Abstract
Serum levels of soluble tumor necrosis factor alpha receptor I (sTNF-RI) were elevated in patients with lepromatous (LL) reactional-state type II leprosy, and sTNF-RII levels were increased in patients with full tuberculoid (TT) or LL type II leprosy. The sTNF-R in sera from patients with type II leprosy, but not other forms of leprosy, inhibited recombinant TNF cytolytic activities in vitro. This suggests that sTNF-R regulatory activities are partially impaired in patients with leprosy.
Leprosy is a chronic infectious disease which presents clinically as a spectrum of symptoms associated with the immune response to the causative pathogen, Mycobacterium leprae. Histologically, the bacteria are focused in lesions which vary from well-organized granulomas containing few mycobacterial organisms at the tuberculoid (TT) end of the spectrum to less-organized lesions where bacilli are found in great numbers at the lepromatous (LL) end. Between the TT and LL poles, tissue responses may present borderline forms (borderline tuberculoid [BT], borderline [BB], and borderline lepromatous [BL]). The development of reactional states in leprosy represent detrimental corollaries of the immune response due to either alterations in cellular immunity (type I reactions) or excessive formation of immune complexes at the sites of antigen deposition (type II reactions).
Marked tumor necrosis factor (TNF) release by macrophages in reactional states indicates that TNF mediates immunopathologic effects and tissue damage in leprosy patients (4, 5, 7, 8, 18, 19). TNF-induced cellular activities are provided by the receptors TNF receptor I (TNF-RI; 55 kDa) and TNF-RII (75 kDa), which can be proteolytically cleaved from the cell surface and found in soluble TNF-R (sTNF-R) forms in serum (2, 11, 16). These sTNF-R forms compete for TNF and thus block or stabilize activities of the latter (2, 17). To determine the potential role of sTNF-R in leprosy forms, we analyzed the levels of sTNF-RI and sTNF-RII and their blocking activities in sera from leprosy patients.
Sera from healthy individuals without any history of mycobacterial infection were obtained from regions of Amazonas State, Brazil, where leprosy is endemic. Sera from leprosy patients (4 females and 32 males; mean age, 31.4 ± 5.6 years) undergoing or not undergoing multidrug therapy (Ofloxacin Multicenter Field Trial) and diagnosed in accordance with the Ridley-Jopling classification (12) were obtained after the patients provided informed consent at the Institute Alfredo da Matta. Peripheral blood obtained through venipuncture was incubated for 2 h at 4°C to achieve complete coagulation and then centrifuged (1,000 × g, 4°C), and serum was stored at −20°C before use.
The biological activity of TNF in sera was measured by cytolytic assays using L929 cells and human recombinant TNF (hTNF; kindly provided by G. R. Adolf, Ernst-Boehringer-Institut, Vienna, Austria) for standard curve values (sensitivity of the assay, 10 pg/ml) (2). Sera from patients with TT, LL, and type II (LL and BL form) leprosy showed a significant increase in TNF titers, in contrast to patients with type I leprosy (BL form) (P < 0.01; Mann-Whitney U test comparison with levels in healthy donors) (Fig. 1). Lower TNF levels in BL type I patients may be due to intrinsic immune response defects in these patients, high levels of TNF inhibitors, or deficient production of cytokines involved in the induction of TNF, such as gamma interferon (7, 10).
FIG. 1.
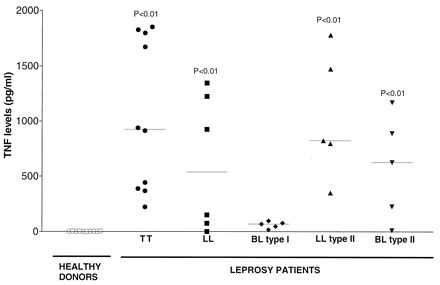
Levels of TNF in sera from healthy donors and leprosy patients (with TT and LL forms and type I and type II reactional states of the disease). The levels of bioactive TNF in human sera were analyzed by cytotoxic assays using L929 cells. Results are expressed as horizontal bars that indicate median values. P values indicate results that are significantly different from results for healthy donors (P < 0.05; Mann-Whitney U test).
We then analyzed the levels of sTNF-RI and sTNF-RII in human sera by an enzyme-linked immunosorbent assay. Briefly, 96-well plates (Nunc, Wiesbaden, Germany) were incubated with inhibitory monoclonal antibodies (MAb) diluted in coating buffer against anti-hTNF-RI (clone 16803.1; 1 μg/ml) (R&D Systems, Wiesbaden-Nordenstadt, Germany) or anti-hTNF-RII (clone 22221.311; 1 μg/ml) (R&D Systems). After washing and blocking of wells, diluted human serum (1:3) was added and the wells were incubated at 23°C for 4 h. The wells were washed, polyclonal goat anti-hTNF-RI (375 ng/ml) or anti-hTNF-RII (750 ng/ml) affinity-purified antibodies (all from R&D Systems) were added, and the wells were incubated for 1 h. The reaction was visualized by adding peroxidase-conjugated mouse anti-goat immunoglobulin G antibodies (1 μg/ml; Dianova, Hamburg, Germany), followed by phenylenediamine-dihydroxide (Sigma, Deisenhofen, Germany) in citrate buffer (pH 5.0), for 10 min. The concentration of sTNF-RI or sTNF-RII was calculated by using a standard curve generated with recombinant hTNF-RI (rTNF-RI) or rTNF-RII (R&D Systems) (sensitivity of the assay, 300 pg/ml). Levels of sTNF-RI were increased only in sera from patients with LL type II reactional-state leprosy, whereas levels of sTNF-RII were elevated in sera from patients with TT and type II reactional-state forms (LL and BL) of the disease, in comparison to levels in healthy donors (P < 0.05) (Fig. 2). It is important to note that no correlation was detected between the levels of TNF and sTNF-RI or sTNF-RII or between the levels of sTNF-RI and sTNF-RII in sera from leprosy patients, suggesting that induction of TNF-RI and TNF-RII are not coregulated.
FIG. 2.
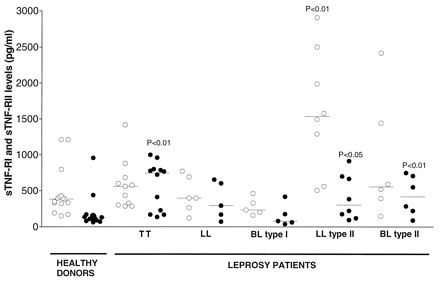
Levels of sTNF-RI and sTNF-RII in sera from healthy donors and leprosy patients (with TT and LL forms and type I and type II reactional states of the disease). The amount of sTNF-RI (○) or sTNF-RII (•) in human sera was analyzed by an enzyme-linked immunosorbent assay. Results are expressed as horizontal bars that indicate median values. P values indicate results that are significantly different from results for healthy donors (P < 0.05; Mann-Whitney U test).
Since sTNF-R binds to and regulates TNF activities (2), we investigated the ability of sTNF-R in human sera to inhibit rTNF functions in vitro (1). The cytocidal activity of TNF was analyzed on L929 cells incubated for 12 h in the presence of rTNF (1 μg/ml) alone or together with diluted serum samples in phosphate-buffered saline (1:10), with or without anti-hTNF-RII and anti-hTNF-RII inhibitory MAb (5 μg/ml; R&D Systems). The percentage of lysis was calculated as follows: % lysis = [(test OD − spontaneous OD)/(OD of sample with 1 μg of rTNF per ml − spontaneous OD)] × 100, where OD is optical density. Figure 3 shows that sera from five patients with type II reactional-state leprosy (LL form) inhibited, at different levels, TNF cytotoxicity (mean ± standard deviation = 40% ± 3%), in contrast to sera from healthy subjects or patients with the TT, LL, or BL type I reactional-state leprosy. In addition, anti-hTNF-R MAb abolished the inhibitory effects of sTNF-R, confirming the specific serum activity of sTNF-R. When mouse rTNF (10 pg/ml; Genzyme Corp., Cambridge, Mass.) was used instead, type II patient sTNF-R decreased mouse rTNF activity four- to fivefold (data not shown) (9).
FIG. 3.
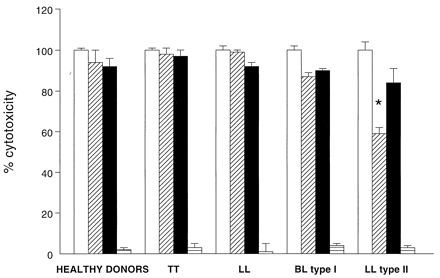
Inhibition of TNF activity by sTNF-R in sera from healthy donors and leprosy patients (with TT and LL forms and BL type I and LL type II reactional states of the disease). The capacity of sTNF-R to inhibit rTNF cytolytic activity (1 μg/ml) was analyzed on L929 cells incubated in the presence of rTNF (□), rTNF plus human sera (▨), rTNF plus human sera together with anti-TNF-RI and anti-TNF-RII MAb (■), or human sera plus anti-TNF-RI and anti-TNF-RII MAb (▤). Results of three independent experiments are expressed as means ± standard deviations of percentages of cytotoxicity. ✶, significantly different (P < 0.05) from values for healthy donors (Mann-Whitney U test).
Our data show increased levels of sTNF-RI in patients with LL type II leprosy and of sTNF-RII in patients with TT and LL type II leprosy, and treatment of these patients with chemotherapy does not seem to change the results. The sTNF-R from the LL type II form, but not from other forms of the disease, inhibited TNF activities in vitro. It is known that the affinity of TNF is higher for sTNF-RII than for sTNF-RI (10). Consistent with this knowledge, elevated levels of sTNF-RII observed in TT patients could regulate TNF activities in vivo. In contrast, it seems that increased sTNF-RII levels in type II patients do not regulate TNF activities in vivo, possibly because sTNF-RII bound to TNF at elevated titers in serum or because functions of sTNF-RII, but not sTNF-RI, were altered in vitro and thus failed to inhibit TNF activities. We assume that in vitro TNF inhibitory effects observed with serum from type II patients is dependent on elevated serum levels of sTNF-RI, which may have better function in vitro than sTNF-RII. This event may be relevant in type II reactional states of the disease.
TNF is a short-range cytokine, and sTNF-Rs make TNF available for systemic effects (2, 15). High concentrations of sTNF-R are found in cases of severe inflammatory diseases, which may counteract harmful effects of TNF (1, 3, 6, 13). However, a 10- to 100-fold molar excess of sTNF-R over TNF is required for complete inhibition of TNF activity (10). Thus, increased levels of TNF detected in sera from patients with reactional-state type II leprosy (Fig. 1) (14) may result from insufficient amounts of inhibitory sTNF-R and/or an increased pool of stabilized sTNF-R–TNF complexes leading to the harmful effects seen in this form of leprosy.
In summary, our data suggest that regulation of TNF activities in leprosy type II reactional states is not as efficient as it is in other leprosy forms. This is consistent with the elevated and harmful TNF effects seen in type II reactional states. Further studies are necessary to correlate levels of sTNF-R with severity of reactional states in leprosy.
Acknowledgments
We thank Silvia Chotzen for excellent technical assistance; G. R. Adolf, Ernst-Boehringer-Institut, for hTNF; and Gert Hausdorf, Humboldt University, Berlin, Germany, for helpful discussions.
Financial support from the German Leprosy Relief Association is gratefully acknowledged.
REFERENCES
- 1.Aderka D, Wysenbeek A, Engelmann H, Cope A P, Brennan F, Molad Y, Hornik V, Levo Y, Maini R N, Feldmann M, Wallach D. Correlation between serum levels of soluble tumor necrosis factor receptor and disease activity in systemic lupus erythematosus. Arthritis Rheum. 1993;36:1111–1120. doi: 10.1002/art.1780360812. [DOI] [PubMed] [Google Scholar]
- 2.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cope A P, Aderka D, Doherty M, Engelmann H, Gibbons D, Jones A C, Brennan F M, Maini R N, Wallach D, Feldmann M. Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 1992;35:1160–1169. doi: 10.1002/art.1780351008. [DOI] [PubMed] [Google Scholar]
- 4.Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- 5.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Girardin E, Roux-Lombard P, Grau G E, Suter P, Gallati H, Dayer J M. Imbalance between tumour necrosis factor-alpha and soluble TNF receptor concentrations in severe meningococcaemia. The J5 Study Group. Immunology. 1992;76:20–23. [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 8.Khanolkar-Young S, Rayment N, Brickell P M, Katz D R, Vinayakumar S, Colston M J, Lockwood D N. Tumour necrosis factor-alpha (TNF-alpha) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis M, Tartaglia L A, Lee A, Bennett G L, Rice G C, Wong G H, Chen E Y, Goeddel D V. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loetscher H, Gentz R, Zulauf M, Lustig A, Tabuchi H, Schlaeger E J, Brockhaus M, Gallati H, Manneberg M, Lesslauer W. Recombinant 55-kDa tumor necrosis factor (TNF) receptor. Stoichiometry of binding to TNF alpha and TNF beta and inhibition of TNF activity. J Biol Chem. 1991;266:18324–18329. [PubMed] [Google Scholar]
- 11.Porteu F, Brockhaus M, Wallach D, Engelmann H, Nathan C F. Human neutrophil elastase releases a ligand-binding fragment from the 75-kDa tumor necrosis factor (TNF) receptor. Comparison with the proteolytic activity responsible for shedding of TNF receptors from stimulated neutrophils. J Biol Chem. 1991;266:18846–18853. [PubMed] [Google Scholar]
- 12.Ridley D S, Jopling W H. Classification of leprosy according to immunity. A five-group system. Int J Lepr. 1966;34:255–273. [PubMed] [Google Scholar]
- 13.Roux-Lombard P, Punzi L, Hasler F, Bas S, Todesco S, Gallati H, Guerne P A, Dayer J M. Soluble tumor necrosis factor receptors in human inflammatory synovial fluids. Arthritis Rheum. 1993;36:485–489. doi: 10.1002/art.1780360408. [DOI] [PubMed] [Google Scholar]
- 14.Sarno E N, Grau G E, Vieira L M, Nery J A. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin Exp Immunol. 1991;84:103–108. [PMC free article] [PubMed] [Google Scholar]
- 15.Spinas G A, Keller U, Brockhaus M. Release of soluble receptors for tumor necrosis factor (TNF) in relation to circulating TNF during experimental endotoxinemia. J Clin Invest. 1992;90:533–536. doi: 10.1172/JCI115891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartaglia L A, Goeddel D V. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 17.Van Zee K J, Kohno T, Fischer E, Rock C S, Moldawer L L, Lowry S F. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 19.Yamamura M, Wang X H, Ohmen J D, Uyemura K, Rea T H, Bloom B R, Modlin R L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–1475. [PubMed] [Google Scholar]


