non-invasive cerebellar-centric neuromodulation strategies (20) can gather a refined mechanistic understanding of brain response mechanisms in psychosis. This can aid in the development of personalized treatment approaches. DN stimulation may be achieved with TMS, although TMS will necessarily induce stimulation in the areas of cerebellar cortex located in between the stimulator and the DN, and will not achieve levels of spatial precision required to stimulate sub-regions of the DN. These two limitations, namely, the inability to avoid the cerebellar cortical surface, and the inability to stimulate specific DN sub-regions, may be both overcome by two emerging methods of non-invasive stimulation. Low-intensity focused ultrasound has shown spatially precise modulation of brain activity in deep brain regions located beneath the cerebral cortex, such as the amygdala (47). Temporally Interfering Electric Fields, another emerging method of non-invasive brain stimulation, has shown successful modulation of deep brain territories such as the hippocampus without affecting neighboring lateral or surface areas (48).
Future directions
Building upon our work, we envision that further exploration of the cerebellar output circuitry will generate valuable contributions to the field of translational and precision psychiatry. Novel gradient-based analysis strategies (49) can be used to complement existing brain mapping approaches (50), and to detect functional abnormalities in psychiatric disorders that may remain hidden using other methods of analysis (51, 52). Cerebellum's interplay with cerebral cortical dynamics is still poorly understood. New results have demonstrated that blocking/stimulating cerebellar cortical output through DN via the thalamus (CTC pathway) can modulate cerebral cortical dynamics as demonstrated by suppression/triggering of movement initiation (53). Extensive disruptions in CTC connectivity has been linked to “cognitive dysmetria” (1, 2) and increased risks for psychosis conversion (6, 7). Future research might investigate the possibility that some of these alterations may be specific to particular psychiatric disorders, while others may be linked to broader domains of psychopathology [as in (51)]. We recently estimated effective connectivity using spectral dynamic causal modeling (DCM) (54) in the Human Connectome Project dataset to examine cerebello-cerebral interactions indexed by FC between the cerebellar and cerebral cortex (55). This work supports the Universal Cerebellar Transform (UCT) theory, which posits that the neurological processes underlying cerebellar modulation of movement, thought and emotion (3, 56, 57) are the same. The existence of a UCT is a fundamental underpinning of the dysmetria of thought theory that may be further interrogated using DN-targeted non-invasive stimulation in the future. Lastly, emerging methods of non-invasive brain stimulation may allow the development of spatially precise targets within DN as tools for the treatment of disease and for the study of cerebellar functional anatomy.
Technological advances in MRI
Blood oxygenation level dependent (BOLD) MRI contrast to noise (CNR) ratio, which is directly proportional to temporal signal-to-noise ratio (tSNR), is remarkably better at ultra-high field strengths. When used in combination with parallel transmit head coils (58) and optimized pulse sequences (59), 7T can offer unprecedented improvements in tSNR and spatiotemporal resolution for fMRI (60). Superior BOLD CNR that comes with these technological advances can be used for investigating the full range of DN-cerebral, DN-thalamic and DN-cerebellar FC. The field of cerebellar functional neuroanatomy is emerging with novel theories, which include functional gradients that dictate the position and relationship between cerebellar FTs. Identifying FTs of the DN with 7T resting-state fMRI and using these functional parcels to better characterize functional abnormalities in cerebellar-linked neuropathology can thus generate valuable contributions to the field of cerebellar neuroscience and translational psychiatry.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Boards at Beth Israel Deaconess Medical Center and also by the Shanghai Mental Health Center. Written informed consent to participate in this study was not obtained since no human studies are present.
Author contributions
SA conceived the presented idea. SA and XG performed the data analysis and verified the analytical methods for the two publications that are highlighted in this perspective piece. SW-G contributed the data needed for the work and fueled the critical thinking needed to accomplish the work. All authors discussed the results and contributed to the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Andreasen NC, O'leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. (1996) 93:9985–90. 10.1073/pnas.93.18.9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasen NC, Paradiso S, O'leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. (1998) 24:203–18. 10.1093/oxfordjournals.schbul.a033321 [DOI] [PubMed] [Google Scholar]
- 3.Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. (1991) 48:1178–87. 10.1001/archneur.1991.00530230086029 [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Wei X, Hu N, Zhang W, Xiao Y, Zeng J, et al. Cerebello-thalamo-cortical hyperconnectivity classifies patients and predicts long-term treatment outcome in first-episode schizophrenia. Schizophr Bull. (2022) 48:505–13. 10.1093/schbul/sbab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. (2014) 24:3116–30. 10.1093/cercor/bht165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. (2015) 72:882–91. 10.1001/jamapsychiatry.2015.0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao H, Chen OY, Chung Y, Forsyth JK, Mcewen SC, Gee DG, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. (2018) 9:3836. 10.1038/s41467-018-06350-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YL, Tu PC, Lee YC, Chen YS, Li CT, Su TP. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr Res. (2013) 149:26–34. 10.1016/j.schres.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 9.Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, Van Den Heuvel MP. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. (2011) 2:73. 10.3389/fpsyt.2011.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W, Liu F, Chen J, Wu R, Zhang Z, Yu M, et al. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci Rep. (2015) 5:17275. 10.1038/srep17275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Zhang F, Liu F, Chen J, Wu R, Chen DQ, et al. Cerebellar abnormalities in first-episode, drug-naive schizophrenia at rest. Psychiatry Res Neuroimaging. (2018) 276:73–9. 10.1016/j.pscychresns.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 12.Kim DJ, Moussa-tooks AB, Bolbecker AR, Apthorp D, Newman SD, O'donnell BF, et al. Cerebellar-cortical dysconnectivity in resting-state associated with sensorimotor tasks in schizophrenia. Hum Brain Mapp. (2020) 41:3119–32. 10.1002/hbm.25002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. (2011) 69:967–73. 10.1016/j.biopsych.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinn AK, Baker JT, Lewandowski KE, Ongur D, Cohen BM. Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front Hum Neurosci. (2015) 9:134. 10.3389/fnhum.2015.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, et al. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. (2005) 128:2097–108. 10.1093/brain/awh556 [DOI] [PubMed] [Google Scholar]
- 16.Sultan F, Hamodeh S, Baizer JS. The human dentate nucleus: a complex shape untangled. Neuroscience. (2010) 167:965–8. 10.1016/j.neuroscience.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 17.Yu X, Ba W, Zhao G, Ma Y, Harding EC, Yin L, et al. Dysfunction of ventral tegmental area GABA neurons causes mania-like behavior. Mol Psychiatry. (2020). 10.1101/684142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voogd J. What we do not know about cerebellar systems neuroscience. Front Syst Neurosci. (2014) 8:227. 10.3389/fnsys.2014.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinberg DA, Setsompop K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J Magn Reson. (2013) 229:90–100. 10.1016/j.jmr.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brady RO, Jr, Gonsalvez I, Lee I, Ongur D, Seidman LJ, Schmahmann JD, et al. Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am J Psychiatry. (2019) 176:512–20. 10.1176/appi.ajp.2018.18040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao H, Cannon TD. Cerebellar dysfunction and schizophrenia: from “cognitive dysmetria” to a potential therapeutic target. Am J Psychiatry. (2019) 176:498–500. 10.1176/appi.ajp.2019.19050480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. (2014) 34:12049–56. 10.1523/JNEUROSCI.1776-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steele CJ, Anwander A, Bazin PL, Trampel R, Schaefer A, Turner R, et al. Human cerebellar sub-millimeter diffusion imaging reveals the motor and non-motor topography of the dentate nucleus. Cereb Cortex. (2017) 27:4537–48. 10.1093/cercor/bhw258 [DOI] [PubMed] [Google Scholar]
- 24.Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. (2010) 20:214–28. 10.1093/cercor/bhp091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Miyachi S, Ito Y, Nambu A, Takada M. Topographic distribution of output neurons in cerebellar nuclei and cortex to somatotopic map of primary motor cortex. Eur J Neurosci. (2007) 25:2374–82. 10.1111/j.1460-9568.2007.05482.x [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto M, Takahara D, Hirata Y, Inoue K, Miyachi S, Nambu A, et al. Motor and non-motor projections from the cerebellum to rostrocaudally distinct sectors of the dorsal premotor cortex in macaques. Eur J Neurosci. (2010) 31:1402–13. 10.1111/j.1460-9568.2010.07151.x [DOI] [PubMed] [Google Scholar]
- 27.Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. (2019) 42:337–64. 10.1146/annurev-neuro-070918-050258 [DOI] [PubMed] [Google Scholar]
- 28.Dietrichs E. The cerebellar corticonuclear and nucleocortical projections in the cat as studied with anterograde and retrograde transport of horseradish peroxidase. IV The paraflocculus Exp Brain Res. (1981) 44:235–42. 10.1007/BF00236560 [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-gonzalez MA, Garcia-cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. (2005) 25:6076–83. 10.1523/JNEUROSCI.0968-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. (1995) 187:583–92. [PMC free article] [PubMed] [Google Scholar]
- 31.Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. (2008) 62:544–50. 10.1002/syn.20525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froudist-walsh S, Bliss DP, Ding X, Rapan L, Niu M, Knoblauch K, et al. A dopamine gradient controls access to distributed working memory in the large-scale monkey cortex. Neuron. (2021) 109:3500–20.e13. 10.1016/j.neuron.2021.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. (1997) 41:31–60. 10.1016/S0074-7742(08)60346-3 [DOI] [PubMed] [Google Scholar]
- 34.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. (2003) 23:8432–44. 10.1523/JNEUROSCI.23-23-08432.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felten DL, O'banion MK, Maida MS, Netter FH. Netter's Atlas of Neuroscience. Philadelphia, PA: Elsevier; (2016). [Google Scholar]
- 36.De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. Microcircuitry and function of the inferior olive. Trends Neurosci. (1998) 21:391–400. 10.1016/S0166-2236(98)01310-1 [DOI] [PubMed] [Google Scholar]
- 37.Schmahmann JD, Pandya DN. Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. J Comp Neurol. (1989) 289:53–73. 10.1002/cne.902890105 [DOI] [PubMed] [Google Scholar]
- 38.Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol. (1991) 308:224–48. 10.1002/cne.903080209 [DOI] [PubMed] [Google Scholar]
- 39.Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. (1997) 17:438–58. 10.1523/JNEUROSCI.17-01-00438.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmahmann JD, Rosene DL, Pandya DN. Motor projections to the basis pontis in rhesus monkey. J Comp Neurol. (2004) 478:248–68. 10.1002/cne.20286 [DOI] [PubMed] [Google Scholar]
- 41.Guell X, D'mello AM, Hubbard NA, Romeo RR, Gabrieli JDE, Whitfield-gabrieli S, et al. Functional territories of human dentate nucleus. Cereb Cortex. (2020) 30:2401–17. 10.1093/cercor/bhz247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A. (2016) 113:12574–9. 10.1073/pnas.1608282113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anteraper SA, Guell X, Collin G, Qi Z, Ren J, Nair A, et al. Abnormal function in dentate nuclei precedes the onset of psychosis: a resting-state fmri study in high-risk individuals. Schizophr Bull. (2021) 47:1421–30. 10.1093/schbul/sbab038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y, Xi Y, Cui LB, Li C, Xu Y, Zhang Y, et al. Altered functional connectivity of the dentate nuclei in patients with schizophrenia. Schizophr Res. (2021) 233:16–23. 10.1016/j.schres.2021.06.035 [DOI] [PubMed] [Google Scholar]
- 45.Collin G, Seidman LJ, Keshavan MS, Stone WS, Qi Z, Zhang T, et al. Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol Psychiatry. (2020) 25:2431–40. 10.1038/s41380-018-0288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collin G, Nieto-castanon A, Shenton ME, Pasternak O, Kelly S, Keshavan MS, et al. Brain functional connectivity data enhance prediction of clinical outcome in youth at risk for psychosis. Neuroimage Clin. (2020) 26:102108. 10.1016/j.nicl.2019.102108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry JF, et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron. (2019) 101:1109–16.e5. 10.1016/j.neuron.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell. (2017) 169:1029–41.e16. 10.1016/j.cell.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guell X. Functional gradients of the cerebellum: a review of practical applications. Cerebellum. (2021). 10.1007/s12311-021-01342-8. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Essen DC, Ugurbil K. The future of the human connectome. Neuroimage. (2012) 62:1299–310. 10.1016/j.neuroimage.2012.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong D, Guell X, Genon S, Wang Y, Chen J, Eickhoff SB, et al. Linking cerebellar functional gradients to transdiagnostic behavioral dimensions of psychopathology. Neuroimage Clin. (2022) 36:103176. 10.1016/j.nicl.2022.103176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong D, Luo C, Guell X, Wang Y, He H, Duan M, et al. Compression of cerebellar functional gradients in schizophrenia. Schizophr Bull. (2020) 46:1282–95. 10.1093/schbul/sbaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dacre J, Colligan M, Clarke T, Ammer JJ, Schiemann J, Chamosa-pino V, et al. A cerebellar-thalamocortical pathway drives behavioral context-dependent movement initiation. Neuron. (2021) 109:2326–38.e8. 10.1016/j.neuron.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Razi A, Kahan J, Rees G, Friston KJ. Construct validation of a DCM for resting state fMRI. Neuroimage. (2015) 106:1–14. 10.1016/j.neuroimage.2014.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bukhari Q, Ruf SF, Guell X, Whitfield-gabrieli S, Anteraper S. Interaction between cerebellum and cerebral cortex, evidence from dynamic causal modeling. Cerebellum. (2021) 21:225–233. 10.1007/s12311-021-01284-1 [DOI] [PubMed] [Google Scholar]
- 56.Guell X, Gabrieli JDE, Schmahmann JD. Embodied cognition and the cerebellum: perspectives from the dysmetria of thought and the universal cerebellar transform theories. Cortex. (2018) 100:140–8. 10.1016/j.cortex.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 57.Guell X, Hoche F, Schmahmann JD. Metalinguistic deficits in patients with cerebellar dysfunction: empirical support for the dysmetria of thought theory. Cerebellum. (2015) 14:50–8. 10.1007/s12311-014-0630-z [DOI] [PubMed] [Google Scholar]
- 58.Wu X, Auerbach EJ, Vu AT, Moeller S, Van De Moortele PF, Yacoub E, et al. Human connectome project-style resting-state functional MRI at 7 Tesla using radiofrequency parallel transmission. Neuroimage. (2019) 184:396–408. 10.1016/j.neuroimage.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. (2010) 63:1144–53. 10.1002/mrm.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raimondo L, Oliveira LAF, Heij J, Priovoulos N, Kundu P, Leoni RF, et al. Advances in resting state fMRI acquisitions for functional connectomics. Neuroimage. (2021) 243:118503. 10.1016/j.neuroimage.2021.118503 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.