The pervasiveness of viral infectious agents, capable of parasitizing living organisms all along the evolutionary spectrum, has obligated hosts to evolve defense mechanisms that control viral infection. Elucidation of host–pathogen interactions in model organisms has been fundamental to the translational understanding of human antiviral immunity. Lower organisms such as unicellular prokaryotes and eukaryotes have been shown to act as viral hosts and, in many cases, overcome infection via primitive immune mechanisms. The most widely studied example of this is the CRISPR/Cas system—a restriction mechanism in bacteria and archaea against highly specific viruses known as bacteriophages [1]. This seminal discovery has led to important advances in genetic engineering and has increased interest in viruses that infect unicellular organisms, notably those that are pathogenic to humans, and has led to an emerging role of viruses in this triangular host–pathogen interaction [2].
Viral infection of lower eukarya: Introduction to endosymbiotic interactions
The first unicellular eukaryotic virus was discovered fortuitously in Entamoeba histolytica, after several reports of viral-like particles in electron micrographs of protist ultrastructure [2,3]. Since this discovery, a remarkable variety of viruses has been reported in all major subgroups of unicellular eukaryotic microorganisms [2].
Studies in lower organisms have been key to our current understanding of viral infectious agents. The discovery of nucleocytoplasmic large double-stranded DNA viruses in the amoeba Acanthamoeba, for example, shifted the definition of viral genomes to account for increased complexity and genomic plasticity [4].
To enable their survival, pathogens have 2 effective options for infection: The first is to kill their host and spread rapidly, while the second is to coexist within their host. This introduces the novel concept that not all viruses are deleterious to their hosts [5,6]. In fact, many lower eukaryotic viruses are recognized as endosymbionts, often either existing neutrally within their host organisms or providing them with greater resistance to environmental stressors. The mutualistic host–pathogen interaction between the eukaryotic cell and its virus can therefore provide an evolutionary advantage to both organisms, explaining in part the ubiquity of such infections [6]. This mutualism can be illustrated by the yeast Saccharomyces cerevisiae, which utilizes toxins of viral endosymbiotic origin to eliminate competing yeast colonies, while being protected through competitive inhibition by its preprotoxin [7]. Thus, the yeast gains a fitness advantage provided by the virus, while the virus can utilize translational machinery [6]. Similar instances of virus-enabled gain of function are omnipresent throughout the biosphere, yet, many of these interactions remain uncharacterized.
Double-stranded RNA viruses of protozoan parasites: Drivers of pathology
The recent advancement of imaging, molecular, and sequencing technologies has enabled researchers to systematically search for, then characterize, endogenous viruses or virus-like particles in a wide array of unicellular protozoa (Table 1) [8]. This group of parasites is of particular interest as it encompasses several neglected tropical diseases, accounting for millions of new infections, deaths, and disability-adjusted life years worldwide [9].
Table 1. Viral endosymbionts of protozoa can affect parasitic pathogenesis.
| Protozoon | Viral endosymbiont | Virus type | Impact on parasite | Effect on parasitic pathogenesis | Proposed mechanism of action |
|---|---|---|---|---|---|
| Cryptosporidium spp. | Cryspovirus (Csp1) [10] | Partitiviridae (dsRNA) | Up-regulation of parasitic fecundity [10]. | Increase | Unknown |
| Trichomonas vaginalis | TVV [11] | Totiviridae (dsRNA) |
Modulation of parasitic metabolism and immunogenicity [12]. | Increase | Interaction with host TLR3 proinflammatory signaling [13] |
| Leptomonas seymouri | Lepsey NLV1 [14] | Narnaviridae (ssRNA+) | Unknown | Unknown | Unknown |
| Phytomonas spp. | PserNV1 [15] | Narnaviridae (ssRNA+) | Unknown | Unknown | Unknown |
| Giardia spp. | GLV [16] | Totiviridae (dsRNA) |
Unknown | None | Unknown |
| Leishmania (Viannia) | LRV1 [17] | Totiviridae (dsRNA) |
Modulation of parasitic metabolism, virulence, and immunogenicity. [18,19] | Increase | Interaction with host TLR3 proinflammatory signaling and modulation of the NLRP3 inflammasome. [17] |
| Leishmania (Leishmania) | LRV2 [20] | Totiviridae (dsRNA) |
Unknown | Unknown | Unknown |
dsRNA, double-stranded RNA; GLV, Giardia lamblia virus; LRV1, Leishmania RNA virus 1; LRV2, Leishmania RNA virus 2; NLV1, narna-like virus 1; PserNV1, Pser Narna virus 1; TLR3, Toll-like receptor 3; TVV, Trichomonas vaginalis virus.
Cryptosporidium, for example, the causative agent of the severe parasitic diarrhoeal infection cryptosporidiosis, can be infected by a bi-segmented double-stranded RNA (dsRNA) viral agent coined Cryspovirus (CspV1) that has been positively correlated to parasitic fecundity, underscoring a potential role of the virus in the apicomplexan’s fitness [10].
Among viruses, Totiviridae seem to be of particular importance as it encompasses most viral endosymbionts identified in pathogenic protozoa. This viral family has evolved significant diversity, with closely related viruses identified in almost all genera of yeasts, fungi, and protozoa studied [5]. This can be explained, in part, by their circumvention of a lytic infectious phase, in favor of long-term symbiotic persistence [21]. Viruses of this family are non-enveloped and contain uncapped dsRNA genomes. Intriguingly, viral endosymbionts of the Totiviridae family have been associated to exacerbated pathology in the context of parasitic infection, despite being noninfectious to mammalian hosts [21].
This phenomenon was first observed in Trichomonas vaginalis, the protozoon responsible for the sexually transmitted infection trichomoniasis. Infection of the parasite with Trichomonas vaginalis virus (TVV) has been shown to induce differential expression of parasitic virulence factors, notably an increase of the major surface immunogenic virulence factor P270, which aids in evasion of the host immune response [11,12]. TVV has also been reported to induce mammalian host Toll-like receptor 3 (TLR3) proinflammatory signaling, causing exacerbation of trichomoniasis lesions—associated to preterm birth and HIV susceptibility [12,13]. Another example of an infected protozoon is Giardia, which causes the parasitic diarrhoeal infection giardiasis and its virus, Giardia lamblia virus (GLV) [16]. Similarly, this interaction is persistent, yet no correlation to pathogenesis has been observed.
Flagellates of the Trypanosomatiadae family seem to be particularly affected by RNA viral infection, with genetically diverse RNA viruses, including several Totiviridae, characterized in Leptomonas seymouri, Phytomonas spp., and Leishmania spp., among others [8,22–24]. While little remains known as to the impact of viral infection on other trypanosomatids, the interaction between Leishmania RNA virus (LRV) and Leishmania is a flagrant and clinically relevant example of effective mutualism and viral-driven parasitic pathogenicity [2].
Leishmania and Leishmania RNA virus: Viral infection and hyperpathogenesis
The vector-borne infection leishmaniasis is caused by the intracellular protozoan Leishmania. Characterized as a neglected tropical disease, this sandfly-transmitted infection can present clinically in 3 distinct forms. While cutaneous leishmaniasis (CL) presents as self-limiting dermal lesions, parasites can metastasize to the mucosa, marking progression to the destructive mucocutaneous (MCL) pathological form [25]. Along with the life-threatening and systemic visceral leishmaniasis (VL), these account for an overall annual burden of about 2 million clinical infections and 30,000 deaths [26].
The cytoplasmic viral endosymbiont LRV, of the Totiviridae family, has been identified as a major driver of leishmaniasis severity and has been associated to progression of CL to MCL, drug treatment failure, and disease relapse [27,28]. The virus is also suspected to contribute to treatment resistance in Leishmania/HIV coinfection [2]. Leishmania RNA virus 1 (LRV1) is frequently identified in species of the Viannia subgenus, in particular in Leishmania v. guyanensis strains endemic to the amazon [29]. This subgenus has highly conserved RNA interference pathways, which was initially thought help establish a balance between viral replication and RNAi-mediated silencing, maintaining viremia under a threshold that would be detrimental to the parasite. However, the more recent discovery of the closely related Leishmania RNA virus 2 (LRV2) within the Leishmania Leishmania subgenera, which lacks functional Argonaute and Dicer, has brought this mechanism into question [27,29,30].
In the absence of its viral endosymbiont, Leishmania establishes infection and chronicity through immunogenic silence. With tropism towards macrophages, neutrophils, and dendritic cells, the parasite inhibits NLRP3 inflammasome activation within host cells—an important component of the antiparasitic response [25]. Leishmania parasites have evolved multiple synergistic mechanisms for immune evasion via inflammasome inhibition. These include the up-regulation of the host protein A20, which is involved in the inhibition of pro-IL-1β maturation and negative regulation of NF-kB [31]. This will then hinder downstream activation of the IL-1 receptor and the MyD88 adaptor protein, both necessary for triggering parasitotoxic oxidative stress [19]. Other mechanisms include GP63-dependent cleavage of inflammasome components and down-regulation of inflammasome gene transcription, such as caspase-1, which all contribute to the modulation of the host’s antiparasitic response [17,19,32]. Thus, Leishmania can circumvent the host immune response long enough to establish a high parasitic load, resulting in tissue damage and lesion progression [25]. In fact, leishmaniasis severity is inversely correlated to inflammasome activation [17].
It follows that LRVs themselves can modulate this immune suppression, capable of dampening inflammasome activation by inhibition of caspase-1 and IL-1β cleavage, while inducing proinflammatory cytokines such as TNFα and IL-12 [17]. Intriguingly, LRVs can simultaneously act as strong innate immunogens, with activation of host endosomal TLR3 signaling in response to the viral dsRNA genome. This triggers TRIF-dependent signaling that contributes to inflammasome inhibition and production of type I IFN, leading to host cell autophagy [17]. In addition, IFN-β production inhibits superoxide-related killing of Leishmania, thus conferring additional fitness to the parasite [33]. Therefore, it is LRV that causes the hyperinflammatory phenotype and increased parasitic resistance that is a hallmark of MCL [34].
LRV transmission and extracellular vesicles: Novel insight into host–pathogen interaction and mechanisms of infection
Extracellular transmission of Totiviridae is rare, previously only documented in GLV, with other viruses in the family presumed to be solely transmitted vertically during host cellular division [33]. Recently, however, it has been shown that the viral endosymbiont LRV1 can be horizontally transmitted between Leishmania parasites of the Viannia subgenus via the parasite’s endosomal sorting complex required for transport (ESCRT) or exosomal pathway [18]. Although utilization of different aspects of the host exosomal pathway had previously been reported in mammalian viruses such as HIV, EBV, and HCV, this discovery is the first of its kind within protozoa [18].
Exosomes are nanosized extracellular vesicles, containing biologically active macromolecules (RNA, proteins, lipids), and are constitutively produced by eukaryotic cells, primarily for intercellular communication [35]. Leishmania exosomes themselves have been reported to play an important role in parasitic virulence, notably via their enrichment in the major surface metalloprotease GP63, which influences several key secondary messengers, contributing to pathology via aforementioned signaling cascades [19,35].
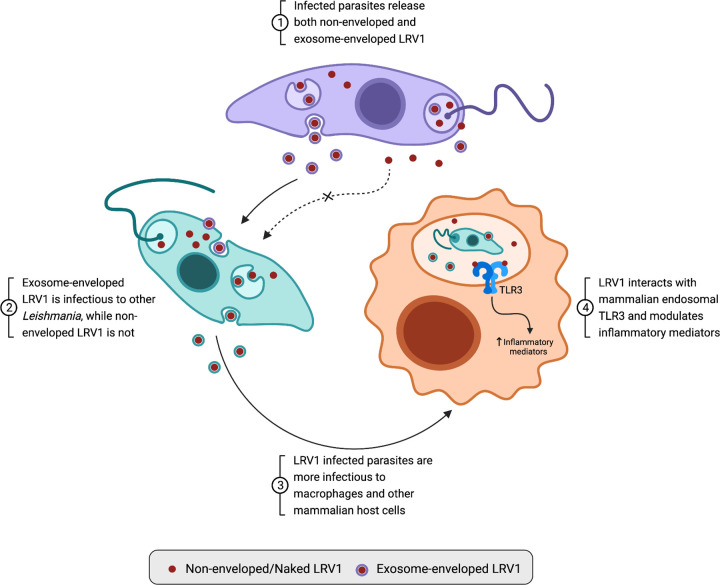
In the context of LRV1 infection, the virus hijacks the parasite’s exosomal pathway to become encapsulated within an extracellular vesicle, creating a viral envelope that protects the virion from hostile extracellular environments, and enables rapid endocytic uptake by surrounding parasites [25]. Strikingly, viruses shed from the parasite through non-exosomal pathways in the flagellar pocket lack this envelope and are unable to infect other parasites, highlighting the key role of leishmanial exosomes in LRV1 pathogenesis [18] (summarized in Fig 1).
Fig 1. Leishmanial exosomal pathway hijacking by LRV1 enables viral transmission and induces TLR3-mediated hyperpathogenesis in mammalian hosts.
LRV1, Leishmania RNA virus 1; TLR3, Toll-like receptor 3.
While the natural host of LRV1 is Leishmania (Viannia) guyanensis, the exosome-enveloped virus can, in fact, infect and persist within the closely related species Leishmania (Viannia) panamensis, while it is rapidly eliminated from the species Leishmania (Leishmania) mexicana [18]. The infection of Leishmania v. panamensis therefore provides a model by which the roles of the virus and the parasite can be untangled in the context of Leishmania/LRV1 coinfection. In fact, LRV1-infected Leishmania panamensis was shown to induce greater severity of lesions within a murine model, comparatively to its uninfected counterpart, highlighting the role of the virus in leishmanial hyperpathogenesis [25]. Evidence that LRV1 infection modulates the leishmanial translational machinery, effectively hijacking ribosomes, indicates that the virus may play a much more complex role in the parasite’s virulence and fitness [18]. Additionally, this same study showed that LRV1-infected Leishmania v. panamensis has the capacity to control the virus to undetectable levels over a 10-week time span, suggesting primitive immune machinery [18].
Future directions in the study of Leishmania and Leishmania RNA virus
While little is currently known surrounding the evolution of complex antiviral immunity, studying protozoa infected by viral endosymbionts may provide insight ancestral immune mechanisms that enable viral control.
Additionally, increasing treatment resistance for different leishmaniases underscores the need for novel therapies and/or prophylaxis for parasitic infection. Considering the significant burden of LRV-positive Leishmania isolates—up to 70% in certain endemic regions—along the virus’ association to treatment failure, LRVs are an obvious target [36]. A significant decrease in leishmaniasis lesion swelling and parasitic load following the immunization of C57BL/6 mice with LRV1 capsid proteins indicates the potential to target a viral endosymbiont to decrease parasitic hyperpathogenicity and generate protective immunity [36]. Thus, this novel vaccine strategy should be further explored as it could potentially be adapted for other Totiviridae of pathogenic protozoa.
Overall, investigation of viral endosymbionts of lower eukarya is of utmost clinical and biological importance in the field of infectious disease.
Funding Statement
MO is supported by the Canadian Institutes of Health Research (CIHR, grant number PJT-159765) and the The Natural Sciences and Engineering Research Council of Canada (NSERC). AL is supported by a MSc studentship from Fonds de Recherche du Québec en Santé (FRQS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science. 2012;337:6096. doi: 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow P, Dujardin JC, Fasel N, Greenwood AD, Osterrieder K, Lomonossoff G, et al. Viruses of protozoan parasites and viral therapy: Is the time now right? Virol J. 2020;17:142. doi: 10.1186/s12985-020-01410-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond LS, Mattern CFT, Bartgis IL. Viruses of Entamoeba histolytica I. Identification of Transmissible Virus-Like Agents. J Virol. 1972;9:326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colson P, La Scola B, Raoult D. Giant Viruses of Amoebae: A Journey Through Innovative Research and Paradigm Changes. Annu Rev Virol. 2017;4:61–85. doi: 10.1146/annurev-virology-101416-041816 [DOI] [PubMed] [Google Scholar]
- 5.Bruenn JA. 7—Viruses of Fungi and Protozoans: Is Everyone Sick? In: Hurst CJ, editor. Viral Ecology. San Diego: Academic Press; 2000. p. 297–317. doi: 10.1016/B978-012362675-2/50008-2 [DOI] [Google Scholar]
- 6.Jagdale SS, Joshi RS. Enemies with benefits: mutualistic interactions of viruses with lower eukaryotes. Arch Virol. 2018;163:821–830. doi: 10.1007/s00705-017-3686-5 [DOI] [PubMed] [Google Scholar]
- 7.Marquina D, Santos A, Peinado J. Biology of killer yeasts. Int Microbiol. 2002;5:65–71. doi: 10.1007/s10123-002-0066-z [DOI] [PubMed] [Google Scholar]
- 8.Grybchuk D, Akopyants NS, Kostygov AY, Konovalovas A, Lye L-F, Dobson DE, et al. Viral discovery and diversity in trypanosomatid protozoa with a focus on relatives of the human parasite Leishmania. Proc Natl Acad Sci U S A. 2018;115:E506–E515. doi: 10.1073/pnas.1717806115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra AK, Mawson AR. Neglected Tropical Diseases: Epidemiology and Global Burden. Trop Med Infect Dis. 2017;2:36. doi: 10.3390/tropicalmed2030036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkins MC, Higgins J, Abrahante JE, Kniel KE, O’Brien C, Trout J, et al. Fecundity of Cryptosporidium parvum is correlated with intracellular levels of the viral symbiont CPV. Int J Parasitol. 2008;38:1051–1055. doi: 10.1016/j.ijpara.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 11.He D, Pengtao G, Ju Y, Jianhua L, He L, Guocai Z, et al. Differential Protein Expressions in Virus-Infected and Uninfected Trichomonas vaginalis. Korean J Parasitol. 2017;55:121–128. doi: 10.3347/kjp.2017.55.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoshnan A, Alderete JF. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J Virol. 1994;68:4035–4038. doi: 10.1128/JVI.68.6.4035-4038.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Gayar EK, Mokhtar AB, Hassan WA. Molecular characterization of double-stranded RNA virus in Trichomonas vaginalis Egyptian isolates and its association with pathogenicity. Parasitol Res. 2016;115:4027–4036. doi: 10.1007/s00436-016-5174-3 [DOI] [PubMed] [Google Scholar]
- 14.Lye L-F, Akopyants NS, Dobson DE, Beverley SM. A Narnavirus-Like Element from the Trypanosomatid Protozoan Parasite Leptomonas seymouri. Genome Announc. 2016;4:e00713–e00716. doi: 10.1128/genomeA.00713-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akopyants NS, Lye L-F, Dobson DE, Lukeš J, Beverley SM. A Narnavirus in the Trypanosomatid Protist Plant Pathogen Phytomonas serpens. Genome Announc. 2016;4:e00711–e00716. doi: 10.1128/genomeA.00711-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang AL, Wang CC. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986;21:269–276. doi: 10.1016/0166-6851(86)90132-5 [DOI] [PubMed] [Google Scholar]
- 17.de Carvalho RVH, Lima-Junior DS, da Silva MVG, Dilucca M, Rodrigues TS, Horta CV, et al. Leishmania RNA virus exacerbates Leishmaniasis by subverting innate immunity via TLR3-mediated NLRP3 inflammasome inhibition. Nat Commun. 2019;10:5273. doi: 10.1038/s41467-019-13356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atayde VD, da Silva Lira Filho A, Chaparro V, Zimmermann A, Martel C, Jaramillo M, et al. Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nat Microbiol. 2019;4:714–723. doi: 10.1038/s41564-018-0352-y [DOI] [PubMed] [Google Scholar]
- 19.Hartley M-A, Eren RO, Rossi M, Prevel F, Castiglioni P, Isorce N, et al. Leishmania guyanensis parasites block the activation of the inflammasome by inhibiting maturation of IL-1β. Microb Cell Graz Austria. 2018;5:137–149. doi: 10.15698/mic2018.03.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleschenko Y, Grybchuk D, Matveeva NS, Macedo DH, Ponirovsky EN, Lukashev AN, et al. Molecular Characterization of Leishmania RNA virus 2 in Leishmania major from Uzbekistan. Genes. 2019;10:830. doi: 10.3390/genes10100830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillman BI, Cohen AB. Totiviruses (Totiviridae)1. In: Bamford DH, Zuckerman M, editors. Encyclopedia of Virology (Fourth Edition). Oxford: Academic Press; 2021. p. 648–657. doi: [DOI] [Google Scholar]
- 22.Sukla S, Roy S, Sundar S, Biswas S. Leptomonas seymouri narna-like virus 1 and not leishmaniaviruses detected in kala-azar samples from India. Arch Virol. 2017;162:3827–3835. doi: 10.1007/s00705-017-3559-y [DOI] [PubMed] [Google Scholar]
- 23.Dollet M, Marche S, Gargani D, Muller E, Baltz T. Virus of Plant Trypanosomes (Phytomonas Spp.). In: Nicole M, Gianinazzi-Pearson V, editors. Histology, Ultrastructure and Molecular Cytology of Plant-Microorganism Interactions. Dordrecht: Springer Netherlands; 1996. p. 227–236. doi: 10.1007/978-94-009-0189-6_13 [DOI] [Google Scholar]
- 24.Adaui V, Lye L-F, Akopyants NS, Zimic M, Llanos-Cuentas A, Garcia L, et al. Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania braziliensis in Peru and Bolivia. J Infect Dis. 2016;213:112–121. doi: 10.1093/infdis/jiv354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivier M, Zamboni DS. Leishmania Viannia guyanensis, LRV1 virus and extracellular vesicles: a dangerous trio influencing the faith of immune response during muco-cutaneous leishmaniasis. Curr Opin Immunol. 2020;66:108–113. doi: 10.1016/j.coi.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 26.Kevric I, Cappel MA, Keeling JH. New World and Old World Leishmania Infections: A Practical Review. Dermatol Clin. 2015;33:579–593. doi: 10.1016/j.det.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 27.Brettmann EA, Shaik JS, Zangger H, Lye L-F, Kuhlmann FM, Akopyants NS, et al. Tilting the balance between RNA interference and replication eradicates Leishmania RNA virus 1 and mitigates the inflammatory response. Proc Natl Acad Sci U S A. 2016;113:11998–12005. doi: 10.1073/pnas.1615085113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Masina S, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331. doi: 10.1126/science.1199326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kariyawasam R, Lau R, Valencia BM, Llanos-Cuentas A, Boggild AK. Leishmania RNA Virus 1 (LRV-1) in Leishmania (Viannia) braziliensis Isolates from Peru: A Description of Demographic and Clinical Correlates. Am J Trop Med Hyg. 2020;102:280–285. doi: 10.4269/ajtmh.19-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abtahi M, Eslami G, Cavallero S, Vakili M, Hosseini SS, Ahmadian S, et al. Relationship of Leishmania RNA Virus (LRV) and treatment failure in clinical isolates of Leishmania major. BMC Res Notes. 2020;13:126. doi: 10.1186/s13104-020-04973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giri S, Shaha C. Leishmania donovani parasite requires Atg8 protein for infectivity and survival under stress. Cell Death Dis. 2019;10:1–19. doi: 10.1038/s41419-019-2038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shio MT, Christian JG, Jung JY, Chang K-P, Olivier M. PKC/ROS-Mediated NLRP3 Inflammasome Activation Is Attenuated by Leishmania Zinc-Metalloprotease during Infection. PLoS Negl Trop Dis. 2015;9:e0003868. doi: 10.1371/journal.pntd.0003868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartley M-A, Ronet C, Zangger H, Beverley SM, Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol. 2012;2. doi: 10.3389/fcimb.2012.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronet C, Beverley SM, Fasel N. Muco-cutaneous leishmaniasis in the New World. Virulence. 2011;2:547–552. doi: 10.4161/viru.2.6.17839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atayde VD, Hassani K, da Silva Lira Filho A, Borges AR, Adhikari A, Martel C, et al. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell Immunol. 2016;309:7–18. doi: 10.1016/j.cellimm.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 36.Castiglioni P, Hartley M-A, Rossi M, Prevel F, Desponds C, Utzschneider DT, et al. Exacerbated Leishmaniasis Caused by a Viral Endosymbiont can be Prevented by Immunization with Its Viral Capsid. PLoS Negl Trop Dis. 2017;11:e0005240. doi: 10.1371/journal.pntd.0005240 [DOI] [PMC free article] [PubMed] [Google Scholar]