Supplemental Digital Content is Available in the Text.
BACKGROUND
OnabotulinumtoxinA safety and efficacy are well established for upper facial lines (UFL), including forehead lines (FHL), glabellar lines (GL), and crow's feet lines (CFL).
OBJECTIVE
To investigate the association of onabotulinumtoxinA efficacy with patient-reported psychological impacts and satisfaction in UFL.
MATERIALS AND METHODS
A pooled analysis of data from 4 pivotal Phase 3 trials (onabotulinumtoxinA vs placebo in FHL ± GL, FHL + GL ± CFL, CFL, and CFL + GL for ≤180 days) evaluated investigator-assessed ≥1-grade severity improvement on the Allergan Facial Wrinkle Scale at Day 30 (responders). Facial Line Outcomes (FLO-11) Questionnaire, Facial Line Satisfaction Questionnaire (FLSQ), and Subject Assessment of Satisfaction of Appearance (SASA) were used to evaluate responder appearance-related psychological impacts and satisfaction.
RESULTS
OnabotulinumtoxinA patients, by primary study focus (FHL, GL, or CFL), totaled 921, 921, and 833, respectively; 786 patients received placebo. Most patients were female, White, and aged 45 to 50 years (median). Through 150 days, >42% FHL, >43% GL, and ≥32% CFL patients were onabotulinumtoxinA responders. Responders reported improvements in appearance-related psychological impacts (FLO-11) and high satisfaction (FLSQ and SASA), sustained through ≥150 days.
CONCLUSION
A ≥1-grade improvement with onabotulinumtoxinA is a clinically meaningful outcome in UFL, associated with long-lasting improved patient-reported psychological impacts and high satisfaction.
Three facial regions that have been identified among patients' top 5 priorities for aesthetic treatment are forehead lines (FHL), glabellar lines (GL; frown lines between the brows), and lateral canthal lines [i.e., crow's feet lines (CFL)].1,2 Collectively described as upper facial lines (UFL), their appearance is known to negatively affect self-esteem, self-perception, and mood; interfere with social interactions; and alter perceptions by others about emotional status and age.3–5 The most commonly used treatment for UFL is neurotoxin injections,6 typically performed during a single visit.7
OnabotulinumtoxinA (Botox Cosmetic; Allergan Aesthetics, an AbbVie Company, Madison, NJ) is indicated for temporary improvement in the appearance of moderate to severe FHL associated with frontalis muscle activity, moderate to severe GL associated with corrugator and/or procerus muscle activity, and moderate to severe CFL associated with orbicularis oculi activity.8 The US FDA approval for GL was based on the results of 2 prospective clinical studies demonstrating efficacy and safety of onabotulinumtoxinA for reducing the severity of GL.9,10 FDA approval for the CFL and FHL indications was based on the results of 4 Phase 3 studies demonstrating the efficacy, safety, and tolerability of onabotulinumtoxinA for reducing the appearance of UFL; the primary efficacy end point in these studies was at least a 2-grade improvement in wrinkle severity.11–14 Efficacy and safety data on onabotulinumtoxinA for improving the appearance of UFL are well established, including patient-reported psychological impact and satisfaction15,16; however, less is known regarding patient perception of treatment among those who achieved at least a 1-grade improvement.
The objective of this analysis was to investigate the relationship between investigator-assessed ≥1-grade improvement in UFL severity with onabotulinumtoxinA and patient-reported psychological impact and satisfaction, using data from 4 Phase 3 registration studies.
Methods
Study Design
This analysis is based on the pooled data from 4 randomized, double-blind, placebo-controlled, multicenter studies of onabotulinumtoxinA (Figure 1), detailed methods of which have been described previously.11–14 The 4 studies consisted of 2 studies investigating FHL treatment in combination with GL treatment (Studies 142 and 143) and 2 investigating CFL treatment with or without GL treatment (Studies 098 and 099). The duration of follow-up assessments varied across the studies (Figure 1).
Figure 1.
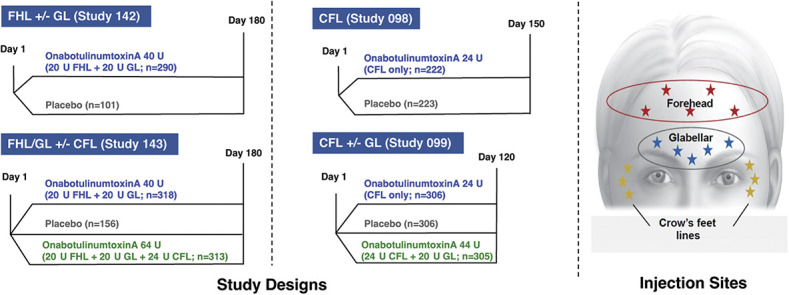
Phase 3 study designs and injection sites: Studies 098,11 099,12 142,13 and 143.14 CFL, crow's feet lines; FHL, forehead lines; GL, glabellar lines.
Study 142 evaluated onabotulinumtoxinA treatment in toxin-naive patients with moderate to severe FHL and GL; patients were randomized 3:1 to receive onabotulinumtoxinA 40 U, distributed between the frontalis muscle (20 U) and glabellar complex (20 U) or placebo, with follow-up assessments performed at Weeks 1 and 2 and Days 30, 60, 90, 120, 150, and 180.13
Study 143 evaluated onabotulinumtoxinA treatment in toxin-naive patients with moderate to severe FHL, GL, and CFL; patients were randomized 2:2:1 to receive onabotulinumtoxinA 40 U, distributed between the frontalis muscle (20 U) and glabellar complex (20 U), with or without simultaneous treatment of the orbicularis oculi muscle to address lines in the lateral canthal areas (24 U), or placebo. Follow-up assessments were performed at Weeks 1 and 2 and Days 30, 60, 90, 120, 150, and 180.14
Study 098 evaluated onabotulinumtoxinA treatment of the orbicularis oculi muscle in toxin-naive patients with moderate to severe, bilaterally symmetrical CFL, randomized 1:1 to receive onabotulinumtoxinA 24 U or placebo; follow-up assessments were performed at Weeks 1 and 2 and Days 30, 60, 90, and 120, with study exit at Day 150.11
Study 099 evaluated onabotulinumtoxinA treatment in toxin-naive patients with moderate to severe GL and moderate to severe, bilaterally symmetrical CFL randomized 1:1:1 to receive onabotulinumtoxinA 44 U (20 U to the GL area; 24 U to CFL areas), 24 U (placebo in the GL area; 12 U per side to CFL areas), or placebo (GL and CFL areas); follow-up assessments were performed at Weeks 1 and 2 and Days 30, 60, 90, and 120.12
Analyses
Responders
Data from Studies 142 and 143 were pooled to assess the effects of onabotulinumtoxinA treatment for FHL and GL. Similarly, data from Studies 098 and 099 were pooled to assess the effects of onabotulinumtoxinA treatment for CFL. Responders were defined as patients who received onabotulinumtoxinA and had an investigator-assessed ≥1-grade improvement from baseline in FHL or GL severity (Studies 142 and 143) or CFL severity (Studies 098 and 099) based on the Allergan Facial Wrinkle Scale (FWS) at the Day 30 visit. The Allergan FWS is a 4-point photonumeric scale, where 0 = none, 1 = mild, 2 = moderate, and 3 = severe. The facial wrinkle score was measured at maximum eyebrow elevation in the FHL studies, at maximum frown in the GL studies, and at maximum smile in the CFL studies.11–14
Appearance-Related Psychological Impact and Patient Satisfaction
Three validated patient-reported outcome (PRO) measures were used to evaluate patient psychological impact and satisfaction with onabotulinumtoxinA treatment in FHL, GL, and CFL responders: the Facial Line Outcomes (FLO-11) Questionnaire, the Facial Line Satisfaction Questionnaire (FLSQ), and the Subject Assessment of Satisfaction of Appearance (SASA).16
The FLO-11 Questionnaire,17 designed to assess patient perception of appearance-related psychological impacts of UFL, was used in all 4 studies. Responses were graded on an 11-point numeric rating scale ranging from 0 (not at all) to 10 (very much), where higher scores indicate greater negative impact. Items 1 (bothered by facial lines), 4 (looking older than actual age), and 5 (looking less attractive) were specified as additional efficacy measures in Studies 142 and 143 and are therefore the focus in this analysis.16 Only responders who had a ≥3-point improvement in CFL or FHL impact from baseline or a ≥4-point improvement in GL impact from baseline were included; these responder definitions are in reference to anchor-based meaningful change analyses conducted for each UFL separately (Data on File, AbbVie).
FLSQ follow-up Items 4 and 518 were used in the FHL and GL studies (Studies 142 and 143) to evaluate patient satisfaction with receiving a natural look (Item 4) and to assess overall satisfaction with treatment (Item 5). These items were chosen as the focus for this analysis because they are validated for use as single items; in addition, Item 4 assesses one of the most relevant UFL concepts as reported by patients (Data on File, AbbVie), and Item 5 is currently addressed in the product label for the FHL indication. Responses were graded on a 5-point Verbal Descriptor Scale (VDS) ranging from −2 (very dissatisfied) to +2 (very satisfied). Responders were defined as those who reported that they were very satisfied or mostly satisfied.
The SASA is a PRO measure used in the CFL studies (Studies 098 and 099) in which patients graded their level of satisfaction with their appearance on a 5-point VDS ranging from 1 (very unsatisfied) to 5 (very satisfied). Responders were defined as those who reported that they were very satisfied or satisfied.
Statistical Analyses
Analyses were performed on the pooled intent-to-treat (ITT) population for each measure. Results were summarized with descriptive statistics (mean, SD, number, and percent). No imputation was applied for missing values. p values were determined by Cochran–Mantel–Haenszel (CMH) tests stratified by investigator site. p values for treatment-by-investigator interaction were derived from Breslow–Day homogeneity of the odds ratio test.
Results
Across the studies, most patients were female, White, and between the ages of 45 and 50 years (See Supplemental Digital Content, Table S1, http://links.lww.com/DSS/B143). Between 48% and 69% of patients had severe UFL at maximum contraction.
Forehead Lines and Glabellar Lines Responders (Studies 142 and 143)
The pooled analysis of the effects of treatment on FHL and GL contained 313 patients who received onabotulinumtoxinA 64 U (for FHL, GL, and CFL), 608 patients who received onabotulinumtoxinA 40 U (for FHL and GL), and 257 patients who received placebo.
Forehead Lines Responders
At Day 30, ≥98% of patients who had received onabotulinumtoxinA in either dose group demonstrated an investigator-assessed ≥1-grade improvement from baseline in FHL severity on the Allergan FWS (referred to as responders), compared with only 13% of patients who received placebo. Nearly 65% of patients demonstrated a ≥1-grade improvement in FHL severity lasting 120 days post-treatment, and more than 42% of patients maintained a ≥1-grade improvement in FHL severity for 150 days post-treatment in both onabotulinumtoxinA dose groups (See Supplemental Digital Content, Figure S1, http://links.lww.com/DSS/B138). Improvement in FHL severity persisted through Day 180 post-treatment in more than 23% of responders.
Appearance-Related Forehead Lines Psychological Impact and Patient Satisfaction
Patient-reported improvements from baseline scores on psychological impacts of FHL remained higher after onabotulinumtoxinA treatment than with placebo at all time points through 180 days for responders in both dose groups, as measured by FLO-11 Items 1, 4, and 5 (See Supplemental Digital Content, Table S2, http://links.lww.com/DSS/B144). More than 81% of responders reported that they were mostly or very satisfied with the effect of onabotulinumtoxinA treatment on FHL up to 180 days post-treatment (Figure 2A), and more than 80% of responders reported that they were mostly or very satisfied with the natural effect of onabotulinumtoxinA treatment on FHL up to 180 days post-treatment (Figure 2B).
Figure 2.
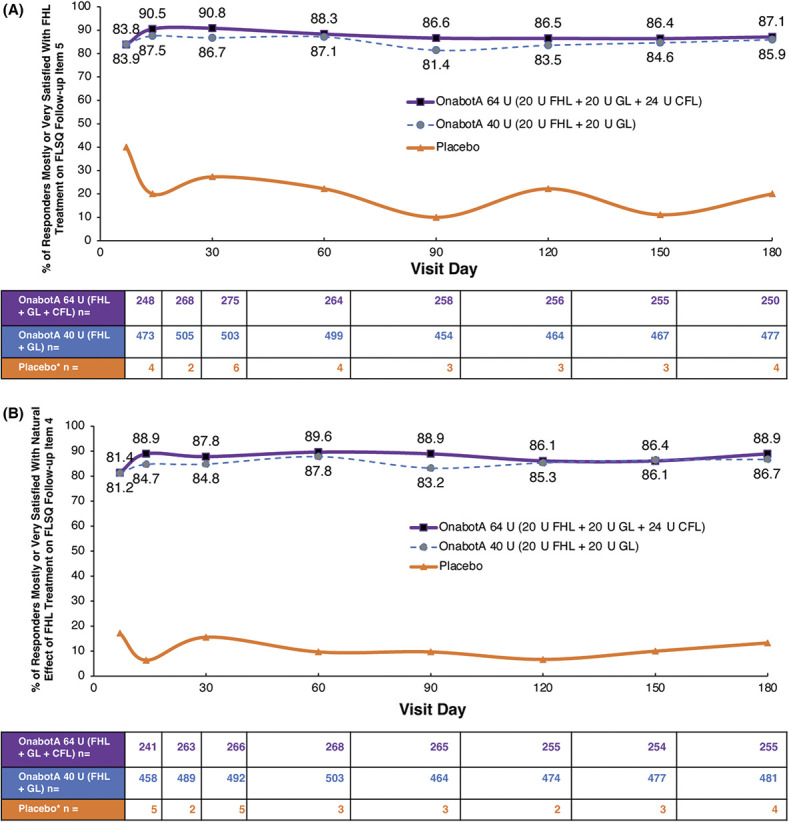
Satisfaction with treatment for FHL. (A) Satisfaction with effect of onabotA treatment on FHL: FLSQ follow-up Item 5 (overall satisfaction with treatment). (B) Satisfaction with the natural effect of onabotA treatment on FHL: FLSQ follow-up Item 4 (satisfaction with receiving a natural look). Responders were patients who received onabotA and had investigator-assessed ≥1-grade improvement from baseline in FHL severity on the FWS at Day 30 (pooled 142/143 studies). *Fewer than 7 patients in the placebo group had a reported ≥1-grade improvement from BL in FHL severity on the FWS at Day 30. BL, baseline; CFL, crow's feet lines; FHL, forehead lines; FLSQ, Facial Line Satisfaction Questionnaire; FWS, Facial Wrinkle Scale; GL, glabellar lines; onabotA, onabotulinumtoxinA.
Glabellar Lines Responders
At Day 30, ≥95% of patients who had received onabotulinumtoxinA (in either dose group) demonstrated an investigator-assessed ≥1-grade improvement from baseline in GL severity on the Allergan FWS (responders), compared with only 14% of patients who received placebo. More than 63% of patients demonstrated a ≥1-grade improvement in GL severity lasting 120 days post-treatment, and more than 43% of patients maintained a ≥1-grade improvement in GL severity for 150 days post-treatment in both onabotulinumtoxinA dose groups (See Supplemental Digital Content, Figure S2, http://links.lww.com/DSS/B139). Improvement in GL severity persisted through Day 180 post-treatment in more than 26% of responders.
Appearance-Related Glabellar Lines Psychological Impact and Patient Satisfaction
Patient-reported improvements from baseline scores on psychological impacts of GL remained higher after onabotulinumtoxinA treatment (both dose groups) than with placebo at all time points through 150 days for FLO-11 Item 5 and 180 days for FLO-11 Items 1 and 4 (See Supplemental Digital Content, Table S2, http://links.lww.com/DSS/B144). More than 80% of responders reported that they were mostly or very satisfied with the effect of onabotulinumtoxinA treatment on GL up to 180 days post-treatment in both dosage groups (Figure 3). Similarly, more than 80% of responders reported that they were mostly or very satisfied with the natural effect of onabotulinumtoxinA treatment on GL up to 180 days post-treatment (See Supplemental Digital Content, Figure S3, http://links.lww.com/DSS/B140).
Figure 3.
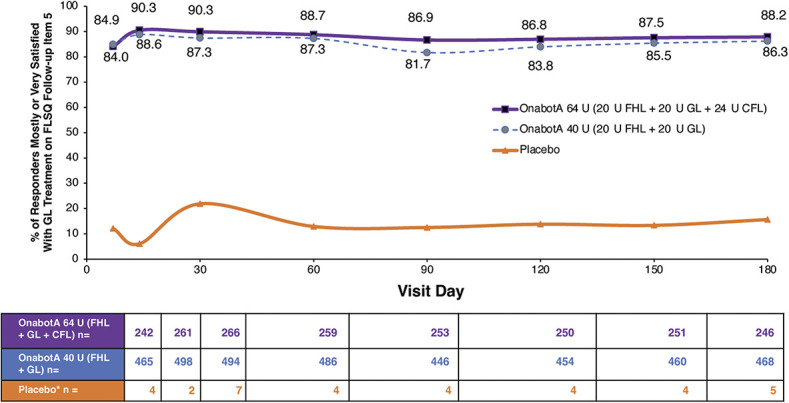
Satisfaction with treatment for GL. FLSQ follow-up Item 5 (overall satisfaction with treatment). Responders were patients who received onabotA and had investigator-assessed ≥1-grade improvement from baseline in GL severity on the FWS at Day 30 (pooled 142/143 studies). *Fewer than 8 patients in the placebo group had a reported ≥1-grade improvement from BL in FHL severity on the FWS at Day 30. BL, baseline; CFL, crow's feet lines; FHL, forehead lines; FLSQ, Facial Line Satisfaction Questionnaire; FWS, Facial Wrinkle Scale; GL, glabellar lines; onabotA, onabotulinumtoxinA.
Crow's Feet Lines (Studies 098 and 099)
The pooled analysis of the effects of treatment on CFL contained 305 patients who received onabotulinumtoxinA 44 U (20 U to GL and 24 U to CFL), 528 patients who received onabotulinumtoxinA 24 U (CFL only), and 529 patients who received placebo.
Crow's Feet Lines Responders
At Day 30, ≥82% of patients who had received onabotulinumtoxinA (either dose group) had an investigator-assessed ≥1-grade improvement from baseline in CFL severity on the Allergan FWS (responders), compared with only 11% of patients who received placebo. More than 32% of patients achieved a ≥1-grade improvement in CFL severity for up to 150 days post-treatment with onabotulinumtoxinA (See Supplemental Digital Content, Figure S4, http://links.lww.com/DSS/B141). Studies 098 and 099 evaluated patients up to Day 150 and 120, respectively11,12; therefore, duration of response through Day 180 was not evaluated as was performed for Studies 142 and 143 (Figure 1).
Appearance-Related Crow's Feet Lines Psychological Impact and Patient Satisfaction
Patient-reported improvements from baseline scores on psychological impacts of CFL remained higher after onabotulinumtoxinA treatment than with placebo at all time points through Day 90 for Items 1 and 5 and through Day 60 for Item 4 (See Supplemental Digital Content, Table S2, http://links.lww.com/DSS/B144). Nearly 30% of responders were satisfied or very satisfied with their appearance for up to 150 days post-treatment with onabotulinumtoxinA (both dose groups) as assessed by SASA (See Supplemental Digital Content, Figure S5, http://links.lww.com/DSS/B142).
Discussion
This subanalysis of pooled data from 4 Phase 3 clinical studies showed that an investigator-assessed ≥1-grade improvement on a 4-point scale in UFL severity at Day 30 was accompanied by patient-assessed improvements in psychological impact and high patient satisfaction after a 1-time onabotulinumtoxinA treatment that lasted throughout the study duration (up to 5 months for CFL and GL and up to 6 months for FHL and GL).
In the pooled 6-month, double-blind, placebo-controlled phase of the FHL studies,13,14 more than 97% of patients demonstrated an investigator-assessed ≥1-grade improvement in FHL severity from baseline at Day 30 (primary time point) after onabotulinumtoxinA treatment; after 150 days post-treatment, almost 50% of patients still retained this improvement. These findings corresponded with higher FLO-11 response rates, indicating improvements in appearance-related psychological impact (i.e., feeling bothered, looking older than actual age, and looking less attractive) with onabotulinumtoxinA treatment to the FHL (20 U, in combination with 20 U to GL with or without 24 U to CFL) than with placebo at all time points through 180 days. Moreover, greater than 80% of FHL responders reported that they were mostly or very satisfied with the effect of treatment and with the natural effect of treatment with onabotulinumtoxinA across all time points through 180 days (6 months) post-treatment. Similar results for both psychological impact and satisfaction were demonstrated with the pooled 6-month GL analysis.
In the pooled 4- and 5-month, double-blind, placebo-controlled phase of the CFL studies,11,12 ≥82% of patients demonstrated an investigator-assessed ≥1-grade improvement in CFL severity from baseline at Day 30 (primary time point) after onabotulinumtoxinA treatment; after 150 days post-treatment, more than 32% of patients still retained this improvement. FLO-11 response rates were higher with onabotulinumtoxinA treatment versus placebo through Day 90 for Items 1 (feeling bothered) and 5 (looking less attractive) and through Day 60 for Item 4 (looking older than actual age) after treatment. Almost 30% of CFL responders who received onabotulinumtoxinA 24 U for their CFL were satisfied or very satisfied with their appearance for up to 150 days (5 months).
The results of the current study are consistent with data from a clinical trial of another botulinum toxin A formulation associating efficacy and duration of effect with high patient satisfaction with treatment of GL and other facial lines. In this Phase 4 study, abobotulinumtoxinA 50 U was reported to provide ≥1-grade improvement in investigator-assessed GL severity on a validated 4-point photographic scale, along with high patient satisfaction for up to 4 months.19
The current findings emphasize that a 1-grade improvement in UFL severity—a seemingly minor change to some—has a substantial impact on patient satisfaction and corresponds with favorable improvements in psychological impacts as reported by patients with validated outcome measures. Altogether, simultaneous treatment of all 3 UFL (FHL, GL, and CFL) with onabotulinumtoxinA can result in lasting, clinically meaningful improvements for up to 180 days (6 months) based on investigator- and patient-reported outcomes.
Limitations
All studies included in the analyses enrolled only treatment-naive patients; thus, the results may not apply to patients with previous exposure to botulinum toxin. The double-blind phases of the studies also varied in length: Studies 142 and 143 were 180 days, Study 098 was 150 days, and Study 099 was 120 days. Therefore, data regarding persistence of response through the full 6-month period were not obtained across all studies (the maximum follow-up period in 2 of the studies was 4 and 5 months).
Not all studies used the same satisfaction questionnaires. The CFL studies were originally conducted between 2010 and 2011, whereas the FHL studies were conducted between 2014 and 2016; at the time of the CFL studies, the FLSQ had not been validated and so was not used. Differences in rating scales and questionnaires used to evaluate efficacy by other manufacturers, as well as product noninterchangeability, make it difficult to compare and contrast the findings of this study with similar data on other botulinum toxin A formulations. In addition, the simultaneous treatment of all 3 areas (FHL, GL, and CFL) is likely associated with a higher level of patient satisfaction compared with treatment of 1 or 2 areas; however, only a subset of patients (in Study 143) received simultaneous UFL treatment. To this end, clinical outcomes, including patient-reported psychological impact and satisfaction, may be further improved by evaluating safety and efficacy studies in which all 3 UFL areas are treated simultaneously. It is worthwhile to note that, as of this writing, onabotulinumtoxinA is the only neurotoxin approved for the treatment of all 3 types of UFL.
Conclusion
Treatment of UFL (FHL, GL, and CFL) with onabotulinumtoxinA leads to long-lasting, clinically meaningful outcomes based on both investigator- and patient-reported outcomes from 4 studies ranging from 4 to 6 months' duration. This pooled analysis demonstrates that a ≥1-grade improvement with onabotulinumtoxinA as assessed by investigators is a clinically meaningful outcome in UFL because it is associated with improved psychological impact and high patient satisfaction for up to 6 months.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
This study was funded by Allergan Aesthetics, an AbbVie Company, Dublin, Ireland. Editorial support for this article was provided by Regina Kelly, MA of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by Allergan Aesthetics, an AbbVie Company.
J. L. Cohen: consultant for Allergan/AbbVie. Steven Fagien: clinical investigator and consultant for Allergan/AbbVie, Patricia Ogilvie: advisor and investigator for AbbVie; principal investigator of a clinical trial analyzed in this report. K. De Boulle: advisor, speaker, and grant/honorarium recipient for Allergan Aesthetics, an AbbVie company. Jean Carruthers: consultant and researcher for Allergan Aesthetics. Sue Ellen Cox: principal investigator, advisory board member, and consultant to AbbVie. R. Kelly: employee of Peloton Advantage, an OPEN Health company, which received funding for medical writing and editorial support for this manuscript. J. K. Garcia: employee of AbbVie. Sara Sangha: employee of AbbVie.
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor.
For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Contributor Information
Joel L. Cohen, Email: jcohenderm@yahoo.com.
Steven Fagien, Email: sfagien@aol.com.
Patricia Ogilvie, Email: Skin-concept@hotmail.de.
Koenraad De Boulle, Email: koen@doctordeboulle.be.
Jean Carruthers, Email: drjean@carruthers.net.
Sue Ellen Cox, Email: sec@aesthetic-solutions.com.
Julia K. Garcia, Email: rkelly@pelotonadvantage.com.
Sara Sangha, Email: sara.sangha@abbvie.com.
References
- 1.Jagdeo J, Keaney T, Narurkar V, Kolodziejczyk J, et al. Facial treatment preferences among aesthetically oriented men. Dermatol Surg 2016;42:1155–63. [DOI] [PubMed] [Google Scholar]
- 2.Narurkar V, Shamban A, Sissins P, Stonehouse A, et al. Facial treatment preferences in aesthetically aware women. Dermatol Surg 2015;41:S153–S60. [DOI] [PubMed] [Google Scholar]
- 3.Finn JC, Cox SE, Earl ML. Social implications of hyperfunctional facial lines. Dermatol Surg 2003;29:450–5. [DOI] [PubMed] [Google Scholar]
- 4.Cox SE, Finn JC. Social implications of hyperdynamic facial lines and patient satisfaction outcomes. Int Ophthalmol Clin 2005;45:13–24. [DOI] [PubMed] [Google Scholar]
- 5.Gupta MA, Gilchrest BA. Psychosocial aspects of aging skin. Dermatol Clin 2005;23:643–8. [DOI] [PubMed] [Google Scholar]
- 6.American Society of Plastic Surgeons. 2019 Plastic Surgery Statistics Report. American Society of Plastic Surgeons; 2020. Available at: https://www.plasticsurgery.org/documents/News/Statistics/2019/plastic-surgery-statistics-full-report-2019.pdf. Accessed July 30, 2020. [Google Scholar]
- 7.Wu WT. Botox facial slimming/facial sculpting: the role of botulinum toxin-A in the treatment of hypertrophic masseteric muscle and parotid enlargement to narrow the lower facial width. Facial Plast Surg Clin North Am 2010;18:133–40. [DOI] [PubMed] [Google Scholar]
- 8.Botox Cosmetic [Package Insert]. Dublin, Ireland: Allergan plc; 2020. [Google Scholar]
- 9.Carruthers JA, Lowe NJ, Menter MA, Gibson J, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol 2002;46:840–9. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers JD, Lowe NJ, Menter MA, Gibson J, et al. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg 2003;112:1089–98. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers A, Bruce S, de Coninck A, Connolly S, et al. Efficacy and safety of onabotulinumtoxinA for the treatment of crow's feet lines: a multicenter, randomized, controlled trial. Dermatol Surg 2014;40:1181–90. [DOI] [PubMed] [Google Scholar]
- 12.Moers-Carpi M, Carruthers J, Fagien S, Lupo M, et al. Efficacy and safety of onabotulinumtoxinA for treating crow's feet lines alone or in combination with glabellar lines: a multicenter, randomized, controlled trial. Dermatol Surg 2015;41:102–12. [DOI] [PubMed] [Google Scholar]
- 13.Fagien S, Cohen JL, Coleman W, Monheit G, et al. Forehead line treatment with onabotulinumtoxinA in subjects with forehead and glabellar facial rhytids: a phase 3 study. Dermatol Surg 2017;43:S274–S84. [DOI] [PubMed] [Google Scholar]
- 14.De Boulle KL, Werschler WP, Gold MH, Bruce S, et al. Phase 3 study of onabotulinumtoxinA distributed between frontalis, glabellar complex, and lateral canthal areas for treatment of upper facial lines. Dermatol Surg 2018;44:1437–48. [DOI] [PubMed] [Google Scholar]
- 15.Rivkin AZ, Ogilvie P, Dayan S, Yoelin SG, et al. OnabotulinumtoxinA for simultaneous treatment of upper facial lines: subject-reported satisfaction and impact from a phase 3 study. Dermatol Surg 2020;46:50–60. [DOI] [PubMed] [Google Scholar]
- 16.Ogilvie P, Rivkin AZ, Dayan S, Yoelin SG, et al. Pooled subject-reported outcomes from 2 phase 3 studies of onabotulinumtoxinA for simultaneous treatment of forehead and glabellar lines. Dermatol Surg 2020;46:950–7. [DOI] [PubMed] [Google Scholar]
- 17.Yaworsky A, Daniels S, Tully S, Beddingfield F, III, et al. The impact of upper facial lines and psychological impact of crow's feet lines: content validation of the Facial Line Outcomes (FLO-11) Questionnaire. J Cosmet Dermatol 2014;13:297–306. [DOI] [PubMed] [Google Scholar]
- 18.Pompilus F, Burgess S, Hudgens S, Banderas B, et al. Development and validation of a novel patient-reported treatment satisfaction measure for hyperfunctional facial lines: Facial Line Satisfaction Questionnaire. J Cosmet Dermatol 2015;14:274–85. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman J, Cohen JL, Peredo MI, Jonas B, et al. Clinical assessment of 2 licensed abobotulinumtoxinA injection volumes for the treatment of glabellar lines. Dermatol Surg 2019;45:1274–84. [DOI] [PubMed] [Google Scholar]


